A ten-year follow-up study of treatment outcome of craniopharyngiomas
DOI: https://doi.org/10.4414/smw.2018.14521
Lukas
Andereggena, Benjamin
Hessb, Robert H.
Andresa, Marwan
El-Koussyc, Luigi
Marianid, Andreas
Raabea, Rolf W.
Seilera, Emanuel
Christbe
aDepartment of Neurosurgery, Bern University Hospital, Inselspital, Switzerland
bDepartment of Endocrinology, Bern University Hospital, Inselspital, Switzerland
cDepartment of Neuroradiology, Bern University Hospital, Inselspital, Switzerland
dDepartment of Neurosurgery, University Hospital of Basel, Switzerland
eDepartment of Endocrinology, Diabetology and Metabolism, University Hospital of Basel, Switzerland
Summary
PURPOSE
Craniopharyngioma-related hypothalamic obesity is a devastating complication with limited data on whether long-term follow-up should focus on problems other than endocrine deficiencies and weight gain. The primary endpoint was the assessment of predictors of hypothalamic obesity development; the secondary endpoint was the assessment of functional outcome (endocrine deficiencies, visual acuity) at long-term follow-up.
METHODS
This retrospective case-note study examined craniopharyngioma patients with at least 2 years of follow-up. Clinical, radiological and biochemical characteristics were assessed at diagnosis, postoperatively, and at last follow-up.
RESULTS
Thirty-two patients met the inclusion criteria. Median follow-up period was 9.8 years (range 2.2–33 years). Longitudinal changes in body mass index (BMI) were substantial (median ΔBMI/year was +0.48 kg/m2/year, interquartile range 0.28–1.33). The prevalence of patients with hypothalamic obesity had significantly increased at last follow-up (45 vs 4%; p = 0.003). Long-term pituitary deficiencies remained high. Diabetes insipidus was common (66% vs 34%, p<0.001), with postoperative diabetes insipidus but not hypothalamic involvement, being an independent predictor for hypothalamic obesity (odds ratio 15.2, 95% confidence interval 1.3–174.8, p = 0.03). Osteodensitometry in two thirds of patients at last follow-up revealed a pathological bone density in 53% of those tested.
CONCLUSIONS
Rates of hypothalamic obesity and long-term pituitary deficiencies are substantial, with postoperative diabetes insipidus being a potential marker for hypothalamic obesity development. Besides long-term monitoring of endocrine deficiencies with consideration of osteodensitometry, early weight control programmes and continuing multidisciplinary care are mandatory in craniopharyngioma patients.
Introduction
Craniopharyngiomas are rare intracranial lesions. Although classified by the World Health Organization (WHO) as grade I tumours, they recur quite often [1]. Morbidity can be significant as a result of mass effect and tumour expansion into adjacent areas. Treatment has been associated with a high likelihood of hypothalamic-pituitary dysfunction [2]. In particular, excessive weight gain in patients with hypothalamic involvement leading to hypothalamic obesity has frequently been associated with craniopharyngioma treatment [3]. Indications for radical resections (gross total resection), with lower recurrence rates but higher risks for morbidity, have to be balanced against indications for subtotal resection to preserve hypothalamic structures. Likewise, adjuvant radiotherapy in this functional anatomic area is critical, especially in younger patients [4]. Despite careful treatment planning, craniopharyngioma patients frequently suffer from increased rates of obesity postoperatively, even following subtotal resection, which has serious consequences for long-term morbidity and mortality [5]. Despite efforts to provide treatment to reduce weight gain and thus cardiovascular diseases, hypothalamic obesity has been shown to be less responsive to any weight control programmes than common obesity [6]. There is currently limited data on whether the long-term follow-up should be focused differently, on specific problems other than endocrine deficiencies and weight gain affecting late morbidity in these patients.
The present study aimed at investigating the surgical long-term outcome, seeking for predictors of hypothalamic obesity development (primary endpoint). The secondary endpoint was the assessment of functional outcome (endocrine deficiencies, visual acuity) at long-term follow-up.
Subjects, material and methods
This study was approved by the Ethics Committee of Bern, Switzerland, and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Patient selection
In this retrospective case-note study, we evaluated the longitudinal medical data records of all patients who were operated on for a histologically confirmed craniopharyngioma. The minimum follow-up period required, in order to document longitudinal changes, was 24 months. All patients were treated at our institution (single-centre study) between 1980 and 2011. Patients’ demographic, endocrinological, neurological and ophthalmological characteristics preoperatively, postoperatively and at last follow-up were analysed.
Assessment of body mass index
Body mass index (BMI) z-scores, or BMI standard deviation scores (SDS), were taken into account in patients with childhood onset (<18 years of age) to calculate the relative weight adjustment according to the child’s age and sex [7]. Patients with a z-score >2 SDS were considered to be suffering from obesity. Standard BMI was calculated for adult patients (adult-onset, at ≥18 years of age). Thereby, patients were categorised according to BMI as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), or obese (>30 kg/m2).
Assessment of hypothalamic obesity and hypothalamic involvement
Craniopharyngioma invasion of hypothalamic structures (hypothalamic involvement) and/or treatment-related lesions of the hypothalamus were assessed by means of magnetic resonance imaging (MRI) of the brain [3]. Hypothalamic obesity was defined as significant obesity and postsurgical weight gain in craniopharyngioma patients with hypothalamic involvement or with postsurgical hypothalamic damage [8].
Endocrinological assessment
The diagnosis of hypothyroidism was based on the presence of low-normal thyroid stimulating hormone (TSH) levels in parallel with low free thyroxine levels (FT4). Secondary adrenal insufficiency was assumed in the presence of low fasting cortisol levels or an inappropriate response of cortisol to adrenocorticotrophic hormone (ACTH), or insulin-induced hypoglycaemia. A gonadotrophin deficiency or central hypogonadism was considered when normal to low levels of gonadotrophins in the presence of low serum testosterone/oestradiol concentrations were found in adult-onset patients. In patients with childhood-onset tumours, prepubertal gonadotrophin deficiency was defined as the necessity for hormonal induction of puberty recorded during follow-up.
Growth hormone deficiency was defined as impaired growth rate in children and low age-adjusted insulin-like growth factor 1 levels (in children and adults) in parallel with impaired function of three additional pituitary hormonal axes. Alternatively, an insulin tolerance test was performed to diagnose growth hormone deficiency.
Visual assessment
Assessment of visual function included documentation of visual acuity using the Snellen chart and visual field assessment with the Goldmann manual perimeters.
Assessment of bone health status
Bone mineral density was assessed at last follow-up with dual-energy X-ray absorptiometry (Hologic QDR 4500A®, Bedford, MA, USA) of the femur, head and/or neck, tibia and spine. According to the WHO classification, a T-score ≥−1 SD was regarded as normal, whereas a T-score of −1 to −2.5 SDs indicated osteopenia, and of <−2.5 SDs osteoporosis. Pathological bone density was considered to be present if one of the aforementioned bones showed a pathological T-score.
Assessment of recurrence
Craniopharyngioma recurrence was defined as the postoperative presence of a radiographically detectable tumour after gross total resection and/or growth of the tumour seen on subsequent radiological follow-up scans if a residual tumour (i.e., subtotal resection) was present during the initial postoperative imaging studies, as reported previously [9].
Statistical analysis
Data were analysed with IBM SPSS statistical software Version 21.0 (IBM Corp., New York, NY, USA) and GraphPad Prism Version 6.03 (GraphPad Software, San Diego, CA, USA). Continuous variables were examined for homogeneity of variance and are expressed as mean ± standard error of the mean (SEM) unless otherwise noted. For comparisons of means between two groups, Student’s t-test was used for normally distributed data, and the Mann–Whitney test for nonparametric data. Odds ratios (ORs) and 95% confidence intervals (CIs) of independent factors associated with hypothalamic obesity at the last follow-up were analysed with univariate and multivariate logistic regression. The variables tested were: age at diagnosis, sex, hypothalamic involvement, histology (i.e., adamantinomatous), hyperprolactinaemia, and diabetes insipidus. The multivariable logistic regression analysis included all dependent risk factors in the univariable regression with a p-value ≤0.3 [10, 11]. Categorical variables were compared by use of Pearson’s chi-square test or Fisher’s exact test, as appropriate. Differences were considered significant when p ≤0.05 and reported p-values are two-sided.
Results
Patients’ characteristics at baseline
Long-term follow-up (≥24 months) was recorded for 32 patients (16 female and 16 male) with histologically proven craniopharyngioma. The median follow-up period was 117 months (range 26–398 months). Hypothalamic involvement was noted in 25% of all patients with craniopharyngioma. Baseline characteristics were not significantly different between patients with and without hypothalamic involvement (table 1). The median age at diagnosis was 33.6 years (rang 3–70 years). Adynamia was noted in 44% of all patients and headache in 46%, with no differences between patients with and without hypothalamic involvement. Visual acuity and visual field deficits were noted in 44% and 59% patients, respectively. Hormonal deficiencies were present in the majority of patients, with gonadotrophin (in 48% patients with adult onset vs 86% patients with childhood onset) and secondary adrenal deficiencies (in 31% of all patients) being the most frequent.
Table 1 Baseline characteristics of patients with and without hypothalamic involvement.
|
No HI
n (%)
|
HI
n (%)
|
All patients
n (%)
|
| Sex |
|
|
|
| Female |
10 (42) |
6 (75) |
16 (50) |
| Male |
14 (58) |
2 (25) |
16 (50) |
| Age at diagnosis in years, median (range) |
36.1 (6–70) |
33.7 (2–61) |
33.6 (3–70) |
| Tumour site |
|
|
|
| Intrasellar |
2 (9) |
0 (0) |
2 (7) |
| Extrasellar |
21 (91) |
8 (100) |
29 (93) |
| Hydrocephalus |
3 (13) |
2 (25) |
5 (16) |
| Primary surgical approach |
|
|
|
| Transcranial |
22 (92) |
7 (88) |
29 (91) |
| Transnasal |
2 (8) |
1 (12) |
3 (9) |
| Extent of resection |
|
|
|
| Subtotal resection |
17 (74) |
6 (75) |
23 (74) |
| Gross total resection |
6 (26) |
2 (25) |
8 (26) |
| Adjuvant radiotherapy |
11 (46) |
4 (50) |
15 (47) |
| Pathological finding |
|
|
|
| Adamantinomatous |
16 (67) |
5 (63) |
21 (66) |
| Papillary |
8 (25) |
3 (9) |
11 (34) |
| Clinical symptoms |
|
|
|
| Adynamia |
9 (38) |
5 (63) |
14 (44) |
| Headache |
9 (50) |
2 (33) |
11 (46) |
| Visual acuity deficits |
11 (46) |
3 (38) |
14 (44) |
| Visual field deficits |
15 (63) |
5 (63) |
20 (63) |
| Affected pituitary axes |
|
|
|
| GH insufficiency |
2 (8) |
1 (13) |
3 (9) |
Gonadotrophin deficiency
Adult onset
Childhood onset |
9 (38)
8 (44)
5 (83) |
4 (50)
4 (57)
1 (100) |
13 (41)
12 (48)
6 (86) |
| Secondary adrenal insufficiency |
7 (29) |
3 (38) |
10 (32) |
| Secondary hypothyroidism |
6 (25) |
2 (25) |
8 (25) |
| Hyperprolactinaemia |
5 (21) |
3 (38) |
8 (25) |
| Diabetes insipidus |
3 (13) |
1 (13) |
4 (13) |
| Follow-up in years, median (range) |
102.5 (26–398) |
157.5 (42–272) |
117 (26–398) |
Tumour location and surgical/radio-oncological management
A solely intrasellar location was noted in two (9%) patients who had craniopharyngioma without hypothalamic involvement and in none of the patients with hypothalamic involvement (p = 0.15). Occlusive hydrocephalus due to tumour extension was noted in two (25%) patients with hypothalamic involvement versus three (13%) patients without hypothalamic involvement.
Gross total resection was attained in 26% of patients. None of the patients were referred for proton therapy. Instead, those assigned to adjuvant radiation therapy (i.e., patients with subtotal resection) were treated with photon therapy. Overall, nine (29%) patients received fractionated radiotherapy and five (16%) patients LINAC-stereotactic radiosurgery on. One patient received intracavitary irradiation with locally implanted beta-emitting radioisotope yttrium-90 (90Y) silicate colloid. There was no difference in the adjuvant therapy following craniopharyngioma treatment between patients treated via the transcranial (45%) or transnasal (33%) approaches (p = 0.99). There was no difference in the extent of craniopharyngioma resection between patients with or without hypothalamic involvement (p = 0.99). Median cumulative doses of radiation were 54 Gy (range 9–56 Gy; on average 2 Gy in 25 fractions).
Histological results
Of the patients with a childhood-onset tumour, six (86%) presented with an adamantinomatous and one (14%) with a papillary craniopharyngioma. In the adult-onset cohort, 14 patients (58%) were diagnosed with an adamantinomatous and 10 (42%) with a papillary craniopharyngioma. Histological diagnosis was not a predictor for long-term hypothalamic obesity development (OR 1.4, 95% CI 0.3–6.4; p = 0.71, table 2).
Table 2 Predictors for long-term hypothalamic obesity development.
|
Risk factors for long-term HO (BMI >30 kg/m2)
|
Univariable analysis
OR (95% CI)
|
p-value
|
Multivariable analyses
OR (95% CI)
|
p-value
|
| Age |
1.0 (0.9–1.0) |
0.29
|
1.0 (0.9–1.1) |
0.34
|
| Follow-up period |
1.0 (0.9–1.0) |
0.73 |
|
|
| Sex (i.e., women) |
2.7 (0.6–12.0) |
0.20
|
5.3 (0.4–67.6) |
0.20
|
| Hypothalamic involvement |
2.7 (0.5–14.5) |
0.25
|
3.6 (0.5–26.6) |
0.22
|
| GTR |
1.3 (0.3–6.5) |
0.78 |
|
|
| Adjuvant radiotherapy |
1.5 (0.3–6.5) |
0.59 |
|
|
| Histology (adamantinomatous) |
1.4 (0.3–6.4) |
0.71 |
|
|
| Postoperative hyperprolactinaemia |
2.9 (0.6–13.5) |
0.18
|
3.2 (0.4–22.8) |
0.26
|
| Postoperative diabetes insipidus |
5.1 (1.0–26.7) |
0.06
|
20.5 (1.5–284.2) |
0.02
|
Postoperative complications
No perioperative mortality was recorded. Surgical complications included cerebrospinal fluid leak in two patients (6%; one patient following the transcranial and one following the transnasal approach), epileptic seizure in one (3%) patient treated via the transcranial approach, peripheral facial nerve paralysis in one (3%) treated with transcranial surgery, and anosmia in three (9%) patients treated with transcranial surgery.
Weight control
Changes in weight gain and the BMI categories (normal, overweight, obese) are depicted in fig. 1. A significant increase in BMI was recorded at long-term follow-up (from 23.1 ± 4.9 to 28.4 ± 4.9 kg/m2; p <0.001, fig. 1A). There was, furthermore, a significant increase in the BMI of patients with hypothalamic involvement (28.9 vs 23.5 kg/m2 at baseline, p <0.001). At diagnosis, 14% of patients with a childhood-onset craniopharyngioma presented with a z-score >2 SDS. Of the patients with an adult-onset craniopharyngioma, 43% were overweight and 3% obese. The proportion of patients with a normal BMI was significantly higher at baseline (53%) compared with last follow-up (24%; p = 0.03); the prevalence of hypothalamic obesity had significantly increased at last follow-up (45 vs 4%; p = 0.003, fig. 1B). At long-term follow-up, overweight (BMI 25–30 kg/m2) was noted in 9 patients (31%) and obesity (BMI ≥ 30 kg/m2) in 13 (45%). Severe obesity (BMI >35 kg/m2) was noted in two (6%) patients.
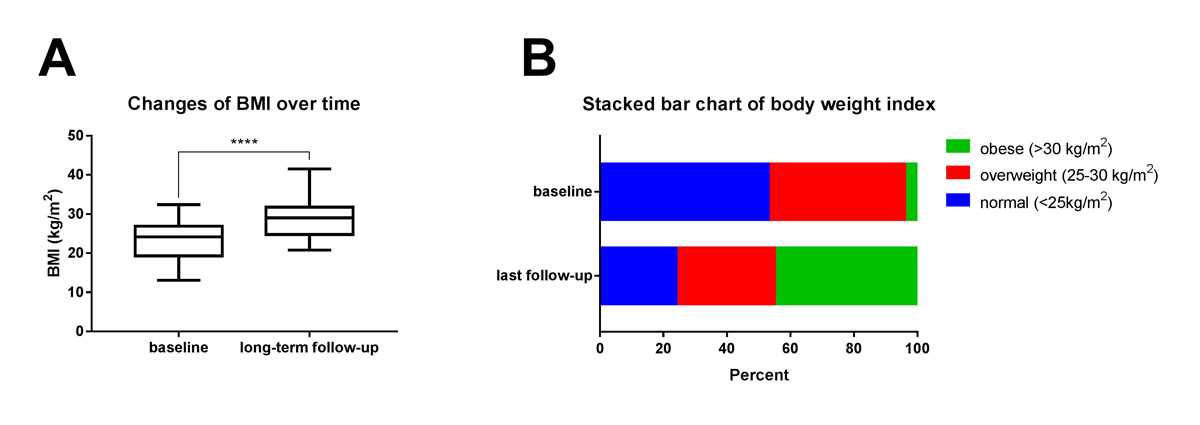
Figure 1 Stacked bar chart of patients’ BMI at baseline and long-term follow-up and changes in BMI over the study period. (A) There was a significant increase in patients’ BMI at long-term follow-up (from 23.1 ± 4.9 to 28.4 ± 4.9 kg/m2; p < 0.001). (B) At baseline, there was a significantly higher prevalence of patients with a normal BMI (53%) than at last follow-up (24%; p = 0.03). In contrast, at last follow-up, the prevalence of hypothalamic obesity significantly increased (45 vs 4%; p = 0.003).
The median ΔBMI/year was +0.48 kg/m2/year, interquartile range (IQR) 0.28–1.33 kg/m2/year. There was no difference in median ΔBMI/year between gross total and subtotal resection (+0.46 kg/m2/year, IQR 0.25–1.43 vs +0.53 kg/m2/year, IQR 0.31–1.2; p = 0.31).
In patients treated with adjuvant radiotherapy, the median ΔBMI/year was +0.73 kg/m2/year (IQR 0.37–3.14) compared with +0.45 kg/m2/year (IQR 0.18–0.96) in those who did not undergo radiotherapy (p = 0.12).
Visual outcome
Visual field and acuity deficits improved after surgery, although not statistically significantly (table 3). Eighteen (56%) patients with craniopharyngioma showed visual field deficits at diagnosis, and one of them improved following surgery. In contrast, 14 (44%) patients showed visual acuity deficits at baseline, and 9 (28%) of them showed persisting deficits at last follow-up (p = 0.29).
Table 3 Long-term functional and endocrine outcomes.
|
Baseline
n (%)
|
Long-term follow-up
n (%)
|
p-value
|
| Clinical presentation, |
|
|
|
| Visual field deficits |
19 (59) |
18 (56) |
0.99 |
| Visual acuity deficits |
14 (44) |
9 (28) |
0.29 |
| Affected pituitary axes |
|
|
|
| Growth hormone insufficiency |
3 (9) |
20 (63) |
<0.001 |
| Gonadotropin deficiency |
13 (41) |
28 (88) |
<0.001 |
| Secondary adrenal insufficiency |
10 (31) |
27 (84) |
<0.001 |
| Secondary hypothyroidism |
8 (25) |
26 (81) |
<0.001 |
| Hyperprolactinaemia |
8 (25) |
13 (41) |
0.29 |
| Diabetes insipidus |
4 (13) |
21 (66) |
<0.001 |
Endocrinological outcome
Hormonal deficiencies at diagnosis and last follow-up are summarised in table 3. Long-term deficits were significantly increased compared with preoperative values, notably in the growth hormone axis (63 vs 9%, p <0.001), ACTH axis (84 vs 31%, p <0.001), TSH axis (81 vs 25%, p <0.001) and diabetes insipidus (66% vs 13%, p <0.001), but not hyperprolactinaemia (41 vs 25%, p = 0.29). The cumulative pituitary dysfunctions – the mean sum score (%) of hormonal axes deficiencies – are depicted in figure 2. It reflects the sum of hormonal axes deficits (%), including growth hormone and secondary adrenal insufficiency, hypothyroidism, gonadotrophin deficiency or central hypogonadism, and hyperprolactinaemia preoperatively, postoperatively (3 months later) and at last follow-up. There was a significant increase in cumulative pituitary axes deficits compared with preoperative values (p <0.001, fig. 2A). Long-term deficits of hormonal axes were not significantly different from postoperative deficiencies (p = 0.62). Long-term endocrine deficiencies remained high and were more severe in male than female patients (p = 0.05, fig. 2B).
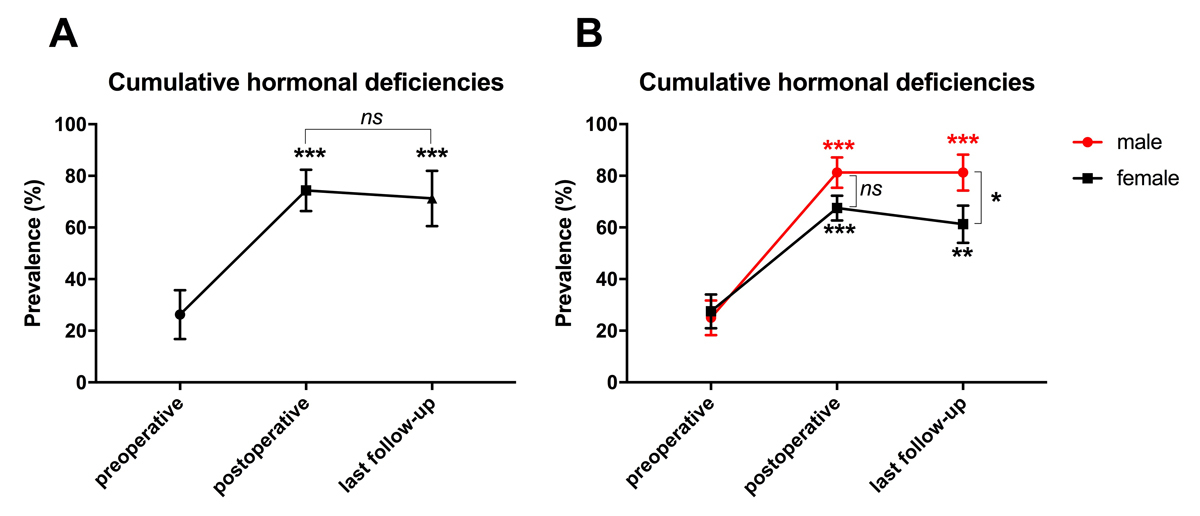
Figure 2 Cumulative pituitary deficiencies over the study period. (A) There was a significant increase in the cumulative pituitary axes deficits compared with baseline (p <0.001) or postoperative values (p <0.001). Long-term deficits in hormonal axes were not significantly different from postoperative deficiencies (p = 0.62). (B) Long-term endocrine deficiencies remained high and were more severe in male than female patients (p = 0.05)
ns = not significant, * p < 0.05, ** p < 0.01, *** p < 0.001
Bone health
At the last follow-up, osteodensitometry was performed in 19 patients (59%), revealing a pathological bone density in 53% of them, namely in one 39-year-old patient who had had childhood-onset craniopharyngioma and in 50% of the patients with adult-onset craniopharyngioma, at a median age of 52 years (range 30–65 years).
Recurrences, tumour growth and disability
None of the craniopharyngiomas treated with gross total resection relapsed or grew, whereas in seven (30%) patients with subtotal resection the tumour recurred (p = 0.15). There was no significant difference in the recurrence rate according to the surgical approach: six (21%) patients undergoing transcranial surgery versus one patient (33%) undergoing transnasal surgery (p = 0.54). At last follow-up, four (21%) women and one (5%) man were claiming disability insurance.
Discussion
The main findings of this study can be summarised as follows. After a median follow-up period of 10 years: (1) a high prevalence of hypothalamic obesity was recorded, with postoperative diabetes insipidus being a potential marker for hypothalamic obesity development; (2) long-term endocrine deficiencies remained significantly high; (3) visual deficits improved, although not significantly; and (4) osteodensitometry revealed impaired bone density in a substantial number of patients.
Hypothalamic obesity
We observed a significant increase in patients’ BMI at long-term follow-up, particularly in those with hypothalamic involvement. An increase in the percentage of patients suffering from hypothalamic obesity over time is an established phenomenon [12]. An increase in patients’ BMI over the long term might partly be related to the normal age-associated increase in body weight [13], but obesity has been shown to be the most common manifestation of hypothalamic damage, with hypothalamic involvement, in turn, being a long-term predictor for hypothalamic obesity development in patients with craniopharyngiomas [14]. Although early preventive measures for weight control have been shown to be beneficial, they are difficult to implement [15]. A recent systematic review and meta-analysis of craniopharyngioma patients with obesity showed that bariatric surgery induced weight loss at 1-year follow-up, but the impact was far less significant than in patients without craniopharyngiomas [15], corroborating the finding that obesity associated with craniopharyngioma is different from primary obesity.
Interestingly, weight gain did not differ between patients treated with subtotal or gross total resection, contrary to previous findings indicating that a more conservative approach reduces the risk of hypothalamic obesity [16]. The relatively low number of patients in our study may account for these controversial findings. Conversely, in patients treated with adjuvant radiotherapy, the average weight gain was higher than in those not treated with radiotherapy. This is in accordance with previous reports that additional high-dose radiotherapy (51–72 Gy) was a risk factor for hypothalamic obesity [17]. The relatively small number of patients studied may explain the lack of statistical significance.
The postoperative occurrence of diabetes insipidus was a significant and independent risk factor for long-term development of hypothalamic obesity, but not hypothalamic involvement. This is an interesting and intriguing finding, which was recently reported by Roth et al. [3]. It can be speculated that diabetes insipidus is a marker for the severity of disease. Given the high prevalence, with about half of all patients (45%) developing hypothalamic obesity at last follow-up (in line with prior studies [18]), the postoperative presence of diabetes insipidus might become an easy and inexpensive marker to assess patients’ risk for hypothalamic obesity development. This in turn would identify patients for whom closer monitoring and potentially aggressive intervention aiming at weight control is warranted.
Endocrine deficiencies
No reversal of pre-existing pituitary hormone deficiencies was noted, which is consistent with previous findings [19]. Van Effenterre noted that most patients with craniopharyngioma presented with insufficient pituitary axes over the study period [20]. Persistence of hypopituitarism might be due to irreversible tumour damage of the hypothalamic-pituitary system in cases of surrounding tissue infiltration, or a consequence of surgery, radiation therapy or tumour recurrence. As shown by Tomlinson et al., patients with hypopituitarism have an excess mortality, with craniopharyngiomas an independent risk factor [21]. However, specific endocrine axis deficiencies, with the exception of untreated gonadotrophin deficiency, did not appreciably worsen the prognosis [21]. In our cohort, the extent of the surgical approach (subtotal or gross total resection) did not result in different outcomes with regard to hormonal deficiencies. Although hypopituitarism is almost universally reported with any approach for treating craniopharyngiomas, a transnasal approach may lead to less morbidity and hormonal deficiency in selected cases [22].
Visual deficits
Preoperative and long-term visual acuity and visual field deficits improved following treatment, although not significantly. This is in contrast to a report by Lee et al., in which preoperative visual field deficits were associated with worse postoperative visual deficits [23]. Recently, Drimtzias and co-workers assessed the natural history and visual outcome of 20 patients with craniopharyngiomas, predominantly treated with subtotal resection. In their study, preoperative visual field deficits and type of surgery were not prognostic indicators for postoperative visual acuity and visual field deficits. Interestingly, type of surgery and preoperative visual field deficits were not associated with craniopharyngioma recurrence, so that monitoring for recurrence of craniopharyngioma with MRI rather than by means of visual field assessment was recommended [24].
Bone health status
Fifty-three percent of patients who underwent osteodensitometry had a pathological bone density at last follow-up. This may reflect normal age-related bone loss. However, tumour-induced hypogonadism has been shown to contribute more than patients’ age to the high prevalence of pathological bone density [25]. In adults with childhood onset craniopharyngiomas, bone mineral density was lower than in healthy control subjects and discontinuation of growth hormone therapy before peak bone mass was achieved is a potential cause for this phenomenon [26]. Patients with hypopituitarism, in particular those with gonadotrophin and growth hormone deficiency, suffer from early bone loss because of their inability to build up their peak bone mass in the first three decades of life [27]. Overweight may protect against bone loss, probably through the increase in fat mass with the action of augmented leptin levels and peripheral conversion of androgens to oestradiol [28]. However, it is conceivable that hypothalamic structures might become insensitive, in particular to the increased levels of endogenous leptin in craniopharyngioma patients [29], with leptin resistance in adolescents being negatively correlated to bone mineral density [30]. The relationship between leptin and bone mineral density is complex, with different effects depending on whether central or peripheral mechanisms are operating [31].
Extent of tumour resection and recurrence rate
None of the craniopharyngiomas recurred after gross total resection compared with 17% following subtotal resection. In addition, recurrence and growth rate were independent of the surgical approach (transnasal vs transcranial). Currently, the optimal surgical approach for craniopharyngiomas is still a matter of debate. Whereas some authors suggest gross total resection only if hypothalamic morbidity is acceptably low [32], others support a primary subtotal resection accompanied by radiotherapy [33]. Excellent rates of disease control with a favourable functional outcome have been reported when radical resection was attempted using a standard or extended transnasal approach [22]. However, even at highly experienced centres, gross total resection can result in hypothalamic disorders with significant morbidities. In our cohort, adjuvant radiotherapy followed subtotal resection in 14 (61%) patients. As a result of progress made in supportive care, radiation therapy and hormone replacement, less invasive approaches have resulted in an improved quality of life [34]. This has led to less radical surgical approaches, with reduced rates of hypothalamic lesions and thus reduced hypothalamic obesity development, being proposed [35]. In the case of subtotal resection of craniopharyngiomas, close follow-up in a referral centre remains indispensable so that adjuvant fractionated radiotherapy, stereotactic radiosurgery or re-operation can be promptly instigated. As mentioned above, monitoring for recurrence of craniopharyngiomas by use of MRI rather than by visual acuity or field assessment was recommended owing to poor correlation of recurrence with the latter [24]. Markers of recurrence have been difficult to identify, with symptoms of intracranial hypertension being the most promising predictor [36]. The recent detection of frequent and clonal mutations in β-catenin (cadherin-associated protein; CTNNB1) in adamantinomatous craniopharyngiomas and the B-RAF proto-oncogene serine/threonine-protein kinase (BRAF) in papillary craniopharyngiomas show promise for testing molecularly guided therapeutics in the treatment of these neoplasms [37].
In conclusion, rates of hypothalamic obesity and long-term endocrine deficiencies in patients with craniopharyngiomas are substantial, with postoperative diabetes insipidus being a potential marker for hypothalamic obesity development. Besides long-term monitoring of endocrine deficiencies, with consideration of osteodensitometry, early weight control programmes and continuing multidisciplinary care are mandatory in all patients suffering from this chronic disease.
Study limitations
This study suffers from the limitations of any retrospective study, the small number of patients, and the single-centre design. Furthermore, this study spans a mean observation period of ≥10 years during which there have been significant advances in neurosurgical techniques and materials, perioperative care and hormone replacement therapy, radiotherapy and radiosurgery, which may affect outcome. The number of patients is relatively small and assessment of multiple independent predictors for hypothalamic obesity development using multiple logistic regressions was thus limited. Statistical uncertainty in this small sample size precluded us from discriminating outcome parameters between childhood- and adult-onset patients. Likewise, the small sample size precluded us from performing any subanalysis in those treated with radiosurgery only. The radiological assessment of hypothalamic involvement, including the degree of surgical resection (gross total or subtotal resection), was not subjected to a blinded review by two independent specialists, but rather was based on the reported findings recorded during the observational period.
Acknowledgment
We are grateful to Professor Rolf W. Seiler for granting access to his personal operative records. The assistance of Ms. Susan Kaplan in editing the manuscript is acknowledged.
References
1
Hussain
I
,
Eloy
JA
,
Carmel
PW
,
Liu
JK
. Molecular oncogenesis of craniopharyngioma: current and future strategies for the development of targeted therapies. J Neurosurg. 2013;119(1):106–12. doi:.https://doi.org/10.3171/2013.3.JNS122214
2
Mortini
P
,
Gagliardi
F
,
Bailo
M
,
Spina
A
,
Parlangeli
A
,
Falini
A
, et al.
Magnetic resonance imaging as predictor of functional outcome in craniopharyngiomas. Endocrine. 2016;51(1):148–62. doi:.https://doi.org/10.1007/s12020-015-0683-x
3
Roth
CL
,
Eslamy
H
,
Werny
D
,
Elfers
C
,
Shaffer
ML
,
Pihoker
C
, et al.
Semiquantitative analysis of hypothalamic damage on MRI predicts risk for hypothalamic obesity. Obesity (Silver Spring). 2015;23(6):1226–33. doi:.https://doi.org/10.1002/oby.21067
4
Clark
AJ
,
Cage
TA
,
Aranda
D
,
Parsa
AT
,
Auguste
KI
,
Gupta
N
. Treatment-related morbidity and the management of pediatric craniopharyngioma: a systematic review. J Neurosurg Pediatr. 2012;10(4):293–301. doi:.https://doi.org/10.3171/2012.7.PEDS11436
5
Heymsfield
SB
,
Avena
NM
,
Baier
L
,
Brantley
P
,
Bray
GA
,
Burnett
LC
, et al.
Hyperphagia: current concepts and future directions proceedings of the 2nd international conference on hyperphagia. Obesity (Silver Spring). 2014;22(S1, Suppl 1):S1–17. doi:.https://doi.org/10.1002/oby.20646
6
Wijnen
M
,
Olsson
DS
,
van den Heuvel-Eibrink
MM
,
Wallenius
V
,
Janssen
JA
,
Delhanty
PJ
, et al.
Efficacy and safety of bariatric surgery for craniopharyngioma-related hypothalamic obesity: a matched case-control study with 2 years of follow-up. Int J Obes. 2017;41(2):210–6. doi:.https://doi.org/10.1038/ijo.2016.195
7
Must
A
,
Anderson
SE
. Body mass index in children and adolescents: considerations for population-based applications. Int J Obes. 2006;30(4):590–4. doi:.https://doi.org/10.1038/sj.ijo.0803300
8
Müller
HL
. Craniopharyngioma and hypothalamic injury: latest insights into consequent eating disorders and obesity. Curr Opin Endocrinol Diabetes Obes. 2016;23(1):81–9. doi:.https://doi.org/10.1097/MED.0000000000000214
9
Weiner
HL
,
Wisoff
JH
,
Rosenberg
ME
,
Kupersmith
MJ
,
Cohen
H
,
Zagzag
D
, et al.
Craniopharyngiomas: a clinicopathological analysis of factors predictive of recurrence and functional outcome. Neurosurgery. 1994;35(6):1001–10, discussion 1010–1. doi:.https://doi.org/10.1227/00006123-199412000-00001
10
Bursac
Z
,
Gauss
CH
,
Williams
DK
,
Hosmer
DW
. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3(1):17. doi:.https://doi.org/10.1186/1751-0473-3-17
11
Mickey
RM
,
Greenland
S
. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–37. doi:.https://doi.org/10.1093/oxfordjournals.aje.a115101
12
Khan
MJ
,
Humayun
KN
,
Donaldson
M
,
Ahmed
SF
,
Shaikh
MG
. Longitudinal changes in body mass index in children with craniopharyngioma. Horm Res Paediatr. 2014;82(6):372–9. doi:.https://doi.org/10.1159/000368798
13
Andereggen
L
,
Frey
J
,
Andres
RH
,
El-Koussy
M
,
Beck
J
,
Seiler
RW
, et al.
Long-term follow-up of primary medical versus surgical treatment of prolactinomas in men: Effects on hyperprolactinemia, hypogonadism and bone health. World Neurosurg. 2017;97:595–602. doi:.https://doi.org/10.1016/j.wneu.2016.10.059
14
Müller
HL
,
Gebhardt
U
,
Teske
C
,
Faldum
A
,
Zwiener
I
,
Warmuth-Metz
M
, et al.; Study Committee of KRANIOPHARYNGEOM 2000. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. Eur J Endocrinol. 2011;165(1):17–24. doi:.https://doi.org/10.1530/EJE-11-0158
15
Bretault
M
,
Boillot
A
,
Muzard
L
,
Poitou
C
,
Oppert
JM
,
Barsamian
C
, et al.
Clinical review: Bariatric surgery following treatment for craniopharyngioma: a systematic review and individual-level data meta-analysis. J Clin Endocrinol Metab. 2013;98(6):2239–46. doi:.https://doi.org/10.1210/jc.2012-4184
16
Elowe-Gruau
E
,
Beltrand
J
,
Brauner
R
,
Pinto
G
,
Samara-Boustani
D
,
Thalassinos
C
, et al.
Childhood craniopharyngioma: hypothalamus-sparing surgery decreases the risk of obesity. J Clin Endocrinol Metab. 2013;98(6):2376–82. doi:.https://doi.org/10.1210/jc.2012-3928
17
Lustig
RH
,
Post
SR
,
Srivannaboon
K
,
Rose
SR
,
Danish
RK
,
Burghen
GA
, et al.
Risk factors for the development of obesity in children surviving brain tumors. J Clin Endocrinol Metab. 2003;88(2):611–6. doi:.https://doi.org/10.1210/jc.2002-021180
18
Sterkenburg
AS
,
Hoffmann
A
,
Gebhardt
U
,
Warmuth-Metz
M
,
Daubenbüchel
AM
,
Müller
HL
. Survival, hypothalamic obesity, and neuropsychological/psychosocial status after childhood-onset craniopharyngioma: newly reported long-term outcomes. Neuro-oncol. 2015;17(7):1029–38. doi:.https://doi.org/10.1093/neuonc/nov044
19
Karavitaki
N
,
Brufani
C
,
Warner
JT
,
Adams
CB
,
Richards
P
,
Ansorge
O
, et al.
Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol (Oxf). 2005;62(4):397–409. doi:.https://doi.org/10.1111/j.1365-2265.2005.02231.x
20
Van Effenterre
R
,
Boch
AL
. Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg. 2002;97(1):3–11. doi:.https://doi.org/10.3171/jns.2002.97.1.0003
21
Tomlinson
JW
,
Holden
N
,
Hills
RK
,
Wheatley
K
,
Clayton
RN
,
Bates
AS
, et al.; West Midlands Prospective Hypopituitary Study Group. Association between premature mortality and hypopituitarism. Lancet. 2001;357(9254):425–31. doi:.https://doi.org/10.1016/S0140-6736(00)04006-X
22
Patel
KS
,
Raza
SM
,
McCoul
ED
,
Patrona
A
,
Greenfield
JP
,
Souweidane
MM
, et al.
Long-term quality of life after endonasal endoscopic resection of adult craniopharyngiomas. J Neurosurg. 2015;123(3):571–80. doi:.https://doi.org/10.3171/2014.12.JNS141591
23
Lee
MJ
,
Hwang
JM
. Initial visual field as a predictor of recurrence and postoperative visual outcome in children with craniopharyngioma. J Pediatr Ophthalmol Strabismus. 2012;49(1):38–42. doi:.https://doi.org/10.3928/01913913-20110208-03
24
Drimtzias
E
,
Falzon
K
,
Picton
S
,
Jeeva
I
,
Guy
D
,
Nelson
O
, et al.
The ophthalmic natural history of paediatric craniopharyngioma: a long-term review. J Neurooncol. 2014;120(3):651–6. doi:.https://doi.org/10.1007/s11060-014-1600-5
25
Kyvernitakis
I
,
Saeger
U
,
Ziller
V
,
Bauer
T
,
Seker-Pektas
B
,
Hadji
P
. The effect of age, sex hormones, and bone turnover markers on calcaneal quantitative ultrasonometry in healthy German men. J Clin Densitom. 2013;16(3):320–8. doi:.https://doi.org/10.1016/j.jocd.2013.01.009
26
Boot
AM
,
van der Sluis
IM
,
Krenning
EP
,
de Muinck Keizer-Schrama
SM
. Bone mineral density and body composition in adolescents with childhood-onset growth hormone deficiency. Horm Res. 2009;71(6):364–71.
27
Shalet
SM
,
Shavrikova
E
,
Cromer
M
,
Child
CJ
,
Keller
E
,
Zapletalová
J
, et al.
Effect of growth hormone (GH) treatment on bone in postpubertal GH-deficient patients: a 2-year randomized, controlled, dose-ranging study. J Clin Endocrinol Metab. 2003;88(9):4124–9. doi:.https://doi.org/10.1210/jc.2003-030126
28
Hamrick
MW
,
Ferrari
SL
. Leptin and the sympathetic connection of fat to bone. Osteoporos Int. 2008;19(7):905–12. doi:.https://doi.org/10.1007/s00198-007-0487-9
29
Roth
C
,
Wilken
B
,
Hanefeld
F
,
Schröter
W
,
Leonhardt
U
. Hyperphagia in children with craniopharyngioma is associated with hyperleptinaemia and a failure in the downregulation of appetite. Eur J Endocrinol. 1998;138(1):89–91. doi:.https://doi.org/10.1530/eje.0.1380089
30
do Prado
WL
,
de Piano
A
,
Lazaretti-Castro
M
,
de Mello
MT
,
Stella
SG
,
Tufik
S
, et al.
Relationship between bone mineral density, leptin and insulin concentration in Brazilian obese adolescents. J Bone Miner Metab. 2009;27(5):613–9. doi:.https://doi.org/10.1007/s00774-009-0082-6
31
Fu
L
,
Patel
MS
,
Bradley
A
,
Wagner
EF
,
Karsenty
G
. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122(5):803–15. doi:.https://doi.org/10.1016/j.cell.2005.06.028
32
Albright
AL
,
Hadjipanayis
CG
,
Lunsford
LD
,
Kondziolka
D
,
Pollack
IF
,
Adelson
PD
. Individualized treatment of pediatric craniopharyngiomas. Childs Nerv Syst. 2005;21(8-9):649–54. doi:.https://doi.org/10.1007/s00381-005-1185-6
33
Puget
S
,
Garnett
M
,
Wray
A
,
Grill
J
,
Habrand
JL
,
Bodaert
N
, et al.
Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg. 2007;106(1, Suppl):3–12.
34
Ali
ZS
,
Bailey
RL
,
Daniels
LB
,
Vakhshori
V
,
Lewis
DJ
,
Hossain
AT
, et al.
Comparative effectiveness of treatment options for pediatric craniopharyngiomas. J Neurosurg Pediatr. 2014;13(2):178–88. doi:.https://doi.org/10.3171/2013.11.PEDS1320
35
Hoffmann
A
,
Warmth-Metz
M
,
Gebhardt
U
,
Pietsch
T
,
Pohl
F
,
Kortmann
RD
, et al.
Childhood craniopharyngioma - changes of treatment strategies in the trials KRANIOPHARYNGEOM 2000/2007. Klin Padiatr. 2014;226(3):161–8. doi:.https://doi.org/10.1055/s-0034-1368785
36
Gautier
A
,
Godbout
A
,
Grosheny
C
,
Tejedor
I
,
Coudert
M
,
Courtillot
C
, et al.; Craniopharyngioma Study Group. Markers of recurrence and long-term morbidity in craniopharyngioma: a systematic analysis of 171 patients. J Clin Endocrinol Metab. 2012;97(4):1258–67. doi:.https://doi.org/10.1210/jc.2011-2817
37
Brastianos
PK
,
Taylor-Weiner
A
,
Manley
PE
,
Jones
RT
,
Dias-Santagata
D
,
Thorner
AR
, et al.
Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet. 2014;46(2):161–5. doi:.https://doi.org/10.1038/ng.2868

