Comparison of ESSDAI and ClinESSDAI in potential optimisation of trial outcomes in primary Sjögren’s syndrome: examination of data from the UK Primary Sjögren’s Syndrome Registry
DOI: https://doi.org/10.4414/smw.2018.14588
Alexandre
Dumuscab, Wan-Fai
Ngcd, Katherine
Jamese, Bridget
Griffithsc, Elizabeth
Pricef, Colin T.
Peaseg, Paul
Emeryh, Peter
Lanyonh, Adrian
Jonesh, Michele
Bombardierii, Nurhan
Sutcliffei, Costantino
Pitzalisi, Monica
Guptaj, John
McLarenk, Annie
Cooperl, Ian
Gilesm, David
Isenbergm, Vadivelu
Saravanann, David
Coadyo, Bhaskar
Dasguptap, Neil
McHughq, Steven
Young-Minr, Robert J.
Mootss, Nagui
Gendit, Mohammed
Akilu, Francesca
Baronev, Benjamin A.
Fisherv, Saaeha
Rauzv, Andrea
Richardsw, Simon J.
Bowmanb, *, on behalf of the UK primary Sjögren’s Syndrome Registry
aUniversity Hospital Lausanne, Switzerland
bUniversity Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom
cNewcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom
dMusculoskeletal Research Group, Institute of Cellular Medicine and Newcastle NIHR Biomedical Research Centre for Ageing and Chronic Diseases, Newcastle University, Newcastle upon Tyne, United Kingdom
eInterdisciplinary Computing and Complex BioSystems research group, School of Computing Science, Newcastle University, Newcastle upon Tyne, United
Kingdom
fGreat Western Hospitals NHS Foundation Trust, Swindon, United Kingdom
gLeeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Chapel Allerton Hospital, Leeds and NIHR Leeds Musculoskeletal Biomedical Research Unit, Leeds Teaching Hospitals Trust, Leeds, United Kingdom
hNottingham University Hospital, Nottingham, United Kingdom
iBarts Health NHS Trust and Barts and the London School of Medicine and Dentistry, United Kingdom
jGartnavel General Hospital, Glasgow, United Kingdom
kNHS Fife, Whyteman's Brae Hospital, Kirkcaldy, United Kingdom
lRoyal Hampshire County Hospital, Winchester, United Kingdom
mUniversity College London Hospitals NHS Foundation Trust, London, United Kingdom
nQueen Elizabeth Hospital, Gateshead, United Kingdom
oSunderland Royal Hospital, Sunderland, UK
pSouthend University Hospital, Southend, United Kingdom
qRoyal National Hospital for Rheumatic Diseases, Bath, United Kingdom
rPortsmouth Hospitals NHS Trust, Portsmouth, United Kingdom
sAintree University Hospitals, Liverpool, United Kingdom
tBasildon Hospital, Basildon, United Kingdom
uRoyal Hallamshire Hospital, Sheffield, United Kingdom
vUniversity of Birmingham, Birmingham, United Kingdom
wBirmingham Dental Hospital, Birmingham, United Kingdom
*Other collaborators in the UKPSSR are listed in appendix 1
Summary
OBJECTIVES
To assess the use of the Clinical EULAR Sjögren’s Syndrome Disease Activity Index (ClinESSDAI), a version of the ESSDAI without the biological domain, for assessing potential eligibility and outcomes for clinical trials in patients with primary Sjögren’s syndrome (pSS), according to the new ACR-EULAR classification criteria, from the UK Primary Sjögren’s Syndrome Registry (UKPSSR).
METHODS
A total of 665 patients from the UKPSSR cohort were analysed at their time of inclusion in the registry. ESSDAI and ClinESSDAI were calculated for each patient.
RESULTS
For different disease activity index cut-off values, more potentially eligible participants were found when ClinESSDAI was used than with ESSDAI. The distribution of patients according to defined disease activity levels did not differ statistically (chi2 p = 0.57) between ESSDAI and ClinESSDAI for moderate disease activity (score ≥5 and <14; ESSDAI 36.4%; ClinESSDA 36.5%) or high disease activity (score ≥14; ESSDAI 5.4%; ClinESSDAI 6.8%). We did not find significant differences between the indexes in terms of activity levels for individual domains, with the exception of the articular domain. We found a good level of agreement between both indexes, and a positive correlation between lymphadenopathy and glandular domains with the use of either index and with different cut-off values. With the use of ClinESSDAI, the minimal clinically important improvement value was more often achievable with a one grade improvement of a single domain than with ESSDAI. We observed similar results when using the new ACR-EULAR classification criteria or the previously used American-European Consensus Group (AECG) classification criteria for pSS.
CONCLUSIONS
In the UKPSSR population, the use of ClinESSDAI instead of ESSDAI did not lead to significant changes in score distribution, potential eligibility or outcome measurement in trials, or in routine care when immunological tests are not available. These results need to be confirmed in other cohorts and with longitudinal data.
Introduction
Primary Sjögren’s syndrome (pSS) is an autoimmune rheumatic disease characterised by inflammation of the exocrine glands, inducing a reduction in tear and salivary production. The clinical presentation varies, ranging from sicca symptoms associated with fatigue and arthralgia to systemic extraglandular manifestations [1]. There is no definitive treatment for pSS, but several clinical trials with biological therapies are ongoing or planned [2–5].
In order to assess systemic disease activity in pSS, the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) has been developed. This index consists of 12 domains, 11 related to organ involvement and one biological domain reflecting B-cell activity [6]. The levels of activity of each domain (ranging from 0 to 3 points) are multiplied by their respective weights (ranging from 1 to 7 points) to obtain the total score. A user guide has been published to help clinicians who use this tool [7]. ESSDAI has been shown to be reliable and sensitive to change [8–10]. Cut-off values of ESSDAI defining moderate (>5 and <14) and severe (>14) systemic disease activity and the reduction in the score that represents the minimal clinically important improvement (MCII) have been proposed [11].
There is an issue when using the biological domain of the ESSDAI in studies aimed at finding new biomarkers for pSS or in clinical trials investigating drugs targeting B-cell activity. This domain comprises hypergammaglobulinaemia, raised IgG or low complement levels and/or cryoglobulinaemia. There is a risk of false positive associations between a potential new biomarker and systemic disease activity assessment because biomarkers are often correlated to B-cell activity, as is the biological domain of ESSDAI, producing a risk of multicollinearity of the data. In other words, there is a rationale for an index detecting clinical changes independently of the biological effects of studied drugs in clinical trials. Such an index would also be useful in monitoring clinical systemic disease activity, without the need for immunological tests, in routine clinical practice.
Therefore, the Clinical EULAR Sjögren’s Syndrome Disease Activity Index (ClinESSDAI) score has recently been developed. ClinESSDAI includes the same domains as the ESSDAI score apart from the biological domain. The weightings of the remaining domains have been recalculated. This score has been validated through the same process used for the ESSDAI with the same data set. The reliability and sensitivity to change of ClinESSDAI are similar to those of ESSDAI. The same cut-off values for levels of disease activity (≥5 moderate activity, ≥14 high activity) and MCII (3 points improvement) as for ESSDAI have been validated [12]. Levels of activity for each domain and their respective weights are summarised in table 1.
Table 1 Levels of activity and respective weights of indexes domains and theoretical illustration of disease activity indexes ability to improve according to selected MCII values.
Does a one grade improvement in one domain permit reaching the MCII when using ESSDAI or ClinESSDAI for selected MCII values?
|
Disease activity score domains
|
Levels of activity
|
ESSDAI
|
ClinESSDAI
|
|
Weight
|
MCII
|
Weight
|
MCII
|
|
3
|
4
|
5
|
|
3
|
4
|
5
|
| Constitutional |
0–2 |
3 |
Yes
|
No |
No |
4 |
Yes
|
Yes
|
No |
| Lymphadenopathy |
0–3 |
4 |
Yes
|
Yes
|
No |
4 |
Yes
|
Yes
|
No |
| Glandular |
0–2 |
2 |
No |
No |
No |
2 |
No |
No |
No |
| Articular |
0–3 |
2 |
No |
No |
No |
3 |
Yes
|
No |
No |
| Cutaneous |
0–3 |
3 |
Yes
|
No |
No |
3 |
Yes
|
No |
No |
| Pulmonary |
0–3 |
5 |
Yes
|
Yes
|
Yes
|
6 |
Yes
|
Yes
|
Yes
|
| Renal |
0–3 |
5 |
Yes
|
Yes
|
Yes
|
6 |
Yes
|
Yes
|
Yes
|
| Muscular |
0–3 |
6 |
Yes
|
Yes
|
Yes
|
7 |
Yes
|
Yes
|
Yes
|
| Peripheral nervous system |
0–3 |
5 |
Yes
|
Yes
|
Yes
|
5 |
Yes
|
Yes
|
Yes
|
| Central nervous system |
0, 1, 3*
|
5 |
Yes
|
Yes
|
Yes
|
5 |
Yes
|
Yes
|
Yes
|
| Haematological |
0–3 |
2 |
No |
No |
No |
2 |
No |
No |
No |
| Biological |
0–2 |
1 |
No |
No |
No |
NR |
NR |
NR |
NR |
The UK Primary Sjögren’s Syndrome Registry (UKPSSR) is a cohort of patients in the UK who fulfil the American-European Consensus Group (AECG) classification criteria for pSS, and are included in a research database and a tissue bank [13, 14]. A previous paper discussed the eligibility of the UKPSSR cohort for clinical trials according to different criteria, including the ESSDAI [15].
The American College of Rheumatology / European League Against Rheumatism classification criteria (ACR-EULAR) for pSS were published in 2016 and will probably supersede the AECG criteria in defining inclusion criteria in future clinical trials [16].
The objective of this study was to assess the impact of using ClinESSDAI, in comparison with ESSDAI, in the UKPSSR on the assessment of potential eligibility and the ability to demonstrate change with treatment for future controlled clinical trials in participants with pSS according to the ACR-EULAR criteria.
Methods
Patients were recruited to the UKPSSR from August 2009. Data used for analysis were extracted from the database registry on 24 June 2013. All participants provided written informed consent to be included in the UKPSSR. Research ethical approval for the UKPSSR was obtained from National Research Ethics Committee North West Haydock. We selected for the primary analysis only participants fulfilling the ACR-EULAR classification criteria (n = 665). In a secondary analysis, we repeated the analysis with all the participants included in the dataset who all fulfilled the AECG classification criteria at inclusion or prior to inclusion in the UKPSSR (n = 668).
In ESSDAI and ClinESSDAI, we arbitrarily divided the domains into two groups. The first, defined as “major systemic” domains, were those whose weightings in the index calculation are 5 or more, and comprised the respiratory, muscular, peripheral nervous system and central nervous system and renal domains. We arbitrarily defined the second group of domains as “general’ domains, comprising constitutional, lymphadenopathy, glandular, articular, cutaneous, haematological and biological domains. This division was based on a subjective view that the major systemic domains, although clinically very important, are individually relatively rare and most are difficult to measure objectively in a clinical trial context. As a consequence, the more common general domains are likely to be of greater use in evaluating the benefit of novel therapies in controlled clinical trials in pSS.
The ClinESSDAI was calculated for every participant. We analysed the distribution of ESSDAI and ClinESSDAI values. The distribution of active domains and their grading were compared between ESSDAI and ClinESSDAI for several cut-off indexes values: ≥5 (moderate systemic disease activity), ≥6, ≥7, >14 (high systemic disease activity). We analysed the level of agreement between total ESSDAI and ClinESSDAI scores. We also did a correlation analysis between individual domains of both indexes.
In an exploratory analysis, we evaluated how the participants were able to reach the MCII, when this was set at different values (3, 4 or 5). More specifically, we assessed how many of them needed a one grade improvement in one, two or three of their active domains to reach the MCII value. We did the calculation for three cut-off values for ESSDAI and ClinESSDAI (≥5, ≥6, ≥7).
Statistical analysis
Data were analysed with Microsoft Excel for descriptive statistics and GraphPad Prism v7.01 for comparative analyses. We used the chi2 test to compare discontinuous variables between groups. We analysed the level of agreement between total ESSDAI and ClinESSDAI scores using the method described by Bland and Altman [17]. Differences between ESSDAI and ClinESSDAI were calculated for each patient and plotted against their average. The standard deviation (SD) of such differences was calculated to estimate the limits of agreement. Spearman’s test was used for correlations analyses. A p-value <0.05 was considered statistically significant. We generally made no corrections for multiple comparisons as the analyses included few comparisons, except for the correlation analyses between domains. As these analyses included many comparisons we made a Bonferroni correction and calculated a p-value of 0.0009 for ClinESSDAI and 0.0007 ESSDAI to be statistically significant.
Results
Eligibility for clinical trials
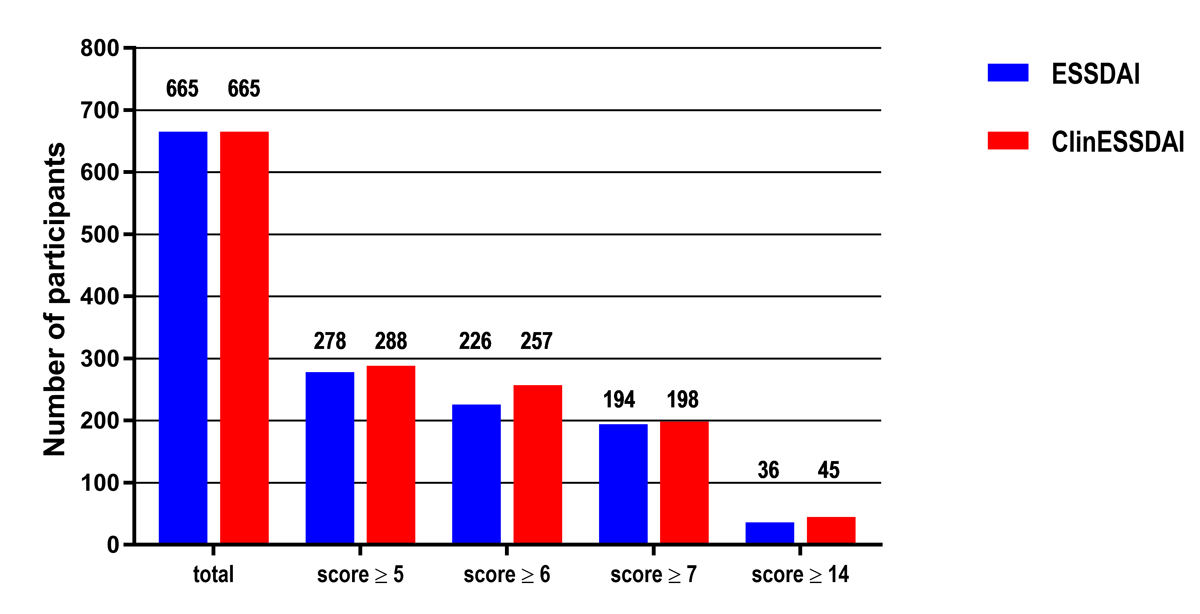
The number of patients potentially eligible for inclusion in a clinical trial according to various disease activity index cut-off values (≥5, ≥6, ≥7, ≥14) for ESSDAI and ClinESSDAI are reported in fig. 1. For each selected cut-off value, there were more patients available for potential trial inclusion when ClinESSDAI was used than with use of ESSDAI. For example, with a score ≥5 (the value defining moderate disease activity), 278 patients were eligible (41.8%) with ESSDAI and 288 (43.3%) with ClinESSDAI; with a score ≥14 (the value defining high disease activity), 36 patients were eligible (5.4%) with ESSDAI and 45 (6.8%) with ClinESSDAI.
Index values and domain activity distribution
In the analysed data from UKPSSR, the ESSDAI mean value was 4.9 (SD 5.0, median 4.0, interquartile range [IQR] 1.0–7.0) and the ClinESSDAI mean value was 4.9 (SD 5.6, median 4.0, IQR 0–7.0). The detailed score distribution of ESSDAI and ClinESSDAI can be found in table 2. The distribution of patients according to defined activity levels did not differ statistically (chi2 p = 0.57) when calculated with ESSDAI (moderate activity 36.4%, high activity 5.4%) or with ClinESSDAI (moderate activity 36.5%, high activity 6.8%). Interestingly, there were 101 more patients with an index value of 0 when ClinESSDAI was used instead of ESSDAI. These patients had only one active domain, which was the biological domain with a level of activity of 1 (n = 58) or 2 (n = 43).
Table 2 Distribution of participants from the UKPSSR according to disease activity score, calculated with ESSDAI or ClinESSDAI, total n = 665.
|
Disease activity index values
|
Number of participants (%)
|
|
ESSDAI
|
ClinESSDAI
|
| 0 |
113 (17.0) |
214 (32.2) |
| 1 |
58 (8.7) |
0 |
| 2 |
102 (15.3) |
61 (9.2) |
| 3 |
59 (8.9) |
56 (8.4) |
| 4 |
55 (8.3) |
46 (6.9) |
| 5 |
52 (7.8) |
31 (4.7) |
| 6 |
32 (4.8) |
59 (8.9) |
| 7 |
43 (6.5) |
34 (5.1) |
| 8 |
32 (4.8) |
25 (3.8) |
| 9 |
21 (3.2) |
31 (4.7) |
| 10 |
17 (2.6) |
23 (3.5) |
| 11 |
18 (2.7) |
9 (1.4) |
| 12 |
14 (2.1) |
16 (2.4) |
| 13 |
13 (2.0) |
15 (2.3) |
| 14 |
7 (1.1) |
7 (1.1) |
| 15 |
7 (1.1) |
11 (1.7) |
| 16 |
5 (0.8) |
3 (0.5) |
| 17 |
1 (0.2) |
3 (0.5) |
| 18 |
1 (0.2) |
2 (0.3) |
| 19 |
2 (0.3) |
4 (0.6) |
| 20 |
3 (0.5) |
2 (0.3) |
| ≥21 |
10 (1.5) |
13 (2.0) |
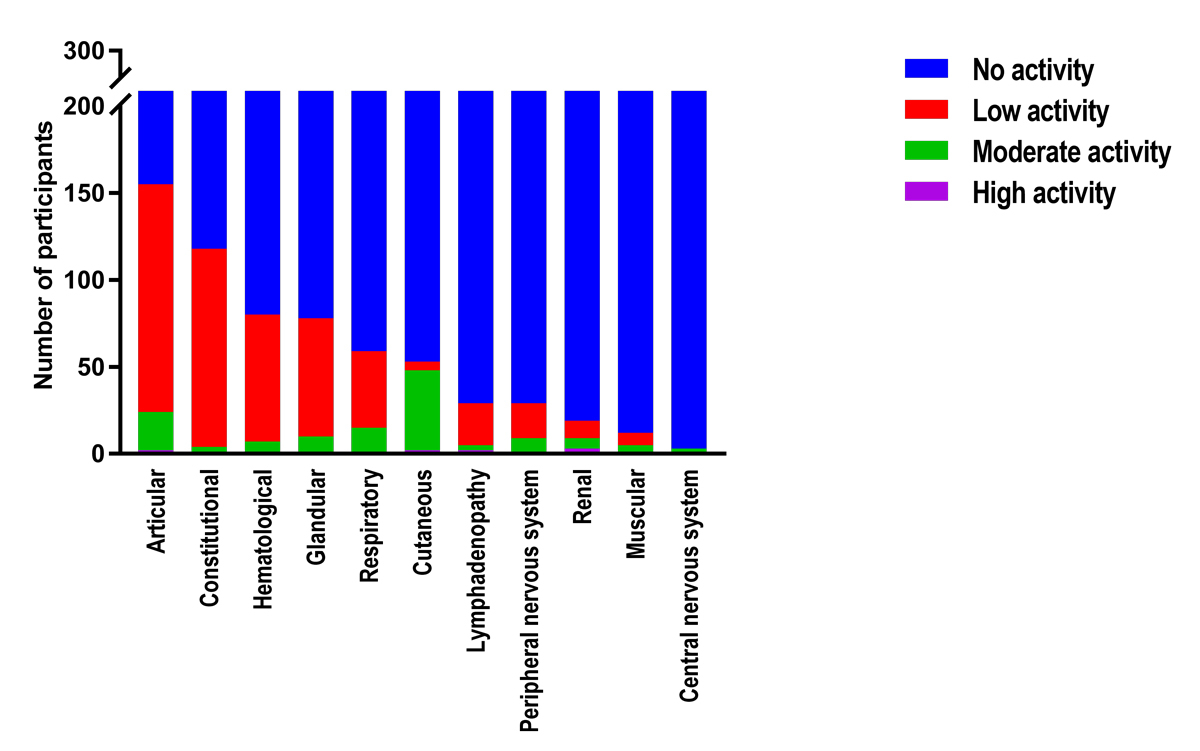
When the frequency of active domains in patients with an ESSDAI or ClinESSDAI score of ≥5, ≥6 or ≥7 were compared, the only statistically significant difference was the proportion of participants with an active articular domain and an ESSDAI ≥7 or ClinESSDAI ≥7 (53 and 41%, respectively; chi2 = 5.36, p = 0.02). The distribution of domain activity levels for participants with a ClinESSDAI ≥5, currently the cut-off value most used for inclusion in clinical trials, can be found in figure 2.
Levels of agreement and correlation analysis
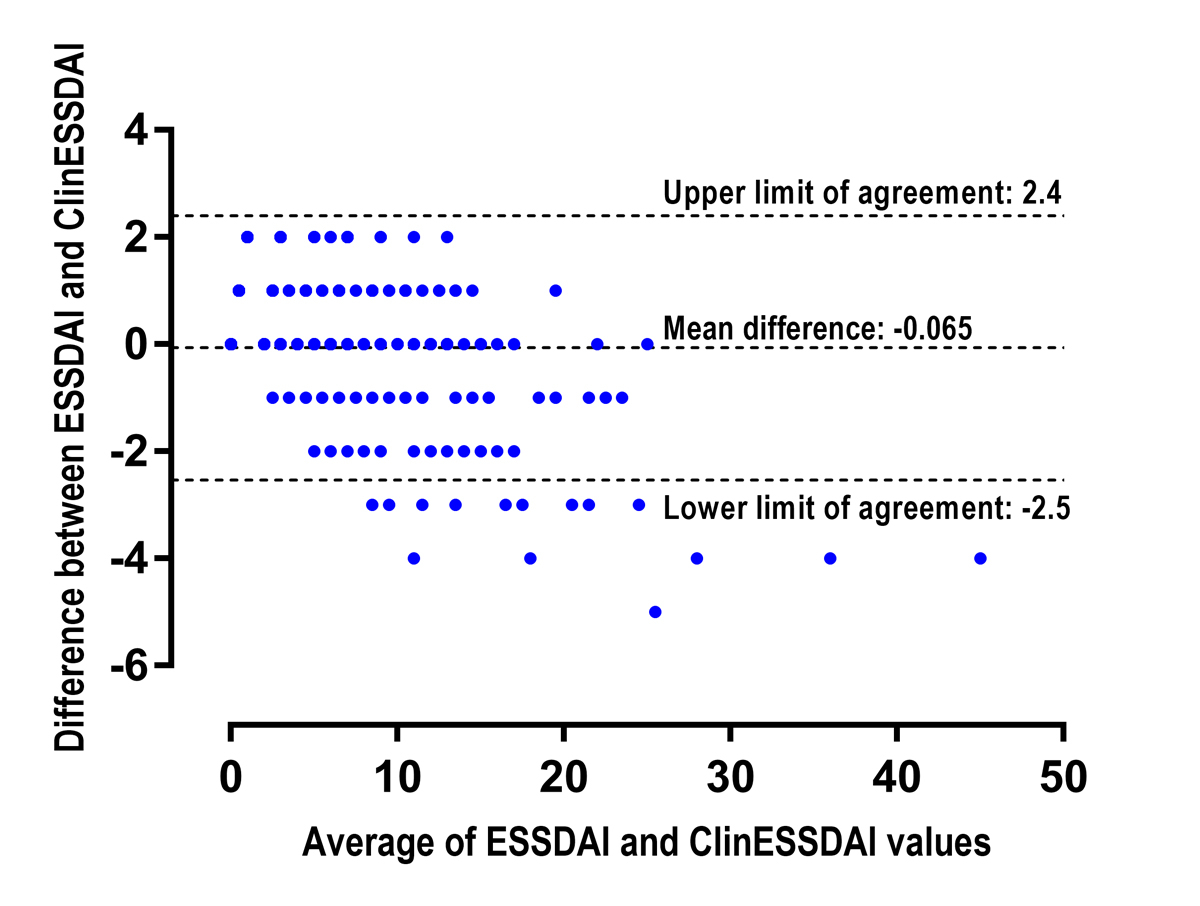
Figure 3 shows the differences between ESSDAI and ClinESSDAI plotted against their average. The mean difference between the two indexes was −0.07. The SD of the differences was 1.3. This value allows calculation of the lower and upper levels of agreement between both indexes, which are −2.5 and 2.4, respectively. The difference between both indexes was outside the levels of agreement for 22 (3.3%) patients, with a maximum difference value of 5. In these cases, ClinESSDAI was always higher than ESSDAI, with values ranging from 10 to 43.
We observed statistically significant correlations between domains when using ESSDAI or ClinESSDAI in the UKPSSR data, summarised for patients with activity index value ≥5 in table 3. The highest positive correlation coefficient was between the glandular and lymphadenopathy domains for ESSDAI and ClinESSDAI for different indexes cut-off values (all participants: rho = 0.196, p <0.0001 for both indexes; index ≥5: rho = 0.218, p = 0.0002, rho = 0.247, p <0.0001; index ≥6: rho = 0.263, p <0.0001, rho = 0.299, p <0.0001; index ≥7: rho = 0.313, p <0.0001, rho = 0.309, p <0.0001, for ESSDAI and ClinESSDAI, respectively, using Spearman’s correlation).
Table 3 Correlation coefficients with p<0.05 between domains in participants in the UKPSSR with an ESSDAI (n = 278) or ClinESSDAI (n = 288) score ≥5.
|
Domain
|
Rho
|
p-value
|
|
ESSDAI ≥5 (n = 278)
|
| Lymphadenopathy |
Glandular |
0.22 |
0.0002*
|
| Biological |
Haematological |
0.19 |
0.002 |
| Biological |
Cutaneous |
0.15 |
0.01 |
| Haematological |
Renal |
0.13 |
0.03 |
| Biological |
Renal |
0.13 |
0.03 |
| Glandular |
PNS |
−0.12 |
0.04 |
| Constitutional |
Biological |
−0.12 |
0.04 |
| Glandular |
Respiratory |
−0.13 |
0.04 |
| Articular |
Cutaneous |
−0.13 |
0.03 |
| Articular |
Muscular |
−0.13 |
0.03 |
| Cutaneous |
Respiratory |
−0.17 |
0.006 |
| Constitutional |
Cutaneous |
−0.23 |
0.0001*
|
| Constitutional |
Respiratory |
−0.23 |
<0.0001*
|
|
ClinESSDAI ≥5 (n = 288)
|
| Lymphadenopathy |
Glandular |
0.25 |
<0.0001*
|
| Haematological |
Renal |
0.13 |
0.03 |
| Glandular |
PNS |
−0.13 |
0.03 |
| Glandular |
Respiratory |
−0.13 |
0.03 |
| Articular |
Respiratory |
−0.14 |
0.02 |
| Articular |
Muscular |
−0.16 |
0.009 |
| Cutaneous |
Respiratory |
−0.16 |
0.009 |
| Articular |
Cutaneous |
−0.19 |
0.001 |
| Constitutional |
Cutaneous |
−0.20 |
0.001 |
| Constitutional |
Respiratory |
−0.20 |
0.001 |
Ability to improve by the minimal clinically important improvement value
An MCII value of 3 has been recommended by the EULAR group who developed the ESSDAI and ClinESSDAI, although higher values could be chosen depending on the clinical trial and eligibility criteria [11]. The number of participants requiring a one grade improvement in one, two or three of their active domains in order to reach several MCII target values and disease activity index values are reported in table 4. To help the reader to understand the reasoning, a theoretical illustration of the ability for individual domains alone to reach certain specified MCII target values can be found in table 1. We observed that when an MCII value of 3 or 4 was chosen, the proportion of patients who need an improvement of only one grade in a single domain to reach the MCII is higher when ClinESSDAI is used instead of ESSDAI: 90.3/99.3, 92.9/99.2, 96.9/100% (MCII = 3); 49.3/73.3, 54.9/80.5, 60.3/88.9% (MCII = 4) for ESSDAI/ClinESSDAI ≥5, ≥ 6, ≥7, respectively.
Table 4 Ability to improve: number of participants from the UKPSSR requiring a one grade improvement in 1, 2, or 3 of their active domains to reach the MCII value according to several MCII target values and disease activity scores.
Egibility criteria
(disease activity score)
|
Response criteria
(MCII)
|
Disease activity score selected for calculation
|
Total of patients fulfilling activity index score
|
Participant distributions according to the number of domains needing a one-grade improvement to reach the selected MCII, n (%)
|
|
1
|
2
|
3
|
MCII not reachable
|
|
≥5
|
MCII = 3 |
ESSDAI |
278 |
251 (90.3) |
27 (9.7) |
0 |
0 |
| ClinESSDAI |
288 |
286 (99.3) |
2 (0.7) |
0 |
0 |
| MCII = 4 |
ESSDAI |
278 |
137 (49.3) |
113 (40.6) |
15 (5.4) |
13 (4.7) |
| ClinESSDAI |
288 |
211 (73.3) |
51 (17.7) |
0 |
26 (9.0) |
| MCII = 5 |
ESSDAI |
278 |
110 (39.6) |
119 (42.8) |
17 (6.1) |
32 (11.5) |
| ClinESSDAI |
288 |
110 (38.2) |
145 (50.3) |
0 |
33 (11.5) |
|
≥6
|
MCII = 3 |
ESSDAI |
226 |
210 (92.9) |
16 (7.1) |
0 |
0 |
| ClinESSDAI |
257 |
255 (99.2) |
2 (0.8) |
0 |
0 |
| MCII = 4 |
ESSDAI |
226 |
124 (54.9) |
85 (37.6) |
8 (3.5) |
9 (4.0) |
| ClinESSDAI |
257 |
207 (80.5) |
24 (9.3) |
0 |
26 (10.1) |
| MCII = 5 |
ESSDAI |
226 |
100 (44.2) |
92 (40.7) |
10 (4.4) |
24 (10.6) |
| ClinESSDAI |
257 |
106 (41.2) |
118 (45.9) |
0 |
33 (12.8) |
|
≥7
|
MCII = 3 |
ESSDAI |
194 |
188 (96.9) |
6 (3.1) |
0 |
0 |
| ClinESSDAI |
198 |
198 (100) |
0 |
0 |
0 |
| MCII = 4 |
ESSDAI |
194 |
117 (60.3) |
74 (38.1) |
3 (1.5) |
0 |
| ClinESSDAI |
198 |
176 (88.9) |
21 (10.6) |
0 |
1 (0.5) |
| MCII = 5 |
ESSDAI |
194 |
96 (49.5) |
79 (40.7) |
5 (2.6) |
14 (7.2) |
| ClinESSDAI |
198 |
93 (47.0) |
99 (50.0) |
0 |
6 (3.0) |
Discussion
We evaluated the ClinESSDAI in comparison with ESSDAI in terms of theoretical patient recruitment and outcome measurement in clinical trials using the UKPSSR dataset in participants fulfilling ACR-EULAR classification criteria, which are likely to be used as inclusion criteria for future trials.
Use of ClinESSDAI potentially modestly increases the number of eligible participants for a clinical trial for any selected index cut-off value.
Participants whose only active domain is the biological domain are attributed an index value of 0 in the ClinESSDAI, as this domain in not part of this activity index. Therefore, the proportion of participants with an index value of 0 is higher with ClinESSDAI than with ESSDAI.
We found good agreement between ClinESSDAI and ESSDAI, with a mean difference between them close to zero and limits of agreement smaller than the MCII of 3. However, we observed that ClinESSDAI tends to be higher than ESSDAI (maximum difference 5) in some patients with a total score of 10 or more and in all patients with a score of more than 25. Thus, caution is advised when using ClinESSDAI in patients with high disease activity; however, this affects only a small proportion of potentially eligible participants in a clinical trial.
We found a consistent positive correlation between the lymphadenopathy and glandular domains, and an inverse correlation between the constitutional and respiratory/cutaneous domains, when using either ESSDAI or ClinESSDAI at different cut-off values. This could suggest the existence of subgroups of patients with different clinical presentations, eventually related to various underlying pathogenesis pathways. As the efficacy of studied medications could differ in these patients, subgroup analysis could be useful in future clinical trials to identify which patients are more likely to respond to different treatments. In an exploratory analysis, we did not find any relationship between the correlation coefficients and the participants’ characteristics (disease duration, age, anti-Ro/La positivity). Nevertheless, these preliminary results need to be confirmed in other cohorts and with longitudinal data.
We assessed how the ability to change is affected by the use of ClinESSDAI. We focused the analysis on patients with scores ≥5, as this cut-off value, defining moderate disease activity, is most often used in controlled studies. In our data, the use of ClinESSDAI made the MCII generally more easily reachable with a one grade improvement in one or two domains than with the use of ESSDAI. These findings can be interpreted in two ways: it could mean that ClinESSDAI will allow a more sensitive analysis and perhaps earlier detection of change in a drug trial or, conversely, it could artificially magnify the improvement of “soft” domains (e.g., articular domain with arthralgia) not necessarily representative of the full clinical picture of pSS.
We attempted to evaluate whether “major systemic” and “general” domains behave differently with the use of ESSDAI and ClinESSDAI. We believe that the activity level of major systemic domains is more difficult to assess than that of general domains. This difficulty is due to the frequent need of complementary investigations, such as imaging or nerve conduction studies, for diagnosis. These domains are often not formally assessed at each follow-up visit in routine care. In addition, investigations such as nerve conduction studies have not been validated for use as outcome tools in clinical trials. We found no significant differences in major systemic domain activity levels with the use of ESSDAI or ClinESSDAI. As major systemic domains are weighted 5 or more in the calculation of the scores, an improvement in any of these domains allows MCII values of 3, 4 or 5 to be achieved with both ESSDAI and ClinESSDAI.
In order to assess the influence of classification criteria on our results, we performed the same analyses including all UKPSSR participants, who all fulfilled the AECG classification criteria at inclusion or prior to inclusion as this is a mandatory criterion for inclusion in the registry. Among the 668 participants, we found that only 3 could be classified as pSS according to AECG criteria but not to the new ACR-EULAR criteria. These patients all showed positivity for anti-La (SSB) and not for anti-Ro (SSA), which are no longer part of ACR-EULAR classification criteria for pSS. Globally, the results are very similar and conclusions are identical with both sets of classification criteria (data not shown). However, the new ACR-EULAR criteria introduce a pSS classification different from the AECG criteria. A patient can be classified as pSS if there is a clinical suspicion of pSS and one active ESSDAI domain even without sicca symptoms. This alternative classification pathway was not assessed in the present study as it is not part of the AECG criteria, which were previously used for inclusion in the registry. Nevertheless, in our experience, the number of participants potentially affected by this alternative classification pathway is likely to be marginal and would not alter the results.
This study is limited by the fact that only baseline data were available for the analysis, with no follow-up data. In the French ASSESS cohort, the most frequent changes at follow-up were seen not only in the biological, articular, haematological and glandular domains, but also in the pulmonary domain [18]. Objective evaluation of this domain may require formal pulmonary function tests. In an Italian longitudinal study with a 12 months’ follow-up, there was a tendency to improvement for the articular and cutaneous domains [19]. Whereas patients included in the UKPSSR are not necessarily representative of pSS patient populations from other countries, this cohort is representative of potentially eligible patients for clinical trials in the UK.
In a context of clinical trials in pSS, ClinESSDAI would probably be used in studies investigating biomarkers or drugs targeting B-cell activity, in order to avoid the issue of collinearity of data. However, ESSDAI could also still be calculated, as biological domain items (immunoglobulins, complement, cryoglobulin) would certainly be recorded as part of the drug safety assessment.
In conclusion, our data suggest that the use of ClinESSDAI, a modification of ESSDAI without the biological domain, is practicable, does not lead to a loss of potentially eligible patients for trials, and does not significantly alter the ability to identify changes of the indexes. However, more studies are needed, especially longitudinal data to confirm that ClinESSDAI could be used as an endpoint for clinical trials studying medications with a B-cell or other systemic effect, or in routine care for monitoring disease activity when immunological tests are not available.
Appendix 1 Other collaborators in the UKPSSR
WFN, SJB and BG are investigators of the UKPSSR. The other UKPSSR members include, in alphabetical order of their affiliations: Helen Frankland, Ayren Mediana, Robert Moots (Aintree University Hospitals); Kuntal Chadravarty, Shamin Lamabadusuriya (Barking, Havering and Redbridge NHS Trust); Michele Bombardieri, Constantino Pitzalis, Nurhan Sutcliffe (Barts Health NHS Foundation Trust and Barts and the London School of Medicine and Dentistry); Nagui Gendi, Rashidat Adeniba (Basildon Hospital); John Hamburger, Jon Higham, Ana Poveda-Galego, Andrea Richards (Birmingham Dental Hospital); Joanne Logan, Diarmuid Mulherin (Cannock Chase Hospital); Jacqueline Andrews, Paul Emery, Alison McManus, Colin Pease (Chapel Allerton Hospital, Leeds); Alison Booth, Marian Regan (Derbyshire Royal Infirmary); Theodoros Dimitroulas, Lucy Kadiki, Daljit Kaur, George Kitas (Dudley Group of Hospitals NHS Foundation Trust); Mark Lloyd, Lisa Moore (Frimley Park Hospital); Esther Gordon, Cathy Lawson (Harrogate District Foundation Trust Hospital); Monica Gupta, John Hunter, Lesley Stirton (Gartnavel General Hospital, Glasgow); Gill Ortiz, Elizabeth Price (Great Western Hospital); Gavin Clunie, Ginny Rose, Sue Cuckow (Ipswich Hospital NHS Trust); Susan Knight, Deborah Symmons, Beverley Jones (Macclesfield District General Hospital and Arthritis Research UK Epidemiology Unit, Manchester); Andrew Carr, Suzanne Edgar, Marco Carrozzo, Francisco Figuereido, Heather Foggo, Colin Gillespie, Vicky Hindmarsh, Dennis Lendrem, Iain Macleod, Sheryl Mitchell, Jessica Tarn (Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University); Adrian Jones, Peter Lanyon, Alice Muir (Nottingham University Hospital); Paula White, Steven Young-Min (Portsmouth Hospitals NHS Trust); Susan Pugmire, Saravanan Vadivelu (Queen Elizabeth Hospital, Gateshead); Annie Cooper, Marianne Watkins (Royal Hampshire County Hospital); Anne Field, Stephen Kaye, Devesh Mewar, Patricia Medcalf, Pamela Tomlinson, Debbie Whiteside (Royal Liverpool University Hospital); Neil McHugh, John Pauling, Julie James, Nike Olaitan (Royal National Hospital for Rheumatic Diseases); Mohammed Akil, Jayne McDermott, Olivia Godia (Royal Hallamshire Hospital, Sheffield); David Coady, Elizabeth Kidd, Lynne Palmer (Royal Sunderland Hospital); Bhaskar Dasgupta, Victoria Katsande, Pamela Long (Southend University Hospital); Usha Chandra, Kirsten MacKay (Torbay Hospital); Stefano Fedele, Ada Ferenkeh-Koroma, Ian Giles, David Isenberg, Helena Marconnell, Stephen Porter (University College Hospital and Eastman Dental Institute); Sue Brailsford (University Hospital Birmingham); Francesca Barone, Ben Fisher, Saaeha Rauz (University of Birmingham); Paul Allcoat, John McLaren (Whyteman’s Brae Hospital, Kirkcaldy).
Acknowledgements
We thank the Medical Research Council (grant G0800629 to WFN, SJB, BG) for funding the UKPSSR. We also thank all the patients who participated in the study and the British Sjögren’s Syndrome Association, the Newcastle NIHR Biomedical Research Centre and the Sir Samuel Scott of Yews Trust for additional support. We also thank Dr Peter Nightingale for expert statistical advice.
References
1
Kassan
SS
,
Moutsopoulos
HM
. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med. 2004;164(12):1275–84. doi:.https://doi.org/10.1001/archinte.164.12.1275
2
Bowman
SJ
,
Everett
CC
,
O’Dwyer
JL
,
Emery
P
,
Pitzalis
C
,
Ng
WF
, et al.
Randomized Controlled Trial of Rituximab and cost-effectiveness analysis in treating fatigue and oral dryness in primary Sjogren’s Syndrome. Arthritis Rheumatol. 2017;69(7):1440–50. doi:.https://doi.org/10.1002/art.40093
3
Devauchelle-Pensec
V
,
Mariette
X
,
Jousse-Joulin
S
,
Berthelot
JM
,
Perdriger
A
,
Puéchal
X
, et al.
Treatment of primary Sjögren syndrome with rituximab: a randomized trial. Ann Intern Med. 2014;160(4):233–42. doi:.https://doi.org/10.7326/M13-1085
4
Mariette
X
,
Ravaud
P
,
Steinfeld
S
,
Baron
G
,
Goetz
J
,
Hachulla
E
, et al.
Inefficacy of infliximab in primary Sjögren’s syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjögren’s Syndrome (TRIPSS). Arthritis Rheum. 2004;50(4):1270–6. doi:.https://doi.org/10.1002/art.20146
5
Mariette
X
,
Seror
R
,
Quartuccio
L
,
Baron
G
,
Salvin
S
,
Fabris
M
, et al.
Efficacy and safety of belimumab in primary Sjögren’s syndrome: results of the BELISS open-label phase II study. Ann Rheum Dis. 2015;74(3):526–31. doi:.https://doi.org/10.1136/annrheumdis-2013-203991
6
Seror
R
,
Ravaud
P
,
Bowman
SJ
,
Baron
G
,
Tzioufas
A
,
Theander
E
, et al.; EULAR Sjögren’s Task Force. EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis. 2010;69(6):1103–9. doi:.https://doi.org/10.1136/ard.2009.110619
7
Seror
R
,
Bowman
SJ
,
Brito-Zeron
P
,
Theander
E
,
Bootsma
H
,
Tzioufas
A
, et al.
EULAR Sjögren’s syndrome disease activity index (ESSDAI): a user guide. RMD Open. 2015;1(1):e000022. doi:.https://doi.org/10.1136/rmdopen-2014-000022
8
Moerman
RV
,
Arends
S
,
Meiners
PM
,
Brouwer
E
,
Spijkervet
FK
,
Kroese
FG
, et al.
EULAR Sjogren’s Syndrome Disease Activity Index (ESSDAI) is sensitive to show efficacy of rituximab treatment in a randomised controlled trial. Ann Rheum Dis. 2014;73(2):472–4. doi:.https://doi.org/10.1136/annrheumdis-2013-203736
9
Seror
R
,
Mariette
X
,
Bowman
S
,
Baron
G
,
Gottenberg
JE
,
Boostma
H
, et al., European League Against Rheumatism Sjögren’s Task Force. Accurate detection of changes in disease activity in primary Sjögren’s syndrome by the European League Against Rheumatism Sjögren’s Syndrome Disease Activity Index. Arthritis Care Res (Hoboken). 2010;62(4):551–8. doi:.https://doi.org/10.1002/acr.20173
10
Seror
R
,
Theander
E
,
Brun
JG
,
Ramos-Casals
M
,
Valim
V
,
Dörner
T
, et al.; EULAR Sjögren’s Task Force. Validation of EULAR primary Sjögren’s syndrome disease activity (ESSDAI) and patient indexes (ESSPRI). Ann Rheum Dis. 2015;74(5):859–66. doi:.https://doi.org/10.1136/annrheumdis-2013-204615
11
Seror
R
,
Bootsma
H
,
Saraux
A
,
Bowman
SJ
,
Theander
E
,
Brun
JG
, et al.; EULAR Sjögren’s Task Force. Defining disease activity states and clinically meaningful improvement in primary Sjögren’s syndrome with EULAR primary Sjögren’s syndrome disease activity (ESSDAI) and patient-reported indexes (ESSPRI). Ann Rheum Dis. 2016;75(2):382–9. doi:.https://doi.org/10.1136/annrheumdis-2014-206008
12
Seror
R
,
Meiners
P
,
Baron
G
,
Bootsma
H
,
Bowman
SJ
,
Vitali
C
, et al.; EULAR Sjögren Task Force. Development of the ClinESSDAI: a clinical score without biological domain. A tool for biological studies. Ann Rheum Dis. 2016;75(11):1945–50. doi:.https://doi.org/10.1136/annrheumdis-2015-208504
13
Vitali
C
,
Bombardieri
S
,
Jonsson
R
,
Moutsopoulos
HM
,
Alexander
EL
,
Carsons
SE
, et al.; European Study Group on Classification Criteria for Sjögren’s Syndrome. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–8. doi:.https://doi.org/10.1136/ard.61.6.554
14
Ng
WF
,
Bowman
SJ
,
Griffiths
B
; UKPSSR study group. United Kingdom Primary Sjogren’s Syndrome Registry--a united effort to tackle an orphan rheumatic disease. Rheumatology (Oxford). 2011;50(1):32–9. doi:.https://doi.org/10.1093/rheumatology/keq240
15
Oni
C
,
Mitchell
S
,
James
K
,
Ng
WF
,
Griffiths
B
,
Hindmarsh
V
, et al.; UK Primary Sjögren’s Syndrome Registry. Eligibility for clinical trials in primary Sjögren’s syndrome: lessons from the UK Primary Sjögren’s Syndrome Registry. Rheumatology (Oxford). 2016;55(3):544–52.
16
Shiboski
CH
,
Shiboski
SC
,
Seror
R
,
Criswell
LA
,
Labetoulle
M
,
Lietman
TM
, et al.; International Sjögren’s Syndrome Criteria Working Group. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76(1):9–16. doi:.https://doi.org/10.1136/annrheumdis-2016-210571
17
Bland
JM
,
Altman
DG
. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327(8476):307–10. doi:.https://doi.org/10.1016/S0140-6736(86)90837-8
18
Gottenberg
JE
,
Seror
R
,
Saraux
A
, et al.
Evolution of Disease Activity over a 5-Year Period in the 395 Patients with Primary Sjögren’s Syndrome of the ASSESS Prospective Cohort
[abstract].
Arthritis Rheumatol. 2016;68(suppl 10):abstract no. 2693.
19
Baldini
C
,
Ferro
F
,
Luciano
N
, et al.
Clinically Meaningful Improvement of ESSDAI and ESSPRI in Patients with Primary Sjögren’s Syndrome in Real Life: A 12-Month Longitudinal Study
[abstract].
Arthritis Rheumatol. 2016;6(suppl 10):abstract no. 2679.