Preventing HIV transmission through blockade of CCR5: rationale, progress and perspectives
DOI: https://doi.org/10.4414/smw.2018.14580
Elsa
Martins, Ilaria
Scurci, Oliver
Hartley
Department of Pathology and Immunology, Faculty of Medicine, University of Geneva, Switzerland
Summary
Of the two million people estimated to be newly infected with human immunodeficiency virus (HIV) every year, 95% live in poorer regions of the world where effective HIV treatment is not universally available. Strategies to reduce the spread of HIV infection, which predominantly occurs via sexual contact, are urgently required. In the absence of an effective vaccine, a number of approaches to prevent HIV infection have been developed. These include using potent anti-HIV drugs prophylactically, either through systemic administration or topical application to the mucosal tissues that HIV initially encounters during sexual transmission. Genetic deficiency of the chemokine receptor CCR5 provides individuals with a remarkable degree of protection from HIV acquisition. This is because CCR5 is the major coreceptor used by HIV to infect new target cells. Since CCR5 deficiency does not appear to carry any health disadvantages, targeting the receptor is a promising strategy for both therapy and prevention of HIV.
In this review we first describe the advantages and limitations of the currently available strategies for HIV prevention, then we focus on strategies targeting CCR5, covering the progress that has been made in developing different classes of CCR5 inhibitors for prophylaxis, and the perspectives for their future development as new weapons in the global fight against HIV/AIDS.
Rationale
Current status of the HIV epidemic and principal transmission mechanisms
According to the latest estimates (aidsinfo.unaids.org), 37 million people are currently living with human immunodeficiency virus (HIV), with two million people newly infected every year. HIV/AIDS disproportionally affects the poorer regions of the world where ~95% of people living with HIV can be found, and a similar proportion of the new infections occur. The situation is particularly severe in sub-Saharan Africa, with ~70% of all cases.
HIV transmission can occur (i) through blood-to-blood contact (transfusion, needle sharing, needle stick), (ii) from mother-to-child (during pregnancy, childbirth or breastfeeding), and (iii) as a consequence of unprotected sexual intercourse, which accounts for the vast majority of new HIV infections in the world (aidsinfo.unaids.org).
Progress with HIV therapy: optimised combined antiretroviral therapy
Until the mid-1990s, use of the relatively few licensed HIV therapeutics was hampered by their low potency, poor bioavailability and pharmacokinetics, toxic side-effects and the rapid emergence in patients of drug-resistant strains [1], and HIV was regarded as an almost universally fatal disease. The advent of new antiviral medicines and improved strategies for their use have transformed the situation, with HIV now considered as a manageable chronic illness [2].
Today, more than 25 different anti-HIV drugs are approved for clinical use, classed according to the step in the viral life cycle that they inhibit: nucleoside-analogue reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), integrase inhibitors, protease inhibitors, fusion inhibitors and coreceptor antagonists [3]. Optimised HIV therapy (known as combined antiretroviral therapy) involves administration of selected combinations of anti-HIV drugs (normally two NRTIs in combination with an NNRTI, an integrase inhibitor or a protease inhibitor), together with regular clinical follow-up to monitor treatment efficacy, side-effects and the emergence of viral escape mutants [4, 5]. Not only does combined antiretroviral therapy suppress viral replication to a level where infected individuals can live essentially free of symptoms [6], but it has also been shown to strongly reduce onward transmission of the virus, providing a clear rationale for treatment of HIV-infected individuals as a means of preventing the spread of the epidemic [7].
Beyond therapy: treatment as prevention
Expanding global access to optimised combined antiretroviral therapy has been put forward as a strategy to end the epidemic by 2030 [8]. Such a venture would be hugely challenging to undertake, however, not only in terms of providing the required diagnostic tests and anti-HIV drugs for many millions of people over many years, but also in terms of upgrading healthcare infrastructure to ensure adequate monitoring. An additional problem relates to the social stigma attached to HIV-positive status in many of the regions at the centre of the epidemic, which negatively impacts campaigns for both testing and treatment of HIV [9–11]. For this reason, the development and adoption of complementary approaches to prevent transmission of HIV from person to person remains a priority. To make an impact, any such approach must be safe enough for use in uninfected people, inexpensive enough to be economically feasible, acceptable enough to ensure sufficiently broad uptake in the population, and designed in such a way as to minimize interference with the procedures used to treat infected people.
No effective HIV vaccine in the near future
The genetic variability of HIV and its capacity to evade the immune system has made the effort to develop an effective HIV vaccine immensely challenging. Hundreds of clinical trials of HIV vaccine candidates have been undertaken, leading to six large-scale clinical trials for different vaccine concepts, none of which showed sufficient efficacy to be approved for clinical use [12–14]. Progress continues to be made, using both theoretical and empirical approaches to vaccine design [13], but it is generally accepted that many more years will be required to develop a suitable HIV vaccine. In the meantime, several other HIV prevention strategies are being elaborated.
Barrier protection for HIV prevention
Condoms can be used not only to prevent HIV transmission but also other sexually transmitted diseases and unwanted pregnancies. When used consistently and correctly, male condoms have been estimated to be between 60% and 80% effective at preventing HIV transmission [15, 16]. Although condoms represent a key component of the HIV prevention arsenal, initiatives to make their use more widespread and consistent are hampered not only by issues of cost and supply, but also those of acceptability including (i) contraceptive activity, (ii) social stigma, (iii) moral or religious objections, and (iv) concerns about impact on sexual pleasure [17].
Male circumcision
The male foreskin provides a surface that can facilitate the acquisition of a range of sexually transmitted pathogens, including HIV [18]. Three clinical trials conducted in Africa demonstrated that male circumcision reduces HIV acquisition from women to men by approximately 60% [19–21], resulting in a joint policy statement from the World Health Organisation and UNAIDS that endorses male circumcision as an approach for HIV prevention [22]. An observational study of a sample of sexually active men in the USA indicated a lower HIV infection rate among circumcised individuals [23], suggesting that the conclusions drawn from the studies carried out in Africa are also applicable to populations elsewhere. It should be noted, however, that the protective benefit to circumcised men is not extended to their sexual partners [24–27].
Pre-exposure prophylaxis
During the 1980s and 1990s, anti-HIV drugs began to be used successfully both in post-exposure prophylaxis, where establishment of infection is prevented by the prompt administration of drugs after a known exposure to the virus [28, 29], and in the prevention of mother-to-child transmission of HIV, where drugs are administered to the mother in the period up to and during birth [30, 31]. At that time, use of anti-HIV drugs to prevent infection of healthy individuals at risk of exposure (referred to as pre-exposure prophylaxis, or PrEP) was not considered feasible owing to concerns about low potency, high toxicity, complicated dosing procedures and the emergence of drug resistance [32].
Oral pre-exposure prophylaxis with TDF-FTC
This perception changed with the approval of two new HIV therapeutics with improved potency and safety profiles: the NRTI tenofovir disoproxil fumarate (TDF, marketed as Viread®) in 2001 [33], and TDF in combination with a second NRTI, emtricitabine (TDF-FTC, marketed as Truvada®), in 2004 [34]. These drugs were rapidly developed for clinical evaluation in PrEP, and several phase III trials involving TDF and TDF-FTC products have now been carried out. TDF-FTC provided statistically significant reductions in HIV infection events in three separate studies in transgender women and men who have sex with men [35–37]. A study with TDF-FTC in a population of intravenous drug users showed a comparable protective benefit [38], and two trials in populations of heterosexual men and women showed statistically significant efficacy [39, 40].
Limitations of TDF-FTC-based oral pre-exposure prophylaxis
Although TDF and TDF-FTC-based PrEP has emerged as a significant breakthrough in the fight against HIV/AIDS [41], it should be noted that two other large-scale trials, both conducted with women at sites in Africa, failed to show efficacy [42, 43]. The main explanation put forward for these trial failures was low adherence to the daily dosing regimen owing to doubts about the efficacy of the products, and social stigma related to HIV status [43], but it has also been observed that during dosing of TDF-FTC products, lower drug concentrations are achieved in vaginal tissue than in rectal tissue, another potential factor in the lower performance of the trials that specifically addressed protection of women [44].
Topical prevention: a first generation of broad-spectrum “microbicides”
During the development of the oral PrEP strategies, topically applied HIV prevention strategies were being pursued in parallel. A first group of products to reach clinical trials were vaginal gels containing simple substances that had been shown to possess some anti-HIV activity in vitro: (i) detergent-based spermicides, (ii) buffers modulating vaginal pH to the lower end of the physiological range, and (iii) anionic polymers that are capable of capturing viruses, such as HIV, that carry positive charges at their surface [45]. Since these agents were expected to act not only on HIV but also on other sensitive sexually transmitted pathogens, they were referred to as “microbicides”. Several large-scale efficacy studies were carried out with these products during the 2000s, but none were successful. Worse, certain products were shown to increase risk of HIV transmission compared with placebo owing to their capacity to elicit local irritation and inflammation, leading to a local influx of HIV target cells [46].
Positive signs with tenofovir vaginal gel
The encouraging results emerging from the development of TDF-based oral PrEP prompted a shift of emphasis towards the development of topical prevention methods based on potent anti-HIV drugs. A first efficacy trial of a tenofovir vaginal gel used in a 24-hour window before or after sexual intercourse provided evidence of modest but statistically significant protection [47]. Neither of the two follow-up studies, one repeating the 24-hour coitally dependent usage [48], the other involving daily gel dosing [43], demonstrated efficacy, however. As with the unsuccessful TDF oral PrEP studies in women, failure in these studies was explained in terms of lack of adherence by the study participants [47, 48].
Success with dapivirine intravaginal rings
At the same time, significant efforts were being made to develop intravaginal rings that provide longer-term release of HIV inhibitors. Intravaginal rings were originally developed to enable hormonal contraceptives to be delivered locally and independently of gastrointestinal absorption over several weeks, thereby reducing systemic exposure to the drugs and facilitating adherence [49]. Work focused on rings containing dapivirine, an NNRTI with a promising safety and potency profile [50], which had been initially developed as an HIV therapeutic [51]. Two large-scale efficacy studies with dapivirine intravaginal rings showed modest but statistically significant protection from HIV infection in women in sub-Saharan Africa [52, 53]. As with the tenofovir gel studies, protection was shown to be highly dependent on the adherence level of the participants, which was considerably lower among younger women [52, 53].
Next-generation strategies that encourage user adherence are required
The success of oral TDF-based products and the dapivirine intravaginal rings has provided important new tools that are now ready to be used in the fight to contain the HIV epidemic. However, neither approach has been shown to provide consistently high levels of protection in populations located in the worst-affected regions of the world. A particular issue is the absence of a strategy shown to strongly protect women in sub-Saharan Africa, where (i) tenofovir-based PrEP trials met with several failures [42, 43], (ii) tenofovir gel failed to show efficacy [47, 48], and (iii) dapivirine rings provided only modest levels of protection [52, 53]. In each of these trials, lack of efficacy was linked to low product adherence rather than low drug potency. Some of the adherence problems with PrEP in sub-Saharan Africa may be reduced as news of its success in other regions spreads and as a consequence of political efforts to change perceptions about its use [54]. In addition, new strategies are being introduced to better incentivise adherence in younger women, for example by offering intravaginal rings that provide additional protection against other prevalent sexually transmitted diseases and/or contraceptive activity [55]. Devices providing longer-term drug release are also under development, including those that are implanted, reducing any risk of removal or loss [56].
The importance of developing rectal products
Receptive anal intercourse is the sexual behaviour that carries the highest risk of HIV transmission [57]. Practiced by many men and women around the world [58], it is probable that unprotected receptive anal intercourse is a major driver in the HIV epidemic. Whereas TDF-based oral PrEP is effective at protecting men who practice unprotected receptive anal sex [35–37], efficacy in women has not been demonstrated. Topical delivery of drugs to the vagina via either vaginal gel or intravaginal ring leads to drug accumulation in rectal tissue, but levels are typically several orders of magnitude lower than in vaginal tissue [59–61], implying that protection from rectal exposure to the virus would be significantly reduced. For this reason, efforts are being made to develop HIV prevention products for rectal or vaginal-plus-rectal use, including gels, enemas and suppositories [62]. The furthest advanced rectal product is a tenofovir gel, which provided indications of acceptable safety and tolerability in a phase II study [63].
Influence of the microbiome at mucosal surfaces
The microbiome on the sexual mucosa, which is known to impact on the HIV transmission process [64], should be taken into account during the development and evaluation of strategies for HIV prevention. In the vagina, microbiomes rich in Lactobacillus correlate with a lower risk of HIV acquisition [65, 66]. This is likely to be due to both (i) the capacity of Lactobacillus to generate a low pH environment, which is detrimental towards HIV virions, and (ii) the low level of baseline inflammation induced by Lactobacillus colonisation compared with other microbial populations [67]. For this reason, candidate HIV prevention agents are typically assessed during preclinical development for any potential to disrupt a protective Lactobacillus-rich vaginal microbiome [68].
In addition there is emerging evidence that the vaginal microbiome can influence the effectiveness of locally applied HIV prevention drugs. Follow-up analysis of the first tenofovir gel field trial showed that women with Lactobacillus-high microbiomes were better protected than women with Lactobacillus-low microbiomes. This observation was linked to higher levels of the drug measured in women with Lactobacillus-high microbiomes than in those with Lactobacillus-low microbiomes, leading to the suggestion that certain of abundant non-Lactobacillus microbes are capable of metabolising tenofovir, lowering its effective concentration in the tissues [69].
The rectal mucosal microbiome also shows striking heterogeneity between individuals [70], and although it has been less well studied than the vaginal mucosa, it is reasonable to assume by analogy that (i) certain microbiomes will provide better intrinsic protection from HIV acquisition then others, (ii) topically applied HIV prevention agents could influence the constitution of the rectal microbiome, and (iii) the rectal microbiome could influence the levels of rectally applied HIV prevention levels by breakdown and/or metabolism.
Integrase inhibitors for long-acting prevention
Cabotegravir is a second-generation integrase strand transfer inhibitor that has shown efficacy as a long-acting injectable PrEP agent in macaque rectal and vaginal challenge models [71–73]. In a recent phase IIa clinical study in men, safety, tolerability and acceptability profiles of long-acting injectable cabotegravir were described as good enough to justify further evaluation of the product as an alternative to topical and orally delivered PrEP [74].
New anti-HIV drugs are needed in the pipeline
The successes achieved with dapivirine, tenofovir, tenofovir-emtricitabine and cabotegravir provide strong support for the rationale of adapting potent anti-HIV drugs for use in prevention, but some limitations remain. First, single point mutations in the reverse transcriptase gene are sufficient for circulating HIV strains to gain significant resistance to both tenofovir and tenofovir-emtricitabine [75], an alarming prospect given that these drugs are used as first-line therapeutics in infected people [76, 77]. HIV has a less rapid route to the acquisition of resistance to dapivirine, but the mutants that emerge are broadly cross-resistant to NNRTI drugs, including those commonly used in therapy [78]. The development of HIV strains resistant to integrase inhibitors has also been noted [75], although there is evidence that cabotegravir might present a higher barrier to escape [79].
Second, although tenofovir has a good safety profile in comparison with earlier anti-HIV drugs from the NRTI family, it is known to be capable of provoking renal toxicity and bone demineralisation [80], and based on evidence from similar NNRTIs and integrase inhibitors that are used in therapy [80, 81], neither dapivirine nor cabotegravir is unlikely to be completely innocuous. The safety versus efficacy balance of medicines is always a concern, but toxicity becomes an even more important issue when medicines are used prophylactically in healthy people, and any concerns about side effects will impact adherence.
It will therefore be important to introduce anti-HIV drugs into the development pipeline as the optimisation of HIV prevention strategies moves ahead. Particularly desirable attributes would be (i) no concurrent use in or development for therapy, (ii) high barriers to the development of resistant escape mutants, (iii) no generation of cross-resistance to inhibitors used in therapy, and (iv) improved efficacy versus safety profiles compared with tenofovir, emtricitabine and dapivirine. In the next part of the review we will discuss the potential use of CCR5 inhibitors in HIV prevention.
Progress
CCR5: a key player in the infection of target cells by HIV
Infection of a new host by HIV requires entry into susceptible target cells. The molecular details of the HIV entry process were established at the end of the 1990s and have been reviewed extensively [82, 83]. Entry is mediated by the HIV envelope glycoprotein complex, comprised of gp120 and gp41. The first step in the process is the engagement of CD4 on host cells by gp120, eliciting a conformational change in the envelope glycoprotein that enables gp120 to interact with a second host cell surface protein known as a coreceptor. Engagement of the coreceptor elicits a second conformational change in the envelope glycoprotein that initiates gp41-mediated virus-host cell membrane fusion.
HIV coreceptors were discovered following two key observations. First, the human chemokine proteins, MIP-1α/CCL3, MIP-1β/CCL4 and RANTES/CCL5 were identified as endogenous suppressors of HIV replication [84]. Subsequently, functional screening of a human cDNA library led to the identification of a chemokine receptor, now known as CXCR4, as an HIV coreceptor [85]. CXCR4 is not the receptor for the previously identified HIV-suppressive chemokines, but a rapid series of studies published in 1996 established that CCR5, the cognate receptor for MIP-1α/CCL3, MIP-1β/CCL4 and RANTES/CCL5, is also a functional HIV coreceptor [86–88], and that SDF-1/CXCL12, the cognate chemokine for CXCR4, also inhibits HIV infection [89, 90].
In the same year, a null allele for CCR5 known as CCR5Δ32, which encodes a truncated protein that is unable to reach the cell surface and function as an HIV coreceptor, was discovered [91, 92]. Groups of rare individuals known to have had multiple exposures to HIV-1 and not infected were genetically characterised and shown to be homozygous for CCR5Δ32 [93]. Importantly, the absence of a chemokine receptor gene in these individuals does not appear to impact on health, presumably as a consequence of the functional promiscuity of the chemokine-chemokine receptor network [94]. The dispensability of CCR5 to the host is underlined by the relatively high frequency of the allele (up to 10%), particularly in populations originating from northern and central Europe [95]. Together, these findings highlight the importance of CCR5 in HIV transmission and suggest that pharmacologically replicating the phenotype of CCR5Δ32 homozygotes would be a promising strategy for HIV prevention.
Mucosal transmission of HIV: a potential role for CCR5
It is not possible to identify and study individuals who are in the process of acquiring an HIV infection, so most of our knowledge about HIV transmission mechanisms has been acquired by use of a combination of human mucosal tissue explant cultures [96] and non-human primate models [97]. Although these models have limitations [97, 98], they provide an experimental system that is as close as currently possible to the actual transmission process in humans. Until now, the main focus of research has been on transmission of the virus following vaginal challenge [97, 99]. The key features of the transmission mechanism as it is currently understood [100–102] are summarised below and in figure 1.
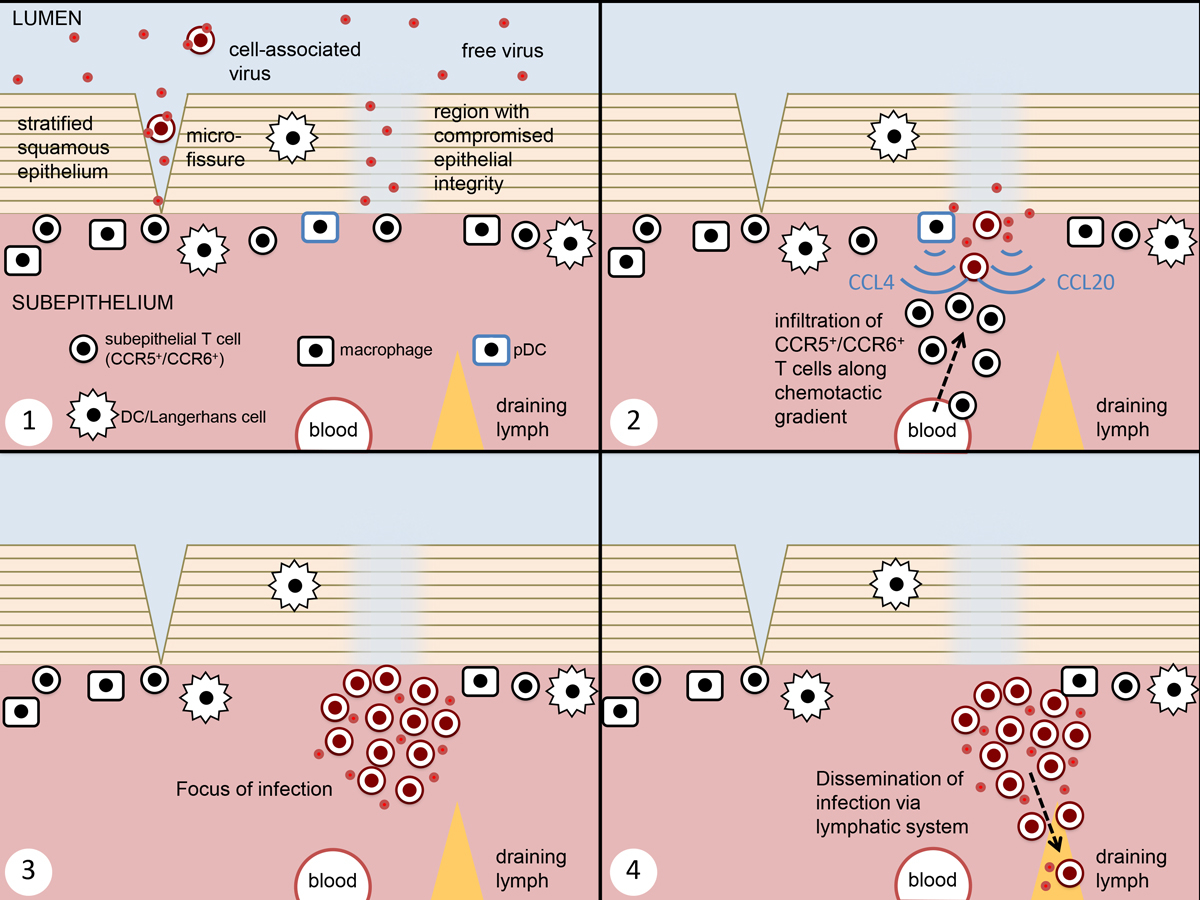
Figure 1
Current understanding of mucosal transmission of HIV. This figure represents infection across a squamous epithelial barrier (e.g. vagina and penile foreskin), the mechanism which has been most extensively studied. 1. Challenge arrives in the form of both cell-free and cell-associated virus, with entry at points of reduced epithelial integrity. 2. The initially infected cells are believed to be submucosal CCR5+ CD4 T cells. At sites of initial infection, both epithelial damage and innate antiviral responses lead to the release of chemokines, leading to recruitment of susceptible target cells. 3. This leads to the formation of a focus of infection, generating a population of founder virus. 4. Free and cell-associated virus from the founder population enters the lymphatic system, leading to the establishment of systemic infection.
Viruses cross the epithelial barrier within hours of arrival in the lumen, exploiting the single layer columnar epithelial layer of the cervix or penetrating the multilayer squamous epithelium of the vagina either at microlesions [102] or in regions where barrier permeability is locally compromised [103]. Dendritic cells, including the subset that reside in the epithelial layer (Langerhans cells), have been shown to be capable of capturing infectious virions and transferring them for infection of T cells in coculture conditions [104]. Although these cells have attributes that make them theoretically capable of playing a role in mucosal transmission, little evidence for their involvement has been found in animal and human tissue explant models so far [100–102].
The first infected target cells that can be detected in the new host are located in the subepithelial layer, giving rise to a small viral “founder population”. Analysis of these infected cells reveals that they are predominantly CD4 T cells of the Th17 lineage expressing the chemokine receptor CCR6 [105], as well as CCR5. Other potential target cells are available in the subepithelial environment, such as CD4 and coreceptor-positive macrophages and dendritic cells, but it has been suggested that CCR6+ T cells are preferentially infected because they have a physiological role in epithelial repair, accumulating at the same sites of compromised epithelial barrier function that are used by HIV as portals for entry [105]. Expansion of the initial founder population is necessary for successful transmission to the new host, and this process is believed to be facilitated by the recruitment of new target cells to the focus of infection. It has been proposed that activated plasmacytoid dendritic cells, having detected the presence of local viral infection, release MIP-1α/CCL3 and MIP-1β/CCL4, which will then attract CCR5-positive T cells [106, 107]. In addition, ongoing disruption to epithelial integrity at the infection focus might elicit the recruitment of further T cells via CCR6 [105]. Within a week, infection spreads to the local lymphatic system, from where the abundance and proximity of target cells ensures that acute systemic infection is established within 2 weeks.
Study of transmitted viruses reveals CCR5 as a “gatekeeper” for transmission
Genetic analysis of HIV strains isolated soon after transmission in humans has revealed that, despite the presence of a genetically diverse swarm of viruses in the infected partner, in the majority of cases only a single viral species is responsible for the initiation of the founder population in the new host [101]. The successfully transmitted viruses have several distinguishing features [101]. Typically, their envelope glycoproteins have fewer glycosylation sites [108] and exhibit an increased capacity to interact directly with α4β7 integrins expressed on T cells, which may promote attachment to target cells, facilitating infection [109]. Strikingly, successfully transmitted viruses are overwhelmingly those that use CCR5, and not CXCR4, as an entry coreceptor [110, 111]. The mechanism underlying such highly stringent selection based on coreceptor usage remains unclear [112], but the observation provides both an explanation for the remarkable levels of resistance to HIV acquisition shown by individuals homozygous for the CCR5Δ32 allele, and a rationale for the development of HIV prevention approaches based on CCR5 blockade.
CCR5 blockade: small molecule inhibitors and monoclonal antibodies
The first small molecule CCR5 inhibitor to be described was TAK-779 [113]. Low oral bioavailability prevented it from being taken into clinical development, but an improved follow-up compound, cenicriviroc [114], which also has inhibitory activity against the chemokine receptor CCR2, is being taken forward for both HIV therapy [115] and non-alcoholic fatty liver disease [116]. Another small molecule CCR5 inhibitor to emerge soon after the identification of CCR5 as an HIV coreceptor was CMPD-167 [117]. Although it was not taken into development as an HIV therapeutic, it has been used extensively in preclinical models of HIV treatment and prevention. The mid-2000s saw the arrival of several other orally available small molecule CCR5 inhibitors at the end of the HIV therapy development pipeline. Aplaviroc [118] was discontinued in 2005 after evidence of hepatotoxicity emerged in phase III studies [119], vicriviroc [120] was discontinued in 2010 owing to lack of efficacy [121], but maraviroc [122] was licensed for use in HIV therapy in 2007 [123], and since then has been the sole CCR5 inhibitor in clinical use.
A large number of monoclonal antibodies specific for CCR5 have been isolated [124], some of which exhibit anti-HIV activity. Two of these have been taken into clinical development. A phase I study for HGS004 was completed in 2006 [125], but no further development has been carried out since. PRO-140 [126] is currently in phase II/III studies, with results expected during 2018 [127].
The antibody-like inhibitor eCD4-Ig consists of two domains of CD4 fused to an IgG Fc region, appended with a tyrosine-sulphated CCR5-mimetic peptide. Capable of simultaneously engaging the highly conserved CD4 and CCR5 binding sites on the HIV envelope glycoprotein, eCD4-Ig is a highly potent inhibitor of a broad range of viral isolates. Although it could in principle be developed as a protein for passive immunisation, efforts so far have focused on long-term delivery using an adeno-associated viral vector. Delivered in this format, eCD4-Ig expression levels remained detectable in the serum of macaques for at least 40 weeks, and provided complete protection from successive intravaginal viral challenges [128]. Before this promising concept can be brought into clinical development, however, it will be necessary to address several concerns related to the use of the adeno-associated viral vector system including (i) pre-existing immunity to the vectors, which limits transduction efficiency, (ii) development of an immune response against eCD4-Ig, and (iii) how to rapidly shut down expression from the vector in the event of an adverse response [129].
CCR5 blockade: nucleic acid-based approaches
Several different nucleic acid-based strategies for achieving CCR5 blockade have been developed. The first approaches involved inducing the expression of modified CCR5 ligands in the biosynthetic pathway so that they immobilise and/or degrade the receptor before it can reach the cell surface [130, 131]. Subsequently, techniques targeting CCR5 mRNA were described, including small interfering RNA [132], small hairpin RNA [133, 134], antisense RNA [135] and ribozymes [136]. Finally, DNA-modifying strategies are being developed based on zinc finger nuclease (ZFN) [137], transcription activator-like effector nuclease (TALEN) [138] and CRISPR/Cas9 [139] technologies.
CCR5 blockade: chemokine analogues
Experiments performed in vitro have shown that the modest anti-HIV activity of native CCR5 chemokines is due to a combination of (i) steric blockade of the HIV envelope-CCR5 interaction at the cell surface, and (ii) agonist-mediated receptor desensitisation, resulting in removal of CCR5 from the cell surface [140]. This inhibitory role is of some importance in vivo, since a lower probability of HIV transmission has been noted in individuals carrying a gene duplication that results in increased circulating concentrations of MIP-1α/CCL3 [141].
Chemokine analogues with potent anti-HIV activity have been developed specifically as agents for use in HIV prevention strategies. Within months of the identification of CCR5 as an HIV coreceptor, a first inhibitor, RANTES(9-68), was described [142]. This truncated version of RANTES/CCL5 did not show improved anti-HIV potency, but since CCR5 is an inflammatory chemokine receptor, its lack of signalling activity was considered advantageous. Shortly afterwards, AOP-RANTES, an analogue featuring a hydrophobic N-terminal extension with anti-HIV potency several orders of magnitude higher than that of the parent chemokine, was described [143]. In a subsequent study, further work on the N-terminal pharmacophore region of AOP-RANTES led to the identification of 50-fold more potent analogue, PSC-RANTES [144]. Like AOP-RANTES, PSC-RANTES is a CCR5 superagonist [145] whose inhibitory activity is due to its capacity to elicit profound and prolonged intracellular sequestration of CCR5 [144].
Perspectives
Several CCR5 inhibitors have been taken towards development as HIV prevention medicines. These efforts are described in the following section, and the efficacies of the drugs concerned in animal models of HIV transmission are summarised in table 1.
Table 1 Preclinical efficacy testing of CCR5 inhibitors in animal models of HIV transmission.
|
Compound
|
Formulation
|
Dose
|
Animals protected
|
Model
|
Reference
|
| CMPD-167 |
Vaginal gel |
1 mM
5 mM |
2/3
8/10 |
Macaque
(SHIV-162P3 single challenge,
30 min after inhibitor delivery |
[146] |
| Vaginal gel |
1 mM |
2/11 |
Macaque
(SHIV-162P4 single challenge,
15 min after inhibitor delivery) |
[147] |
| Maraviroc |
Vaginal gel |
60 mM, 3.3% (w/w) |
4/4 |
Macaque
(SHIV-162P3 single challenge,
30 min after inhibitor delivery) |
[60] |
| Oral |
44 mg/kg |
1/6 |
Macaque
(weekly challenge of SHIV-162P3, weekly exposure to inhibitor 24 hours before and 2 hours after virus exposure) |
[148] |
| Vaginal gel |
5 mM |
7/7 |
RAG-hu humanised mouse
(HIV-1 BaL single challenge,
1 hour after inhibitor delivery) |
[149] |
| Vaginal gel |
6 mM
2 mM
0.6 mM
0.2 mM |
6/7
3/4
3/4
2/4 |
Macaque
(SHIV-162P3 single challenge,
30 min after inhibitor delivery) |
[150] |
| Oral |
61.5 mg/kg |
6/6 |
RAG-hu humanised mouse
(HIV-1 single challenge on day 4 of 7-day treatment) |
[151] |
| 5P12-RANTES |
Vaginal solution |
1 mM |
5/5 |
Macaque
(SHIV-162P3 single challenge,
30 min after inhibitor delivery) |
[152] |
CCR5 inhibitors in oral PrEP
Research into the use of orally administered CCR5 inhibitors for pre-exposure prophylaxis began in the early 2000s, when the first orally available small molecule CCR5 inhibitors emerged. Oral daily dosing of CMPD-167 provided partial but statistically significant protection in a macaque vaginal challenge model [153]. Orally delivered maraviroc in a humanised mouse model provided full protection against vaginal HIV-1 challenge [151], but it was not effective at protecting macaques in a rectal challenge model, despite the accumulation of drug concentrations estimated to be 40-fold high then those required to prevent infection of macaque leucocytes in vitro [148].
In initial clinical studies, orally delivered maraviroc was safe and well tolerated, as expected, but did not prevent infection of mucosal explants from the subjects [154, 155], despite accumulation of high doses in the tissues concerned [155]. A recently completed phase II study of oral PrEP with maraviroc in men who have sex with men again showed that it was safe and well-tolerated, but the study was not powered to determine efficacy [156].
CCR5 inhibitors in topical PrEP: CMPD-167
At the beginning of the 2000s, a prevailing model for mucosal HIV transmission involved infection-independent uptake of virions by epithelial dendritic cells, which would then migrate to draining lymph nodes and allow the captured virus to infect T cells. In this way, the site of the first CCR5-dependent infection event would be too far from the epithelial surface to allow topically applied CCR5 inhibitors to provide any protective benefit. In line with this model, gel-formulated CMPD-167 at a concentration (1 mM) several orders of magnitude in excess of its in vitro potency, was capable of protecting only 2/11 animals in a macaque vaginal challenge model [147]. However, the demonstration that topically applied PSC-RANTES achieves full protection at the same concentration in the same model provided an important proof-of-concept that topical blockade of CCR5 is indeed a feasible strategy for HIV prevention [157], and when CMPD-167 was tested at a higher concentration (5 mM) in a subsequent study it protected 8/10 macaques in the vaginal challenge model [146]. Further development of CMPD-167 in the form of both gels [60] and intravaginal rings [60, 158] was initiated, but as a candidate small molecule CCR5 inhibitor for use in topical prevention it has been superseded by maraviroc, which was licensed by Pfizer to the International Partnership for Microbicides for this purpose in 2008.
CCR5 inhibitors in topical PrEP: maraviroc
Maraviroc was first tested as a vaginal gel in the macaque vaginal challenge model, where it protected 6/7 macaques when used at 6 mM concentration [150]. In a subsequent study, full protection in the model was achieved at a concentration approximately 10-fold higher [159]. Maraviroc gels have also been shown to be active in standardised human rectal and vaginal explant tissue challenge models [160]. Maraviroc has also been developed for topical prevention in the form of vaginal rings, both alone [158] or coformulated with dapivirine [161]. A phase I study of maraviroc and maraviroc-dapivirine rings indicated that whereas rings containing maraviroc are both safe and well-tolerated, they did not confer protection on explanted cervical tissue in challenge experiments, an observation that was explained in terms of the low cervical tissue levels achieved in this study [162].
CCR5 inhibitors in topical PrEP: 5P12-RANTES
Despite the breakthrough success achieved with PSC-RANTES in the macaque vaginal challenge model [157], there were concerns that the high topical doses required for full protection would prevent a chemically synthesised macromolecule such as PSC-RANTES from being affordable for use in developing countries [163]. This, together with concern over potential inflammatory activity due to its CCR5 signalling activity, prompted the search for a new generation of fully recombinant analogues (featuring only natural, coded amino acids) using a modified phage display approach [164, 165]. In this way, 5P12-RANTES, a chemokine analogue with potency [166] and efficacy [152] as high as that of PSC-RANTES, but lacking any detectable signalling activity [166], was discovered. As a fully recombinant protein, 5P12-RANTES can be produced to clinical grade using a low-cost microbial fermentation approach [167]. Many proteins would be expected to be unstable outside the cold chain, away from neutral pH and in the enzyme-rich environment around mucosal tissue, but 5P12-RANTES shows remarkable stability to temperature, vaginal pH, and incubation with samples of human semen, cervicovaginal lavage and rectal lavage [168, 169]. Finally, 5P12-RANTES presents a remarkably high barrier to the development of resistant mutants, and is active against HIV variants resistant to other CCR5 inhibitors including maraviroc [170]. Hence 5P12-RANTES represents a promising candidate for further development for use in topical HIV prevention, with current efforts focusing on a range of formulation options including gel [171, 172], vaginal ring [173] and silk fibroin disks [174].
Outlook
Will CCR5 fulfil its promise as a target for HIV prevention? Although the efficacy of a CCR5-targeting HIV inhibitor has yet to be tested clinically, studies of maraviroc in both oral PrEP [156] and intravaginal ring [162] strategies have shown it to be safe and well tolerated. It is far from clear that the investment required to take maraviroc-based products further in development will be made, however. For oral PrEP, the results obtained in mucosal explant challenge studies from study volunteers were not encouraging [154, 155], even in cases where drug levels in tissues were in the hoped-for range [155]. These results correlate with the difficulty in achieving full protection with maraviroc-based strategies in non-human primate models of mucosal challenge [148, 150], providing an indication that maraviroc might not be a sufficiently potent CCR5 inhibitor for use in HIV prevention. The International Partnership for Microbicides has suspended development of maraviroc-based topical prevention strategies [175].
If maraviroc is not taken further into clinical development for HIV prevention, what are the alternatives? Nucleic acid-based methods could in principle be used for CCR5 blockade, but the DNA editing strategies currently being developed as cell therapies in the hope of curing HIV infection would not be appropriate for use in uninfected people, and the topical strategies involving RNA interference have not progressed beyond studies in cultured cells.
Protein-based inhibitors, either monoclonal antibodies or chemokine analogues, represent a promising alternative. As a class of medicines, biologics are much less likely to cause off-target side-effects than small molecule drugs. Furthermore, for the most advanced candidate biologics in development for HIV prevention, the key issues of stability [168, 169] and costs of production [167, 176, 177] appear to have been successfully addressed.
Anti-CCR5 antibodies, in particular PRO-140, which is currently under evaluation as an HIV therapeutic, could in principle be adapted for use in either topical or systemic prevention in line with the initiative for the development of HIV envelope-specific antibodies such as 4E10, 2F5, 2G12 and VRC01 [178].
Another promising option is 5P12-RANTES, developed especially for use in HIV prevention. It has yet to be tested in humans, and significant time and resources would be required to bring it to the stage of clinical development currently occupied by maraviroc. Aside from providing indications of safety, initial clinical studies will be required to ascertain whether, as a modified host protein, 5P12-RANTES poses problems of immunogenicity. Importantly, there are indications that 5P12-RANTES might be a more potent inhibitor of mucosal HIV infection than maraviroc. Although 5P12-RANTES showed full protection in the macaque vaginal challenge model when used at a 1 mM concentration (0.8%) [152], a substantially higher concentration of maraviroc (60 mM, 3.3%) was required for full protection in the same model [159].
In conclusion, a large number of studies point to the importance of CCR5 in the transmission of HIV and its potential utility as a target for HIV prevention strategies. Maraviroc, primarily an HIV therapeutic, was the first CCR5-targeting drug to be ready for use in clinical studies of HIV prevention, but it appears not to have the all attributes required of an effective HIV prevention agent. Other molecules are available earlier in the developmental pipeline, but quite significant investments will be required to determine whether they are capable of exploiting the opportunity presented by CCR5 blockade in HIV prevention.
Acknowledgements
The authors are grateful to the Swiss National Science Foundation for support (grant 310030_163085.
Author contributions
Elsa Martins and Ilaria Scurci made equal contributions to this article:
References
1
De Clercq
E
. Toward improved anti-HIV chemotherapy: therapeutic strategies for intervention with HIV infections. J Med Chem. 1995;38(14):2491–517. Published online July 07, 1995. doi:.https://doi.org/10.1021/jm00014a001
2
Hawkins
T
. Understanding and managing the adverse effects of antiretroviral therapy. Antiviral Res. 2010;85(1):201–9. Published online October 28, 2009. doi:.https://doi.org/10.1016/j.antiviral.2009.10.016
3
Tseng
A
,
Seet
J
,
Phillips
EJ
. The evolution of three decades of antiretroviral therapy: challenges, triumphs and the promise of the future. Br J Clin Pharmacol. 2015;79(2):182–94. doi:.https://doi.org/10.1111/bcp.12403
4Guidelines WHO, Approved by the Guidelines Review Committee. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: World Health Organization; 2013.
5
Günthard
HF
,
Saag
MS
,
Benson
CA
,
del Rio
C
,
Eron
JJ
,
Gallant
JE
, et al.
Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2016 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316(2):191–210. doi:.https://doi.org/10.1001/jama.2016.8900
6
Lee
FJ
,
Amin
J
,
Carr
A
. Efficacy of initial antiretroviral therapy for HIV-1 infection in adults: a systematic review and meta-analysis of 114 studies with up to 144 weeks’ follow-up. PLoS One. 2014;9(5):e97482. doi:.https://doi.org/10.1371/journal.pone.0097482
7
Cohen
MS
,
Chen
YQ
,
McCauley
M
,
Gamble
T
,
Hosseinipour
MC
,
Kumarasamy
N
, et al.; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi:.https://doi.org/10.1056/NEJMoa1105243
8UNAIDS. Fast-Track: ending the AIDS epidemic by 2030. Geneva: Joint United Nations Programme on HIV/AIDS, 2014.
9
Jamieson
D
,
Kellerman
SE
. The 90 90 90 strategy to end the HIV Pandemic by 2030: Can the supply chain handle it?
J Int AIDS Soc. 2016;19(1):20917. doi:.https://doi.org/10.7448/IAS.19.1.20917
10
Bain
LE
,
Nkoke
C
,
Noubiap
JJN
. UNAIDS 90-90-90 targets to end the AIDS epidemic by 2020 are not realistic: comment on “Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades”. BMJ Glob Health. 2017;2(2):e000227. doi:.https://doi.org/10.1136/bmjgh-2016-000227
11
Corey
L
,
Gray
GE
. Preventing acquisition of HIV is the only path to an AIDS-free generation. Proc Natl Acad Sci USA. 2017;114(15):3798–800. doi:.https://doi.org/10.1073/pnas.1703236114
12
Esparza
J
. A brief history of the global effort to develop a preventive HIV vaccine. Vaccine. 2013;31(35):3502–18. doi:.https://doi.org/10.1016/j.vaccine.2013.05.018
13
Fauci
AS
,
Marston
HD
. Toward an HIV vaccine: A scientific journey. Science. 2015;349(6246):386–7. doi:.https://doi.org/10.1126/science.aac6300
14
Haynes
BF
,
Mascola
JR
. The quest for an antibody-based HIV vaccine. Immunol Rev. 2017;275(1):5–10. doi:.https://doi.org/10.1111/imr.12517
15
Weller
S
,
Davis
K
. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev. 2002;(1):CD003255. Published online March 01, 2002. doi:.https://doi.org/10.1002/14651858.cd003255
16
Smith
DK
,
Herbst
JH
,
Zhang
X
,
Rose
CE
. Condom effectiveness for HIV prevention by consistency of use among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2015;68(3):337–44. Published online December 04, 2014. doi:.https://doi.org/10.1097/QAI.0000000000000461
17
Sarkar
NN
. Barriers to condom use. Eur J Contracept Reprod Health Care. 2008;13(2):114–22. Published online May 10, 2008. doi:.https://doi.org/10.1080/13625180802011302
18
Morris
BJ
,
Wamai
RG
. Biological basis for the protective effect conferred by male circumcision against HIV infection. Int J STD AIDS. 2012;23(3):153–9. Published online May 15, 2012. doi:.https://doi.org/10.1258/ijsa.2011.011228
19
Auvert
B
,
Taljaard
D
,
Lagarde
E
,
Sobngwi-Tambekou
J
,
Sitta
R
,
Puren
A
. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2(11):e298. doi:. Correction in: PLoS Med;3(5):e226. doi:https://doi.org/10.1371/journal.pmed.0020298
20
Bailey
RC
,
Moses
S
,
Parker
CB
,
Agot
K
,
Maclean
I
,
Krieger
JN
, et al.
Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–56. Published online February 27, 2007. doi:.https://doi.org/10.1016/S0140-6736(07)60312-2
21
Gray
RH
,
Kigozi
G
,
Serwadda
D
,
Makumbi
F
,
Watya
S
,
Nalugoda
F
, et al.
Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–66. Published online February 27, 2007. doi:.https://doi.org/10.1016/S0140-6736(07)60313-4
22WHO/UNAIDS, ed. New data on male circumcision and HIV prevention: policy and programme implications. WHO/UNAIDS Technical Consultation, Male Circumcision and HIV Prevention: Research Implications for Policy and Programming; 2007; Geneva, Switzerland: World Health Organization.
23
Warner
L
,
Ghanem
KG
,
Newman
DR
,
Macaluso
M
,
Sullivan
PS
,
Erbelding
EJ
. Male circumcision and risk of HIV infection among heterosexual African American men attending Baltimore sexually transmitted disease clinics. J Infect Dis. 2009;199(1):59–65. Published online December 18, 2008. doi:.https://doi.org/10.1086/595569
24
Wawer
MJ
,
Makumbi
F
,
Kigozi
G
,
Serwadda
D
,
Watya
S
,
Nalugoda
F
, et al.
Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet. 2009;374(9685):229–37. doi:.https://doi.org/10.1016/S0140-6736(09)60998-3
25
Baeten
JM
,
Donnell
D
,
Kapiga
SH
,
Ronald
A
,
John-Stewart
G
,
Inambao
M
, et al.; Partners in Prevention HSV/HIV Transmission Study Team. Male circumcision and risk of male-to-female HIV-1 transmission: a multinational prospective study in African HIV-1-serodiscordant couples. AIDS. 2010;24(5):737–44. doi:.https://doi.org/10.1097/QAD.0b013e32833616e0
26
Sánchez
J
,
Sal Y Rosas
VG
,
Hughes
JP
,
Baeten
JM
,
Fuchs
J
,
Buchbinder
SP
, et al.
Male circumcision and risk of HIV acquisition among MSM. AIDS. 2011;25(4):519–23. doi:.https://doi.org/10.1097/QAD.0b013e328340fd81
27
Wiysonge
CS
,
Kongnyuy
EJ
,
Shey
M
,
Muula
AS
,
Navti
OB
,
Akl
EA
, et al.
Male circumcision for prevention of homosexual acquisition of HIV in men. Cochrane Database Syst Rev. 2011;(6):CD007496. Published online June 17, 2011. doi:.https://doi.org/10.1002/14651858.CD007496.pub2
28
Gerberding
JL
. Prophylaxis for occupational exposure to HIV. Ann Intern Med. 1996;125(6):497–501. doi:.https://doi.org/10.7326/0003-4819-125-6-199609150-00011
29
Meylan
PR
,
Francioli
P
,
Decrey
H
,
Chave
JP
,
Glauser
MP
. Post-exposure prophylaxis against HIV infection in health care workers. Lancet. 1988;331(8583):481. Published online February 27, 1988. doi:.https://doi.org/10.1016/S0140-6736(88)91282-2
30
Melvin
AJ
,
Frenkel
LM
. Prevention of mother-to-infant transmission of HIV-1. Mol Med Today. 1997;3(6):242–5. Published online June 01, 1997. doi:.https://doi.org/10.1016/S1357-4310(97)01029-0
31
De Santis
M
,
Noia
G
,
Caruso
A
,
Mancuso
S
. Guidelines for the use of zidovudine in pregnant women with HIV infection. Drugs. 1995;50(1):43–7. Published online July 01, 1995. doi:.https://doi.org/10.2165/00003495-199550010-00004
32
Paxton
LA
,
Hope
T
,
Jaffe
HW
. Pre-exposure prophylaxis for HIV infection: what if it works?
Lancet. 2007;370(9581):89–93. Published online July 10, 2007. doi:.https://doi.org/10.1016/S0140-6736(07)61053-8
33
James
JS
. Tenofovir approved: broad indication. AIDS Treat News. 2001;(373):2–3. Published online January 05, 2002.
34
Two-once-daily fixed-dose NRTI combinations for HIV. Med Lett Drugs Ther. 2005;47(1203):19–20. Published online March 16, 2005.
35
Grant
RM
,
Lama
JR
,
Anderson
PL
,
McMahan
V
,
Liu
AY
,
Vargas
L
, et al.; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi:.https://doi.org/10.1056/NEJMoa1011205
36
Molina
JM
,
Capitant
C
,
Spire
B
,
Pialoux
G
,
Cotte
L
,
Charreau
I
, et al.; ANRS IPERGAY Study Group. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med. 2015;373(23):2237–46. Published online December 02, 2015. doi:.https://doi.org/10.1056/NEJMoa1506273
37
McCormack
S
,
Dunn
DT
,
Desai
M
,
Dolling
DI
,
Gafos
M
,
Gilson
R
, et al.
Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi:.https://doi.org/10.1016/S0140-6736(15)00056-2
38
Choopanya
K
,
Martin
M
,
Suntharasamai
P
,
Sangkum
U
,
Mock
PA
,
Leethochawalit
M
, et al.; Bangkok Tenofovir Study Group. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–90. Published online June 19, 2013. doi:.https://doi.org/10.1016/S0140-6736(13)61127-7
39
Baeten
JM
,
Donnell
D
,
Ndase
P
,
Mugo
NR
,
Campbell
JD
,
Wangisi
J
, et al.; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi:.https://doi.org/10.1056/NEJMoa1108524
40
Thigpen
MC
,
Kebaabetswe
PM
,
Paxton
LA
,
Smith
DK
,
Rose
CE
,
Segolodi
TM
, et al.; TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. Published online July 13, 2012. doi:.https://doi.org/10.1056/NEJMoa1110711
41
Cáceres
CF
,
Borquez
A
,
Klausner
JD
,
Baggaley
R
,
Beyrer
C
. Implementation of pre-exposure prophylaxis for human immunodeficiency virus infection: progress and emerging issues in research and policy. J Int AIDS Soc. 2016;19(7(Suppl 6), Suppl 6):21108.
42
Van Damme
L
,
Corneli
A
,
Ahmed
K
,
Agot
K
,
Lombaard
J
,
Kapiga
S
, et al.; FEM-PrEP Study Group. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. doi:.https://doi.org/10.1056/NEJMoa1202614
43
Marrazzo
JM
,
Ramjee
G
,
Richardson
BA
,
Gomez
K
,
Mgodi
N
,
Nair
G
, et al.; VOICE Study Team. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–18. doi:.https://doi.org/10.1056/NEJMoa1402269
44
Patterson
KB
,
Prince
HA
,
Kraft
E
,
Jenkins
AJ
,
Shaheen
NJ
,
Rooney
JF
, et al.
Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3(112):112re4. doi:.https://doi.org/10.1126/scitranslmed.3003174
45
D’Cruz
OJ
,
Uckun
FM
. Clinical development of microbicides for the prevention of HIV infection. Curr Pharm Des. 2004;10(3):315–36. doi:.https://doi.org/10.2174/1381612043386374
46
Grant
RM
,
Hamer
D
,
Hope
T
,
Johnston
R
,
Lange
J
,
Lederman
MM
, et al.
Whither or wither microbicides?
Science. 2008;321(5888):532–4. doi:.https://doi.org/10.1126/science.1160355
47
Abdool Karim
Q
,
Abdool Karim
SS
,
Frohlich
JA
,
Grobler
AC
,
Baxter
C
,
Mansoor
LE
, et al.; CAPRISA 004 Trial Group. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. doi:.https://doi.org/10.1126/science.1193748
48Rees H, Delany-Moretlwe S, Baron D, Lombard C, Gray G, Myer L, et al., eds. FACTS 001 phase III trial of pericoital tenofovir 1% gel for HIV prevention in women. Conference on Retroviruses and Opportunistic Infections (CROI); 2015.
49
Brache
V
,
Faundes
A
. Contraceptive vaginal rings: a review. Contraception. 2010;82(5):418–27. doi:.https://doi.org/10.1016/j.contraception.2010.04.012
50
Van Herrewege
Y
,
Michiels
J
,
Van Roey
J
,
Fransen
K
,
Kestens
L
,
Balzarini
J
, et al.
In vitro evaluation of nonnucleoside reverse transcriptase inhibitors UC-781 and TMC120-R147681 as human immunodeficiency virus microbicides. Antimicrob Agents Chemother. 2004;48(1):337–9. doi:.https://doi.org/10.1128/AAC.48.1.337-339.2004
51Gruzdev B, Horban A, Boron-Kaczmarska A, Gille D, Van’t Klooster G, Pauwels R, eds. TMC120, a new non-nucleoside reverse transcriptase inhibitor, is a potent antiretroviral in treatment naive, HIV-1 infected subjects. 8th Conference on Retroviruses and Opportunistic Infections, Chicago; 2001.
52
Baeten
JM
,
Palanee-Phillips
T
,
Brown
ER
,
Schwartz
K
,
Soto-Torres
LE
,
Govender
V
, et al.; MTN-020–ASPIRE Study Team. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N Engl J Med. 2016;375(22):2121–32. doi:.https://doi.org/10.1056/NEJMoa1506110
53
Nel
A
,
van Niekerk
N
,
Kapiga
S
,
Bekker
L-G
,
Gama
C
,
Gill
K
, et al.; Ring Study Team. Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women. N Engl J Med. 2016;375(22):2133–43. doi:.https://doi.org/10.1056/NEJMoa1602046
54
Mugo
NR
,
Ngure
K
,
Kiragu
M
,
Irungu
E
,
Kilonzo
N
. The preexposure prophylaxis revolution; from clinical trials to programmatic implementation. Curr Opin HIV AIDS. 2016;11(1):80–6. doi:.https://doi.org/10.1097/COH.0000000000000224
55
Fernández-Romero
JA
,
Deal
C
,
Herold
BC
,
Schiller
J
,
Patton
D
,
Zydowsky
T
, et al.
Multipurpose prevention technologies: the future of HIV and STI protection. Trends Microbiol. 2015;23(7):429–36. doi:.https://doi.org/10.1016/j.tim.2015.02.006
56
Landovitz
RJ
,
Kofron
R
,
McCauley
M
. The promise and pitfalls of long-acting injectable agents for HIV prevention. Curr Opin HIV AIDS. 2016;11(1):122–8. doi:.https://doi.org/10.1097/COH.0000000000000219
57
Baggaley
RF
,
White
RG
,
Boily
MC
. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39(4):1048–63. Published online April 22, 2010. doi:.https://doi.org/10.1093/ije/dyq057
58
McBride
KR
,
Fortenberry
JD
. Heterosexual anal sexuality and anal sex behaviors: a review. J Sex Res. 2010;47(2-3):123–36. doi:.https://doi.org/10.1080/00224490903402538
59
Nuttall
J
,
Kashuba
A
,
Wang
R
,
White
N
,
Allen
P
,
Roberts
J
, et al.
Pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques. Antimicrob Agents Chemother. 2012;56(1):103–9. doi:.https://doi.org/10.1128/AAC.00597-11
60
Malcolm
RK
,
Lowry
D
,
Boyd
P
,
Geer
L
,
Veazey
RS
,
Goldman
L
, et al.
Pharmacokinetics of a CCR5 inhibitor in rhesus macaques following vaginal, rectal and oral application. J Antimicrob Chemother. 2014;69(5):1325–9. doi:.https://doi.org/10.1093/jac/dkt506
61
Holt
JD
,
Cameron
D
,
Dias
N
,
Holding
J
,
Muntendam
A
,
Oostebring
F
, et al.
The sheep as a model of preclinical safety and pharmacokinetic evaluations of candidate microbicides. Antimicrob Agents Chemother. 2015;59(7):3761–70. doi:.https://doi.org/10.1128/AAC.04954-14
62
McGowan
I
. The development of rectal microbicides for HIV prevention. Expert Opin Drug Deliv. 2014;11(1):69–82. Published online November 26, 2013. doi:.https://doi.org/10.1517/17425247.2013.860132
63
Cranston
RD
,
Lama
JR
,
Richardson
BA
,
Carballo-Diéguez
A
,
Kunjara Na Ayudhya
RP
,
Liu
K
, et al.; MTN-017 Protocol Team. MTN-017: A Rectal Phase 2 Extended Safety and Acceptability Study of Tenofovir Reduced-Glycerin 1% Gel. Clin Infect Dis. 2017;64(5):614–20. Published online December 18, 2016. doi:.https://doi.org/10.1093/cid/ciw832
64
Saxena
D
,
Li
Y
,
Yang
L
,
Pei
Z
,
Poles
M
,
Abrams
WR
, et al.
Human microbiome and HIV/AIDS. Curr HIV/AIDS Rep. 2012;9(1):44–51. doi:.https://doi.org/10.1007/s11904-011-0103-7
65
Sewankambo
N
,
Gray
RH
,
Wawer
MJ
,
Paxton
L
,
McNairn
D
,
Wabwire-Mangen
F
, et al.
HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350(9077):546–50. doi:.https://doi.org/10.1016/S0140-6736(97)01063-5
66
Brotman
RM
. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest. 2011;121(12):4610–7. doi:.https://doi.org/10.1172/JCI57172
67
Mirmonsef
P
,
Spear
GT
. The barrier to HIV transmission provided by genital tract Lactobacillus colonization. Am J Reprod Immunol. 2014;71(6):531–6. Published online March 26, 2014. doi:.https://doi.org/10.1111/aji.12232
68
Lard-Whiteford
SL
,
Matecka
D
,
O’Rear
JJ
,
Yuen
IS
,
Litterst
C
,
Reichelderfer
P
; International Working Group on Microbicides. Recommendations for the nonclinical development of topical microbicides for prevention of HIV transmission: an update. J Acquir Immune Defic Syndr. 2004;36(1):541–52. doi:.https://doi.org/10.1097/00126334-200405010-00001
69
Klatt
NR
,
Cheu
R
,
Birse
K
,
Zevin
AS
,
Perner
M
,
Noël-Romas
L
, et al.
Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science. 2017;356(6341):938–45. Published online June 03, 2017. doi:.https://doi.org/10.1126/science.aai9383
70
Eckburg
PB
,
Bik
EM
,
Bernstein
CN
,
Purdom
E
,
Dethlefsen
L
,
Sargent
M
, et al.
Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–8. doi:.https://doi.org/10.1126/science.1110591
71
Whitfield
T
,
Torkington
A
,
van Halsema
C
. Profile of cabotegravir and its potential in the treatment and prevention of HIV-1 infection: evidence to date. HIV AIDS (Auckl). 2016;8:157–64. doi:.https://doi.org/10.2147/HIV.S97920
72
Andrews
CD
,
Yueh
YL
,
Spreen
WR
,
St Bernard
L
,
Boente-Carrera
M
,
Rodriguez
K
, et al.
A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med. 2015;7(270):270ra4. doi:.https://doi.org/10.1126/scitranslmed.3010298
73
Andrews
CD
,
Spreen
WR
,
Mohri
H
,
Moss
L
,
Ford
S
,
Gettie
A
, et al.
Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science. 2014;343(6175):1151–4. doi:.https://doi.org/10.1126/science.1248707
74
Markowitz
M
,
Frank
I
,
Grant
RM
,
Mayer
KH
,
Elion
R
,
Goldstein
D
, et al.
Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV. 2017;4(8):e331–40. Published online May 27, 2017. doi:.https://doi.org/10.1016/S2352-3018(17)30068-1
75
Shafer
RW
. Human Immunodeficiency Virus Type 1 Drug Resistance Mutations Update. J Infect Dis. 2017;216(suppl_9):S843–6. Published online October 03, 2017. doi:.https://doi.org/10.1093/infdis/jix398
76
TenoRes Study Group. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis. 2016;16(5):565–75. doi:.https://doi.org/10.1016/S1473-3099(15)00536-8
77
Parikh
UM
,
Mellors
JW
. Should we fear resistance from tenofovir/emtricitabine preexposure prophylaxis?
Curr Opin HIV AIDS. 2016;11(1):49–55. doi:.https://doi.org/10.1097/COH.0000000000000209
78
Selhorst
P
,
Vazquez
AC
,
Terrazas-Aranda
K
,
Michiels
J
,
Vereecken
K
,
Heyndrickx
L
, et al.
Human immunodeficiency virus type 1 resistance or cross-resistance to nonnucleoside reverse transcriptase inhibitors currently under development as microbicides. Antimicrob Agents Chemother. 2011;55(4):1403–13. doi:.https://doi.org/10.1128/AAC.01426-10
79
Yoshinaga
T
,
Kobayashi
M
,
Seki
T
,
Miki
S
,
Wakasa-Morimoto
C
,
Suyama-Kagitani
A
, et al.
Antiviral characteristics of GSK1265744, an HIV integrase inhibitor dosed orally or by long-acting injection. Antimicrob Agents Chemother. 2015;59(1):397–406. doi:.https://doi.org/10.1128/AAC.03909-14
80
Margolis
AM
,
Heverling
H
,
Pham
PA
,
Stolbach
A
. A review of the toxicity of HIV medications. J Med Toxicol. 2014;10(1):26–39. doi:.https://doi.org/10.1007/s13181-013-0325-8
81
Peñafiel
J
,
de Lazzari
E
,
Padilla
M
,
Rojas
J
,
Gonzalez-Cordon
A
,
Blanco
JL
, et al.
Tolerability of integrase inhibitors in a real-life setting. J Antimicrob Chemother. 2017;72(6):1752–9. Published online March 24, 2017. doi:.https://doi.org/10.1093/jac/dkx053
82
Doms
RW
,
Moore
JP
. HIV-1 membrane fusion: targets of opportunity. J Cell Biol. 2000;151(2):F9–14. doi:.https://doi.org/10.1083/jcb.151.2.F9
83
Chan
DC
,
Kim
PS
. HIV entry and its inhibition. Cell. 1998;93(5):681–4. Published online June 18, 1998. doi:.https://doi.org/10.1016/S0092-8674(00)81430-0
84
Cocchi
F
,
DeVico
AL
,
Garzino-Demo
A
,
Arya
SK
,
Gallo
RC
,
Lusso
P
. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells
[see comments].
Science. 1995;270(5243):1811–5. doi:.https://doi.org/10.1126/science.270.5243.1811
85
Feng
Y
,
Broder
CC
,
Kennedy
PE
,
Berger
EA
. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272(5263):872–7. doi:.https://doi.org/10.1126/science.272.5263.872
86
Deng
H
,
Liu
R
,
Ellmeier
W
,
Choe
S
,
Unutmaz
D
,
Burkhart
M
, et al.
Identification of a major co-receptor for primary isolates of HIV-1
[see comments].
Nature. 1996;381(6584):661–6. doi:.https://doi.org/10.1038/381661a0
87
Dragic
T
,
Litwin
V
,
Allaway
GP
,
Martin
SR
,
Huang
Y
,
Nagashima
KA
, et al.
HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381(6584):667–73. doi:.https://doi.org/10.1038/381667a0
88
Alkhatib
G
,
Combadiere
C
,
Broder
CC
,
Feng
Y
,
Kennedy
PE
,
Murphy
PM
, et al.
CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272(5270):1955–8. doi:.https://doi.org/10.1126/science.272.5270.1955
89
Oberlin
E
,
Amara
A
,
Bachelerie
F
,
Bessia
C
,
Virelizier
JL
,
Arenzana-Seisdedos
F
, et al.
The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382(6594):833–5. doi:.https://doi.org/10.1038/382833a0
90
Bleul
CC
,
Farzan
M
,
Choe
H
,
Parolin
C
,
Clark-Lewis
I
,
Sodroski
J
, et al.
The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382(6594):829–33. doi:.https://doi.org/10.1038/382829a0
91
Samson
M
,
Libert
F
,
Doranz
BJ
,
Rucker
J
,
Liesnard
C
,
Farber
CM
, et al.
Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382(6593):722–5. Published online August 22, 1996. doi:.https://doi.org/10.1038/382722a0
92
Liu
R
,
Paxton
WA
,
Choe
S
,
Ceradini
D
,
Martin
SR
,
Horuk
R
, et al.
Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86(3):367–77. Published online August 09, 1996. doi:.https://doi.org/10.1016/S0092-8674(00)80110-5
93
Dean
M
,
Carrington
M
,
Winkler
C
,
Huttley
GA
,
Smith
MW
,
Allikmets
R
, et al.; Multicenter AIDS Cohort Study; Multicenter Hemophilia Cohort Study; San Francisco City Cohort; ALIVE Study. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study
[see comments]. [published erratum appears in Science 1996 Nov 15;274(5290):1069].
Science. 1996;273(5283):1856–62. doi:.https://doi.org/10.1126/science.273.5283.1856
94
Bachelerie
F
,
Ben-Baruch
A
,
Burkhardt
AM
,
Combadiere
C
,
Farber
JM
,
Graham
GJ
, et al.
International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2013;66(1):1–79. doi:.https://doi.org/10.1124/pr.113.007724
95
Novembre
J
,
Galvani
AP
,
Slatkin
M
. The geographic spread of the CCR5 Delta32 HIV-resistance allele. PLoS Biol. 2005;3(11):e339. doi:.https://doi.org/10.1371/journal.pbio.0030339
96
Dezzutti
CS
,
Hladik
F
. Use of human mucosal tissue to study HIV-1 pathogenesis and evaluate HIV-1 prevention modalities. Curr HIV/AIDS Rep. 2013;10(1):12–20. doi:.https://doi.org/10.1007/s11904-012-0148-2
97
Garcia-Tellez
T
,
Huot
N
,
Ploquin
MJ
,
Rascle
P
,
Jacquelin
B
,
Müller-Trutwin
M
. Non-human primates in HIV research: Achievements, limits and alternatives. Infect Genet Evol. 2016;46:324–32. Published online July 30, 2016. doi:.https://doi.org/10.1016/j.meegid.2016.07.012
98
Dezzutti
CS
. Animal and human mucosal tissue models to study HIV biomedical interventions: can we predict success?
J Int AIDS Soc. 2015;18(1):20301. doi:.https://doi.org/10.7448/IAS.18.1.20301
99
Hatziioannou
T
,
Evans
DT
. Animal models for HIV/AIDS research. Nat Rev Microbiol. 2012;10(12):852–67. doi:.https://doi.org/10.1038/nrmicro2911
100
Haase
AT
. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62(1):127–39. Published online November 09, 2010. doi:.https://doi.org/10.1146/annurev-med-080709-124959
101
Ronen
K
,
Sharma
A
,
Overbaugh
J
. HIV transmission biology: translation for HIV prevention. AIDS. 2015;29(17):2219–27. doi:.https://doi.org/10.1097/QAD.0000000000000845
102
Hladik
F
,
Hope
TJ
. HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep. 2009;6(1):20–8. Published online January 20, 2009. doi:.https://doi.org/10.1007/s11904-009-0004-1
103
Carias
AM
,
McCoombe
S
,
McRaven
M
,
Anderson
M
,
Galloway
N
,
Vandergrift
N
, et al.
Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. J Virol. 2013;87(21):11388–400. doi:.https://doi.org/10.1128/JVI.01377-13
104
Wu
L
,
KewalRamani
VN
. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6(11):859–68. doi:.https://doi.org/10.1038/nri1960
105
Stieh
DJ
,
Matias
E
,
Xu
H
,
Fought
AJ
,
Blanchard
JL
,
Marx
PA
, et al.
Th17 Cells Are Preferentially Infected Very Early after Vaginal Transmission of SIV in Macaques. Cell Host Microbe. 2016;19(4):529–40. doi:.https://doi.org/10.1016/j.chom.2016.03.005
106
Li
Q
,
Estes
JD
,
Schlievert
PM
,
Duan
L
,
Brosnahan
AJ
,
Southern
PJ
, et al.
Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458(7241):1034–8. doi:.https://doi.org/10.1038/nature07831
107
Shang
L
,
Duan
L
,
Perkey
KE
,
Wietgrefe
S
,
Zupancic
M
,
Smith
AJ
, et al.
Epithelium-innate immune cell axis in mucosal responses to SIV. Mucosal Immunol. 2017;10(2):508–19. doi:.https://doi.org/10.1038/mi.2016.62
108
Sagar
M
. HIV-1 transmission biology: selection and characteristics of infecting viruses. J Infect Dis. 2010;202(S2, Suppl 2):S289–96. doi:.https://doi.org/10.1086/655656
109
Nawaz
F
,
Cicala
C
,
Van Ryk
D
,
Block
KE
,
Jelicic
K
,
McNally
JP
, et al.
The genotype of early-transmitting HIV gp120s promotes α (4) β(7)-reactivity, revealing α (4) β(7) +/CD4+ T cells as key targets in mucosal transmission. PLoS Pathog. 2011;7(2):e1001301. doi:.https://doi.org/10.1371/journal.ppat.1001301
110
Salazar-Gonzalez
JF
,
Salazar
MG
,
Keele
BF
,
Learn
GH
,
Giorgi
EE
,
Li
H
, et al.
Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206(6):1273–89. doi:.https://doi.org/10.1084/jem.20090378
111
Keele
BF
,
Giorgi
EE
,
Salazar-Gonzalez
JF
,
Decker
JM
,
Pham
KT
,
Salazar
MG
, et al.
Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105(21):7552–7. doi:.https://doi.org/10.1073/pnas.0802203105
112
Grivel
JC
,
Shattock
RJ
,
Margolis
LB
. Selective transmission of R5 HIV-1 variants: where is the gatekeeper?
J Transl Med. 2011;9(Suppl 1):S6. doi:.https://doi.org/10.1186/1479-5876-9-S1-S6
113
Baba
M
,
Nishimura
O
,
Kanzaki
N
,
Okamoto
M
,
Sawada
H
,
Iizawa
Y
, et al.
A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci USA. 1999;96(10):5698–703. doi:.https://doi.org/10.1073/pnas.96.10.5698
114
Baba
M
,
Takashima
K
,
Miyake
H
,
Kanzaki
N
,
Teshima
K
,
Wang
X
, et al.
TAK-652 inhibits CCR5-mediated human immunodeficiency virus type 1 infection in vitro and has favorable pharmacokinetics in humans. Antimicrob Agents Chemother. 2005;49(11):4584–91. doi:.https://doi.org/10.1128/AAC.49.11.4584-4591.2005
115
Kim
MB
,
Giesler
KE
,
Tahirovic
YA
,
Truax
VM
,
Liotta
DC
,
Wilson
LJ
. CCR5 receptor antagonists in preclinical to phase II clinical development for treatment of HIV. Expert Opin Investig Drugs. 2016;25(12):1377–92. Published online October 30, 2016. doi:.https://doi.org/10.1080/13543784.2016.1254615
116
Rotman
Y
,
Sanyal
AJ
. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66(1):180–90. Published online September 21, 2016. doi:.https://doi.org/10.1136/gutjnl-2016-312431
117
Wolinsky
SM
,
Veazey
RS
,
Kunstman
KJ
,
Klasse
PJ
,
Dufour
J
,
Marozsan
AJ
, et al.
Effect of a CCR5 inhibitor on viral loads in macaques dual-infected with R5 and X4 primate immunodeficiency viruses. Virology. 2004;328(1):19–29. doi:.https://doi.org/10.1016/j.virol.2004.07.021
118
Maeda
K
,
Nakata
H
,
Koh
Y
,
Miyakawa
T
,
Ogata
H
,
Takaoka
Y
, et al.
Spirodiketopiperazine-based CCR5 inhibitor which preserves CC-chemokine/CCR5 interactions and exerts potent activity against R5 human immunodeficiency virus type 1 in vitro. J Virol. 2004;78(16):8654–62. doi:.https://doi.org/10.1128/JVI.78.16.8654-8662.2004
119
Crabb
C
. GlaxoSmithKline ends aplaviroc trials. AIDS. 2006;20(5):641. doi:.https://doi.org/10.1097/01.aids.0000216362.59657.96
120
Strizki
JM
,
Tremblay
C
,
Xu
S
,
Wojcik
L
,
Wagner
N
,
Gonsiorek
W
, et al.
Discovery and characterization of vicriviroc (SCH 417690), a CCR5 antagonist with potent activity against human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2005;49(12):4911–9. doi:.https://doi.org/10.1128/AAC.49.12.4911-4919.2005
121Dunkle L. Merck Research Laboratories. Vicriviroc Discontinued Investigator Letter. National AIDS Treatment Advocacy Project (NATAP): News Updates; 2010. Available from: http://www.natap.org/2010/newsUpdates/071510_02.html.
122
Dorr
P
,
Westby
M
,
Dobbs
S
,
Griffin
P
,
Irvine
B
,
Macartney
M
, et al.
Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49(11):4721–32. doi:.https://doi.org/10.1128/AAC.49.11.4721-4732.2005
123
Kuritzkes
D
,
Kar
S
,
Kirkpatrick
P
. Fresh from the Pipeline: Maraviroc. Nat Rev Drug Discov. 2008;7(1):15–6. doi:https://doi.org/10.1038/nrd2490
124
Olson
WC
,
Jacobson
JM
. CCR5 monoclonal antibodies for HIV-1 therapy. Curr Opin HIV AIDS. 2009;4(2):104–11. doi:.https://doi.org/10.1097/COH.0b013e3283224015
125Lalezari J, Lederman M, Yadavalli G, Para M, DeJesus E, Searle J, et al., eds. A phase 1, dose-escalation, placebo controlled study of a fully human monoclonal antibody (CCR5mAb004) against CCR5 in patients with CCR5 tropic HIV-1 infection. The 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 September 27-30; San Francisco, CA.
126
Olson
WC
,
Rabut
GE
,
Nagashima
KA
,
Tran
DN
,
Anselma
DJ
,
Monard
SP
, et al.
Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J Virol. 1999;73(5):4145–55.
127
Reichert
JM
. Antibodies to watch in 2017. MAbs. 2017;9(2):167–81. doi:.https://doi.org/10.1080/19420862.2016.1269580
128
Gardner
MR
,
Kattenhorn
LM
,
Kondur
HR
,
von Schaewen
M
,
Dorfman
T
,
Chiang
JJ
, et al.
AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature. 2015;519(7541):87–91. doi:.https://doi.org/10.1038/nature14264
129
Gardner
MR
,
Farzan
M
. Engineering antibody-like inhibitors to prevent and treat HIV-1 infection. Curr Opin HIV AIDS. 2017;12(3):294–301. doi:.https://doi.org/10.1097/COH.0000000000000367
130
Yang
AG
,
Bai
X
,
Huang
XF
,
Yao
C
,
Chen
S
. Phenotypic knockout of HIV type 1 chemokine coreceptor CCR-5 by intrakines as potential therapeutic approach for HIV-1 infection. Proc Natl Acad Sci USA. 1997;94(21):11567–72. doi:.https://doi.org/10.1073/pnas.94.21.11567
131
Steinberger
P
,
Andris-Widhopf
J
,
Bühler
B
,
Torbett
BE
,
Barbas
CF, 3rd
. Functional deletion of the CCR5 receptor by intracellular immunization produces cells that are refractory to CCR5-dependent HIV-1 infection and cell fusion. Proc Natl Acad Sci USA. 2000;97(2):805–10. doi:.https://doi.org/10.1073/pnas.97.2.805
132
Martínez
MA
,
Gutiérrez
A
,
Armand-Ugón
M
,
Blanco
J
,
Parera
M
,
Gómez
J
, et al.
Suppression of chemokine receptor expression by RNA interference allows for inhibition of HIV-1 replication. AIDS. 2002;16(18):2385–90. Published online December 04, 2002. doi:.https://doi.org/10.1097/00002030-200212060-00002
133
Anderson
J
,
Banerjea
A
,
Akkina
R
. Bispecific short hairpin siRNA constructs targeted to CD4, CXCR4, and CCR5 confer HIV-1 resistance. Oligonucleotides. 2003;13(5):303–12. Published online March 06, 2004. doi:.https://doi.org/10.1089/154545703322616989
134
Wolstein
O
,
Boyd
M
,
Millington
M
,
Impey
H
,
Boyer
J
,
Howe
A
, et al.
Preclinical safety and efficacy of an anti-HIV-1 lentiviral vector containing a short hairpin RNA to CCR5 and the C46 fusion inhibitor. Mol Ther Methods Clin Dev. 2014;1:11. doi:.https://doi.org/10.1038/mtm.2013.11
135
Li
W
,
Yu
M
,
Bai
L
,
Bu
D
,
Xu
X
. Downregulation of CCR5 expression on cells by recombinant adenovirus containing antisense CCR5, a possible measure to prevent HIV-1 from entering target cells. J Acquir Immune Defic Syndr. 2006;43(5):516–22. Published online October 05, 2006. doi:.https://doi.org/10.1097/01.qai.0000243102.95640.92
136
Bai
J
,
Gorantla
S
,
Banda
N
,
Cagnon
L
,
Rossi
J
,
Akkina
R
. Characterization of anti-CCR5 ribozyme-transduced CD34+ hematopoietic progenitor cells in vitro and in a SCID-hu mouse model in vivo. Mol Ther. 2000;1(3):244–54. Published online August 10, 2000. doi:.https://doi.org/10.1006/mthe.2000.0038
137
Mani
M
,
Kandavelou
K
,
Dy
FJ
,
Durai
S
,
Chandrasegaran
S
. Design, engineering, and characterization of zinc finger nucleases. Biochem Biophys Res Commun. 2005;335(2):447–57. Published online August 09, 2005. doi:.https://doi.org/10.1016/j.bbrc.2005.07.089
138
Mussolino
C
,
Morbitzer
R
,
Lütge
F
,
Dannemann
N
,
Lahaye
T
,
Cathomen
T
. A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res. 2011;39(21):9283–93. doi:.https://doi.org/10.1093/nar/gkr597
139
Cho
SW
,
Kim
S
,
Kim
JM
,
Kim
JS
. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31(3):230–2. Published online January 31, 2013. doi:.https://doi.org/10.1038/nbt.2507
140
Trkola
A
,
Paxton
WA
,
Monard
SP
,
Hoxie
JA
,
Siani
MA
,
Thompson
DA
, et al.
Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J Virol. 1998;72(1):396–404.
141
Gonzalez
E
,
Kulkarni
H
,
Bolivar
H
,
Mangano
A
,
Sanchez
R
,
Catano
G
, et al.
The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307(5714):1434–40. doi:.https://doi.org/10.1126/science.1101160
142
Arenzana-Seisdedos
F
,
Virelizier
JL
,
Rousset
D
,
Clark-Lewis
I
,
Loetscher
P
,
Moser
B
, et al.
HIV blocked by chemokine antagonist. Nature. 1996;383(6599):400. doi:.https://doi.org/10.1038/383400a0
143
Simmons
G
,
Clapham
PR
,
Picard
L
,
Offord
RE
,
Rosenkilde
MM
,
Schwartz
TW
, et al.
Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276(5310):276–9. doi:.https://doi.org/10.1126/science.276.5310.276
144
Hartley
O
,
Gaertner
H
,
Wilken
J
,
Thompson
D
,
Fish
R
,
Ramos
A
, et al.
Medicinal chemistry applied to a synthetic protein: development of highly potent HIV entry inhibitors. Proc Natl Acad Sci USA. 2004;101(47):16460–5. doi:.https://doi.org/10.1073/pnas.0404802101
145
Gaertner
H
,
Lebeau
O
,
Borlat
I
,
Cerini
F
,
Dufour
B
,
Kuenzi
G
, et al.
Highly potent HIV inhibition: engineering a key anti-HIV structure from PSC-RANTES into MIP-1 beta/CCL4. Protein Eng Des Sel. 2008;21(2):65–72. doi:.https://doi.org/10.1093/protein/gzm079
146
Veazey
RS
,
Klasse
PJ
,
Schader
SM
,
Hu
Q
,
Ketas
TJ
,
Lu
M
, et al.
Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438(7064):99–102. doi:.https://doi.org/10.1038/nature04055
147
Veazey
RS
,
Klasse
PJ
,
Ketas
TJ
,
Reeves
JD
,
Piatak
M, Jr
,
Kunstman
K
, et al.
Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J Exp Med. 2003;198(10):1551–62. doi:.https://doi.org/10.1084/jem.20031266
148
Massud
I
,
Aung
W
,
Martin
A
,
Bachman
S
,
Mitchell
J
,
Aubert
R
, et al.
Lack of prophylactic efficacy of oral maraviroc in macaques despite high drug concentrations in rectal tissues. J Virol. 2013;87(16):8952–61. Published online June 07, 2013. doi:.https://doi.org/10.1128/JVI.01204-13
149
Neff
CP
,
Kurisu
T
,
Ndolo
T
,
Fox
K
,
Akkina
R
. A topical microbicide gel formulation of CCR5 antagonist maraviroc prevents HIV-1 vaginal transmission in humanized RAG-hu mice. PLoS One. 2011;6(6):e20209. Published online June 16, 2011. doi:.https://doi.org/10.1371/journal.pone.0020209
150
Veazey
RS
,
Ketas
TJ
,
Dufour
J
,
Moroney-Rasmussen
T
,
Green
LC
,
Klasse
PJ
, et al.
Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis. 2010;202(5):739–44. Published online July 16, 2010. doi:.https://doi.org/10.1086/655661
151
Neff
CP
,
Ndolo
T
,
Tandon
A
,
Habu
Y
,
Akkina
R
. Oral pre-exposure prophylaxis by anti-retrovirals raltegravir and maraviroc protects against HIV-1 vaginal transmission in a humanized mouse model. PLoS One. 2010;5(12):e15257. doi:.https://doi.org/10.1371/journal.pone.0015257
152
Veazey
RS
,
Ling
B
,
Green
LC
,
Ribka
EP
,
Lifson
JD
,
Piatak
M, Jr
, et al.
Topically applied recombinant chemokine analogues fully protect macaques from vaginal simian-human immunodeficiency virus challenge. J Infect Dis. 2009;199(10):1525–7. doi:.https://doi.org/10.1086/598685
153
Veazey
RS
,
Springer
MS
,
Marx
PA
,
Dufour
J
,
Klasse
PJ
,
Moore
JP
. Protection of macaques from vaginal SHIV challenge by an orally delivered CCR5 inhibitor. Nat Med. 2005;11(12):1293–4. doi:.https://doi.org/10.1038/nm1321
154
Coll
J
,
Moltó
J
,
Boix
J
,
Gómez-Mora
E
,
Else
L
,
García
E
, et al.
Single oral dose of maraviroc does not prevent ex-vivo HIV infection of rectal mucosa in HIV-1 negative human volunteers. AIDS. 2015;29(16):2149–54. Published online November 07, 2015. doi:.https://doi.org/10.1097/QAD.0000000000000769
155
Fox
J
,
Tiraboschi
JM
,
Herrera
C
,
Else
L
,
Egan
D
,
Dickinson
L
, et al.
Brief Report: Pharmacokinetic/Pharmacodynamic Investigation of Single-Dose Oral Maraviroc in the Context of HIV-1 Pre-exposure Prophylaxis. J Acquir Immune Defic Syndr. 2016;73(3):252–7. Published online October 12, 2016. doi:.https://doi.org/10.1097/QAI.0000000000001108
156
Gulick
RM
,
Wilkin
TJ
,
Chen
YQ
,
Landovitz
RJ
,
Amico
KR
,
Young
AM
, et al.
Phase 2 Study of the Safety and Tolerability of Maraviroc-Containing Regimens to Prevent HIV Infection in Men Who Have Sex With Men (HPTN 069/ACTG A5305). J Infect Dis. 2017;215(2):238–46. Published online November 05, 2016. doi:.https://doi.org/10.1093/infdis/jiw525
157
Lederman
MM
,
Veazey
RS
,
Offord
R
,
Mosier
DE
,
Dufour
J
,
Mefford
M
, et al.
Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306(5695):485–7. doi:.https://doi.org/10.1126/science.1099288
158
Malcolm
RK
,
Veazey
RS
,
Geer
L
,
Lowry
D
,
Fetherston
SM
,
Murphy
DJ
, et al.
Sustained release of the CCR5 inhibitors CMPD167 and maraviroc from vaginal rings in rhesus macaques. Antimicrob Agents Chemother. 2012;56(5):2251–8. doi:.https://doi.org/10.1128/AAC.05810-11
159
Malcolm
RK
,
Forbes
CJ
,
Geer
L
,
Veazey
RS
,
Goldman
L
,
Klasse
PJ
, et al.
Pharmacokinetics and efficacy of a vaginally administered maraviroc gel in rhesus macaques. J Antimicrob Chemother. 2013;68(3):678–83. doi:.https://doi.org/10.1093/jac/dks422
160
Dezzutti
CS
,
Yandura
S
,
Wang
L
,
Moncla
B
,
Teeple
EA
,
Devlin
B
, et al.
Pharmacodynamic Activity of Dapivirine and Maraviroc Single Entity and Combination Topical Gels for HIV-1 Prevention. Pharm Res. 2015;32(11):3768–81. doi:.https://doi.org/10.1007/s11095-015-1738-7
161
Fetherston
SM
,
Boyd
P
,
McCoy
CF
,
McBride
MC
,
Edwards
KL
,
Ampofo
S
, et al.
A silicone elastomer vaginal ring for HIV prevention containing two microbicides with different mechanisms of action. Eur J Pharm Sci. 2013;48(3):406–15. Published online December 26, 2012. doi:.https://doi.org/10.1016/j.ejps.2012.12.002
162
Chen
BA
,
Panther
L
,
Marzinke
MA
,
Hendrix
CW
,
Hoesley
CJ
,
van der Straten
A
, et al.
Phase 1 Safety, Pharmacokinetics, and Pharmacodynamics of Dapivirine and Maraviroc Vaginal Rings: A Double-Blind Randomized Trial. J Acquir Immune Defic Syndr. 2015;70(3):242–9. doi:.https://doi.org/10.1097/QAI.0000000000000702
163
Moore
JP
. Topical microbicides become topical. N Engl J Med. 2005;352(3):298–300. Published online January 22, 2005. doi:.https://doi.org/10.1056/NEJMcibr043727
164
Hartley
O
,
Dorgham
K
,
Perez-Bercoff
D
,
Cerini
F
,
Heimann
A
,
Gaertner
H
, et al.
Human immunodeficiency virus type 1 entry inhibitors selected on living cells from a library of phage chemokines. J Virol. 2003;77(12):6637–44. doi:.https://doi.org/10.1128/JVI.77.12.6637-6644.2003
165
Dorgham
K
,
Cerini
F
,
Gaertner
H
,
Melotti
A
,
Rossitto-Borlat
I
,
Gorochov
G
, et al.
Generating Chemokine Analogs with Enhanced Pharmacological Properties Using Phage Display. Methods Enzymol. 2016;570:47–72. doi:.https://doi.org/10.1016/bs.mie.2015.09.014
166
Gaertner
H
,
Cerini
F
,
Escola
JM
,
Kuenzi
G
,
Melotti
A
,
Offord
R
, et al.
Highly potent, fully recombinant anti-HIV chemokines: reengineering a low-cost microbicide. Proc Natl Acad Sci USA. 2008;105(46):17706–11. doi:.https://doi.org/10.1073/pnas.0805098105
167
Cerini
F
,
Gaertner
H
,
Madden
K
,
Tolstorukov
I
,
Brown
S
,
Laukens
B
, et al.
A scalable low-cost cGMP process for clinical grade production of the HIV inhibitor 5P12-RANTES in Pichia pastoris. Protein Expr Purif. 2016;119:1–10. doi:.https://doi.org/10.1016/j.pep.2015.10.011
168
Cerini
F
,
Offord
R
,
McGowan
I
,
Hartley
O
. Stability of 5P12-RANTES, A Candidate Rectal Microbicide, in Human Rectal Lavage. AIDS Res Hum Retroviruses. 2017;33(8):768–77. doi:.https://doi.org/10.1089/aid.2016.0199
169
Cerini
F
,
Landay
A
,
Gichinga
C
,
Lederman
MM
,
Flyckt
R
,
Starks
D
, et al.
Chemokine analogues show suitable stability for development as microbicides. J Acquir Immune Defic Syndr. 2008;49(5):472–6. doi:.https://doi.org/10.1097/QAI.0b013e31818c953f
170
Nedellec
R
,
Coetzer
M
,
Lederman
MM
,
Offord
RE
,
Hartley
O
,
Mosier
DE
. Resistance to the CCR5 inhibitor 5P12-RANTES requires a difficult evolution from CCR5 to CXCR4 coreceptor use. PLoS One. 2011;6(7):e22020. doi:.https://doi.org/10.1371/journal.pone.0022020
171
McBride
JW
,
Dias
N
,
Cameron
D
,
Offord
RE
,
Hartley
O
,
Boyd
P
, et al.
Pharmacokinetics of the protein microbicide 5P12-RANTES in sheep following single-dose vaginal gel administration. Antimicrob Agents Chemother. 2017;61(10):e00965-17. Published online August 09, 2017. doi:.https://doi.org/10.1128/AAC.00965-17
172
Dezzutti
C
,
Tirabassi
D
,
Graebing
P
,
Wang
L
,
Rohan
L
,
Ayudhya
RKN
, et al.
Abstracts of the HIV Research for Prevention Meeting, HIVR4P, 17-20 October, 2016, Chicago, USA. AIDS Res Hum Retroviruses. 2016;32(S1):1–409. doi:.https://doi.org/10.1089/aid.2016.5000.abstracts
173
McBride
J
,
Boyd
P
,
Offord
R
,
Hartley
O
,
Kett
V
,
Malcolm
K
. Abstracts of the HIV Research for Prevention Meeting, HIVR4P, 17-20 October, 2016, Chicago, USA. AIDS Res Hum Retroviruses. 2016;32(S1):1–409. doi:.https://doi.org/10.1089/aid.2016.5000.abstracts
174
Zhang
L
,
Herrera
C
,
Coburn
J
,
Olejniczak
N
,
Ziprin
P
,
Kaplan
DL
, et al. Stabilization and sustained release of HIV inhibitors by encapsulation in silk fibroin disks. ACS Biomaterials Science & Engineering. 2017.
175
http://www.ipmglobal.org/our-work/arvs-in-the-pipeline/maraviroc. [July 5th 2017].
176
Fuqua
JL
,
Wanga
V
,
Palmer
KE
. Improving the large scale purification of the HIV microbicide, griffithsin. BMC Biotechnol. 2015;15(1):12. doi:.https://doi.org/10.1186/s12896-015-0120-5
177
Ramessar
K
,
Rademacher
T
,
Sack
M
,
Stadlmann
J
,
Platis
D
,
Stiegler
G
, et al.
Cost-effective production of a vaginal protein microbicide to prevent HIV transmission. Proc Natl Acad Sci USA. 2008;105(10):3727–32. doi:.https://doi.org/10.1073/pnas.0708841104
178
Anderson
DJ
,
Politch
JA
,
Zeitlin
L
,
Hiatt
A
,
Kadasia
K
,
Mayer
KH
, et al.
Systemic and topical use of monoclonal antibodies to prevent the sexual transmission of HIV. AIDS. 2017;31(11):1505–17. Published online May 04, 2017. doi:.https://doi.org/10.1097/QAD.0000000000001521
