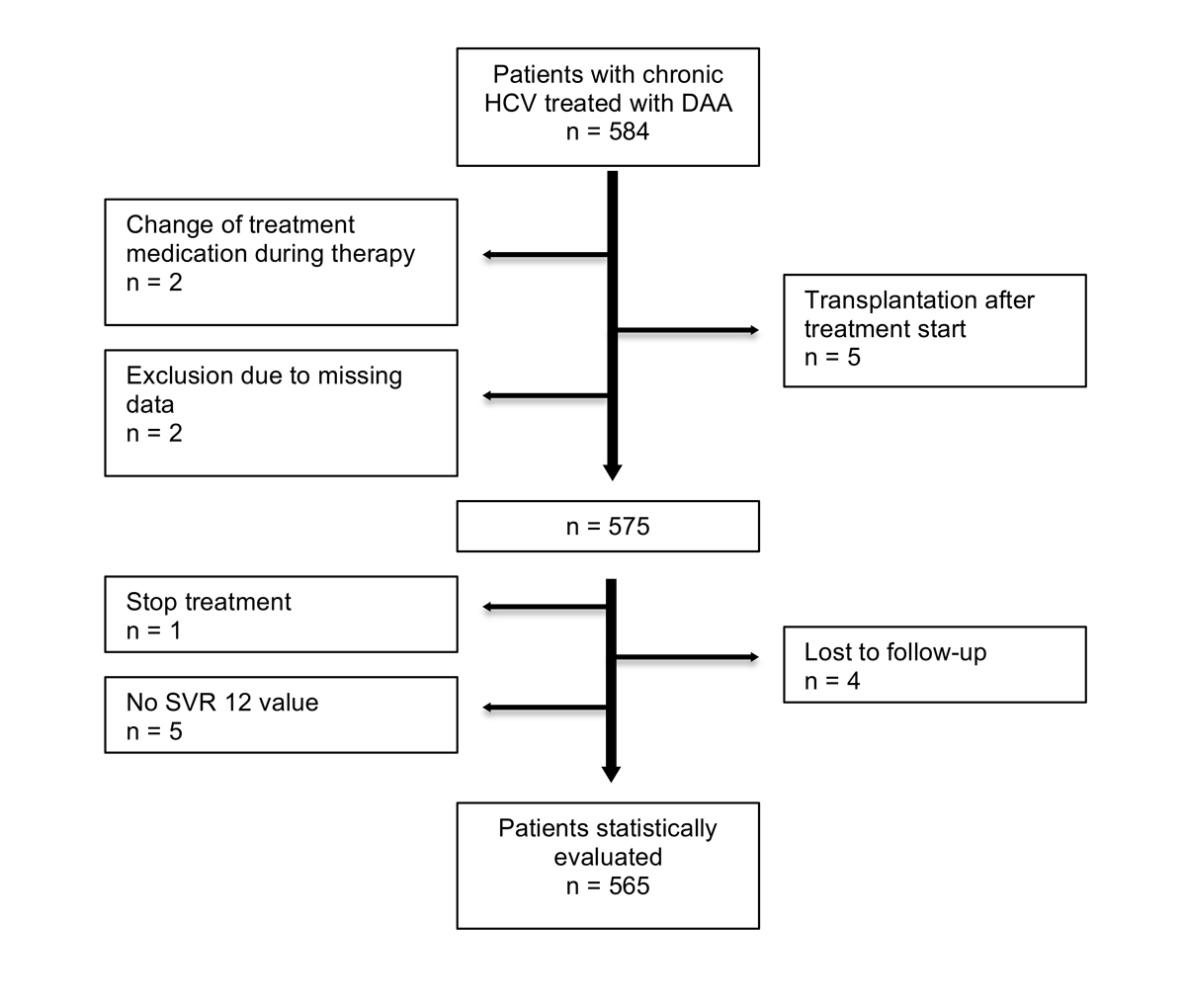
Figure 1 Flow chart of patient recruitment.
DOI: https://doi.org/10.4414/smw.2018.14560
chronic hepatitis C
direct acting antivirals
European Association for the Study of the Liver
hepatitis B virus
hepatitis C virus
intention to treat
pegylated interferon
resistance associated substitutions
sustained virological response
Interferon-free direct antiviral agents (DAAs) for the treatment of chronic hepatitis C (CHC) became available in Switzerland in December 2013. In large registration studies, DAA treatment resulted in sustained virological response (SVR) rates of more than 90% in many patient populations with minimal side effects [1–3]. Based on these registration studies, recommendations of the major national and international societies quickly adopted the novel treatment regimens for most patients, rendering previous pegylated-interferon (PEG-IFN)-based regimens obsolete (summarised in the EASL and AASLD guidelines on the treatment of chronic hepatitis C virus [HCV] infection) [4, 5].
With the widespread use of DAA in diverse clinical settings and patient populations, initial registration study results have been challenged by so-called “real life” data that, in several cases, were clearly inferior to the SVR rates expected on the basis of phase III trials [6, 7]. These data remain controversial, with other studies finding SVR rates similar to registration trials (e.g., treatment in age >65 [8] or human immunodeficiency virus [HIV] co-infected [9]).
Given the significant cost and burden to healthcare systems due to the high large number of CHC patients and the high costs of DAA, the success rate and performance of these novel drugs in a clinical situation outside of registration studies is of major interest. Furthermore, it allows for the evaluation of recommended treatment regimens as well as critical evaluation of current local access to medication and reimbursement restrictions.
The aim of this study was to assess treatment regimens and success rates of patients with CHC in Switzerland, and to review access and reimbursement limitations with regards to treatment outcome.
This was an observational study assessing treatment regimens and treatment outcome of a patient cohort suffering from CHC. Reporting of data follows the STROBE Statement (Strengthening the Reporting of Observational studies in Epidemiology) for observational epidemiological studies [10].
The present study was conducted at three Swiss tertiary care centres (University Hospital Zurich, Kantonsspital St Gallen, Epatocentro Ticino, Lugano). Data collection was performed retrospectively as well as prospectively. Data were analysed per intention-to-treat (ITT) analysis, and per modified ITT analysis with respect to HCV genotype, regimen of DAA therapy, treatment outcome as well as re-treatment in patients who did not achieve SVR after the first course of DAA. Patients were recruited between November 2013 and June 2016. Most patients are participants in the Swiss Hepatitis C Cohort Study [11].
Five hundred and eighty-five patients with CHC undergoing DAA treatment between November 2013 and June 2016 were evaluated. DAA treatment regimens were chosen according to current recommendations of the European Association for the study of the Liver (EASL), Swiss expert opinion statement and/or treatment according to availability, and reimbursement limitations of the Swiss health authorities.
Inclusion criteria were (1) age older than 18 years (2), a documented HCV infection (3), a DAA-based therapy for CHC (4), available data on HCV treatment including laboratory data and documented HCV viral load (HCV RNA by PCR [COBAS 4800; Roche Diagnostics, Rotkreuz, Schweiz]), and (5) patient informed consent. Patients not meeting these criteria were not included in the study (see fig. 1). Concomitant liver diseases and co-infections were recorded if available.
Patients were divided into five groups according to HCV genotype and subtype (1a, 1b, 2–4). Patients with HCV genotype 1 were divided into the subgroups 1a and 1b, since the treatment regimen differs for the two subtypes. In contrast, the other HCV genotypes (2, 3 and 4) were treated with a genotype-specific regimen regardless of subtype. Because of the scarcity of these genotypes in Switzerland, no patients with HCV genotype 5 or 6 infection were included in the study. Successful treatment was defined as negative HCV-PCR 12 weeks after end of treatment (SVR12). Treatment failure was defined as again positive HCV-PCR during antiviral therapy (breakthrough) or after end of treatment (relapse).
HCV genotyping was performed by a line probe assay (Versant HCV Genotype 2.0 Assay, Siemens/Realtime HCV Genotype II, Abbott) and assessment of viral load was performed by a PCR-based test (COBAS 4800; Roche Diagnostics, Rotkreuz, Schweiz). SVR was defined as undetectable HCV RNA by PCR at week 12 after treatment. The lower limit of detection of the HCV-PCR assay was 10 IU/ml at University Hospital Zurich and 15 IU/ml at Kantosspital St Gallen and Epatocentro Ticino. Liver fibrosis was assessed histologically by means of liver biopsy or non-invasively by transient elastography (Fibroscan®). Results were graded according to Metavir F stages with transient elastography values of >9.5 kPa corresponding to Metavir F3 while transient elastography values >12.5 equalling Metavir F4 [12]. If both biopsy and transient elastography results were available, transient elastography values were only used for analysis if more recent than biopsy.
To control for a selection bias, the study had defined inclusion and exclusion criteria, which reflects the Swiss CHC population undergoing antiviral treatment.
All patients with CHC undergoing DAA treatment between November 2013 and June 2016 from three tertiary Swiss hepatology centres were screened for study participation.
Statistical analysis was performed using SPSS 22.0 (IBM Corp, Armonk, NY USA). Graphs were generated using PowerPoint (Version 14.6.9, Microsoft PowerPoint Mac OS X 2011). Patients lost to follow-up were excluded before performing statistical evaluations (see fig. 1).
The study protocol was designed according to the ethical guidelines of the 1975 Helsinki Declaration. The ethics committee of the Canton of Zurich (BASEC 2016-00341) and the ethics committees of the participating centres (BASEC 2016-00171) approved the present study. Written informed consent of all patients was obtained. For the statistical analysis all clinical data were anonymised.
A total of 584 patients from three centres undergoing DAA treatment for CHC were evaluated. Eighteen patients did not meet inclusion criteria and were therefore excluded from further analysis (fig. 1). The 565 remaining patients that underwent DAA treatment with a minimal post-treatment follow-up of 12 weeks were included and a modified ITT analysis was performed. Characteristics of the cohort are shown in table 1. Seventy-one percent (403 of 565, 71.3%) of patients had Metavir F3 or F4 fibrosis as determined by biopsy or transient elastography. Two hundred and fifty-seven patients in our study suffered from liver cirrhosis (liver fibrosis stage F4). Among those cirrhotic patients there were 197 patients with Child-Pugh Class A (197 of 257, 77%) and 18 patients with Child-Pugh Class B (18 of 257, 7%). There was no cirrhotic patient with Child-Pugh Class C. Five-hundred-twelve patients received an interferon-free DAA combination ± ribavirin (512 of 565, 90.6%) and 53 patients a combination of PEG-IFN, ribavirin and sofosbuvir (53 of 565, 9.4%). Mean treatment duration for all patients was 15 weeks. Overall SVR rate was 94% (530 of 565, 93.8%, 95% CI 92–96%) for all patients treated (fig. 2). Of the patients without cirrhosis (fibrosis stage F1–F3), 95% reached SVR (286 of 300, 95% CI 93–97%). SVR rate in patients with liver cirrhosis (fibrosis stage F4) was lower (236 of 259, 92%, 95% CI 88–94%). Cirrhotic patients with Child-Pugh Class A reached SVR in 91% (179 of 197, 95% CI 87–95%) and cirrhotic patients with Child-Pugh Class B reached SVR in 83% (15 of 18, 95% CI 65–100%]) of cases. Fifty-five percent of all patients were treatment naïve (310 of 565, 54.9%), 46 patients had a concomitant HIV infection (46 of 565, 8.1%), 6 patients had a hepatitis B virus (HBV) infection (6 of 565, 1%) and 2 patients suffered from HIV and HBV co-infection (2 of 565, 0.4%). There was one case of each of the following: primary biliary cholangitis, primary sclerosing cholangitis, haemochromatosis, autoimmune hepatitis and Wilson’s disease (1 of 565, 0.2%).

Figure 1 Flow chart of patient recruitment.
Table 1 Patients' baseline characteristics (n = 565).
| Age (years), median (range) | 56 (21–86) |
| Male sex, n (%) | 356 (63.0) |
| Body mass index (kg/m 2 ), mean (range)] | 25.34 (16.0–41.9) |
| HCV genotype, n (%) | |
| 1 | 350 (62.0) |
| 2 | 51 (9.0) |
| 3 | 107 (18.9) |
| 4 | 57 (10.1) |
| HCV RNA (log10 IU/ml), median (range) | 6.08 (1.5–7.5) |
| Liver stiffness (kPa), median (range) | 12.10 (3.1–75.0) |
| Treatment history, n (%) | |
| Treatment naive | 310 (54.9) |
| Relapse | 128 (22.7) |
| Breakthrough | 19 (3.4) |
| Non-response | 85 (15.0) |
| Premature discontinuation | 21 (3.7) |
| Other | 2 (0.4) |
| Co-infections, n (%) | |
| HIV | 46 (8.1) |
| HBV | 6 (1.1) |
| HIV + HBV | 2 (0.4) |
| No co-infection | 511 (90.4) |
| Metavir, n (%) | |
| F1 (≤7.5) | 78 (13.8) |
| F2 (7.6–9.5) | 76 (13.5) |
| F3 (9.6–12.5) | 146 (25.8) |
| F4 (≥12.6) | 257 (45.5) |
| No data | 8 (1.4) |
HBV = hepatitis B virus; HCV = hepatitis C virus; HIV = human immunodeficiency virus
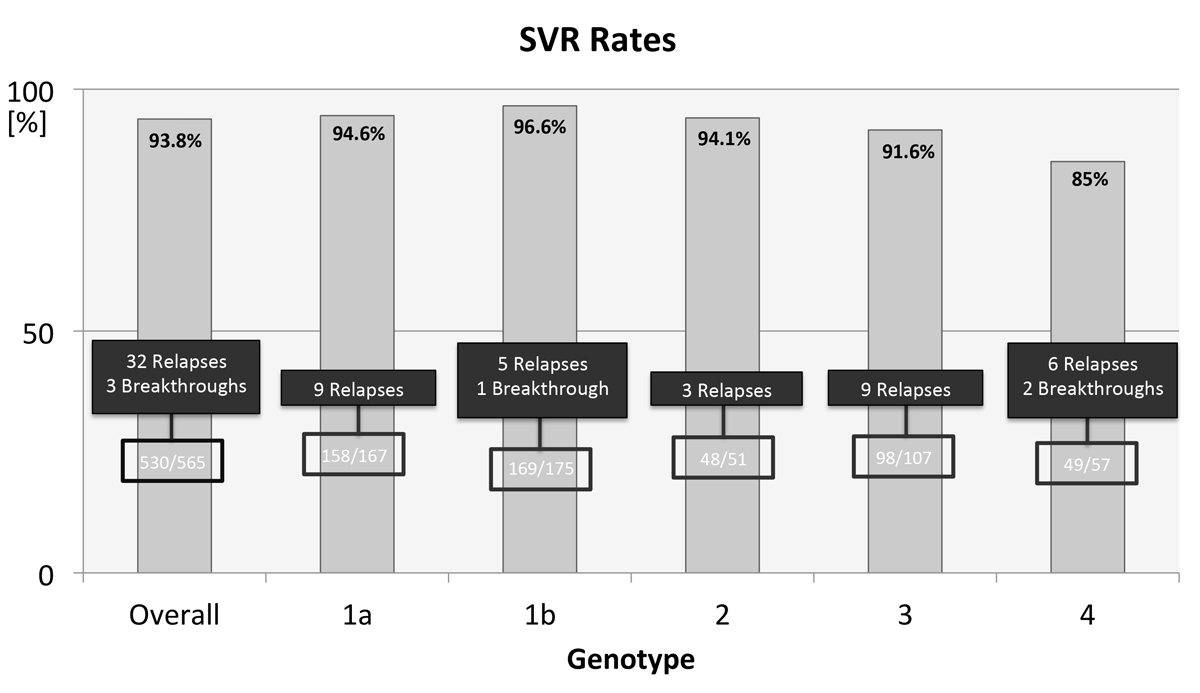
Figure 2 Overall SVR rate and SVR rates for individual HCV genotypes. Eight patients with not further specified genotype 1 are not included in this figure.
Reasons for exclusion from the final analysis were liver transplantation during the course of therapy (5 of 584, 0.9%), change in HCV treatment (2 of 584, 0.3%) and missing data (2 of 584, 0.3%). One patient stopped the treatment because of reasons other than adverse events (1 of 584, 0.2%). There were no SVR data for five patients at the time of analysis (5 of 584, 0.9%) and four patients (4 of 584, 0.7%) were lost to follow-up.
Reporting SVR results by ITT analysis, overall SVR rates were (530 of 575, 92%, 95% CI 90–94%). In this analysis the following 10 patients are included: one patient who stopped the treatment (1 of 10, 10%), five patients who had no SVR value (5 of 10, 50%) and four patients who were lost to follow-up (4 of 10, 40%). The following results on treatment outcome are from a modified ITT from which the previously mentioned 10 patients were excluded.
Patients with HCV genotype 1a (n = 167; supplementary fig. S1a in appendix 1) had an overall SVR rate of 95% (158 of 167, 95% CI 92–98%). Seventy-one percent (118 of 167) of patients with genotype 1a had advanced fibrosis (Metavir F3/4), and 94% (111 of 118, 95% CI 90–98%) of these achieved SVR. Genotype 1a patients received sofosbuvir-containing treatment in 81% (136 of 167), while 19% (31 of 167) were treated with a combination of ritonavir-boosted paritaprevir with ombitasvir and dasabuvir with (n = 26) or without (n = 5) ribavirin for 12 weeks. In the patient group that received paritaprevir/ritonavir/ombitasvir and dasabuvir, 3 out of 31 patients (9.6%) did not reach SVR. Sofosbuvir was most frequently combined with ledipasvir (95 of 136 sofosbuvir treatments, 70%), daclatasvir (13 of 135 sofosbuvir treatments, 9.5%) and simeprevir (11 of 136 sofosbuvir treatments, 8%). Seventeen patients were treated with sofosbuvir and ribavirin only (17 of 136 sofosbuvir treatments, 12.5%). Overall SVR rate of sofosbuvir-containing regimens was 95% (130 of 136, 95% CI 91–99%). Most relapses (5 out of 95, 5%) were seen in patients receiving a combination of sofosbuvir/ledipasvir with or without ribavirin for 8 weeks (one relapse without ribavirin; see fig. 3), 12 weeks (three relapses) or 24 weeks (one relapse). All patients who received sofosbuvir/ribavirin for 12 or 24 weeks (n = 17) or sofosbuvir/daclatasvir with or without ribavirin for 24 weeks (n = 13) reached SVR, while there was one relapse among the 11 patients treated with sofosbuvir/simeprevir/ribavirin for 12 weeks (1 of 11, 9%). Of all relapses, most patients were cirrhotic and/or had prior treatment failure (7 of 9, 78%).
Genotype 1b patients (fig. S1b) were treated with sofosbuvir-containing regimens in 69% (120 of 175). Most of these patients had a combination of sofosbuvir with ledipasvir with or without ribavirin (89 of 120, 74%). Twelve patients had a combination of sofosbuvir plus ribavirin (12 of 120, 10%), 8 patients received sofosbuvir plus daclatasvir with or without ribavirin (8 of 120, 7%) and 11 patients were treated with sofosbuvir plus simeprevir with or without ribavirin (11 of 120, 9%). Fifty-four patients were treated with paritaprevir/ritonavir/ombitasvir and dasabuvir with or without ribavirin in 31% of cases (54 of 175). One patient received a combination of daclatasvir/simeprevir for 12 weeks but did have a viral breakthrough. All other genotype 1b relapses occurred in patients treated with sofosbuvir containing regimens (5 of 120; 4%), while all patients that received paritaprevir/ritonavir/ombitasvir and dasabuvir with or without ribavirin for 12 weeks or 24 weeks reached SVR.
For genotype 2 HCV patients (fig. S2), the only DAA regimen available in Switzerland until 2016 was sofosbuvir/ribavirin for 12, 16, 20 or 24 weeks. Thirty-six were treated for 12 weeks, 5 for 16 weeks, 7 for 20 weeks and 3 for 24 weeks. Three out of 51 patients (6%) did not achieve SVR. One patient was a treatment-naïve cirrhotic and received sofosbuvir/ribavirin for 12 weeks, the other two patients were treatment-experienced. One of them was cirrhotic and was treated for 20 weeks.
Similarly, all 107 genotype 3 patients (fig. S3) received a sofosbuvir-based treatment. For most patients sofosbuvir, was combined with daclatasvir with or without ribavirin for 12 or 24 weeks (52 of 107; 49%) or sofosbuvir was given with ribavirin for 12, 24 or 36 weeks (50 of 107; 47%). Relapse rate in the sofosbuvir/ribavirin group was 6% (3 of 50, 6%), with one relapse after 12 weeks and two after 24 weeks of therapy. Four patients were treated with sofosbuvir/ledipasvir and one of these (25%) experienced a relapse. One patient received a combination with sofosbuvir and simeprevir without ribavirin for 12 weeks and did achieve SVR.
The majority of genotype 4 patients (fig. S4) also received sofosbuvir as part of the DAA treatment regimen (52 out of 57; 91%). The majority received sofosbuvir in combination with ribavirin (22 out of 52, 42%), followed by sofosbuvir/ledipasvir (20 out of 52, 38%). Seven patients were treated with a sofosbuvir/simeprevir regimen with ribavirin (5 of 7, 71%) for 12 weeks or without ribavirin (1 of 7, 14%) for 24 weeks. One patient (1 of 7, 14%) was treated with sofosbuvir/simeprevir for 12 weeks without ribavirin and still reached SVR. Three patients received sofosbuvir/daclatasvir with (1 out of 3, 33%) or without ribavirin (2 out of 3, 67%) for 24 weeks. The remaining 9% (5 out of 57, 9%) were treated with paritaprevir/ritonavir/ombitasvir and ribavirin for 12 weeks.
Of all HCV genotypes, patients with genotype 4 had the largest number of relapses (8 out of 57; 14%). Of all genotype 4 patients 56% (32 out of 57) were treatment-experienced and 53% (30 out of 57) were cirrhotic. Genotype 4 without SVR were treatment-experienced in 63% (5 of 8) and cirrhotic in 63% (5 of 8) of cases. Most relapses occurred in the group that received sofosbuvir/ribavirin for 24 weeks, with 5 (5 out of 22, 23%) patients relapsing and one (1 out of 22, 5%) showing a breakthrough during therapy. In contrast, all patients that received sofosbuvir/ledipasvir (± ribavirin for 12 or 24 weeks) (20 of 20, 100%) and all three that received sofosbuvir/daclatasvir (± ribavirin for 24 weeks) reached SVR. Of 5 patients who received paritaprevir/ritonavir/ombitasvir with ribavirin for 12 weeks, 4 patients achieved SVR (4 out of 5, 80%) with 1 breakthrough.
Overall, 53 patients received their DAA treatment in combination with PEG-IFN. Sixteen patients (16 out of 167; 10%) with genotype 1a received a sofosbuvir/ribavirin therapy with PEG-IFN for 12 weeks (15 out of 16) or 24 weeks (1 out of 16). All of them reached SVR. In the genotype 1b group only 11 patients (11 out of 175; 6%) received a sofosbuvir/ribavirin/PEG-IFN combination for 12 weeks. Two of them did not reach SVR (2 out of 11). Of all genotypes, genotype 3 had the largest number of patients treated with PEG-IFN (20 out of 107; 19%). Ten patients received a sofosbuvir/ribavirin/PEG-IFN combination for 12 or 24 weeks, and 10 received a sofosbuvir/daclatasvir/PEG-IFN combination with or without ribavirin. Two (10%) of the genotype 3 patients that received DAA plus PEG-IFN did not achieve SVR. Six patients in the genotype 4 group also received a combination with sofosbuvir/ribavirin and PEG-IFN for 12 weeks (6 of 57; 11%) and they all reached SVR.
As shown in figure 2, 35 patients in total did not achieve an SVR (32 relapses, 3 breakthroughs). If a relapse occurred, it usually became apparent within the first four weeks of the end of treatment. Later relapses were only seen in 3 out of 35 cases. Patients who did relapse had advanced fibrosis in 89% (31 out of 35) versus 68% in the group of patients that did achieve SVR (358 out of 530). Of all 35 patients who experienced treatment failure, 17 were treatment-naïve (49%). Of all 530 patients who reached SVR, 293 were treatment naïve (293 out of 530, 55%).
Resistance-associated substitutions (RAS) were analysed in 14 out of 35 (40%) treatment failure patients. The most frequent RAS occurred in the NS5A gene (9 out of 14; 60%). This is in concordance with international studies on RAS incidence [13–15]
Re-treatment of this group was successful in a large proportion of patients (fig. 3). Of 16 patients that did receive re-treatment, 14 patients achieved SVR (88%). For several patients, reimbursement of re-treatment is currently under revision. Only one patient did not have any further treatment options based on his RAS profile.
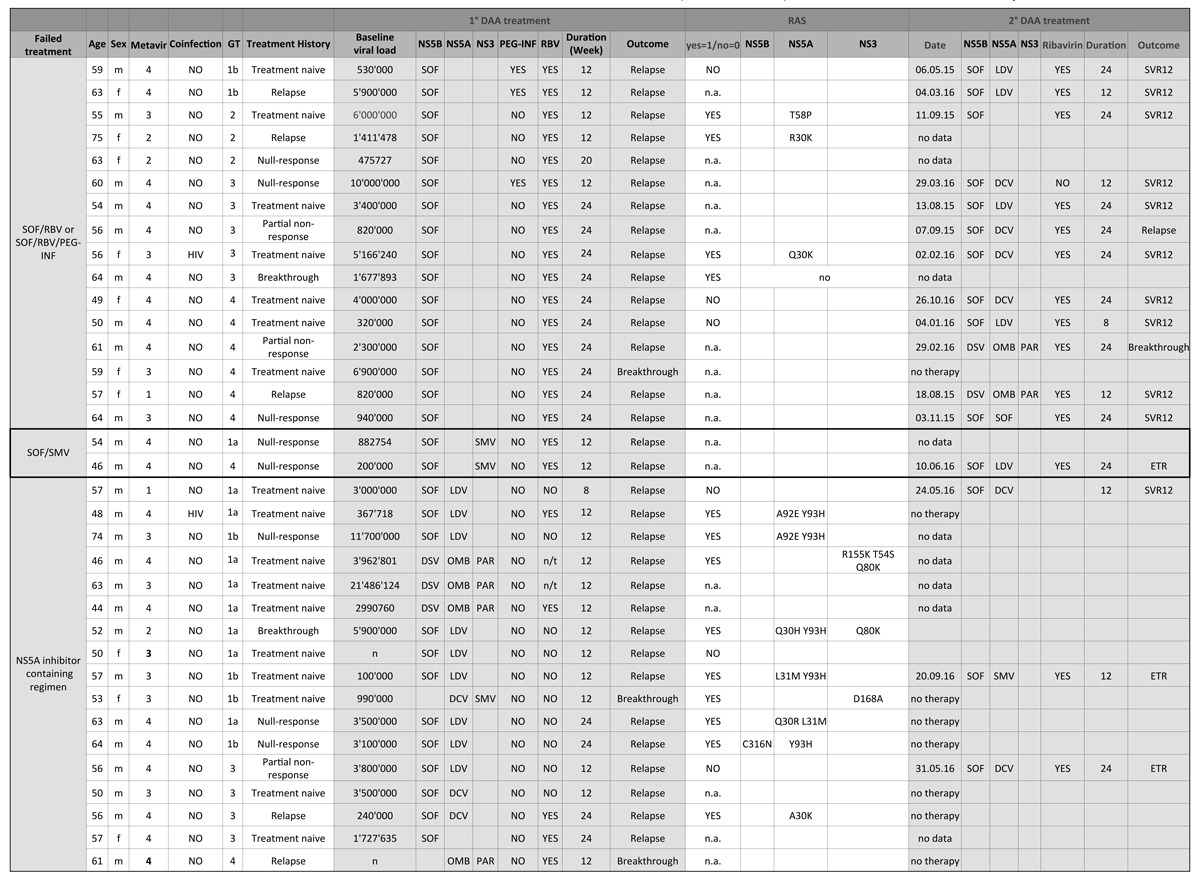
Figure 3 Treatment and re-treatment of relapsing patients and clinically relevant RAS.
DCV = daclatasvir; DSV = dasabuvir; ETR = end-of-treatment response; GT = genotype; HIV = human immunodeficiency virus; LDV = ledipasvir; n.a. = test results not available or no RAS test was performed; n/t = not taken (patient did not take RBV); PAR = paritaprevir; OMB = ombitasvir; PEG-INF = pegylated interferon; RAS = resistance associated substitutions; RBV = ribavirin; SMV = simeprevir; SOF = sofosbuvir; SVR = sustained virological response; SVR12 = negative HCV-PCR 12 weeks after end of treatment; SVR24 = negative HCV-PCR 12 weeks after end of treatment.
* No RAS detected.
This Swiss data for novel DAA treatment of CHC shows SVR rates very similar to those from the registration trials [1–3, 16]. This is in accordance with other studies that also reproduce the generally excellent SVR rates, especially among patients with genotype 1 CHC [17–19]. For the observed time period between 2014 and mid-2016, genotypes 2 and especially 4 had limited treatment options. The data reported here do not take into account the introduction of novel DAAs such as elbasvir/grazoprevir (April 2016) and sofosbuvir/velpatasvir (January 2017) that were licensed after this study and should significantly improve the SVR rates in these patient groups [20, 21]
Most relapses were detected early in the post-treatment period, usually within the first 4 weeks. Fortunately, re-treatment was successful in most patients.
Access to DAA treatment and reimbursement of the very high therapy costs is a worldwide challenge for HCV patients as well as for healthcare systems [22–25]. Limitations of access and reimbursement have been introduced in many countries [26]. Beginning in 2014, the reimbursement of DAA therapies in Switzerland was limited to patients that had histologically verified advanced fibrosis or cirrhosis (Metavir F3 or F4) or showed transient elastography values >9.5 kPa in two consecutive measurements with a minimum interval of 3 months [27]. Moreover, several of the recommended therapeutics were not approved or reimbursed. This made modifications and deviations from the treatment recommendations by EASL necessary in 2014 and early 2015. Thirty percent of patients that did suffer a relapse were not treated according to international guidelines due to limitations of availability or reimbursement. Until the market introduction of paritaprevir/ritonavir/ombitasvir plus ribavirin, and grazoprevir/elbasvir in mid-2016, there was no approved interferon-free treatment regimen for HCV genotype 4 available in Switzerland, and alternative treatments required case-by-case approval of reimbursement by health insurance providers.
Since the analysis of our data, several pan-genotypic treatments have been approved that show high efficacy and SVR rates. Moreover, limitation for treatment reimbursement according to fibrosis grade has been lifted meanwhile. These new circumstances expand the population of HCV patients that will be treated. Future studies will need to include data on these new patient populations and medications.
This study highlights the importance of the early adoption of internationally accepted treatment guidelines, if possible. Moreover, approved but suboptimal treatment regimens that did comply with national drug availability and reimbursement limitations may have generated excessive costs for the healthcare system.
Our study has limitations. Patients were only included from three referral centres in this non-randomised observational study with a retro- and prospective follow-up. Therefore, the study population might not reflect the standard Swiss HCV population. But at the same time, patients with advanced fibrosis and cirrhosis might be overrepresented in this tertiary care population. As we have shown, the SVR rate in this population is lower, and therefore these excellent SVR results might nevertheless be representative for Switzerland. Finally, the median follow-up period is relatively short, but it is well documented that late relapses are extremely uncommon [28]. Accordingly, this short follow-up has no relevant impact on the SVR reported in this study
In summary, data from Switzerland for DAA treatment in CHC confirm the excellent efficacy of these drugs. These results are novel and have not been reported for Switzerland so far. They are in line with other national and international studies reporting excellent overall outcomes for DAA in a post-approval real-life setting. Patients who do suffer a relapse can in most cases be successfully re-treated. Limitations of access prevented optimal therapy in some patients.
Patients with relapse or breakthrough are marked in orange. Patients marked in yellow received PEG-IFN in addition to their DAA therapy. The number of patients receiving PEG-IFN is marked with *.
16 patients with genotype 1a and 11 patients with genotype 1b were treated with DAA plus PEG-IFN. None of the patients with genotype 2 were treated with additional PEG-IFN. Twenty patients with genotype 3 and 6 patients with genotype 4 were treated with DAA plus PEG-IFN. Eight patients with HCV genotype 1 were excluded from analysis becaue of lack of HCV subtyping.
NON = no second DAA; n/t = not taken (patient did not take RBV); GT = genotype; BT = breakthrough; DCV = daclatasvir; DSV = dasabuvir; GT = genotype; LDV = ledipasvir; PTV/r/OBV = paritaprevir/ritonavir/ombitasvir; RBV = ribavirin; SMV = simeprevir; SOF = sofosbuvir; PEG-IFN = pegylated interferon.
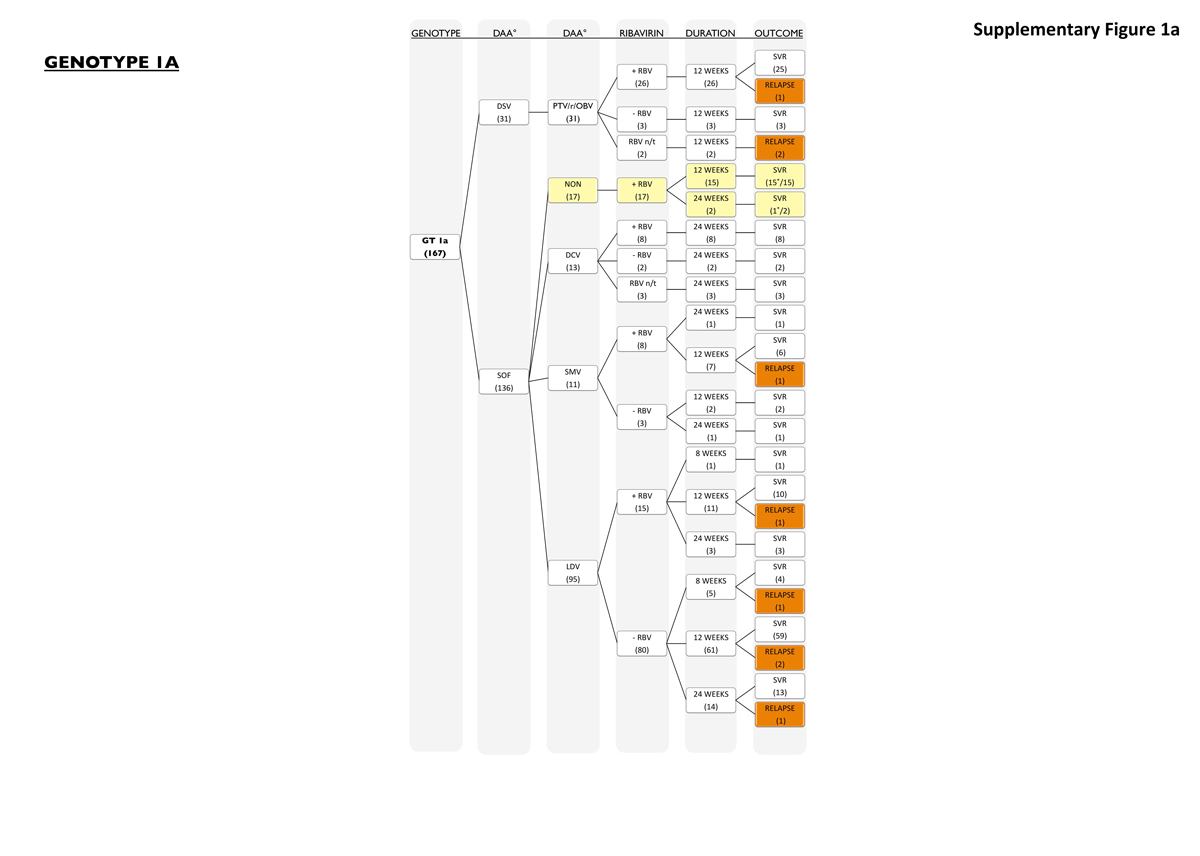
Figure S1a Patients with genotype 1a.
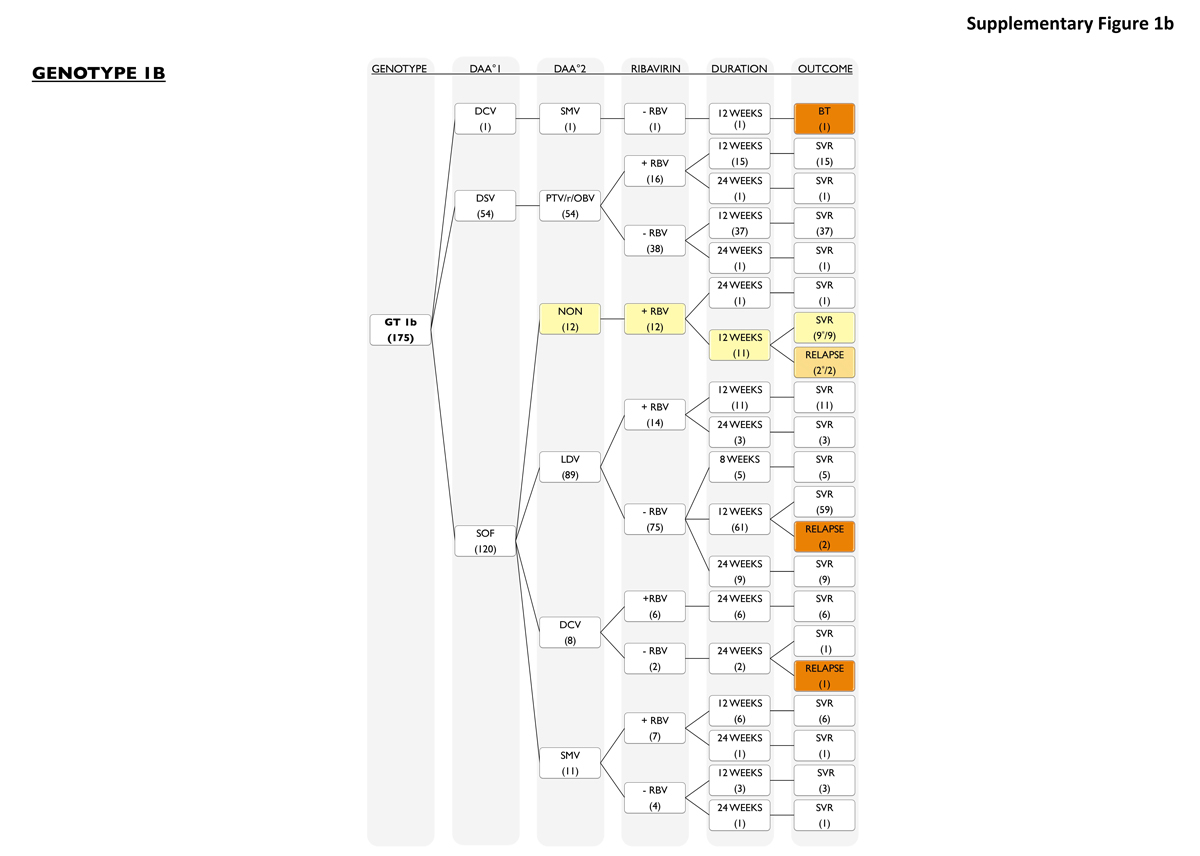
Figure S1b Patients with genotype 1b.
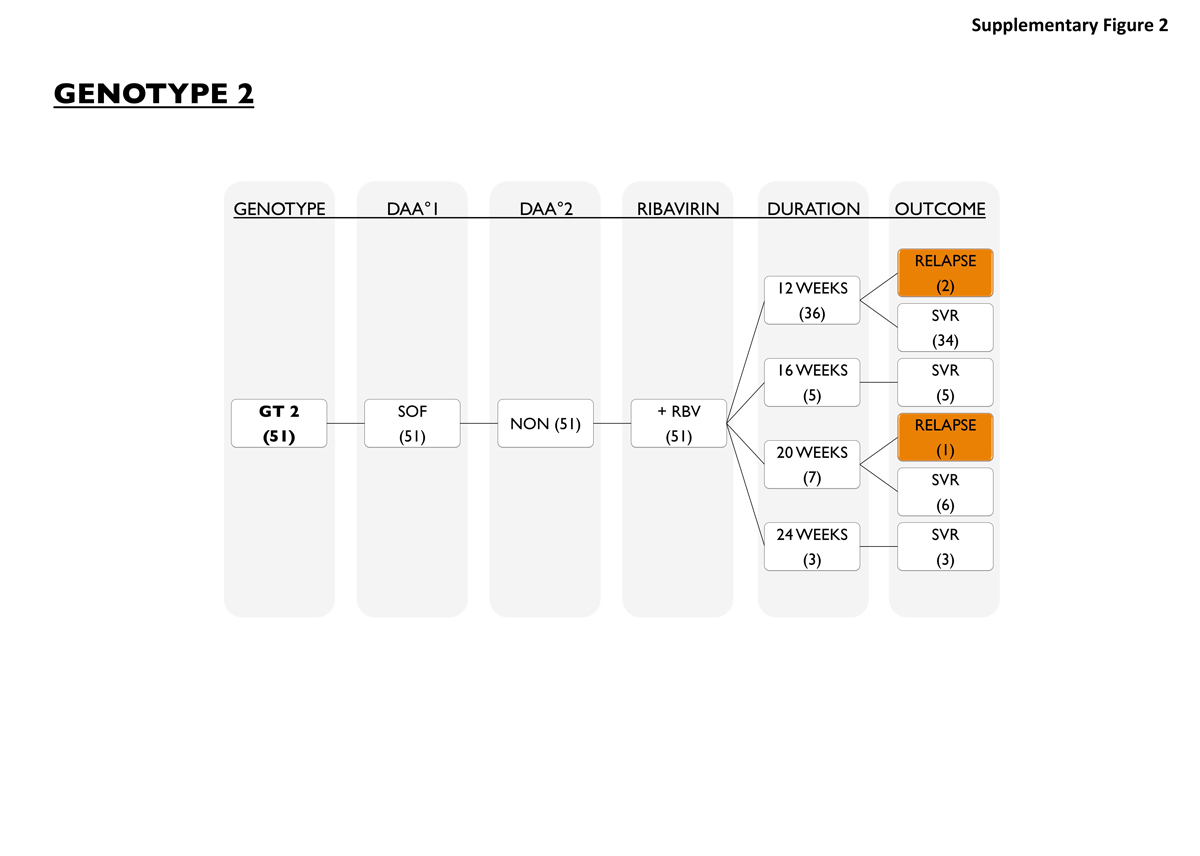
Figure S2 Patients with genotype 2.
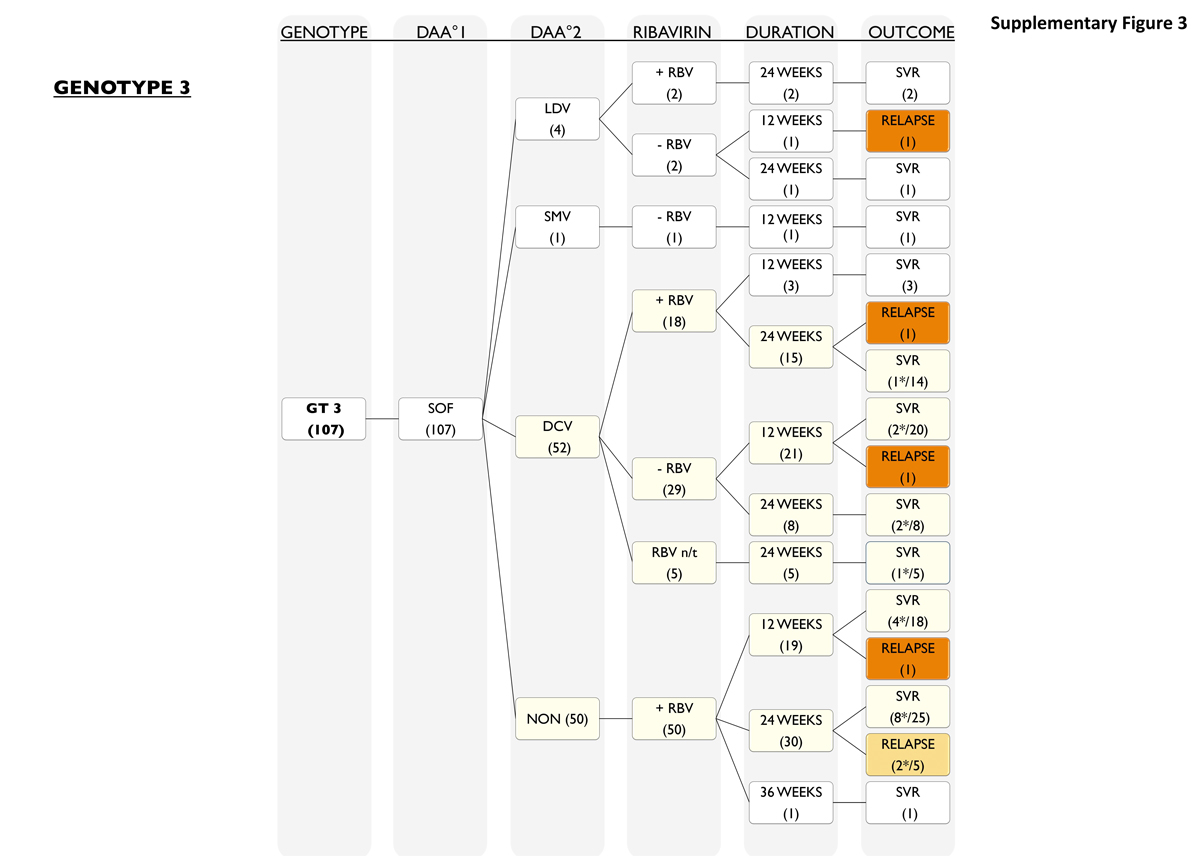
Figure S3 Patients with genotype 3.
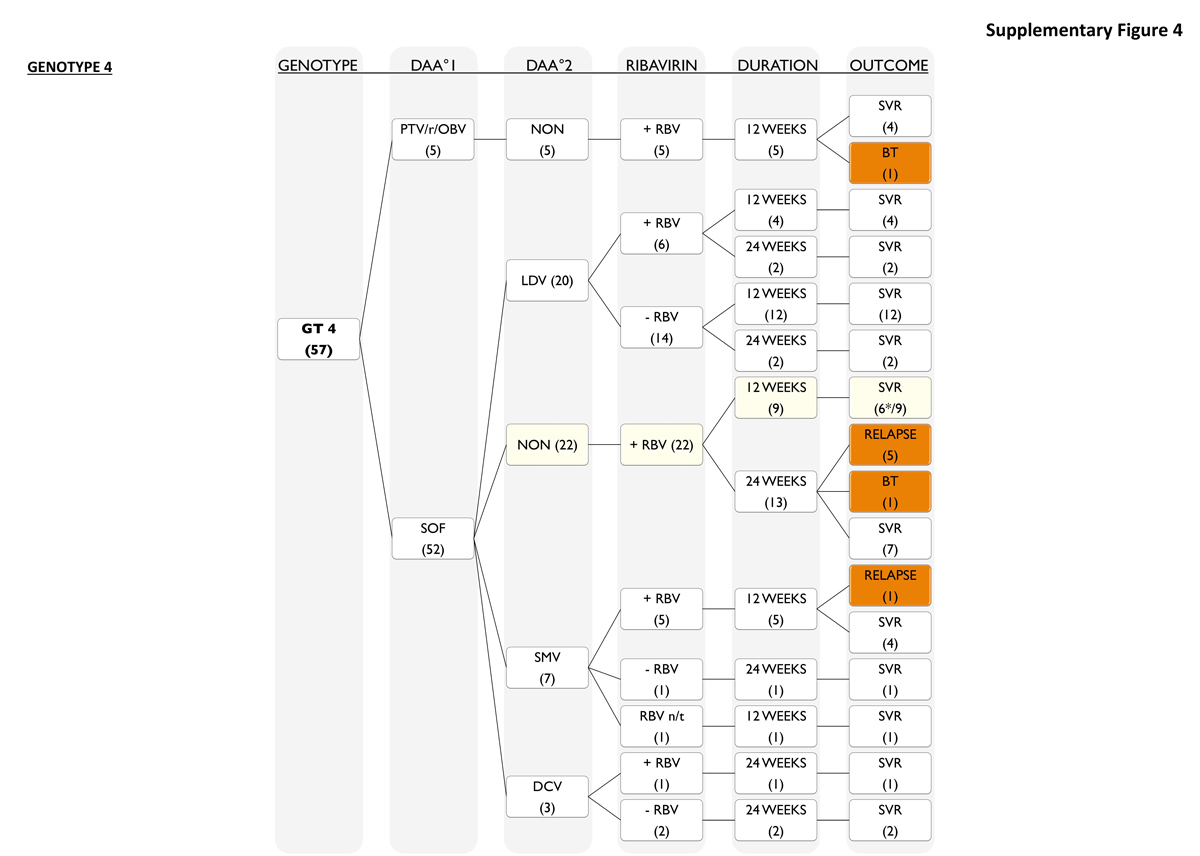
Figure S4 Patients with genotype 4.
We would like to thank Fondazione Epatocentro Ticino for their support of this research.
This study was supported by Gilead Pharmaceuticals Inc. and Abbvie Pharmaceuticals.
JM has received consulting honoraria from Abbvie, BMS, Gilead, Janssen, Merck and MSD. BM has received consulting honoraria from Abbvie, BMS, Gilead, Janssen, Merck and MSD. PVV has received consulting honoraria from Gilead. PK has received consulting honoraria from Abbvie and Gilead. AK has received consulting honoraria from Gilead. LM has received consulting honoraria from Abbvie, Gilead, BMS, MSD and Janssen. BTBP has received consulting honoraria from BMS.
1 Lawitz E , Mangia A , Wyles D , Rodriguez-Torres M , Hassanein T , Gordon SC , et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–87. doi:.https://doi.org/10.1056/NEJMoa1214853
2 Zeuzem S , Dusheiko GM , Salupere R , Mangia A , Flisiak R , Hyland RH , et al.; VALENCE Investigators. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993–2001. doi:.https://doi.org/10.1056/NEJMoa1316145
3 Lawitz E , Sulkowski MS , Ghalib R , Rodriguez-Torres M , Younossi ZM , Corregidor A , et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384(9956):1756–65. doi:.https://doi.org/10.1016/S0140-6736(14)61036-9
4 European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63(1):199–236. doi:.https://doi.org/10.1016/j.jhep.2015.03.025
5 AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–54. doi:.https://doi.org/10.1002/hep.27950
6 Butt AA , Yan P , Shaikh OS , Freiberg MS , Lo Re V, 3rd , Justice AC , et al.; ERCHIVES (Electronically Retrieved Cohort of HCV Infected Veterans) Study Team. Virologic response and haematologic toxicity of boceprevir- and telaprevir-containing regimens in actual clinical settings. J Viral Hepat. 2015;22(9):691–700. doi:.https://doi.org/10.1111/jvh.12375
7 Backus LI , Belperio PS , Shahoumian TA , Cheung R , Mole LA . Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39(1):93–103. doi:.https://doi.org/10.1111/apt.12546
8 Conti F , Brillanti S , Buonfiglioli F , Vukotic R , Morelli MC , Lalanne C , et al. Safety and efficacy of direct acting antivirals for the treatment of chronic hepatitis C in a real-world population aged 65 years and older. J Viral Hepat. 2017;24(6):454–63.
9 Milazzo L , Lai A , Calvi E , Ronzi P , Micheli V , Binda F , et al. Direct-acting antivirals in hepatitis C virus (HCV)-infected and HCV/HIV-coinfected patients: real-life safety and efficacy. HIV Med. 2017;18(4):284–91.
10 von Elm E , Altman DG , Egger M , Pocock SJ , Gøtzsche PC , Vandenbroucke JP ; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7. doi:.https://doi.org/10.7326/0003-4819-147-8-200710160-00010
11 Prasad L , Spicher VM , Zwahlen M , Rickenbach M , Helbling B , Negro F ; Swiss Hepatitis C Cohort Study Group. Cohort Profile: the Swiss Hepatitis C Cohort Study (SCCS). Int J Epidemiol. 2007;36(4):731–7. doi:.https://doi.org/10.1093/ije/dym096
12 Castera L , Forns X , Alberti A . Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48(5):835–47. doi:.https://doi.org/10.1016/j.jhep.2008.02.008
13 Parczewski M , Leszczyszyn-Pynka M , Urbańska A . Differences in the integrase and reverse transcriptase transmitted resistance patterns in Northern Poland. Infect Genet Evol. 2017;49:122–9. doi:.https://doi.org/10.1016/j.meegid.2016.12.019
14 Peres-da-Silva A , Brandão-Mello CE , Lampe E . Prevalence of sofosbuvir resistance-associated variants in Brazilian and worldwide NS5B sequences of genotype-1 HCV. Antivir Ther. 2017;22(5):447–51. doi:.https://doi.org/10.3851/IMP3131
15 Ogawa E , Furusyo N , Nomura H , Dohmen K , Higashi N , Takahashi K , et al. NS5A resistance-associated variants undermine the effectiveness of ledipasvir and sofosbuvir for cirrhotic patients infected with HCV genotype 1b. J Gastroenterol. 2017;52(7):845–54.
16 Andreone P , Colombo MG , Enejosa JV , Koksal I , Ferenci P , Maieron A , et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147(2):359–365.e1. doi:.https://doi.org/10.1053/j.gastro.2014.04.045
17 Belli LS , Berenguer M , Cortesi PA , Strazzabosco M , Rockenschaub SR , Martini S , et al.; European Liver and Intestine Association (ELITA). Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study. J Hepatol. 2016;65(3):524–31. doi:.https://doi.org/10.1016/j.jhep.2016.05.010
18 d’Arminio Monforte A , Cozzi-Lepri A , Ceccherini-Silberstein F , De Luca A , Lo Caputo S , Castagna A , et al.; Icona Foundation and HepaIcona Study Group. Access and response to direct antiviral agents (DAA) in HIV-HCV co-infected patients in Italy: Data from the Icona cohort. PLoS One. 2017;12(5):e0177402. doi:.https://doi.org/10.1371/journal.pone.0177402
19 Bielen R , Moreno C , Van Vlierberghe H , Bourgeois S , Mulkay JP , Vanwolleghem T , et al. Belgian experience with direct acting antivirals in people who inject drugs. Drug Alcohol Depend. 2017;177:214–20. doi:.https://doi.org/10.1016/j.drugalcdep.2017.04.003
20 Afdhal N , Zeuzem S , Kwo P , Chojkier M , Gitlin N , Puoti M , et al.; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–98. doi:.https://doi.org/10.1056/NEJMoa1402454
21 Foster GR , Afdhal N , Roberts SK , Bräu N , Gane EJ , Pianko S , et al.; ASTRAL-2 Investigators; ASTRAL-3 Investigators. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015;373(27):2608–17. doi:.https://doi.org/10.1056/NEJMoa1512612
22 Kim DY , Han KH , Jun B , Kim TH , Park S , Ward T , et al. Estimating the Cost-Effectiveness of One-Time Screening and Treatment for Hepatitis C in Korea. PLoS One. 2017;12(1):e0167770. doi:.https://doi.org/10.1371/journal.pone.0167770
23 Ayoub HH , Abu-Raddad LJ . Impact of treatment on hepatitis C virus transmission and incidence in Egypt: A case for treatment as prevention. J Viral Hepat. 2017;24(6):486–95.
24 Ward T , Gordon J , Bennett H , Webster S , Sugrue D , Jones B , et al. Tackling the burden of the hepatitis C virus in the UK: characterizing and assessing the clinical and economic consequences. Public Health. 2016;141:42–51. doi:.https://doi.org/10.1016/j.puhe.2016.08.002
25 Bach TA , Zaiken K . Real-World Drug Costs of Treating Hepatitis C Genotypes 1-4 with Direct-Acting Antivirals: Initiating Treatment at Fibrosis 0-2 and 3-4. J Manag Care Spec Pharm. 2016;22(12):1437–45. doi:.https://doi.org/10.18553/jmcp.2016.22.12.1437
26 Gentile I , Maraolo AE , Niola M , Graziano V , Borgia G , Paternoster M . Limiting the access to direct-acting antivirals against HCV: an ethical dilemma. Expert Rev Gastroenterol Hepatol. 2016;10(11):1227–34. doi:.https://doi.org/10.1080/17474124.2016.1234375
27 Bachofner JA , Valli PV , Kroger A , Bergamin I , Kunzler P , Baserga A , et al. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int. 2017;37(3):369–76.
28 Reddy KR , Pol S , Thuluvath PJ , Kumada H , Toyota J , Chayama K , et al. Long-term follow-up of clinical trial patients treated for chronic HCV infection with daclatasvir-based regimens. Liver Int. 2017 Sep 21 [Epub ahead of print] doi:.https://doi.org/10.1111/liv.13596
JAB and PVV contributed equally to the study. The manuscript has been reviewed and approved by all authors. The manuscript was endorsed by the Swiss Hepatitis C Cohort Study (SCCS).
This study was supported by Gilead Pharmaceuticals Inc. and Abbvie Pharmaceuticals.
JM has received consulting honoraria from Abbvie, BMS, Gilead, Janssen, Merck and MSD. BM has received consulting honoraria from Abbvie, BMS, Gilead, Janssen, Merck and MSD. PVV has received consulting honoraria from Gilead. PK has received consulting honoraria from Abbvie and Gilead. AK has received consulting honoraria from Gilead. LM has received consulting honoraria from Abbvie, Gilead, BMS, MSD and Janssen. BTBP has received consulting honoraria from BMS.