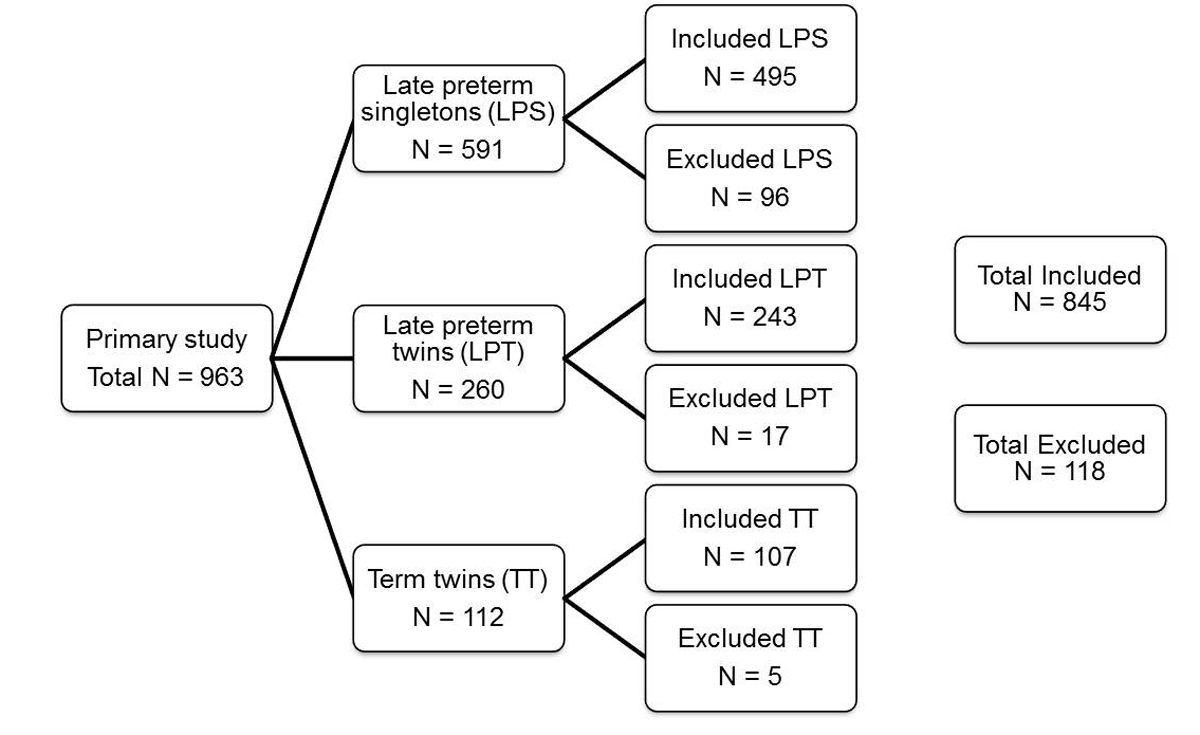
Figure 1 Study recruitment and exclusions.
DOI: https://doi.org/10.4414/smw.2018.14581
Late preterm infants are defined as infants born between 34 0/7 and 36 6/7 weeks of gestation [1–3]. In Switzerland, approximately 6% of live born infants are late preterm infants, which corresponds to about 70% of all prematurely born infants. In the past, late preterm infants have been regarded as almost term infants and their neonatal morbidity was therefore as assumed to be equal to that of term infants. Increasingly, however, it has been shown that late preterm infants have a higher risk for postnatal complications due to their immaturity which requires longer hospitalisation [1, 3–5]. Complications include respiratory distress syndrome, transient tachypnoea of the newborn, hyperbilirubinaemia, hypoglycaemia, feeding problems, instability in body temperature apnoea, sepsis and intraventricular haemorrhage [6–12].
More than half of multiple pregnancies are associated with preterm birth, most of them for medical reasons [13]. The number of multiple pregnancies has increased in recent years [13–15]. Compared with singleton pregnancies, multiple pregnancies have an overall higher risk for complications in pregnancy, including preeclampsia or preterm premature rupture of membranes (PPROM). It can be assumed that late preterm twins (LPT) are likely to have an even higher risk for postnatal complications, such as respiratory distress, hypoglycaemia or hyperbilirubinaemia, than late preterm singletons (LPS) [5, 16].
The objective of this study was to examine if LPT require longer postnatal hospitalisation and if LPT have a higher neonatal morbidity and mortality compared with LPS and term twins (TT).
This was a single centre retrospective analysis. The University Hospital Inselspital Bern is one of five Swiss university hospitals and accommodates one of nine Swiss neonatal units providing tertiary neonatal care. Annually, around 1600 infants are born at our hospital, including 120–140 very preterm infants with a gestational age below 32 weeks, and around 100 twins. The unit of obstetrics has special expertise in the treatment of monochorionic twins with prenatal laser therapy in twins with imminent feto-fetal transfusion syndrome.
All late preterm infants (twins and singletons) as well as all TT from 37 0/7 to 41 6/7 weeks of gestation who were born between January 2009 and December 2013 at the University Hospital Inselspital Bern, Switzerland, were eligible for this study. Twins were only included when both infants were born alive. Each twin was analysed separately with regard to the exclusion criteria. If one twin fulfilled the exclusion criteria, he or she was excluded. Exclusion of one twin, however, did not automatically mean exclusion of the other as long as they did not meet any exclusion criterion.
All patients or mothers with incomplete documentation were excluded. Infants with fetal malformations that required prolonged hospitalisation and patients suffering from postnatal drug withdrawal were excluded.
Data were retrieved from local databases as well as hospital discharge reports, and collected in a password-protected electronic database. Data were collected for the interval between birth and discharge home. The study was approved by the local Institutional Ethics Board.
Gestational age was defined according to the last menstrual period and confirmed by first trimester ultrasound. HELLP syndrome (haemolysis, elevated liver enzyme levels, low platelet count) was defined by the International Society for the Study of Hypertension in Pregnancy [17]. Maternal infection included all women with a known urogenital infection sub partu. Cases where women had completed antibiotic therapy before birth or had an unknown group B streptococcus status were not classified as infections.
Swiss recommendations concerning treatment of late preterm infants state that neonates with a gestational age of 34–35 weeks and/or a birth weight of less than 1800–2000 g should be transferred to a neonatal unit. Neither Swiss nor local recommendations changed during the observation period.
Hyperbilirubinaemia was defined according to the Swiss Neonatal Guidelines [18]. Hypoglycaemia was defined as serum glucose levels below 2.5 mmol/l [19]. Respiratory distress syndrome was defined as clinical signs of respiratory distress, such as grunting, nasal flaring, retractions, tachypnoea or additional oxygen demand requiring therapeutic intervention or hospital admission. Antibiotic therapy was administered in cases were an infection was suspected on the basis of clinical signs and/or relevant changes in laboratory parameters. Sepsis was defined as clinical or laboratory signs of sepsis or positive blood culture and was treated with antibiotics for 5–7 days or longer. Patients with a positive blood culture were included; however, a positive blood culture was not mandatory for diagnosis of sepsis. Necrotising enterocolitis was classified according to the Bell classification [20]. For statistical analysis, only necrotising enterocolitis of stage II or higher was defined as presence of necrotising enterocolitis.
In our hospital, cranial ultrasound is performed only if a clinical indication is present, including microcephaly, traumatic birth, large cephalic hematoma, prenatally suspected pathology. Intraventricular haemorrhage was defined according to the Papile classification [21].
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 21. For analysis between LPS and LPT, we used Fisher’s test and/or χ2 test for categorical variables and Mann-Whitney U test for continuous variables. Likewise, for analysis between LPT and TT, we used Fisher’s test and/or χ2 test for categorical variables and Mann-Whitney U-test for continuous variables. Normal distribution was assumed for all variables. Probability levels below <0.05 were considered significant.
Overall, 845 of 963 (87.7%) eligible newborn infants were included during the 5-year study period (fig. 1); 118 newborn infants were excluded because of congenital comorbidities, neonatal drug withdrawal syndrome or missing data, as defined in the exclusion criteria. Of the included newborns (n = 845), 495 (58.6%) were LPS, 243 (28.7%) LPT and 107 (12.7%) TT. Outcomes at birth of LPS and LPT are summarised in table 1. The mean gestational age of LPS was significantly higher with 35 3/7 weeks than the mean gestational age of LPT with 35 2/7 weeks (p = 0.035). The LPS group included 53.5% males, and the LPT group 51.0% males. Significantly more LPT than LPS were born via caesarean section (p <0.001). Compared with LPS, LPT had a significantly lower mean birth weight (2214 vs 2399 g, p <0.001). Outcomes at birth of LPT compared with TT at birth are summarised in table 2. The mean gestational age of TT was 37 5/7 weeks. There were significantly more monochorionic pregnancies in the LPT group than in the TT group (83.2 vs 70.8%, p = 0.016). Both LPT and TT were more frequently delivered via caesarean section than born spontaneously. With a mean birth weight of 2731g, TT were significantly heavier at birth (p <0.001).

Figure 1 Study recruitment and exclusions.
Table 1 Characteristics at birth of late preterm singletons compared with late preterm twins.
| Variable |
LPT
(n = 243) |
LPS
(n = 495) |
p-value |
|---|---|---|---|
| Gestational age at delivery (weeks)† | 35.2 ± 0.9 | 35.3 ± 0.9 | 0.035* |
| Male gender | 51.0% | 53.5% | 0.531 |
| Spontaneous delivery | 13.6% | 35.8% | <0.001* |
| Vaginal operative delivery | 4.5% | 4.6% | 1.000 |
| Caesarean-section | 81.9% | 59.0% | <0.001* |
| Apgar score (1 min) | 7.2 ±1.7 (7–8) |
7.1 ±1.9 (6–8) |
0.384 |
| Apgar score (5 min) | 8.5 ±1.1 (8–9) |
8.4 ±1.3 (8–9) |
0.181 |
| Apgar score (10 min) | 9.3 ± 0.9 (9–10) |
9.1 ±1.0 (9–10) |
0.013* |
| pH umbilical artery† | 7.30 ± 0.06 (7.28–7.33) |
7.29 ± 0.07 (7.26–7.33) |
0.111 |
| Birth weight (g)† | 2214 ± 376 (1960–2470) |
2399 ±474 (2070–2670) |
<0.001* |
| Length (cm)† | 45.7 ± 2.2 (44.0–47.0) |
46.0 ± 2.4 (44.0–47.5) |
0.096 |
| Head circumference (cm)† | 32.4 ± 1.4 (31.5–33.5) |
32.5 ± 1.6 (31.5–33.7) |
0.353 |
LPS = late preterm singletons; LPT = late preterm twins * p <0.05 † mean + standard deviation (interquartile range)
Table 2 Characteristics at birth of late preterm twins compared with term twins.
| Variable |
LPT
(n = 243) |
TT
(n = 107) |
p-value |
|---|---|---|---|
| Gestational age at delivery (weeks)† | 35.2 ± 0.9 | 37.8 ± 0.5 | <0.001* |
| Male gender | 51.0% | 54.2% | 0.643 |
| Spontaneous delivery | 13.6% | 17.8% | 0.330 |
| Vaginal operative delivery | 4.5% | 3.7% | 1.000 |
| Caesarean-section | 81.9% | 78.5% | 0.464 |
| Apgar score (1 min) | 7.2 ±1.7 (7–8) |
7.5 ±1.7 (7–9) |
0.025* |
| Apgar score (5 min) | 8.5 ±1.1 (8–9) |
8.6 ±1.3 (8–9) |
0.396 |
| Apgar score (10 min) | 9.3 ±0.9 (9–10) |
9.4 ±0.9 (9–10) |
0.084 |
| pH umbilical artery† | 7.30 ± 0.06 (7.28–7.33) |
7.28 ± 0.09 (7.26–7.33) |
0.207 |
| Birth weight (g)† | 2214 ± 376 (1960–2470) |
2731 ± 356 (2470–2970) |
<0.001* |
| Birth length (cm)† | 45.7 ± 2.2 (44.0–47.0) |
47.3 ± 1.9 (46.0–49.0) |
<0.001* |
| Head circumference (cm)† | 32.4 ± 1.4 (31.5–33.5) |
33.6 ± 1.2 (33.0–34.6) |
<0.001* |
LPT = late preterm twins; TT = term twins * p <0.05 † mean + standard deviation (interquartile range)
There were no significant differences regarding neonatal morbidity between LPT and TT when stratified for chorionicity, except for duration of neonatal hospitalisation and Apgar score at 10 minutes. Dichorionic LPT had a significantly lower Apgar at 10 minutes than dichorionic TT (p = 0.048). Both monochorionic and dichorionic LPT were hospitalised significantly more frequent (p ≤0.001) and longer (p ≤0.001) than their respective TT counterparts.
Maternal characteristics of the three groups are summarised in table 3. PPROM was significantly more common in the LPS group than in the LPT group (p = 0.002). HELLP syndrome and maternal infection at birth, however, occurred more frequently in the LPT group than in the LPS group. In LPT mothers, preeclampsia/eclampsia (p = 0.006) as well as PPROM (p <0.001) occurred significantly more often than in TT mothers. There were no significant differences regarding diabetes or maternal infection between LPS and LPT as well as LPT and TT, respectively.
Table 3 Maternal characteristics for all three groups.
| Variable |
LPT
(n = 125) |
LPS
(n = 495) |
TT
(n = 56) |
p-value |
|---|---|---|---|---|
| Preeclampsia/eclampsia | 12.0% | 12.5% | 0.0% | 0.019* |
| HELLP | 4.8% | 2.6% | 0.0% | 0.174 |
| Gestational diabetes mellitus | 9.6% | 8.9% | 8.9% | 0.969 |
| Diabetes mellitus type I | 0.0% | 1.2% | 0.0% | 0.330 |
| Diabetes mellitus type II | 0.0% | 0.4% | 0.0% | 0.693 |
| PPROM | 26.4% | 41.4% | 0.0% | <0.001** |
| Infection | 14.4% | 11.3% | 7.1% | 0.352 |
| Dichorionic-diamniotic | 70.8% | n/a | 83.2% | 0.016 |
| Monochorionic-diamniotic | 27.2% | n/a | 16.8% | 0.042 |
| Monochorionic-monoamniotic | 1.2% | n/a | 0.0% | 0.556 |
HELLP = Haemolysis, Elevated Liver enzyme levels, Low Platelet count; n/a = not applicable; PPROM = preterm premature rupture of membranes; LPS = late preterm singletons; LPT = late preterm twins; TT = term twins; * LPT vs LPS p = 0.006 ** LPT vs LPS p = 0.002 and LPT vs TT p<0.001
The primary outcome is shown in tables 4 and 5 . LPT were hospitalised approximately one day longer than LPS (13.5 ± 8.0 vs 12.6 ± 8.6 days, p <0.001). However, there was no significant difference between these two groups regarding incidence of hospitalisation in the neonatology unit. Likewise, there was no difference between the two groups with regard to the postmenstrual age at discharge. Overall, only one patient in the LPS group died during postnatal hospitalisation. This was an infant who died on the sixth day of life from perinatal asphyxia and was therefore not included in the calculation of the average hospital stay.
Table 4 Neonatal outcomes of late preterm twins compared with late preterm singletons.
| Outcome |
LPT
(n = 243) |
LPS
(n = 495) |
p value |
|---|---|---|---|
| Hospitalisation neonatology | 72.4% | 75.4% | 0.420 |
| Length of stay (d)† | 13.5 ± 8.01 (7–20) |
12.6 ± 8.6 (6–17) |
0.011* |
| Hyperbilirubinaemia | 29.2% | 49.7% | <0.001* |
| Days of phototherapy (d)† | 1.45 ± 0.94 | 1.99 ± 1.40 | <0.001* |
| Exchange transfusion | 0.4% | 0.6% | 1.000 |
| Rhesus incompatibility | 7.0% | 10.1% | 0.175 |
| Hypoglycaemia | 23.5% | 23.8% | 0.927 |
| Lowest glucose level (mmol/l)† | 2.02 ± 0.38 | 1.91 ± 0.47 | 0.233 |
| Respiratory distress syndrome | 25.1% | 25.1% | 1.000 |
| Supplemental oxygen | 9.5% | 10.5% | 0.699 |
| CPAP | 14.4% | 19.2% | 0.123 |
| Duration of CPAP (h)† | 34.3 ± 49.5 (3–37) |
32.2 ± 40.2 (6–48) |
0.515 |
| Mechanical ventilation | 1.2% | 3.4% | 0.095 |
| Duration of mechanical ventilation (h) | 41.3 ± 49.7 (5–98) |
41.1 ± 51.8 (5–77) |
0.832 |
| Sepsis | 0.4% | 0.6% | 1.000 |
| Need for antibiotics | 10.3% | 10.1% | 1.000 |
| Duration of administration (d)† | 4.68 ± 1.95 | 5.02 ± 2.74 | 0.962 |
| Necrotising enterocolitis | 0.8% | 0.4% | 0.602 |
| Intraventricular haemorrhage 1° | 0.0% | 0.4% | 1.000 |
| Intraventricular haemorrhage 4° | 0.0% | 0.2% | 1.000 |
| Periventricular leukomalacia | 0.0% | 0.2% | 1.000 |
| Postmenstrual age at discharge (weeks)† | 37.1 ± 0.8 | 37.1 ± 1.0 | 0.611 |
| Discharge weight (g)† | 2356 ± 310 (2130–2550) |
2482 ± 394 (2210–2728) |
<0.001* |
| Discharge length (cm)† | 45.9 ± 4.4 (44.5–47.5) |
46.8 ± 2.5 (45.0–48.0) |
0.038* |
| Discharge head circumference (cm)† | 32.8 ± 1.2 (32.0–33.5) |
32.9 ± 1.4 (32.0–33.8) |
0.574 |
CPAP = continuous positive airway pressure; LPS = late preterm singletons; LPT = late preterm twins * p <0.05 † mean ± standard deviation (interquartile range)
Table 5 Neonatal outcome of late preterm twins compared with term twins.
| Outcome |
LPT
(n = 243) |
TT
(n = 107) |
p-value |
|---|---|---|---|
| Hospitalisation neonatology | 72.4% | 15.9% | <0.001* |
| Length of stay (d)† | 13.5 ± 8.01 (7–20) |
6.29 ± 1.99 (5–7) |
<0.001* |
| Hyperbilirubinaemia | 29.2% | 3.7% | <0.001* |
| Days of phototherapy (d)† | 1.45 ± 0.94 | 1.5 ± 1 | 0.976 |
| Exchange transfusion | 0.4% | 0.0% | 1.000 |
| Rhesus incompatibility | 7.0% | 0.9% | 0.017* |
| Hypoglycaemia | 23.5% | 4.7% | <0.001* |
| Lowest glucose level (mmol/l)† | 2.02 ± 0.38 | 2.18 ± 0.18 | 0.521 |
| Respiratory distress syndrome | 25.1% | 2.8% | <0.001* |
| Supplemental oxygen | 9.5% | 0.9% | 0.002* |
| CPAP | 14.4% | 5.6% | 0.019* |
| Duration of CPAP (h)† | 34.3 ± 49.5 (3–37) |
7.83 ± 9.81 (1–14.8) |
0.110 |
| Mechanical ventilation | 1.2% | 0.0% | 0.556 |
| Duration of mechanical ventilation (h) | 41.3 ± 49.7 (5–98) |
0.0% (0–0) |
<0.001* |
| Sepsis | 0.4% | 0.9% | 0.519 |
| Need for antibiotics | 10.3% | 0.9% | 0.001* |
| Duration of administration (d)† | 4.68 ± 1.95 | 7.0 ± 0 | 0.298 |
| Necrotising enterocolitis | 0.8% | 0.0% | 1.000 |
| Intraventricular haemorrhage | 0.0% | 0.0% | - |
| Periventricular leukomalacia | 0.0% | 0.0% | 1.000 |
| Postmenstrual age at discharge (weeks)† | 37.1 ± 0.8 | 38.7 ± 0.6 | <0.001* |
| Discharge weight (g)† | 2356 ± 310 (2130–2550) |
2652 ± 345 (2390–2885) |
<0.001* |
| Discharge length (cm)† | 45.9 ± 4.4 (44.5–47.5) |
47.5 ± 2.5 (44.8–49.5) |
0.029* |
| Discharge head circumference (cm)† | 32.8 ± 1.2 (32.0–33.5) |
33.4 ± 1.1 (33.0–34.0) |
<0.001* |
CPAP = continuous positive airway pressure; LPT = late preterm twins; TT = term twins * p <0.05 † mean ± standard deviation (interquartile range)
With a mean length of hospitalisation of 13.5 days, LPT were hospitalised more than twice as long as TT, who were hospitalised for a mean duration of 6.3 days (p <0.001). In addition, significantly more LPT were hospitalised in the neonatal unit compared to TT (72.4 vs 15.9%, p <0.001). Overall, LPT were hospitalised significantly longer than LPS and TT.
Secondary outcomes are summarised in tables 4 and 5 . LPT had a reduced risk of hyperbilirubinaemia (29.2 vs 49.7%, p <0.001) and a shorter duration of phototherapy than LPS (1.45 vs 1.99 days, p <0.001). All other examined outcome criteria regarding neonatal morbidity showed no significant differences between LPS and LPT.
Comparisons of LPT and TT revealed that LPT had significantly higher rates of hyperbilirubinaemia (29.2 vs 3.7%, p <0.001), hypoglycaemia (23.5 vs 4.7%, p <0.001), rhesus incompatibility (7.0 vs 0.9%, p = 0.017) need for antibiotics (10.3 vs 0.9%, p <0.001) as well as respiratory distress syndrome (25.1 vs 2.8%, p <0.001), and therefore also required supplemental oxygen more frequently (9.5 vs 0.9%, p = 0.002) and continuous positive airway pressure (CPAP) (14.4 vs 5.6%, p = 0.019) than TT. At discharge, LPT were shorter (p = 0.038) and lighter (p<0.001) than LPS, but had a comparable head circumference (p = 0.574). Compared with TT, LPT had lower weight (p <0.001), length (p = 0.029) and head circumference (p <0.001) at discharge.
To our knowledge, this is the first study comparing neonatal outcome, especially duration of neonatal hospitalisation between late preterm singletons, late preterm twins and term twins.
In our retrospective study, we were able to show that length of neonatal hospitalisation for LPT is significantly longer than for LPS. This may be because LPT were born on average one day earlier than LPS, resulting in a similar gestational age at discharge. When we designed this study we expected multiple pregnancy to increase the risk for neonatal morbidities. Therefore, we were surprised to find only a small difference in duration of hospitalisation. Clinically, the results might have only a small impact as it will be difficult to considerably modify discharge planning.
The longer duration of hospitalisation of LPT compared with TT seems obvious, since preterm infants are faced with frequent complications of prematurity that could be responsible for prolonged hospitalisation, in particular respiratory morbidity, hypoglycaemia and hyperbilirubinaemia. In general, our results of comparisons between LPT and TT were as expected, since late preterm infants were compared with term infants. Previously, a direct comparison of LPS and term singletons showed that LPS have more morbidities and require longer hospitalisation than term singletons, with hyperbilirubinaemia being the most frequent complication [4]. In line with these results, our study showed that hyperbilirubinaemia was the most common complication followed by respiratory distress syndrome in both LPT and LPS, while hypoglycaemia followed by hyperbilirubinaemia and respiratory distress syndrome were the most common complications in TT.
An interesting result of our study is a reduced incidence of hyperbilirubinaemia in LPT compared with LPS. This significant finding supports the results of a recent study that also reported a substantial decreased occurrence of hyperbilirubinaemia in LPT [22]. This study hypothesised that LPS suffer more frequently from lactational jaundice than LPT, since LPS are breastfed earlier than LPT. However, as twin pregnancies are prone to complications that are associated with an increased risk of hyperbilirubinaemia, such as the feto-fetal transfusion syndrome, we expected hyperbilirubinaemia to occur more frequently in premature twins than singletons. To the best of our knowledge, no pathophysiological mechanism has been proposed to date to explain this discrepancy. A recent study reported postnatal haemoglobin levels immediately after birth to be higher in twins and higher order multiples than in singletons [23]. We speculate that an unknown factor induces the expression of UDP-glucuronosyltransferase, the key enzyme responsible for neonatal jaundice. Prenatal stress in terms of increased levels of cortisol and catecholamines is an unlikely key factor, because significantly more LPT than LPS were born via caesarean section. It is well known that postnatal respiratory function and the induction of epithelial sodium channels in the lungs is influenced by prenatal stress, but our results show that all examined respiratory parameters (respiratory distress syndrome, CPAP, duration of CPAP, intubation and duration of mechanical ventilation) did not differ between LPS and LPT.
Several studies showed respiratory problems to be the most common complication in late prematurely born infants [5, 24]. This underlines the fact that respiratory immaturity is common in this age group and might include an immature respiratory drive as well as impaired gas exchange in terms of wet lung or surfactant deficiency. Furthermore, twins are frequently born via caesarean section, which in itself increases the risk for respiratory problems, irrespective of maturity [25, 26]. Indeed, in our study LPT were delivered by caesarean section more frequently than LPS. In the literature, rates of mechanical ventilation in LPT are reported to range between 6.2% and 24.7% [5, 27, 28]. This is considerably higher than in our study, which showed an intubation rate of 3.4% in LPS and 1.2% in LPT (combined 2.7%). The most likely explanation seems that our treatment strategies aim for avoidance of mechanical ventilation and favour CPAP instead.
LPT had lower gestational age at birth compared with LPS. This was as expected, since the risk for complications increases towards the end of pregnancy in twins, especially in monochorionic twins. Current recommendations with regard to time of delivery are no later than 38 weeks of gestation for an uncomplicated dichorionic pregnancy and between 34 and 37 weeks of gestation for uncomplicated monochorionic twin pregnancies [13, 29]. Furthermore, twin pregnancies, in particular monochorionic ones, are reported to have a higher rate of maternal complications such as HELLP and preeclampsia/eclampsia, which is one of the most common indications for caesarean section in premature twin pregnancies [30]. This is mirrored by our results, which showed that pregnancies with LPT have a higher incidence of preeclampsia/eclampsia than TT and also have a higher incidence of PPROM than LPS or TT. The specific risks for morbidities in monochorionic and dichorionic pregnancies are beyond the scope of this paper and are therefore not discussed in detail.
Congruent with well-known evidence showing that birth weight in twins is lower than in singletons, our study showed that LPS were significantly heavier than LPT. The small difference in gestational age at birth of one day cannot explain the weight difference. However, multiple birth is the most plausible explanation [31]. Regarding mortality, only one infant in the LPS group died in our entire study population. Therefore, we cannot draw any conclusions concerning mortality rates of LPT in comparison with LPS or TT based on our data.
One major strength of our study is the size of our study population. In addition, this study was performed in a single centre, which guarantees more uniform therapies and treatment strategies of patients than a multicentre study. Weaknesses include the retrospective study design. In addition, data on neurocognitive outcome are not routinely assessed in late preterm infants in our hospital; hence, no further analysis was possible to that end. Several studies have demonstrated an association of late preterm birth with cognitive impairment and behavioural deficits [32–34].
In summary, as late preterm infants, especially twins, are faced with a higher incidence of neonatal morbidity and an increased length of hospitalisation, the indication for preterm delivery should be well justified and avoided if not safe for mother and infant.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Engle WA , Tomashek KM , Wallman C ; Committee on Fetus and Newborn, American Academy of Pediatrics. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120(6):1390–401. doi:.https://doi.org/10.1542/peds.2007-2952
2 Raju TN . Epidemiology of late preterm (near-term) births. Clin Perinatol. 2006;33(4):751–63, abstract vii. doi:.https://doi.org/10.1016/j.clp.2006.09.009
3 Cheong JL , Doyle LW . Increasing rates of prematurity and epidemiology of late preterm birth. J Paediatr Child Health. 2012;48(9):784–8. doi:.https://doi.org/10.1111/j.1440-1754.2012.02536.x
4 Leone A , Ersfeld P , Adams M , Meyer Schiffer P , Bucher HU , Arlettaz R . Neonatal morbidity in singleton late preterm infants compared with full-term infants. Acta Paediatr. 2012;101(1):e6–10. doi:.https://doi.org/10.1111/j.1651-2227.2011.02459.x
5 Refuerzo JS , Momirova V , Peaceman AM , Sciscione A , Rouse DJ , Caritis SN , et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Neonatal outcomes in twin pregnancies delivered moderately preterm, late preterm, and term. Am J Perinatol. 2010;27(7):537–42. doi:.https://doi.org/10.1055/s-0030-1248940
6 Bhutani VK . Multidisciplinary guidelines for the care of late preterm infants. J Perinatol. 2014;34(1):81. doi:.https://doi.org/10.1038/jp.2013.126
7 Cheng YW , Kaimal AJ , Bruckner TA , Halloran DR , Caughey AB . Perinatal morbidity associated with late preterm deliveries compared with deliveries between 37 and 40 weeks of gestation. BJOG. 2011;118(12):1446–54. doi:.https://doi.org/10.1111/j.1471-0528.2011.03045.x
8 Melamed N , Klinger G , Tenenbaum-Gavish K , Herscovici T , Linder N , Hod M , et al. Short-term neonatal outcome in low-risk, spontaneous, singleton, late preterm deliveries. Obstet Gynecol. 2009;114(2 Pt 1):253–60. doi:.https://doi.org/10.1097/AOG.0b013e3181af6931
9 McIntire DD , Leveno KJ . Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111(1):35–41. doi:.https://doi.org/10.1097/01.AOG.0000297311.33046.73
10 Raju TN . Developmental physiology of late and moderate prematurity. Semin Fetal Neonatal Med. 2012;17(3):126–31. doi:.https://doi.org/10.1016/j.siny.2012.01.010
11 Shapiro-Mendoza CK , Lackritz EM . Epidemiology of late and moderate preterm birth. Semin Fetal Neonatal Med. 2012;17(3):120–5. doi:.https://doi.org/10.1016/j.siny.2012.01.007
12 Shapiro-Mendoza CK , Tomashek KM , Kotelchuck M , Barfield W , Weiss J , Evans S . Risk factors for neonatal morbidity and mortality among “healthy,” late preterm newborns. Semin Perinatol. 2006;30(2):54–60. doi:.https://doi.org/10.1053/j.semperi.2006.02.002
13 Refuerzo JS . Impact of multiple births on late and moderate prematurity. Semin Fetal Neonatal Med. 2012;17(3):143–5. doi:.https://doi.org/10.1016/j.siny.2012.01.012
14 Beemsterboer SN , Homburg R , Gorter NA , Schats R , Hompes PG , Lambalk CB . The paradox of declining fertility but increasing twinning rates with advancing maternal age. Hum Reprod. 2006;21(6):1531–2. doi:.https://doi.org/10.1093/humrep/del009
15 Mally PV , Bailey S , Hendricks-Muñoz KD . Clinical issues in the management of late preterm infants. Curr Probl Pediatr Adolesc Health Care. 2010;40(9):218–33. doi:.https://doi.org/10.1016/j.cppeds.2010.07.005
16 Lee YM , Cleary-Goldman J , D’Alton ME . The impact of multiple gestations on late preterm (near-term) births. Clin Perinatol. 2006;33(4):777–92, abstract viii. doi:.https://doi.org/10.1016/j.clp.2006.09.008
17 Tranquilli AL . Introduction to ISSHP new classification of preeclampsia. Pregnancy Hypertens. 2013;3(2):58–9. doi:.https://doi.org/10.1016/j.preghy.2013.04.006
18 Arlettaz RBA , Buetti L , Fahnenstich H , Mieth D , Roth-Kleiner M . Evaluation and treatment of hyperbilirubinaemia in near term/full term neonates (gestational age of at least 35 0/7 weeks). Paediatrica. 2006;17(3):26–9.
19Berger TMD-KS, Pfister RE, Pfister R, Stocker M, Zimmermann U. Prevention and therapy of hypoglycemia in infants with a gestational age above 34 0/7 weeks in maternity wards http://www.neonet.ch/files/4114/2597/8496/2007_Hypoglykaemie_e.pdf
20 Bell MJ , Ternberg JL , Feigin RD , Keating JP , Marshall R , Barton L , et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi:.https://doi.org/10.1097/00000658-197801000-00001
21 Papile LA , Burstein J , Burstein R , Koffler H . Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–34. doi:.https://doi.org/10.1016/S0022-3476(78)80282-0
22 Ribicic R , Kranjcec I , Borosak J , Tumbri J , Mihovilovic Prajz L , Ribicic T . Perinatal outcome of singleton versus twin late preterm infants: do twins mature faster than singletons? J Matern Fetal Neonatal Med. 2016;29(9):1520–4. doi:.https://doi.org/10.3109/14767058.2015.1053449
23 Shah J , Shah PS , Jefferies A . Hematological parameters immediately after birth in twins and higher order multiples. Am J Perinatol. 2015;32(7):653–8. doi:.https://doi.org/10.1055/s-0034-1390353
24 Kitsommart R , Janes M , Mahajan V , Rahman A , Seidlitz W , Wilson J , et al. Outcomes of late-preterm infants: a retrospective, single-center, Canadian study. Clin Pediatr (Phila). 2009;48(8):844–50. doi:.https://doi.org/10.1177/0009922809340432
25 Barrett JF , Hannah ME , Hutton EK , Willan AR , Allen AC , Armson BA , et al.; Twin Birth Study Collaborative Group. A randomized trial of planned cesarean or vaginal delivery for twin pregnancy. N Engl J Med. 2013;369(14):1295–305. doi:.https://doi.org/10.1056/NEJMoa1214939
26 Kolås T , Saugstad OD , Daltveit AK , Nilsen ST , Øian P . Planned cesarean versus planned vaginal delivery at term: comparison of newborn infant outcomes. Am J Obstet Gynecol. 2006;195(6):1538–43. doi:.https://doi.org/10.1016/j.ajog.2006.05.005
27 Kitsommart R , Phatihattakorn C , Pornladnun P , Paes B . A prospective study of the severity of early respiratory distress in late preterms compared to term infants. J Matern Fetal Neonatal Med. 2016;29(2):207–12. doi:.https://doi.org/10.3109/14767058.2014.992335
28 Vachharajani AJ , Vachharajani NA , Dawson JG . Comparison of short-term outcomes of late preterm singletons and multiple births: an institutional experience. Clin Pediatr (Phila). 2009;48(9):922–5. doi:.https://doi.org/10.1177/0009922809336359
29 Breathnach FM , McAuliffe FM , Geary M , Daly S , Higgins JR , Dornan J , et al.; Perinatal Ireland Research Consortium. Optimum timing for planned delivery of uncomplicated monochorionic and dichorionic twin pregnancies. Obstet Gynecol. 2012;119(1):50–9. doi:.https://doi.org/10.1097/AOG.0b013e31823d7b06
30 Langenveld J , Ravelli AC , van Kaam AH , van der Ham DP , van Pampus MG , Porath M , et al. Neonatal outcome of pregnancies complicated by hypertensive disorders between 34 and 37 weeks of gestation: a 7 year retrospective analysis of a national registry. Am J Obstet Gynecol. 2011;205(6):540.e1–7. doi:.https://doi.org/10.1016/j.ajog.2011.07.003
31 Baumann MU , Marti M , Durrer L , Koumoutsakos P , Angelikopoulos P , Bolla D , et al. Placental plasticity in monochorionic twins: Impact on birth weight and placental weight. Placenta. 2015;36(9):1018–23. doi:.https://doi.org/10.1016/j.placenta.2015.07.120
32 Talge NM , Holzman C , Wang J , Lucia V , Gardiner J , Breslau N . Late-preterm birth and its association with cognitive and socioemotional outcomes at 6 years of age. Pediatrics. 2010;126(6):1124–31. doi:.https://doi.org/10.1542/peds.2010-1536
33 Woythaler MA , McCormick MC , Smith VC . Late preterm infants have worse 24-month neurodevelopmental outcomes than term infants. Pediatrics. 2011;127(3):e622–9. doi:.https://doi.org/10.1542/peds.2009-3598
34 Guy A , Seaton SE , Boyle EM , Draper ES , Field DJ , Manktelow BN , et al. Infants born late/moderately preterm are at increased risk for a positive autism screen at 2 years of age. J Pediatr. 2015;166(2):269–75.e3. doi:.https://doi.org/10.1016/j.jpeds.2014.10.053
Jarmila A. Zdanowicz and Eliane Sommer contributed equally to this work.
No financial support and no other potential conflict of interest relevant to this article was reported.