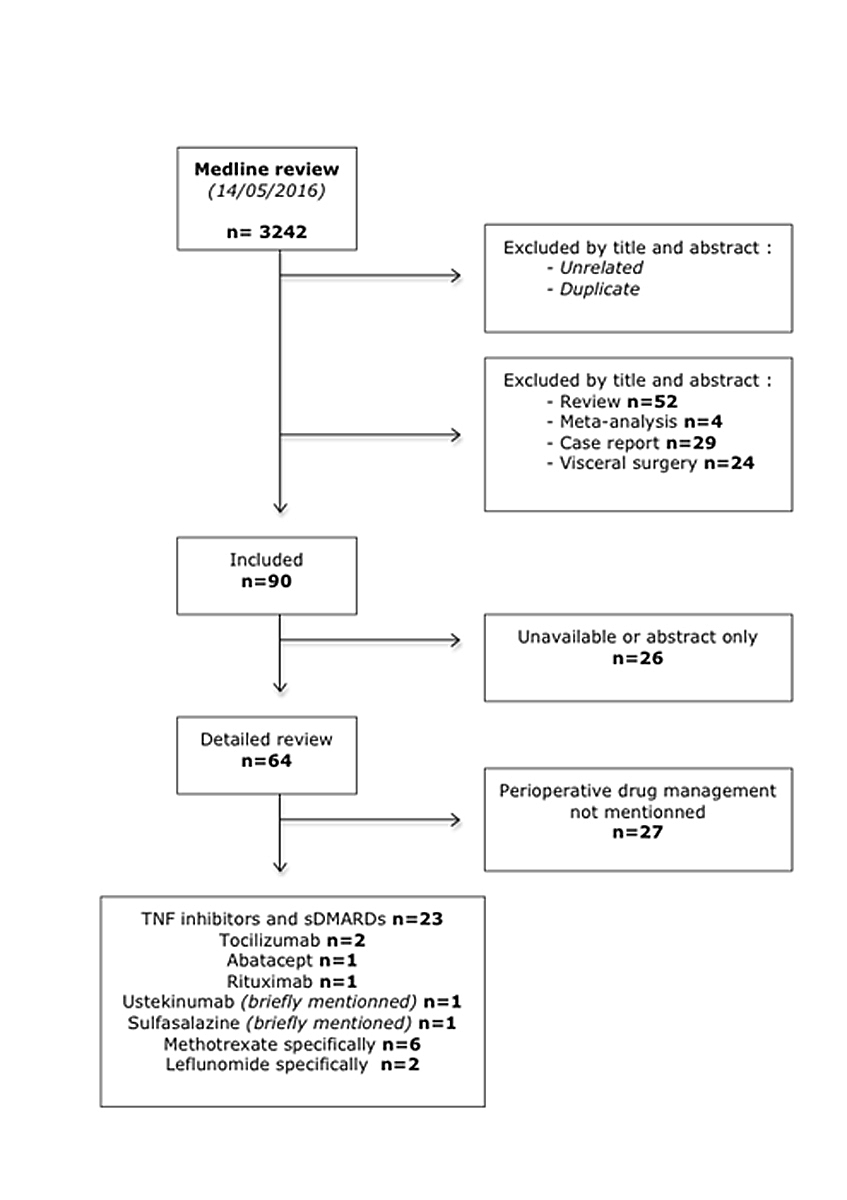
Figure 1 Medline search strategy.
sDMARD = synthetic disease modifying antirheumatic drug; TNF = tumour necrosis factor
DOI: https://doi.org/10.4414/smw.2017.14563
The first-line treatment of rheumatoid arthritis (RA) comprises various conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) [1]. Methotrexate is the cornerstone csDMARD that is used either as monotherapy or in combination with other DMARDs. If low disease activity or remission is not achieved with csDMARDs, treatment is escalated with the use of biological DMARDs (bDMARDs), including monoclonal antibodies or soluble receptors acting on specific cytokines, co-stimulatory signals and B cells. More recently, targeted synthetic DMARDs (tsDMARDs) blocking cell signalling such as Janus kinase (JAK) inhibitors have been introduced for the management of RA. In addition to their pathogenic role in immune-mediated inflammatory diseases, the pathways targeted by bDMARDs and tsDMARDs contribute to hosting defence against pathogens [2]. As an example, the use of tumour necrosis factor (TNF) inhibitors is associated with an enhanced risk of infectious complications including various bacterial and viral infections as well as opportunistic infections [3]. Despite recent treatment progresses, some RA patients experience end-stage joint disease with structural damage requiring surgery. One critical question is how to manage immunosuppressive agents in patients who will require surgery, in order to avoid the occurrence of complications after surgery, but also to limit perioperative disease flares. This article aims to review the evidence regarding the use of DMARDs during the perioperative period and provide some guidance to the clinicians caring for patients with RA and other auto-immune diseases. To do this, the Medline database was screened to address the question of drug discontinuation and occurrence of perioperative complications (infection and delayed wound healing) in RA patients treated with cs- or bDMARDs undergoing orthopaedic surgery.
Several studies have attempted to examine surgical site complications (wound infection, delayed healing, and deep infection) among RA patients, unfortunately without always taking into account on-going DMARDs treatment. Even so, one would have to deal with factors such as disease activity that cannot be dissociated from DMARDs use, immunity impairment inherent to the disease, and concomitant glucocorticoid use. Table 1 summarises the conclusions of studies that have tried to give an overview of infection risks in RA patients, and aims to reflect the postoperative complications seen in various centres when no drug interruption guidelines are involved.
Table 1 Rheumatoid arthritis as a risk factor for postoperative infection.
| Studies | Surgical interventions on RA patients | Control group | Orthopaedic procedures | Infection rate | Increased infection risk |
|---|---|---|---|---|---|
| Koyama et al. [4] | 47 | None | Spinal surgery | 14.89% | Could not be proven |
| Kang et al. [5] | 40 | 134 | Spinal surgery | 47.5 vs 17.1% | Yes |
| Lo Verde et al. [6] | 159 | 318 | TKA | Deep infection: 0 vs 0% Minor infection: 8.2 vs 13.2% |
No |
| Schrama et al. [7] | 13 384 | 377 287 | THA | 1995–2001: RR 1.1 (95% CI 0.8–1.5; p = 0.5) | Yes, from 2001 |
| Singh et al. [8] | 496 | 33 815 | THA, TKA | Readmission within 90 d: 8.5 vs 6.7% Major readmission cause infection: 10.2 vs 5.7% |
Yes |
| Ravi et al. [9] | 3855 | 97 445 OA 14 490 inflammatory arthritis |
THA, TKA | RA: 1.3%; inflammatory arthritis: 1.13%; OA: 0.94% | Yes for TKA Not proven for THA |
| Pedersen et al. [10] | 50 | 50 | Foot and ankle surgery | Deep infection: 2 vs 0% Minor infection: 2 vs 0% |
No |
| Carrol et al. [11] | 58 | 906 | THA, TKA | Superficial wound complication OR 2.56 (95% CI 1.17–5.58; p = 0.018) | Yes |
| Stundner et al. [12] | 11 755 | 339 348 | TKA | 4.5 vs 3.8% p = 0.001 | Yes |
| Hsieh et al. [13] | 46 | 300 | THA, TKA | 44 vs 32% p = 0.14 | Could not be proven Worse outcomes among RA patients |
| Pahys et al. [14] | 36 | 965 | Cervical spine surgery | Infection OR 6.3 (95% CI 1.5–26.1; p = 0.027) | Yes |
| Michaud et al. [15] | 839 | 33 685 | THA, TKA | 4.17 vs 4.15% | No |
| Bhatia et al. [16] | 41 | 59 | Occipito-cervical fixation | 5 vs 5.1% Deep infection: 4 vs 0% |
No |
| da Cunha et al. [17] | 75 | 131 | THA, TKA | Superficial infection: 8 vs 2.3% Systemic infection: 5.3 vs 7.7% |
No No significant result |
| Soohoo et al. [18] | 5565 | 132 834 | THA | 90-day infection risk OR 1.47 (95% CI 0.90–2.41; p = 0.12) | Could not be proven |
| Schrama et al. [19] | 6629 | 102 157 | THA, TKA | 0.86 vs 0.64% | Yes for TKA Yes for THA only from 2001 |
| van der Heide et al. [20] | 58 | None | Total ankle arthroplasty | 6.90% Infection most important implant cause of failure |
Could not be proven |
| Jämsen et al. [21] | 3290 | 39 859 | TKA | 1.32% for primary TKA, 3.24% for revision HR 1.86 (95% CI 1.31–2.63) for primary |
Yes |
| Bongartz et al. [22] | 462 | 462 | THA, TKA | HR 4.08 (95% CI 1.35–12.33) | Yes |
| Himanen et al. [23] | 751 | None | TKA | 1.33% | Could not be proven |
| Ljung et al. [24] | 50 | None | Capitello-condylar prosthesis | Deep infection: 2% Delayed wound healing: 4% |
Could not be proven |
| Kristensen et al. [25] | 71 | None | TKA | 7% 4 haematogenous /1 postop. skin infection |
Could not be proven |
| Wymenga et al. [26] | No data | 3013 in total | THA, TKA | THA: 17/2651 TKA: 9/362 RR infection in TKA: 4.8 (95% CI 1.2–19) |
Yes for TKA |
| Wilson et al. [27] | 2076 | 1857 | TKA | 2.2 vs 1% (p <0.0001) | Yes |
| Poss et al. [28] | 275 | 382 | THA | 1.46 vs 1.57% | No |
| Garner et al. [29] | 100 | 100 | All except spinal surgery | 13 vs 8% (p >0.4) | No |
| Luessenhop et al. [30] | 51 primary prosthesis infection RA prosthesis | None | THA, TKA, SA | 19 RA patients among the 27 secondary infections | Yes |
CI = confidence interval; OA = osteoarthritis; OR = odds ratio; RA = rheumatoid arthritis; RR = relative risk; SA = shoulder arthroplasty; THA = total hip arthroplasty; TKA = total knee arthroplasty
The largest study on total knee arthroplasties performed between 2006 and 2010 compared the number of infections after surgery in 11 755 RA and 339 348 osteoarthritis (OA) patients and showed that the risk was higher in RA patients (4.5% vs 3.8% infections, p <0.001) [12]. The results from the Nordic Arthroplasty Register Association included 13 384 total hip arthroplasties in RA and in 377 287 OA patients [7]. The relative risk (RR) of infectious complications was higher in RA patients who underwent surgery between 2002 and 2010 (RR 1.4, 95% confidence interval [CI] 1.0–1.8; p = 0.05) as compared with those who had surgery between 1995 and 2001 (RR 1.1, 95% CI 0.8–1.5; p = 0.5). Although not formally proven, the authors raise the fact that this slight increase could be related to the introduction of TNF inhibitors in Scandinavian countries. The Norwegian Arthroplasty Registry [19] reported a significantly increased RR of 1.6 (95% CI 1.06–2.38; p = 0.027) of infectious complications in RA versus OA patients undergoing total hip or knee arthroplasty. In contrast, the risk of infections within 90 days following total hip arthroplasty was not significantly higher in 5565 RA compared with 132 834 non-RA patients (odds ratio [OR] for infection risk 1.47, 95% CI 0.90–2.41; p = 0.12) in another study [18]. Despite these contradictory data, the larger studies consistently show a small but significant increase in risk of postoperative infections in RA compared to OA patients.
We used a combination of the following MeSH terms: surgery, arthroplasty, infection, wound healing, surgical wound, rheumatoid arthritis, postoperative complications, perioperative period, orthopaedic surgery, anti-tumour necrosis factor, tocilizumab, abatacept, rituximab, belimumab, anakinra, tofacitinib, apremilast, methotrexate, leflunomide, sulfasalazine, detailed in appendix 1. We included 37 articles based on the exclusion criteria summarised in figure 1. Based on the titles and the abstracts, our first selection process allowed us to exclude all the studies that did not discuss the link between perioperative complications and immunosuppressive drugs. We did not find any publications on the perioperative use of some DMARDs, such as apremilast, tofacitinib, belimumab and anakinra.

Figure 1 Medline search strategy.
sDMARD = synthetic disease modifying antirheumatic drug; TNF = tumour necrosis factor
A study examining the perioperative use of methotrexate (median dose 10 mg, 2.5–25 mg) included 388 RA patients who were prospectively randomised into three groups: 88 patients in whom methotrexate was continued, 72 patients in whom methotrexate was discontinued two weeks prior to surgery and resumed two weeks after surgery, and a control group of 228 patients without methotrexate treatment [31]. After a follow-up of one year, there was a significant reduction in the incident rate of infections in patients in whom methotrexate was maintained (2%), as compared to those in whom methotrexate was discontinued (15%, p <0.003) and the control group (10.5%, p = 0.026). Within six weeks of surgery, RA flares were numerically more frequent in the methotrexate discontinuation group (8%) and the control group (4%) than in the methotrexate maintenance group (0%). A further logistic regression analysis showed a significant risk of infection with use of D-penicillamine, ciclosporin, hydroxychloroquine, chloroquine and prednisone.
Sixty-five patients included in this study were reviewed ten years later, including 23 patients from the methotrexate continuation, 8 from the methotrexate interruption, and 34 from the control groups. This long-term follow-up did not reveal any case of delayed infections [32].
A retrospective study included 122 RA patients undergoing 201 orthopaedic procedures [33]. Methotrexate was maintained in 77 cases and discontinued for more than one week prior to surgery in 21 cases. The remaining patients (103 surgeries) had not been treated with methotrexate. There was no significant difference in terms of postoperative infection (3.9, 4.8 and 3.9, respectively) or occurrence of delayed wound healing (1.3, 9.5 and 7.8%, respectively). There was also no significant difference regarding the incidence of disease flares in the three groups (3.9, 14.3 and 6.8%, respectively). Eighty RA patients undergoing 129 hand surgical procedures without perioperative drug interruption were divided into various treatment groups, including methotrexate monotherapy, glucocorticoids only, methotrexate plus glucocorticoid, and no therapy [34]. The authors observed four wound infections in the methotrexate groups (5%), and two in the group without therapy (4%).
A randomised non-blind prospective study examined the frequency of postoperative complications in 64 RA patients separated into two groups. In group A (32 patients, 50 surgical procedures) methotrexate was discontinued seven days before surgery; in group B (32 patients, 39 procedures), methotrexate was maintained [35]. There was no postoperative infection but prolonged wound healing in six and four cases in groups A and B, respectively (not significant).
A prospective non-randomised study included 32 RA patients undergoing elective total joint arthroplasty who were divided into two groups. In group one, methotrexate was discontinued one week prior to and after surgery (n = 19 with 26 procedures), whereas in group two, methotrexate was maintained throughout the perioperative period (n = 13 with 16 procedures) [36]. Four postoperative infectious complications occurred in group two, including two prosthetic infections, one infected joint fusion, and one deep wound infection, but none was observed in group one (p = 0.03). No disease flare was observed in either group.
A systematic review including the studies described above concluded that low dose methotrexate is safe during the perioperative period, and associated with significantly fewer disease flares [37].
Two studies provided conflicting results regarding the perioperative safety of leflunomide. A case-control study including 41 RA patients treated with leflunomide (82 orthopaedic procedures) versus 41 patients in whom leflunomide was discontinued four weeks prior to surgery (79 orthopaedic procedures) showed no difference in the infection percentage (6.1 vs 6.3% local infections, respectively) [38].
A more recent prospective study included 201 patients with RA and psoriatic arthritis who underwent orthopaedic surgery and were treated with various combinations of methotrexate, leflunomide, etanercept, infliximab and glucocorticoids [39]. An increased number of postoperative complications was observed, including infections, impaired wound healing and necrotic scars in patients treated with leflunomide compared to methotrexate (40.6 vs 13.6%). A logistic regression analysis confirmed that the complication risk was significantly higher for leflunomide (OR 3.48, 95% CI 1.31–9.24; p = 0.01), but not for the other drugs. Based on these results, the authors recommended stopping leflunomide without providing any specific indication regarding the timing and duration of treatment discontinuation. They also suggested that preoperative cholestyramine administration could help to reduce circulating levels of leflunomide.
There is no specific study on the safety of sulfasalazine in patients with inflammatory arthritis undergoing orthopaedic surgery. However, a retrospective cohort study compared the number of surgical site infections following 1219 orthopaedic procedures in RA patients, in whom TNF inhibitors were maintained or discontinued four drug half-lives before surgery (infliximab 39 d, etanercept 12 d, adalimumab 56 d) [40]. There was no significant effect of TNF inhibitors on the risk for infection (OR 1.5, 95% CI 0.45–5.2; p = 0.53). Interestingly, the risk of infection was lower among those treated concomitantly with sulfasalazine (OR 0.21, 95% CI 0.05–0.89; p = 0.035). The authors attributed the protective effect of sulfasalazine to its action on bacterial folic acid synthesis.
Hydroxychloroquine did not show any statistically significant association with either delayed healing or postoperative infections [41].
The effect of TNF inhibitors on the risk of infectious complications in patients undergoing orthopaedic surgery has been examined in several studies. Most of these are retrospective and very heterogeneous regarding their design and conclusions. They include mainly RA patients and occasionally patients with other rheumatic diseases. Most of these studies compared the rates of infections in patients treated with TNF inhibitors versus csDMARDs without specifying whether the treatment was discontinued during the perioperative period. There is only one prospective randomised clinical trial that specifically compared the perioperative discontinuation versus maintenance of TNF inhibitors [42]. We have arbitrarily divided the presentation of the results into two parts: the first includes all the studies in which the use of TNF inhibitors was associated with an increased risk of infections, and the second describes those that did not find any difference in the number of infectious complications. All these studies are summarised in table 2.
Table 2 Overview of studies with TNF inhibitors.
| Study | Design | Population | Treatment | Endpoint | Follow-up | Results | Conclusion |
|---|---|---|---|---|---|---|---|
| Studies showing increased risk of infection | |||||||
| Giles et al. 2006 [43] | Retrospective | 91 patients 35/91 on TNFi, 56 naive |
Infection | Infection while on treatment 7/91 Infection in naive 3/91 |
Interruption recommended | ||
| Kawakami et al. 2010 [44] | Retrospective | RA, orthopaedic surgery 64 TNFi interrupted (ETN: 2–4 w, IFX: 4 w) vs 64 csDMARDs |
Infection DVT Flare |
Infection on TNFi OR 21.8 (95% CI 1.231–386.1; p = 0.036) DVT with TNFi OR 2.83 (95% CI 1.10–7.25; p = 0.03) 31% flares with ETN, 5.7% with IFX |
|||
| Galloway et al. 2011 [45] | Prospective | 11 881 TNFi 3673 csDMARDs |
Septic arthritis | aHR 2.3 (95% CI 1.2–4.4) for TNFi | |||
| Momohara et al. 2011 [46] | Retrospective | RA Orthopaedic surgery |
Septic arthritis | Use of bDMARDs OR 5.69 (95% CI 2.07–15.61) Longer RA duration OR 1.09 (95% CI 1.04–1.14) |
|||
| Suzuki et al. 2011 [47] | Prospective multicentre study | RA Orthopaedic surgery | bDMARDs | Prevalence of postoperative complications | January 2004 to November 2008 | Use of bDMARDs OR 2.12 (95% CI 1.48–3.03; p <0.0001). Infection prevalence: 2.1% for bDMARDs, 1.0% for csDMARDs |
|
| Johnson et al. 2013 [48] | Retrospective | RA TKA |
bDMARDs csDMARDs | Prevalence of postoperative complications | 7.3% AE including 4.3% infections: with TNFi 2.10%, local infections with csDMARDs | ||
| Scherrer et al. 2013 [49] | Retrospective | Inflammatory rheumatic diseases OA Orthopaedic surgery |
bDMARDs csDMARDs |
Infection | OA group: 0.8% Inflammatory rheumatic disease group: 2.0% OR 2.58 (95% CI 1.81–3.67; p = 0.001) TNFi given <1 administration interval OR 10.05 (95% CI 1.17–86.29; p = 0.035) |
||
| Studies not showing increased risk of infection | |||||||
| Bibbo et al. 2004 [42] | Randomised prospective | 31 patients: 141 elective foot procedures Gr 1: 16 pts with TNFi continued, 72 operations Gr 2: 15 pts without TNFi, 69 operations |
Etanercept: 2.6 d (1–10 d) Infliximab: 20.2 d mean (2–45 d) csDMARD continued in both groups |
Wound healing Infection | 1 year | Total complications with TNFi < without TNFi Gr 1: 1 superficial infection Gr 2: 3 delayed cicatrisations, 2 nonunions, 1 delayed union, 1 osteomyelitis No respiratory or urinary infection |
No interruption |
| Talwalkar et al. 2005 [50] | Retrospective | 11 patients: 16 orthopaedic procedures |
Gr A: 4 with TNFi continued Gr B: 12 with TNFi stopped |
No systemic or local infection in groups A and B | |||
| Wendling et al. 2005 [51] | Retrospective | 30 patients: 50 procedures, mainly orthopaedic 18 TNFi stopped 32 TNFi continued |
Infection Flare |
No complication in either group Flares more common in interruption group |
No interruption | ||
| Den Broeder et al. 2007 [40] | Retrospective | 768 patients, 1219 orthopaedic procedures | Gp 1: no TNFi Gp 2A: TNFi stopped 4 half-lives before Gp 2B: TNFi continued |
Infection | Infection while on TNFi OR 1.5 (95% CI 0.45–5.2) | No interruption Risk factors: skin infection, elbow, ankle, foot surgery Sulfasalazine could be protective OR 0.21 (95% CI 0.05–0.89) |
|
| Ruyssen-Wittrand et al. 2007 [52] | Retrospective | 127 procedures (mainly orthopaedic), 97 patients (77% RA) | Surgery type Timing of discontinuation |
Trend towards more infections with shorter delay before surgery | |||
| Corrao et al. 2007 [53] | Case series | 5 RA (ETN) | Without stop | No infection | |||
| Hirano et al. 2010 [54] | Retrospective | RA Orthopaedic surgery 39 on TNFi 74 no TNFi |
Wound healing Fever Inflammatory markers |
TNFi: 5.1% adverse events on surgical wounds csDMARDs: 6.8% (not statistically significant) |
No interruption | ||
| Gilson et al. 2010 [55] | Retrospective | RA Orthopaedic surgery 20 cases (infection while on TNFi) 40 controls (TNFi) TNFi interrupted 30 d ADA, 14 d ETN |
Risk factors for infection | 5 mg/d of GC plus TNFi OR 5 (CI 95% 1.1–21.6; p = 0.03) Previous arthroplasty infection OR 88.3 (95% CI 1.1–7071; p = 0.04) |
Minimum doses Interruption recommended |
||
| Hayata et al. 2011 [56] | Retrospective | RA Orthopaedic surgery 52 procedures IFX stopped 4 w before surgery |
Infection Wound healing |
3.8% postoperative infection (2/52) | |||
| Barnard et al. 2012 [57] | Retrospective | RA Hand surgery ETN postponed 2–3 w before surgery |
bDMARDs csDMARDs |
Infection Wound healing Flare |
No infection | Continue csDMARDs Stop bDMARDs |
|
| Berthold et al. 2013 [58] | Observational | TNFi Rheumatic patients Hand and general orthopaedic procedures |
Gp A: 872 procedures stopped TNFi (IFX 8 w, ETN 1 w, ADA 4 w.) Gp B: 671 procedures without stopping TNFi |
Infection | No significant difference in infection rates | ||
| Somayaji et al. 2013 [59] | Retrospective | RA Orthopaedic surgery |
Infection | No significant difference in infection rates, except for prednisone use OR 21 (95% CI 3.5–127.2; p<0.001) for doses >15 mg/day) | |||
| Kubota et al. 2014 [60] | Retrospective | RA Orthopaedic surgery | bDMARDs with drug interruption 2–4 w before surgery (principally TNFi) Non-biologic (300 joints) |
Infection | No significant difference in infection rates | ||
| Abou Zahr et al. 2015 [61] | Retrospective | RA | csDMARDs bDMARDs according to algorithm |
Infection | No significant difference in infection rates But increased risk of generalised infection with bDMARDs interrupted after surgery OR 9.2 (95% CI 1.99–42.60; p = 0.005) Increased risk of wound infection OR 14.15 (95% CI 1.76–113.76; p = 0.013) |
||
| Tada et al. 2016 [62] | Retrospective | RA Orthopaedic surgery |
csDMARDs bDMARDs with drug interruption 2–4 w before surgery |
Infection Wound healing |
No significant difference in infection rates | ||
| Kadota et al. 2016 [63] | Retrospective | RA Orthopaedic surgery |
bDMARDs (TNFi) with drug interruption 2–4 w before surgery |
Infection Wound healing |
No significant difference in infection rates Higher infection risk with foot surgery OR 3.167 (95% CI 1.256–7.986; p = 0.015) Delayed wound healing with TKA OR 4.044 (95% CI 1.436–11.389; p = 0.008) |
||
ADA = adalimumab; AE = adverse event; aHR = adjusted hazard ratio; bDMARD = biologic DMARD; DVT = deep vein thrombosis; CI = confidence interval; csDMARD = conventional synthetic DMARD; DMARD = disease-modifying antirheumatic drug; ETA = etanercept; GC = glucocorticoid; IFX = infliximab; OR = odds ratio; TNFi = tumour necrosis factor inhibitor;
In summary, there are six retrospective [43, 44, 46–49] and one prospective study [45] showing an increased risk for infection. Most of them do not provide information on whether TNF inhibitors treatment was discontinued before surgery. First of all, we describe the prospective study and then the retrospective ones.
A prospective observational non-randomised study evaluated the risk of septic arthritis among patients treated with TNF inhibitors (11 881) or csDMARDs (3673) [45]. Patients treated with TNF inhibitors were more likely to develop septic arthritis than patients on csDMARDs (HR 2.3, 95% CI 1.2–4.4), with 20 events in the csDMARD group and 179 events in the TNF inhibitor group. A subsequent analysis showed that previous arthroplasty was associated with an increased risk of septic arthritis (HR 2.45, 95% CI 1.90–3.17) in both groups.
In a retrospective nonrandomised multicentre study, a significant increase in infectious complications was observed in RA patients treated by bDMARDs (TNF inhibitors, abatacept, tocilizumab) undergoing arthroplasties (OR 2.12, 95% CI 1.48–3.03; p <0.0001) [47]. The prevalence of infections was 2.1% for bDMARDs (out of a total of 1626 arthroplasties) versus 1.0% in patients treated with csDMARDs (on a total of 29 903 arthroplasties). In contrast, the prevalence of infections was the same for other surgical procedures in the bDMARD and csDMARD groups (1.6 vs 1.0%). Timing of discontinuation before surgery was 26.4 ± 9.1 for infliximab and 14.1 ± 6.7 for etanercept. Number of surgical procedures on infliximab was 1616 and on etanercept 1686, with a number surgical site infections of 21 (1.3%) for infliximab and 23 (1.4%) for etanercept.
Ten infections were observed in a retrospective study including 91 RA patients who underwent orthopaedic procedures [43]. 35 patients were on TNF inhibitors at the time of surgery (no information on treatment discontinuation) and 56/91 were TNF inhibitor-naive. Seven out of these ten infectious complications occurred in patients on TNF inhibitors. In the multivariate analysis, the use of TNF inhibitors was significantly associated with serious infections (OR 5.3, 95% CI 1.1–24.9).
Another retrospective case control study comparing the rates of infectious complications, deep venous thrombosis and RA flares included 64 orthopaedic procedures in 49 patients on TNF inhibitors (35 on infliximab and 29 on etanercept) versus 64 cases of surgery in 63 patients treated with csDMARDs (mainly methotrexate or sulfasalazine) [44]. Etanercept and infliximab were discontinued between two and four weeks, and four weeks, before surgery, as per the British Society of Rheumatology [64] and the Japanese College of Rheumatology [65] guidelines, whereas csDMARDs were not discontinued. The results showed that infections occurred more frequently in patients treated with TNF inhibitors (OR 21.8, 95% CI 1.231–386.1; p = 0.036). Likewise, deep venous thrombosis was more frequent in patients treated with TNF inhibitors (OR 2.83, 95% CI 1.10–7.25; p = 0.03). Disease flares were observed in 17.2% of all patients and more specifically in 31% of patients treated with etanercept versus 5.7% with infliximab. This latter result must be interpreted with caution, as csDMARDs were not interrupted perioperatively. Finally, the authors did not observe any delay in wound healing.
A retrospective study analysed all consecutive total hip and total knee arthroplasties. 5.7% developed a superficial wound infection and 0.7% suffered a serious infection leading to removal of the prosthesis [46]. The use of bDMARDs (OR 5.69, 95% CI 2.07–15.61) and longer RA duration (OR 1.09, 95% CI 1.04–1.14) were associated with prosthetic joint infections.
A retrospective cohort study including 268 total knee arthroplasties in 248 RA patients reported adverse events in 7.3% of cases, including an infection rate of 4.3%, more specifically 3/92 (3.26%) local infections and one deep infection in patients on TNF inhibitors, and 3/143 (2.10%) local infections in patients treated with csDMARDs [48].
In a Swiss retrospective study covering 50 359 orthopaedic procedures (inflammatory rheumatic diseases and OA), 373 infections in 47 887 (0.8%) occurred in OA patients, and 49 infections in 2472 (2.0%) in patients with inflammatory rheumatic diseases (OR 2.58, 95% CI 1.81–3.67; p = 0.001) [49]. Within the inflammatory rheumatic disease group, the risk of infection was higher in TNF inhibitor than in csDMARD users, with an OR of 2.54 (95% CI 1.08–5.97; p = 0.032) with TNF inhibitors and an OR of 2.49 (95% CI 1.06–5.84; p = 0.036) for csDMARDs. This risk increased when the TNF inhibitors had been given within one administration interval (i.e., without stop) with an OR of 10.05 (95% CI 1.17–86.29; p = 0.035). Furthermore, the highest rate of infections was observed in cases of elbow and foot surgery.
Goodman et al. performed a meta-analysis in 2016 including eight observational and three case-control studies, covering a total of 3681 patients exposed to TNF inhibitors and 4310 with no recent exposure [66]. Results showed a higher risk of infection in the TNF inhibitor group with an OR of 2.47 (95% CI 1.66–3.68; p<0.0001). They did not analyse the continuation or stop of the TNF inhibitors before the operation.
We found three prospective studies – two studies in which TNF inhibitors were maintained [42, 58] and another that compared TNF inhibitor discontinuation with a group of patients not receiving a TNF inhibitor [60] – and 11 retrospective studies including three maintaining the TNF inhibitor [40, 50, 51], seven discontinuing [52, 56, 57, 59, 61–63], and one without further specification [54]. First of all, we describe the three prospective studies, and then the retrospective ones.
A prospective, randomised study included 31 RA patients who underwent 141 elective foot and ankle procedures [42]. Patients were divided into two groups: 16 patients on TNF inhibitors and 15 TNF inhibitor-naive patients who underwent 4.5 and 4.6 mean procedures per patient, respectively, without stopping their treatment (bDMARDs and csDMARDs). Three soft-tissue healing complications were reported (two non-unions and one delayed union), and two cases of infections: one superficial infection in group one, and one osteomyelitis in a TNF inhibitor-naive patient. There was no deep venous thrombosis reported. Except for a higher number of smokers in TNF inhibitor group, both groups were well matched for demographic characteristics. The authors concluded that TNF inhibitors could be safely administrated during the perioperative period because of the same complication number between the two groups.
Rheumatic patients on TNF inhibitors undergoing hand and other orthopaedic procedures were divided in a prospective observational study into the following two groups: the first group (group A: 872 procedures) between 2003 and 2005 in which the TNF inhibitor was stopped (infliximab 8 w, etanercept 1 w, adalimumab 4 w) and another group (group B: 671 procedures) from 2006 to 2009 in which the TNF inhibitor was continued [58]. No significant association was observed between infection rates (total deep and superficial infections) and medical treatment, although 3% infections in the group A compared with 5.3% in the group B were reported.
A study divided RA patients undergoing orthopaedic surgery (total joint arthroplasty) into two groups; one biologic (267 joints) with drug interruption between two and four weeks before surgery according to the Japanese guidelines [65] (principally TNF inhibitors), and one non-biologic (300 joints) [60]. No significant differences in the incidence of complications were observed between the two groups (TNF inhibitor stopped vs csDMARDs continued) in terms of superficial and deep infections, which were observed in 0.37 and 1%, respectively in the biologic group vs 0.67 and 0% in the non-biologic group.
A retrospective study reported 11 patients (10 RA and one psoriatic arthritis) who underwent 16 operations. The TNF inhibitor was discontinued in 12 patients and maintained in four during the perioperative period. There were no serious systemic or wound infections. There was one episode of disease flare in a patient who stopped therapy [50].
In a retrospective study that included 50 surgical procedures in 30 RA patients, TNF inhibitors were maintained in 32 cases and withheld prior to surgery in 18 cases [51]. There was no major complication, but disease flares occurred more frequently in patients who discontinued therapy (p = 0.02).
A retrospective parallel cohort included 768 RA patients with 1219 orthopaedic procedures who were divided as follows: group 1, TNF inhibitor-naïve; group 2A, TNF inhibitors stopped (four half-lives), and group 2B, TNF inhibitors continued [40]. There was no significant difference in the occurrence of infections between groups (OR 1.5, 95% CI 0.45–5.2). However, surgical procedures on the elbow, foot or ankle, as well as previous episodes of skin or wound infections, were associated with an increased risk of infectious complications (OR 4.1, 95% CI 1.6–10.1; OR 3.2, 95% CI 1.6–6.5; OR 13.8, 95% CI 5.2–36.7, respectively). Wound dehiscence (RR 2.4, 95% CI 1.1–5.0) and bleeding were more frequent in the TNF inhibitor group.
One retrospective study included 770 patients on biologics, 92 of whom underwent surgery (RA n = 71, ankylosing spondylitis n = 18) [52]. TNF inhibitors included infliximab, etanercept and adalimumab. Patients underwent 127 surgical procedures (orthopaedic n = 107, 85%; abdominal n = 6, 4.7%; gynaecological n = 2, 1.6%; and other n = 12, 9.4%). Surgical procedures were separated into five groups based on the risk of infections and other complications such as deep venous thrombosis and rate of wound healing. Complication rates were compared based on the type of surgery and timing of TNF inhibitor discontinuation (less than two and up to five circulating half-lives prior to surgery). The total complications rate was 18.9% (24 cases), including 12 infections, 1 deep venous thrombosis and six delayed healings. Patients in whom therapy was discontinued less than two half-lives before surgery showed a higher rate of complications than those in whom discontinuation occurred with more than two (up to five) half-lives (30% vs 16.3, 19.4%), but the difference was not statistically significant (p = 0.24).
A retrospective study including 39 RA patients on TNF inhibitors and 74 patients on csDMARDs undergoing orthopaedic surgery (ankle arthrodesis; total hip, knee, elbow or shoulder arthroplasty) found no differences in overall adverse events in wounds (csDMARDs: 6.8%, TNF inhibitors 5.1%, p = 1.0), nor in duration of wound healing and fever. This study, however, does not provide sufficient data on discontinuation timing [54].
A case-control study performed within the French national registry RATIO (Research Axed on Tolerance of bIOtherapies) included 20 patients with various rheumatic diseases (RA 90%, ankylosing spondylitis, psoriatic arthritis) treated with TNF inhibitors [55]. Patients who presented with infection after a total arthroplasty of a large joint were compared with 40 controls. A rate of 67% of flares was observed. Increased steroid intake was associated with infection (OR 5, 95% CI 1.1–21.6; p = 0.03) as was previous arthroplasty infection (OR 88.3, 95% CI 1.1–7071; p = 0.04). Neither TNF inhibitor use nor duration of exposure showed a statistically significant increase of infection rate, but infections had a worse outcome in patients on TNF inhibitors.
A retrospective study including RA patients who stopped infliximab four weeks prior to 52 orthopaedic procedures (total hip, knee, elbow or shoulder arthroplasty; foot/hand surgery, arthroscopic synovectomy and fractures) showed that infliximab did not increase the risk of superficial and deep infections [56].
In a study on hand surgery, authors reviewed 35 Swanson’s metacarpophalangeal joint arthroplasties performed on RA patients [57]. They only interrupted their biologic treatment (TNF inhibitor, etanercept) two or three weeks prior to surgery and resumed after wound healing, while maintaining the other synthetic DMARDs, steroids or nonsteroidal anti-inflammatory drugs (NSAIDs). In addition, antibiotic prophylaxis was used. They observed no deep infection, only one superficial infection, one late suture granuloma, and one presumed rheumatoid flare.
A multicentre retrospective study reviewed 381 hip and knee arthroplasties performed on RA patients [59]. No further details were provided on the timing protocol for withholding biological agents (94% TNF inhibitors) or synthetic DMARDs except that 67.5% of patients were being treated at the time of surgery. Based on the small numbers of surgical site infections [7], the authors did not find any significant association between treatments and infection, except for prednisone use exceeding 15 mg/day (OR 21, 95% CI 3.5–127.2; p <0.001).
A large retrospective study examined use of DMARDs and biological agents in 6024 RA patients undergoing surgery from the US database of the Veteran Health Administration [61]. To determine the drug-withhold timing, they developed an algorithm. The authors did not report any significant increase in risk of surgical site infection or wound infection between perioperative drug continuation and interruption. However, they found an increased risk of generalised infection with bDMARD interruption after surgery (OR 9.2, 95% CI 1.99–42.60; p = 0.005), which may be explained by drug interruption because of an on-going postoperative complication, as well as a significantly higher risk of postoperative wound infection with an OR of 14.15 (95% CI 1.76–113.76; p = 0.013).
Risk factors for surgical site infection and delayed wound healing were retrospectively analysed among 332 elective orthopaedic procedures performed on RA patients in various Japanese centres [62]. Their bDMARDs (etanercept, adalimumab, infliximab, tocilizumab, abatacept) and csDMARDs were interrupted prior surgery as per the Japanese College of Rheumatology guidelines [65]. The authors did not find any association of infection or delayed wound healing with bDMARD or csDMARD use. Unfortunately, the small number of patients on new biological drugs (abatacept or tocilizumab) does not allow for any firm conclusions.
Another recent retrospective study of a larger sample examined risk factors for the same parameters (infection and wound healing) after 1036 various orthopaedic procedures on RA patients [63]. TNF inhibitors (etanercept, adalimumab, infliximab, golimumab) were interrupted two to four weeks before surgery, again according to the Japanese guidelines. There was no significant correlation between infection or delayed wound healing and bDMARDs. A higher risk of infection with foot surgery (OR 3.167, 95% CI 1.256–7.986; p = 0.015) and delayed wound healing with total knee arthroplasty (OR 4.044, 95% CI 1.436–11.389; p = 0.008) was observed.
A retrospective case-control study compared wound healing and inflammatory state in 44 RA patients treated with either tocilizumab or csDMARDs [67] who underwent upper and lower limb total arthroplasties. The mean time from last infusion of tocilizumab was 16.1 days (range 3–27). There were no differences in terms of C-reactive protein (CRP) levels (p<0.001), white blood cell counts, temperature, superficial or deep infections and no wound healing delays.
More recently, a multicentre retrospective study collected 161 cases of orthopaedic surgery (mainly arthroplasties: 89/161 cases) among 122 RA patients treated with tocilizumab [68]. According to the Japanese guidelines [69], treatment was interrupted prior to surgery but with some variability among patients. The mean time between the last infusion and surgery was 23.5 ± 12.7 days, and the time for resuming therapy was 24.8 ± 12.7 days after surgery. The authors reported a small number of surgical site infections – two superficial (1.9%) and one deep (0.6%) infection – as well as a few cases of wound healing delays (14%) and disease flares (22.4%). In a multiple logistic regression analysis, no correlation was found between the duration of treatment interruption and disease flares (OR 1.033, 95% CI 1.013–1.055; p = 0.0015). Delays in wound healing were mostly related to concomitant use of prednisolone (OR 5.494, 95% CI 1.026–101.926; p = 0.0459). There were also significant correlations between foot and spinal surgery and delayed wound healing with ORs of 3.066 (95% CI 1.009–9.327; p = 0.0483) and 158.750 (95% CI 10.521–6993.007; p = 0.0002).
The French Auto-Immunity and Rituximab (AIR) registry analysed data that included 94 orthopaedic and 23 abdominal procedures in RA patients [70]. The mean time between the last rituximab infusion and surgery was 6.4 months (4.3–8.7). There were 12 postoperative complications in nine patients: eight surgical site infections, and one fatal case of septic shock. In a univariate analysis, spine surgery was significantly associated with postoperative complications (p = 0.048), but no association was reported regarding the timeframe between surgery and the last rituximab infusion.
A retrospective study included seven RA patients undergoing eight orthopaedic procedures (knee, hand, foot and vertebral surgery) [71]. Abatacept was discontinued on average 15.9 days prior to surgery (mean of 33.1 days in the perioperative period) but concomitant medications (prednisolone, methotrexate, sulfasalazine) were maintained. There was no case of postoperative surgical site infection, delayed wound healing or disease flare.
A retrospective study was performed on 131 psoriasis patients who underwent various surgical procedures (orthopaedic, cardiovascular, dermatological, dental extraction and neurosurgery) [72]. Only 13 patients were treated with ustekinumab, and 118 patients were on TNF inhibitors (infliximab, adalimumab or etanercept). The treatment (TNF inhibitor or ustekinumab) was maintained in 87 patients and discontinued in 44 patients according to the guidelines of the British Association of Dermatologists, which advises that ustekinumab be interrupted 12 weeks prior to surgery (four half-lives) [73]. Three wound infections were observed without knowing the type of treatment: two in the discontinuation group (knee arthroplasty), and one in the maintenance group (dental implant). Given its small sample, this study seems reassuring as far as the perioperative safety of TNF inhibitors is concerned, but does not really provide much information on perioperative ustekinumab use.
Our review of the current literature shows that the number of clinical studies on the effect of DMARDs during the perioperative period is scarce. Furthermore, the limited numbers of patients included in most studies, as well as the sometimes poor study design, are major limitations to making recommendations with a good level of evidence. Finally, as several studies were observational, confounding factors may have introduced major bias leading to conflicting results and sometimes, wrong conclusions.
Methotrexate is by far the best studied among all csDMARDs, in particular in prospective randomised clinical trials addressing the question of treatment maintenance during the perioperative period. Based on the results of these studies, it seems reasonable to continue methotrexate administration during the perioperative period. This recommendation is not only supported by the absence of major safety signals, but also aims to avoid disease flares that may ultimately lead to use corticosteroids or an increase their dosage, thus promoting a higher risk of complications. However, one should also bear in mind that low median doses of methotrexate (10 mg/week) were used in most of the studies, and that the safety of larger doses, as currently used, has not been formally examined. The evidence currently suggests that other csDMARDs (HCQ, sulfasalazine) can also continue to be safely administered during the perioperative period.
National recommendations on the use of bDMARDs in patients undergoing surgical procedures have been based primarily on limited evidence and mostly on clinical experience and expert opinions. Some of the recommendations, which vary greatly between countries, are based essentially on bDMARDs half-lives (table 3). In France, the CRI (Club Rhumatisme et Inflammation) adjusts its recommendations to discontinue TNF inhibitors according to the surgical site: two half-lives for interventions in a sterile milieu such as cataracts, to four to five half-lives for high-risk procedures such as abdominal surgery [74]. In the UK and in Japan, guidelines advise stopping TNF inhibitors between three and five half-lives prior to any surgical procedure [69, 75]. In Spain [76], like in Switzerland (Commission des médicaments des pharmaciens suisses) [77], guidelines recommend stopping administration without any further detail regarding the timing of discontinuation or the type of surgery. The German Rheumatology Society gives further recommendations on synthetic DMARDs, mainly based on expert opinion and drug half-lives. Preoperative interruption is only recommended for ciclosporin (1–2 days before surgery), mycophenolate mofetil (1–2 days before surgery) and azathioprine (1–2 days before surgery) [78]. In 2017, The American College of Rheumatology published new recommendations with low and moderate level of evidence: they suggest not stopping csDMARDs and stopping bDMARDs based on the timing of administration rather than the half-life, i.e., schedule surgery one week after normal administration, for example 9 weeks for infliximab, and 3 weeks for adalimumab [79].
Table 3 Half-lives of disease-modifying antirheumatic drugs.
| Drug | Half-life |
|---|---|
| bDMARDs | |
| TNF inhibitors | |
| Infliximab | 8–9.5 days |
| Etanercept | 70 hours (between 7 and 300 hours) |
| Adalimumab | 2 weeks |
| Golimumab | 12 ± 3 days |
| Certolizumab pegol | 14 days |
| IL6 receptor antagonist | |
| Tocilizumab | Dependent on concentration: between 6 and 16 days |
| Anti-CD20 | |
| Rituximab | 22 days, range 6.1–52 days |
| CTLA4-IgG | |
| Abatacept | 13.1 days, range 8–25 days |
| Anti-p40 antibody targeting both IL-12 and IL-23 | |
| Ustekinumab | Median 3 weeks, range 15–32 days |
| IL1 receptor antagonist | |
| Anakinra | Between 4 and 6 hours |
| csDMARDs | Half-live |
| Methotrexate | 6–7 hours, range 3–17 hours |
| Leflunomide | 1–4 weeks |
bDMARD = biologic DMARD; csDMARD = conventional synthetic DMARD; DMARD = disease-modifying antirheumatic drug; IL = interleukin Source: medicaments.gouv.fr
Various case reports [80, 81] and a case-control study comparing postoperative inflammation [82] reported a lack of inflammatory response (CRP and fever) in patients treated with tocilizumab. These findings are consistent with the well-described role of interleukin (IL)-6 in the control of acute-phase protein production by hepatocytes and body temperature. Thus, physicians should be aware of the specificity of tocilizumab’s mode of action when assessing a patient with a postoperative complication. Notably, they should not rely on CRP as a marker of infection since CRP elevation is blunted by IL-6 inhibition. Furthermore, and regardless of surgery, severe infections can occur in tocilizumab-treated patients with an OR of 1.78 (95% CI 0.98–3.23), including mainly skin and subcutaneous infections, when tocilizumab is used in combination with methotrexate. Adverse events were also noticed with tocilizumab 8 mg/kg in combination with csDMARDs, as compared with controls treated with csDMARDs, with an OR of 1.53 (95% CI 1.26–1.86) [83].
Regarding bDMARDs, the preoperative withdrawal will depend on several parameters. Decisions should ideally be made in a multidisciplinary setting involving the rheumatologist, surgeon, and specialist in infectious diseases in certain cases. Points to consider should include individual patient and disease characteristics, including the presence of comorbidities, previous infectious complications, the type of immunosuppressive therapy and the severity of the disease. High disease activity is a risk factor for infection [84], as is the presence of prosthetic joint [45]. The current literature does not clearly address whether bDMARDs should be discontinued according to their half-life or administration interval, and it seems difficult to completely dissociate one from the other. However, the administration interval has the advantage of being easy to use in routine practice. The intrinsic risk of infectious complications associated with the type and site of surgical procedures should be assessed. Particular attention should be given to an articular pathology that is known to be a risk factor for subsequent postoperative complications [85]. The same applies to the type and severity of the autoimmune disease. Indeed, uncontrolled systemic lupus erythematosus may have major consequences on morbidity or even mortality that may preclude the complete cessation of immunosuppressive therapy. The decision of bDMARD discontinuation will also depend on whether bDMARDs are used as monotherapy or in combination with methotrexate or other csDMARDs, since in the latter case the disease may still be controlled by the administration of csDMARDs, whereas patients left without therapy in the former situation are at risk of flares. Finally, it is common practice to withhold bDMARDs before surgery because of the increased risk (or fear) of infection and delayed wound healing. However, the benefits of such an approach should be carefully weighed against the side effects of increasing glucocorticoid doses to treat flares.
Pubmed database up to 14 May 2016
((surgery) AND infection) AND rheumatoid arthritis:
Total : 1841
Selected: 56
Specific drug
Methotrexate: 6
Leflunomide: 2
Tocilizumab: 2
Sulfasalazine: 1
Meta-analysis: 4
Case report: 18
Review: 25
(“post-operative complications”) AND “tumor necrosis factor alpha”
Total: 593
Selected: 4
Specific drug: Ustekinumab: 1
Already included with previous MeSH terms: 3
Visceral surgery: 23
Review: 5
Case report: 1
Unrelated: 1
“anti tumor necrosis factor AND orthopedic surgery”:
Total: 271
Selected: 1
Already included with previous MeSH terms: 6
Review: 4
Case report: 4
Unrelated: 2
“anti tumor necrosis factor AND orthopedic surgery” AND “wound healing”:
Total: 11
Selected: 0
Already included with previous MeSH terms: 3
Case report: 1
Review: 2
((surgery) AND infection) AND tocilizumab:
Total: 17
Selected: 0
Already included with previous MeSH terms: 1
Case report: 3
Review: 1
((((“surgery”) OR “arthroplasty”) AND “infection”) AND “wound healing”) AND “anti tumor necrosis factor”
Total: 2
Selected: 0
Already included with previous MeSH terms: 2
(abatacept) AND perioperative period
Total: 10
Selected: 1
Review: 1
((rituximab) AND rheumatoid arthritis) AND operation
Total: 52
Selected: 1
Review: 2
((“surgery”) AND “infection”) AND “rituximab”:
Total: 124
Selected: 0
Review: 3
(rituximab) AND perioperative period
Total: 18
Selected: 0
Review: 2
(rituximab) AND wound healing
Tota : 13
Selected: 0
((rituximab) OR “anti CD20”) AND “wound healing”
Total: 8
Selected: 0
(rituximab) AND “orthopedic/surgery”
Total: 0
((((belimumab) OR anakinra) OR tofacitinib) OR apremilast) AND perioperative period
Total: 20
Selected: 0
Review: 1
Unrelated: 1
((((belimumab) OR anakinra) OR tofacitinib) OR apremilast) AND “surgical wound”
Total: 8
Selected: 0
Already included with previous MeSH terms: 1
Review: 1
((“methotrexate”) AND “surgery”) AND “infection”
Total: 212
Selected: 0
Already included with previous MeSH terms: 13
Review: 5
Visceral surgery: 1
Case report: 2
Unrelated: 1
((“leflunomide”) AND “surgery”) AND “infection”
Total: 27
Selected: 0
Already included with previous MeSH terms: 2
((“sulfasalazine”) AND “surgery”) AND “infection”
Total: 14
Selected: 0
Already included with previous MeSH terms: 1
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Smolen JS , Landewé R , Bijlsma J , Burmester G , Chatzidionysiou K , Dougados M , et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–77. doi:.https://doi.org/10.1136/annrheumdis-2016-210715
2 Kalliolias GD , Ivashkiv LB . TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12(1):49–62. doi:.https://doi.org/10.1038/nrrheum.2015.169
3 Singh JA , Cameron C , Noorbaloochi S , Cullis T , Tucker M , Christensen R , et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet. 2015;386(9990):258–65. doi:.https://doi.org/10.1016/S0140-6736(14)61704-9
4 Koyama K , Ohba T , Ebata S , Haro H . Postoperative Surgical Infection After Spinal Surgery in Rheumatoid Arthritis. Orthopedics. 2016;39(3):e430–3. doi:.https://doi.org/10.3928/01477447-20160404-05
5 Kang C-N , Kim C-W , Moon J-K . The outcomes of instrumented posterolateral lumbar fusion in patients with rheumatoid arthritis. Bone Joint J. 2016;98-B(1):102–8. doi:.https://doi.org/10.1302/0301-620X.98B1.36247
6 LoVerde ZJ , Mandl LA , Johnson BK , Figgie MP , Boettner F , Lee Y-Y , et al. Rheumatoid Arthritis Does Not Increase Risk of Short-term Adverse Events after Total Knee Arthroplasty: A Retrospective Case-control Study. J Rheumatol. 2015;42(7):1123–30. doi:.https://doi.org/10.3899/jrheum.141251
7 Schrama JC , Fenstad AM , Dale H , Havelin L , Hallan G , Overgaard S , et al. Increased risk of revision for infection in rheumatoid arthritis patients with total hip replacements. Acta Orthop. 2015;86(4):469–76. doi:.https://doi.org/10.3109/17453674.2015.1017793
8 Singh JA , Inacio MCS , Namba RS , Paxton EW . Rheumatoid arthritis is associated with higher ninety-day hospital readmission rates compared to osteoarthritis after hip or knee arthroplasty: a cohort study. Arthritis Care Res (Hoboken). 2015;67(5):718–24. doi:.https://doi.org/10.1002/acr.22497
9 Ravi B , Croxford R , Hollands S , Paterson JM , Bogoch E , Kreder H , et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254–63. doi:.https://doi.org/10.1002/art.38231
10 Pedersen E , Pinsker E , Younger ASE , Penner MJ , Wing KJ , Dryden PJ , et al. Outcome of total ankle arthroplasty in patients with rheumatoid arthritis and noninflammatory arthritis. A multicenter cohort study comparing clinical outcome and safety. J Bone Joint Surg Am. 2014;96(21):1768–75. doi:.https://doi.org/10.2106/JBJS.M.01164
11 Carroll K , Dowsey M , Choong P , Peel T . Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2014;20(2):130–5. doi:.https://doi.org/10.1111/1469-0691.12209
12 Stundner O , Danninger T , Chiu Y-L , Sun X , Goodman SM , Russell LA , et al. Rheumatoid arthritis vs osteoarthritis in patients receiving total knee arthroplasty: perioperative outcomes. J Arthroplasty. 2014;29(2):308–13. doi:.https://doi.org/10.1016/j.arth.2013.05.008
13 Hsieh P-H , Huang K-C , Shih H-N . Prosthetic joint infection in patients with rheumatoid arthritis: an outcome analysis compared with controls. PLoS One. 2013;8(8):e71666. doi:.https://doi.org/10.1371/journal.pone.0071666
14 Pahys JM , Pahys JR , Cho SK , Kang MM , Zebala LP , Hawasli AH , et al. Methods to decrease postoperative infections following posterior cervical spine surgery. J Bone Joint Surg Am. 2013;95(6):549–54. doi:.https://doi.org/10.2106/JBJS.K.00756
15 Michaud K , Fehringer EV , Garvin K , O’Dell JR , Mikuls TR . Rheumatoid arthritis patients are not at increased risk for 30-day cardiovascular events, infections, or mortality after total joint arthroplasty. Arthritis Res Ther. 2013;15(6):R195. doi:.https://doi.org/10.1186/ar4385
16 Bhatia R , Desouza RM , Bull J , Casey ATH . Rigid occipitocervical fixation: indications, outcomes, and complications in the modern era. J Neurosurg Spine. 2013;18(4):333–9. doi:.https://doi.org/10.3171/2013.1.SPINE12645
17 da Cunha BM , de Oliveira SB , Santos-Neto L . Incidence of infectious complications in hip and knee arthroplasties in rheumatoid arthritis and osteoarthritis patients. Rev Bras Reumatol. 2011;51(6):609–15.
18 SooHoo NF , Farng E , Lieberman JR , Chambers L , Zingmond DS . Factors that predict short-term complication rates after total hip arthroplasty. Clin Orthop Relat Res. 2010;468(9):2363–71. doi:.https://doi.org/10.1007/s11999-010-1354-0
19 Schrama JC , Espehaug B , Hallan G , Engesaeter LB , Furnes O , Havelin LI , et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res (Hoboken). 2010;62(4):473–9. doi:.https://doi.org/10.1002/acr.20036
20 van der Heide HJL , Schutte B , Louwerens JWK , van den Hoogen FHJ , de Waal Malefijt MC . Total ankle prostheses in rheumatoid arthropathy: Outcome in 52 patients followed for 1-9 years. Acta Orthop. 2009;80(4):440–4. doi:.https://doi.org/10.3109/17453670903153568
21 Jämsen E , Huhtala H , Puolakka T , Moilanen T . Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38–47. doi:.https://doi.org/10.2106/JBJS.G.01686
22 Bongartz T , Halligan CS , Osmon DR , Reinalda MS , Bamlet WR , Crowson CS , et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713–20. doi:.https://doi.org/10.1002/art.24060
23 Himanen A-K , Belt EA , Lehto MUK , Hämäläinen MMJ . A comparison of survival of moulded monoblock and modular tibial components of 751 AGC total knee replacements in the treatment of rheumatoid arthritis. J Bone Joint Surg Br. 2007;89-B(5):609–14. doi:.https://doi.org/10.1302/0301-620X.89B5.17950
24 Ljung P , Jonsson K , Rydholm U . Short-term complications of the lateral approach for non-constrained elbow replacement. Follow-up of 50 rheumatoid elbows. J Bone Joint Surg Br. 1995;77(6):937–42.
25 Kristensen O , Nafei A , Kjaersgaard-Andersen P , Hvid I , Jensen J . Long-term results of total condylar knee arthroplasty in rheumatoid arthritis. J Bone Joint Surg Br. 1992;74(6):803–6.
26 Wymenga AB , van Horn JR , Theeuwes A , Tmuytjens HL , Slooff TJ . Perioperative factors associated with septic arthritis after arthroplasty. Prospective multicenter study of 362 knee and 2,651 hip operations. Acta Orthop Scand. 1992;63(6):665–71. doi:.https://doi.org/10.1080/17453679209169732
27 Wilson MG , Kelley K , Thornhill TS . Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72(6):878–83. doi:.https://doi.org/10.2106/00004623-199072060-00013
28 Poss R , Ewald FC , Thomas WH , Sledge CB . Complications of total hip-replacement arthorplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 1976;58(8):1130–3. doi:.https://doi.org/10.2106/00004623-197658080-00016
29 Garner RW , Mowat AG , Hazleman BL . Wound healing after operations of patients with rheumatoid arthritis. J Bone Joint Surg Br. 1973;55(1):134–44.
30 Luessenhop CP , Higgins LD , Brause BD , Ranawat CS . Multiple prosthetic infections after total joint arthroplasty. Risk factor analysis. J Arthroplasty. 1996;11(7):862–8. doi:.https://doi.org/10.1016/S0883-5403(96)80189-6
31 Grennan DM , Gray J , Loudon J , Fear S . Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214–7. doi:.https://doi.org/10.1136/ard.60.3.214
32 Sreekumar R , Gray J , Kay P , Grennan DM . Methotrexate and post operative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery--a ten year follow-up. Acta Orthop Belg. 2011;77(6):823–6.
33 Murata K , Yasuda T , Ito H , Yoshida M , Shimizu M , Nakamura T . Lack of increase in postoperative complications with low-dose methotrexate therapy in patients with rheumatoid arthritis undergoing elective orthopedic surgery. Mod Rheumatol. 2006;16(1):14–9. doi:.https://doi.org/10.3109/s10165-005-0444-4
34 Jain A , Witbreuk M , Ball C , Nanchahal J . Influence of steroids and methotrexate on wound complications after elective rheumatoid hand and wrist surgery. J Hand Surg Am. 2002;27(3):449–55. doi:.https://doi.org/10.1053/jhsu.2002.32958
35 Sany J , Anaya JM , Canovas F , Combe B , Jorgensen C , Saker S , et al. Influence of methotrexate on the frequency of postoperative infectious complications in patients with rheumatoid arthritis. J Rheumatol. 1993;20(7):1129–32.
36 Carpenter MT , West SG , Vogelgesang SA , Casey Jones DE . Postoperative joint infections in rheumatoid arthritis patients on methotrexate therapy. Orthopedics. 1996;19(3):207–10.
37 Loza E , Martinez-Lopez JA , Carmona L . A systematic review on the optimum management of the use of methotrexate in rheumatoid arthritis patients in the perioperative period to minimize perioperative morbidity and maintain disease control. Clin Exp Rheumatol. 2009;27(5):856–62.
38 Tanaka N , Sakahashi H , Sato E , Hirose K , Ishima T , Ishii S . Examination of the risk of continuous leflunomide treatment on the incidence of infectious complications after joint arthroplasty in patients with rheumatoid arthritis. J Clin Rheumatol. 2003;9(2):115–8. doi:.https://doi.org/10.1097/01.RHU.0000062514.54375.bd
39 Fuerst M , Möhl H , Baumgärtel K , Rüther W . Leflunomide increases the risk of early healing complications in patients with rheumatoid arthritis undergoing elective orthopedic surgery. Rheumatol Int. 2006;26(12):1138–42. doi:.https://doi.org/10.1007/s00296-006-0138-z
40 den Broeder AA , Creemers MCW , Fransen J , de Jong E , de Rooij D-JR , Wymenga A , et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study. J Rheumatol. 2007;34(4):689–95.
41 Bibbo C , Anderson RB , Davis WH , Norton J . The influence of rheumatoid chemotherapy, age, and presence of rheumatoid nodules on postoperative complications in rheumatoid foot and ankle surgery: analysis of 725 procedures in 104 patients [corrected] [corrected]. Foot Ankle Int. 2003;24(1):40–4. doi:.https://doi.org/10.1177/107110070302400106
42 Bibbo C , Goldberg JW . Infectious and healing complications after elective orthopaedic foot and ankle surgery during tumor necrosis factor-alpha inhibition therapy. Foot Ankle Int. 2004;25(5):331–5. doi:.https://doi.org/10.1177/107110070402500510
43 Giles JT , Bartlett SJ , Gelber AC , Nanda S , Fontaine K , Ruffing V , et al. Tumor necrosis factor inhibitor therapy and risk of serious postoperative orthopedic infection in rheumatoid arthritis. Arthritis Rheum. 2006;55(2):333–7. doi:.https://doi.org/10.1002/art.21841
44 Kawakami K , Ikari K , Kawamura K , Tsukahara S , Iwamoto T , Yano K , et al. Complications and features after joint surgery in rheumatoid arthritis patients treated with tumour necrosis factor-alpha blockers: perioperative interruption of tumour necrosis factor-alpha blockers decreases complications? Rheumatology (Oxford). 2010;49(2):341–7. doi:.https://doi.org/10.1093/rheumatology/kep376
45 Galloway JB , Hyrich KL , Mercer LK , Dixon WG , Ustianowski AP , Helbert M , et al.; BSR Biologics Register. Risk of septic arthritis in patients with rheumatoid arthritis and the effect of anti-TNF therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70(10):1810–4. doi:.https://doi.org/10.1136/ard.2011.152769
46 Momohara S , Kawakami K , Iwamoto T , Yano K , Sakuma Y , Hiroshima R , et al. Prosthetic joint infection after total hip or knee arthroplasty in rheumatoid arthritis patients treated with nonbiologic and biologic disease-modifying antirheumatic drugs. Mod Rheumatol. 2011;21(5):469–75. doi:.https://doi.org/10.3109/s10165-011-0423-x
47 Suzuki M , Nishida K , Soen S , Oda H , Inoue H , Kaneko A , et al. Risk of postoperative complications in rheumatoid arthritis relevant to treatment with biologic agents: a report from the Committee on Arthritis of the Japanese Orthopaedic Association. J Orthop Sci. 2011;16(6):778–84. doi:.https://doi.org/10.1007/s00776-011-0142-3
48 Johnson BK , Goodman SM , Alexiades MM , Figgie MP , Demmer RT , Mandl LA . Patterns and associated risk of perioperative use of anti-tumor necrosis factor in patients with rheumatoid arthritis undergoing total knee replacement. J Rheumatol. 2013;40(5):617–23. doi:.https://doi.org/10.3899/jrheum.121171
49 Scherrer CB , Mannion AF , Kyburz D , Vogt M , Kramers-de Quervain IA . Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res (Hoboken). 2013;65(12):2032–40. doi:.https://doi.org/10.1002/acr.22077
50 Talwalkar SC , Grennan DM , Gray J , Johnson P , Hayton MJ . Tumour necrosis factor α antagonists and early postoperative complications in patients with inflammatory joint disease undergoing elective orthopaedic surgery. Ann Rheum Dis. 2005;64(4):650–1. doi:.https://doi.org/10.1136/ard.2004.028365
51 Wendling D , Balblanc JC , Brousse A , Lohse A , Lehuede G , Garbuio P , et al. Surgery in patients receiving anti-tumour necrosis factor α treatment in rheumatoid arthritis: an observational study on 50 surgical procedures. Ann Rheum Dis. 2005;64(9):1378–9. doi:.https://doi.org/10.1136/ard.2005.037762
52 Ruyssen-Witrand A , Gossec L , Salliot C , Luc M , Duclos M , Guignard S , et al. Complication rates of 127 surgical procedures performed in rheumatic patients receiving tumor necrosis factor alpha blockers. Clin Exp Rheumatol. 2007;25(3):430–6.
53 Corrao S , Pistone G , Arnone S , Calvo L , Scaglione R , Licata G . Safety of etanercept therapy in rheumatoid patients undergoing surgery: preliminary report. Clin Rheumatol. 2007;26(9):1513–5. doi:.https://doi.org/10.1007/s10067-007-0534-0
54 Hirano Y , Kojima T , Kanayama Y , Shioura T , Hayashi M , Kida D , et al. Influences of anti-tumour necrosis factor agents on postoperative recovery in patients with rheumatoid arthritis. Clin Rheumatol. 2010;29(5):495–500. doi:.https://doi.org/10.1007/s10067-009-1346-1
55 Gilson M , Gossec L , Mariette X , Gherissi D , Guyot M-H , Berthelot J-M , et al. Risk factors for total joint arthroplasty infection in patients receiving tumor necrosis factor α-blockers: a case-control study. Arthritis Res Ther. 2010;12(4):R145. doi:.https://doi.org/10.1186/ar3087
56 Hayata K , Kanbe K , Chiba J , Nakamura A , Inoue Y , Hobo K . Clinical factors related to the efficacy and complications of orthopedic surgery for rheumatoid arthritis with infliximab. Int J Rheum Dis. 2011;14(1):31–6. doi:.https://doi.org/10.1111/j.1756-185X.2010.01579.x
57 Barnard AR , Regan M , Burke FD , Chung KC , Wilgis EFS . Wound healing with medications for rheumatoid arthritis in hand surgery. ISRN Rheumatol. 2012;2012:251962. doi:.https://doi.org/10.5402/2012/251962
58 Berthold E , Geborek P , Gülfe A . Continuation of TNF blockade in patients with inflammatory rheumatic disease. An observational study on surgical site infections in 1,596 elective orthopedic and hand surgery procedures. Acta Orthop. 2013;84(5):495–501. doi:.https://doi.org/10.3109/17453674.2013.842431
59 Somayaji R , Barnabe C , Martin L . Risk factors for infection following total joint arthroplasty in rheumatoid arthritis. Open Rheumatol J. 2013;7(1):119–24. doi:.https://doi.org/10.2174/1874312920131210005
60 Kubota A , Sekiguchi M , Nakamura T , Miyazaki Y , Suguro T . Does use of a biologic agent increase the incidence of postoperative infection in surgery for rheumatoid arthritis after total joint arthroplasty? Mod Rheumatol. 2014;24(3):430–3. doi:.https://doi.org/10.3109/14397595.2013.844387
61 Abou Zahr Z , Spiegelman A , Cantu M , Ng B . Perioperative use of anti-rheumatic agents does not increase early postoperative infection risks: a Veteran Affairs’ administrative database study. Rheumatol Int. 2015;35(2):265–72. doi:.https://doi.org/10.1007/s00296-014-3121-0
62 Tada M , Inui K , Sugioka Y , Mamoto K , Okano T , Kinoshita T , et al. Delayed wound healing and postoperative surgical site infections in patients with rheumatoid arthritis treated with or without biological disease-modifying antirheumatic drugs. Clin Rheumatol. 2016;35(6):1475–81. doi:.https://doi.org/10.1007/s10067-016-3274-1
63 Kadota Y , Nishida K , Hashizume K , Nasu Y , Nakahara R , Kanazawa T , et al. Risk factors for surgical site infection and delayed wound healing after orthopedic surgery in rheumatoid arthritis patients. Mod Rheumatol. 2016;26(1):68–74. doi:.https://doi.org/10.3109/14397595.2015.1073133
64 Filippou G , Tacchini D , Adinolfi A , Bertoldi I , Picerno V , Toscano C , et al. Histology of the synovial membrane of patients affected by osteoarthritis and calcium pyrophosphate dihydrate crystal deposition disease vs. osteoarthritis alone: a pilot study. Scand J Rheumatol. 2016;45(6):538–9. doi:.https://doi.org/10.3109/03009742.2016.1150508
65 Koike R , Takeuchi T , Eguchi K , Miyasaka N ; Japan College of Rheumatology. Update on the Japanese guidelines for the use of infliximab and etanercept in rheumatoid arthritis. Mod Rheumatol. 2007;17(6):451–8. doi:.https://doi.org/10.3109/s10165-007-0626-3
66 Goodman SM , Menon I , Christos PJ , Smethurst R , Bykerk VP . Management of perioperative tumour necrosis factor α inhibitors in rheumatoid arthritis patients undergoing arthroplasty: a systematic review and meta-analysis. Rheumatology (Oxford). 2016;55(3):573–82.
67 Hirao M , Hashimoto J , Tsuboi H , Nampei A , Nakahara H , Yoshio N , et al. Laboratory and febrile features after joint surgery in patients with rheumatoid arthritis treated with tocilizumab. Ann Rheum Dis. 2009;68(5):654–7. doi:.https://doi.org/10.1136/ard.2008.090068
68 Momohara S , Hashimoto J , Tsuboi H , Miyahara H , Nakagawa N , Kaneko A , et al. Analysis of perioperative clinical features and complications after orthopaedic surgery in rheumatoid arthritis patients treated with tocilizumab in a real-world setting: results from the multicentre TOcilizumab in Perioperative Period (TOPP) study. Mod Rheumatol. 2013;23(3):440–9. doi:.https://doi.org/10.3109/s10165-012-0683-0
69 Koike R , Harigai M , Atsumi T , Amano K , Kawai S , Saito K , et al. Japan College of Rheumatology 2009 guidelines for the use of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, in rheumatoid arthritis. Mod Rheumatol. 2009;19(4):351–7. doi:.https://doi.org/10.3109/s10165-009-0197-6
70 Godot S , Gottenberg J-E , Paternotte S , Pane I , Combe B , Sibilia J , et al. Safety of surgery after rituximab therapy in 133 patients with rheumatoid arthritis: data from the autoimmunity and rituximab registry. Arthritis Care Res (Hoboken). 2013;65(11):1874–9. doi:.https://doi.org/10.1002/acr.22056
71 Nishida K , Nasu Y , Hashizume K , Nakahara R , Ozawa M , Harada R , et al. Abatacept management during the perioperative period in patients with rheumatoid arthritis: report on eight orthopaedic procedures. Mod Rheumatol. 2014;24(3):544–5. doi:.https://doi.org/10.3109/14397595.2013.874758
72 Fabiano A , De Simone C , Gisondi P , Piaserico S , Lasagni C , Pellacani G , et al. Management of patients with psoriasis treated with biological drugs needing a surgical treatment. Drug Dev Res. 2014;75(Suppl 1):S24–6. doi:.https://doi.org/10.1002/ddr.21189
73 Smith CH , Anstey AV , Barker JNWN , Burden AD , Chalmers RJG , Chandler DA , et al.; Chair of Guideline Group. British Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009. Br J Dermatol. 2009;161(5):987–1019. doi:.https://doi.org/10.1111/j.1365-2133.2009.09505.x
74 Fautrel B , Constantin A , Morel J , Vittecoq O , Cantagrel A , Combe B , et al.; Rheumatism and Inflammation Club (CRI) and of the French Society for Rheumatology. Recommendations of the French Society for Rheumatology. TNFalpha antagonist therapy in rheumatoid arthritis. Joint Bone Spine. 2006;73(4):433–41. doi:.https://doi.org/10.1016/j.jbspin.2006.04.001
75 Ding T , Ledingham J , Luqmani R , Westlake S , Hyrich K , Lunt M , et al.; Standards, Audit and Guidelines Working Group of BSR Clinical Affairs Committee; BHPR. BSR and BHPR rheumatoid arthritis guidelines on safety of anti-TNF therapies. Rheumatology (Oxford). 2010;49(11):2217–9. doi:.https://doi.org/10.1093/rheumatology/keq249a
76 Gómez Reino J , Loza E , Andreu JL , Balsa A , Batlle E , Cañete JD , et al.; Sociedad Española de Reumatología. [Consensus statement of the Spanish Society of Rheumatology on risk management of biologic therapy in rheumatic patients]. Reumatol Clin. 2011;7(5):284–98. Article in Spanish.
77pharmaSuisse. Articles de la CMPS 2016 [Available from: http://www.pharmasuisse.org/fr/Dienstleistungen/publikationen/Pages/AKA.aspx.
78 Nunes BP , Camargo-Figuera FA , Guttier M , de Oliveira PD , Munhoz TN , Matijasevich A , et al. Multimorbidity in adults from a southern Brazilian city: occurrence and patterns. Int J Public Health. 2016;61(9):1013–20. doi:.https://doi.org/10.1007/s00038-016-0819-7
79 Goodman SM , Springer B , Guyatt G , Abdel MP , Dasa V , George M , et al. 2017 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients With Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty. Arthritis Rheumatol. 2017;69(8):1538–51. doi:.https://doi.org/10.1002/art.40149
80 Makino T , Kaito T , Tsuboi H , Fujiwara H , Yonenobu K . Late-onset deep surgical-site infection after posterior lumbar interbody fusion in a patient treated with tocilizumab; unusual changes in inflammatory markers. Eur Spine J. 2014;23(S2, Suppl 2):296–301. doi:.https://doi.org/10.1007/s00586-014-3317-8
81 Wakabayashi H , Takigawa S , Hasegawa M , Kakimoto T , Yoshida K , Sudo A . Polyarticular late infection of total joint arthroplasties in a patient with rheumatoid arthritis treated with anti-interleukin-6 therapy. Rheumatology (Oxford). 2014;53(6):1150–1. doi:.https://doi.org/10.1093/rheumatology/ket379
82 Hiroshima R , Kawakami K , Iwamoto T , Tokita A , Yano K , Sakuma Y , et al. Analysis of C-reactive protein levels and febrile tendency after joint surgery in rheumatoid arthritis patients treated with a perioperative 4-week interruption of tocilizumab. Mod Rheumatol. 2011;21(1):109–11. doi:.https://doi.org/10.3109/s10165-010-0343-1
83 Campbell L , Chen C , Bhagat SS , Parker RA , Östör AJK . Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology (Oxford). 2011;50(3):552–62. doi:.https://doi.org/10.1093/rheumatology/keq343
84 Au K , Reed G , Curtis JR , Kremer JM , Greenberg JD , Strand V , et al.; CORRONA Investigators. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785–91. doi:.https://doi.org/10.1136/ard.2010.128637
85 Mathews CJ , Weston VC , Jones A , Field M , Coakley G . Bacterial septic arthritis in adults. Lancet. 2010;375(9717):846–55. doi:.https://doi.org/10.1016/S0140-6736(09)61595-6
No financial support and no other potential conflict of interest relevant to this article was reported.