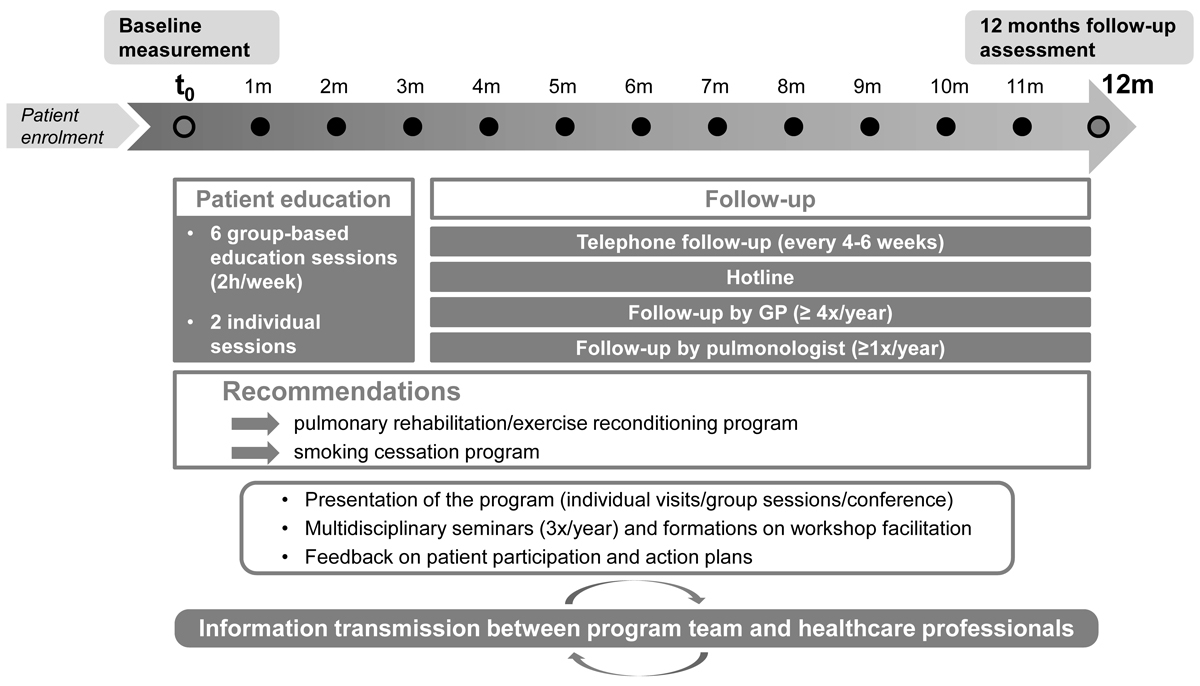
Figure 1 Life cycle of the programme.
DOI: https://doi.org/10.4414/smw.2017.14567
Chronic obstructive pulmonary disease (COPD), which presents as a progressive airflow obstruction associated with systemic comorbidities such as sarcopenia, and ischaemic heart and metabolic diseases, is now the third cause of mortality worldwide [1]. COPD is characterised by acute exacerbations, which are responsible for frequent emergency visits and hospitalisations, as well as deaths. These acute exacerbations also durably reduce quality of life and put a high burden on the healthcare system, not only in terms of health but also in terms of healthcare costs [2].
Current care for COPD is usually based on inhaled pharmacotherapy for symptom relief and prevention of exacerbations, but often neglects the systemic impact of COPD and patients’ perspectives on care. In addition, the quality of COPD care is suboptimal and does not always reflect current guidelines. For example, Swiss and Canadian studies have shown that pulmonary rehabilitation is largely underprescribed and inhaled corticosteroids are prescribed to patients despite disease severity not requiring them [3–5]. A study conducted in the United States reported that COPD patients received only 58% of recommended care [6]. Studies have suggested several explanations, at the healthcare professional and patient levels, for inappropriate care and poor outcomes. At the healthcare professional level, primary care physicians may lack familiarity with clinical evidence, they may lack confidence in diagnosing and staging COPD, and they may face time constraints [7]. At the patient level, patients may lack information and skills to perform self-care, as well as confidence and motivation, especially when it comes to engaging in regular physical activity and perform recommended self-care activities. In addition, depression and anxiety, which are associated with low self-efficacy, have been shown to predict low levels of physical activity in COPD [8].
In this context, integrated care programmes, based on the Chronic Care Model [9, 10], can contribute to overcoming patient and healthcare professional barriers to appropriate care, and, hopefully, to closing the observed quality care gap. Integrated care is a polymorphous concept that was developed within the context of an ageing population and the rise in long-term conditions, and which promotes patient-centeredness and care coordination [11]. Integrated care programmes, which focus on long term behavioural change and self-management support, foster formal contacts between healthcare providers and multidisciplinary care, promote evidence-based care such as early detection and management of acute exacerbations, and encourage physical activity, healthy diet, smoking cessation and adherence to medication. These programmes have been shown to improve health-related quality of life, exercise capacity and hospital admissions in COPD patients [12–14]. In addition, and despite their heterogeneity, integrated care programmes for COPD were found to be cost-effective [15].
Whereas European countries such as the Netherlands, the UK, Germany and Spain started implementing integrated programmes almost two decades ago, developments in Switzerland are more recent and programmes often lack resources for appropriate implementation and evaluation [16]. However, an ongoing study, aimed at identifying and describing integrated care initiatives in Switzerland, identified 162 integrated care initiatives, two of which specifically targeted COPD (Schusselé-Fillietaz, personnal communication, October 2016). Based on the Chronic Care Model and Bourbeau’s “Living Well with COPD” programme [17, 18], two integrated care programmes were implemented in two different Swiss regions, the cantons of Zurich [19] and Valais.
The canton of Valais is a rural and alpine region characterised by a homogeneous but geographically dispersed population, a low population density, and lower density of general practitioners and specialised healthcare providers compared with the rest of Switzerland. Solo practices remain the norm, and medical homes and care networks did not exist at the time of the programme implementation. Implementing a multidisciplinary integrated care programme in such a setting was an innovative challenge. Hence, the aims of the present evaluation were: first, to assess the feasibility and acceptability of the implementation of a pilot COPD evidence-based integrated care programme in an alpine and rural Swiss canton, and second, to explore its effectiveness.
For this process and outcome evaluation, we used a mixed-methods approach. The qualitative part consisted of focus groups of COPD patients and healthcare professionals to assess the feasibility and acceptability of the programme. The quantitative part consisted in the data collection of both clinical and self-reported survey measures to assess the effectiveness of the programme. We conducted pre-post analyses on these latter measures, with a matched control group for three comparisons.
The study protocol was reviewed and approved by the Cantonal Ethics Committee of the Canton of Valais (December 2012; No. CCVEM 046/12).
Healthcare professionals residing in the French-speaking part of the canton of Valais, Switzerland, were recruited through individual visits to their practice or group information sessions, during which information on programme participation, programme implementation, and guidelines for treatment and management of COPD were provided. General practitioners and pulmonologists were in charge of recruiting patients, meeting them regularly and encouraging them to follow the medical recommendations. The pulmonologists were also invited to collaborate in the development of the patient action plan and could intervene during education sessions, along with physiotherapists and pharmacists. Pharmacists were also prompted to distribute flyers at their pharmacies, strengthen the smoking cessation message and offer polypharmacy consultations. Finally, all healthcare professionals were encouraged to take part in the multidisciplinary seminars and training sessions organised within the context of the programme.
Patients recruited by healthcare professionals were contacted by the programme coordinator, who gave them information about the programme, verified eligibility criteria and asked for written consent. Patients with a diagnosis of COPD (GOLD stage I–IV or B-C-D), aged 35 years and over, not institutionalised and residing in the French-speaking part of the canton of Valais in Switzerland were eligible to participate in the pilot programme. Patients were excluded if they had cognitive problems, their level of French was insufficient, or their life expectancy was less than 12 months. Patients in the control group were sampled from the Swiss COPD Cohort [3, 4] and matched with the programme participants on age, gender and COPD Assessment Test (CAT) scores.
The pilot programme, called “Soins intégrés BPCO Valais – Mieux vivre avec une BPCO”, was based on the Chronic Care Model (CCM) and the Canadian programme “Living Well with COPD: A Plan of Action for Life” [18, 20, 21]. Before the implementation of the programme, we conducted focus groups with healthcare professionals to discuss the appropriateness and feasibility of the key components of the programme [9, 10, 22].The programme, as implemented in 2013 and 2014, included the following elements (fig. 1):

Figure 1 Life cycle of the programme.
Qualitative data were collected via focus groups conducted with patients and healthcare professionals during and at the end of the programme, and quantitative data were collected at baseline (during the first visit of the programme) and after 12 months of participation. Baseline data included various self-reported health-related and personal variables and medical data reported by the patient’s physician. At 12 months, self-reported data and data on physical activity were collected during an individual session.
For the control group, we extracted data from the Swiss COPD Cohort database.
Based on the literature [24–27], we decided to measure four process indicators: reach, dosage, fidelity to assess the programme’s feasibility (defined as the extent to which an intervention can be carried out in a particular setting) and acceptability.
In contrast to “efficacy” trials, which are conducted in settings that maximise the management of and the control over the research process, “effectiveness” evaluations aim “to measure the impact of an intervention when it is tested within a population that is representative of the intended target audience.” [28]. An effective programme is thus defined as “a programme [that] does more good than harm when delivered under real-world conditions” [29]. We assessed effectiveness by conducting pre-post analyses (before and after the intervention) on several primary and secondary outcomes, and comparing three of these outcomes with those in a matched control group.
Our two primary outcomes were health-related quality of life and hospital admissions in the past 12 months, two key indicators in the field of integrated care programme generally [30] and COPD management more specifically [13, 14].
Health-related quality of life (HRQoL) was measured using two self-administered questionnaires: (1) the Chronic Respiratory Questionnaire (CRQ), a disease-specific instrument comprising 20 questions on a seven point Likert-type scale, producing four domain scores for dyspnoea, mastery, fatigue and emotion (scores ranged from 0 to 7, a higher score indicating better quality of life) [31, 32]; (2) the 36-Item Short Form Health Survey (SF-36), a generic instrument measuring eight dimensions of health: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, mental health (scores ranged from 0 to 100, with 100 indicating better quality of life) [33–35].
We used two indicators for hospital admissions: the proportion of patients with one or more hospitalisations (all-causes) in the past 12 months and the proportion of patients with one or more hospitalisations for acute exacerbations in the past 12 months.
We also measured the following secondary outcomes:
We also collected sociodemographic data (age, gender, relationship status, living situation, education, employment status, type of residence, insurance status, nationality) and health status data (body mass index, lung function).
The focus groups, conducted with a pre-established interview guide, were recorded and fully transcribed; content analysis [45, 46] was used to extract the arguments associated with each topic under discussion.
Descriptive analyses were carried out first, with data reported as means or percentages for continuous or categorical variables, respectively. We then used bivariate analyses to compare before and after measures of all primary and secondary outcomes; paired t-tests and McNemar tests were considered for continuous and categorical variables, respectively. Only data from patients who participated in the evaluation at 12 months were included in the analyses. In order to compare the results between the patients participating in the programme and the patients from the Swiss COPD Cohort, we first selected a control group (two controls per case), performing a propensity score matching for age, gender and CAT score [47]. We then analysed overtime differences within groups for three commonly available outcome variables: dyspnoea (mMRC score), the proportion of patients reporting ≥1 exacerbations during the last 6/12 months, and smoking status. The confidence intervals (CIs) for mean paired differences were computed with the Student distribution for continuous variables and a method developed by Newcombe [48] for dichotomous variables. We then computed the between group differences of within group differences. For continuous variables, we used a Student confidence interval for the mean difference of two independent samples and for the dichotomous variables we used a method developed by Newcombe [49] to compare differences of paired differences of proportions. Statistical significance was set at p <0.05, and all analyses were performed using Stata 14.
For this pilot phase of the programme, we aimed to include between 30 and 50 patients over 2 years (2013–2014), with a follow-up of 12 months. Between 2013 and 2014, 83 patients were identified during the recruitment process. Of these, 57 patients were eligible and consented to participate in the study; 3 did not meet the inclusion criteria and 23 declined to participate at a first telephone contact or after a first meeting with a programme coordinator (specialised physiotherapist or nurse). The target of 30 to 50 participants for the pilot phase of the programme was therefore achieved. The average participation rate in the education sessions was 83.6%; patients participated in five sessions out of six on average. Three participants were not present at the individual session that followed the six group sessions, and 46 out of 57 agreed to be assessed at the 12-month follow-up. The 11 non-adherent patients at the 12-month evaluation did not differ significantly from the participating patients on key demographics, but were less likely to live alone and presented lower forced expiratory volume in 1 second (FEV1) and Tiffeneau index.
Baseline characteristics of the participants are presented in table 1. On average, patients were 66 years old and 56% were male. Most patients lived with a partner and half were retired. Two thirds of patients had at least one exacerbation in the previous year and most had moderate COPD. Demographics of the participants were similar to the population of Swiss patients living with COPD and were typical of the population targeted by comparable interventions [50].
Table 1 Baseline characteristics of participants (n = 57 patients with COPD).
| Sociodemographic and general health characteristics | ||
| Age | (n = 57) | 66.0 ± 8.3 |
| Female | (n = 57) | (43.9) |
| Marital status | (n = 57) | |
| Single | 1(1.8) | |
| Married or living with partner | 37 (64.9) | |
| Separated, divorced, widowed | 19 (33.3) | |
| Education | (n = 56) | |
| Primary | 18 (32.1) | |
| Secondary | 25 (44.7) | |
| Tertiary | 13 (23.2) | |
| Employment status | (n = 56) | |
| Employed | 8 (14.2) | |
| Reduced working time because of health problem | 14 (25.0) | |
| Unemployed, house wife | 6 (10.8) | |
| Retired | 28 (50.0) | |
| Living alone | (n = 57) | 15 (26.3) |
| Habitation | (n = 56) | |
| Rural | 37 (66.1) | |
| Urban | 19 (33.9) | |
| Current smoker | (n = 51) | 22 (43.1) |
| Body mass index | (n = 57) | |
| Overweight (25–29.9 kg/m2) | 17 (29.8) | |
| Obese (≥30 kg/m2) | 14 (24.6) | |
| COPD | ||
| Lung function | (n = 45) | |
| FEV1, % predicted | 57.5 ± 18.9 | |
| FEV1/FVC | 49.9 ± 13.4 | |
| GOLD stage* | (n = 54) | |
| 1: mild (FEV1 ≥ 80% predicted) | 5 (9.3) | |
| 2: moderate (50% ≤ FEV1 < 80% predicted) | 30 (55.5) | |
| 3: severe (30% ≤ FEV1 < 50% predicted) | 13 (24.1) | |
| 4: very severe (FEV1 < 30% predicted) | 6 (11.1) | |
| GOLD stage, ABCD classification† | (n = 54) | |
| A: low risk, less symptoms | 2 (3.7) | |
| B: low risk, more symptoms | 21 (38.9) | |
| C: high risk, less symptoms | 3 (5.6) | |
| D: high risk, more symptoms | 28 (51.9) | |
| mMRC score ‡ | (n = 57) | |
| 0 | 3 (5.3) | |
| 1 | 13 (22.8) | |
| 2 | 23 (40.3) | |
| 3 | 14 (24.6) | |
| 4 | 4 (7.0) | |
| CAT score | (n = 57) | 17.3 ± 7.5 |
| ≥1 exacerbations in previous year | (n = 46) | 31 (67.4) |
| ≥1 hospitalizations in previous year | (n = 56) | 24 (42.9) |
| Respiratory treatments | (n = 50) | |
| Short-acting β2 agonist | 22(44) | |
| Short-acting anticholinergic | 4(8) | |
| Long-acting β2 agonist | 15(30) | |
| Long-acting anticholinergic | 38(76) | |
| Combined long-acting β2 agonist and inhaled corticosteroids | 26(52) | |
| Inhaled corticosteroids | 3(6) | |
CAT = COPD Assessment Test; FEV = forced expiratory volume; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; mMRC=modified Medical Research Council. Data are presented as mean ± SD or n (%). * From lung function tests. † From lung function tests, number of exacerbations (past 12 months), CAT score and mMRC score. ‡ mMRC scale is divided into five categories: 0 = breathless with strenuous exercise, 1 = short of breath when hurrying on level ground or walking up a slight hill, 2 = walks slower than people of the same age because of breathlessness, or has to stop for breath when walking at own pace, 3 = has to stop for breath after walking about 100 yards or after a few minutes, 4 = too breathless to leave the house or breathless while dressing.
Healthcare professionals participated in patient enrolment to various degrees: pulmonologists, general practitioners (GPs) and, pharmacists enrolled 34, 14, 1 patients, respectively. The GP participation rate was weaker than expected: during the first year of the programme, of the 11 general practitioners who agreed to meet the programme coordinator, only three finally recruited patients. Furthermore, the response rate to the GP questionnaire was so low that we stopped sending it to GPs in 2014. On the other hand, the six half-day seminars on COPD management were successful, with a mean of 60 participants each time coming from a wide range of professional backgrounds.
During the first 2 years of the programme, eight series of group-based education sessions took place in three different locations in the canton of Valais (Sion, Martigny, Monthey). During each series, all six group-based education sessions were delivered. The teaching material was printed and handed out to all participants prior to education sessions (469 workbooks and action plans in total for 57 patients and 10 healthcare professionals). Two thirds of patients received an action plan for acute COPD exacerbations, whereas all patients were offered an individual session at home or at the hospital before and after the group sessions. Patients received telephone calls from the physiotherapist / specialised nurse every 4 to 6 weeks according to their needs, and the hotline was active five days a week and was used 42 times by the patients during the 2 years of the pilot phase. Furthermore, an information campaign was implemented in order to communicate on the programme and inform on the COPD management guidelines. Flyers were distributed in pharmacies and medical offices, articles were published in the local newspaper, and several conferences and workshops were held.
Intervention reports and monitoring data showed good adherence to the programme protocol. However, the programme required several adaptations in order to respond to local constraints. First, we revised the recruiting method to be more efficient, by extending the range of healthcare professionals authorised to recruit patients and replacing individual visits to GPs and pulmonologists by group information sessions. Second, the multidisciplinarity component was underdeveloped in the first 2 years of the pilot phase of the programme, mainly because considerable effort had been dedicated to the preparation of the material and the group-based education sessions instead. Although the protocol aimed to foster collaboration between the different healthcare professionals involved in the management of patients with COPD, in practice, information transfer was not optimal and GPs’ involvement not sufficient. Third, we had to stop conducting education sessions in one of the regions because it was difficult to access and it registered low attendance rates in the first year of the programme. The group sessions took place in three different towns in Valais, instead of four as originally planned.
Patient and healthcare professionals perceptions collected via focus groups at the end of the intervention are summarised in table 2. Overall, patients and professionals were satisfied with the programme. Both mentioned better knowledge and know-how about COPD, and peer contact was unanimously regarded as helpful and a potential source of motivation for patients. Some areas required improvement nevertheless. The major criticism made by patients and healthcare professionals related to communication. The information provided to stakeholders and the community was deemed insufficient. Patients were faced with practitioners outside the programme who were not aware of it or were critical of it. They were also confused about the evaluation and monitoring aspect intrinsically linked to the pilot programme. Healthcare professionals said they wanted more information about their patients’ follow-up. A general lack of coordination between healthcare professionals was also mentioned. Finally, GPs expressed difficulties in motivating patients to follow medical recommendations and change life habits. Despite these criticisms, qualitative data revealed that the intervention was well received by patients and healthcare professionals, confirming the acceptability of the programme. The results from the patient satisfaction questionnaire at the end of the programme were consistent with this conclusion, with 96% of patients giving a positive evaluation to the programme, and 85% stating they would recommend the programme to another person living with COPD (n = 46).
Table 2 Patients’ and healthcare professionals’ feedback on the programme.
| Positive feedback | Negative feedback |
|---|---|
| Patients | |
| Improved knowledge on COPD mechanism and medications | Objectives of the programme unclear / research side of the project confusing |
| Raised awareness of the presence of COPD (if denial) and the need to change lifestyles | External healthcare professionals poorly informed about the programme |
| Acquired breathing techniques and stress management skills | Lack of communication and coordination between healthcare professionals |
| Peer support | Lack of impact on motivation to change lifestyles |
| Clarity of the education material | Organisational problems (during the first year) |
| Healthcare professionals | |
| Focus on healthy lifestyles (physical activity and smoking cessation) | Lack of information about the programme |
| Peer support | Difficulty in recruiting patients |
| Improved self-efficacy | Poor transmission of information between healthcare professionals |
| Duration of the programme | Absence of feedback on patients monitoring |
| Action plan for acute COPD exacerbations | Measures to help patients with tobacco cessation could be improved |
Primary and secondary outcomes before (baseline) and after (12 months) the intervention are presented in detail in table 3. Only patients with complete data were included in these analyses (n = 46).
Table 3 Primary and secondary outcomes.
| Primary outcomes | Baseline | 12 months | p-value | |
|---|---|---|---|---|
| Disease specific quality of life (CRQ) | (n = 46) | |||
| Dyspnoea | 4.8 ± 1.5 | 5.1 ± 1.5 | 0.12 | |
| Fatigue | 4.0 ± 1.5 | 3.9 ± 1.4 | 0.70 | |
| Emotion | 4.3 ± 1.5 | 4.4 ± 1.5 | 0.39 | |
| Mastery | 4.7 ± 1.6 | 5.2 ± 1.4 | 0.01 | |
| Generic health-related quality of life (SF-36) | ||||
| Physical functioning | (n = 44) | 55.5 ± 26.1 | 54.7 ± 25.5 | 0.76 |
| Role physical | (n = 44) | 50.0 ± 39.3 | 50.6 ± 40.5 | 0.93 |
| Bodily pain | (n = 46) | 54.8 ± 26.5 | 57.1 ± 24.8 | 0.56 |
| General health | (n = 44) | 42.9 ± 21.9 | 45.5 ± 19.0 | 0.23 |
| Vitality | (n = 46) | 47.4 ± 21.9 | 45.4 ± 20.6 | 0.47 |
| Social functioning | (n = 45) | 59.7 ± 30.3 | 70.0 ± 26.4 | <0.01 |
| Role emotional | (n = 45) | 48.0 ± 44.1 | 62.8 ± 41.6 | 0.02 |
| Mental health | (n = 46) | 56.3 ± 23.8 | 60.5 ± 22.8 | 0.09 |
| Hospitalisations | ||||
| ≥1 hospitalisations (all-causes) in previous year | (n = 45) | 18 (40.0) | 12 (26.7) | 0.21 |
| ≥1 hospitalisations for acute exacerbations in previous year | (n = 38) | 8 (21.1) | 7 (18.4) | 1.00 |
| Secondary outcomes | ||||
| Self-efficacy | (n = 45) | 7.9 ± 1.4 | 8.5 ± 1.2 | <0.01 |
| Symptoms and acute exacerbations | ||||
| CAT score | (n = 46) | 17.3 ± 7.4 | 17.6 ± 8.3 | 0.73 |
| mMRC score | (n = 46) | 2.0 ± 1.1 | 1.8 ± 1.1 | 0.20 |
| Number of exacerbations in previous year | (n = 38) | 1.53 ± 1.6 | 1.58 ± 1.7 | 0.87 |
| ≥1 exacerbations in previous year | (n = 38) | 24 (63.2) | 25 (65.8) | 1.00 |
| Exercise capacity | ||||
| 6 min walking test, m | (n = 31) | 429.6 ± 115.1 | 449.6 ± 102.2 | 0.15 |
| Sit-to-stand test | (n = 37) | 20.6 ± 6.4 | 23.1 ± 6.7 | <0.01 |
| Preventative measures | ||||
| Current smoker* | (n = 42) | 18 (42.9) | 16 (38.1) | 0.69 |
| Influenza vaccination in previous year | (n = 46) | 32 (69.6) | 40 (87.0) | <0.01 |
| Pneumococcal vaccination in the last 5 years* | (n = 37) | 22 (59.5) | 26 (70.3) | 0.22 |
| Healthcare utilisation | ||||
| ≥1 ER visits / unscheduled physician visits in previous year | (n = 45) | 19 (42.2) | 17 (37.8) | 0.82 |
| Family physician visits in the previous year | (n = 45) | 6.5 ± 4.9 | 6.5 ± 4.7 | 1.00 |
| ≥1 pulmonologist visits in the previous year | (n = 46) | 34 (73.9) | 38 (82.6) | 0.34 |
| Congruence with CCM (PACIC score) | (n = 46) | 2.2 ± 0.9 | 3.4 ± 1.0 | <0.01 |
CAT = COPD Assessment Test; CCM = Chronic Care Model; CRQ = Chronic Respiratory Questionnaire; ER = emergency room; mMRC = modified Medical Research Council; PACIC = Patient Assessment of Chronic Illness Care; SF-36 = Short Form 36. Data are presented as mean ± SD or n (%). Bold values are marking statistically significant difference (p <0.05). * Data reported by GPs at baseline and by patients at 12 months follow-up.
The mastery domain of the disease-specific quality-of-life score (CRQ) increased significantly at 12 months (+0.5; p = 0.01). A significant improvement on two generic HRQoL dimensions was also observed: social functioning (+10.3; p <0.01) and role limitations due to emotional problems (+14.8; p = 0.02). Whereas these three dimensions presented changes that can be considered clinically significant, the other dimensions or domains of (health-related) quality of life remained stable.
The proportion of patients with one or more hospitalisations (all causes) and the proportion of patients with one or more hospitalisations for acute exacerbations were not statistically different between baseline and follow-up, even though the proportion of patients with one or more hospitalisations (all-causes) decreased from 40% to 27%.
Both the mean self-efficacy score – corresponding to the patients’ beliefs in their capabilities to manage COPD – and the results of the sit-to-stand test increased significantly between baseline and 12 months (+0.6, p<0.01 and +2.5, p<0.01, respectively). Severity of symptoms, measured as the CAT and mMRC scores, as well as the frequency of exacerbations, remained stable at 12 months. No change was observed in the number of emergency department visits / unscheduled physician visits. Regarding risk factors, results showed that influenza vaccination coverage improved significantly at 12 months (70% at baseline vs 87% at 12 months), whereas there was no significant change in the proportion of current smokers between baseline and 12-month follow-up (43 vs 38%).
The demographic and medical characteristics of both groups were similar except for lung function and number of exacerbations, which were lower in the intervention group, and for inhaled corticosteroid use which was much higher in the intervention group. Evolution differences of dyspnoea measures (mMRC), number of exacerbations and smoking status are shown in table 4. These results suggest that (1) the proportion of patients reporting at least one exacerbation in the previous year or in the previous six months increased less strongly in the intervention group (not statistically significant); (2) the proportion of current smokers decreased more sharply in the intervention group (not statistically significant).
Table 4 mMRC score, number of exacerbations and proportion of current smoker: within and between group differences at 12 months.
| Intervention group | Control group | Difference in differences | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | Difference | Baseline | 12 months | Difference | |||
| mMRC score | n = 46 | 2.0 ± 1.1 | 1.8 ± 1.1 | −0.2 (−0.5–0.1) | 1.8 ± 0.9 | 1.9 ± 0.9 | 0.1 (0.0–0.3) | −0.3 (−0.7–0.0) |
| ≥1 exacerbations in last 6/12 months | n = 38 | 63.2 ± 7.8% | 65.8 ± 7.7% | 2.6% (−19.1–24.0%) | 21.1 ± 4.7% | 28.9 ± 5.2% | 7.9% (−4.9–20.4%) | −5.3% (−30.4–19.7%) |
| Current smoker | n = 42 | 42.9 ± 7.6% | 38.1 ± 7.5% | −4.8% (−16.5–7.2%) | 38.1 ± 5.3% | 35.7 ± 5.2% | −2.4% (−7.6–2.8%) | −2.4% (−15.2–10.7%) |
The results of this mixed methods evaluation showed that the implementation of a pilot COPD integrated care programme emphasising self-management was feasible in an alpine canton of Switzerland. The investigation of the implementation process showed a suitable reach, considering that the programme was in its pilot phase, and a good dosage with regard to the self-management sessions, a core component of the programme; multidisciplinarity was in contrast less successful. In addition, whereas the evaluation of fidelity showed that the intervention was delivered according to the protocol, notwithstanding a few adjustments linked to the results of the focus groups and field constraints; acceptability among stakeholders was moderately high whereas participants were highly satisfied with the programme. Finally, exploration of the effectiveness of this evidence-based pilot programme showed that health-related quality of life, self-efficacy, exercise capacity, immunisation coverage and PACIC score improved after 12 months of participation in the programme, and comparisons with a matched control group suggested trends towards improvements despite the absence of statistically significant differences.
The qualitative part of the evaluation truly added value to the quantitative evaluation. Feedback from participating patients and healthcare professionals helped improve and adapt the programme to the needs of those concerned. For example, once we realised that healthcare professionals outside the programme were poorly informed about it and sometimes even reluctant to show interest and accompany their participating patients, and that healthcare professionals involved in the programme reported insufficient feedback from the programme coordinator regarding their own patients, we decided to send formal letters containing patient data to patients, pharmacists, referring GPs and pulmonologists. Sent at entry into the programme, after the self-management education sessions and at 12 months, these letters included a summary table containing results of pulmonary function tests, quality of life, physical activity level, exacerbation rate, CAT score, smoking status and quality of inhaled medication techniques. Healthcare professionals seemed to appreciate this easy-to-read and valuable information.
Another example of programme improvement following the process evaluation relates to the use of action plans in cases of COPD acute exacerbations. Upon finding that only two thirds of patients had received an individualised action plan during the pilot phase, which is a key element for reducing healthcare utilisation [18, 51], we decided to change the procedure. At first, action plan drafting and distribution were left to the referring practitioner. Now, they are prepared by the programme’s team involved in self-management education sessions, who have a good understanding of patients’ self-efficacy. This approach, similar to that of Benzo et al. in other settings [52], reduced practitioners’ workload and increased the number of action plans delivered to patients.
Negative feedback allowed us to improve the intervention, but positive aspects highlighted during the evaluation are promising. Patients’ reports of improved COPD knowledge, improved breathing techniques skills to manage dyspnoea, usefulness of peer support and recognition of the clarity of the “Living Well with COPD” course material, confirmed the appropriateness and utility of the self-management education group sessions. Our pilot programme also appears effective in term of quality of life and exercise capacity, which is consistent with results of recent meta-analyses on self-management COPD programmes [13, 14]. Furthermore, participants increased their self-efficacy score, showing better confidence in their capabilities to manage their disease, thus confirming the effectiveness of self-management education as reported by patients and demonstrated before by Stellefson et al. [53]. Our pilot study did not allow us to confirm a reduction of hospitalisations, unlike other studies on implementation of the “Living Well with COPD” programme [18, 20]. This might be due to the low prevalence of severe exacerbations before entry into the programme. For safety reasons, we choose in the first year of our programme to include only patients in a stable condition, excluding those recently admitted for COPD exacerbations.
Effectiveness of integrated care programmes might be improved by good training of the programme staff and fidelity to the intervention. Regular evaluations, involvement of the programme leaders, formal feedbacks about strengths and weaknesses, individualised multidisciplinary assessment of patients and a centralised database with patients’ electronic reports to insure easy quality control represent some necessary elements. Future developments of the programme will target multidisciplinarity, a component that did not receive enough attention in the pilot phase; this is particularly important since the development of mutual trust and clarity around each actor’s role is a complex process that requires time. Up to now, the promotion of the programme during multidisciplinary seminars, physician-specific meetings and via traditional media and regional newspapers led to its better acceptability, and also improved interprofessional collaboration between healthcare professionals involved in COPD care; in addition, it increased the coverage of the programme.
The main strengths of this project were that it was conducted under real-world conditions, that it combined both a process (feasibility and acceptability) and an outcome (effectiveness) evaluation, and that it took advantage of both qualitative and quantitative methods. This choice enabled the continual adaptation and improvement of the programme, and confirmed that COPD patients participating in the programme benefited from it. Our results need nevertheless to be interpreted with caution on account of the following main limitations. First, the control group was not randomly selected. However, we used propensity score matching to compare the intervention group to the control group (the Swiss COPD Cohort), which allowed us to adjust for patient age, gender and CAT score, but not for other variables that could be associated with outcomes. Therefore, our results related to the effectiveness of the programme need to be interpreted with caution. Second, the sample size for the effectiveness analyses (46 observations) was small, conferring low power to detect differences and make robust conclusions about effectiveness. However, this is not problematic, since the primary target of the evaluation was not to assess its effectiveness, because the programme was in its pilot phase and only included a small number of patients. In addition, the effectiveness of integrated care programmes for COPD patients has been reported in other studies [13, 14]. Third, clinical measures were collected as part of routine clinical assessment of COPD patients to monitor their evolution; they were not collected to prove the effectiveness of COPD integrated care programmes per se. In that context, the positive trends towards effectiveness, which are in line with previous results, are encouraging, even if they need to be interpreted carefully. Fourth, besides limitations associated with pre-post study design, selection bias cannot be excluded, since patients participating in this programme were volunteers and patients lost to follow-up were on average less symptomatic. Finally, the implementation of complex interventions makes the identification of causal effects very difficult, as we cannot associate an outcome to a specific component. However, systematic reviews have shown that the implementation of one single or two components of the Chronic Care Model are associated with significantly improved clinical and process outcomes [54, 55].
This evaluation demonstrated that a community-based COPD integrated care programme emphasising self-management education is both feasible and acceptable in an alpine and rural canton of Switzerland. Improvement in social and emotional dimensions of health-related quality of life, self-efficacy, exercise capacity, COPD knowledge and breathing technique skills tend to confirm the known benefits of integrated care with self-management education for COPD patients. Considering the specificities of the canton of Valais in term of geographical spread, fragmentation of care and lack of familiarity of primary care physicians with similar programmes for other chronic diseases, the implementation of the programme can be considered as successful overall even if some components, such as multidisciplinarity and information transfer, need to be further developed. An extension of the programme to the German-speaking part of the canton is planned, and a nation-wide programme, led by the Swiss Lung League and involving a variety of healthcare stakeholders, is currently being developed. To achieve successful implementation in other settings, rigorous organisation is required, and fidelity to the designed intervention should be secured and regularly verified, while leaving some leeway to adapt if necessary. It is also important to allow integrated care programmes to run their course, since the lack of familiarity with nonpharmacological interventions among healthcare professionals means that changes in care take time.
We would like to thank all patients with COPD and healthcare providers who participated in the programme. We also thank all those who have helped in the development, implementation and evaluation of the programme, either directly or indirectly.
This project was funded by the Swiss Academy of Medical Sciences (SAMS), the Gottfried und Julia Bangerter-Rhyner Foundation, and the Valais Pulmonary League. The Swiss COPD Cohort, from which comparison data was drawn, is funded by Boehringer Ingelheim GmbH Switzerland and Novartis AG Switzerland. I.P.-B. was supported by a grant from the Swiss National Science Foundation (PROSPER Grant 32333B-123817 and Grant 32333B-139789) and, since August 2013, by the Swiss School of Public Health (SSPH+ Assistant Professorship grant). The funders had no role in study design, data collection, analysis and interpretation, decision to publish results, and/or preparation of the manuscript.
MK has received consultancy fees from Roche, Novartis, Astra-Zeneca, Boehringer Ingelheim and CSL Boheim in the previous 24 months.
1Institute for Health Metrics and Evaluation (IHME). GBD Compare Data Visualization. Seattle WI, University of Washington, 2016. Available from http://vizhub.healthdata.org/gbd-compare. (Accessed 17 Oct.16).
2 Samyshkin Y , Schlunegger M , Haefliger S , Ledderhose S , Radford M . Cost-effectiveness of roflumilast in combination with bronchodilator therapies in patients with severe and very severe COPD in Switzerland. Int J Chron Obstruct Pulmon Dis. 2013;8:79–87. doi:.https://doi.org/10.2147/COPD.S37486
3 Jochmann A , Scherr A , Jochmann DC , Miedinger D , Török SS , Chhajed PN , et al. Impact of adherence to the GOLD guidelines on symptom prevalence, lung function decline and exacerbation rate in the Swiss COPD cohort. Swiss Med Wkly. 2012;142:w13567. doi:.https://doi.org/10.4414/smw.2012.13567
4 Jochmann A , Neubauer F , Miedinger D , Schafroth S , Tamm M , Leuppi JD . General practitioner’s adherence to the COPD GOLD guidelines: baseline data of the Swiss COPD Cohort Study. Swiss Med Wkly. 2010;140:w12988.
5 Bourbeau J , Sebaldt RJ , Day A , Bouchard J , Kaplan A , Hernandez P , et al. Practice patterns in the management of chronic obstructive pulmonary disease in primary practice: the CAGE study. Can Respir J. 2008;15(1):13–9. doi:.https://doi.org/10.1155/2008/173904
6 McGlynn EA , Asch SM , Adams J , Keesey J , Hicks J , DeCristofaro A , et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–45. doi:.https://doi.org/10.1056/NEJMsa022615
7 Perez X , Wisnivesky JP , Lurslurchachai L , Kleinman LC , Kronish IM . Barriers to adherence to COPD guidelines among primary care providers. Respir Med. 2012;106(3):374–81. doi:.https://doi.org/10.1016/j.rmed.2011.09.010
8 Miravitlles M , Cantoni J , Naberan K . Factors associated with a low level of physical activity in patients with chronic obstructive pulmonary disease. Lung. 2014;192(2):259–65. doi:.https://doi.org/10.1007/s00408-014-9557-x
9 Epping-Jordan JE , Pruitt SD , Bengoa R , Wagner EH . Improving the quality of health care for chronic conditions. Qual Saf Health Care. 2004;13(4):299–305. doi:.https://doi.org/10.1136/qshc.2004.010744
10 Wagner EH , Austin BT , Davis C , Hindmarsh M , Schaefer J , Bonomi A . Improving chronic illness care: translating evidence into action. Health Aff (Millwood). 2001;20(6):64–78. doi:.https://doi.org/10.1377/hlthaff.20.6.64
11Goodwin N. What Is Integrated Care? In: Amelung V, Stein, V., Goodwin, N., Balicer, R., Nolte, E., Suter, E., editor. Handbook Integrated Care. Springer; 2017.
12 Mantoani LC , Rubio N , McKinstry B , MacNee W , Rabinovich RA . Interventions to modify physical activity in patients with COPD: a systematic review. Eur Respir J. 2016;48(1):69–81. doi:.https://doi.org/10.1183/13993003.01744-2015
13 Kruis AL , Smidt N , Assendelft WJ , Gussekloo J , Boland MR , Rutten-van Mölken M , et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;10(10):CD009437. doi:.https://doi.org/10.1002/14651858.CD009437.pub2
14 Peytremann-Bridevaux I , Staeger P , Bridevaux PO , Ghali WA , Burnand B . Effectiveness of chronic obstructive pulmonary disease-management programmes: systematic review and meta-analysis. Am J Med. 2008;121(5):433–443.e4. doi:.https://doi.org/10.1016/j.amjmed.2008.02.009
15 Boland MR , Tsiachristas A , Kruis AL , Chavannes NH , Rutten-van Mölken MP . The health economic impact of disease management programmes for COPD: a systematic literature review and meta-analysis. BMC Pulm Med. 2013;13(1):40. doi:.https://doi.org/10.1186/1471-2466-13-40
16Ebert S, Peytremann-Bridevaux I, Senn N. Les programmemes de prise en charge des maladies chroniques et de la multimorbidité en Suisse (Obsan Dossier 44). Neuchâtel: Observatoire suisse de la santé; 2015. Available from: http://www.obsan.admin.ch/fr/publications/les-programmemes-de-prise-en-charge-des-maladies-chroniques-et-de-la-multimorbidite-en
17Living Well with COPD. http://www.livingwellwithcopd.com/. Accessed 19 Jan.17.
18 Bourbeau J , Julien M , Maltais F , Rouleau M , Beaupré A , Bégin R , et al., Chronic Obstructive Pulmonary Disease axis of the Respiratory Network Fonds de la Recherche en Santé du Québec. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163(5):585–91. doi:.https://doi.org/10.1001/archinte.163.5.585
19 Steurer-Stey C , Markun S , Lana KD , Frei A , Held U , Wensing M , et al. The improving care in chronic obstructive lung disease study: CAROL improving processes of care and quality of life of COPD patients in primary care: study protocol for a randomized controlled trial. Trials. 2014;15(1):96. doi:.https://doi.org/10.1186/1745-6215-15-96
20 Gadoury MA , Schwartzman K , Rouleau M , Maltais F , Julien M , Beaupré A , et al.; Chronic Obstructive Pulmonary Disease axis of the Respiratory Health Network, Fonds de la recherche en santé du Québec (FRSQ). Self-management reduces both short- and long-term hospitalisation in COPD. Eur Respir J. 2005;26(5):853–7. doi:.https://doi.org/10.1183/09031936.05.00093204
21 Bourbeau J , Collet JP , Schwartzman K , Ducruet T , Nault D , Bradley C . Economic benefits of self-management education in COPD. Chest. 2006;130(6):1704–11. doi:.https://doi.org/10.1378/chest.130.6.1704
22 Wagner EH . Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1(1):2–4.
23Global Initiative for Chronic Obstructive Lung Disease. http://www.goldcopd.org/. Accessed 16 Mar. 2016.
24 Proctor E , Silmere H , Raghavan R , Hovmand P , Aarons G , Bunger A , et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65–76. doi:.https://doi.org/10.1007/s10488-010-0319-7
25 Peters DH , Adam T , Alonge O , Agyepong IA , Tran N . Implementation research: what it is and how to do it. BMJ. 2013;347:f6753. doi:.https://doi.org/10.1136/bmj.f6753
26 Saunders RP , Evans MH , Joshi P . Developing a process-evaluation plan for assessing health promotion programme implementation: a how-to guide. Health Promot Pract. 2005;6(2):134–47. doi:.https://doi.org/10.1177/1524839904273387
27Steckler L, Linnan L. Process evaluation for public health interventions and research - an overview. Process Evaluation for Public Health Interventions and Research. San Fransisco: Jossey-Bass; 2002.
28 Glasgow RE , Lichtenstein E , Marcus AC . Why don’t we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition. Am J Public Health. 2003;93(8):1261–7. doi:.https://doi.org/10.2105/AJPH.93.8.1261
29 Flay BR . Efficacy and effectiveness trials (and other phases of research) in the development of health promotion programmes. Prev Med. 1986;15(5):451–74. doi:.https://doi.org/10.1016/0091-7435(86)90024-1
30 Ouwens M , Wollersheim H , Hermens R , Hulscher M , Grol R . Integrated care programmemes for chronically ill patients: a review of systematic reviews. Int J Qual Health Care. 2005;17(2):141–6. doi:.https://doi.org/10.1093/intqhc/mzi016
31 Wijkstra PJ , TenVergert EM , Van Altena R , Otten V , Postma DS , Kraan J , et al. Reliability and validity of the chronic respiratory questionnaire (CRQ). Thorax. 1994;49(5):465–7. doi:.https://doi.org/10.1136/thx.49.5.465
32 Williams JE , Singh SJ , Sewell L , Guyatt GH , Morgan MD . Development of a self-reported Chronic Respiratory Questionnaire (CRQ-SR). Thorax. 2001;56(12):954–9. doi:.https://doi.org/10.1136/thorax.56.12.954
33 Ware JE, Jr , Sherbourne CD . The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. doi:.https://doi.org/10.1097/00005650-199206000-00002
34 Perneger TV , Leplège A , Etter JF , Rougemont A . Validation of a French-language version of the MOS 36-Item Short Form Health Survey (SF-36) in young healthy adults. J Clin Epidemiol. 1995;48(8):1051–60. doi:.https://doi.org/10.1016/0895-4356(94)00227-H
35 Leplège A , Ecosse E , Verdier A , Perneger TV . The French SF-36 Health Survey: translation, cultural adaptation and preliminary psychometric evaluation. J Clin Epidemiol. 1998;51(11):1013–23. doi:.https://doi.org/10.1016/S0895-4356(98)00093-6
36 Lorig KR , Sobel DS , Ritter PL , Laurent D , Hobbs M . Effect of a self-management programme on patients with chronic disease. Eff Clin Pract. 2001;4(6):256–62.
37 Jones PW , Harding G , Berry P , Wiklund I , Chen WH , Kline Leidy N . Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–54. doi:.https://doi.org/10.1183/09031936.00102509
38 Mahler DA , Wells CK . Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–6. doi:.https://doi.org/10.1378/chest.93.3.580
39 Brooks SMc. Task group on surveillance for respiratory hazards in the occupational setting. ATS News. 1982;8:12–6.
40 ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. doi:.https://doi.org/10.1164/ajrccm.166.1.at1102
41 Ozalevli S , Ozden A , Itil O , Akkoclu A . Comparison of the Sit-to-Stand Test with 6 min walk test in patients with chronic obstructive pulmonary disease. Respir Med. 2007;101(2):286–93. doi:.https://doi.org/10.1016/j.rmed.2006.05.007
42 Puhan MA , Siebeling L , Zoller M , Muggensturm P , ter Riet G . Simple functional performance tests and mortality in COPD. Eur Respir J. 2013;42(4):956–63. doi:.https://doi.org/10.1183/09031936.00131612
43 Glasgow RE , Wagner EH , Schaefer J , Mahoney LD , Reid RJ , Greene SM . Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Med Care. 2005;43(5):436–44. doi:.https://doi.org/10.1097/01.mlr.0000160375.47920.8c
44 Iglesias K , Burnand B , Peytremann-Bridevaux I . PACIC Instrument: disentangling dimensions using published validation models. Int J Qual Health Care. 2014;26(3):250–60. doi:.https://doi.org/10.1093/intqhc/mzu042
45 L’Ecuyer R . L’analyse développementale du contenu. Revue de l’Association pour la Recherche Qualitative. 1989;1:51–80.
46Berelson B. Content analysis in communication research. Glencoe, Ill: Free Press; 1952.
47 Rosenbaum PR , Rubin DB . The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi:.https://doi.org/10.1093/biomet/70.1.41
48 Newcombe RG . Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med. 1998;17(22):2635–50. doi:.https://doi.org/10.1002/(SICI)1097-0258(19981130)17:22<2635::AID-SIM954>3.0.CO;2-C
49 Newcombe RG . Estimating the difference between differences: measurement of additive scale interaction for proportions. Stat Med. 2001;20(19):2885–93. doi:.https://doi.org/10.1002/sim.925
50 Bridevaux PO , Probst-Hensch NM , Schindler C , Curjuric I , Felber Dietrich D , Braendli O , et al. Prevalence of airflow obstruction in smokers and never-smokers in Switzerland. Eur Respir J. 2010;36(6):1259–69. doi:.https://doi.org/10.1183/09031936.00004110
51 Rea H , McAuley S , Stewart A , Lamont C , Roseman P , Didsbury P . A chronic disease management programmeme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern Med J. 2004;34(11):608–14. doi:.https://doi.org/10.1111/j.1445-5994.2004.00672.x
52 Benzo R , Vickers K , Novotny PJ , Tucker S , Hoult J , Neuenfeldt P , et al. Health Coaching and Chronic Obstructive Pulmonary Disease Rehospitalization. A Randomized Study. Am J Respir Crit Care Med. 2016;194(6):672–80. doi:.https://doi.org/10.1164/rccm.201512-2503OC
53 Stellefson M , Tennant B , Chaney JD . A Critical Review of Effects of COPD Self-Management Education on Self-Efficacy. ISRN Public Health. 2012;2012:10.
54 Tsai AC , Morton SC , Mangione CM , Keeler EB . A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11(8):478–88.
55 Adams SG , Smith PK , Allan PF , Anzueto A , Pugh JA , Cornell JE . Systematic review of the chronic care model in chronic obstructive pulmonary disease prevention and management. Arch Intern Med. 2007;167(6):551–61. doi:.https://doi.org/10.1001/archinte.167.6.551
This project was funded by the Swiss Academy of Medical Sciences (SAMS), the Gottfried und Julia Bangerter-Rhyner Foundation, and the Valais Pulmonary League. The Swiss COPD Cohort, from which comparison data was drawn, is funded by Boehringer Ingelheim GmbH Switzerland and Novartis AG Switzerland. I.P.-B. was supported by a grant from the Swiss National Science Foundation (PROSPER Grant 32333B-123817 and Grant 32333B-139789) and, since August 2013, by the Swiss School of Public Health (SSPH+ Assistant Professorship grant). The funders had no role in study design, data collection, analysis and interpretation, decision to publish results, and/or preparation of the manuscript.
MK has received consultancy fees from Roche, Novartis, Astra-Zeneca, Boehringer Ingelheim and CSL Boheim in the previous 24 months.