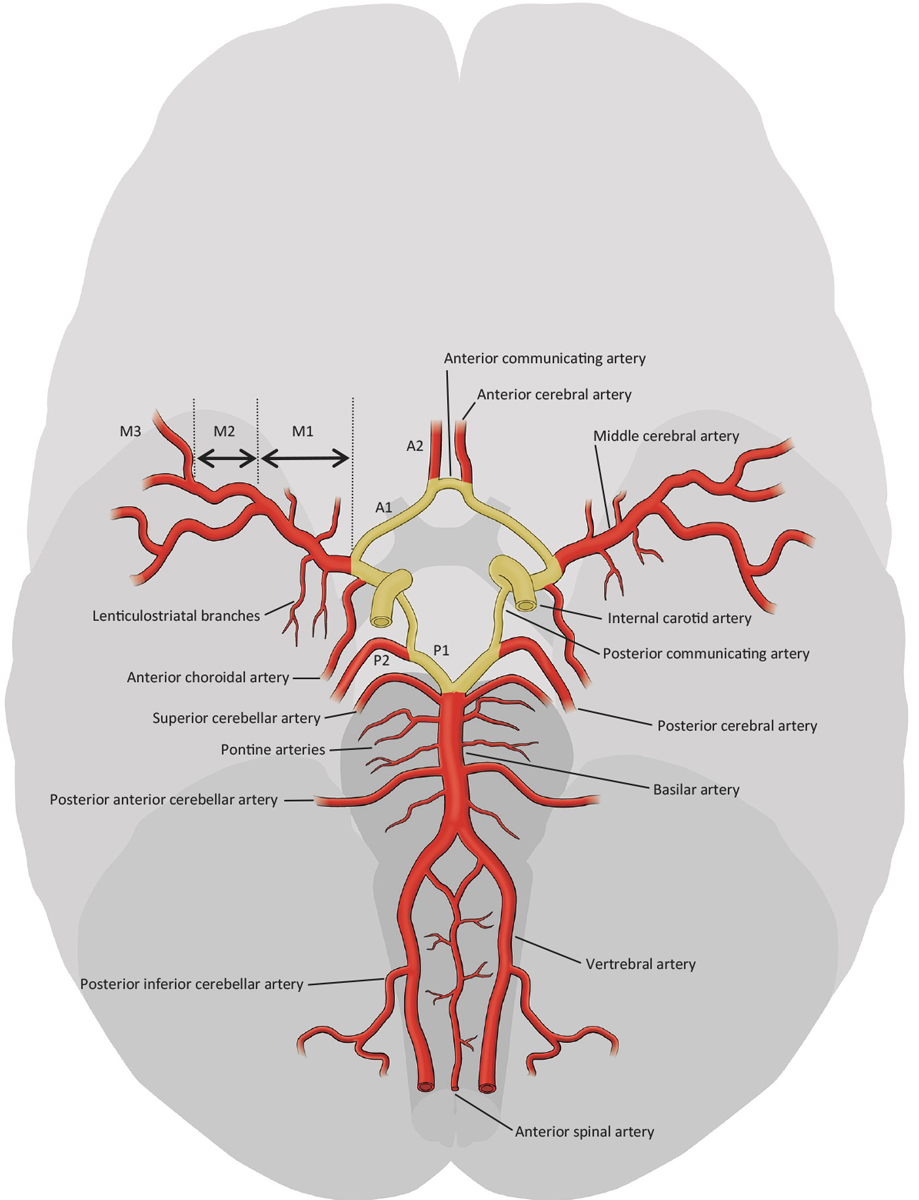
Figure 1 Intracranial vessels. Yellow: Circle of Willis (adapted from the Stroke Guidelines of the University Hospital of Bern 2017, www.strokecenter.ch).
DOI: https://doi.org/10.4414/smw.2017.14538
Collateral blood circulation is common in most species as a system of vascular redundancy designed to preserve blood supply in the event of failure of the primary blood supplying system. In humans, collateral circulation can be found in most organs, and the blood supply to the brain especially is secured by an extensive collateral circulation system. The collateral circuits to the brain can be divided into primary and secondary routes. The primary route consists of the Circle of Willis, which mainly links the anterior with the posterior circulation and the nearby main cerebral arteries to each other (fig. 1). The secondary routes include all external to internal carotid artery connections through facial, maxillary, middle meningeal, and occipital arteries. The most common one is the connection through the ophthalmic artery: retrograde blood flow via the ophthalmic artery allows supply from the external carotid artery to the internal carotid artery (ICA) in the case of proximal occlusion of the ICA. In addition, the secondary routes comprise leptomeningeal collaterals, perforator collaterals, the tectal plexus and the ophthalmic plexus. Leptomeningeal collaterals are small pial arterioles connecting the territories of the middle cerebral artery (MCA) with those of the anterior (ACA) and posterior cerebral artery (PCA) (fig. 2). Leptomeningeal collaterals are very important in occlusions of the intracranial arteries. Perforator collaterals connect lenticulostriate and thalamostriate arteries with branches of the MCA and PCA and can be recruited in addition to leptomeningeal collaterals in MCA occlusions.

Figure 1 Intracranial vessels. Yellow: Circle of Willis (adapted from the Stroke Guidelines of the University Hospital of Bern 2017, www.strokecenter.ch).
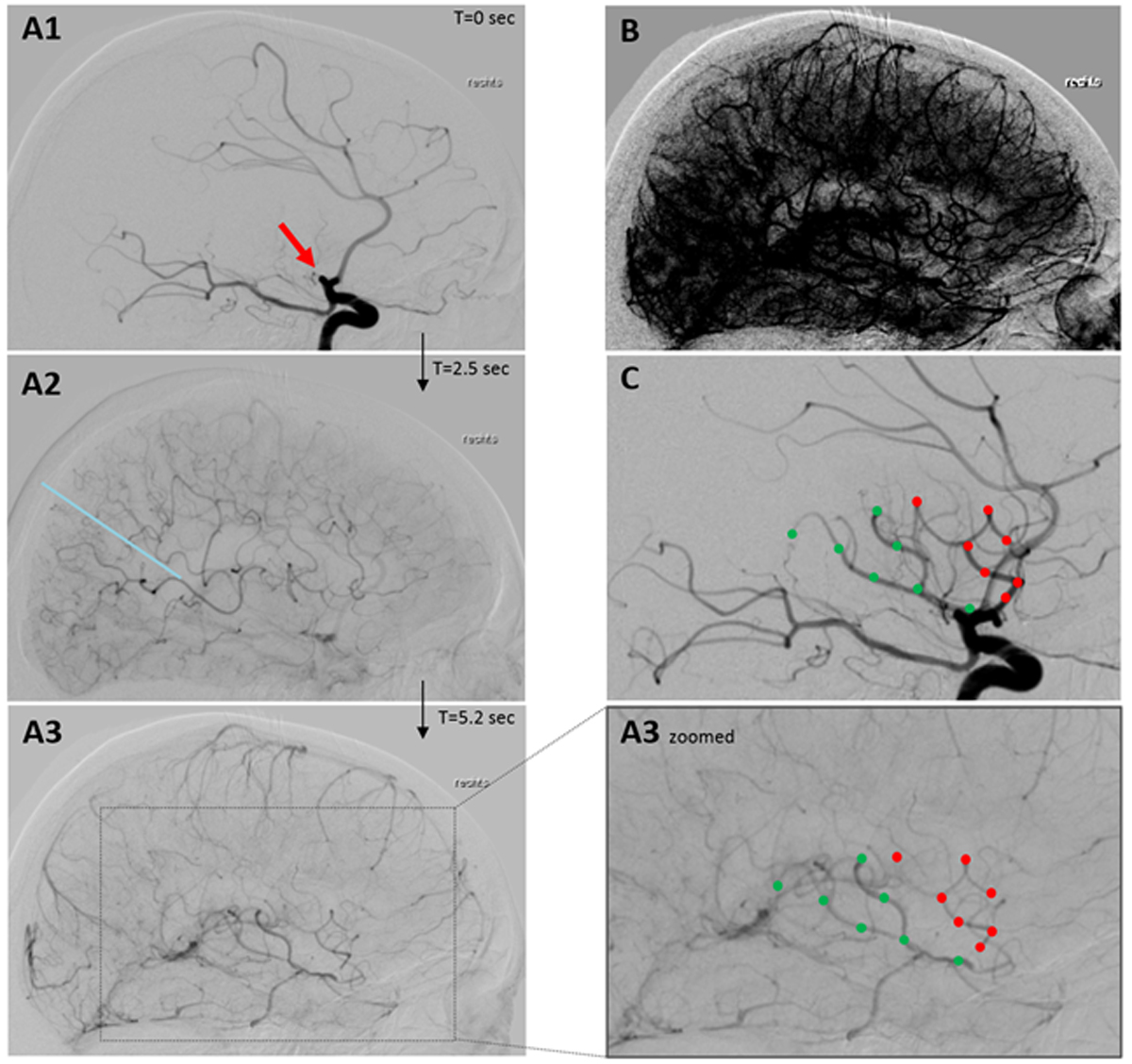
Figure 2 Leptomeningeal collateral flow in occlusion of the proximal middle cerebral artery as seen in conventional angiography (sagittal plane). (A1–A3): serial images after contrast agent application into the internal carotid artery showing proximal middle cerebral artery occlusion (red arrow in A1) with subsequent filling of leptomeningeal collaterals 2.5 sec thereafter fed by the anterior (above and right of blue line in A2) and posterior circulation. After 5.3 sec retrograde collateral flow filled the superior (red dots) and inferior branch (green dots) of the occluded middle cerebral artery (A3 + A3 zoomed). (B) After windowing A3 capillary filling visualised supply of the whole middle cerebral artery territory by collaterals. (C) The same branches filled retrogradely by collaterals in A3 are filled anterogradely after reperfusion of the occlusion. (Pictures: Stroke Centre Bern)
The extent of collaterals is highly variable between individuals, but there is also intraindividual variability during the lifetime: rarefaction of the collaterals is observed with aging and as a result of vascular risk factors [1].
Intravenous thrombolysis (IVT) and endovascular treatment (EVT) have been proven to have considerable therapeutic effect in acute ischaemic stroke in several large clinical trials [2–10]. The treatment effect of these reperfusion therapies is based on the penumbral concept, which assumes the existence of salvageable tissue due to gradual infarct growth within the territory of the occluded vessel (fig. 3) [11, 12]. Theoretical considerations quantify this gradual infarct growth to be 1.9 million neurones dying every minute in occlusion of one of the large arteries [13]. This is fast – but why do neurones not die all at once after a few minutes, given results from in-vitro experiments that have shown that neuronal cell death occurs already minutes after interruption of energy supply? Neuronal cell death can only be delayed by ongoing energy supply – that is, the whole penumbral concept as well as the therapeutic effect of reperfusion therapies relies on collateral blood flow. Depending on the extent of collateral blood flow, neuronal death can be delayed in a variable manner or even prevented. The extent of collateral flow is highly variable between individuals, but it is usually greatest in the case of occlusion of the extracranial vessels: almost 60% of patients tolerate even complete ICA occlusion without infarct development because of sufficient blood supply through primary and secondary collateral circuits [14, 15]. The recruitment of inactive, vasoconstricted collaterals can take place extremely fast: it takes only 12 seconds after ICA occlusion in rats until maximal dilatation of the leptomeningeal collaterals [16].
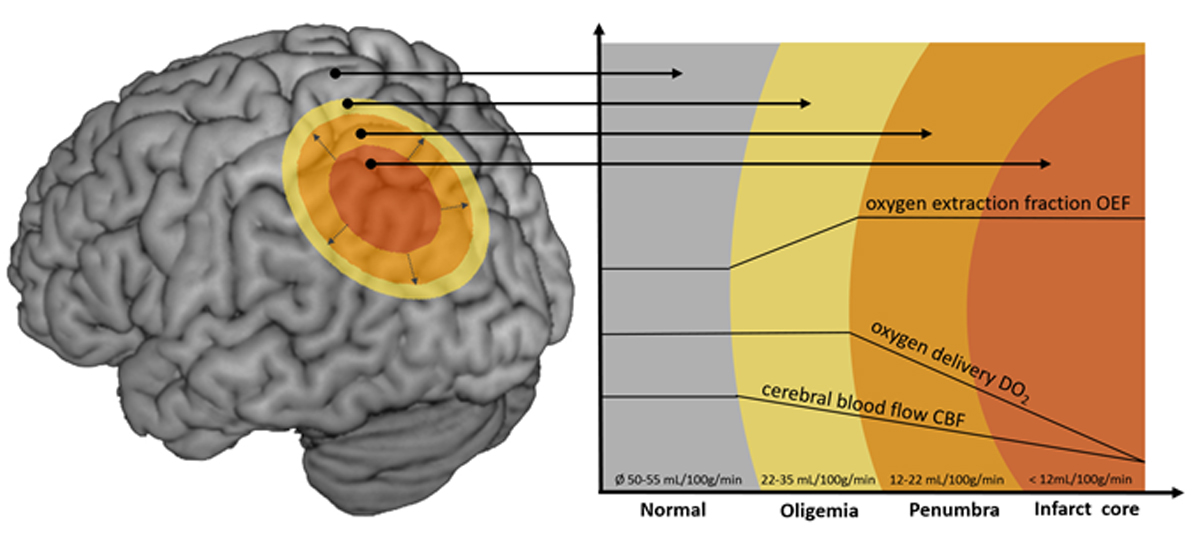
Figure 3 Illustration of the penumbra concept. Infarct core (red): infarcted tissue. Penumbra (orange): salvageable tissue at risk for infarction in case of persistence vessel occlusion. Oligaemia (yellow): hypoperfused tissue without risk for infarction. Cerebral blood flow decreases in direction to the infarct core. Decreased blood flow can be compensated by an increased oxygen extraction fraction and vasodilation of collateral vessels sufficiently enough in the oligaemia but not in the penumbra. (Picture: Stroke Centre Bern)
The extent of collateral flow has major impact on the outcome of conservatively treated patients and of patients treated with IVT or EVT. Even at hospital admission, the severity of neurological deficits already depends on the extent of collaterals [17, 18], which is revealed by variable infarct sizes in baseline imaging, depending on the collateral quality [19, 20]. Several studies demonstrated that the outcome after IVT and EVT is also highly dependent on the extent of collateral flow [21–33]: not only the volume of the final infarct [19, 20, 24, 34–38], but also the success of reperfusion [20, 27, 39, 40] are influenced by the collateral quality. In addition, the risk for symptomatic bleeding after reperfusion therapy seems to rise with poor collateral flow [30, 41, 42].
Reperfusion therapy by means of IVT and EVT is highly effective. In large randomised trials only 5–14 patients had to be treated with IVT and 3–7 patients with EVT to prevent one patient from death or dependency [2–10]. The therapeutic effect of these therapies is not only dependent on the success of reperfusion, but is also highly time dependent. Treatment time windows of 4.5 hours for IVT and 7.3 hours for EVT have been defined by pooled analysis of the patients treated in the large trials [43, 44]. Although patients on average may not benefit from later therapy, patient selection based on these time windows would neglect the large interindividual variability of the extent of collateral flow. This regimen would lead to the exclusion from therapy of many patients who would, in fact, benefit. Indeed, infarct growth is much less dependent on the elapsed time than on the quality of collaterals: good collaterals slow down infarct growth and poor collaterals accelerate it [19, 45–48]. Of course reperfusion treatment should always be initiated as early as possible because “time is brain”, but the collaterals set the pace of neuronal loss, and with this the individual time window until infarction of all tissue at risk. Figure 4 illustrates two patients in whom the approximated velocity of neuronal cell loss differed between 9000 neurones/min and more than 460 billion neurones/min. This large variability of the velocity of neuronal cell loss implies that there are probably many patients eligible for therapy far beyond time windows that were calculated on the average of patients. Patient selection based on restricted time windows would not only exclude from therapy eligible patients outside the time windows, but also all patients with wake-up stroke and with unknown symptom onset, who account for at least one third of all stroke patients. Therefore, patient selection for reperfusion therapy should be individualised by imaging salvageable tissue, rather than being restricted by strict time windows.
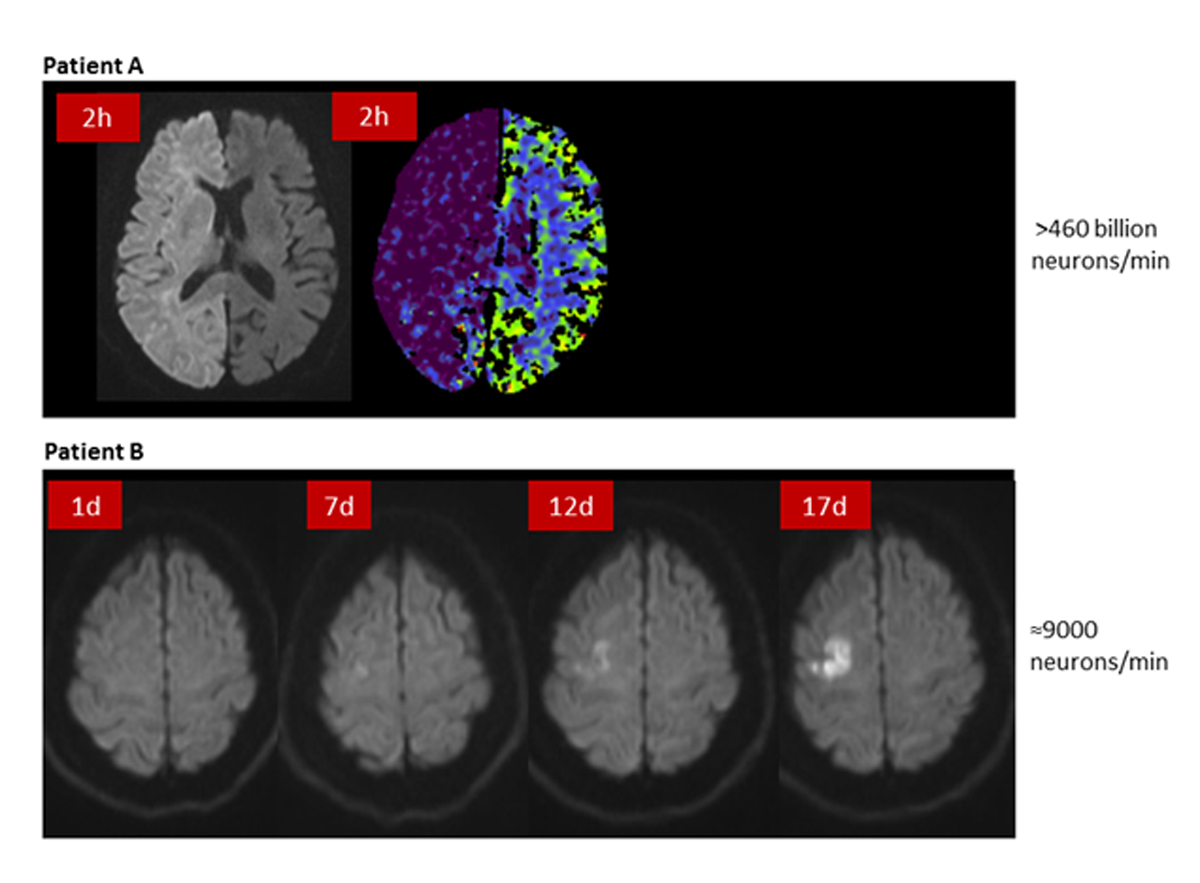
Figure 4 Variable velocities of infarct growth. Patient A: carotid T occlusion and fetal type posterior cerebral artery, 2 hours after symptom onset hyperintensity of the whole hemisphere in diffusion-weighted imaging (DWI) with corresponding hypodensity in CT perfusion (CBV). Patient B: persistent carotid occlusion, slow infarct growth in DWI over 17 days. Neuronal cell loss was estimated based on volumetric measurement of the lesion and the average neuronal density described in Saver et al. [13] (Pictures: Stroke Centre Bern)
That imaging-based patient selection is a promising concept has recently been demonstrated by the DAWN trial (not yet published), which included patients with large artery occlusion between 6 and 24 hours after symptom onset for EVT, if they presented with a so called clinical-image mismatch: patients had to be clinically severely affected (NIHSS ≥10) but infarct core had to be restricted (<21 cc if patients was ≥80 years, <31 cc if patients was <80 years and NIHSS ≥10 or <51 cc if patient was <80 years and NIHSS ≥20). The trial revealed a clear effect of reperfusion therapy in these selected patients: the number needed to treat was reported to be 2.8 for 90-day functional independence. Future trials will hopefully allow the inclusion of even more patients by a more detailed visualisation of salvageable tissue.
Collaterals can be visualised most accurately by use of conventional angiography, which is the gold standard due to its high temporal and spatial resolution [49–51]. Unfortunately, there are several systems for grading collateral flow, which hampers the comparability of study results. One of the scoring systems most often used is the one described by the Society of NeuroInterventional Surgery (formally ASITN/SIR) [52]. In clinical practice, therapeutic decisions are usually made in advance of conventional angiography, on the basis of noninvasive imaging with magnetic resonance (MR) or computed tomography (CT). Although grading of collaterals is also possible by CT and MR imaging, it is less accurate [53, 54]. Furthermore, there is more a need for an accurate visualisation of salvageable tissue at risk for infarction, rather than pure information on collateral flow.
Positron emission tomography (PET) is the gold standard for imaging of the penumbra because it allows the most accurate visualisation of the infarct core and the hypoperfused tissue [55]. A cerebral blood flow (CBF) below 12 ml/100g/min defines the area of infarct core, a flow of 12–22 ml/100g/min identifies the tissue at risk for infarction in the case of persistence vessel occlusion, and a flow greater than 22 ml/100g/min defines an area of oligaemia, i.e., hypoperfused tissue without risk for infarction [56]. Although very accurate, PET imaging is not a practicable technique in the acute setting of stroke. Both MR and CT allow approximation of the penumbra, but show a relevant inaccuracy of the imaging techniques used up to now [56–58]. The core is defined with MR imaging of the lesion in diffusion-weighted imaging (DWI) and the hypoperfused area by perfusion imaging. With CT, the core is visualised by means of cerebral blood volume (CBV) and the hypoperfused area by perfusion imaging. Visualisation of the infarct core with MRI is more accurate than with CT, but diffusion lesion reversal can occur because the DWI lesion may overestimate the infarct core and contain up to 25% false positive, surviving, tissue [59]. Definition of the core by use of quantitative analysis of the calculated Apparent Diffusion Coefficient (ADC) maps is more accurate [59].
Imaging of the hypoperfused tissue at risk by use of MR and CT perfusion imaging relies on surrogates of perfusion parameters calculated from nondeconvolved (e.g, time to peak TTP]) or deconvolved (e.g. CBF, mean transit time [MTT], time to maximum [Tmax]) tissue residue function (time contrast curve) of the intravascular contrast bolus [56]. Different maps computed from MR and CT imaging have been developed and compared with PET measurements. Recent studies indicate that Tmax thresholds of >6 sec improves the prediction of salvageable tissue compared with previous maps [60]. Nevertheless, estimation of the tissue at risk based on maps derived from simple thresholding is prone to errors in about 25% of patients, with variations of the predicted penumbra of up to 100 ml [61]. In most cases the penumbra is overestimated owing to inclusion of parts of the oligaemia. Newer studies applying machine learning techniques instead of using only the somewhat arbitrary thresholds of surrogate parameters have shown first promising results with improved accuracy of the penumbra imaging [62].
Given the association of poor collateral flow with poor outcome, and larger and faster infarct growth, therapeutic promotion of collateral flow may offer the chance to improve outcome beyond efforts to raise reperfusion success rates and to minimise treatment delay. Up to now, mainly in-vivo data support the potential benefit from promoting collateral flow [63, 64]. Possible treatment options include induced hypertension (e.g. by phenylephrine), selective cerebral vasodilation (e.g., by nitric oxide or sphenopalatine ganglion stimulation), external counterpulsation, and endovascular partial occlusion of the abdominal aorta [63, 64]. On the one hand, induced hypertension and partial occlusion of the abdominal aorta aim to improve collateral circulation by raising the perfusion pressure through an increased systemic pressure and flow diversion, respectively. External counterpulsation aims also to raise the perfusion pressure of collaterals, but through augmented diastolic flow. On the other hand, selective cerebral vasodilatation aims to increase collateral flow by reducing the vessel resistance within collateral arterioles.
Jan Gralla is the Global Principal Investigator of the SWIFT DIRECT Trial. No other potential conflict of interest relevant to this article was reported.
1 Menon BK , Smith EE , Coutts SB , Welsh DG , Faber JE , Goyal M , et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol. 2013;74(2):241–8. doi:http://doi.wiley.com/10.1002/ana.23906.
2 National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–8. doi:.https://doi.org/10.1056/NEJM199512143332401
3 Hacke W , Kaste M , Bluhmki E , Brozman M , Dávalos A , Guidetti D , et al.; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–29. doi:.https://doi.org/10.1056/NEJMoa0804656
4 Goyal M , Demchuk AM , Menon BK , Eesa M , Rempel JL , Thornton J , et al.; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30. doi:.https://doi.org/10.1056/NEJMoa1414905
5 Jovin TG , Chamorro A , Cobo E , de Miquel MA , Molina CA , Rovira A , et al.; REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–306. doi:.https://doi.org/10.1056/NEJMoa1503780
6 Saver JL , Goyal M , Bonafe A , Diener HC , Levy EI , Pereira VM , et al.; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–95. doi:.https://doi.org/10.1056/NEJMoa1415061
7 Berkhemer OA , Fransen PS , Beumer D , van den Berg LA , Lingsma HF , Yoo AJ , et al.; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20.
8 Campbell BCV , Mitchell PJ , Kleinig TJ , Dewey HM , Churilov L , Yassi N , et al.; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18. doi:.https://doi.org/10.1056/NEJMoa1414792
9 Bracard S , Ducrocq X , Mas JL , Soudant M , Oppenheim C , Moulin T , et al.; THRACE investigators. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138–47. doi:.https://doi.org/10.1016/S1474-4422(16)30177-6
10 Mocco J , Zaidat OO , von Kummer R , Yoo AJ , Gupta R , Lopes D , et al.; THERAPY Trial Investigators*. Aspiration Thrombectomy After Intravenous Alteplase Versus Intravenous Alteplase Alone. Stroke. 2016;47(9):2331–8. doi:.https://doi.org/10.1161/STROKEAHA.116.013372
11 Baron JC . Mapping the ischaemic penumbra with PET: implications for acute stroke treatment. Cerebrovasc Dis. 1999;9(4):193–201. doi:.https://doi.org/10.1159/000015955
12 Astrup J , Siesjö BK , Symon L . Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12(6):723–5. doi:.https://doi.org/10.1161/01.STR.12.6.723
13 Saver JL . Time is brain--quantified. Stroke. 2006;37(1):263–6. doi:.https://doi.org/10.1161/01.STR.0000196957.55928.ab
14 Powers WJ . Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29(3):231–40. doi:.https://doi.org/10.1002/ana.410290302
15 Arnold M , Slezak A , El-Koussy M , Lüdi R , Findling O , Mono ML , et al. Occlusion Location of Middle Cerebral Artery Stroke and Outcome after Endovascular Treatment. Eur Neurol. 2015;74(5-6):315–21. doi:.https://doi.org/10.1159/000441445
16 Morita Y , Fukuuchi Y , Koto A , Suzuki N , Isozumi K , Gotoh J , et al. Rapid changes in pial arterial diameter and cerebral blood flow caused by ipsilateral carotid artery occlusion in rats. Keio J Med. 1997;46(3):120–7. doi:.https://doi.org/10.2302/kjm.46.120
17 Roberts HC , Dillon WP , Furlan AJ , Wechsler LR , Rowley HA , Fischbein NJ , et al. Computed tomographic findings in patients undergoing intra-arterial thrombolysis for acute ischemic stroke due to middle cerebral artery occlusion: results from the PROACT II trial. Stroke. 2002;33(6):1557–65. doi:.https://doi.org/10.1161/01.STR.0000018011.66817.41
18 Marks MP , Lansberg MG , Mlynash M , Olivot JM , Straka M , Kemp S , et al.; Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2 Investigators. Effect of collateral blood flow on patients undergoing endovascular therapy for acute ischemic stroke. Stroke. 2014;45(4):1035–9. doi:.https://doi.org/10.1161/STROKEAHA.113.004085
19 Jung S , Gilgen M , Slotboom J , El-Koussy M , Zubler C , Kiefer C , et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain. 2013;136(Pt 12):3554–60. doi:.https://doi.org/10.1093/brain/awt246
20 Marks MP , Lansberg MG , Mlynash M , Olivot JM , Straka M , Kemp S , et al.; Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2 Investigators. Effect of collateral blood flow on patients undergoing endovascular therapy for acute ischemic stroke. Stroke. 2014;45(4):1035–9. doi:.https://doi.org/10.1161/STROKEAHA.113.004085
21 Galimanis A , Jung S , Mono M-L , Fischer U , Findling O , Weck A , et al. Endovascular therapy of 623 patients with anterior circulation stroke. Stroke. 2012;43(4):1052–7. doi:.https://doi.org/10.1161/STROKEAHA.111.639112
22 Kucinski T , Koch C , Eckert B , Becker V , Krömer H , Heesen C , et al. Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology. 2003;45(1):11–8. doi:.https://doi.org/10.1007/s00234-002-0881-0
23 Toni D , Fiorelli M , Bastianello S , Falcou A , Sette G , Ceschin V , et al. Acute ischemic strokes improving during the first 48 hours of onset: predictability, outcome, and possible mechanisms. A comparison with early deteriorating strokes. Stroke. 1997;28(1):10–4. doi:.https://doi.org/10.1161/01.STR.28.1.10
24 Rosenthal ES , Schwamm LH , Roccatagliata L , Coutts SB , Demchuk AM , Schaefer PW , et al. Role of recanalization in acute stroke outcome: rationale for a CT angiogram-based “benefit of recanalization” model. AJNR Am J Neuroradiol. 2008;29(8):1471–5. doi:.https://doi.org/10.3174/ajnr.A1153
25 Tan JC , Dillon WP , Liu S , Adler F , Smith WS , Wintermark M . Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol. 2007;61(6):533–43. doi:.https://doi.org/10.1002/ana.21130
26 Tan IYL , Demchuk AM , Hopyan J , Zhang L , Gladstone D , Wong K , et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30(3):525–31. doi:.https://doi.org/10.3174/ajnr.A1408
27 Bang OY , Saver JL , Kim SJ , Kim GM , Chung CS , Ovbiagele B , et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42(3):693–9. doi:.https://doi.org/10.1161/STROKEAHA.110.595256
28 Gerber JC , Petrova M , Krukowski P , Kuhn M , Abramyuk A , Bodechtel U , et al. Collateral state and the effect of endovascular reperfusion therapy on clinical outcome in ischemic stroke patients. Brain Behav. 2016;6(9):e00513. doi:.https://doi.org/10.1002/brb3.513
29 Leng X , Lan L , Liu L , Leung TW , Wong KS . Good collateral circulation predicts favorable outcomes in intravenous thrombolysis: a systematic review and meta-analysis. Eur J Neurol. 2016;23(12):1738–49. doi:.https://doi.org/10.1111/ene.13111
30 Leng X , Fang H , Leung TWH , Mao C , Miao Z , Liu L , et al. Impact of collaterals on the efficacy and safety of endovascular treatment in acute ischaemic stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87(5):537–44. doi:.https://doi.org/10.1136/jnnp-2015-310965
31 Liebeskind DS , Jahan R , Nogueira RG , Zaidat OO , Saver JL ; SWIFT Investigators. Impact of collaterals on successful revascularization in Solitaire FR with the intention for thrombectomy. Stroke. 2014;45(7):2036–40. doi:.https://doi.org/10.1161/STROKEAHA.114.004781
32 Goyal M , Demchuk AM , Menon BK , Eesa M , Rempel JL , Thornton J , et al.; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30. doi:.https://doi.org/10.1056/NEJMoa1414905
33 Tan BYQ , Wan-Yee K , Paliwal P , Gopinathan A , Nadarajah M , Ting E , et al. Good Intracranial Collaterals Trump Poor ASPECTS (Alberta Stroke Program Early CT Score) for Intravenous Thrombolysis in Anterior Circulation Acute Ischemic Stroke. Stroke. 2016;47(9):2292–8. doi:.https://doi.org/10.1161/STROKEAHA.116.013879
34 Ovbiagele B , Buck BH , Liebeskind DS , Starkman S , Bang OY , Ali LK , et al. Prior antiplatelet use and infarct volume in ischemic stroke. J Neurol Sci. 2008;264(1-2):140–4. doi:.https://doi.org/10.1016/j.jns.2007.08.033
35 Boers AM , Jansen IG , Berkhemer OA , Yoo AJ , Lingsma HF , Slump CH , et al.; MR CLEAN trial investigators †. Collateral status and tissue outcome after intra-arterial therapy for patients with acute ischemic stroke. J Cereb Blood Flow Metab. 2016;Jan 1:X16678874. doi:http://journals.sagepub.com/doi/10.1177/0271678X16678874.
36 Christoforidis GA , Vakil P , Ansari SA , Dehkordi FH , Carroll TJ . Impact of pial collaterals on infarct growth rate in experimental acute ischemic stroke. AJNR Am J Neuroradiol. 2017;38(2):270–5. doi:.https://doi.org/10.3174/ajnr.A5003
37 Son JP , Lee MJ , Kim SJ , Chung JW , Cha J , Kim GM , et al. Impact of Slow Blood Filling via Collaterals on Infarct Growth: Comparison of Mismatch and Collateral Status. J Stroke. 2017;19(1):88–96. doi:.https://doi.org/10.5853/jos.2016.00955
38 Vagal A , Menon BK , Foster LD , Livorine A , Yeatts SD , Qazi E , et al. Association Between CT Angiogram Collaterals and CT Perfusion in the Interventional Management of Stroke III Trial. Stroke. 2016;47(2):535–8. doi:.https://doi.org/10.1161/STROKEAHA.115.011461
39 Leng X , Fang H , Leung TWH , Mao C , Xu Y , Miao Z , et al. Impact of Collateral Status on Successful Revascularization in Endovascular Treatment: A Systematic Review and Meta-Analysis. Cerebrovasc Dis. 2016;41(1-2):27–34. doi:.https://doi.org/10.1159/000441803
40 Liebeskind DS , Jahan R , Nogueira RG , Zaidat OO , Saver JL ; SWIFT Investigators. Impact of collaterals on successful revascularization in Solitaire FR with the intention for thrombectomy. Stroke. 2014;45(7):2036–40. doi:.https://doi.org/10.1161/STROKEAHA.114.004781
41 Bang OY , Saver JL , Kim SJ , Kim GM , Chung CS , Ovbiagele B , et al.; UCLA-Samsung Stroke Collaborators. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42(8):2235–9. doi:.https://doi.org/10.1161/STROKEAHA.110.604603
42 Liebeskind DS , Tomsick TA , Foster LD , Yeatts SD , Carrozzella J , Demchuk AM , et al.; IMS III Investigators. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke. 2014;45(3):759–64. doi:.https://doi.org/10.1161/STROKEAHA.113.004072
43 Hacke W , Donnan G , Fieschi C , Kaste M , von Kummer R , Broderick JP , et al., NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768–74. doi:.https://doi.org/10.1016/S0140-6736(04)15692-4
44 Saver JL , Goyal M , van der Lugt A , Menon BK , Majoie CB , Dippel DW , et al.; HERMES Collaborators. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA. 2016;316(12):1279–88. doi:.https://doi.org/10.1001/jama.2016.13647
45 Cheng-Ching E , Frontera JA , Man S , Aoki J , Tateishi Y , Hui FK , et al. Degree of collaterals and not time is the determining factor of core infarct volume within 6 hours of stroke onset. AJNR Am J Neuroradiol. 2015;36(7):1272–6. doi:.https://doi.org/10.3174/ajnr.A4274
46 Cheripelli BK , Huang X , McVerry F , Muir KW . What is the relationship among penumbra volume, collaterals, and time since onset in the first 6 h after acute ischemic stroke? Int J Stroke. 2016;11(3):338–46. doi:.https://doi.org/10.1177/1747493015620807
47 Hwang Y-H , Kang D-H , Kim Y-W , Kim Y-S , Park S-P , Liebeskind DS . Impact of time-to-reperfusion on outcome in patients with poor collaterals. AJNR Am J Neuroradiol. 2015;36(3):495–500. doi:.https://doi.org/10.3174/ajnr.A4151
48 Maurer CJ , Egger K , Dempfle AK , Reinhard M , Meckel S , Urbach H . Facing the Time Window in Acute Ischemic Stroke: The Infarct Core. Clin Neuroradiol. 2016;26(2):153–8. doi:.https://doi.org/10.1007/s00062-016-0501-8
49 McVerry F , Liebeskind DS , Muir KW . Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am J Neuroradiol. 2012;33(3):576–82. doi:.https://doi.org/10.3174/ajnr.A2794
50 Martinon E , Lefevre PH , Thouant P , Osseby GV , Ricolfi F , Chavent A . Collateral circulation in acute stroke: assessing methods and impact: a literature review. J Neuroradiol. 2014;41(2):97–107. doi:.https://doi.org/10.1016/j.neurad.2014.02.001
51 Raymond SB , Schaefer PW . Imaging Brain Collaterals: Quantification, Scoring, and Potential Significance. Top Magn Reson Imaging. 2017;26(2):67–75. doi:.https://doi.org/10.1097/RMR.0000000000000123
52 Higashida RT , Furlan AJ , Roberts H , Tomsick T , Connors B , Barr J , et al.; Technology Assessment Committee of the American Society of Interventional and Therapeutic Neuroradiology; Technology Assessment Committee of the Society of Interventional Radiology. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34(8):e109–37. doi:.https://doi.org/10.1161/01.STR.0000082721.62796.09
53 Baird AE , Warach S . Magnetic resonance imaging of acute stroke. J Cereb Blood Flow Metab. 1998;18(6):583–609. doi:.https://doi.org/10.1097/00004647-199806000-00001
54 Wintermark M , Reichhart M , Thiran J-P , Maeder P , Chalaron M , Schnyder P , et al. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann Neurol. 2002;51(4):417–32. doi:.https://doi.org/10.1002/ana.10136
55 Heiss W-D . PET imaging in ischemic cerebrovascular disease: current status and future directions. Neurosci Bull. 2014;30(5):713–32. doi:.https://doi.org/10.1007/s12264-014-1463-y
56 Heiss W-D , Zaro Weber O . Validation of MRI Determination of the Penumbra by PET Measurements in Ischemic Stroke. J Nucl Med. 2017;58(2):187–93. doi:.https://doi.org/10.2967/jnumed.116.185975
57 Donnan GA , Baron JC , Ma H , Davis SM . Penumbral selection of patients for trials of acute stroke therapy. Lancet Neurol. 2009;8(3):261–9. doi:.https://doi.org/10.1016/S1474-4422(09)70041-9
58 Heit JJ , Wintermark M . Perfusion Computed Tomography for the Evaluation of Acute Ischemic Stroke: Strengths and Pitfalls. Stroke. 2016;47(4):1153–8. doi:.https://doi.org/10.1161/STROKEAHA.116.011873
59 Heiss W-D , Sobesky J , Smekal U , Kracht LW , Lehnhardt FG , Thiel A , et al. Probability of cortical infarction predicted by flumazenil binding and diffusion-weighted imaging signal intensity: a comparative positron emission tomography/magnetic resonance imaging study in early ischemic stroke. Stroke. 2004;35(8):1892–8. doi:.https://doi.org/10.1161/01.STR.0000134746.93535.9b
60 Olivot JM , Mlynash M , Thijs VN , Kemp S , Lansberg MG , Wechsler L , et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40(2):469–75. doi:.https://doi.org/10.1161/STROKEAHA.108.526954
61 Forkert ND , Kaesemann P , Treszl A , Siemonsen S , Cheng B , Handels H , et al. Comparison of 10 TTP and Tmax estimation techniques for MR perfusion-diffusion mismatch quantification in acute stroke. AJNR Am J Neuroradiol. 2013;34(9):1697–703. doi:.https://doi.org/10.3174/ajnr.A3460
62 McKinley R , Häni L , Gralla J , El-Koussy M , Bauer S , Arnold M , et al. Fully automated stroke tissue estimation using random forest classifiers (FASTER). J Cereb Blood Flow Metab. 2017;37(8):2728–41. doi:.https://doi.org/10.1177/0271678X16674221
63 Cuccione E , Padovano G , Versace A , Ferrarese C , Beretta S . Cerebral collateral circulation in experimental ischemic stroke. Exp Transl Stroke Med. 2016;8(1):2. doi:.https://doi.org/10.1186/s13231-016-0015-0
64 Nishijima Y , Akamatsu Y , Weinstein PR , Liu J . Collaterals: Implications in cerebral ischemic diseases and therapeutic interventions. Brain Res. 2015;1623:18–29. doi:.https://doi.org/10.1016/j.brainres.2015.03.006
Jan Gralla is the Global Principal Investigator of the SWIFT DIRECT Trial. No other potential conflict of interest relevant to this article was reported.