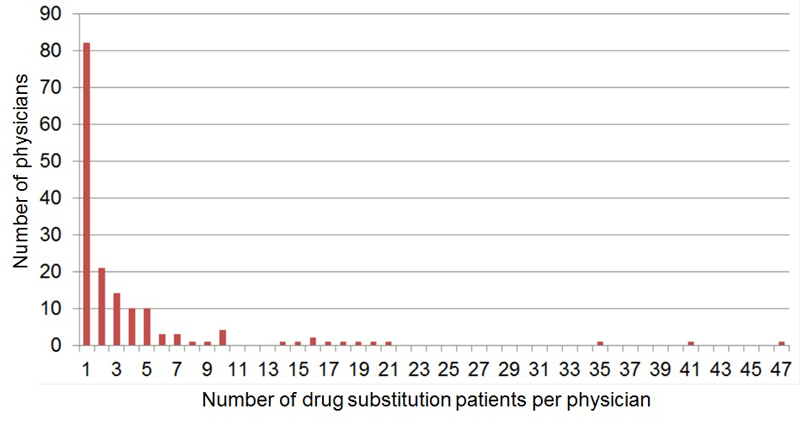
Figure 1 Number of drug substitution patients per physician in the canton Aargau (631 patients, 161 physicians).
DOI: https://doi.org/10.4414/smw.2017.14544
Hepatitis C is a blood-borne viral infection highly prevalent in patients with current or former intravenous drug use as a result of sharing of injection equipment. Globally, hepatitis C virus (HCV) seroprevalence in people who inject drugs (PWID) is 67.5% [1]. Given a ~25% spontaneous clearance rate, ~50% of the intravenous drug users have chronic HCV infection, corresponding to 8 million people [2].
HCV seroprevalence in Switzerland is 0.7–1.75% in the general population, 26–48% in those on oral opioid substitution treatment and 60–80% in heroin substitution programmes [3]. It increases with the length of heroin use (55% after 2–4 years, 75% after 5–9 years, 90% after 10–15 years, 98% after >15 years) [4]. Of the 22 000–27 000 opioid addicts in Switzerland [5], 18 000 are in a methadone/buprenorphine programme and 1600 in a heroin substitution programme [6]. In about 60%, substitution treatment is provided by a general practitioner (GP) [6].
About 75% of HCV infected people develop chronic infection [7]. Being mostly asymptomatic, hepatitis C may remain undetected for years and become a “silent killer” [8]. About 20% of chronically infected individuals develop liver cirrhosis after 20 years [9], with an annual risk of hepatocellular carcinoma of 1–5% and of hepatic decompensation of 3–6%. After an episode of decompensation, the risk of death in the following year is 15–20% [10]. Factors associated with accelerated liver fibrosis progression (alcohol [11], cannabis [12, 13], nicotine [14], human immunodeficiency virus (HIV) co-infection [15, 16]) are common among PWID.
HCV infection can be cured with a therapy course of limited duration. With the new direct-acting antivirals, 90–100% reach sustained virological response after 12 (8–24) weeks of treatment [17–19]. In Italian PWID (one third on opioid substitution treatment), the sustained virological response rate with direct-acting antivirals was 93% [20]. Owing to its simplicity and safety, interferon-free direct-acting antiviral therapy reduces the time required for HCV treatment from 27 to 9 hours, tripling treatment capacity [21]. In Canada, compared with people receiving interferon-based therapy, people receiving interferon-free direct-acting antiviral therapy were more likely to be HIV co-infected, PWID or on opioid substitution treatment, demonstrating the feasibility of the new HCV treatments in previously excluded groups [22].
As a result of high prices, reimbursement for direct-acting antiviral therapy is often restricted. In April 2017, 32 of 34 European countries required specialists to prescribe direct-acting antivirals; 38% required stage ≥F2 fibrosis and 24% ≥F3 fibrosis, whereas 26% had no fibrosis stage restrictions (e.g., Germany, France). Nine percent had additional HCV genotype requirements and 18% drug or alcohol use limitations (e.g., abstinence prior to treatment) [23]. In Switzerland, direct-acting antiviral prescription was restricted to specialists and ≥F2 fibrosis, unless extrahepatic symptoms were present. Since 1 May 2017, HIV or hepatitis B virus (HBV) co-infected patients and PWID can be treated irrespective of fibrosis stage [24]. Based on significant price reductions, in July, August and October 2017, reimbursement restrictions have been abandoned for several direct-acting antiviral agents [25].
Early diagnosis and successful treatment not only prevent HCV-related complications (individual benefit), but also stop infectiousness (community benefit).
Although PWID are the main source of new HCV infections [26], and their adherence, side effects and treatment outcome are not different from people who do not use injectable drugs [27–29], they are commonly underdiagnosed and undertreated [30–33]. Only 10–50% of all PWID worldwide receive HCV testing and <10% have access to assessment and treatment [2], although there are evidence-based interventions to enhance: (1) HCV diagnosis/case-finding, (2) linkage to care, (3) pretherapeutic evaluation / treatment initiation, and (4) treatment adherence [34]. In Scotland, the introduction of dried blood spot testing within the Hepatitis C Action Plan resulted in a 3-fold increase in testing and 12-fold increase in positive individuals in drug services [35].
Within our study, we offered free HIV/HCV rapid tests on capillary blood from the finger and free noninvasive liver fibrosis assessment with Fibroscan® to remove two known barriers to HCV diagnosis and treatment in PWID: destroyed peripheral veins after long-term intravenous drug use and fear of liver biopsy. Based on test results and information from a questionnaire, an infectious disease specialist provided the treating physician with guideline-based, patient-customised recommendations for further care, including referral for HCV treatment if indicated.
For PWID, various successful hepatitis C care models have been described, including multidisciplinary approaches in opioid substitution clinics, GP-based models, integrated HCV management in secondary/tertiary care settings, directly observed therapy and peer-based models [36]. However, the different models have not been directly compared with each other so far.
National [30] and international [37] guidelines for the management of hepatitis C in PWID and those on opioid substitution therapy recommend: (1) yearly HIV and HCV screening of seronegative individuals, (2) HCV RNA determination in HCV-positive patients, (3) yearly HCV RNA screening in HCV-positive patients with negative HCV RNA, (4) HCV genotyping and treatment evaluation in HCV RNA-positive individuals, (5) antiretroviral treatment of all HIV/HCV co-infected patients with chronic hepatitis C, irrespective of CD4 count, and (6) vaccination against hepatitis A and B in the absence of immunity.
It can be hypothesised that compliance with guidelines is better in specialised, centralised settings than unspecialised, decentralised settings.
The aim of our study was to compare the current state of HCV management in centralised and decentralised drug substitution programmes of the canton Aargau. Objectives were to quantify HIV/HCV prevalence, compliance with guidelines and gaps in the HCV cascade (to develop further strategies to improve linkage to care), as well as feasibility/acceptance/validity of HIV/HCV rapid tests that use finger-prick blood and noninvasive liver fibrosis assessment with Fibroscan®.
The study was approved by the cantonal ethical committee (AG/SO 2012/091). All participants gave written informed consent before any disclosure of personal data.
The cantonal physician provided a list of all 161 physicians in the canton Aargau providing opioid substitution treatment and the respective number of drug substitution patients they cared for.
For the cross-sectional study, in June 2013, patient informed consent forms, questionnaires and free rapid tests for HIV (Determine®, Alere) and HCV (OraQuick®, Orasure) [38] using capillary blood (one finger-stick for both tests, results after 20 minutes) were sent to the 161 physicians who provided drug substitution treatment for 631 patients. For data protection reasons, study material was sent by the cantonal physician to the treating physicians without identifying the patient names to the study team. Flyers inviting study participation were distributed to drug substitution patients in pharmacies.
Physicians received CHF 50 per patient for obtaining informed consent, performing the rapid tests and completing the questionnaire.
Liver fibrosis was measured with mobile Fibroscan® 402 (Echosens) [38–42] by a member of the study team (result after ~5 minutes). Once the study team received the completed questionnaire and the rapid test results, the Fibroscan® was scheduled – in most cases in the Infectious Diseases Outpatient Clinic of the Cantonal Hospital Aarau, where the Fibroscan® was based. Rarely, (when ≥10 patients were recruited from the same family practice) the Fibroscan® was offered in the family practice on an appointed day.
Patients were also directly recruited by the study team in the Infectious Diseases Outpatient Clinic of the Cantonal Hospital Aarau, as well as in the heroin substitution programme and several addiction clinics, visited every 4–6 months. For the visits, the mobile Fibroscan® was transported to the heroin substitution programme and the addiction clinics. Thus, patients in the centralised setting had the questionnaire, rapid tests and Fibroscan® in the same session – all performed by a member of the study team.
Based on test results and information from the questionnaire, an infectious disease specialist provided the treating physician with guideline-based, patient-customised recommendations for further care, including referral for HCV treatment if indicated.
HCV positivity/infection means a positive HCV antibody test. Active/chronic HCV infection was defined as detectable HCV RNA (at least 6 months after infection/diagnosis).
The spontaneous HCV clearance rate was calculated as the ratio between patients HCV RNA-negative without treatment and all HCV antibody-positive patients with available HCV RNA.
Nonresponse to HCV treatment was defined as HCV RNA decrease <2 log U/ml after 12 weeks or detectable HCV RNA after 24 weeks of treatment.
Daily alcohol intake of >40g was considered as alcohol overconsumption.
Categorical variables were compared using chi-squared or Fisher’s exact test. Continuous variables were analysed with the Wilcoxon rank sum test. A two-sided p-value <0.05 was considered statistically significant.
Risk ratios (RRs) were calculated by dividing the risk in exposed by the risk in the unexposed. Risks were considered significantly different at the 5% level if the 95% confidence interval (CI) of the RR did not include 1.
Statistical analyses were performed with Stata Version 12.0.
In the drug substitution programme of the canton Aargau 161 physicians were caring for 631 patients (fig. 1). Half of the physicians (82/161) had one and >90% (149/161) ≤10 drug substitution patients. There were nine physicians with 14 to 21 patients and three with 35 to 47 patients.

Figure 1 Number of drug substitution patients per physician in the canton Aargau (631 patients, 161 physicians).
Between July 2013 and July 2015, 205 (32.5%) of the 631 patients living and receiving opioid substitution in the canton Aargau were enrolled, 192 (93.7%) with HIV/HCV rapid tests and 167 (81.5%) with Fibroscan®. From the first year (July 2013 to June 2014, n = 117) to the second year (July 2014 to July 2015, n = 88), the source of patient recruitment changed: family practice from 71.8 to 19.3%, heroin substitution programme from 15.4 to 37.5%, addiction clinic from 3.4 to 28.4%, Infectious Diseases Outpatient Clinic of the Cantonal Hospital Aarau from 9.4 to 11.4%, pharmacy from 0 to 3.4%.
Overall, 50.7% of the study population was recruited in a decentralised setting (family practice / pharmacy) and 49.3% in a centralised setting (heroin substitution programme, addiction clinic, Infectious Diseases Outpatient Clinic of the Cantonal Hospital Aarau) (table 1).
Table 1 Baseline patient characteristics.
|
Total
(n = 205) |
Decentralised*
(n = 104) |
Centralised†
(n = 101) |
p-value | |
|---|---|---|---|---|
| Male | 79.5% (163) | 72.1% (75) | 87.1% (88) | 0.008 |
| Median age (y) (IQR) | 38.5 (32.4–44.7) | 39.5 (33.8–45.4) | 37.9 (31.2–44.6) | 0.110 |
| Median BMI (kg/m2) (IQR) | 24.4 (21.3–27.8) (n = 195) |
24.4 (21.1–26.6) (n = 97) |
24.3 (21.6–28.2) (n = 98) |
0.535 |
| Attendance: | ||||
| Daily (5–7 times/week) | 54.0% (108/200) | 35.3% (36/102) | 73.5% (72/98) | <0.001 |
| ≤1 time/week | 32.5% (65/200) | 49.0% (50/102) | 15.3% (15/98) | <0.001 |
| Drug substitution: | <0.001 | |||
| Methadone | 63.9% (131) | 82.7% (86) | 44.6% (45) | |
| Heroin | 8.3% (17) | 0% (0) | 16.8% (17) | |
| Methadone + heroin | 4.4% (9) | 0% (0) | 8.9% (9) | |
| Buprenorphine | 18.0% (37) | 16.4% (17) | 19.8% (20) | |
| Retarded morphine | 5.4% (11) | 1.0% (1) | 9.9% (10) | |
| Median time (y) in programme (IQR) | 3 (0.5–8.2) (n = 191) |
5.9 (2.9–12) (n = 92) |
0.6 (0.2–4) (n = 99) |
<0.001 |
| Ever IDU | 77.8% (158/203) | 70.6% (72/102) | 85.2% (86/101) | 0.013 |
| Median age (y) at first IDU (IQR) | 20 (17-24) (n = 148) |
20 (17-25) (n = 63) |
19 (17-23) (n = 85) |
0.658 |
| Ever intranasal drug use | 89.5% (179/200) | 85.2% (86/101) | 93.9% (93/99) | 0.043 |
| Ever consumption of: | ||||
| Heroin | 98.5% (201/204) | 99.0% (102/103) | 98.0% (99/101) | 0.549 |
| Cocaine | 91.6% (186/203) | 87.3% (89/102) | 96.0% (97/101) | 0.024 |
| Benzodiazepines | 71.1% (140/197) | 59.8% (58/97) | 82.0% (82/100) | 0.001 |
| Cannabis | 90.5% (181/200) | 90.0% (90/100) | 91.0% (91/100) | 0.809 |
| Current alcohol consumption >40 g/d | 24.9% (50/201) | 27.5% (28/102) | 22.2% (22/99) | 0.391 |
| Depression | 46.0% (93/202) | 42.2% (43/102) | 50.0% (50/100) | 0.263 |
| Ever suicide attempt | 22.6% (45/199) | 17.8% (18/101) | 27.6% (27/98) | 0.101 |
BMI = body mass index; IDU = intravenous drug use; IQR = interquartile range * Recruitment in family practice (n=101) or pharmacy (n = 3) † Recruitment in heroin substitution programme (n = 51), addiction clinic (n = 29) or Infectious Diseases Outpatient Clinic of the Cantonal Hospital Aarau (n = 21)
Compared with patients from centralised settings, those from decentralised settings were more likely to be female, were predominantly receiving methadone substitution (16.4% buprenorphine, but no heroin), had fewer visits per week and had been longer in the substitution programme. The proportion with ever-use of intravenous drugs, cocaine and benzodiazepines was lower. Overconsumption of alcohol (24.9%) and depression (46.0%) were highly prevalent without setting-specific differences.
Prior to HCV rapid tests and Fibroscans®, we identified four diagnostic- and two treatment-related gaps in the HCV cascade (fig. 2): 23.9% (49/205) had never been screened for HCV before (table 2); 18.9% of the 95 HCV-positive patients had no HCV RNA test; of the 61 patients developing chronic HCV infection, 19.7% were not HCV genotyped, 52.5% had no liver fibrosis assessment and 54.1% never received treatment; 25.0% of the 28 patients ever treated did not achieve a sustained virological response.
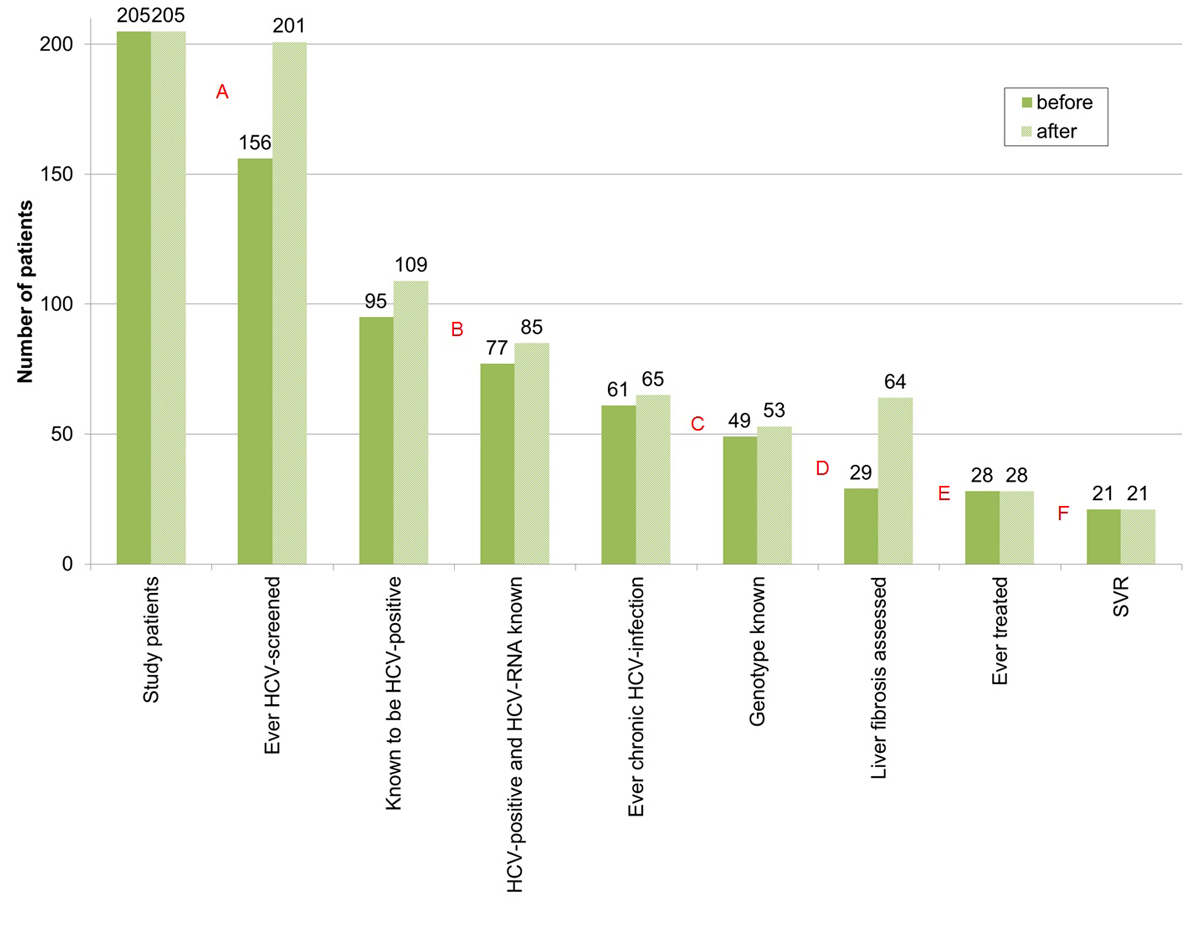
Figure 2 Diagnostic and treatment-related gaps in the HCV cascade (total study population), before and after HCV rapid tests and Fibroscan® within our study.
HCV = hepatitis C virus; SVR = sustained virological response; before = before HCV rapid tests and Fibroscan® within our study; after = after HCV–rapid tests and Fibroscan® within our study
A = first diagnostic gap – no HCV antibody screening: 23.9% (49/205) → 2.0% (2/205); B = second diagnostic gap – no HCV RNA determination, if HCV positive: 18.9% (18/95) → 22% (24/109); C = third diagnostic gap – HCV genotype unknown, if ever chronic HCV infection: 19.7% (12/61) → 18.5% (12/65); D = fourth diagnostic gap – no liver fibrosis assessment, if ever chronic HCV infection: 52.5% (32/61) → 1.5% (1/65). E = treatment uptake gap – never treated, if ever chronic HCV infection: 54.1% (33/61) → 56.9% (37/65). F = treatment success gap – treated, but no sustained virological response: 25.0% (7/28) → 25.0% (7/28).
Table 2 HCV management – decentralised vs centralised setting.
|
Total
(n = 205) |
Decentralised*
(n = 104) |
Centralised†
(n = 101) |
p-value | ||||
|---|---|---|---|---|---|---|---|
| HIV/HCV screening prior to the study | |||||||
| HIV test prior to study | 74.1% (152) | 65.4% (68) | 83.2% (84) | 0.004 | |||
| If HIV negative: | |||||||
| Never tested before | 26.7% (50/187) | 34.0% (33/97) | 18.9% (17/90) | 0.020 | |||
| Last test ≤1 year ago | 19.8% (37/187) | 10.3% (10/97) | 30.0% (27/90) | <0.001 | |||
| HCV antibody test prior to study | 76.1% (156) | 66.3% (69) | 86.1% (87) | <0.001 | |||
| If HCV negative: | |||||||
| Never tested before | 39.4% (37/94) | 48.2% (26/54) | 27.5% (11/40) | 0.043 | |||
| Last test ≤1 year ago | 23.4% (22/94) | 14.8% (8/54) | 35.0% (14/40) | 0.022 | |||
| If HCV positive and HCV-RNA negative: | |||||||
| HCV-RNA ≤1 year ago | 40.0% (24/40) | 31.6% (6/19) | 47.6% (10/21) | 0.301 | |||
| HCV rapid test (performed during the study) | |||||||
| HCV rapid test performed | 93.7% (192) | 93.3% (97) | 94.1% (95) | 0.816 | |||
| New HCV diagnosis | 7.3% (14/192) | 9.3% (9/97) | 5.3% (5/95) | 0.285 | |||
| False negative | 5.2% (10/192) | 6.2% (6/97) | 4.2% (4/95) | 0.538 | |||
| HIV/HCV-prevalence | |||||||
| HIV seropositivity | 7.4% (15/202) | 4.0% (4/101) | 10.9% (11/101) | 0.060 | |||
| HCV seropositivity | 53.7% (109/203) | 47.1% (48/102) | 60.4% (61/101) | 0.057 | |||
| HCV mono-infected | 46.0% (93/202) | 42.6% (43/101) | 49.5% (50/101) | 0.323 | |||
| HIV-HCV co-infected | 7.4% (15/202) | 4.0% (4/101) | 10.9% (11/101) | 0.060 | |||
| HIV and HCV negative | 46.5% (94/202) | 53.5% (54/101) | 39.6% (40/101) | 0.048 | |||
| Liver biopsy prior to the study | |||||||
| Liver biopsy, if ever chronic HCV infection | 47.5% (29/61) | 57.1% (12/21) | 42.5% (17/40) | 0.277 | |||
| ≥F2, if result available | 57.1% (12/21) | 50.0% (4/8) | 61.5% (8/13) | 0.604 | |||
| Liver biopsy, if currently HCV-RNA positive | 36.4% (16/44) | 38.5% (5/13) | 35.5% (11/31) | 0.851 | |||
| ≥F2, if result available | 50.0% (7/14) | 40.0% (2/5) | 55.6% (5/9) | 0.577 | |||
| Fibroscan® (performed during the study) | |||||||
| Fibroscan® performed | 81.5% (167) | 69.2% (72) | 94.1% (95) | <0.001 | |||
| Sufficient quality‡ | 94.6% (158/167) | 93.1% (67/72) | 95.8% (91/95) | 0.438 | |||
| F0/1 (≤7.0 kPa) | 71.5% (113/158) | 65.7% (44/67) | 75.8% (69/91) | 0.162 | |||
| F2 (>7.0 and ≤9.5 kPa) | 10.1% (16/158) | 13.4% (9/67) | 7.7% (7/91) | 0.237 | |||
| F3/4 (>9.5 kPa) | 18.4% (29/158) | 20.9% (14/67) | 16.5% (15/91) | 0.479 | |||
| Fibroscan® performed, if currently HCV-RNA positive | 93.2% (41/44) | 76.9% (10/13) | 100% (31/31) | 0.006 | |||
| Sufficient quality‡ | 95.1% (39/41) | 100% (10/10) | 93.5% (2/31) | 0.410 | |||
| F0/1 (≤7.0 kPa) | 59.0% (23/39) | 40% (4/10) | 65.5% (19/29) | 0.157 | |||
| F2 (>7.0 and ≤9.5 kPa) | 10.3% (4/39) | 20% (2/10) | 7.0% (2/29) | 0.239 | |||
| F3/4 (>9.5 kPa) | 30.8% (12/39) | 40% (4/10) | 27.6% (8/29) | 0.463 | |||
| ≥7.5 kPa | 38.5% (15/39) | 60% (6/10) | 31.0% (9/29) | 0.105 | |||
| HCV – further evaluation and treatment | |||||||
| Median age (y) at HCV diagnosis (IQR) | 29 (24-38) (n = 102) |
34 (26-39) (n = 45) |
28 (23-33) (n = 57) |
0.076 | |||
| Years between first IDU and HCV diagnosis (IQR) | 9 (4-16) (n = 92) |
9 (6-22) (n = 35) |
8 (3-13) (n = 57) |
0.088 | |||
| HCV-RNA test, if HCV positive | 78.0% (85/109) | 68.8% (33/48) | 85.2% (52/61) | 0.039 | |||
| Currently RNA positive | 51.8% (44/85) | 39.4% (13/33) | 59.6% (31/52) | 0.069 | |||
| Genotype available | 86.4% (38/44) | 76.9% (10/13) | 90.3% (28/31) | 0.237 | |||
| Genotype distribution: | 0.703 | ||||||
| Genotype 1 | 47.4% (18/38) | 60% (6/10) | 42.9% (12/28) | ||||
| Genotype 2 | 2.6% (1/38) | 0% (0/10) | 3.6% (1/28) | ||||
| Genotype 3 | 31.6% (12/38) | 20% (2/10) | 35.7% (10/28) | ||||
| Genotype 4 | 18.4% (7/38) | 20% (2/10) | 17.9% (5/28) | ||||
| Spontaneous clearance | 23.5% (20/85) | 33.3% (11/33) | 17.3% (9/52) | 0.090 | |||
| HCV treatment-uptake, if no SC | 43.1% (28/65) | 45.5% (10/22) | 41.9% (18/43) | 0.782 | |||
| SVR | 75.0% (21/28) | 80% (8/10) | 72.2% (13/18) | 0.649 | |||
| Hepatitis A/B | |||||||
| HAV serology available | 38.5% (79) | 32.7% (34) | 44.6% (45) | 0.081 | |||
| Immunity (after infection or vaccination) | 54.4% (43/79) | 55.9% (19/34) | 53.3% (24/45) | 0.821 | |||
| Interpretable HBV serology available§ | 48.8% (100) | 41.3% (43) | 56.4% (57) | 0.031 | |||
| Immunity | |||||||
| After vaccination¶ | 21.0% (21/100) | 16.3% (7/43) | 24.6% (14/57) | 0.314 | |||
| After infection** | 40.0% (40/100) | 39.5% (17/43) | 40.4% (23/57) | 0.934 | |||
| Unclear†† | 6.0% (6/100) | 11.6% (5/43) | 1.8% (1/57) | 0.040 | |||
| No‡‡ | 33.0% (33/100) | 32.6% (14/43) | 33.3% (19/57) | 0.935 | |||
HAV = hepatitis A virus; HBV = hepatitis B virus; HCV = hepatitis C virus; HIV = human immunodeficiency virus; IQR = interquartile range; IDU = intravenous drug use; SC = spontaneous clearance; SVR = sustained virological response * Recruitment in family practice (n = 101) or pharmacy (n = 3) † Recruitment in heroin substitution programme (n = 51), addiction clinic (n = 29) or Infectious Diseases Outpatient Clinic of the Cantonal Hospital Aarau (n = 21) ‡ Success rate >60% and IQR/median <30% § 13 HBV serology results not interpretable regarding immunity (decentralised: 12, centralised: 1) ¶ Anti-HBs positive/>10 U/l and anti-HBc negative ** anti-HBc positive †† Immunity after vaccination or infection (anti-HBs positive/>10 U/l and anti-HBc unknown) ‡‡ Anti-HBs and anti-HBc negative
Performing HCV rapid tests and Fibroscans® within our study, we reduced the percentage without HCV screening to 2.0% (4/205) and the proportion of ever chronically HCV infected without liver fibrosis assessment to 1.5% (1/65) (fig. 3). Fourteen patients were newly diagnosed with the HCV rapid test (tables 3, 4 and 5 ), increasing the number known to be HCV positive to 109. Of the newly diagnosed patients, eight had immediate further evaluation with HCV RNA testing. HCV genotype was determined in all four HCV RNA-positive patients.
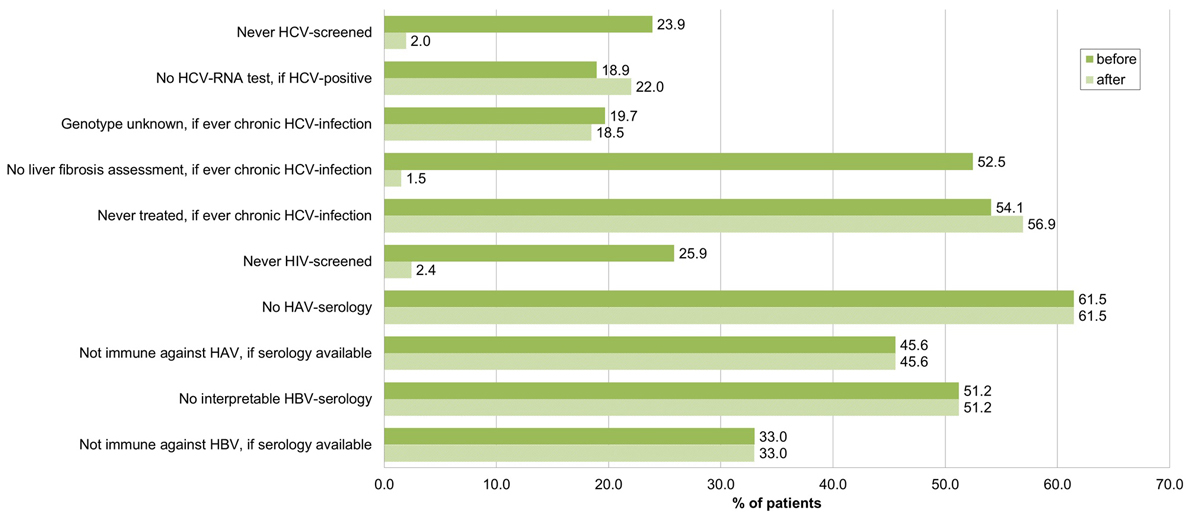
Figure 3 Proportion of patients not evaluated according to guidelines (total study population, n = 205), before and after HCV–rapid tests and Fibroscan® within our study.
HCV = hepatitis C virus; HIV = human immunodeficiency virus; HAV = hepatitis A virus; HBV = hepatitis B virus; before = before HCV rapid tests and Fibroscan® within our study, after = after HCV rapid tests and Fibroscan® within our study
Table 3 Characteristics of the 14 new HCV diagnoses made by rapid test.
| Age (y) | Sex | Ever IDU | First IDU | First HCV diagnosis | Prior negative HCV test | HCV RNA (Genotype) | Fibroscan® (kPa) |
|---|---|---|---|---|---|---|---|
| 34 | M | 1 | ? | 2013 (D) | 2008 | ? | 6.1 |
| 44 | M | 1 | 1990 | 2013 (D) | – | 14468 (1a) | – |
| 42 | F | 1 | 1988 | 2013 (D) | – | 0 | – |
| 47 | F | 0 | – | 2013 (D) | – | 0 | 7.5 |
| 38 | M | 1 | 1992 | 2013 (D) | – | ? | 6.4 |
| 29 | M | 1 | 2007 | 2013 (D) | 2012 | ? | – |
| 46 | M | 0 | – | 2013 (D) | – | 0 | 6.1 |
| 22 | M | 1 | 2003 | 2013 (C) | – | ? | 5.4 |
| 42 | M | 1 | 1984 | 2014 (C) | 2013 | 636000 (1a) | 4.0 |
| 38 | M | 1 | 2000 | 2014 (D) | – | ? | – |
| 45 | M | 1 | 1989 | 2014 (C) | 2000 | 2580000 (3) | 6.8 |
| 51 | M | 1 | 1987 | 2014 (C) | – | 0 | 4.9 |
| 39 | M | 1 | 1991 | 2015 (D) | – | ? | 6.9 |
| 27 | M | 1 | 2015 | 2015 (C) | – | 3120 (4) | 6.8 |
M = male, F = female, IDU = intravenous drug use, 1 = yes, 0 = no, HCV = hepatitis C virus, (D) = decentralised setting, (C) = centralised setting, ? = unknown All 14 patients were human immunodeficiency virus negative.
Table 4 Characteristics of the 10 patients with false negative HCV rapid tests.
| Age (y) | Sex | Ever IDU | First IDU | First HCV-diagnosis | HCV treatment | HCV–RNA |
|---|---|---|---|---|---|---|
| 35 | F | 1 | 1996 | 2012 | – | 0 |
| 50 | M | 1 | 1981 | 1997 | – | 0 |
| 40 | M | 0 | – | 2001 | – | 0 |
| 42 | F | 1 | 1987 | 2012 | ? | 0 |
| 48 | F | 1 | 1983 | 1992 | – | 0 |
| 40 | M | 1 | ? | 1991 | 1993, SVR | 0 |
| 43 | M | 1 | 1996 | 2012 | – | 0 |
| 48 | M | 1 | ? | 1997 | – | ? |
| 40 | F | 1 | 1995 | 1996 | 1996, SVR | 0 |
| 39 | M | 1 | 2001 | 2009 | – | ? |
M = male, F = female, IDU = intravenous drug use, 1 = yes, 0 = no, HCV = hepatitis C virus, ? = unknown, SVR = sustained virological response All 10 patients were HIV negative.
Table 5 Performance of the HCV antibody rapid test (OraQuick®).
| HCV serology | ||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| HCV rapid test | Positive | (a) 94 | (b) 0 | 94 |
| Negative | (c) 10 | (d) 88 | 98 | |
| Total | 104 | 88 | 192 | |
Prevalence = (a+c)/(a+b+c+d) = 104/192 = 54.2% (95% CI 47.1–61.1%) Sensitivity = a/(a+c) = 94/104 = 90.4% (95% CI 84.7–96.1%) (proportion of HCV antibody positives, correctly identified by the rapid test) Specificity = d/(b+d) = 88/88 =100% (proportion of HCV antibody negatives, correctly identified by the rapid test) PPV (positive predictive value) = a/(a+b) = 94/94 = 100% (proportion of individuals with a positive rapid test result, who is actually HCV antibody positive) NPV (negative predictive value) = d/(c+d) = 88/98 = 89.8% (95% CI 83.8–95.8%) (proportion of individuals with a negative rapid test result, who is actually HCV antibody negative)
Sixty-five patients who had ever had chronic HCV infection minus 21 successfully treated resulted in 44 patients still HCV RNA positive and thus in need of treatment (supplementary fig. S1 in appendix 1). For 6 (13.6%), the HCV genotype was unknown. Forty-three (97.7%) had either liver biopsy or Fibroscan®, but only 15 (34.1%) had fibrosis stage ≥F2 or Fibroscan® ≥7.5 kPa (requirement for direct-acting antiviral reimbursement in Switzerland) (table 6). However, all but one (97.7%) reported former or current intravenous drug use, which since May 2017 qualifies for direct-acting antiviral reimbursement irrespective of liver fibrosis stage.
Table 6 Currently HCV–RNA positive patients qualifying for reimbursement of interferon–free DAA (Direct–acting antivirals) treatment in Switzerland.
| Currently HCV RNA positive patients | p–value | |||
|---|---|---|---|---|
|
Total
(n = 44) |
Decentralised*
(n = 13) |
Centralised†
(n = 31) |
||
| Fibroscan® ≥7.5 kPa or Metavir ≥F2 in liver biopsy | 34.1% (15) | 46.2% (6) | 29.0% (9) | 0.274 |
| Irrespective of liver fibrosis: | ||||
| IDU‡ | 97.7% (43) | 92.3% (12) | 100.0% (31) | 0.118 |
| HIV‡ | 22.7% (10) | 23.1% (3) | 22.6% (7) | 0.971 |
| Genotype 1 or 4§ | 56.8% (25) | 61.5% (8) | 54.8% (17) | 0.682 |
n = number, IDU = intravenous drug use, HCV = Hepatitis C virus, HIV = Human immunodeficiency virus, DAA = Direct–acting antivirals * Recruitment in family practice (n = 12) or pharmacy (n = 1) † Recruitment in heroin substitution program (n = 11), addiction clinic (n = 8) or Infectious Diseases Outpatient Clinic of the Cantonal Hospital Aarau (n = 12) ‡ Since May 2017 § Since July 2017
The proportion never before screened for HCV was significantly higher in the decentralised setting (33.7 vs 13.9%, p <0.001) (figs 4 and 5 , table 2). Of the 14 new HCV diagnoses (table 3), 9 (64.3%) were from the decentralised and 5 (35.7%) from the centralised setting. Four of five patients in the centralised setting, but only four of nine patients in the decentralised setting were immediately further evaluated with HCV RNA testing, increasing the proportion of HCV-positive individuals without an HCV RNA test in the decentralised setting to 31.3%, compared with 14.8% in the centralised setting (p = 0.039). Including the four HCV RNA-positive new HCV diagnoses with known HCV genotype (three from the centralised and one from the decentralised setting), the percentage of HCV RNA-positive patients with unknown HCV genotype was significantly higher in the decentralised setting (31.8 vs 11.6%, p = 0.047). Concerning liver fibrosis assessment, and HCV treatment uptake and outcome, there were no setting-specific differences.
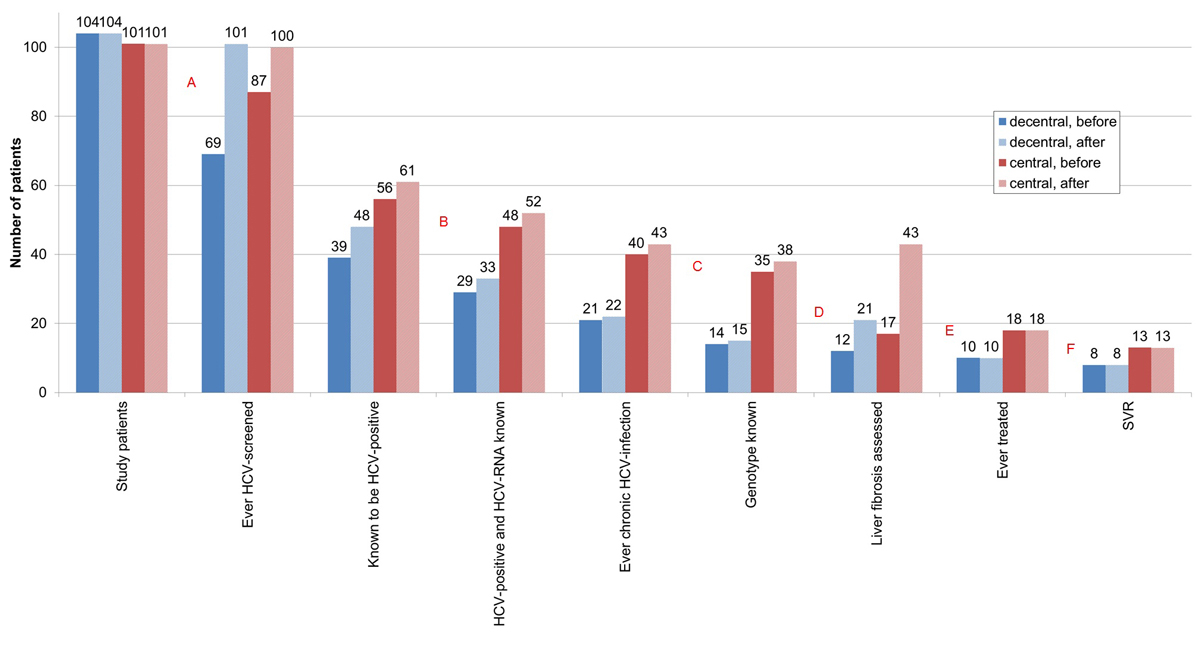
Figure 4 HCV cascade according to place of recruitment (decentralised versus centralised setting), before and after HCV rapid tests and Fibroscan® within our study.
HCV = hepatitis C virus; SVR = sustained virological response; before = before HCV rapid tests and Fibroscan® within our study; after = after HCV rapid tests and Fibroscan® within our study
A = first diagnostic gap – no HCV antibody screening: 33.7% (35/104) vs 13.9% (14/101) (p<0.001) → 2.9% (3/104) vs 1.0% (1/101) (p=0.327); B = second diagnostic gap – no HCV RNA determination, if HCV positive: 25.6% (10/39) vs 14.3% (8/56) (p = 0.165) → 31.3% (15/48) vs 14.8% (9/61) (p=0.039); C = third diagnostic gap – HCV genotype unknown, if ever chronic HCV infection: 33.3% (7/21) vs 12.5% (5/40) (p=0.052) → 31.8% (7/22) vs 11.6% (5/43) (p=0.047); D = fourth diagnostic gap – no liver fibrosis assessment, if ever chronic HCV infection: 42.9% (9/21) vs 57.5% (23/40) (p = 0.277) → 4.5% (1/22) vs 0.0% (0/43) (p = 0.159). E = treatment uptake gap – never treated, if ever chronic HCV infection: 52.4% (11/21) vs 55.0% (22/40) (p = 0.845) → 54.5% (12/22) vs 58.1% (25/43) (p = 0.782). F = treatment success gap – treated, but no sustained virological response: 20.0% (2/10) vs 27.8% (5/18) (p = 0.649) → 20.0% (2/10) vs 27.8% (5/18) (p = 0.649).
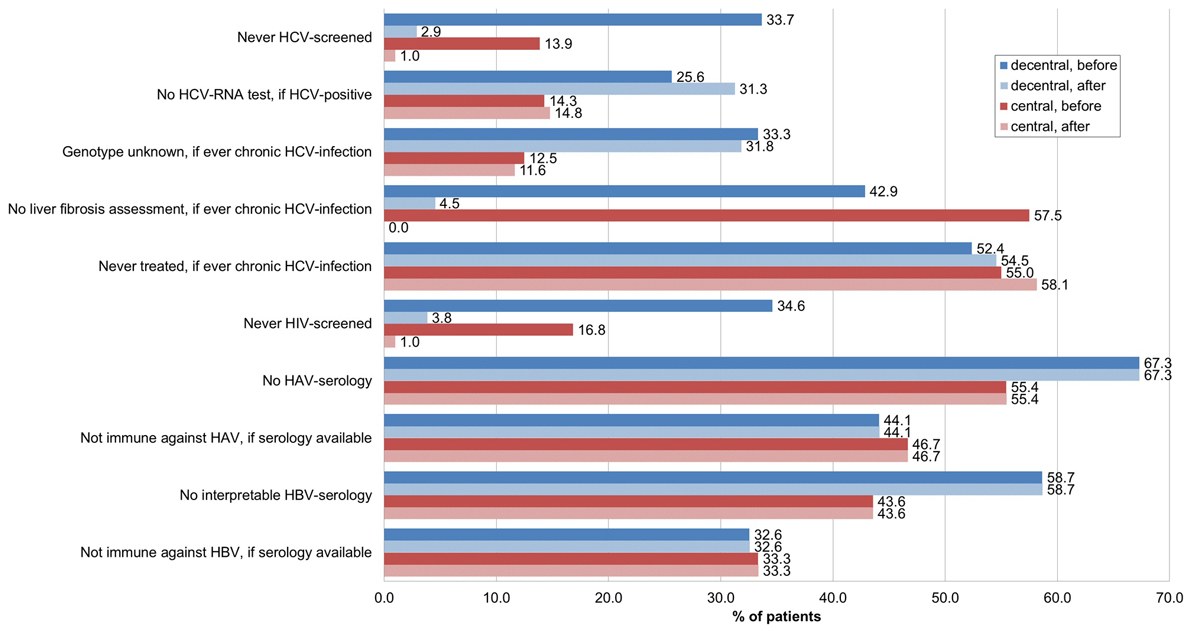
Figure 5 Noncompliance with guidelines according to place of recruitment – decentralised (n = 104) versus centralised setting (n = 101) – before and after HCV rapid tests and Fibroscan® within our study.
HCV = hepatitis C virus; HIV = human immunodeficiency virus; HAV = hepatitis A virus; HBV = hepatitis B virus; decentral = decentralised setting (n=104); central = centralised setting (n=101); before = before HCV rapid tests and Fibroscan® within our study; after = after HCV rapid tests and Fibroscan® within our study
In the decentralised setting, 22 patients ever chronically HCV infected minus 8 with sustained virological response resulted in 13 (rather than 14) patients currently HCV RNA positive (supplementary fig. S1), because one patient who prematurely stopped HCV treatment was HCV RNA negative after 3 months of treatment (last HCV RNA available), but no check-up for sustained viral response was performed. In the centralised setting, 43 patients who had ever had chronic HCV infection minus 13 with sustained virological response resulted in 31 (rather than 30) patients currently HCV RNA-positive, because one patient with a sustained virological response was reinfected.
To investigate whether the quality of HCV work-up depends on caseload, we compared the HCV cascades of patients cared for by physicians with <10 versus ≥10 drug substitution patients in the decentralised setting (supplementary table S1 in appendix 1). With the physicians having <10 drug substitution patients (90.1% of all 161 physicians and 49.4% of all 631 patients), a higher proportion of patients had HCV RNA testing if HCV positive (100 vs 64.3%, p = 0.022) and liver fibrosis assessment by liver biopsy if ever chronically HCV infected (85.7 vs 42.9%, p = 0.061). However, patients and physicians from the <10 drug substitution patients/physician group were underrepresented in the decentralised setting (table S2).
Overall, 25.9% (53/205) had never been HIV screened before (fig. 3). The proportion was twice as high in the decentralised than in the centralised setting (34.6 vs 16.8%, p = 0.004) (fig. 5, table 2). Offering HIV rapid tests within our study, we reduced the proportion never HIV screened to 2.4% (5/205).
Yearly HIV and HCV screening in seronegative patients was also more rarely performed in the decentralised setting (10.3 vs 30.0%, p <0.001 and 14.8 vs 35.0%, p = 0.022, respectively) (table 2). Of the HCV-positive patients with negative HCV RNA in the decentralised setting, 36.6% had had an HCV RNA determination ≤1 year ago, compared with 47.6% in the centralised setting (p = 0.301).
The proportion with known hepatitis A virus (HAV) and hepatitis B virus (HBV) serostatus was lower in the decentralised setting (32.7 vs 44.6%, p = 0.081 and 41.3 vs 56.4%, p = 0.031, respectively) (table 2, fig. 5). Among those with available HAV/HBV serology, 54.4% were immune to HAV and 67.0% to HBV (59.7% of them after infection, only 31.3% after vaccination), with no setting-specific differences. None of the patients had chronic hepatitis B (positive HBs-antigen).
Of the 192 HIV rapid tests, all 13 from patients with known HIV infection were positive (6.8%) (2 HIV positive patients not tested). There were no new diagnoses.
Of the 192 HCV rapid tests, 94 (49.0%) were positive. Fourteen HCV infections were newly diagnosed (table 3). Ten test results were false negatives (table 4).
In 8/10 patients with a false negative HCV rapid test, HCV RNA was undetectable (2 unknown), either due to spontaneous clearance or previous successful HCV treatment. The presumed date of infection (in most cases shortly after the first intravenous drug use [43, 44]) was more than 10 years ago.
Due to 10 false negative results, sensitivity was 90.4% (95% CI 84.7–96.1%) and the negative predictive value 89.8% (95% CI 83.8–95.8%), whereas specificity and positive predictive value were 100% (no false positive results) (table 5).
HIV and HCV seroprevalence was 7.4 and 53.7%, respectively (table 2). Both were higher in the centralised setting (10.9 vs 4.0%, p = 0.060 and 60.4 vs 47.1%, p = 0.057, respectively). HIV infection only occurred as HIV/HCV co-infection (no HIV mono-infection) and without any new diagnoses since 2008.
The proportion of HIV/HCV-negative patients was higher in the decentralised setting (53.5 vs 39.6%, p = 0.048).
Of the 167 Fibroscans® performed, 158 (94.6%) were of sufficient quality (success rate >60% and interquartile range/median <30%). For all 8 patients with a success rate ≤60%, the body mass index (BMI) was elevated (four overweight [BMI 25–29.9 kg/m2], one with class I obesity [BMI 30–34.9 kg/m2], one with class II obesity [BMI 35–39.9 kg/m2], two with class III obesity [BMI ≥40 kg/m2]).
Overall (n = 158), 71.5% had no/mild fibrosis (F0/1), 10.1% significant fibrosis (F2), 7.0% severe fibrosis (F3) and 11.4% cirrhosis (F4) (table 2, fig. 6).

Figure 6 Fibroscan® results and proportion with severe fibrosis/cirrhosis (Metavir F3/4; liver stiffness >9.5 kPa) in different risk groups.
HCV ab– = hepatitis C virus antibody negative; HCV ab+ = hepatitis C virus antibody positive; HIV ab– = human immunodeficiency virus antibody negative; HIV ab+ = human immunodeficiency virus antibody positive; Alc ≤40 = alcohol intake ≤40 g/d; Alc >40 = alcohol intake >40 g/d
F3/4 prevalence was five-fold higher in HCV antibody-positive patients (28.4 vs 5.7%, p <0.001) and three-fold higher with current alcohol consumption >40 g/d (35.7 vs 12.3%, p <0.001). It was highest with HIV/HCV co-infection (50.0%) and HCV infection plus current alcohol consumption >40 g/d (44.8%). Both HIV co-infection and alcohol overconsumption doubled the risk of severe fibrosis/cirrhosis in HCV positive individuals (RR 2.06, 95% CI 1.06–3.98 and RR 2.17, 95% CI 1.14–4.13, respectively).
Among patients currently HCV RNA positive (n = 39), F3/4-prevalence was 30.8%, 10.3% had F2 and 59.0% F0/1.
In the centralised setting (questionnaire, rapid tests and Fibroscan® during the same session), Fibroscan® coverage was 94.1%, compared with 69.2% in the decentralised setting (Fibroscan® at a separate appointment, mostly in a different place).
Overall, there were 15 HIV-infected patients (diagnosed 1986–2008), 11 in the centralised and 4 in the decentralised setting. All were HCV co-infected and under antiretroviral therapy. HIV RNA was fully suppressed in 13 (86.7%). One patient had 143 copies/ml (blip) and another 5020 copies/ml (antiretroviral therapy restarted). In all patients, last CD4 count was ≥350/µl, in 86.7%, it was ≥500/µl.
Although the median age at first intravenous drug use was similar in the decentralised and centralised setting (20 vs 19 years; table 1), the median age at HCV diagnosis was 6 years higher in the decentralised setting (34 vs 28 years, p = 0.076) (table 2).
Including the 14 new HCV diagnoses, 24/109 (22.0%) HCV seropositive patients had no HCV-RNA test, and 6/44 (13.6%) currently HCV-RNA positive individuals no HCV genotyping.
The spontaneous HCV clearance rate was higher in the decentralised setting (33.3 vs 17.3%, p = 0.090), reflecting a higher proportion of women with higher spontaneous clearance rates (36.8% [7/19] vs 19.7% [13/66]) and a lower proportion of HIV-positive patients with lower spontaneous clearance rates (13.3% [2/15] vs 25.7% [18/70]). In those without spontaneous clearance, HCV treatment uptake was 43.1%, with no significant differences regarding setting, gender and HIV serostatus.
All 28 HCV treatments (6 HIV co-infected patients) consisted of (pegylated) interferon with or without ribavirin. Two patients with HCV genotype 1 additionally received telaprevir and boceprevir, respectively. Overall, treatment outcome was: 75.0% sustained virological response (3 HIV co-infected), 7.1% relapse (1 HIV co-infected), 3.6% viral breakthrough, 3.6% nonresponse and 10.7% preterm stop (2 HIV co-infected). There were no gender-specific differences regarding the sustained virological response rate, but it was lower in HIV-positive individuals: 50.0% (3/6) vs 81.8% (18/22), p = 0.111. Sustained virological response rates according to genotype were: genotype 1 72.7% (8/11), genotype 3 87.5% (7/8) and genotype 4 0% (0/2) (in 7 genotype was unknown).
Among currently HCV RNA-positive patients (still in need of treatment), genotype distribution (n = 38) was: 47.4% genotype 1 (12 1a, 2 1b, 4 subtype unknown, 15/18 treatment-naïve, 6 HIV co-infected), 2.6% genotype 2 (1 treatment-naïve HIV co-infected), 31.6% genotype 3 (10/12 treatment-naïve, 3 HIV co-infected) and 18.4% genotype 4 (5/7 treatment-naïve).
Our study in decentralised and centralised drug substitution programmes of the canton Aargau, a mixed urban and rural area in Northwestern Switzerland with about 650 000 inhabitants, revealed four diagnostic- and two treatment-related gaps in the HCV cascade: 23.9% had never been HCV screened before; 18.9% of the HCV positive patients had no HCV RNA test; Of the chronically HCV-infected patients, 19.7% were not HCV genotyped, 52.5% had no liver fibrosis assessment, and 54.1% never received treatment; 25.0% of the treated patients did not achieve sustained virological response.
Gaps in the HCV cascade are also described in other parts of the world. In Southern China, HCV test uptake in 45 methadone clinics was 78% [45]. In British Columbia, Canada, 74% of HCV-positive persons had an HCV RNA test and of those who were HCV RNA positive, 85% were genotyped, 28% treated and 17% achieved sustained virological response [46]. In the US in 2014, 50% of the estimated number of people with chronic HCV infection were diagnosed, 27% HCV RNA confirmed, 17% liver fibrosis staged and 16% treated, and 9% had sustained virological response [47]. In Australia, 75% of the estimated number of the chronically HCV infected were diagnosed, 20% ever treated and 11% cured [48].
Offering free HCV antibody rapid tests (OraQuick®) [38] on capillary blood (finger-stick), we could easily close the first diagnostic gap “no HCV antibody screening”. With 14 newly diagnosed HCV infections among 102 patients assumed to be HCV negative, the number needed to test was 8. For 8 of the 10 HCV-infected patients missed by this test (sensitivity: 90.4%), HCV RNA was known. All were HCV RNA negative, due to either spontaneous clearance of the virus or successful treatment, and thus neither infectious nor in need of treatment. The presumed date of infection, shortly after first intravenous drug use [43, 44], was more than 10 years ago. Assuming early viral clearance, it is imaginable that HCV antibody titres had decreased below the limit of detection in the absence of HCV RNA as an immunostimulant [49–51].
The second diagnostic gap “no HCV RNA determination, if HCV positive” still needs to be closed. Possible methods using capillary blood are dried blood spot [52] and GeneXpert® point-of-care testing [53–55]. Providing results within 60–105 minutes, the latter might improve linkage to care [56, 57]. None of these methods has been approved so far, restricting access to study settings.
In the context of direct-acting antivirals effective against all HCV genotypes, such as sofosbuvir/velpatasvir [58], the third diagnostic gap “no HCV genotype, if HCV RNA positive” becomes less important [59].
The fourth diagnostic gap refers to liver fibrosis assessment. Liver biopsy is an invasive method with relevant adverse effects: 20% pain, 0.5% major complications (e.g., bleeding, haemobilia), and 0.01–0.1% mortality [60, 61], and thus was often refused or not repeated. Only 36.4% of the 44 currently HCV RNA-positive patients had undergone liver biopsy, with >50% of the results being >3 years old. Offering noninvasive liver fibrosis staging with mobile Fibroscan®, we could efficiently close this diagnostic gap. The acceptance rate was especially high (94.1%) if the examination was offered in one session together with questionnaire and rapid tests (“one-stop strategy”).
At 45.9%, HCV treatment uptake was higher than the 23.1% in three drug substitution programmes in St Gallen in 2009, comparable to the 37.5% among drug substitution patients in the Swiss Hepatitis C Cohort Study (SCCS) in 2010, but lower than the 64.5% among nonusers of injectable drugs in the SCCS in 2010 [33]. Low treatment success rates (40–80%) and side effects of the (pegylated) interferon / ribavirin treatment [62] explain the low treatment uptake in earlier years. Besides, PWID have long been perceived to have lower adherence, more side effects and lower treatment response rates than nonusers of injectable drugs, which is not true [27–29]. Even in patients with active intravenous drug use, the reinfection rate is as low as 2–6/100 patient-years [63]. In 2002, international consensus guidelines for the first time did not advise against HCV treatment in PWID and heroin/methadone recipients [64–66]. In the 2016 guidelines, treatment of patients with active intravenous drug use is even a priority because of their risk of transmitting HCV [67].
The WHO aims at eliminating hepatitis C as a public health threat by the year 2030 [68]. Mathematic models have shown that needle and syringe programmes and opioid substitution therapy alone are not sufficient to prevent new infections, but more PWID must be treated [69]. According to the WHO, treatment uptake should be increased to 80% [68].
Increased treatment uptake is facilitated by the availability of new potent drugs (directly acting antivirals) that close the treatment success gap (90–100% sustained virological response for all genotypes) with virtually no side effects, shorter duration of treatment (8–12 instead of 24–48 weeks) and the convenience of one pill once daily [17, 70].
Reimbursement restrictions are another important barrier to HCV treatment [23]. Until recently, access to direct-acting antivirals in Switzerland was universally restricted to patients with at least significant fibrosis (Metavir F2) documented either once in a liver biopsy or twice with Fibroscan® (≥7.5 kPa) at least 3 months apart. About two thirds of our currently HCV RNA-positive patients did not fulfil this criterion. Since 1 May 2017, direct-acting antiviral reimbursement in Switzerland is extended to HIV- or HBV-co-infected patients and to PWID irrespective of fibrosis stage. For injectable drug users, opioid substitution /addiction therapy and directly observed therapy are required [24] (www.spezialitätenliste.ch). Thus, 97.9% of our currently HCV RNA-positive patients can now be treated.
Compliance with guidelines was worse in the decentralised setting (family practices / pharmacies) than in the centralised setting (heroin substitution programme, addiction clinics, Infectious Diseases Outpatient Clinic). A higher proportion of patients had no HIV and HCV screening test, no HCV RNA test if HCV positive, no HCV genotype if HCV RNA positive, and no HAV and HBV serology. Independent of setting, 45.6% lacked immunity against HAV and 33.0% against HBV, and thus should be vaccinated.
Care in the decentralised setting was remarkably stable. At 5.9 vs 0.6 years, the median time spent in the programme was significantly longer and half of the patients appeared only once a week or even less frequently to pick up the substitution drugs. In a qualitative study in the UK, HCV diagnosis delay was attributed to, among other factors, GP inaction. Diagnosis involved a change of healthcare providers or a chance medical encounter [71]. To counter this problem, the yearly renewal of opioid substitute prescription could be linked to an obligation to screen and document patients for HIV and viral hepatitis.
In our patients (77.8% ever intravenous drug users), HCV antibody prevalence was 53.7% and HCV RNA prevalence 27.8%. HIV infection only occurred as HIV/HCV co-infection and had a prevalence of 7.4%. The lower proportion of ever intravenous drug users in the decentralised setting correlated with a lower HCV and HIV prevalence. Remarkably, there were no HIV diagnoses in our patients since 2008, suggesting that opioid substitution, needle and syringe programmes, and antiretroviral therapy (100% of our patients) efficiently prevent HIV transmission. In contrast, HCV transmission is still ongoing. Two of the 14 newly diagnosed HCV infections had occurred during the previous year. In addition to sharing contaminated needles and syringes, HCV can also be transmitted by sharing spoons, water, filter [72] or snorting straws for intranasal drug use [73].
Of the 631 patients living and receiving opioid substitution in the canton Aargau, only one third could be enrolled. It is unclear whether time constraints on the treating physicians, difficulties with the provided capillary blood tests, the unwillingness of the patients or other reasons resulted in this low recruitment rate. It must be assumed that providers of opioid substitution therapy participating in our study are more involved in the management of hepatitis C than those not participating (response bias). Thus, compliance with guidelines is rather overestimated and might be worse in nonresponders.
Patients in the decentralised setting were mainly recruited during the first year (July 2013 to June 2014) and patients in the centralised setting during the second year (July 2014 to June 2015). Although, the first interferon-free direct-acting antiviral treatments were approved in Switzerland during the second year – sofosbuvir in August 2014, ledipasvir/sofosbuvir in February 2015, ritonavir-boosted paritaprevir, ombitasvir and dasabuvir in February 2015 [17] – none of the patients was treated with these drugs.
For patients recruited by GPs, we had no access to the medical records to verify the information given in the questionnaire allowing reporting bias.
Another ~170 patients live in the canton Aargau, but receive their opioid substitution in another canton, and have therefore not been considered for this study.
Data of our cross-sectional study as well as follow-up data contribute to the Swiss Association for the Medical Management in Substance User (SAMMSU) cohort study (www.sammsu.ch), which will provide a nationwide picture of the hepatitis C management in drug substitution programs.
Overall, 53.7% of our drug substitution patients were HCV-antibody positive, with a 27.8% prevalence of active infection. Whereas HCV transmission is still ongoing, HIV has not been newly diagnosed since 2008.
Measured against the guidelines, management of hepatitis C was worse in the decentralised setting (family practices / pharmacies) than in the centralised setting (heroin substitution programme, addiction clinics, Infectious Diseases Outpatient Clinic). Access to these patients needs improvement, ideally linked to the yearly renewal of the opioid substitution therapy prescription.
HCV screening with the HCV antibody rapid test Oraquick® using finger-prick blood and liver fibrosis assessment with mobile Fibroscan® are convenient methods to close two of four diagnostic gaps in the HCV cascade. Acceptance of these minimally invasive point-of-care diagnostics is highest if they are offered as a “one-stop strategy”. HCV RNA determination in capillary blood is still an unmet need in this population with destroyed peripheral veins.
The new direct acting antivirals, characterised by shorter treatment duration, virtually no side effects, 90 to 100% sustained virological response rate for all genotypes, irrespective of HIV status [74], high convenience (one pill a day) and pangenotypic activity, can close the treatment success gap and facilitate treatment-uptake. Restricting reimbursement to advanced liver fibrosis withholds treatment from two thirds of the currently HCV RNA positive individuals and makes it impossible to reach the WHO goal of 80% treatment uptake necessary to eliminate hepatitis C as a public health threat by 2030.
Table S1 HCV cascade of patients cared for by physicians with <10 versus ≥10 drug substitution patients in the decentralised setting, before and after HCV rapid tests and Fibroscan®.
| Before HCV rapid tests and Fibroscan® | After HCV rapid tests and Fibroscan® | |||||
|---|---|---|---|---|---|---|
|
<10 drug substitution patients/physician
(21 physicians) (30 patients) |
≥10 drug substitution patients/physician
(7 physicians) (74 patients) |
p–value |
<10 drug substitution patients/physician
(21 physicians) (30 patients) |
≥10 drug substitution patients/physician
(7 physicians) (74 patients) |
p–value | |
| Physicians | 75.0% (21/28) | 25.0% (7/28) | 75.0% (21/28) | 25.0% (7/28) | ||
| Study patients | 28.8% (30/104) | 71.2% (74/104) | 28.8% (30/104) | 71.2% (74/104) | ||
| Ever HCV screened | 70.0% (21/30) | 64.9% (48/74) | 0.616 | 100.0% (30/30) | 95.9% (71/74) | 0.263 |
| HCV antibody positive | 36.7% (11/30) | 37.8% (28/74) | 0.911 | 50.0% (15/30) | 44.6% (33/74) | 0.616 |
| 52.4% (11/21) | 58.3% (28/48) | 0.646 | 50.0% (15/30) | 46.5% (33/71) | 0.746 | |
| HCV RNA test, if HCV positive | 100.0% (11/11) | 64.3% (18/28) | 0.022 | 86.7% (13/15) | 60.6% (20/33) | 0.071 |
| Ever chronic HCV infection | 23.3% (7/30) | 18.9% (14/74) | 0.611 | 26.7% (8/30) | 18.9% (14/74) | 0.381 |
| 63.6% (7/11) | 77.8% (14/18) | 0.408 | 61.5% (8/13) | 70.0% (14/20) | 0.614 | |
| Genotype known | 71.4% (5/7) | 64.3% (9/14) | 0.743 | 75.0% (6/8) | 64.3% (9/14) | 0.604 |
| Liver fibrosis assessed | 85.7% (6/7) | 42.9% (6/14) | 0.061 | 87.5% (7/8) | 100% (14/14) | 0.176 |
| Ever treated | 57.1% (4/7) | 42.9% (6/14) | 0.537 | 50.0% (4/8) | 42.9% (6/14) | 0.746 |
| SVR | 75.0% (3/4) | 83.3% (5/6) | 0.747 | 75.0% (3/4) | 83.3% (5/6) | 0.747 |
HCV = hepatitis C virus, SVR = sustained virological response
Table S2 Patients and physicians recruited in the decentralised setting.
|
<10 drug substitution patients/physician
(145 physicians) (312 patients) |
≥10 drug substitution patients/physician
(16 physicians) (319 patients) |
p–value | |
|---|---|---|---|
| All physicians (n = 161) | 90.1% (145/161) | 0.024 | |
| Physicians recruited in the decentralised setting (n=28) | 75.0% (21/28) | ||
| All patients (n = 631) | 49.4% (312/631) | <0.001 | |
| Patients recruited in the decentralised setting (n = 104) | 28.8% (30/104) | ||
| Physicians recruited in the decentralised setting | 14.5% (21/145) | 43.8% (7/16) | 0.015 |
| Patients recruited in the decentralised setting | 9.6% (30/312) | 23.2% (74/319) | <0.001 |
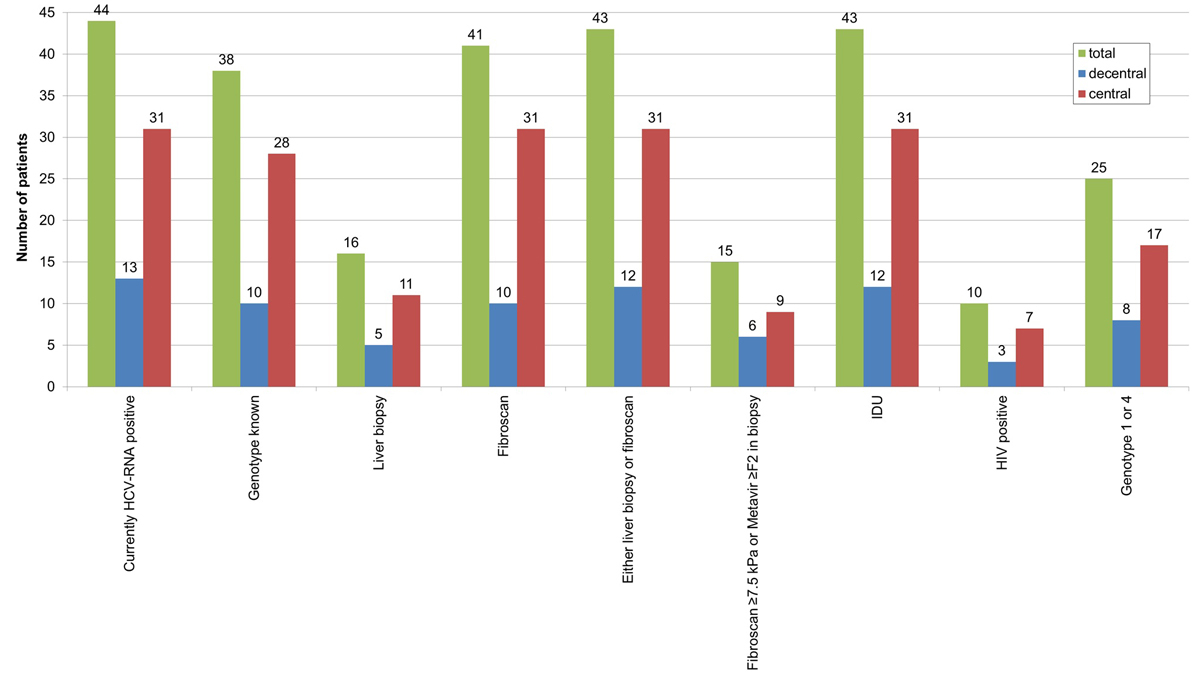
Figure S1 Patients currently HCV RNA positive, i.e., still in need of treatment: current state of evaluation and fulfilment of criteria for reimbursement of interferon-free direct-acting antivirals treatment in Switzerland.
HCV = hepatitis C virus; IDU = intravenous drug use; HIV = human immunodeficiency virus; total = total study population (n = 205); decentral = decentralised setting (n = 104); central = centralised setting (n = 101)
There is no conflict of interest. The study was financed by the Cantonal Hospital Aarau.
1 Nelson PK , Mathers BM , Cowie B , Hagan H , Des Jarlais D , Horyniak D , et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571–83. doi:.https://doi.org/10.1016/S0140-6736(11)61097-0
2 Bruggmann P , Grebely J . Prevention, treatment and care of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26(Suppl 1):S22–6. doi:.https://doi.org/10.1016/j.drugpo.2014.08.014
3Cominetti F, Simonson T, Dubois-Arber F, Gervasoni JP, Schaub M, Monnat M. Analyse der Hepatitis-C-Situation bei den drogenkonsumierenden Personen in der Schweiz. Lausanne: Institut universitaire de médecine sociale et préventive; 2015. (Raisons de santé 234b) Available from: https://www.iumsp.ch/Publications/pdf/rds234b_de.pdf
4 Steffen T , Blättler R , Gutzwiller F , Zwahlen M . HIV and hepatitis virus infections among injecting drug users in a medically controlled heroin prescription programme. Eur J Public Health. 2001;11(4):425–30. doi:.https://doi.org/10.1093/eurpub/11.4.425
5Bundesamt für Gesundheit (BAG). Substitutionsgestützte Behandlungen bei Opioidabhängigkeit Revision July 2013
6Bundesamt für Gesundheit (BAG). Substitutionsgestützte Behandlungen bei Opioidabhängigkeit (revised: 28 Dec. 2016), https://www.bag.admin.ch/bag/de/home/themen/mensch-gesundheit/sucht/suchtberatung-therapie/substitutionsgestuetzte-behandlung.html (Accessed: 24 Jan. 2017)
7 Grebely J , Page K , Sacks-Davis R , van der Loeff MS , Rice TM , Bruneau J , et al.; InC3 Study Group. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology. 2014;59(1):109–20. doi:.https://doi.org/10.1002/hep.26639
8 Edlin BR . Perspective: test and treat this silent killer. Nature. 2011;474(7350):S18–9. doi:.https://doi.org/10.1038/474S18a
9 Thein HH , Yi Q , Dore GJ , Krahn MD . Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48(2):418–31. doi:.https://doi.org/10.1002/hep.22375
10 Westbrook RH , Dusheiko G . Natural history of hepatitis C. J Hepatol. 2014;61(1, Suppl):S58–68. doi:.https://doi.org/10.1016/j.jhep.2014.07.012
11 Wiley TE , McCarthy M , Breidi L , McCarthy M , Layden TJ . Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28(3):805–9. doi:.https://doi.org/10.1002/hep.510280330
12 Hézode C , Roudot-Thoraval F , Nguyen S , Grenard P , Julien B , Zafrani ES , et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63–71. doi:.https://doi.org/10.1002/hep.20733
13 Ishida JH , Peters MG , Jin C , Louie K , Tan V , Bacchetti P , et al. Influence of cannabis use on severity of hepatitis C disease. Clin Gastroenterol Hepatol. 2008;6(1):69–75. doi:.https://doi.org/10.1016/j.cgh.2007.10.021
14 Missiha SB , Ostrowski M , Heathcote EJ . Disease progression in chronic hepatitis C: modifiable and nonmodifiable factors. Gastroenterology. 2008;134(6):1699–714. doi:.https://doi.org/10.1053/j.gastro.2008.02.069
15 Martin-Carbonero L , Benhamou Y , Puoti M , Berenguer J , Mallolas J , Quereda C , et al. Incidence and Predictors of Severe Liver Fibrosis in Human Immunodeficiency Virus–Infected Patients with Chronic Hepatitis C: A European Collaborative Study. Clin Infect Dis. 2004;38(1):128–33. doi:.https://doi.org/10.1086/380130
16 Martinez-Sierra C , Arizcorreta A , Diaz F , Roldan R , Martin-Herrera L , Perez-Guzman L , et al. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and Human Immunodeficiency Virus. Clin Infect Dis. 2003;36:491–8. doi:.https://doi.org/10.1086/367643
17Müllhaupt B, Fehr J, Moradpour D, Rauch A. Treatment of Chronic Hepatitis C – August 2017 Update - SASL - SSI Expert Opinion Statement. http://www.sginf.ch/files/sasl-ssi_hepc_eos_aug2017.pdf (Accessed: 21 Sept. 2017)
18 European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63(1):199–236. doi:.https://doi.org/10.1016/j.jhep.2015.03.025
19 AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–54. doi:.https://doi.org/10.1002/hep.27950
20 Boglione L , Mornese Pinna S , De Nicolò A , Cusato J , Cariti G , Di Perri G , et al. Treatment with direct-acting antiviral agents of hepatitis C virus infection in injecting drug users: A prospective study. J Viral Hepat. 2017;24(10):850–7; Epub ahead of print. doi:.https://doi.org/10.1111/jvh.12711
21 Kaan IA , Jones T , McCaughan GW . Have we significantly underestimated the capacity in the Australian health system to treat chronic hepatitis C infection in an interferon-free era? Intern Med J. 2017;47(3):269–74. doi:.https://doi.org/10.1111/imj.13262
22 Janjua NZ , Islam N , Wong J , Yoshida EM , Ramji A , Samji H , et al. Shift in disparities in hepatitis C treatment from interferon to DAA era: A population-based cohort study. J Viral Hepat. 2017;24(8):624–30; Epub ahead of print. doi:.https://doi.org/10.1111/jvh.12684
23Marshall AD, Nielsen S, Cunningham EB, Aghemo A, Alho H, Backmund M, et al. Restrictions for reimbursement of interferon-free direct acting antiviral therapies for HCV infection in Europe (LBP-505). The International Liver Congress 2017 April 19-23, Amsterdam, The Netherlends
24Bundesamt für Gesundheit (BAG). Stellungnahme des Bundesamtes für Gesundheit zum Bericht Situationsanalyse Hepatitis B und C. (06 Apr. 2017), https://www.bag.admin.ch/dam/bag/de/dokumente/mt/forschungsberichte/situationsanalyse-hepatitis-stellungnahme.pdf.download.pdf/situationsanalyse-hepatitis-stellungnahme-de.pdf (Accessed: 23 May 2017)
25Hepatitis Schweiz [Website]. Keine Limitatio mehr (12 Dec. 2017), http://www.hepatitis-schweiz.ch/de/newsreader/hepatitis-c-medikamente-sind-bald-fuer-alle-betroffenen-verfuegbar (Accessed: 21 Sept. 2017)
26 Broers B , Helbling B , François A , Schmid P , Chuard C , Hadengue A , et al.; Swiss Association for the Study of the Liver (SASL 18). Barriers to interferon-α therapy are higher in intravenous drug users than in other patients with acute hepatitis C. J Hepatol. 2005;42(3):323–8. doi:.https://doi.org/10.1016/j.jhep.2004.11.018
27 Belfiori B , Ciliegi P , Chiodera A , Bacosi D , Tosti A , Baldelli F , et al. Peginterferon plus Ribavirin for chronic hepatitis C in opiate addicts on methadone/buprenorphine maintenance therapy. Dig Liver Dis. 2009;41(4):303–7. doi:.https://doi.org/10.1016/j.dld.2008.08.009
28 Grebely J , deVlaming S , Duncan F , Viljoen M , Conway B . Current approaches to HCV infection in current and former injection drug users. J Addict Dis. 2008;27(2):25–35. doi:.https://doi.org/10.1300/J069v27n02_04
29 Bruggmann P , Falcato L , Dober S , Helbling B , Keiser O , Negro F , et al.; Swiss Hepatitis C Cohort Study. Active intravenous drug use during chronic hepatitis C therapy does not reduce sustained virological response rates in adherent patients. J Viral Hepat. 2008;15(10):747–52. doi:.https://doi.org/10.1111/j.1365-2893.2008.01010.x
30Bruggmann P, Broers B, Meili D. Hepatitis C-Therapie bei Patienten unter Opioidsusbstitution. Empfehlungen der Schweizerischen Gesellschaft für Suchtmedizin (SSAM). Schweiz Med Forum. 2007;7:916–9
31 Prasad L , Spicher VM , Zwahlen M , Rickenbach M , Helbling B , Negro F ; Swiss Hepatitis C Cohort Study Group. Cohort Profile: the Swiss Hepatitis C Cohort Study (SCCS). Int J Epidemiol. 2007;36(4):731–7. doi:.https://doi.org/10.1093/ije/dym096
32 Mehta SH , Genberg BL , Astemborski J , Kavasery R , Kirk GD , Vlahov D , et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33(3):126–33. doi:.https://doi.org/10.1007/s10900-007-9083-3
33 Witteck A , Schmid P , Hensel-Koch K , Thurnheer MC , Bruggmann P , Vernazza P ; Swiss Hepatitis C and HIV Cohort Studies. Management of hepatitis C virus (HCV) infection in drug substitution programs. Swiss Med Wkly. 2011;141:w13193. https://smw.ch/en/article/doi/smw.2011.13193/
34 Meyer JP , Moghimi Y , Marcus R , Lim JK , Litwin AH , Altice FL . Evidence-based interventions to enhance assessment, treatment, and adherence in the chronic Hepatitis C care continuum. Int J Drug Policy. 2015;26(10):922–35. doi:.https://doi.org/10.1016/j.drugpo.2015.05.002
35 McLeod A , Weir A , Aitken C , Gunson R , Templeton K , Molyneaux P , et al. Rise in testing and diagnosis associated with Scotland’s Action Plan on Hepatitis C and introduction of dried blood spot testing. J Epidemiol Community Health. 2014;68(12):1182–8. doi:.https://doi.org/10.1136/jech-2014-204451
36 Bruggmann P , Litwin AH . Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis. 2013;57(Suppl 2):S56–61. doi:.https://doi.org/10.1093/cid/cit271
37 Grebely J , Robaeys G , Bruggmann P , Aghemo A , Backmund M , Bruneau J , et al.; International Network for Hepatitis in Substance Users. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26(10):1028–38. doi:.https://doi.org/10.1016/j.drugpo.2015.07.005
38 Lee SR , Kardos KW , Schiff E , Berne CA , Mounzer K , Banks AT , et al. Evaluation of a new, rapid test for detecting HCV infection, suitable for use with blood or oral fluid. J Virol Methods. 2011;172(1-2):27–31. doi:.https://doi.org/10.1016/j.jviromet.2010.12.009
39 Friedrich-Rust M , Ong MF , Martens S , Sarrazin C , Bojunga J , Zeuzem S , et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134(4):960–74. doi:.https://doi.org/10.1053/j.gastro.2008.01.034
40 Castéra L , Vergniol J , Foucher J , Le Bail B , Chanteloup E , Haaser M , et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–50. doi:.https://doi.org/10.1053/j.gastro.2004.11.018
41 Castera L , Forns X , Alberti A . Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48(5):835–47. doi:.https://doi.org/10.1016/j.jhep.2008.02.008
42 Roulot D , Czernichow S , Le Clésiau H , Costes JL , Vergnaud AC , Beaugrand M . Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48(4):606–13. doi:.https://doi.org/10.1016/j.jhep.2007.11.020
43 Garfein RS , Vlahov D , Galai N , Doherty MC , Nelson KE . Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86(5):655–61. doi:.https://doi.org/10.2105/AJPH.86.5.655
44 Thorpe LE , Ouellet LJ , Levy JR , Williams IT , Monterroso ER . Hepatitis C virus infection: prevalence, risk factors, and prevention opportunities among young injection drug users in Chicago, 1997-1999. J Infect Dis. 2000;182(6):1588–94. doi:.https://doi.org/10.1086/317607
45 Xia YH , Chen W , Tucker JD , Wang C , Ling L . HIV and hepatitis C virus test uptake at methadone clinics in Southern China: opportunities for expanding detection of bloodborne infections. BMC Public Health. 2013;13(1):899. doi:.https://doi.org/10.1186/1471-2458-13-899
46 Janjua NZ , Kuo M , Yu A , Alvarez M , Wong S , Cook D , et al. The Population Level Cascade of Care for Hepatitis C in British Columbia, Canada: The BC Hepatitis Testers Cohort (BC-HTC). EBioMedicine. 2016;12:189–95. doi:.https://doi.org/10.1016/j.ebiom.2016.08.035
47 Yehia BR , Schranz AJ , Umscheid CA , Lo Re V, 3rd . The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One. 2014;9(7):e101554. doi:.https://doi.org/10.1371/journal.pone.0101554
48 Hajarizadeh B , Grebely J , McManus H , Estes C , Razavi H , Gray RT , et al. Chronic hepatitis C burden and care cascade in Australia in the era of interferon-based treatment. J Gastroenterol Hepatol. 2017;32(1):229–36. doi:.https://doi.org/10.1111/jgh.13453
49 Vanhommerig JW , Thomas XV , van der Meer JT , Geskus RB , Bruisten SM , Molenkamp R , et al.; MOSAIC (MSM Observational Study for Acute Infection with hepatitis C) Study Group. Hepatitis C virus (HCV) antibody dynamics following acute HCV infection and reinfection among HIV-infected men who have sex with men. Clin Infect Dis. 2014;59(12):1678–85. doi:.https://doi.org/10.1093/cid/ciu695
50 Kee KM , Wang JH , Hung CH , Chen CH , Lee CM , Chang KC , et al. Decreased anti-hepatitis C virus titer and associated factors in chronic hepatitis C patients after sustained virological response: a prospective study. J Gastroenterol Hepatol. 2012;27(6):1106–11. doi:.https://doi.org/10.1111/j.1440-1746.2011.06946.x
51 Toyoda H , Kumada T , Kiriyama S , Sone Y , Tanikawa M , Hisanaga Y , et al. Changes in hepatitis C virus (HCV) antibody status in patients with chronic hepatitis C after eradication of HCV infection by interferon therapy. Clin Infect Dis. 2005;40(6):e49–54. doi:.https://doi.org/10.1086/428128
52 Tuaillon E , Mondain AM , Meroueh F , Ottomani L , Picot MC , Nagot N , et al. Dried blood spot for hepatitis C virus serology and molecular testing. Hepatology. 2010;51(3):752–8.
53Cepheid® [Website] Xpert HCV Viral Load Brochure - CE-IVD. http://www.cepheid.com/administrator/components/com_productcatalog/library-files/615085cee2245c0c5e547ebff5052119-Xpert-HCV-Viral-Load-Brochure-CEIVD-3043-02.pdf (Accessed: 30 Jan. 2017)
54 Grebely J , Lamoury FMJ , Hajarizadeh B , Mowat Y , Marshall AD , Bajis S , et al.; LiveRLife Study Group. Evaluation of the Xpert HCV Viral Load point-of-care assay from venepuncture-collected and finger-stick capillary whole-blood samples: a cohort study. Lancet Gastroenterol Hepatol. 2017;2(7):514–20. doi:.https://doi.org/10.1016/S2468-1253(17)30075-4
55Bregenzer A, Warmann N, Ottiger C, Fux CA. Validation of a point-of-care quantitative HCV-RNA test using capillary blood from the finger (Xpert® HCV Viral Load, Cepheid®) (P59). Joint Annual Meeting 2017 August 30 - September 01, Basel, Switzerland, https://sginf2017.congress-imk.ch/frontend/imk/media/IMSDKG17/IMSDKG17_Abstractbook_03.pdf (Accessed: 21 Sept. 2017)
56 Bottero J , Boyd A , Gozlan J , Carrat F , Nau J , Pauti MD , et al. Simultaneous Human Immunodeficiency Virus-Hepatitis B-Hepatitis C Point-of-Care Tests Improve Outcomes in Linkage-to-Care: Results of a Randomized Control Trial in Persons Without Healthcare Coverage. Open Forum Infect Dis. 2015;2(4):ofv162. doi:.https://doi.org/10.1093/ofid/ofv162
57 Cooper C . Rapid HCV RNA testing: removing the final obstacle to elimination. Lancet Gastroenterol Hepatol. 2017;2(7):468–9. doi:.https://doi.org/10.1016/S2468-1253(17)30086-9
58 Chahine EB , Sucher AJ , Hemstreet BA . Sofosbuvir/Velpatasvir: The First Pangenotypic Direct-Acting Antiviral Combination for Hepatitis C. Ann Pharmacother. 2016:1060028016668897. [Epub ahead of print].
59 Bruggmann P . Die Hepatitis-C-Epidemiologie in der Schweiz und die Rolle der Grundversorgung. Praxis (Bern). 2016;105(15):885–9. doi:.https://doi.org/10.1024/1661-8157/a002424
60 Cadranel JF , Rufat P , Degos F ; For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Practices of liver biopsy in France: results of a prospective nationwide survey. Hepatology. 2000;32(3):477–81. doi:.https://doi.org/10.1053/jhep.2000.16602
61 Stauber R . Nichtinvasive Diagnose der Leberfibrose bei chronischen Hepatopathien. J Gastroenterol Hepatol Erkr. 2009;7(4):12–7. Available at: www.kup.at/kup/pdf/8531.pdf.
62Witteck A, Schmid P. Hepatitis C – Update 2010. Schweiz Med Forum. 2010;10(42):729–36.
63 Grady BP , Schinkel J , Thomas XV , Dalgard O . Hepatitis C virus reinfection following treatment among people who use drugs. Clin Infect Dis. 2013;57(Suppl 2):S105–10. doi:.https://doi.org/10.1093/cid/cit301
64 National Institute of Health. NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19(3):1–46.
65 Dhumeaux D , Marcellin P , Lerebours E . Treatment of hepatitis C. The 2002 French consensus. Gut. 2003;52(12):1784–7. doi:.https://doi.org/10.1136/gut.52.12.1784
66 Overbeck K , Dufour JF , Müllhaupt B , Helbling B , Borovicka J , Malinverni R , et al. Impact of international consensus guidelines on antiviral therapy of chronic hepatitis C patients in Switzerland. Swiss Med Wkly. 2010;140(9-10):146–52.
67 European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol. 2017;66(1):153–94. doi:.https://doi.org/10.1016/j.jhep.2016.09.001
68WHO. 05/2016, Combating hepatitis B and C to reach elimination by 2030 - Advocacy brief; http://apps.who.int/iris/bitstream/10665/206453/1/WHO_HIV_2016.04_eng.pdf (Accessed: 21 Nov. 2016)
69 Martin NK , Vickerman P , Grebely J , Hellard M , Hutchinson SJ , Lima VD , et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–609. doi:.https://doi.org/10.1002/hep.26431
70Moradpour D, Müllhaupt B. Hepatitis C: aktuelle Therapie. Schweiz Med Forum. 2015;15(17):366-370
71 Harris M , Ward E , Gore C . Finding the undiagnosed: a qualitative exploration of hepatitis C diagnosis delay in the United Kingdom. J Viral Hepat. 2016;23(6):479–86. doi:.https://doi.org/10.1111/jvh.12513
72 Doerrbecker J , Behrendt P , Mateu-Gelabert P , Ciesek S , Riebesehl N , Wilhelm C , et al. Transmission of hepatitis C virus among people who inject drugs: viral stability and association with drug preparation equipment. J Infect Dis. 2013;207(2):281–7. doi:.https://doi.org/10.1093/infdis/jis677
73 Fernandez N , Towers CV , Wolfe L , Hennessy MD , Weitz B , Porter S . Sharing of Snorting Straws and Hepatitis C Virus Infection in Pregnant Women. Obstet Gynecol. 2016;128(2):234–7. doi:.https://doi.org/10.1097/AOG.0000000000001507
74 Karageorgopoulos DE , Allen J , Bhagani S . Hepatitis C in human immunodeficiency virus co-infected individuals: Is this still a “special population”? World J Hepatol. 2015;7(15):1936–52. doi:.https://doi.org/10.4254/wjh.v7.i15.1936
There is no conflict of interest. The study was financed by the Cantonal Hospital Aarau.