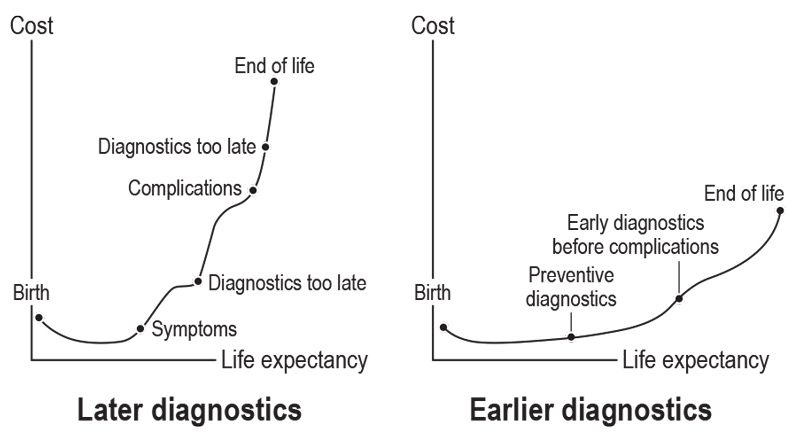
Figure 1 Generic diagram of the consequences of early vs late diagnostic measures with regard to life expectancy and associated costs.
DOI: https://doi.org/10.4414/smw.2017.14546
Healthcare expenditure as a proportion of gross domestic product (GDP) is on the rise. In the US, for example, healthcare spending is expected to grow 1.3 percentage points faster than GDP annually, rising to comprise 20.1% of GDP by 2025 [1]. Across all Organization for Economic Co-operation and Development member states, total public healthcare and long-term care expenditures are projected to double by 2060 (from 6.2 to 13.9% of GDP) if the pace of the last decade continues [2]. Even with concerted efforts at cost containment, expenditures are projected to grow by 50% (to 9.5% of GDP) [2]. Therefore, the importance of improvement to the ratio of healthcare resource utilisation to overall health outcomes is becoming increasingly clear [3].
In-vitro diagnostics (IVDs) play a critical role in driving clinical decision-making, and their true impact includes cost savings and increased efficiencies in downstream activities [4–6]. IVD testing can answer crucial questions about a patient’s health status, including risk or predisposition for developing a certain condition; the stage of disease; the chances of therapy response; and the prognosis for progression/remission under therapy [7].
Evidence suggests that the potential of IVDs is currently underexploited and undervalued. Recent research indicates that IVDs account for 2.3% and 1.4% of total healthcare expenditure in the US and Germany respectively, while driving 66% of clinical decision-making [8]. Nonetheless, the reality surrounding the use of IVDs is different. A watershed analysis of the US healthcare system showed that physicians followed diagnostic best practices only 62% of the time [9], highlighting that ~38% of patients may not have received the best care. This lack of utilisation has a ripple effect. The US National Committee for Quality Assurance linked low compliance with diagnostics-based quality measures for diabetes, colorectal cancer, and breast cancer with 56 200 avoidable adverse health events, nearly 34 000 avoidable deaths, and $899 million in avoidable healthcare costs [10]. Adding insult to injury, a recent meta-analysis of 42 IVD utilisation studies worldwide (evaluating 1.6 million tests) found underuse to be a much bigger problem than overuse [11]. The overall mean rate of underutilisation (IVD tests indicated but not ordered) was 44.8%, more than double the 20.6% rate of overuse (IVD tests ordered but not indicated) [11]. This might be indicative of the fact that in budget-constrained healthcare systems, it seems to be more acceptable to reduce laboratory testing volumes than to ask clinicians to cut down on treatment [12].
Cutting down on IVDs is often favoured as an ad-hoc solution to counteract increasing healthcare costs for payers, mainly because of the methodological inability to measure the cost of not doing something (e.g., not ordering a test). If a payer captures laboratory testing costs but not long-term savings, diagnostic testing will always appear as a net cost. The unpleasant side effect of focusing on this short-term solution is that it aggravates the fundamental problem, namely it further increases society’s overall expenditure on healthcare resources as a result of a delay, inappropriate selection, or complete lack of therapy.
The following tangible examples might be considered: The World Health Organization (WHO) recently completed a longitudinal study of tuberculosis (TB) control using data from 21 European countries. Because of shrinking public health budgets during the 2008–2011 economic downturn, IVD testing rates decreased and rates of TB case detection fell by 5.22% across Europe [13]. Interestingly, at the same time, the WHO projected that the prevalence of TB and TB-attributable mortality would increase by as much as 3% for over a decade after the recession ended [13]. The number of TB-associated deaths was estimated to be approximately 1.5 million in 2014, although the WHO aims to reduce the TB-associated mortality rate to 2 per 100 000 population before 2030 [14]. However, providing sufficient financial means is still the bottleneck in fighting the global burden of TB. Approximately US$8 billion is required annually to cover the costs of detection and treatment on a global scale, but only approximately US$6.4 billion is available [15]. Without the funding to address these needs, TB incidence rates are falling by only 1.5% per year [15].
In oncology, reducing the number of molecular tests performed follows a similar logic. These tests, which cost US$100–3000 each, could help avoid the use of expensive anticancer drugs costing US$600–28 000 per patient [16], and would improve individualised patient care.
To overcome this problem, we propose four conceptual interventions for clinical practice. These are: (1) fostering prevention, screening, early diagnosis, and therapy; promoting (2) comprehensive and (3) stratified disease management, as well as (4) the targeted delivery of treatment alongside companion diagnostics. Based on our argumentation, we conclude with the political actions that should be taken into consideration to pave the way towards more sustainable healthcare systems.
A recent study by Cancer Research UK found that late diagnosis is a major driver of the UK’s National Health Service (NHS) cancer treatment costs [17]. Treatment for stage 3 and 4 colon, rectal, lung, and ovarian cancer costs the NHS nearly 2.5 times the amount spent treating stage 1 and 2 cancers. The report estimated that the financial dividend of earlier diagnosis amounts to 5% of the total UK treatment budget for these four cancers. Extrapolated to a global scale, these data hint at the magnitude of savings that could be made through early detection. Although survival rates differ markedly between cancers, early-stage diagnosis is consistently associated with longer survival. Among patients diagnosed with stage 1 lung, ovarian, or colorectal cancer between 2002 and 2006, five-year survival rates in the east of England were 35, 90 and 95%, respectively; among those diagnosed at stage 4, five-year survival dropped to 1, 2 and 5%, respectively [17]. A recent US Food and Drug Administration (FDA) report estimated that the cost to society is US $775 278 when a patient with high-risk early-stage breast cancer does not receive timely life-saving therapy, and they lose three years of life as a result (fig. 1) [18]. Furthermore, early diagnosis has a downstream effect on the patient’s quality of life during adjuvant chemotherapy, as treatment regimens for metastatic disease require further add-on agents that may increase the adverse-event burden.

Figure 1 Generic diagram of the consequences of early vs late diagnostic measures with regard to life expectancy and associated costs.
Another example is cervical cancer, which has one major known causative factor – human papillomavirus (HPV). The global number of deaths due to cervical cancer was estimated to be approximately 266 000 in 2012 [19]; HPV is prevalent in 99.7% of cervical carcinomas [20]. In the US, the estimated annual cost of treatment of cervical carcinoma and its precursor condition is approximately $1.2 billion [21]. If cervical cancer is diagnosed early, the US National Cancer Institute estimates a five-year survival rate of 91.5%; if diagnosed late, the five-year survival rate drops to 16.5% [22]. Because there are no early-stage symptoms, screening is the primary mode of detection. Ultimately, primary prevention of cervical cancer through HPV vaccination should be encouraged. The cost-effectiveness ratio of vaccination in the US was estimated to be US $43 000 per quality-adjusted life-year compared with current screening practice, if performed at the age of 12 years in girls and if lifelong immunity is ensured [23].
Comprehensive disease management refers to the rigour applied to best support diagnosis and treatment of a given disease during every stage of its lifecycle to help patients and physicians in their clinical decision-making. Again, the treatment of cervical cancer and associated HPV infection provides an example. Of all the high-risk HPV genotypes, HPV16 and HPV18 account for the largest numbers of cervical cancer cases [24], although their impact on disease-free survival and prognosis is still controversial [25].
After its introduction in the 1940s, the Pap smear quickly became the gold standard for cervical-cancer screening and prevention, dramatically decreasing mortality rates. However, a single Pap test has limited ability to detect cases of cervical cancer and cervical intra-epithelial neoplasia. Studies show that up to one-third of cervical cancers occur in women with normal results from a traditional Pap smear, which has a significant diagnostic false-negative rate [26]. To compensate, clinicians perform Pap smears annually, and set a low threshold for follow-up procedures, including colposcopy. The repeated testing and subsequent (often unnecessary) colposcopies are expensive.
An IVD that more accurately identifies women at greatest risk of advanced disease, and thus qualifying for colposcopy, as opposed to those at intermediate risk, maximises the benefits of cervical cancer screening while minimising the potential harm (and cost) of overtreatment.
To better stratify patients by risk status, an HPV screening test with simultaneous genotyping for the high-risk HPV strains 16 and 18 has become available recently [27].
In a trial involving 34 254 patients, primary screening via the test plus triage of HPV-positive women based on HPV16/18 status and Pap smear provided a good balance between maximising sensitivity (benefit) and specificity by limiting the number of colposcopies (potential harm) [28]. Germany showed that using the new test could reduce the annual incidence of cervical cancers by 30% and annual mortality by 70% [4]. Furthermore, primary screening and triage reduced the total cost per patient screened per year by 7%, resulting in annualised payer budget savings of more than €9.5 million [4]. Figure 2 summarises the comprehensive management of HPV and cervical cancer.

Figure 2 Holistic disease management in the diagnosis of cervical cancer and its associated risk factor of HPV infection.
HPV = human papillomavirus; PCR = polymerase chain reaction.
Stratified disease management refers to the allocation of patients to risk groups for either developing a certain disease or for progressing towards a predicted outcome.
In the case of preeclampsia, the use of a biomarker combination can facilitate the prediction of occurrence during pregnancy and ensure that high-risk patients are identified for monitoring while others receive routine care. The ratio of soluble FMS-like tyrosine kinase-1 (sFlt-1) to placental growth factor (PlGF) has been proposed as an indicator of preeclampsia [29–35], as it is elevated in pregnant women 4–5 weeks before the clinical onset of preeclampsia [31].
The Prediction of Short-Term Outcome in Pregnant Women with Suspected Preeclampsia Study (PROGNOSIS) was a large, non-interventional, multi-centre trial that established and validated a threshold-based prediction model using the sFlt-1:PlGF ratio [36]. Managing patients with suspected preeclampsia using the sFlt-1:PlGF ratio could help prevent unnecessary hospitalisations, with concomitant economic benefits for healthcare providers [37].
A further example is found in cardiology, where healthcare expenditures are increasing. In the US alone, healthcare costs attributed to cardiovascular disease are expected to triple between 2010 and 2030 [38]. About 80% of heart-failure-related costs in Europe are attributable to recurrent hospitalisations. Each re-hospitalisation costs almost €7893 [39]. The ability to diagnose and prognosticate worsening heart failure could have great utility in preventing avoidable hospitalisations. By measuring and stratifying levels of N-terminal pro-brain natriuretic peptide (NT-proBNP), clinicians can confirm suspected heart failure more accurately, reduce the use of echocardiography by up to 58%, prevent 13% of initial hospitalisations, and reduce hospital stays by 12% [40]. Indeed, the UK’s National Institute for Health and Clinical Excellence updated its guidelines in 2010 to adopt NT-proBNP biomarker IVDs as “rule-out” tests for suspected heart failure in order to limit unnecessary referrals to echocardiography [41].
Broadly, the concept of targeted delivery of treatment can be summarised as personalised healthcare (PHC) [42, 43], allowing patients to be stratified into responder and non-responder groups. In PHC, IVDs enable clinicians to identify and stratify patients who will benefit from or, possibly, be harmed by a particular therapy. This allows effective treatment of patients while safeguarding the sustainability of finite healthcare resources.
PHC has led to an increase in response rates over the past decade, particularly in cancer [44]. PHC IVDs do not necessarily need to be developed de novo, and evidence shows that a combination of existing biomarkers can be used to formulate algorithms that are sufficient to help the healthcare system safely direct healthcare costs and resources [45].
The human epidermal growth factor receptor 2 (HER2) gene, for example, is known to be linked to certain breast and ovarian cancers [46]. HER2 overexpression is associated with more aggressive disease, making standard chemotherapy less effective [47]. Trastuzumab, which is an effective treatment only for patients with tumours that overexpress HER2 (HER2-positive tumours), was first approved by the FDA in 1998 for use in HER2-positive metastatic breast cancer, and subsequently in 2006 for early-stage HER2-positive disease. Today, it is on the WHO’s Model List of Essential Medicines, a formulary of the most important – and cost-effective – medications needed in a basic health system [48].
Despite its clear utility in selecting patients suitable for treatment with trastuzumab, the HER2 IVD test struggled to gain reimbursement across the European Union, mainly because of heterogeneous regulatory and reimbursement environments, further complicated by the fact that most drugs undergo national-level reviews [49]. In France, the HER2 test was approved in 2000, but reimbursement has only been available since 2007 [49].
The budgetary impact of inappropriate treatment is not the only consideration. The importance of avoiding treating a patient with an agent that will be ineffective is further highlighted when trastuzumab’s side effects are considered, as they include cardiomyopathy, infusion reactions, embryo-fetal toxicity, pulmonary toxicity and exacerbation of chemotherapy-induced neutropenia [50].
Table 1 provides an overview of selected compounds and their companion diagnostics in oncology and beyond.
Table 1 Overview of selected compounds and their companion diagnostics in and beyond the field of oncology.
|
Drug name
(trade name) |
Producer | Swissmedic approval | Indication(s) | Biomarker | Companion IVD(s) |
|---|---|---|---|---|---|
| Trastuzumab (Herceptin®) | Roche | 1999 | Early and metastatic breast cancer Metastatic gastric cancer ALK rearrangements |
HER2 overexpression | INFORM HER-2/NEU PathVysion HER-2 DNA probe kit PATHWAY ANTI-HER-2/NEU (4B5) rabbit Mab HercepTestTM |
| Imatinib (Gleevec®) | Novartis | 2001 | Ph+ chronic myelogenous leukaemia with aggressive systemic mastocytosis | KIT D816V mutation |
KIT
D816V mutation detection DAKO C-KIT PharmDx |
| Myelodysplastic syndrome/ myeloproliferative disease | PDGFRB gene rearrangement | PDGFRB FISH | |||
| Cetuximab (Erbitux®) | Roche | 2003 | Metastatic colorectal cancer | KRAS mutation | cobas® EGFR mutation test therascreen® KRAS RGQ PCR kit EGFR pharmDx assay |
| Gefitinib (Iressa®) |
AstraZeneca | 2004 | Metastatic NSCLC | EGFR exon 19 deletions and exon 21 (L858R) substitution mutations | therascreen® EGFR RGQ PCR kit |
| Deferasirox (Exjade®) | Novartis | 2005 | Non-transfusion-dependent thalassaemia | Liver iron concentration | FerriScan® R2-MRI analysis system |
| Pertuzumab (Perjeta®) | Roche | 2012 | Metastatic breast cancer | HER2 overexpression | HercepTestTM |
| Crizotinib (Xalkori®) | Pfizer | 2012 | Metastatic NSCLC | ALK rearrangements | Ventana ALK (D5F3) CDx assay Vysis ALK Break Apart FISH probe kit |
| Venetoclax (Venclexta®) | AbbVie and Roche | – | CLL | Deletion of LSI TP53 probe target (17p-) | Vysis CLL FISH probe kit |
| Pembrolizumab (Keytruda®) | Merck | 2015 | NSCLC; advanced melanoma | PD-L1 protein | PD-L1 IHC 22C3 pharmDx |
| Osimertinib (Tagrisso®) | AstraZeneca | 2016 | Locally advanced or metastatic EGFR T790 mutation-positive NSCLC | EGFR mutations (exon 19 deletion and L858R; T790M) | cobas® EGFR mutation test |
| Olaparib (Lynparza®) |
AstraZeneca | 2016 | Ovarian cancer | BRCA1/BRCA2 variants in protein coding regions and intron/exon boundaries | BRACAnalysis CDx |
| Afatinib (Gilotrif®) |
Boehringer Ingelheim | 2016 | NSCLC | EGFR exon 19 deletions and exon 21 (L858R) substitution mutations | therascreen® EGFR RGQ PCR kit |
ALK = anaplastic lymphoma kinase gene; BRCA1/2 = breast cancer 1/2 gene; CLL = chronic lymphocytic leukaemia; EGFR = epidermal growth factor receptor gene; FISH = fluorescence in situ hybridization; IHC = immunohistochemistry; HER2 = epidermal growth factor receptor 2; IVD = in vitro diagnostic; MAb, monoclonal antibody; NSCLC = non-small cell lung cancer; PCR = polymerase chain reaction PDGFRB = platelet-derived growth factor receptor-beta gene; PD-L1 = programmed cell death 1 ligand 1; Ph+ = Philadelphia chromosome-positive
The majority of healthcare systems pay for running a diagnostic test but do not offer an incentive for choosing the right diagnostic test. A decision not to use a certain diagnostic test that could allow earlier diagnosis and treatment may lead to productivity losses and increased healthcare costs. To reduce the overall costs related to a specific disease, current legislation mandating fee-for-service delivery should be revised to emphasise the payer’s need for more effective patient management. Catering for customer value is a concept that has been discussed for many decades in the business literature [51], and it is equally applicable in healthcare.
For example, Porter suggested value propositions to describe what outcome measures should be rewarded [52]. They produced a tiered system highlighting the domains of (1) survival, (2) time to recovery and return to routine, and (3) sustainability of health (table 2).
Table 2 Application of the outcome measures hierarchical tier system described by Porter to reinforcement interventions proposed as solutions to the shifting the burden archetype in the in-vitro diagnostics arena [52].
| Tiering | Tier 1 | Tier 2 | Tier 3 |
|---|---|---|---|
| Criterion | Survival | Time to recovery and return to routine | Sustainability of health or recovery |
| Expression | Degree of health or recovery | Disutility of care and treatment process (diagnostic errors, ineffective care, treatment-related discomfort, complications, adverse effects, etc.) | Long-term consequences of therapy (induced illness) |
| Reinforcement through | Fostering screening, early diagnosis and therapy | Comprehensive and integrated disease management | Targeted delivery of treatment |
Unfortunately, unlike pharmaceuticals, the reimbursement of IVDs is still not guided by the value these tests generate [53]. The reimbursement of pharmaceuticals, on the other hand, follows strict guidance based on medical evidence. The UK has one of the oldest outcome-based systems; more recently, Germany introduced its Act on the Restructuring of the Pharmaceutical Market (Arzneimittelmarkt-Neuordnungsgesetz). This legislation was introduced in 2011 after a substantial increase in expenditure on healthcare drugs. The law aims to maintain a balance between innovation and affordable medicines by introducing a rigorous system that requires the manufacturer of an agent to submit evidence of added value from the patient’s perspective [54]. This law is expected to generate cost savings of approximately €2 billion annually. The pharmaceutical industry has already reacted to this important trend in healthcare policies. For example, after the rejection of reimbursement for bortezomib (Velcade®) in the UK in 2007, Johnson and Johnson offered the Velcade Response Scheme to the NHS, which used response to treatment for multiple myeloma (based on serum M-protein levels) in the pricing algorithm. Under this plan, the full cost of treatment for multiple myeloma would be completely covered by the NHS if the patient’s serum M-protein level reduced by ≥25% within the first four cycles of therapy. If the patient did not respond, Johnson and Johnson agreed to cover the costs [55]. Other manufacturers quickly followed with similarly programs e.g., Merck Serono reimburses the costs of its metastatic colorectal cancer drug cetuximab (Erbitux®) if patients fail to respond to therapy at six weeks [56].
The principle of cost containment through value-based rewards is also followed by several non-governmental organisations, including accountable-care organisations (ACOs). According to the Centers for Medicare and Medicaid Services, an ACO is “an organisation of health care practitioners that agrees to be accountable for the quality, cost, and overall care of Medicare beneficiaries who are enrolled in the traditional fee-for-service program who are assigned to it” [57]. ACOs aim to ensure that patients receive the right care at the right time, while avoiding unnecessary duplication of services [58] by linking payments to quality metrics and the cost of care.
In this conceptual paper, we argue that the problem of IVD underutilisation is partly linked to the inability of healthcare systems to track patients longitudinally, and as a result, evidence of the direct health-economic effects of IVDs on patient outcomes is scarce [8, 59, 60]. To overcome this problem, policy-makers need to develop a deep understanding of the underlying problem and make follow-ups and outcomes more measurable. We also described, from a diagnostic perspective, four possible interventions with relevance for clinical practice and cost containment. In particular, we argued that an emphasis on prevention, screening, early diagnosis, and therapy is the means to limit rising healthcare expenditure and to improve patient management. Failure to detect diseases early can result in expensive, late-stage treatments, overuse of procedures and therapies, poor disease management, and possibly the onset of additional complications.
In light of this, it is important to encourage patients and payers to actively engage in screening programmes and to incentivise positive behaviour [61]. Patient education plays a large role in encouraging engagement. Incentives might include reductions in annual premiums or enrolment on loyalty programmes linked to screening, with a downstream effect on patients’ medication costs in the scenario of a positive diagnosis. In South Africa, for example, a private health plan has introduced a voluntary incentive programme in which participants earn points when they receive preventive care. These points can be traded for discounts or goods. This programme led to a significantly higher likelihood of receiving preventive care [62].
In Switzerland, voluntary screening programmes for breast cancer have been introduced in some cantons, where women age 50–74 years receive an invitation for mammography covered by their insurance every other year [63]. Furthermore, the health insurance covers stool testing every two years and colonoscopy every 10 years for people age 50–69 years to screen for colon cancer [64]. Another incentive for higher screening rates could be to facilitate enrolment on clinical trials, which will have a downstream effect on patients’ medication costs.
In order to demonstrate the cost-effectiveness of screening and early diagnosis, and given the increasing quality and availability of electronic health-record data [65], more longitudinal and comprehensive real-world patient data are needed [66].
We further proposed that both comprehensive and stratified patient management have the potential to reduce costs and improve medical outcomes by enabling earlier, individualised interventions that can diminish subsequent health problems [6], avert adverse outcomes [67, 68], reduce or prevent hospitalisations [64], and avoid the cost of late-stage or unnecessary treatment [16]. Figure 3 summarises the potential consequences of all interventions on the current use of IVDs.

Figure 3 How interventions affect the current use of in vitro diagnostics.
+ = amplifying feedback; – = attenuating feedback.
Finally, we favour a move towards individualised treatment (PHC). Individualised treatment allows patients to receive exactly the medication that is needed and avoids inappropriate therapies with consequent high healthcare expenditures, and prevents undesired outcomes [6, 69, 70].
In order to implement these interventions, we argued that shifting healthcare incentives from a service-based to a value-based approach is a conditio sine qua non, as it not only underscores the importance of regulating IVDs, but also serves as an incentive for developers to continue to innovate [49]. Validation of assays should follow quality parameters, such as clear definition of the intended use, thresholds, optimisation, standardisation, repeatability, analytical/diagnostic sensitivity, and specificity [71]. Full validation is required when there is no suitable performance specification available, and should be performed in comparison with the currently available “gold standard” [72]. For IVDs, value-based reimbursement criteria could be potentially based on the following conditions:
In a scenario with transparent and meaningful reimbursement policies for assays with a clearly defined intended use and proven clinical utility, more innovation and investment will happen on the industry side, paired with meaningful patient outcomes, and better resource utilisation [73–76].
In 2012, the American Board of Internal Medicine launched the “Choosing Wisely” initiative to avoid unnecessary medical tests, treatments, and procedures [77]. In 2014, the Swiss Society of Internal Medicine launched a similar campaign called “Smarter Medicine” [78]. These initiatives, indicative of a move towards value-based reimbursement for pharmaceutical agents, have not yet been implemented in the diagnostics field.
Implementing changes to the current utilisation of IVDs will be necessary to provide broad, cost-efficient, state-of-the-art healthcare in the future. It is key to creating awareness that IVDs are often misunderstood as being part of the problem. Those in charge of managing the limited resources available for healthcare are called on to take into account the possible consequences of removing the tools that could help overcome the vicious circle of ever-increasing healthcare costs, and should act accordingly. Considering recent developments in the pharmaceutical sector, we believe that there is an unmet need for politicians to create policies for the use of IVD that reward outcome instead of service. Conversely, those developing and providing IVDs are challenged to focus on medically relevant, innovative and validated diagnostics. Other than that, diagnostics companies should continuously work on innovative reimbursement solutions for their products. This way, the promise of living a longer, healthier life can be kept for generations to come.
In addition to their clinical work, MS and HHS are employees of Roche Diagnostics, Medical and Scientific Affairs; CB was an employee of Roche Diagnostics at the time of preparing the manuscript. The opinion expressed in this article does not reflect the opinion of F. Hoffmann-La Roche Ltd.
1Centers for Medicare & Medicaid Services. National Health Expenditure Fact Sheet. 2016. [Cited August 15, 2016.] Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet.html.
2de la Maisonneuve C, Martins J. Public spending on health and long-term care: a new set of projections. OECD Economic Policy Papers 6. 2013. [Cited July 12, 2016.] Available from: http://www.oecd.org/eco/growth/Health%20FINAL.pdf.
3 Kaplan RS , Porter ME . How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46–52, 54, 56–61 passim.
4Petry U, Huang J, Hertz D, Taggart C, Armstrong S, McQuilling C, et al. Modelling the health economic impact of HPV primary screening with p16/Ki67 dual-stain cytology triage in Germany. Abstract HPV15-0258 presented at 30th International Papillomavirus Conference & Clinical Public Health Workshops. 2015. [Cited July 12, 2016.] Available from: http://www.hpv2015.org/Documents/HPV15%20Abstracts%20for%20after%20conference%20unlocked.pdf.
5 Sharma P , Scotland G , Cruickshank M , Tassie E , Fraser C , Burton C , et al. The clinical effectiveness and cost-effectiveness of point-of-care tests (CoaguChek system, INRatio2 PT/INR monitor and ProTime Microcoagulation system) for the self-monitoring of the coagulation status of people receiving long-term vitamin K antagonist therapy, compared with standard UK practice: systematic review and economic evaluation. Health Technol Assess. 2015;19(48):1–172. doi:.https://doi.org/10.3310/hta19480
6 Vyberg M , Nielsen S , Røge R , Sheppard B , Ranger-Moore J , Walk E , et al. Immunohistochemical expression of HER2 in breast cancer: socioeconomic impact of inaccurate tests. BMC Health Serv Res. 2015;15(1):352. doi:.https://doi.org/10.1186/s12913-015-1018-6
7Cheng C-M, Kuan C-M, Chen C-F. In-vitro diagnostic devices: Introduction to current point-of-care. 2016. Switzerland: Springer International Publishing AG.
8 Rohr UP , Binder C , Dieterle T , Giusti F , Messina CG , Toerien E , et al. The value of in vitro diagnostic testing in medical practice: a status report. PLoS One. 2016;11(3):e0149856. doi:. Correction in: PLoS ONE11(4): e0154008. https://doi.org/10.1371/journal.pone.0149856
9 McGlynn EA , Asch SM , Adams J , Keesey J , Hicks J , DeCristofaro A , et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–45. doi:.https://doi.org/10.1056/NEJMsa022615
10Lewin Group for the Advanced Medical Technology Association (AdvaMed). The value of diagnostics innovation, adoption and diffusion into health care. 2005. [Cited July 12, 2016.] Available from: http://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/ValueofDiagnostics.pdf.
11 Zhi M , Ding EL , Theisen-Toupal J , Whelan J , Arnaout R . The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One. 2013;8(11):e78962. doi:.https://doi.org/10.1371/journal.pone.0078962
12 Lippi G , Mattiuzzi C . Testing volume is not synonymous of cost, value and efficacy in laboratory diagnostics. Clin Chem Lab Med. 2013;51(2):243–5. doi:.https://doi.org/10.1515/cclm-2012-0502
13 Reeves A , Basu S , McKee M , Sandgren A , Stuckler D , Semenza JC . Tuberculosis control and economic recession: longitudinal study of data from 21 European countries, 1991-2012. Bull World Health Organ. 2015;93(6):369–79. doi:.https://doi.org/10.2471/BLT.14.142356
14World Health Organization. Implementing the End TB strategy: the essentials. WHO/HTM/TB/2015.31. 2015. Geneva, Switzerland; World Health Organization. Available from: http://www.who.int/entity/tb/publications/2015/end_tb_essential.pdf.
15 Raviglione M , Sulis G . Tuberculosis 2015: Burden, challenges and strategy for control and elimination. Infect Dis Rep. 2016;8(2):6570. doi:.https://doi.org/10.4081/idr.2016.6570
16 Davis JC , Furstenthal L , Desai AA , Norris T , Sutaria S , Fleming E , et al. The microeconomics of personalized medicine: today’s challenge and tomorrow’s promise. Nat Rev Drug Discov. 2009;8(4):279–86. doi:.https://doi.org/10.1038/nrd2825
17Cancer Research UK. Saving lives, averting costs: An analysis of the financial implications of achieving earlier diagnosis of colorectal, lung and ovarian cancer. 2014. [Cited July 12, 2016.] Available from: http://www.cancerresearchuk.org/sites/default/files/saving_lives_averting_costs.pdf.
18Food and Drug Administration The public health evidence for FDA oversight of laboratory developed tests: 20 case studies. 2015. Rockville, MD: US Food and Drug Administration. [Cited July 12, 2016.] Available from: http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Reports/UCM472777.pdf.
19GLOBOCAN. Cervical cancer: estimated incidence, mortality and prevalence worldwide in 2012. Available from: http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp.
20 Walboomers JM , Jacobs MV , Manos MM , Bosch FX , Kummer JA , Shah KV , et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9. doi:.https://doi.org/10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F
21 Chesson HW , Ekwueme DU , Saraiya M , Watson M , Lowy DR , Markowitz LE . Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine. 2012;30(42):6016–9. doi:.https://doi.org/10.1016/j.vaccine.2012.07.056
22National Cancer Institute. National Cancer Institute Surveillance Epidemiology and End Results (SEER). Program Cancer of the Cervix Uteri – SEER Stat Fact Sheets. 2016. [Cited July 12, 2016.] Available from: http://seer.cancer.gov/statfacts/html/cervix.html.
23 Kim JJ , Goldie SJ . Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359(8):821–32. doi:.https://doi.org/10.1056/NEJMsa0707052
24 Bosch FX , Lorincz A , Muñoz N , Meijer CJ , Shah KV . The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55(4):244–65. doi:.https://doi.org/10.1136/jcp.55.4.244
25 Cuschieri K , Brewster DH , Graham C , Nicoll S , Williams AR , Murray GI , et al. Influence of HPV type on prognosis in patients diagnosed with invasive cervical cancer. Int J Cancer. 2014;135(11):2721–6. doi:.https://doi.org/10.1002/ijc.28902
26 Leyden WA , Manos MM , Geiger AM , Weinmann S , Mouchawar J , Bischoff K , et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. 2005;97(9):675–83. doi:.https://doi.org/10.1093/jnci/dji115
27 Wright TC, Jr , Stoler MH , Sharma A , Zhang G , Behrens C , Wright TL ; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol. 2011;136(4):578–86. doi:.https://doi.org/10.1309/AJCPTUS5EXAS6DKZ
28 Cox JT , Castle PE , Behrens CM , Sharma A , Wright TC, Jr , Cuzick J ; Athena HPV Study Group. Comparison of cervical cancer screening strategies incorporating different combinations of cytology, HPV testing, and genotyping for HPV 16/18: results from the ATHENA HPV study. Am J Obstet Gynecol. 2013;208(3):184.e1–11. doi:.https://doi.org/10.1016/j.ajog.2012.11.020
29 Levine RJ , Maynard SE , Qian C , Lim KH , England LJ , Yu KF , et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–83. doi:.https://doi.org/10.1056/NEJMoa031884
30 Levine RJ , Lam C , Qian C , Yu KF , Maynard SE , Sachs BP , et al.; CPEP Study Group. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. doi:.https://doi.org/10.1056/NEJMoa055352
31 Vatten LJ , Eskild A , Nilsen TI , Jeansson S , Jenum PA , Staff AC . Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol. 2007;196(3):239.e1–6. doi:.https://doi.org/10.1016/j.ajog.2006.10.909
32 Verlohren S , Galindo A , Schlembach D , Zeisler H , Herraiz I , Moertl MG , et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202(2):161.e1–11. doi:.https://doi.org/10.1016/j.ajog.2009.09.016
33 Verlohren S , Herraiz I , Lapaire O , Schlembach D , Moertl M , Zeisler H , et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206(1):58.e1–8. doi:.https://doi.org/10.1016/j.ajog.2011.07.037
34 Villa PM , Hämäläinen E , Mäki A , Räikkönen K , Pesonen AK , Taipale P , et al. Vasoactive agents for the prediction of early- and late-onset preeclampsia in a high-risk cohort. BMC Pregnancy Childbirth. 2013;13(1):110. doi:.https://doi.org/10.1186/1471-2393-13-110
35 Rana S , Powe CE , Salahuddin S , Verlohren S , Perschel FH , Levine RJ , et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125(7):911–9. doi:.https://doi.org/10.1161/CIRCULATIONAHA.111.054361
36 Zeisler H , Llurba E , Chantraine F , Vatish M , Staff AC , Sennström M , et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374(1):13–22. doi:.https://doi.org/10.1056/NEJMoa1414838
37 Seely EW , Solomon CG . Improving the prediction of preeclampsia. N Engl J Med. 2016;374(1):83–4. doi:.https://doi.org/10.1056/NEJMe1515223
38 Heidenreich PA , Trogdon JG , Khavjou OA , Butler J , Dracup K , Ezekowitz MD , et al.; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44. doi:.https://doi.org/10.1161/CIR.0b013e31820a55f5
39 Adlbrecht C , Huelsmann M , Berger R , Moertl D , Strunk G , Oesterle A , et al. Cost analysis and cost-effectiveness of NT-proBNP-guided heart failure specialist care in addition to home-based nurse care. Eur J Clin Invest. 2011;41(3):315–22. doi:.https://doi.org/10.1111/j.1365-2362.2010.02412.x
40 Siebert U , Januzzi JL, Jr , Beinfeld MT , Cameron R , Gazelle GS . Cost-effectiveness of using N-terminal pro-brain natriuretic peptide to guide the diagnostic assessment and management of dyspneic patients in the emergency department. Am J Cardiol. 2006;98(6):800–5. doi:.https://doi.org/10.1016/j.amjcard.2006.06.005
41National Institute for Health and Clinical Excellence. Chronic heart failure in adults: management. NICE Guideline CG10]. 2010. [Cited July 12, 2016.] Available from: https://www.nice.org.uk/guidance/Cg108.
42 Vargas GA . Personalized healthcare: how to improve outcomes by increasing benefit and decreasing risk through the use of biomarkers. Biomarkers Med. 2009;3(6):701–9. doi:.https://doi.org/10.2217/bmm.09.74
43 Tezak Z , Kondratovich MV , Mansfield EUS . FDA and Personalized Medicine: In vitro Diagnostic Regulatory Perspective. Per Med. 2010;7(5):517–30. doi:.https://doi.org/10.2217/pme.10.53
44 Schwaederle M , Zhao M , Lee JJ , Eggermont AM , Schilsky RL , Mendelsohn J , et al. Impact of precision medicine in diverse cancers: A meta-analysis of phase II clinical trials. J Clin Oncol. 2015;33(32):3817–25. doi:.https://doi.org/10.1200/JCO.2015.61.5997
45 Lopez-Chavez A , Thomas A , Rajan A , Raffeld M , Morrow B , Kelly R , et al. Molecular profiling and targeted therapy for advanced thoracic malignancies: a biomarker-derived, multiarm, multihistology phase II basket trial. J Clin Oncol. 2015;33(9):1000–7. doi:.https://doi.org/10.1200/JCO.2014.58.2007
46 Slamon DJ , Godolphin W , Jones LA , Holt JA , Wong SG , Keith DE , et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–12. doi:.https://doi.org/10.1126/science.2470152
47 Slamon DJ , Clark GM , Wong SG , Levin WJ , Ullrich A , McGuire WL . Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi:.https://doi.org/10.1126/science.3798106
48World Health Organization. WHO Model List of Essential Medicines 19th List (April 2015). (Amended August 2015). 2015. [Cited July 12, 2016.] Available from: http://www.who.int/medicines/publications/essentialmedicines/EML2015_8-May-15.pdf.
49 Miller I , Ashton-Chess J , Spolders H , Fert V , Ferrara J , Kroll W , et al. Market access challenges in the EU for high medical value diagnostic tests. Per Med. 2011;8(2):137–48. doi:.https://doi.org/10.2217/pme.11.2
50Genentech Inc. Herceptin package insert. May 11, 2017. Available from: http://medlibrary.org/lib/rx/meds/herceptin.
51 de Ruyter KD , Wetzels M , Lemmink J , Mattson J . The dynamics of the service delivery process: a value-based approach. Int J Res Mark. 1997;14(3):231–43. doi:.https://doi.org/10.1016/S0167-8116(97)00004-9
52 Porter ME . What is value in health care? N Engl J Med. 2010;363(26):2477–81. doi:.https://doi.org/10.1056/NEJMp1011024
53 Desiere F , Gutjahr TS , Rohr UP . Developing companion diagnostics for delivering personalised medicine: opportunities and challenges. Drug Discov Today Ther Strateg. 2013;10(4):e175–81. doi:.https://doi.org/10.1016/j.ddstr.2013.05.002
54 Fischer KE , Stargardt T . Early benefit assessment of pharmaceuticals in Germany: manufacturers’ expectations versus the Federal Joint Committee’s decisions. Med Decis Making. 2014;34(8):1030–47. doi:.https://doi.org/10.1177/0272989X14546377
55National Institute for Health and Clinical Excellence. Summary of VELCADE Response Scheme. 2007. [Cited July 12, 2016.] Available from: https://www.nice.org.uk/guidance/ta129/documents/department-of-health-summary-of-responder-scheme2.
56Sotiropoulos A. Pricing pharmaceuticals by outcome. 2011. [Cited August 8, 2016.] Available from: http://www.2020publicservicestrust.org/downloads/7_Pricing_Pharmaceuticals_by_Outcome.pdf.
57Centers for Medicare & Medicaid Services. Medicare “Accountable Care Organizations” Shared Savings Program – New Section 1899 of Title XVIII, Preliminary Questions & Answers. [Cited April 18, 2015.] Available from: https://www.aamc.org/download/131932/data/acoqa.pdf.
58Centers for Medicare & Medicaid Services. (2015). Accountable Care Organizations (ACO). [Cited August 15, 2016.] Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ACO/index.html?redirect=/aco.
59 Hallworth MJ . Improving clinical outcomes - towards patient-centred laboratory medicine. Ann Clin Biochem. 2015;52(Pt 6):715–6. doi:.https://doi.org/10.1177/0004563215595431
60Lewin Group for the American Clinical Laboratory Association and the Advanced Medical Technology Association (AdvaMed). The value of laboratory screening and diagnostic tests for prevention and health care improvement. 2009. [Cited August 16, 2016.] Available from: http://www.chi.org/uploadedFiles/Industry_at_a_glance/Lewin%20Report%20on%20Dx%20Tests%20(2009).pdf.
61Kane RL, Johnson PE, Town RJ, Butler M. Economic Incentives for Preventive Care. 2004. Rockville, MD, USA: Agency for Healthcare Research and Quality (US); 1998–2005. Available from: https://www.ncbi.nlm.nih.gov/books/NBK11845/.
62 Mehrotra A , An R , Patel DN , Sturm R . Impact of a patient incentive program on receipt of preventive care. Am J Manag Care. 2014;20(6):494–501.
63Swiss Cancer Screening. Available from: https://www.swisscancerscreening.ch/.
64Krebsliga. Darmkrebs-Screening-Programm. June 25, 2013. Available from: https://www.krebsliga.ch/krebs-vorbeugen/krebs-frueh-erkennen-und-vorbeugen/darmkrebs/darmkrebs-screening-programm/
65 Sperrin M , Thew S , Weatherall J , Dixon W , Buchan I . Quantifying the longitudinal value of healthcare record collections for pharmacoepidemiology. AMIA Annu Symp Proc. 2011;2011:1318–25.
66Deloite, AdvaMed, AdvaMedDx. Framework for Comprehensive Assessment of the Value of Diagnostic Tests. Available from: https://www.advamed.org/sites/default/files/resource/advameddiagnosticframeworkreport_09.pdf.
67 Lippi G , Plebani M , Graber ML . Building a bridge to safe diagnosis in health care. The role of the clinical laboratory. Clin Chem Lab Med. 2016;54(1):1–3. doi:.https://doi.org/10.1515/cclm-2015-1135
68 Piva E , Pelloso M , Penello L , Plebani M . Laboratory critical values: automated notification supports effective clinical decision making. Clin Biochem. 2014;47(13-14):1163–8. doi:.https://doi.org/10.1016/j.clinbiochem.2014.05.056
69 Schwartz A , Weiner SJ , Weaver F , Yudkowsky R , Sharma G , Binns-Calvey A , et al. Uncharted territory: measuring costs of diagnostic errors outside the medical record. BMJ Qual Saf. 2012;21(11):918–24. doi:.https://doi.org/10.1136/bmjqs-2012-000832
70 Newman-Toker DE , Pronovost PJ . Diagnostic errors--the next frontier for patient safety. JAMA. 2009;301(10):1060–2. doi:.https://doi.org/10.1001/jama.2009.249
71Organisation Mondiale de la Santé Animale. Chapter 1.1.2: Principles and methods of validation of diagnostic assays for infectious diseases. In: Manual of Diagnostic Tests for Aquatic Animals. 2017. Available at: http://www.oie.int/fileadmin/Home/eng/Health_standards/aahm/current/chapitre_validation_diagnostics_assays.pdf
72 Mattocks CJ , Morris MA , Matthijs G , Swinnen E , Corveleyn A , Dequeker E , et al.; EuroGentest Validation Group. A standardized framework for the validation and verification of clinical molecular genetic tests. Eur J Hum Genet. 2010;18(12):1276–88. doi:.https://doi.org/10.1038/ejhg.2010.101
73 Ferrante di Ruffano L , Davenport C , Eisinga A , Hyde C , Deeks JJ . A capture-recapture analysis demonstrated that randomized controlled trials evaluating the impact of diagnostic tests on patient outcomes are rare. J Clin Epidemiol. 2012;65(3):282–7. doi:.https://doi.org/10.1016/j.jclinepi.2011.07.003
74 Hallworth MJ , Epner PL , Ebert C , Fantz CR , Faye SA , Higgins TN , et al.; IFCC Task Force on the Impact of Laboratory Medicine on Clinical Management and Outcomes. Current evidence and future perspectives on the effective practice of patient-centered laboratory medicine. Clin Chem. 2015;61(4):589–99. doi:.https://doi.org/10.1373/clinchem.2014.232629
75 Schäfer H , Filser L , Rohr U , Laubender R , Dieterle T , Maitland R , et al. Medical value as a new strategy to increase corporate viability: Market chances and limitations in the diagnostic industry. Journal of Entrepreneurship & Organization Management. 2015;4:1000131.
76 Schäfer H , Filser L , Rohr U , Messina C , Laubender R , Dieterle T , et al. The diagnostic industry in a changing health care environment: a focus on the value component. J Manag Res. 2015;15:91–100.
77American Board of Internal Medicine. Choosing wisely. [Cited July 12, 2016.] Available from: http://www.choosingwisely.org/.
78Swiss Society of General Internal Medicine. Smarter Medicine. 2014. [Cited July 12, 2016.] Available from: www.smartermedicine.ch.
In addition to their clinical work, MS and HHS are employees of Roche Diagnostics, Medical and Scientific Affairs; CB was an employee of Roche Diagnostics at the time of preparing the manuscript. The opinion expressed in this article does not reflect the opinion of F. Hoffmann-La Roche Ltd.