Is there a role for procalcitonin in differentiating uncomplicated and complicated diverticulitis in order to reduce antibiotic therapy? A prospective diagnostic cohort study: This article was corrected and republished online on September 24, 2018. Please see Correction ‒ September 24, 2018.
DOI: https://doi.org/10.4414/smw.2017.14555
Victor
Jegerab, Roxana
Popa, Farschad
Forudastand, Jean Pierre
Barrasd, Markus
Zuberc, Rein Jan
Pisoa
aDepartment of Internal Medicine, Kantonsspital Olten soH, Switzerland
bDepartment of Internal Medicine, University Hospital Zurich, Switzerland
cDepartment of Surgery, Kantonsspital Olten soH, Switzerland
dDepartment of Surgery, Bürgerspital Solothurn soH, Switzerland
Summary
AIMS OF THE STUDY
While studies show that antibiotic treatment for uncomplicated diverticulitis seems to have no benefit, most experts advocate antimicrobial therapy for complicated diverticulitis. However, even for uncomplicated diverticulitis, most clinicians are very reluctant to withhold antibiotics. Biomarkers could help to guide antibiotic therapy as this approach has been shown to be effective for acute respiratory infections. In this diagnostic cohort study we evaluated whether procalcitonin could be a biomarker to distinguish complicated from uncomplicated cases of diverticulitis.
METHODS
Complicated diverticulitis was defined as having abscess formation or perforation diagnosed by abdominal computed tomography (CT) scan. In all patients with suspected diverticulitis, procalcitonin values were measured at admission and on day 2. These values were blinded for clinicians, and treatment was carried out according to the physician’s judgement. Two groups (complicated vs uncomplicated diverticulitis) were defined. Patients who had received antibiotic treatment before admission were excluded. Difference in procalcitonin values was calculated for both groups using the Mann-Whitney test. Receiver operating characteristics (ROC) were calculated to determine cut-off values for procalcitonin according to the gold standard (abdominal CT scans).
RESULTS
115 patients were included for analysis. 35 patients (30%) suffered from complicated diverticulitis. The median procalcitonin value for uncomplicated diverticulitis was significantly lower compared to complicated diverticulitis (median 0.05, interquartile range [IQR] 0.05–0.06 µg/l vs median 0.13, IQR 0.05–0.23 µg/l; p <0.0001). In the ROC analysis, the sensitivity and specificity were 81% and 91% when the highest procalcitonin value (days 1 and 2) was considered, with a cut-off value of 0.1 µg/l.
CONCLUSION
Procalcitonin was able to differentiate with a high sensitivity and specificity between complicated and uncomplicated cases of diverticulitis when combined with abdominal CT scans. As most clinicians still treat uncomplicated diverticulitis with antibiotics, procalcitonin could be an interesting parameter for guiding therapy and decreasing antibiotic usage. This should be further evaluated in randomised trials.
Introduction
Although there is growing evidence to promote the avoidance of antibiotic therapy in uncomplicated diverticulitis [1, 2], physicians often continue to prescribe antibiotics in any case of diverticulitis. Availability of biomarkers would be useful to differentiate between complicated and uncomplicated diverticulitis and thereby guide antibiotic therapy. Complicated diverticulitis is usually defined as Hinchey >Ib [3].
Procalcitonin has been identified as a biomarker in the diagnosis of bacterial infections in the setting of acute respiratory infections [4] and has been implemented in guidelines for antibiotic therapy in respective cases [4]. For abdominal infections, the data on reliable biomarkers are rather scarce. For appendicitis, procalcitonin failed to improve diagnosis and its sensitivity was even lower than C-reactive protein (CRP) [5]. However, procalcitonin was able to identify more complicated cases of appendicitis [5]. In sepsis in general, procalcitonin may be helpful early in the course of this heterogeneous disease to differentiate between bacterial infection and, e.g., post-operative inflammation [6]. However the role of procalcitonin in sepsis remains controversial and its trend (persistently high values vs faster clearing kinetics) rather than isolated values seem to have a higher clinical relevance [7].
Available reports on biomarkers in diverticulitis have been reviewed recently [8]. Mainly CRP has been evaluated in small cohort studies, which show only moderate sensitivity and specificity, and cannot be used as a single parameter. Recent data indicate a role for foetal calprotectin or matrix metalloproteinase in detecting diverticular disease, but it has not yet been clarified whether these parameters are useful for differentiation between complicated and uncomplicated courses of disease [8]. The role of procalcitonin has not been assessed so far.
The aim of the present prospective observational study was to evaluate if procalcitonin could differentiate between complicated and uncomplicated cases of diverticulitis compared to the gold-standard abdominal computed tomography (CT) scan. The aim was that results would guide further prospective trials for the more restrictive use of antibiotic therapy in cases of uncomplicated diverticulitis with appropriate procalcitonin cut-off levels.
Material and methods
We conducted a prospective diagnostic cohort study in two level-three emergency rooms and surgical wards in Switzerland (Kantonsspital Olten, Switzerland, and Bürgerspital Solothurn, Switzerland). The study was approved by the local ethics committee (north-western Switzerland, Licence no.: EKNZ 2015-135) in accordance with the Helsinki Declaration and the Swiss law for clinical studies. All patients gave written informed consent to a physician not involved in the study. Patients were enrolled into the study from September 2015 to December 2016.
Inclusion criteria were: suspected diverticulitis according to the treating physician (e.g., lower left abdominal pain, fever, referral from a general practitioner due to suspected diverticulitis), age >18 years, abdominal CT scan (requested by a physician responsible for patient care but independent of the study).
Exclusion criteria were severe immunodeficiency (on-going chemotherapy; corticosteroid prescription >50 mg/d and >14 days; acute malignant haematological disease, human immunodeficiency virus [HIV] stage C3), inflammatory bowel disease.
Blood was drawn by nurses on admission to the emergency room and in the course of routine blood sampling the following day on the ward. Procalcitonin was determined in addition to routine blood parameters (blood count, electrolytes, creatinine, blood urea nitrogen, liver enzymes, C-reactive protein). At the time of admission, two pairs of blood cultures were collected. Physicians were blinded for procalcitonin values, as it is not the current standard of care to assess procalcitonin in cases of suspected abdominal infection.
CT scans of the abdomen were ordered according to the decision of the physician in charge and reviewed by specialist radiologists. CT scans of the abdomen were available 24 hours a day, 7 days a week and were reviewed on site or online (at night). Radiologists were blinded to the procalcitonin value. According to the radiology report, patients were scored as uncomplicated diverticulitis (Hinchey Score 0–Ia) or complicated diverticulitis (with abscess formation, perforation, peritonitis; Hinchey Score Ib–IV).
Patient data, including clinical parameters as well as pre-hospital treatment, were obtained retrospectively from the patient chart.
Procalcitonin was measured with a one-step sandwich immunoassay with fluorescent detection (Vidas® BRAHMS PCTTM). The lowest detection threshold was 0.05 µg/l.
Statistics
Patient data from complicated or uncomplicated diverticulitis were compared using the non-parametric Wilcoxon matched-pairs signed rank test for laboratory parameters at day 1 (admission) and day 2. Parameters between groups were compared using a Mann-Whitney test. Due to the dynamic kinetics of procalcitonin [9], the highest values of either day 1 (admission) or day 2 were compared. A p-value of 0.05 was considered significant. In case of multiple testing, p-values were corrected following the Bonferroni method. Receiver operating characteristics were calculated to determine cut-off values for procalcitonin according to the gold standard (abdominal CT scans). Prism 6 (GraphPad Software Inc, La Jolla, CA, USA) was used to calculate the statistics and draw the figures that appear in this paper. ROC curves of paired data were compared according to DeLong’s Method using R.
Results
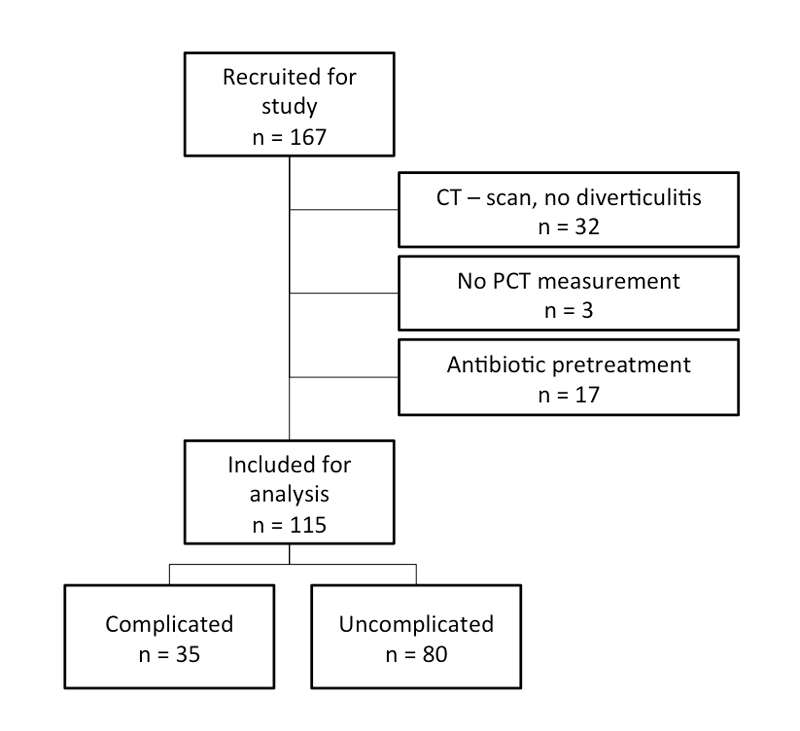
From 167 recruited patients, 32 (19%) were excluded due to already established antibiotic therapy in the outpatient setting, 17 (10%) patients had no confirmation of diverticulitis and 3 (2%) had missing procalcitonin values at admission to the emergency room. Finally, 115 patients were included for analysis (fig. 1). 35 (30%) suffered from complicated diverticulitis. These patients had a longer duration of intravenous antibiotic treatment as well as a longer duration of hospitalisation (table 1). Even in uncomplicated cases, almost all patients received antibiotic therapy (details of antibiotic therapy are shown in table 1). Only one out of 19 blood cultures drawn on admission to the emergency room from complicated cases revealed a positive culture result (Escherichia coli and Streptococcus anginosus).
Table 1 Demographics and characterisation of antibiotic (AB) treatment of complicated and uncomplicated cases of diverticulitis.
|
Complicated
|
Uncomplicated
|
|
|
% |
|
% |
p-value |
| Cases (n) |
35 |
30% |
80 |
69% |
|
| Age (years, median, range) |
62 (31–87) |
|
61 (33–90) |
|
0.962 |
| Gender (m/f) |
16/19 |
46%/54% |
36/44 |
45%/55% |
|
| Hospitalisation (days, median, range) |
7 (3–45) |
|
4 (1–17) |
|
<0.0001 |
| Positive blood culture |
(1/19) |
|
(0/18) |
|
|
| Antibiotic therapy (n) |
35 |
100% |
78 |
98% |
|
| Intravenous AB therapy |
35 |
100% |
68 |
85% |
|
| Days of AB therapy intravenous (median, range) |
6 (3–25) |
|
4 (0–14) |
|
<0.0001 |
| Type |
|
|
|
|
|
| Tazobactam/piperacillin |
16 |
46% |
41 |
51% |
|
| Ceftriaxone/metronidazole |
14 |
40% |
24 |
30% |
|
| Ciprofloxacin/metronidazole |
3 |
9% |
2 |
3% |
|
| Amoxicillin-clavulanic acid |
0 |
0% |
1 |
1% |
|
| Meropenem |
1 |
3% |
0 |
0% |
|
| Cefepime/metronidazole |
1 |
3% |
0 |
0% |
|
| Days of oral AB therapy (median, range) |
7 (0–15) |
|
7 (3–13) |
|
0.391 |
| Type |
|
|
|
|
|
| Amoxicillin-clavulanic acid |
16 |
46% |
55 |
69% |
|
| Ciprofloxacin/metronidazole |
11 |
31% |
20 |
25% |
|
| Amoxicillin |
1 |
3% |
0 |
0% |
|
| Amoxicillin-clavulanic acid/metronidazole |
0 |
0% |
3 |
4% |
|
| Cefuroxime/metronidazole |
1 |
3% |
0 |
0% |
|
| Levofloxacin/metronidazole |
1 |
3% |
0 |
0% |
|
| Cotrimoxazole |
1 |
3% |
0 |
0% |
|
| None |
4 |
11% |
0 |
0% |
|
Procalcitonin at admission (day 1) was significantly higher in patients with complicated diverticulitis compared to patients with uncomplicated diverticulitis (median 0.13, interquartile range [IQR] 0.05–0.23 µg/l vs median 0.05, IQR 0.05–0.06; p <0.0001). If the highest procalcitonin values of day 1 or day 2 were compared between the two groups, a significant difference was observed (median 0.21, IQR 0.12–0.75 µg/l vs median 0.05, IQR 0.05–0.07; p <0.0001). CRP and leucocyte count of complicated and uncomplicated diverticulitis were not significantly different at time of admission (table 2).
Table 2 Procalcitonin, C-reactive protein and leucocyte count on admission (day 1) and day 2.
|
Uncomplicated
|
Complicated
|
p-value
|
|
Admission
|
Day 2
|
Admission
|
Day 2
|
|
Median
|
IQR
|
Median
|
IQR
|
Median
|
IQR
|
Median
|
IQR
|
Uncomplicated; admission vs day 2
|
Complicated; admission vs day 2
|
Admission; uncomplicated vs complicated
|
Day 2; uncomplicated vs complicated
|
| PCT (µg/l) |
0.05 |
0.05–0.06 |
0.05 |
0.05–0.06 |
0.13 |
0.05–0.23 |
0.16 |
0.12–0.63 |
0.712 |
0.022 |
<0.0001
|
<0.0001
|
| CRP (mg/l) |
79 |
45–113 |
94 |
55–121 |
123 |
53–201 |
149 |
115–239 |
0.05 |
0.004
|
0.022 |
<0.0001
|
| leucocytes (G/l) |
11.4 |
9.1–14.2 |
8.4 |
6.5–11.0 |
12.6 |
10.4–14.9 |
10.4 |
8.3–13.3 |
<0.0001
|
0.004
|
0.292 |
0.002
|
Compared to the gold standard (abdominal CT scan), ROC curves of procalcitonin show moderate sensitivity (80%) and high specificity (91%) at a cut-off point of 0.1 µg/l when the highest value was considered (measured at admission or day 2) (area under the curve [AUC] 0.867; confidence interval [CI] 0.791–0.942). The procalcitonin (AUC 0.742; CI 0.633–0.852) as well as the CRP value (AUC 0.635; CI 0.509–0.761) at admission showed only moderate sensitivity and specificity (fig. 2). The ROC curve and AUC for highest procalcitonin was not significantly different compared to the AUC of highest CRP (p = 0.144; AUC for highest CRP 0.782; CI 0.682–0.881).
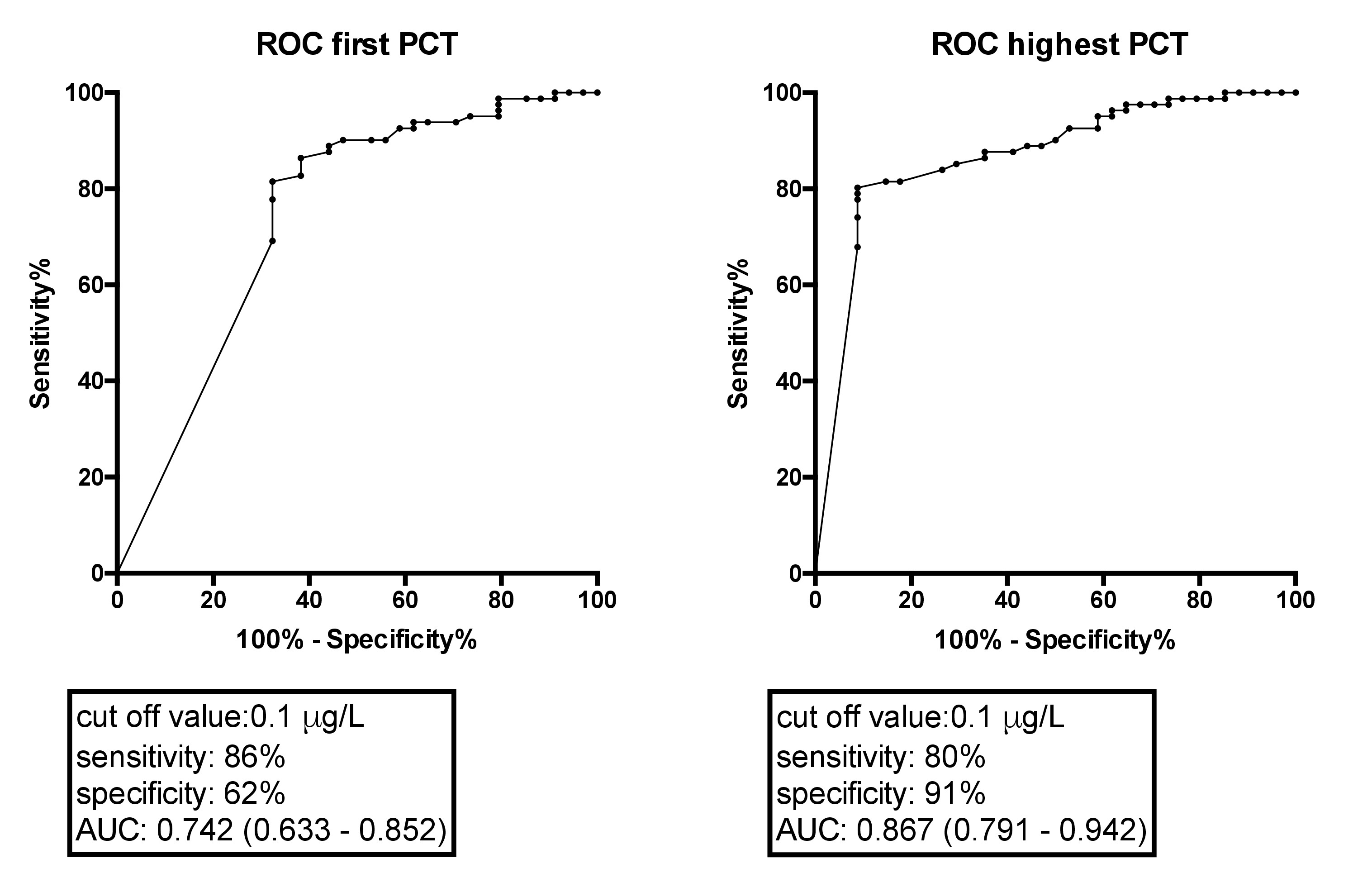
Figure 2 ROC (receiver operating characteristic) curve of procalcitonin against the gold standard of abdominal CT scan. The highest procalcitonin serum concentration of day 1 (admission) or day 2 showed a sensitivity of 80% and a specificity of 91%. AUC is indicated with 95% confidence interval in brackets.
Discussion
Diverticulitis is a frequent diagnosis, with about 75 hospital admissions per 100,000 persons per year, and has a low mortality rate of less than 1% [10]. The true incidence may be even higher, as many cases are treated outside hospitals by family physicians.
Although it is generally believed that diverticulitis results from microperforation of the diverticulum, some authors have hypothesised that diverticulitis is a form of inflammatory bowel disease [11]. The indication for antibiotic treatment has therefore been a challenge for many years [12]. Specific biomarkers for abdominal infections are missing. Mainly CRP and calprotectin have been assessed for diverticulitis but with only moderate specificity and sensitivity [8]. No reports on procalcitonin and diverticulitis are currently available. However, procalcitonin has been evaluated in cases of appendicitis where it showed a strong diagnostic value if abscess formation or perforation was present [13]. However, procalcitonin was not useful as a single parameter to diagnose appendicitis. In a recent published study, the authors studied the role of procalcitonin as a marker to guide antibiotic therapy in cases of peritonitis after surgery [14]. Subgroup analysis revealed a trend for shorter duration of therapy in the procalcitonin group, but the study was underpowered due to small sample size. It is therefore not clear whether procalcitonin is able to guide antibiotic therapy in the case of bacterial peritonitis following surgery.
The use of procalcitonin as a biomarker has been shown to decrease antibiotic consumption in respiratory infections [9]. We believe that treatment and clinical course of diverticulitis have some similarities to respiratory infections.
Even if procalcitonin did not show superiority compared to other biomarkers in abdominal infections [5], it could have its place in the case of diverticulitis. Similar to respiratory infections, most cases of diverticulitis are of non-bacterial origin [15], but in complicated diverticulitis, which often leads to peritonitis, patients may benefit from antibiotic treatment. Similar to non-bacterial respiratory infections, most clinicians might be very reluctant to omit antibiotic treatment for uncomplicated diverticulitis to avoid and prevent complications due to a possible bacterial infection. This was the case in our present study, where most patients with uncomplicated diverticulitis were treated with antibiotics.
Our results indicate that procalcitonin may be useful to differentiate uncomplicated from complicated diverticulitis when compared to the gold standard of abdominal CT scans. Procalcitonin values had a tendency to higher sensitivity and specificity compared to CRP in discerning complicated from uncomplicated diverticulitis, but their AUC were not significantly different, probably due to the small sample size. In 9% of the patients with complicated diverticulitis, procalcitonin values were below 0.1 µg/l, which therefore makes this method of analysis unsuitable as a replacement for CT scans. In addition, the procalcitonin value on admission had only moderate sensitivity and specificity, so a follow- up procalcitonin value on day 2 seems to be necessary. This may be due to the dynamic kinetics of procalcitonin [9] and the delay between onset of infection and measurement of procalcitonin. In summary, we do not conclude that measurement of procalcitonin could replace CT scans or that it should be used as a single parameter, but in the case of uncomplicated diverticulitis, it could have the potential to rule out antibiotic treatment. We hypothesise that in the patient population of the present study, procalcitonin-guided treatment would have decreased antibiotic treatment by 80%. This is a similar percentage compared to studies using procalcitonin in respiratory infections, where a marked reduction of antibiotic consumption was observed [9]. If laboratory tests support clinicians in omitting antibiotic treatment for uncomplicated diverticulitis, its use would be justified in addition to radiological imaging.
We are aware that our study is rather small. The aim was to gain indications of whether procalcitonin is able to differentiate between complicated and uncomplicated diverticulitis. Our results show that procalcitonin in addition to CT could support clinicians’ decisions on whether antibiotic treatment is necessary.
Further studies need to be done to answer this question in order to reduce antibiotic treatment in uncomplicated diverticulitis. These studies should be performed as multi-centre studies using larger sample sizes to obtain strong evidence for the use of procalcitonin in guidance of antibiotic therapy in diverticulitis.
References
1
Shabanzadeh
DM
,
Wille-Jørgensen
P
. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
2
Daniels
L
,
Ünlü
Ç
,
de Korte
N
,
van Dieren
S
,
Stockmann
HB
,
Vrouenraets
BC
, et al.; Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104(1):52–61. doi:.https://doi.org/10.1002/bjs.10309
3
Bolkenstein
HE
,
van de Wall
BJM
,
Consten
ECJ
,
Broeders
IAMJ
,
Draaisma
WA
. Risk factors for complicated diverticulitis: systematic review and meta-analysis. Int J Colorectal Dis. 2017;32(10):1375–83. doi:.https://doi.org/10.1007/s00384-017-2872-y
4
Schuetz
P
,
Müller
B
,
Christ-Crain
M
,
Stolz
D
,
Tamm
M
,
Bouadma
L
, et al.
Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
5
Yu
CW
,
Juan
LI
,
Wu
MH
,
Shen
CJ
,
Wu
JY
,
Lee
CC
. Systematic review and meta-analysis of the diagnostic accuracy of procalcitonin, C-reactive protein and white blood cell count for suspected acute appendicitis. Br J Surg. 2013;100(3):322–9. doi:.https://doi.org/10.1002/bjs.9008
6
Wacker
C
,
Prkno
A
,
Brunkhorst
FM
,
Schlattmann
P
. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(5):426–35. doi:.https://doi.org/10.1016/S1473-3099(12)70323-7
7
Xiao
Z
,
Wilson
C
,
Robertson
HL
,
Roberts
DJ
,
Ball
CG
,
Jenne
CN
, et al.
Inflammatory mediators in intra-abdominal sepsis or injury - a scoping review. Crit Care. 2015;19(1):373. doi:.https://doi.org/10.1186/s13054-015-1093-4
8
Gallo
A
,
Ianiro
G
,
Montalto
M
,
Cammarota
G
. The Role of Biomarkers in Diverticular Disease. J Clin Gastroenterol. 2016;50(Suppl 1):S26–8. doi:.https://doi.org/10.1097/MCG.0000000000000648
9
Sager
R
,
Kutz
A
,
Mueller
B
,
Schuetz
P
. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15(1):15. doi:.https://doi.org/10.1186/s12916-017-0795-7
10
Humes
DJ
,
Spiller
RC
. Review article: The pathogenesis and management of acute colonic diverticulitis. Aliment Pharmacol Ther. 2014;39(4):359–70. doi:.https://doi.org/10.1111/apt.12596
11
Floch
MH
. A hypothesis: is diverticulitis a type of inflammatory bowel disease?
J Clin Gastroenterol. 2006;40(Suppl 3):S121–5. doi:.https://doi.org/10.1097/01.mcg.0000225502.29498.ba
12
Hjern
F
,
Josephson
T
,
Altman
D
,
Holmström
B
,
Mellgren
A
,
Pollack
J
, et al.
Conservative treatment of acute colonic diverticulitis: are antibiotics always mandatory?
Scand J Gastroenterol. 2007;42(1):41–7. doi:.https://doi.org/10.1080/00365520600780650
13
Yamashita
H
,
Yuasa
N
,
Takeuchi
E
,
Goto
Y
,
Miyake
H
,
Miyata
K
, et al.
Diagnostic value of procalcitonin for acute complicated appendicitis. Nagoya J Med Sci. 2016;78(1):79–88.
14
Slieker
JC
,
Aellen
S
,
Eggimann
P
,
Guarnero
V
,
Schäfer
M
,
Demartines
N
. Procalcitonin-Guided Antibiotics after Surgery for Peritonitis: A Randomized Controlled Study. Gastroenterol Res Pract. 2017;2017:3457614. doi:.https://doi.org/10.1155/2017/3457614
15
Heise
CP
. Epidemiology and pathogenesis of diverticular disease. J Gastrointest Surg. 2008;12(8):1309–11. doi:.https://doi.org/10.1007/s11605-008-0492-0