Temporal trends of postpartum haemorrhage in Switzerland: a 22-year retrospective population-based cohort study
DOI: https://doi.org/10.4414/smw.2017.14551
Andrea
Kaelin Agtena, Daniel
Passwegb, Stephanie
von Orellib, Nancy
Ringelc, Ruedi
Tschudid, Boris
Tutscheke
aFetal Medicine Unit, St George’s University Hospital NHS, London, United Kingdom
bDepartment of Obstetrics and Gynaecology, City Hospital Triemli Zurich, Switzerland
cDepartment of Obstetrics and Gynecology, New York University School of Medicine, USA
dSevisa AG, Ermatingen, Switzerland
ePränatal Zürich, Zurich, Switzerland, and Medical Faculty, Heinrich Heine University, Düsseldorf, Germany
Summary
AIM
Postpartum haemorrhage (PPH) is one of the leading causes of maternal morbidity and mortality. Studies have reported an increase in incidence of postpartum haemorrhage in recent years. Our goal was to investigate changes in the incidence of postpartum haemorrhage (PPH) and its risk factors in Switzerland from 1993 to 2014.
METHODS
This population-based retrospective cohort study used data from the national Swiss Hospital in-patient database for obstetric and gynaecological hospital admissions – “Arbeitsgemeinschaft Schweizer Frauenkliniken” (ASF Statistik). All patients with deliveries between January 1993 and December 2014 were included. We used the database codes to identify patients with PPH, maternal factors, pregnancy-related and delivery-related factors. Significant changes in temporal trends were determined using Mantel-Haenszel test for trend. Multivariable logistic regression analyses were conducted to assess PPH and risk factors.
RESULTS
Births complicated by PPH in Switzerland increased from 2.5% in 1993 to 4.5% in 2014 (p <0.001), paralleled by an increase in uterine atony. Failure to progress during the second stage of labour (odds ratio [OR] 1.5, 95% confidence interval [CI] 1.5–1.6), oxytocin augmentation (OR 1.2, 95% CI 1.2–1.3), vacuum extraction (OR 1.1, 95% CI 1.1–1.2), and especially abnormally invasive placenta (OR 10.4, 95% CI 9.5–11.5) and placenta praevia (OR 4.9, 95% CI 432–5.6) were factors with the highest risk for postpartum haemorrhage.
CONCLUSIONS
Postpartum haemorrhage is a relatively common and potentially dangerous obstetric complication with increasing incidence over the last two decades in Switzerland. Its increase over time has been paralleled by an increase in uterine atony.
Introduction
Postpartum haemorrhage (PPH) is a leading cause of maternal morbidity and mortality worldwide [1, 2]. The World Health Organization (WHO) definition of postpartum haemorrhage encompasses all blood losses over 500 ml within 24 hours after birth [3]. The most common causes associated with postpartum haemorrhage are uterine atony, retained placenta and postpartum coagulation defects [4, 5]. Known risk factors include advanced maternal age, hypertensive disorders, chorioamnionitis, placenta praevia, polyhydramnios, labour induction, prolonged and augmented labour, and vaginal operative delivery [4, 6–8].
Population-based studies from Ireland, Canada, Australia and the USA have demonstrated an increase in the incidence of postpartum haemorrhage during the past decade [9–12]. Changes in maternal demographics and obstetric practice may have contributed to the rising numbers of postpartum haemorrhages, such as older maternal age or the rising numbers of caesarean deliveries [5]. The objective of this study was two-fold: to investigate the incidence of postpartum haemorrhage and to assess changes in risk factors in Switzerland during the last two decades.
Materials and methods
This was a retrospective population-based cohort study using de-identified data from the national Swiss Hospital in-patient database for obstetric and gynaecological hospital admissions – “Arbeitsgemeinschaft Schweizer Frauenkliniken” (ASF) database – from January 1993 to December 2014. This database contains detailed individual patient demographics, hospital admission data, and discharge and delivery data of approximately 40% of all deliveries in Switzerland, including rural and urban hospitals as well as teaching hospitals. The database is primarily used for clinical services auditing by the participating hospitals. The Swiss Society of Obstetrics and Gynaecology is responsible for the data, which is maintained by Sevisa AG. Data for this database are collected by the responsible physicians and then anonymously submitted to Sevisa AG. Between January 1993 and December 2004, all diagnoses and procedures were coded according to an ASF-database specific code. Beginning in January 2005, the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10), was also added for most items in the database. ICD-10 codes are provided in the text in brackets after the corresponding items if applicable.
We extracted all patients from the ASF database who delivered between January 1993 and December 2014, including preterm deliveries (>24+0) and multiple pregnancies. The sample comprised all modes of delivery (vaginal delivery, instrumental delivery and caesarean section). Individual patient data sets were assessed. We identified patients with postpartum haemorrhage using the ASF data and/or ICD-10 codes as follows: Overall postpartum haemorrhage (O72), postpartum haemorrhage resulting from uterine atony (O72.1 ± O43.2), retained, trapped or adherent placenta (O72.0), delayed secondary postpartum haemorrhage (O72.2), and postpartum haemorrhage associated with postpartum coagulation defects (O72.3). In the ASF database, “postpartum haemorrhage” is defined as an estimated postpartum blood loss over 500 ml as defined by the WHO [3]. In addition, for the purposes of our study we defined “severe postpartum haemorrhage” as postpartum haemorrhage requiring blood transfusion and/or emergency hysterectomy. The dataset provided information on whether a patient required packed red blood cell (RBC) transfusion. We only used data on postpartum anaemia defined as haemoglobin over 80 g/dl collected from 2005 to 2014 because of a change in the definition of postpartum anaemia in the ASF database. Before 2005 postpartum anaemia in the ASF database was defined as haemoglobin below 100 g/dl.
Maternal factors
We derived maternal demographics, such as age, parity and history of caesarean section form the ASF data set.
Pregnancy-related factors
Pregnancy-related factors such as gestational hypertension (O13), preeclampsia (O14), polyhydramnios (O40), chorioamnionitis (O41.1), placenta praevia (O44.0), placental abruption (O45), twin pregnancy (O30.0), and abnormally invasive placenta (O43.2) were derived from the ASF dataset. Fetal macrosomia was defined as fetal weight above 4000 g.
Delivery-related factors
Delivery-related factors included induction of labour, mode of delivery (planned caesarean section (O82), emergency caesarean section in labour (O82), operative vaginal delivery (O81), and spontaneous vaginal delivery (O80)), bleeding from vaginal/perineal laceration, episiotomy, use of epidural anaesthesia, prolonged first stage of labour (defined as >12h) (O63), prolonged second stage of labour (defined as over one hour full dilation in primipara and in multipara, with or without peridural anaesthesia) (O63), labour augmentation using oxytocin, fever in labour (>38°C) (O75.2), blood transfusion, and hysterectomy.
Statistical analysis
Data were analysed after collecting all maternal factors, pregnancy-related factors and delivery-related factors of all extracted patients. Postpartum haemorrhage and related factors were reported per 100 deliveries and analysed over the 22-year period from 1993 until 2014. Temporal trends were assessed using the Linear-by-Linear Association (LLA; Mantel-Haenszel test for trend). The LLA test for trend equals (N-1)*r^2, with N as sample size and r being the Pearson correlation between the two variables. A p-value <0.05 for the test is consistent with the presence of a statistically significant trend. Year of delivery was used as the independent variable. Risk factors for overall and severe postpartum haemorrhage were analysed using cross-tabulations and logistic regression analysis to estimate unadjusted and adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs). Covariates adjusted for in the multivariable logistic regression models included maternal age (≤19, 20–34, 35–39, and ≥40 years) and other maternal factors, and the pregnancy-related and delivery-related factors derived from the ASF database as mentioned above. The covariates are known or potential risk factors for PPH, based on published literature [6–9]. All analyses were conducted using SPSS Statistics Version 22 (IBM Corp., Armonk, NY). The study was exempt from institutional board review because only de-identified, anonymised data were used.
Results
Postpartum haemorrhage
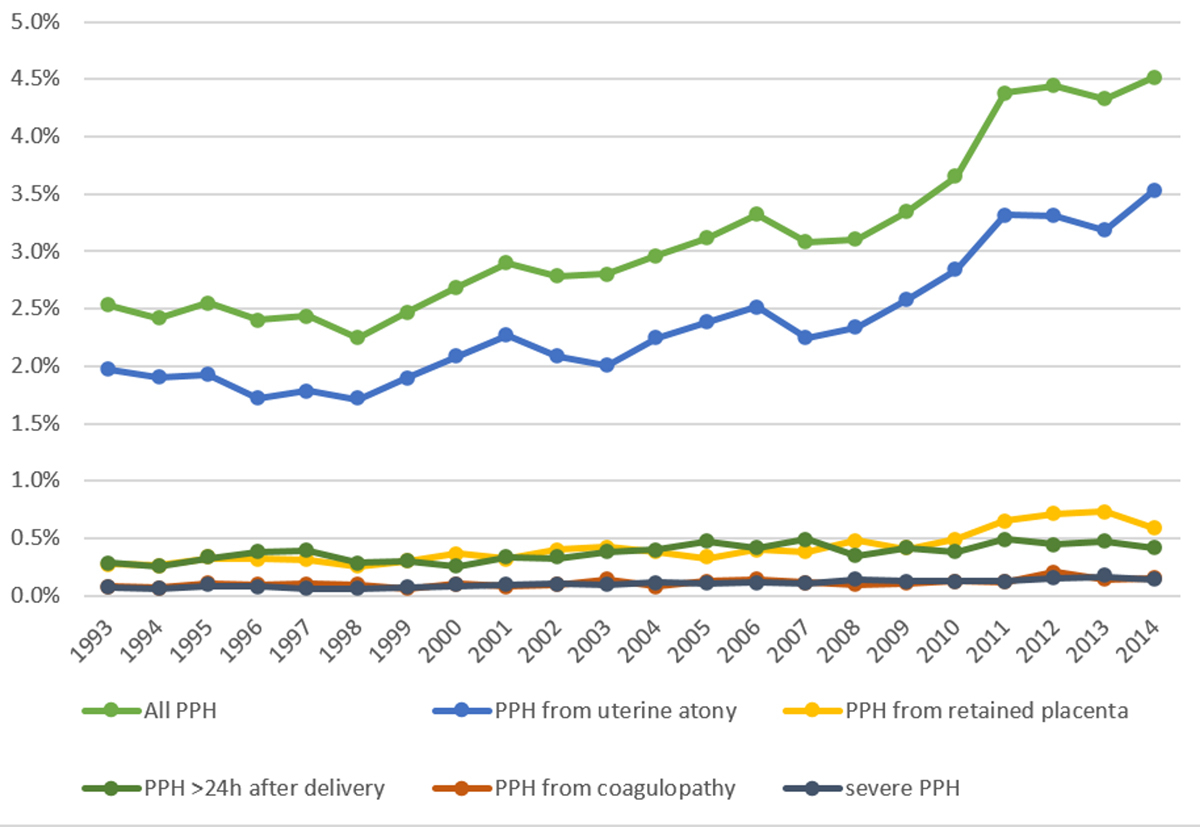
Over the 22-year period, 739 444 deliveries were documented in the ASF database. Postpartum haemorrhage occurred in 22 940 deliveries (3.1%). The postpartum haemorrhage rate was 2.5% in 1993 and increased significantly over time to 4.5% in 2014 (p <0.001) (fig. 1). A total of 761 severe postpartum haemorrhages was noted (0.1%). The rate of severe postpartum haemorrhage doubled from 0.07% in 1993 to 0.14% in 2014 (p <0.001). Postpartum haemorrhage resulting from uterine atony increased from 2% in 1993 to 3.5% in 2014 (p <0.001). Postpartum haemorrhage due to retained placenta (from 0.27 to 0.58%, p <0.001), from postpartum coagulation defects (0.05 to 0.15%, p = 0.002) and delayed postpartum haemorrhage (from 0.29 to 0.42%; p <0.001) increased over time. The numbers of patients requiring red blood cell transfusion increased from 0.75 to 0.97% (p <0.001). The numbers of patients with postpartum anaemia remained unchanged between the years 2005 and 2014 (2.9 vs 3.7%, trend p = 0.778).
Maternal factors
Maternal, pregnancy and delivery-related factors for 1993 and 2004 are shown in table 1. Maternal age during the study period showed a steady increase (p <0.001), especially the proportion of women older than 35 years. In 1993 only 1.3% of all mothers were 40 years or older; this number rose to 3.3% in 2014. The incidence of primiparous women of 40 years and older increased from 0.3 to 1.7% (p <0.001). The numbers of women with history of caesarean section almost doubled in the same time period, from 7.4 to 14.0% (p <0.001). Maternal age at parturition was not associated with a risk of PPH.
Table 1 Risk factors associated with postpartum haemorrhage.
|
1993
(n = 33 465)
%
|
2014
(n = 33 612)
%
|
p-value for trend
|
Odds ratio
(95% CI)
|
Adjusted odds ratio (95% CI)
|
|
Maternal factors
|
|
|
|
|
|
| Age groups
|
|
|
|
|
|
| Age <19 |
1.4 |
1.1 |
<0.001 ↓ |
0.9 (0.8–1.0) |
0.9 (0.8–1.0) |
| Age 20–34 |
87.8 |
78.4 |
<0.001 ↓ |
1.0 (1.0–1.0) |
1.0 (reference) |
| Age 35–39 |
9.5 |
17.2 |
<0.001 ↑ |
1.0 (1.0–1.0) |
1.0 (1.0–1.0) |
| Age >40 |
1.3 |
3.3 |
<0.001 ↑ |
1.1 (1.0–1.2) |
1.1 (1.0–1.2)* |
| Prior caesarean delivery |
7.4 |
14 |
<0.001 ↑ |
0.7 (0.7–0.8) |
1.0 (0.9–1.0) |
| Primiparity >40years |
0.3 |
1.7 |
<0.001 ↑ |
1.0 (0.9–1.2) |
0.9 (0.8–1.1) |
| Multiparity |
56.2 |
52.1 |
<0.001 ↓ |
.9 (0.9–1.0) |
1.0 (1.0–1.0) |
|
Pregnancy-related factors
|
|
|
|
|
|
| Fetal macrosomia |
9.4 |
8.3 |
<0.001 ↓ |
1.6 (1.6–1.7) |
1.6 (1.6–1.7)* |
| Hypertension / pre-eclampsia / eclampsia / HELLP |
3.3 |
3.0 |
0.531 → |
1.7 (1.6–1.8) |
1.8 (1.7–1.9)* |
| Polyhydramnios |
0.3 |
0.9 |
<0.001 ↑ |
1.4 (1.2–1.7) |
1.3 (1.2–1.6)* |
| Chorioamnionitis |
0.2 |
0.3 |
0.513 → |
1.1 (0.9–1.5) |
1.0 (0.8–1.4) |
| Placenta praevia |
0.3 |
0.5 |
<0.001 ↑ |
3.6 (3.1–4.0) |
4.9 (4.3–5.6)* |
| Placental abruption |
1.3 |
1.5 |
<0.001 ↑ |
1.7 (1.6–1.9) |
1.8 (1.7–2.0)* |
| Twin-pregnancy |
0.8 |
1.6 |
<0.001 ↑ |
1.9 (1.7–2.0) |
2.5 (2.2–2.7)* |
| Abnormally invasive placenta |
0.2 |
0.3 |
0.035 ↑ |
11.1 (10.1–12.2) |
10.4 (9.5–11.5)* |
|
Delivery-related factors
|
|
|
|
|
|
| Induction of labour |
15.4 |
19.0 |
0.002 ↑ |
1.4 (1.4–1.5) |
1.3 (1.2–1.3)* |
| Delivery Mode
|
|
|
|
|
|
| Spontaneous vaginal |
77.0 |
58.6 |
<0.001 ↓ |
1.2 (1.1–1.2) |
1.0 (reference) |
| Operative vaginal |
9.5 |
10.5 |
<0.001 ↑ |
1.6 (1.6–1.7) |
1.1 (1.1–1.2)* |
| Elective caesarean |
7.0 |
16.0 |
<0.001 ↑ |
0.5 (0.5–0.5) |
0.4 (0.4–0.5)* |
| Emergency caesarean |
6.5 |
14.9 |
<0.001 ↑ |
0.7 (0.7–0.8) |
0.6 (0.5–0.6)* |
| Vaginal/perineal laceration |
0.3 |
0.6 |
<0.001 ↑ |
9.6 (8.8–10.5) |
7.6 (6.9–8.3)* |
| Episiotomy |
59.9 |
13.1 |
<0.001 ↓ |
1.2 (1.2–1.2) |
0.9 (0.8–0.9)* |
| Epidural anaesthesia |
9.9 |
27.3 |
<0.001 ↑ |
1.2 (1.1–1.2) |
1.0 (0.9–1.0)* |
| Prolonged 1st stage |
2.1 |
2.4 |
0.004 ↑ |
1.4 (1.3–1.5) |
1.2 (1.1–1.3)* |
| Prolonged 2nd stage |
4.5 |
8.4 |
<0.001 ↑ |
2.0 (1.9–2.1) |
1.5 (1.5–1.6)* |
| Labour augmentation, using oxytocin |
24.6 |
26.2 |
0.277 → |
1.5 (1.4–1.5) |
1.2 (1.2–1.3)* |
| Fever during labour (>38°C) |
0.4 |
0.5 |
<0.001 ↑ |
1.7 (1.5–2.0) |
1.6 (1.4–1.9)* |
Pregnancy-related factors
Incidence of most pregnancy-related risk factors increased over time as well (table 1). The risk of postpartum haemorrhage was significantly higher in patients with placenta praevia (adjusted OR 4.8, 95% CI 4.2–5.5) and especially in patients with abnormally invasive placenta (adjusted OR 10.2, 95% CI 9.3–11.3). The incidence of placenta praevia increased from 0.3 to 0.5% (p <0.001) (fig. 2). The numbers of abnormally invasive placentation increased from 0.2 to 0.3% (p = 0.035) (fig. 2). Chorioamnionitis did not increase the risk of PPH.
Delivery-related factors
Delivery-related factors also changed over time (table 1). Induction of labour was more frequently applied over the years, in 15.4% of deliveries in 1993 to 19.0% in 2014 (trend p = 0.002). Prolonged first and second stages of labour was more frequent from 2.1 to 2.5% (trend p = 0.004), and 4.5 to 8.4% (trend p <0.001), respectively. Oxytocin use for augmentation of contractions remained stable (24.6 vs 26.2%, trend p = 0.277). The proportion of deliveries with episiotomies decreased from 59.9 to 13.1% during the study period (trend p <0.001). The use of epidural anaesthesia increased greatly from 9.9 to 27.3% (trend p <0.001). In 1993, around three quarters (77%) of women had a spontaneous vaginal delivery. In 2014 only 58.6% of women delivered spontaneously. Caesarean deliveries showed a substantial increase from 13.5% in 1993 to 30.9% in 2014 with increasing rates of both elective and emergency caesarean sections (table 1). Operative vaginal deliveries also showed an increase from 9.5 to 10.5% over time. All changes in delivery modes over time showed statistically significant trends (all p <0.001).
The incidence of postpartum haemorrhage increased over all delivery modes during the study period (fig. 3). Operative vaginal delivery was associated with the highest rate of postpartum haemorrhage, followed by spontaneous vaginal delivery, emergency caesarean delivery and elective caesarean delivery.
Discussion
The incidence of postpartum haemorrhage in Switzerland almost doubled from 1993 to 2014. This substantial increase was paralleled with increasing numbers of uterine atony. Seventy-seven percent of all postpartum haemorrhages were associated with uterine atony. Although abnormally invasive placentation and placenta praevia accounted for the highest risk of postpartum haemorrhage, their overall numbers in the population remained relatively low, suggesting that these factors cannot be made accountable for the increase in postpartum haemorrhage. The use of blood transfusions paralleled the increasing incidence of postpartum haemorrhage. Interestingly, the number of patients with postpartum anaemia remained stable over the years, which indicates a timely diagnosis of postpartum haemorrhage and adequate intervention. Furthermore, changes in maternal demographics or delivery mode could not sufficiently explain the increase in postpartum haemorrhage.
During the study period, progressively longer durations of labour were accepted by healthcare professionals, both in the first and in the second stage of labour. The wide availability of epidural anaesthesia paralleled longer delivery durations. Perinatal interventions to shorten the duration of the second stage, such as labour augmentation with oxytocin and operative vaginal delivery were associated with higher risks of postpartum haemorrhage. Our results underline that postpartum haemorrhage is a relatively common obstetric complication and the documented increase in incidence over time may be partially attributed to a more liberal approach regarding the duration of labour, meaning longer time intervals until intervention such as operative vaginal interventions or caesarean section are generally accepted.
The strengths of our study were the long timespan of 22 years, whereas most previous studies on this topic have been restricted to only a few years of observation, and the population-based design that included approximately 40% of all hospital deliveries in Switzerland [5, 10]. Furthermore, our data source included deliveries from rural hospitals, urban teaching hospitals, and urban non-teaching hospitals, whereas other studies were limited to tertiary care centres.
Similar to other studies, we showed an increasing incidence of risk factors for postpartum haemorrhage, such as placenta praevia and abnormally invasive placenta, as well as an increase in maternal age [5, 10–21]. Changes in perinatal care issues with a more liberal approach to the duration of labour, especially the second stage, and the rising numbers of labour inductions may be associated with the rising numbers of postpartum haemorrhage. Similarly, studies from France, Israel, Australia and the USA showed the significance of these factors for the development of postpartum haemorrhage [6–8, 22–26]. A Norwegian study “blamed” the increasing numbers of caesarean deliveries for the rise in postpartum haemorrhage [27]. Combs et al. demonstrated that risk factors for haemorrhage during caesarean delivery were a protracted active phase of labour and second-stage arrest [24]. In contrast, in our data caesarean sections (elective and emergency) showed odds ratios smaller than 1, suggesting some protective potential for postpartum haemorrhage compared to spontaneous vaginal delivery. This result is most likely skewed and might reflect at least to some degree low quality in the data acquisition in the first place. Visual estimation of blood loss in vaginal deliveries is difficult and not objective. For caesarean deliveries, postpartum haemorrhage is sometimes defined as blood loss of greater than 1000 ml, making a direct comparison between delivery modes difficult [28].
A major limitation of our study was the underlying blood loss estimation in the participating hospitals, which then translated into some uncertainty in the ASF database. There might be under- or over-diagnosing of postpartum haemorrhage because of clinical diagnostic subjectivity. Nevertheless, the results of our study are consistent with previous reports and clinical expectations, suggesting that our findings are likely to be valid [5, 10]. Lutomski et al. highlighted the need for further research focusing on aetiological factors of postpartum haemorrhage and preventative measures [12]. Contributing factors for the increased number of prolonged second stage of labour could be the more frequent use of epidural analgesia and the increased proportion of primiparous women. Nevertheless, prolonged second stage was an independent risk factor for postpartum haemorrhage, even after correction for both of these factors. Another limitation was the change of the questionnaire in 2004/2005, which is a potential source of bias. Since the definition of postpartum haemorrhage did not change, we believe that our results are not substantially affected by that change.
In conclusion, postpartum haemorrhage is a relatively common and potentially dangerous obstetrical complication with increasing incidence over the last two decades in Switzerland. Its increase over time may be partially attributed to a more liberal approach regarding longer durations of labour and subsequent increase in uterine atony.
References
1
Khan
KS
,
Wojdyla
D
,
Say
L
,
Gülmezoglu
AM
,
Van Look
PF
. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–74. doi:.https://doi.org/10.1016/S0140-6736(06)68397-9
2
Berg
CJ
,
Callaghan
WM
,
Syverson
C
,
Henderson
Z
. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116(6):1302–9. doi:.https://doi.org/10.1097/AOG.0b013e3181fdfb11
3World Health Organization. WHO guidelines for the management of postpartum haemorrhage and retained placenta. Geneva; World Health Organization; 2009.
4
Oyelese
Y
,
Ananth
CV
. Postpartum hemorrhage: epidemiology, risk factors, and causes. Clin Obstet Gynecol. 2010;53(1):147–56. doi:.https://doi.org/10.1097/GRF.0b013e3181cc406d
5
Bateman
BT
,
Berman
MF
,
Riley
LE
,
Leffert
LR
. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–73. doi:.https://doi.org/10.1213/ANE.0b013e3181d74898
6
Combs
CA
,
Murphy
EL
,
Laros
RK, Jr
. Factors associated with postpartum hemorrhage with vaginal birth. Obstet Gynecol. 1991;77(1):69–76.
7
Sheiner
E
,
Sarid
L
,
Levy
A
,
Seidman
DS
,
Hallak
M
. Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population-based study. J Matern Fetal Neonatal Med. 2005;18(3):149–54. doi:.https://doi.org/10.1080/14767050500170088
8
Magann
EF
,
Evans
S
,
Hutchinson
M
,
Collins
R
,
Howard
BC
,
Morrison
JC
. Postpartum hemorrhage after vaginal birth: an analysis of risk factors. South Med J. 2005;98(4):419–22. doi:.https://doi.org/10.1097/01.SMJ.0000152760.34443.86
9
Cameron
CA
,
Roberts
CL
,
Olive
EC
,
Ford
JB
,
Fischer
WE
. Trends in postpartum haemorrhage. Aust N Z J Public Health. 2006;30(2):151–6. doi:.https://doi.org/10.1111/j.1467-842X.2006.tb00109.x
10
Ford
JB
,
Roberts
CL
,
Simpson
JM
,
Vaughan
J
,
Cameron
CA
. Increased postpartum hemorrhage rates in Australia. Int J Gynaecol Obstet. 2007;98(3):237–43. doi:.https://doi.org/10.1016/j.ijgo.2007.03.011
11
Joseph
KS
,
Rouleau
J
,
Kramer
MS
,
Young
DC
,
Liston
RM
,
Baskett
TF
; Maternal Health Study Group of the Canadian Perinatal Surveillance System. Investigation of an increase in postpartum haemorrhage in Canada. BJOG. 2007;114(6):751–9. doi:.https://doi.org/10.1111/j.1471-0528.2007.01316.x
12
Lutomski
JE
,
Byrne
BM
,
Devane
D
,
Greene
RA
. Increasing trends in atonic postpartum haemorrhage in Ireland: an 11-year population-based cohort study. BJOG. 2012;119(3):306–14. doi:.https://doi.org/10.1111/j.1471-0528.2011.03198.x
13
Chantraine
F
,
Braun
T
,
Gonser
M
,
Henrich
W
,
Tutschek
B
. Prenatal diagnosis of abnormally invasive placenta reduces maternal peripartum hemorrhage and morbidity. Acta Obstet Gynecol Scand. 2013;92(4):439–44. doi:.https://doi.org/10.1111/aogs.12081
14
Callaghan
WM
,
Kuklina
EV
,
Berg
CJ
. Trends in postpartum hemorrhage: United States, 1994-2006. Am J Obstet Gynecol. 2010;202(4):353.e1–6. doi:.https://doi.org/10.1016/j.ajog.2010.01.011
15
Mercier
FJ
,
Van de Velde
M
. Major obstetric hemorrhage. Anesthesiol Clin. 2008;26(1):53–66, vi. doi:.https://doi.org/10.1016/j.anclin.2007.11.008
16
Smulian
JC
,
Ananth
CV
,
Kinzler
WL
,
Kontopoulos
E
,
Vintzileos
AM
. Twin deliveries in the United States over three decades: an age-period-cohort analysis. Obstet Gynecol. 2004;104(2):278–85. doi:.https://doi.org/10.1097/01.AOG.0000134524.58795.bd
17
Al-Zirqi
I
,
Vangen
S
,
Forsen
L
,
Stray-Pedersen
B
. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115(10):1265–72. doi:.https://doi.org/10.1111/j.1471-0528.2008.01859.x
18
Green
L
,
Knight
M
,
Seeney
FM
,
Hopkinson
C
,
Collins
PW
,
Collis
RE
, et al.
The epidemiology and outcomes of women with postpartum haemorrhage requiring massive transfusion with eight or more units of red cells: a national cross-sectional study. BJOG. 2016;123(13):2164–70.
19
Thurn
L
,
Lindqvist
PG
,
Jakobsson
M
,
Colmorn
LB
,
Klungsoyr
K
,
Bjarnadóttir
RI
, et al.
Abnormally invasive placenta-prevalence, risk factors and antenatal suspicion: results from a large population-based pregnancy cohort study in the Nordic countries. BJOG. 2016;123(8):1348–55. doi:.https://doi.org/10.1111/1471-0528.13547
20
Kayem
G
,
Dupont
C
,
Bouvier-Colle
MH
,
Rudigoz
RC
,
Deneux-Tharaux
C
. Invasive therapies for primary postpartum haemorrhage: a population-based study in France. BJOG. 2016;123(4):598–605. doi:.https://doi.org/10.1111/1471-0528.13477
21
Briley
A
,
Seed
PT
,
Tydeman
G
,
Ballard
H
,
Waterstone
M
,
Sandall
J
, et al.
Reporting errors, incidence and risk factors for postpartum haemorrhage and progression to severe PPH: a prospective observational study. BJOG. 2014;121(7):876–88. doi:.https://doi.org/10.1111/1471-0528.12588
22
Dionne
MD
,
Deneux-Tharaux
C
,
Dupont
C
,
Basso
O
,
Rudigoz
RC
,
Bouvier-Colle
MH
, et al.
Duration of Expulsive Efforts and Risk of Postpartum Hemorrhage in Nulliparous Women: A Population-Based Study. PLoS One. 2015;10(11):e0142171. doi:.https://doi.org/10.1371/journal.pone.0142171
23
Driessen
M
,
Bouvier-Colle
MH
,
Dupont
C
,
Khoshnood
B
,
Rudigoz
RC
,
Deneux-Tharaux
C
; Pithagore6 Group. Postpartum hemorrhage resulting from uterine atony after vaginal delivery: factors associated with severity. Obstet Gynecol. 2011;117(1):21–31. doi:.https://doi.org/10.1097/AOG.0b013e318202c845
24
Combs
CA
,
Murphy
EL
,
Laros
RK, Jr
. Factors associated with hemorrhage in cesarean deliveries. Obstet Gynecol. 1991;77(1):77–82.
25
Rouse
DJ
,
Leindecker
S
,
Landon
M
,
Bloom
SL
,
Varner
MW
,
Moawad
AH
, et al.; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. The MFMU Cesarean Registry: uterine atony after primary cesarean delivery. Am J Obstet Gynecol. 2005;193(3):1056–60. doi:.https://doi.org/10.1016/j.ajog.2005.07.077
26
Vendittelli
F
,
Barasinski
C
,
Pereira
B
,
Lémery
D
; HERA Group. Incidence of immediate postpartum hemorrhages in French maternity units: a prospective observational study (HERA study). BMC Pregnancy Childbirth. 2016;16(1):242. doi:.https://doi.org/10.1186/s12884-016-1008-7
27
Rossen
J
,
Okland
I
,
Nilsen
OB
,
Eggebø
TM
. Is there an increase of postpartum hemorrhage, and is severe hemorrhage associated with more frequent use of obstetric interventions?
Acta Obstet Gynecol Scand. 2010;89(10):1248–55. doi:.https://doi.org/10.3109/00016349.2010.514324
28
Natrella
M
,
Di Naro
E
,
Loverro
M
,
Benshalom-Tirosh
N
,
Trojano
G
,
Tirosh
D
, et al.
The more you lose the more you miss: accuracy of postpartum blood loss visual estimation. A systematic review of the literature. J Matern Fetal Neonatal Med. 2017 Jan 12. Epub ahead of print. doi:.https://doi.org/10.1080/14767058.2016.1274302