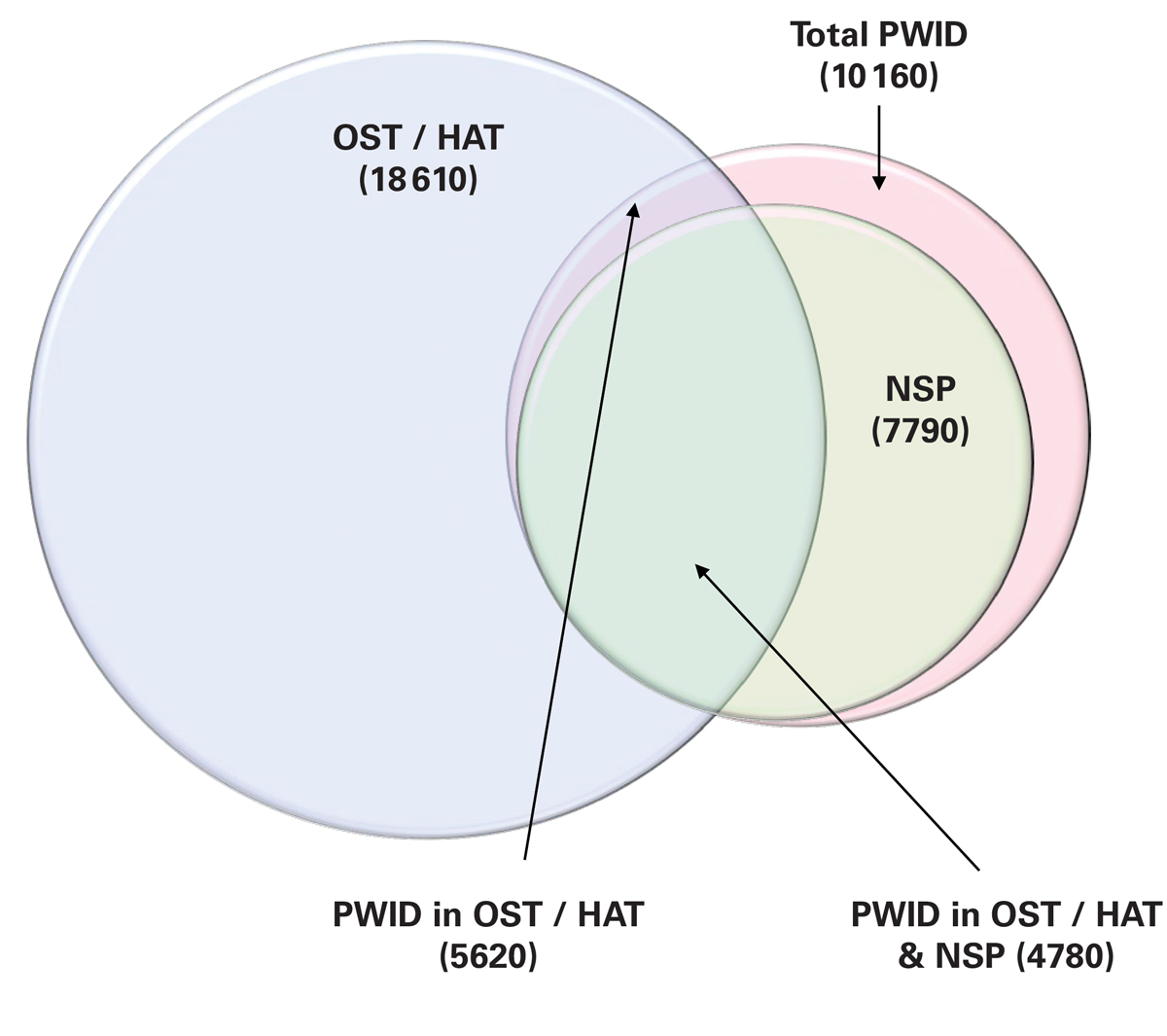
Figure 1 Size of the OST/HAT, NSP and PWID populations, 2015.
HAT = heroin assisted therapy; NSP = needle and syringe programme; OST = opiate substitution therapy; PWID = people who inject drugs
DOI: https://doi.org/10.4414/smw.2017.14543
Modelling predicted that in 2013 there were nearly 83 000 viraemic hepatitis C virus (HCV) infections in Switzerland, a prevalence rate of approximately 1.0% [1]. Of those, between 34 and 67% had a history of injection drug use [2]. HCV-related complications are projected to increase in Switzerland over the next 15 years unless measures are taken to reduce progression to advanced hepatic fibrosis [1]. Focusing treatment on patients with advanced HCV disease may be effective at preventing HCV-related complications; however, it will do little to prevent new infections. Treatment of HCV-infected people who inject drugs (PWID) has been proposed as a method of preventing the spread of HCV and a key step in the elimination of the disease; however, more information is needed to understand the impact that treatment within this population will have on HCV incidence and prevalence.
In the wake of a disastrous escalation in illicit drug use during the 1980s, Switzerland extended its strategic three-pillar concept of prevention, treatment and law enforcement to include a fourth pillar – harm reduction. This four-fold policy has since become a success story. Its strategic objectives include the decriminalisation of drug use, education to encourage young people away from drugs, and medical and social assistance for drug addicts. The main components of the harm reduction measures are needle and syringe exchange programmes (NSPs) and consumption rooms run by major cities where users can inject or smoke their drugs using sterile tools [3]. Although opioid substitution treatment (OST) and heroin-assisted therapy (HAT) are part of the treatment pillar, for the purposes of this analysis they are considered to be a harm reduction measure because of their ability to reduce injection frequency and prevent the transmission of HCV [4].
The percentage of injectors among PWID in Switzerland has been decreasing as a result of these harm reduction activities and the aging PWID population (average age 44 years) [5–7]. Provision of methadone substitution therapy for opiate addicts has been covered by Swiss law since 1975, but also falls under the jurisdiction of local cantonal statutes [8]. Methadone statistics are collected annually by cantonal medical offices and provided to the Swiss Federal Office of Public Health (FOPH), where an annual registry is maintained [8]. HAT is available as a last-resort treatment for adult long-term opioid users who have failed at least two previous attempts at treatment [9]. HAT was first introduced in Switzerland in 1994 and has been covered by the Federal Law on Narcotics since 2008 [9, 10]. HAT participation has been monitored by the Swiss Research Institute for Public Health since 2000, via the Swiss monitoring system of HAT [9, 11].
Additionally, NSPs are available to all PWID in Switzerland, although uptake of services is difficult to estimate. Alongside the provision of sterile needles and syringes, NSPs provide sterile filters, water and cookers. This equipment is provided through safe injecting rooms, low threshold facilities, methadone clinics, general practitioners, pharmacies and in some cities can be purchased through vending machines. NSPs are also provided in some prisons; however, this practice does not occur nationwide.
Understanding the impact of harm reduction efforts and treatment on the HCV infected PWID population requires robust epidemiological modelling. The goals of this project were twofold: (1) to develop a simple model to estimate the number of new HCV infections using available data on PWID; (2) to examine the impact of intervention strategies (prevention and treatment) on new and total HCV infections among PWID.
In order to model the PWID population, standard definitions were necessary. For the purposes of this analysis, PWID referred to an individual who has injected illicit drugs within the past 12 months. OST participant referred to an individual receiving opiate substitution to manage past or present opioid addiction. Subsets of OST participants continue to use illicit drugs (parallel consumption), and among those who use illicit drugs, some do so by means of injection. High coverage NSP is defined as one or more sterile syringes received for every injection. This was estimated using the number of syringes distributed and the average number of injections per day.
The HCV transmission model was developed as an add-on module to a previously described HCV disease burden model [1, 12, 13]. Among PWID, the population was defined by HCV infection (susceptible or infected) and risk-behaviour status (sharing needles or not sharing). PWID were modelled to move across segments in a limited number of directions. Behaviour changes resulting in needle sharing (among those previously not sharing) or in a discontinuation of needle sharing (among those previously sharing) could occur. Additionally, PWID who shared needles and were previously not infected with HCV could become infected. And finally, PWID who were HCV infected (regardless of sharing behaviours) could be treated and cured of their HCV.
PWID exited the model as a result of mortality (estimated at an annual rate of 1.38%) [5] or cessation of injection drug use. The rate of cessation was calculated on an annual basis to match historical trends in the overall PWID population size. The number of new PWID was calculated to balance the number of PWID leaving the population. Additionally, participation in high-coverage NSPs, OST, and NSPs plus OST was modelled. After 2015, all inputs were modelled to remain constant.
New infections were calculated as the probability of infection multiplied by the size of the PWID population that was sharing needles but not currently HCV infected. The estimated probability of infection was calculated from the average annual number of unsafe injections, HCV prevalence among shared syringes and the probability of HCV transmission from a contaminated needle (1.8%, range 0.0–10.3%) [14–16]. Among PWID who did not share injection equipment, the probability of getting infected was dependent on the background HCV prevalence (1% among adults in Switzerland) alone. Engagement in an NSP or OST was assumed to result in a 59% reduction in unsafe injections, and simultaneous involvement in an NSP and OST was assumed to result in an 81% reduction in unsafe injections [17–22]. Among HAT participants, a 75% reduction in unsafe injections was estimated since heroin was dispensed and tied to stationary consumption with clean syringes (expert consensus).
There are 8000–12 000 active PWID in Switzerland, including those who inject while engaged in OST and NSP programmes (personal communication, Dr Jean-Pierre Gervasoni of Centre Hospitalier Universitaire Vaudois, and expert consensus). The number of PWID has been decreasing over the last 20 years owing to harm reduction efforts and the aging PWID population (average age 44 years in 2015) [23].
In a 2015 report analysing the HCV situation among drug users in Switzerland, it was estimated that 56% (27–58%) of PWID are HCV antibody positive, although prevalence rates vary by harm reduction segment as well as by age [24, 25]. On the basis of a spontaneous clearance rate of 25% [26], the viraemic prevalence in the PWID population was 42%, compared with a 1% viraemic prevalence in the general adult population [1]. On average, new PWID were assumed to become HCV infected within 2 years of initiating injecting. As of 2011, approximately 20–30 currently injecting PWID have received antiviral treatment annually for HCV, all of whom were enrolled in OST or HAT (expert consensus).
Since 1999, there have been an average of 17 693 participants enrolled in OST (range 16 960 in 2013 to 18 393 in 2000) [8, 24]. In 2014, there were 17 008 OST participants, among whom an estimated 27.4% (8.3–47.1%) continued to inject illicit drugs while on treatment [8, 24, 27–29]. The percent of OST participants who continued to inject on treatment was estimated from the ARUD centres in Zurich (25.5–28.2% between 2010 and 2013) as well as through OST registries in Zurich (13.5% with a range of 8.3–47.1% in 2013) and in Vaud (36%, or 601 of 1666 patients, between 2001 and 2008) [27–29]. Additionally, in 2013, 1598 individuals were enrolled in HAT [30, 31], of whom 54–66% had been prescribed injectable (or combination oral/injectable) heroin [27, 30]. HAT participation has increased nearly 10% over the past 10 years (1464 participants in 2003) (personal communication, Swiss Research Institute for Public Health).
The average monthly distribution of sterile syringes has been decreasing since the programme’s inception in 1993 [24]. In 2012, approximately 300 000 sterile syringes were distributed per month [24]. Assuming an average use of 1.3 sterile syringes per PWID per day, approximately 7790 PWID were actively engaged with an NSP, based on the estimation that the number of needles per PWID per month is between 29.9 and 76.5 [23]. OST and HAT participants were assumed to use an average of 29.9 sterile needles per month, while PWID not involved in these programmes were assumed to use an average of 53.2 sterile needles per month (average of 29.9 and 76.5) (expert consensus). A midpoint was chosen for the population of PWID not engaged with harm reduction services, to account for PWID who may inject with greater frequency, but not necessarily with a sterile syringe on each occasion (expert consensus).
Scenarios were developed to assess the impact of providing HCV treatment with direct acting antivirals. Projected sustained viral response rates in Switzerland have been described previously [1]. Treatment of 1, 5, 10 or 15% of the total HCV+ PWID population was modelled from 2016, and the resulting impact on total HCV infections and secondary infections was calculated. In the absence of behavioural changes, cured PWID remained susceptible to secondary infection with HCV, with an average time to infection of 2 to 4 years among PWID outside of harm reduction [24].
Additionally, two sub-scenarios were considered under an aggressive treatment paradigm to examine the impact of (1) targeting treatment to OST/HAT programme participants only; (2) providing counselling to result in a 10, 30 or 50% reduction in risk behaviour following sustained viral response.
The uncertainty in inputs, as detailed in table 1, was captured as a range using Beta-PERT. Monte Carlo simulations were run to determine 95% uncertainty intervals (UIs) using Crystal Ball, an Excel add-in by Oracle.
Table 1 Model input data.
| Category | Item | Source | Year | Base-case | Range |
|---|---|---|---|---|---|
| PWID population | Active PWID | [25] | 2000 | 18 415 | 15 900–31 800 |
| [23] | 2006 | 15 113 | 10 950–21 900 | ||
| Dr. Gervasoni; consensus | 2014 | 10 160 | 8000–12 000 | ||
| Mortality rate | [5] | 1.38% | 1.25–58% | ||
| HCV among PWID | Anti-HCV prevalence | [24, 25] | 2011 | 56% | 27–58% |
| Spontaneous clearance rate | [26] | 25% | |||
| Treated | Consensus estimate | 2015 | 25 | 20–30 | |
| Probability of transmission from a contaminated needle | [14–16] | 1.80% | 1.00–3.75% | ||
| Opiate substitution therapy | Participants enrolled | [8] | 1999–2013 | 17 693 | 16 960 |
| 2014 | 17 008 | ||||
| Participants injecting | [8, 24, 27–29] | 2014 | 27.4% | 8.3–47.1% | |
| Reduction in unsafe injections while on OST | [17–22] | 59.0% | 54.0–66.0% | ||
| Heroin assisted therapy | Participants enrolled | [31] | 2003 | 1464 | |
| [30, 31] | 2013 | 1598 | |||
| Participants injecting | [27, 30] | 54–66% | |||
| Needle and syringe programmes | Sterile syringes distributed per month (yearly average) | [24] | 2012 | 300 000 | |
| Needles used per PWID per month | [23]; consensus estimate | 2015 | 53.2 | 29.9–76.5 | |
| Reduction in unsafe injections while on NSP | [17–22] | 59.0% | 54.0–66.0% |
HCV = hepatitis C virus; NSP = needle and syringe programme; OST = opiate substitution therapy; PWID = people who inject drugs
In 2015, there were an estimated 10 160 PWID in Switzerland, more than 85% of whom were engaged in harm reduction efforts including NSPs and/or OST or HAT. Although 18 610 individuals were enrolled in OST or HAT, only 5620 continued to inject. NSP services were accessed by 7790 PWID, and approximately 4780 PWID on an NSP were also being treated with OST or HAT (fig. 1). Overall, 4200 PWID were HCV infected, with an estimated 55 new viraemic HCV infections occurring in 2015 (12.9 per 1000 PWID).

Figure 1 Size of the OST/HAT, NSP and PWID populations, 2015.
HAT = heroin assisted therapy; NSP = needle and syringe programme; OST = opiate substitution therapy; PWID = people who inject drugs
The highest annual number of new viraemic infections occurred in the segment of the PWID population that was not involved in harm reduction (25, 95% UI 23–30, new cases or an infection rate of 19 per 1000 PWID), followed by 20 (95% UI 18–24) cases occurring among PWID engaged in both NSP and OST/HAT (infection rate of 4 per 1000 PWID) (fig. 2). Treating approximately 25 PWID engaged in both NSPs and OST/HAT annually resulted in fewer than five secondary infections annually. Over the next 15 years, the HCV infected PWID population was estimated to decrease by 60% (95% UI 56–61%), probably owing to an aging population and current harm reduction efforts.
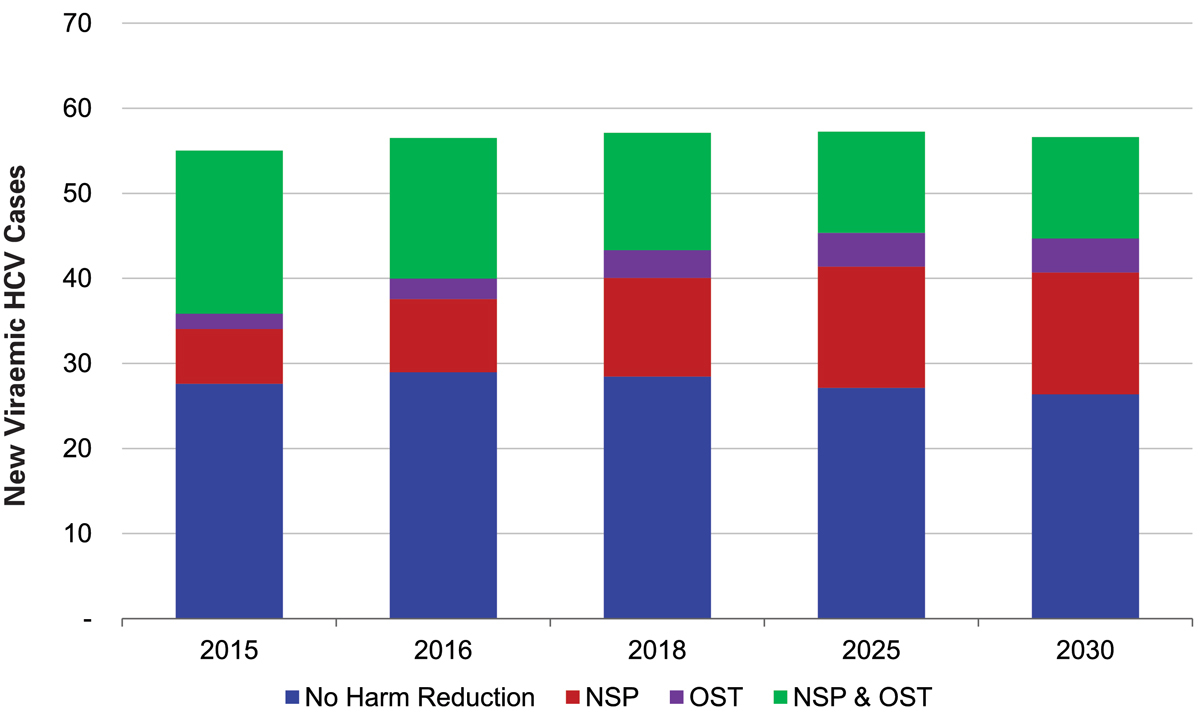
Figure 2 Distribution of new HCV infections among PWID, by segment, 2015-2030.
HAT = heroin assisted therapy; HCV = hepatitis C virus; NSP = needle and syringe programme; OST = opiate substitution therapy; PWID = people who inject drugs
Treatment of 42 patients annually (1% of the HCV+ PWID population) resulted in a 65% decrease in total HCV infections among PWID by 2030 (fig. 3). Secondary infections among treated and cured HCV patients were projected to peak in 2027 at 13 annually and remain constant until 2030. Under this scenario, a total of 660 patients would be treated (including re-treatments) between 2015 and 2030, with a total of 155 secondary infections observed (25.8 secondary infections per 100 treated patients) (fig. 4).
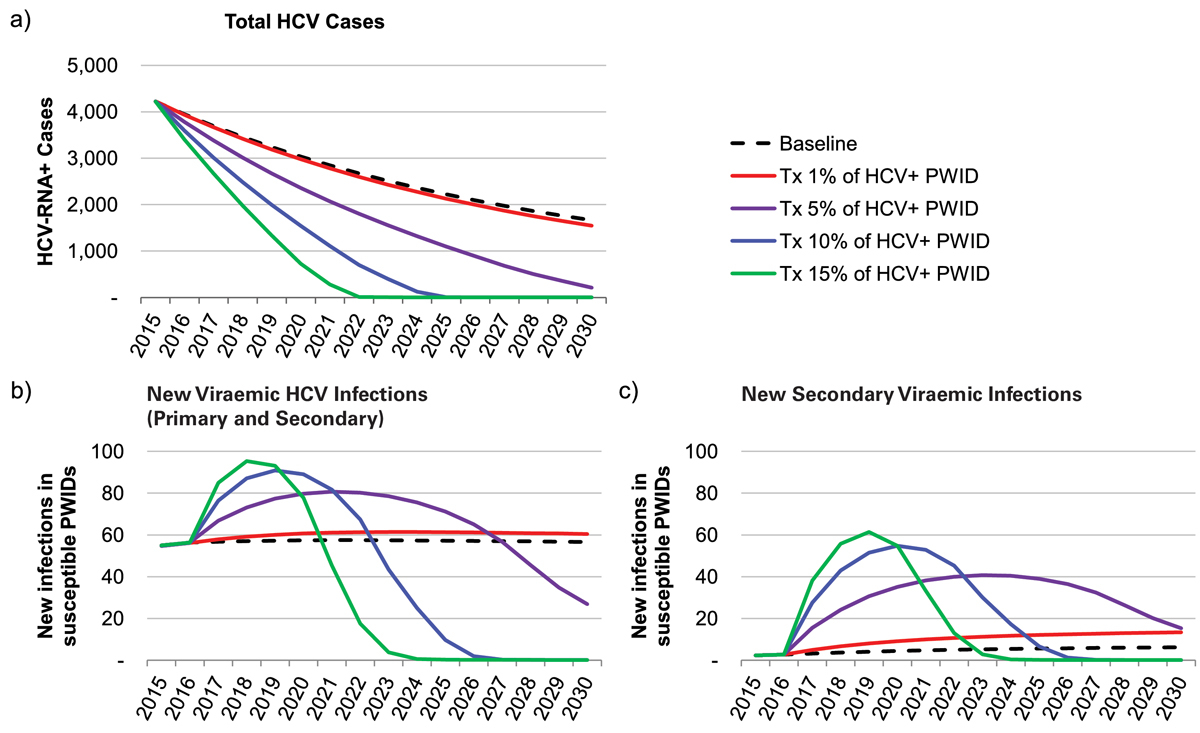
Figure 3 (a) Total HCV cases; (b) viraemic new infections (primary and secondary infections); and (c) viraemic secondary infections, by percent of the total HCV+ PWID population treated, 2015–2030.
HCV = hepatitis C virus; PWID = people who inject drugs
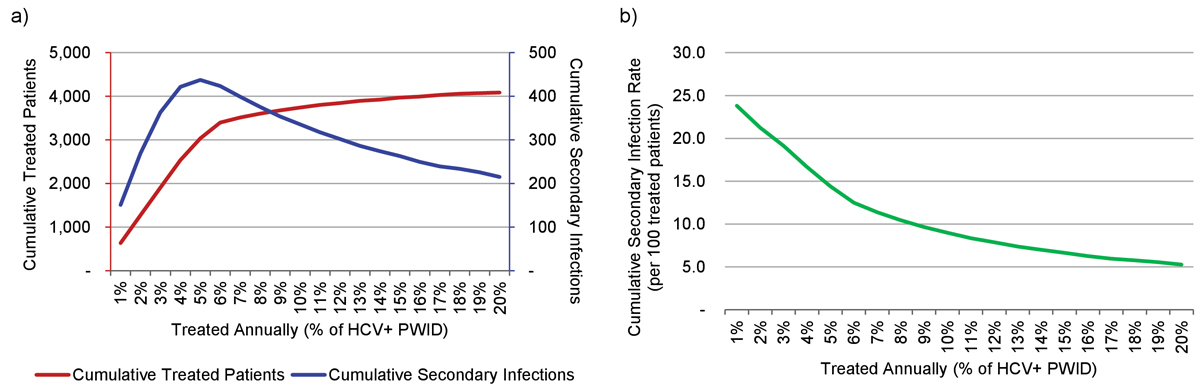
Figure 4 (a) Cumulative treated patients and secondary infections by percent of the HCV+ PWID population treated, 2016–2030; (b) cumulative secondary infection rate (per 100 patients treated) by percent of the HCV-RNA+ PWID population treated, 2016-2030.
HCV = hepatitis C virus; PWID = people who inject drugs
Treatment of 210 patients annually (5% of the HCV+ PWID population) resulted in a 95% reduction in total HCV infections among PWID by 2030. Secondary infections were projected to peak in 2023 at 40, before decreasing to 15 annually by 2030 (fig. 3). Under this scenario, a total of 3055 patients would be treated (including re-treatments) between 2015 and 2030, with a cumulative 440 secondary infections projected (14.4 secondary infections per 100 treated patients) (fig. 4).
Treatment of 425 patients annually (10% of the PWID population) resulted in a greater than 99% reduction in total HCV infections among PWID by 2025. Secondary infections were projected to peak in 2024 at 55, before decreasing to nearly zero annually by 2026 (fig. 3). Under this scenario, treatment of 425 patients annually could be sustained only until 2022, after which point the model ran out of patients and treatment numbers were reduced to fewer than five by 2027. From 2016 to 2030, a total of 3730 patients would be treated (including re-treatments) with a cumulative 335 secondary infections projected (8.9 secondary infections per 100 treated patients) (fig. 4).
Treating 15% of the HCV+ PWID population annually (635 patients annually or 3920 total) achieved >99% reduction one year earlier (2024) than the 10% scenario (fig. 3). Secondary infections peaked earlier (2019) and higher (n = 60), and amounted to a lower cumulative number (n = 260, or 6.6 secondary infections per 100 treated patients) (fig. 4).
Within the 10% treatment scenario, two sub-scenarios were considered.
The aim of this analysis was to estimate the size and distribution of the HCV infected PWID population in Switzerland and to assess the impact of treatment on the total number of HCV infections, as well as the number of secondary infections following a HCV cure. Harm reduction measures including needle and syringe programmes and opiate substitution therapy are estimated to cover 85% of PWID in Switzerland, and the HCV RNA prevalence has been decreasing over time [24]. Assuming constant levels of treatment and harm reduction, the HCV infected PWID population is anticipated to decrease over the next 15 years. Increasing treatment across all segments of PWID (whether engaged in harm reduction programmes or not) will reduce the prevalence of HCV among PWID at every level of treatment. In the absence of behavioural changes, an increase in secondary infections should be anticipated as a result of re-exposure to the HCV virus within an injecting circle. This continued exposure to virus through high-risk activities has been identified, historically, when an individual is found positive for more than one HCV genotype. Although an increase in secondary infections is to be expected, it is only a temporary concern, assuming treatment levels exceed 5% of HCV+ PWID.
PWID engaged in harm reduction programmes had a lower infection rate (4 per 1000 PWID per year, compared with 19 per 1000 PWID per year) than those outside of harm reduction programmes. Treatment of OST/HAT participants alone, however, would not be sufficient to reduce the number of new infections at a national level. From a modelling perspective, this is primarily due to the assumption that some PWID on OST/HAT will inject with individuals who are not engaged in harm reduction, providing a consistent re-introduction of virus into the injecting networks of patients on harm reduction. Providing counselling opportunities to promote reductions in risk behaviour was found to be effective at reducing the number of new infections, even when a low success rate (10%) was assumed.
HCV transmission occurs primarily as a result of intravenous contact with HCV infected blood. In the absence of high-risk behaviours, including the sharing of injection paraphernalia, the risk of HCV transmission is assumed to be negligible. Thus, a history of injection drug use, or even current injection drug use, alone should not be considered contraindications for HCV treatment. Only one quarter of people with a history of injection drug use are estimated to continue injecting, and only 10% of PWID are estimated to share injecting materials. Furthermore, approximately 40% of PWID are HCV-RNA infected. If this relatively small subset of the population (active injection drug users who share injecting materials and are HCV-RNA+) carries the largest risk of acquiring or transmitting HCV, then the remainder of HCV-RNA+ individuals (former injection drug users or active injection drug users who do not share injecting materials) would be recommended for treatment. Because of the complex and unique situation of active drug users, the decision to treat HCV among PWID should be at the discretion of the provider who best knows the individual. Furthermore, a comprehensive approach to treatment, including counselling, linkage to harm reduction services and treatment follow-up should be considered as the best chance to prevent HCV re-exposure.
A few limitations exist within the analysis and the assumptions. Wherever possible, uncertainty in estimates has been captured as a range to inform sensitivity analysis. It is difficult, however, to estimate the size of the hidden PWID population (the population not in contact with harm reduction services) and predict future changes in this population size. Although historical trends show that injection drug use has been decreasing in Switzerland, future uptake of injection drugs remains unknown. In the absence of better information, the total number of PWID was assumed to remain constant after 2015. This could overestimate the number of new infections in the future, as well as the number of treatments needed to curb HCV in this population.
Additionally, changing behaviours among individuals are often lost within aggregated analyses. In this instance, assumptions such as needle sharing and injection frequency are captured as averages and take 1 year to fully implement. However, in real life the individuals contributing to these averages may be at very different risks for HCV. Preferentially directing treatment to PWID who are at highest risk of transmitting HCV owing to sharing behaviours would allow for a more substantial reduction in new infections; however, needle sharing is frequently underestimated as a result of concerns about stigma. Thus, it is important that treatment decisions be made at the provider level, on the basis of the individual, but with adequate systematic resources (including counselling, harm reduction and follow-up care) to prevent re-infection.
Another limitation is that the risk of transmitting or acquiring HCV was considered negligible among PWID who had discontinued injecting, or who were injecting but not sharing injecting equipment. This approach may have underestimated the number of new cases occurring; however, given the lower risk of transmission, it is unlikely that the number of new cases would be substantially increased.
Finally, the ability to model increased treatment does not reflect the availability of resources to provide the treatment. Currently, HCV treatment is provided to PWID at some (but not all) OST centres, as well as at hepatology or infectious disease units based at the secondary and tertiary care level. The present system is not equipped for a large increase in patients, and novel approaches to treatment should be considered. These approaches could include the expansion of treatment to all OST providers and centres and/or the provision of treatment in consumption rooms. It will be important to evaluate the capacity of physicians and harm reduction service providers before recommendations can be made to scale up efforts.
A strategy to treat PWID should carefully consider the impact of time and scale. Rapid aggressive scale-ups in treatment, combined with harm reduction programmes, offer the opportunity to avoid secondary infections in the mid-term, as well as to lower the cumulative number of patients treated. Of course, treatment among PWID who are injecting safely, or among individuals who have discontinued injecting, is always recommended. Cost of treatment with direct acting antivirals and cost savings incurred through prevention of secondary HCV related clinical complications avoided have not been taken into account.
The transmission model used for this study was developed by the Centre for Disease Analysis, and was evaluated in Switzerland by a multi-disciplinary panel of local experts using a Delphi method to achieve consensus. The panel members met in person on four occasions to discuss the study and outcomes, and fulfilled the following criteria for authorship:
1. Contributing substantially to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work.
2. Drafting the work or revising it critically for important intellectual content.
3. Giving final approval of the version of the manuscript to be submitted.
4. Agreeing to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The Swiss expert panel members are listed below and as authors on this study.
− Philip Bruggmann,
− Pierre Deltenre
− Jan Fehr
− Roger Kouyos
− Daniel Lavanchy
− Bat Müllhaupt
− Andri Rauch
− Patrick Schmid
− David Semela
− Martin Stöckle
− Francesco Negro
Additionally, the authors would like to acknowledge the following people for their valuable contributions of data and insight upon request:
− Dr Jean-Pierre Gervasoni, Centre Hospitalier Universitaire Vaudois, Switzerland
− Dr Carlos Nordt, Psychiatric University Hospital, Zurich, Switzerland
The authors acknowledge the contribution to this work of the Swiss Federal Office of Public Health.
This work was supported by Gilead Sciences. Gilead Sciences had no input on the content, the study design, data selection, decision to publish or preparation of the manuscript. Philip Bruggmann has received honoraria for advisory board and speaker fees as well as research and project grants from Abbvie, BMS, Gilead Sciences and MSD. Jan Fehr received grants from BMS, Gilead Sciences, Janssen and MSD. Roger Koynos received travel grants and honoraria from Gilead Sciences. Beat Müllhaupt has served as an advisory board member for MSD, Janssen Therapeutics, Abbvie, Boehringer Ingelheim, Gilead Sciences and BMS; as a consultant for Gilead Sciences and Abbvie; and has received research grants from Gilead Sciences. Andri Rauch has received honoraria for advisory boards and/or travel grants from Janssen-Cilag, MSD, Gilead Sciences and Abbvie. Marcel Stöckle has served as an advisory board member for Abbvie, Gilead Sciences and MSD. Franco Nero was advisor or consultant for Gilead Sciences, Abbvie and Merck and has received research grants from Gilead Sciences and Abbvie.
1 Müllhaupt B , Bruggmann P , Bihl F , Blach S , Lavanchy D , Razavi H , et al. Modeling the Health and Economic Burden of Hepatitis C Virus in Switzerland. PLoS One. 2015;10(6):e0125214. doi:.https://doi.org/10.1371/journal.pone.0125214
2Health SFOoP. Source of infection among mandatory notified cases of hepatitis C, Switzerland 1993-2011: FOPH/MT/EPI/RIC. 2012.
3 Bruggmann P , Kormann A , Meili D . Heroin substitution: an exception or an expanded feasibility for providing hepatitis treatment to drug users? Hot Topics in Viral Hepatitis. 2009;5(13):27–33.
4 Leask JD , Dillon JF . Review article: treatment as prevention - targeting people who inject drugs as a pathway towards hepatitis C eradication. Aliment Pharmacol Ther. 2016;44(2):145–56. doi:.https://doi.org/10.1111/apt.13673
5 Nordt C , Stohler R . Combined effects of law enforcement and substitution treatment on heroin mortality. Drug Alcohol Rev. 2010;29(5):540–5. doi:.https://doi.org/10.1111/j.1465-3362.2009.00167.x
6 Balthasar H , Huissoud T , Zobel F , Arnaud S , Samitca S , Jeannin A . [Evolution of the consumption and practices in risk of transmission of HIV and HCV in injection drug users in Switzerland, 1993-2006]. Bulletin of the Swiss Federal Office of Public Health. 2007;45:804–9.
7Lociciro S, Gervasoni JP, Jeannin A, Dubois-Arber F. [Survey of drug users, clients of low-threshold facilities (SBS) in Switzerland. 1993-2011 trends.]. Lausanne, Switzerland: University Institute for Social and Preventive Medicine; 2013.
8The National Methadone Statistics 1999-2014 [Internet]. 2015 [cited 1/20/2015]. Available from: http://www.bag.admin.ch/themen/drogen/00042/00632/06217/index.html?lang=de.
9 Dickson-Spillmann M , Haug S , Uchtenhagen A , Bruggmann P , Schaub MP . Rates of HIV and Hepatitis Infections in Clients Entering Heroin-Assisted Treatment between 2003 and 2013 and Risk Factors for Hepatitis C Infection. Eur Addict Res. 2016;22(4):181–91. doi:.https://doi.org/10.1159/000441973
10 Uchtenhagen A . Heroin-assisted treatment in Switzerland: a case study in policy change. Addiction. 2010;105(1):29–37. doi:.https://doi.org/10.1111/j.1360-0443.2009.02741.x
11 Gschwend P , Rehm J , Lezzi S , Blättler R , Steffen T , Gutzwiller F , et al. Development of a monitoring system for heroin-assisted substitution treatment in Switzerland. Soz Praventivmed. 2002;47(1):33–8. doi:.https://doi.org/10.1007/BF01318403
12 Razavi H , Elkhoury AC , Elbasha E , Estes C , Pasini K , Poynard T , et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57(6):2164–70. doi:.https://doi.org/10.1002/hep.26218
13 Razavi H , Waked I , Sarrazin C , Myers RP , Idilman R , Calinas F , et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21(Suppl 1):34–59. doi:.https://doi.org/10.1111/jvh.12248
14 Centers for Disease Control and Prevention (CDC). Recommendations for follow-up of health-care workers after occupational exposure to hepatitis C virus. MMWR Morb Mortal Wkly Rep. 1997;46(26):603–6.
15 Mitsui T , Iwano K , Masuko K , Yamazaki C , Okamoto H , Tsuda F , et al. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology. 1992;16(5):1109–14. doi:.https://doi.org/10.1002/hep.1840160502
16 Hasan F , Askar H , Al Khalidi J , Al Shamali M , Al Kalaoui M , Al Nakib B . Lack of transmission of hepatitis C virus following needlestick accidents. Hepatogastroenterology. 1999;46(27):1678–81.
17 Tsui JI , Evans JL , Lum PJ , Hahn JA , Page K . Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med. 2014;174(12):1974–81. doi:.https://doi.org/10.1001/jamainternmed.2014.5416
18 Turner KM , Hutchinson S , Vickerman P , Hope V , Craine N , Palmateer N , et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106(11):1978–88. doi:.https://doi.org/10.1111/j.1360-0443.2011.03515.x
19 Van Den Berg C , Smit C , Van Brussel G , Coutinho R , Prins M ; Amsterdam Cohort. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102(9):1454–62. doi:.https://doi.org/10.1111/j.1360-0443.2007.01912.x
20 White B , Dore GJ , Lloyd AR , Rawlinson WD , Maher L . Opioid substitution therapy protects against hepatitis C virus acquisition in people who inject drugs: the HITS-c study. Med J Aust. 2014;201(6):326–9. doi:.https://doi.org/10.5694/mja13.00153
21 Hagan H , Pouget ER , Des Jarlais DC . A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis. 2011;204(1):74–83. doi:.https://doi.org/10.1093/infdis/jir196
22 MacArthur GJ , van Velzen E , Palmateer N , Kimber J , Pharris A , Hope V , et al. Interventions to prevent HIV and Hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. Int J Drug Policy. 2014;25(1):34–52. doi:.https://doi.org/10.1016/j.drugpo.2013.07.001
23 Arnaud S , Jeannin A , Dubois-Arber F . Estimating national-level syringe availability to injecting drug users and injection coverage: Switzerland, 1996-2006. Int J Drug Policy. 2011;22(3):226–32. doi:.https://doi.org/10.1016/j.drugpo.2011.03.008
24Cominetti F, Simonson T, Dubois-Arber F, IUMSP Gervasoni JP, ISGF Schaub M, SSP Monnat M. [Analysis of the HCV situation in drug users in Switzerland]. Lausanne, Switzerland; /2015. Report No.: Raisons de sante 2334a.
25 Dubois-Arber F , Balthasar H , Huissoud T , Zobel F , Arnaud S , Samitca S , et al. Trends in drug consumption and risk of transmission of HIV and hepatitis C virus among injecting drug users in Switzerland, 1993-2006. Euro Surveill. 2008;13(21):18881. doi:.https://doi.org/10.2807/ese.13.21.18881-en
26 Micallef JM , Kaldor JM , Dore GJ . Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13(1):34–41. doi:.https://doi.org/10.1111/j.1365-2893.2005.00651.x
27Bruggmann P. OST and injecting, ARUD centers, 2010-2013. In: Blach S, editor. 2015.
28Nordt C. OST and injecting in Zurich. In: Blach S, editor. 2015.
29 Huissoud T , Rousson V , Dubois-Arber F . Methadone treatments in a Swiss region, 2001-2008: a registry-based analysis. BMC Psychiatry. 2012;12(1):238. doi:.https://doi.org/10.1186/1471-244X-12-238
30Health FOoP. Affairs FDoH, Program DoNP. [Treatment with prescription heroin/diacetylmorphine (HeGeBe), in 2009 and 2010]. Laussane; 2011.
31Dickson-Spillman M, Hiltebrand D, Bollinger H, Schaub M. Heroin-assisted treatment in Switzerland: Results of the survey in 2013. Zurich; 2014.
See appendix 1.
This work was supported by Gilead Sciences. Gilead Sciences had no input on the content, the study design, data selection, decision to publish or preparation of the manuscript. Philip Bruggmann has received honoraria for advisory board and speaker fees as well as research and project grants from Abbvie, BMS, Gilead Sciences and MSD. Jan Fehr received grants from BMS, Gilead Sciences, Janssen and MSD. Roger Koynos received travel grants and honoraria from Gilead Sciences. Beat Müllhaupt has served as an advisory board member for MSD, Janssen Therapeutics, Abbvie, Boehringer Ingelheim, Gilead Sciences and BMS; as a consultant for Gilead Sciences and Abbvie; and has received research grants from Gilead Sciences. Andri Rauch has received honoraria for advisory boards and/or travel grants from Janssen-Cilag, MSD, Gilead Sciences and Abbvie. Marcel Stöckle has served as an advisory board member for Abbvie, Gilead Sciences and MSD. Franco Nero was advisor or consultant for Gilead Sciences, Abbvie and Merck and has received research grants from Gilead Sciences and Abbvie.