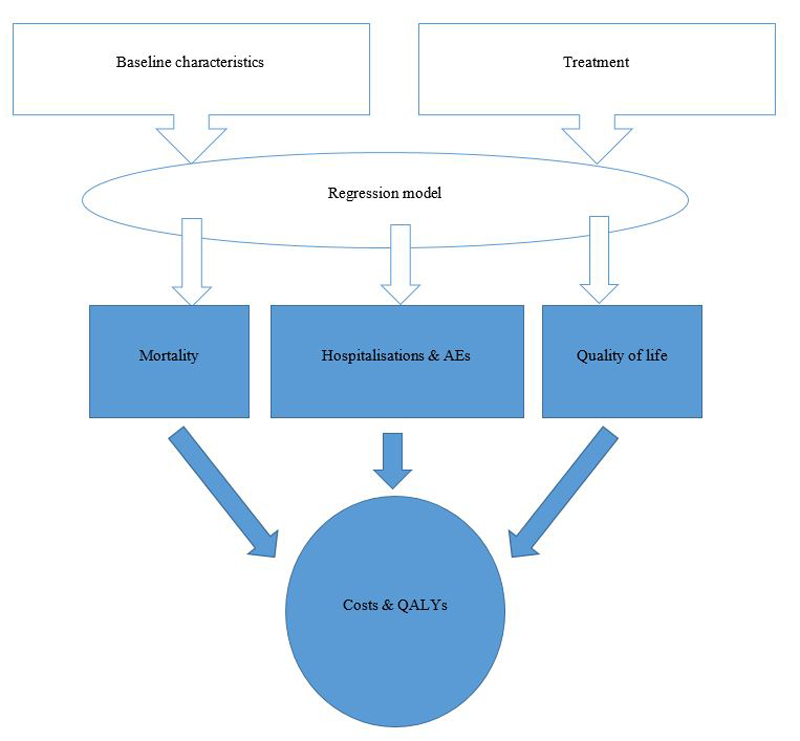
Figure 1 Model structure.
AEs = adverse events; QALYs = quality adjusted life years
DOI: https://doi.org/10.4414/smw.2017.14533
Heart failure is a progressive and incurable disease, with high morbidity and mortality in high-income countries including Switzerland. The reported prevalence of heart failure varies from between 1 and 2%, and increases for individuals aged above 65 years [1]. Estimates for 2010 expected 15 million people with heart failure in Europe and 6.6 million in the United States [2, 3]. Chronic heart failure has a prevalence of 1 to 2% and heart failure with reduced ejection fraction (HFrEF) accounts for about 50% of all heart failure cases [4]. In general, the condition requires complex management and treatment protocols that require substantial effort from patients, care givers, and healthcare services, and therefore poses a high cost burden on society [5]. Morbidity is very prominent in terms of severity of symptoms, reduced quality of life, hospitalisations and continuous need for treatment [6, 7]. Previous guidelines recommend angiotensin-converting enzyme inhibitors (ACEIs) and beta-blockers as initial treatment, as well as diuretics if there is a fluid overload [8]. These treatments appear to reduce the risk of death and improve exercise capacity. Angiotensin receptor blockers (ARBs) are controversial and less well tolerated than ACEIs, but remain a treatment option where ACEIs are not tolerated. Other treatments such as anti-platelets and lipid-lowering agents are added if necessary [9]. Advances in chronic heart failure treatment have been quite limited in the last decade.
Sacubitril/valsartan, an angiotensin-receptor-neprilysin-inhibitor (ARNI), is a novel oral therapy proposed in the current guidelines for the treatment of heart failure in patients with reduced left ventricular ejection fraction (LVEF) [9]. The phase-III prospective double-blind randomised controlled trial PARADIGM-HF (prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure) compared morbidity and mortality between sacubitril/valsartan and the ACEI enalapril in a population with HFrEF [10]. The primary outcome was a composite of death from cardiovascular causes or hospitalisation for heart failure. After a median follow-up of 27 months, sacubitril/valsartan was associated with a significant reduction in time to the primary outcome (hazard ratio [HR] 0.80, 95% confidence interval [95% CI] 0.73–0.87; p <0.001), all-cause mortality (HR 0.84, 95% CI 0.76–0.93; p <0.001) and cardiovascular mortality (HR 0.80, 95% CI 0.71–0.89; p <0.001). In addition, sacubitril/valsartan was also associated with a reduced risk of hospitalisation for heart failure of 21% (p <0.001) and a reduction in the symptoms and physical limitations of heart failure (p = 0.001) [10].
The aim of this study was to assess the clinical effectiveness in terms of quality-adjusted life years (QALYs) gained, the direct medical cost, and the cost-effectiveness of sacubitril/valsartan (in addition to standard care) compared to ACEIs (in addition to standard care) from the perspective of the Swiss healthcare system.
A model-based cost-utility analysis was undertaken comparing sacubitril/valsartan and standard care to ACEI and standard care. The incremental cost-effectiveness ratio (ICER) was expressed as cost per QALY gained. The analysis was conducted from the perspective of the Swiss healthcare system. Costs and effects occurring after one year were discounted by 3% in the base-case analysis.
A two-state Markov model [11] was implemented for the current analyses. In brief, the model is structured as a two-state Markov model (with health states “alive” and “dead”). Regression models were used to predict events and outcomes such as mortality, hospitalisations, adverse events and health-related quality of life over the lifelong time horizon of the model, based on patient characteristics and treatment received (fig. 1). This type of model was chosen as the benefits of treatment and costs continue to accrue beyond the observation period of the PARADIGM-HF trial. Cycle length is one month and a half-cycle correction is applied. The model permits both deterministic (DSA) and probabilistic sensitivity analyses (PSA). Death can occur at any point in time. Model outcomes include survival time (i.e., life years), QALYs, medical resource utilisation, accrued lifetime and total and disaggregated costs, and other clinical events such as number of hospitalisations and adverse events.

Figure 1 Model structure.
AEs = adverse events; QALYs = quality adjusted life years
The patient population considered for the economic model was the same as that enrolled on the PARADIGM-HF trial [10] i.e., adult HErEF and a mean age of 64 years. The following eligibility criteria were applied: age of at least 18 years, NYHA class II–IV symptoms, ejection fraction of 40% or less (which was changed to 35% or less) [12], and plasma B-type natriuretic peptide (BNP) level of at least 150 pg/ml or hospitalisation for heart failure within the previous 12 months and a BNP of at least 100 pg/ml. Patients taking stable doses of ACEIs or ARBs four weeks before screening were considered for participation in the study. Implantable cardioverter-defibrillators (ICDs) and cardiac resynchronisation therapy (CRT) are increasingly used in patients with HFrEF. In the PARADIGM-HF trial [10], 1,857 (22%) of the eligible patients used either ICDs or CRT at baseline. After screening, patients had a run-in phase with enalapril or sacubitril/valsartan, which was followed by the main double-blind randomised treatment phase [13]. Of 8,442 patients randomised, 43 patients were excluded for the full analysis set (FAS) due to invalid randomisation (n = 6) and good clinical practice (GCP) violations (n = 37). The analysis population consisted of 4187 patients receiving sacubitril/valsartan and 4212 patients receiving enalapril. The baseline characteristics of the trial population are presented in supplementary table S1 in appendix 2.
The average daily dose at the end of the PARADIGM-HF trial [10] for sacubitril/valsartan (in addition to standard care) was 375 mg compared to a treatment strategy with a daily dose of the ACEI enalapril (in addition to standard care) of 18.9 mg. Standard care included the use of diuretics, beta-blockers, aldosterone antagonists, digoxin, anticoagulants, aspirin, adenosine diphosphate antagonists and lipid-lowering medications. The choice of standard care is based on medication classes observed in the PARADIGM-HF trial [10].
Clinical information regarding all-cause mortality, hospitalisation rates, health-related quality of life and adverse events was obtained from the PARADIGM-HF trial [10].
The base-case analysis used a multivariable parametric survival model of all-cause mortality, which was based on the treatment arm, baseline characteristics of the patients, and time since randomisation (supplementary table S2 in appendix 2).
An alternative scenario analysis used multivariable parametric survival for cardiovascular mortality from the PARADIGM-HF trial [10] (supplementary table S3), and non-cardiovascular mortality from Swiss national life-tables (table S6). The monthly probability of non-cardiovascular mortality was obtained by subtracting the probability of cardiovascular mortality from the probability of all-cause mortality as calculated with data provided by the Swiss Federal Office of Public Health (SFOPH) [14] and the Swiss Federal Office of Statistics (SFOS) tables [15]. A death rate including cardiovascular death for five-year age bands was calculated by dividing the number of deaths obtained from Swiss life tables [15] by the number of persons in the relevant age group sourced from the SFOPH [14]. The death rates were converted to yearly probabilities of death using the formula p = 1 − e − central death rate*time (time is 1/12 years in this case, as we derived monthly probabilities). All-cause mortality and cardiovascular mortality were assumed to be constant within the 5-year age bands provided by the SFOS, and constant in the age group of persons aged 85 years or above, as we had no additional data for this age category. Additional information about Swiss population and related mortality is available in tables S4, S5 and S6 .
The model predicted the risk of all-cause hospitalisation beyond the PARADIGM-HF trial using negative binomial regression [11]. Briefly, predicted hospitalisation rates were adjusted for baseline characteristics of the subjects included in the PARADIGM-HF trial such as age, race, and region, and were dependent on the treatment arm. The model for all-cause hospitalisation showed that a treatment strategy with a daily dose of sacubitril/valsartan compared to ACEI treatment reduced all-cause hospitalisation (supplementary table S7).
More serious adverse events were considered to be covered by all-cause hospitalisations (table S7), whereas less serious adverse events were considered independently. Rates of these adverse events (hypotension, elevated serum creatinine and potassium, cough and non-severe angio-oedema) were estimated from the PARADIGM-HF trial) [10]. Occurrence of less serious adverse events can be found in the additional material provided in table S8.
A mixed-effects regression model derived from the PARADIGM-HF trial based on patient-level EQ-5D data was estimated to allow the prediction of the EQ-5D-based utility values as a function of baseline characteristics (including baseline EQ-5D), hospitalisations, adverse events, treatment arm and time since randomisation. The EQ-5D 3-level questionnaire was administered at baseline and at months 4, 8, 12, 24, 36, and end of study. The UK EQ-5D tariff published by Dolan et al. was applied to EQ-5D patient responses [16]. Details are available in table S9. Hospitalisations in the 30 days before EQ-5D measurement (to capture the acute effect of hospitalisation), and hospitalisations 30–90 days before EQ-5D measurement (to capture any long term effect during rehabilitation) were implemented. Utility decrements in the model were applied to subjects experiencing hospitalisations or adverse events.
Drug dosage (primary and background drug therapy) data from the PARADIGM-HF trial were validated by using the recommendations of the Swiss Heart Failure Working Group of the Swiss Society of Cardiology [17], which are based on 2012 European Society of Cardiology (ESC) guidelines [8]. Drug dosages used for the Swiss model can be found in supplementary table S10. The proportional occurrence of hospitalisations for surgical procedures (4.0%), interventional procedures (8.0%), or medical management only (88.0%), was obtained from the Western European population of the PARADIGM-HF trial (table S11).
A background medical resource utilisation per unit of time was assumed to be the same in both treatment strategies of the model. We assumed that patients with heart failure would need to have at least 12 primary-care physician (PCP) visits per year. This was based on an article by Muntwyler et al. [18], which measured the quality of the diagnosis and management of heart failure in primary care in 1999 in Switzerland. Over 82 PCPs from all over Switzerland participated in the study. A total of 474 patients were included.
Milder adverse events reported in the PARADIGM-HF trial [10] were modelled separately, as mentioned previously. The following assumptions were applied for resource use associated with adverse events; (a) if a patient experiences hypotension, he/she needs 2 additional PCP visits, (b) if a patient experiences cough, he/she needs 2 additional PCP visits and blood tests, (c) in the case of angio-oedema, patients can experience milder or severe angio-oedema. Milder angio-oedema patients require use of antihistamines and 2 additional outpatient visits, while patients experiencing more severe angio-oedema need 2 additional outpatient visits and use of glucocorticoids, (d) if patients show signs of elevated serum creatinine, they need 2 additional PCP visits and a blood test, (e) if patients show signs of elevated serum potassium, they need 2 additional PCP visits and a blood test.
The cost of sacubitril/valsartan per day in the base-case analyses was CHF 5.79 (375 mg per day), and unit costs of background therapies were sourced from SFOPH data (Spezialitätenliste) relevant to 2015 [19]. For each reported therapeutic substance used in the PARADIGM-HF trial, we collected and mapped drugs representing the same substance, based on the number of available producers in the Swiss pharmaceutical market. For example, if there were three pharmaceutical producers of enalapril 10 mg on the market, then the average cost per tablet strength was calculated. Monthly costs were calculated by multiplying the daily costs by 365.25/12. Daily costs of primary therapies and background therapies can be found in supplementary table S10.
Unit costs of hospitalisations were estimated on the basis of diagnosis-related group (DRG) costs, and by mapping each reported hospitalisation in the PARADIGM-HF trial to relevant Swiss DRG codes [20]. For this mapping procedure, we used the proportional occurrence of hospitalisations involving surgery, interventional procedures or medical management. Where several suitable Swiss DRG codes were identified, the weighted mean was used based on their activity and cost as reported for 2012, which is when the latest data was published [20]. Details about hospitalisations, the proportional occurrence of diagnoses reported in the PARADIGM-HF trial, and Swiss DRGs assigned are provided in table S11. The weighted mean cost per hospitalisation provided information on the unit cost per hospitalisation event rather than cost per day in hospital. For the year 2012, the average cost per hospitalisation was CHF 13 847; these costs were then updated to 2014 values using the Swiss consumer price index [21]. The consumer price index values for 2012 and 2014 were 99.9 and 98.1. The resulting cost per hospitalisation in 2014 was CHF 13 598.
As described previously, the estimated number of PCP visits per year was informed by the European IMPROVEMENT-HF study [18]. The unit costs of a PCP visit were derived from the santésuisse web page, and amounted to CHF 113 in 2007. Based on the consumer price index [21] the updated value for one PCP visit in 2014 was CHF 110.30. Unit costs for the treatment of each relevant type of adverse event were estimated from Swiss literature and information publicly available from the SFOPH, Tarmed, and santésuisse websites [19] [22].
Subgroup analyses based on the a priori subgroups in PARADIGM-HF were undertaken to understand variation of the main results between subgroups of patients enrolled in the PARADIGM-HF trial.
To assess the impact of different assumptions on the model results, a series of scenario analyses were performed. Some were of general relevance. Additional analyses were regarded as specifically relevant for the Swiss setting (see appendix 1 for description). A series of deterministic sensitivity analyses (DSA) were performed to assess the impact of uncertainty surrounding key input parameters. Important parameters were varied independently over plausible ranges determined by the 95% confidence intervals (CI) surrounding point estimates. Where 95% CIs were not available, upper and lower values of ±25% surrounding point estimates were used (supplementary table S12). The ICERs resulting from each analysis were recorded for the upper and lower value and are presented in a Tornado diagram.
Probabilistic sensitivity analysis was undertaken to explore joint parameter uncertainty (details about their respective distributions in table S12). A total of 10 000 iterations were run and the results are shown as a cost-effectiveness plan.
In the base-case analysis, the sacubitril/valsartan strategy compared to enalapril showed a decrease in the number of hospitalisations (6.0%/year absolute reduction) and lifetime hospital costs by 8.0% (discounted). Total QALYs per person over a lifetime horizon were 4.99 and 4.56 in the sacubitril/valsartan and ACEI treatment strategies respectively (table 1). This led to an incremental difference of 0.425 QALYs. The total incremental costs difference was CHF 10 926 (table 1) and the ICER for sacubitril/valsartan treatment versus ACEI was CHF 25 684 per QALY gained. Alternatively, the use of Swiss life-tables for non-cardiovascular mortality and cardiovascular mortality rates from the PARADIGM-HF trial led to an ICER of CHF 24 490.
Table 1 Base case results (all costs are expressed in CHF).
| Parameter | Sacubitril/valsartan | ACEI | Difference |
|---|---|---|---|
| Clinical effectiveness parameters (discounted) | |||
| Total life years | 6.67 | 6.17 | 0.50 |
| Total QALYs | 4.99 | 4.565 | 0.4254 |
| Cost parameters (discounted) | |||
| Primary therapy | 14 119 | 1757 | 12 362 |
| Titration | 220 | 0.00 | 220 |
| Adverse events | 307 | 290 | 17 |
| Background drug therapy | 8072 | 7467 | 605 |
| Management of HF by physicians | 8830 | 8168 | 662 |
| Hospitalisation | 32 857 | 35 797 | −2940 |
| Total costs | 69 683 | 53 479 | 10 926 |
| Cost-effectiveness parameters | |||
| Cost per LYG | CHF21 855 | ||
| Cost per QALY gained | CHF25 684 | ||
ACEI = angiotensin-converting enzyme; HF = chronic heart failure patients; LYG = life year gained; QALY = quality adjusted life year
Cost-effectiveness results for subgroups of patients are presented in full in supplementary table S13 in appendix 2. The ICER was quite stable, with ±1–11% variation from the base-case result. In brief, if the baseline eGFR was <60, the ICER decreased by 8.0%. No use of beta-blockers at baseline decreased the ICER by 11.0%, and where ≤1 year since diagnosis of heart failure was recorded, this increased the ICER by 8.0%.
The most influential parameters in univariate sensitivity analysis were related to all-cause mortality, hospitalisations and HRQoL (fig. 2). Table 2 shows the scenario analysis results. An ICER of > CHF 48 000 per QALY occurred if all treatment effects of sacubitril/valsartan were assumed to cease after year 5 (while the treatment costs of sacubitril/valsartan continued for life). An analysis based on two years of follow-up led to an increased ICER of CHF 58 679. An ICER of CHF 30 812 per QALY gained was observed where there was assumed to be no effect of sacubitril/valsartan on HRQoL. Using the German and French EQ-5D value sets instead of that for the UK led to slightly more favourable ICER results. Other scenario analyses did not have a major impact on the ICER.
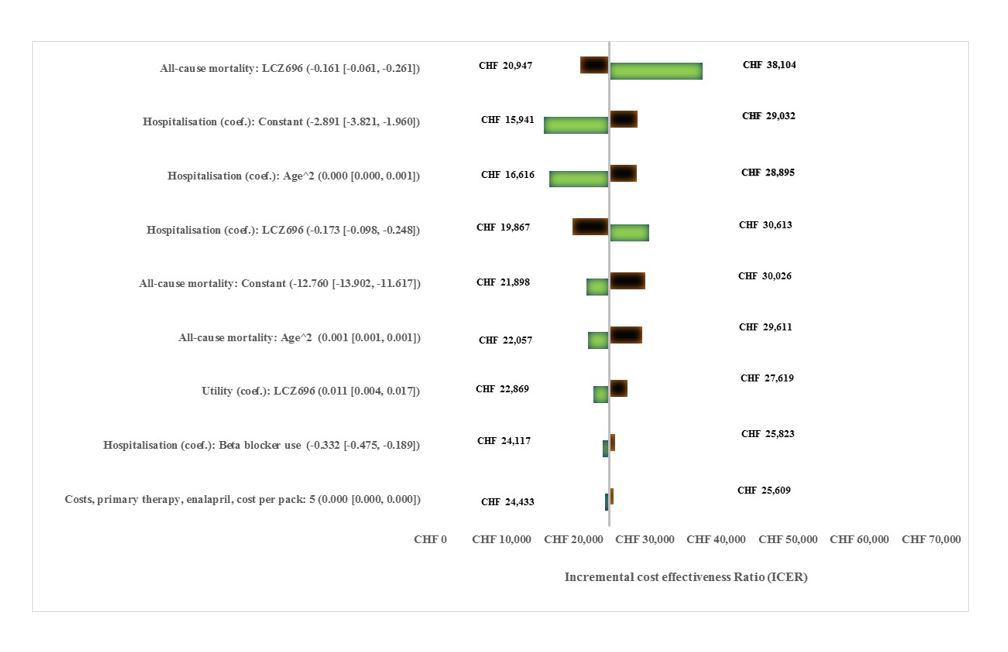
Figure 2 Tornado diagram summarising univariate sensitivity analysis results.
Table 2 Results of scenario analyses.
| Area of uncertainty | Scenario |
ICER
(cost per QALY in CHF) |
|---|---|---|
| Base case (patient level data) | 25 684 | |
| Discount rate | Discount rate: 1.5% benefits; 6% costs | 18 951 |
| Time horizon | 2 years | 58 679 |
| CV mortality PARADIGM, non-CV mortality life tables | 24 490 | |
| HRQL time trend | Time trend halved | 24 648 |
| HRQL time trend | Time trend doubled | 28 041 |
| HRQL time trend | No decrease in HRQL | 23 693 |
| HRQL time trend | HRQL constant at 5 years | 24 648 |
| HRQL time trend | HRQL constant at 10 years | 25 311 |
| Treatment effect on HRQL | No absolute benefit in HRQL for sacubitril/valsartan | 30 812 |
| Treatment effect on hospitalisation | sacubitril/valsartan treatment effect applied only to HF hospitalisations (rather than CV mortality and utility) | 36 472 |
| Effect of hospitalisation on HRQL | Decrements for hospitalisation set to zero | 25 810 |
| Extrapolation of treatment effects | All treatment effects cease at year 5 | 47 062 |
| Extrapolation of treatment effects | All treatment effects cease at year 10 | 30 132 |
| Discontinuation | Include discontinuation as seen in PARADIGM-HF | 25 242 |
| Discontinuation | No discontinuation after year 3 | 25 455 |
| Hospitalisation costs | Double cost per hospitalisation | 25 684 |
| Adverse event rates | All adverse event rates set to zero | 25 621 |
| Cost of primary therapies | Cost of ACEI/ sacubitril/valsartan based on PARADIGM-HF target doses | 26 245 |
| French EQ-5D tariff used | Using EQ-5D tariff instead of UK tariffs | 23 359 |
| German EQ-5D tariff used | Using EQ-5D German tariffs instead of UK tariff | 24 038 |
| NT-pronBNP test inclusion | 26 159 | |
| HF management outpatient visits (40) | 4.6 visits per year | 25 200 |
CV = cardiovascular; HF = heart failure; EQ-5D = European quality of life-5 dimensions; HQRL = health-related quality of life; ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin-receptor blocker; NT-pronBNP = N-terminal pro-brain natriuretic peptide; PCP = primary care physician
PSA results are presented in figure 3 as a cost-effectiveness plane. All simulation fell within the northeast quadrant of the cost-effectiveness plane (meaning in all simulations sacubitril/valsartan was both more effective and costlier than enalapril), with a 95% confidence interval range of CHF 18 798 to CHF 43 974 per QALY gained. The cost-effectiveness threshold of CHF 30 000 per QALY gained was met in 78.0% of 10 000 runs, and threshold of CHF 50 000 per QALY gained was met in 99.0% of 10 000 runs.
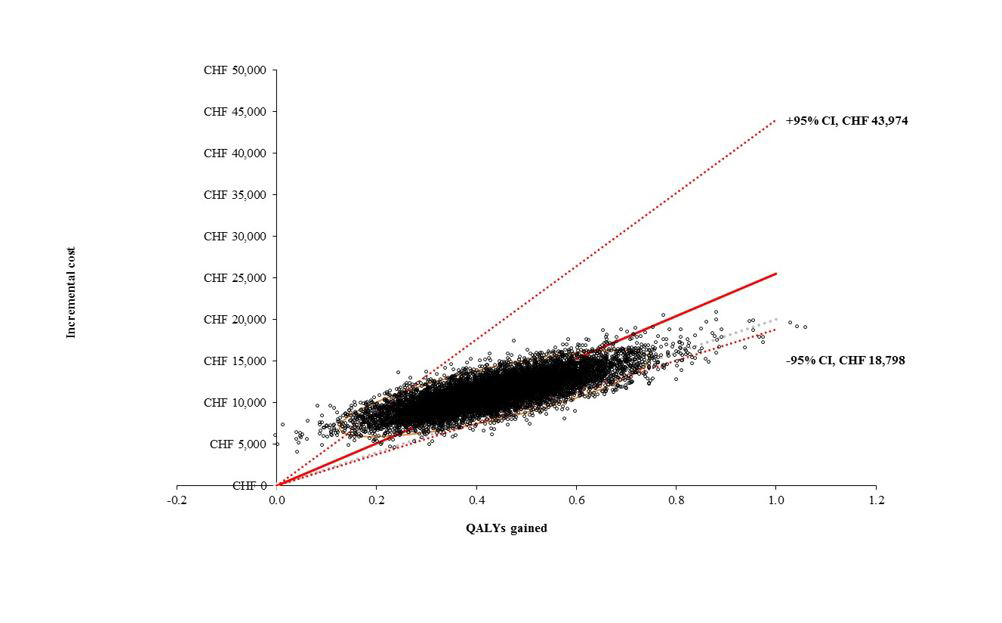
Figure 3 Cost-effectiveness scatterplot and 95% confidence range (10 000 simulations).
Given limited healthcare budgets throughout the world, the health economic aspects of new drug evaluations can be as important as efficacy, safety and the ability to serve important medical needs under routine clinical practice. In most developed countries, heart failure poses a great economic burden. Estimates show that management of heart failure accounts for 2–5% [2, 3] of total healthcare budgets. Long-term drug treatment is a cornerstone of heart failure therapy.
The cost-effectiveness of sacubitril/valsartan plus standard care compared to enalapril plus standard care has been assessed, from the perspective of the Swiss health care system. The base-case analysis indicated an ICER of CHF 25 684 per QALY gained.
The findings presented were robust to changes in assumptions, and the ICER results were similar across multiple patient subgroups. When model input parameters were varied on the basis of their 95% confidence intervals (as observed in the PARADIGM-HF trial or estimated in regression analyses based thereupon), the ICER remained below CHF 50 000 per QALY gained in most of the cases. In the scenario analyses performed, the ICER also remained below CHF 50 000 per QALY gained, except in the extreme scenarios of the treatment effect of sacubitril/valsartan that persisted for only two or five years.
It should be noted that there is no formally accepted cost-effectiveness threshold in Switzerland. In this study, we tentatively assume a threshold of CHF 30 000 and CHF 50 000 per QALY gained to distinguish between favourable and unfavourable ICER results [23, 24]. This threshold level is similar to the upper limit of the threshold range of £20 000–£30 000 accepted in the United Kingdom (UK) [25]. A few years ago, a court in Switzerland hinted at a CHF 100 000 per QALY threshold [26].
Findings were similar to the results of three previously published cost-effectiveness analyses in the United States [27–29]. These studies (Gaziano et al., King et al. and Sandhu et al.) found sacubitril/valsartan to be cost-effective. The first published economic analysis for the US [27] used the same analytical framework over a 30-year time horizon and displayed an ICER of US$45 017 per QALY gained. Differences observed with our study affected costs and quality of life. Incremental costs and effects were higher in the US population. For example, the monthly cost for sacubitril/valsartan in the US was $375, whereas in Switzerland it was CHF 176. The cost of heart failure hospitalisation were $18 158 in the US and CHF 13 599 in Switzerland. Incremental QALYs gained were 0.78 for the US population and 0.42 for the Swiss population.
The second set of cost-effectiveness analyses undertaken by King et al. [28] found similar results with an ICER of $50 959 per QALY gained over a lifetime. However, their model included population with NYHA class I [28], whereas NYHA class I population was excluded from the PARADIGM-HF trial. The third economic evaluation by Sadhu et al. [29] displayed a cost per QALY gained of $47 053, and differences with our study were mainly due to modelling techniques and input parameters. Monthly cost for sacubitril/valsartan in the study by Sadhu et al. [29] was assumed to be $380, and heart failure hospitalisation costs were assumed as $11 829. In terms of health outcomes, the study by Sadhu et al. [29] displayed a higher incremental QALY gained of 0.62, as compared to that of the Swiss population (0.42).
Another recent study from the Netherlands, using a Markov model and using the effectiveness data from the PARADIGM-HF trial over a lifetime horizon, showed that sacubitril/valsartan was considered cost effective at an ICER of 19 113 per QALY gained [30]. Differences observed with our study were mainly in terms of model structure, but also in terms of input parameters, such as quality of life. The Dutch study was not able to utilise patient level PARADIGM-HF trial data. The reported incremental QALY gained was 0.29 for the Dutch population, which was lower than that for the Swiss population (0.42). However, the monthly cost for sacubitril/valsartan in the Dutch and Swiss models was quite similar; the amounts were €161.7 and CHF 176 respectively.
In recent years, there have been a number of cost-effectiveness studies undertaken for heart failure patients. The interventions considered included ivabradine, eplerenone, ACEI, and beta blockers. These were compared with placebo or standard of care. In particular, ivabradine was one of the most studied drugs in heart failure patients. Lifetime ICERs ranged from €7634 in Poland [31] to $53 710 in Mexico [32] within these studies [31, 33–38]. ICERs for sacubitril/valsartan appear to be in a comparable range.
Patient-level data from a large international randomised clinical trial formed the basis of this analysis. Use of data from PARADIGM-HF, a large randomized controlled trial comparing sacubitril/valsartan to a real-world standard of care, allowed for a high level of internal consistency in the model. Multivariate risk equations allowed us to characterise and take into account between-patient heterogeneity. A relatively novel approach was used to predict quality of life, by extrapolating EQ-5D utility values based on time trends observed in PARADIGM-HF. The use of local data with regards to non-cardiovascular deaths and unit costs allowed for adjustment to the Swiss healthcare environment. Consistency across countries with regards to medical resource use was partially improved by using the Western European part of the PARADIGM-HF population for the calculation of some parameters, such as hospitalisations. However, the transferability of clinical trial results is necessarily affected by simplifications and assumptions, as these are required in all health economic models.
The main limitation was extrapolation of the treatment effect beyond the observation period of the PARADIGM-HF trial. This is a common limitation shared by most economic evaluation studies when the lifetime impact of an emerging treatment is assessed. Assumptions made with regards to the long-term effects of treatment on mortality, health-related quality of life and all-cause hospitalisation were addressed by performing state-of-the-art sensitivity and scenario analyses. When these assumptions were changed one at a time, they were found to have a relatively modest impact on the final results. Hence, results derived in the base-case analysis seem to be realistic based upon assumptions in the economic evaluation.
The mean age of patients treated in the PARADIGM–HF trial was 64 years, while the mean age of patients in the Swiss population might be higher. This may have led to an over- or underestimation of the true differences between sacubitril/valsartan and standard treatment to be expected for Switzerland, with unclear implications for the ICER. However, in a subgroup analysis including only PARADIGM-HF [39] patients aged at least 75 at baseline, the resulting ICER was very similar to the base case ICER.
The model used Swiss input data where relevant and available, but some approximations were required due to lack of data. One related limitation was the lack of information with regards to medical resource use in heart failure patients. This highlights the need for high-quality Swiss data to cover the aspects of resource utilisation. In the absence of such data, we have adopted resource-use estimates from the UK and verified these with the Swiss literature published as far as possible. This approach may have led to an underestimation of the medical resource use of Swiss CHF patients, which is expected to have a relatively unclear impact on this ICER. In addition, we were not able to capture out-of-pocket expenses incurred by patients themselves with regards to heart failure, and this information should be included in future assessments of costs from the perspective of the Swiss healthcare system.
From the perspective of the Swiss healthcare system, sacubitril/valsartan represents a cost-effective treatment option in patients with HFrEF versus enalapril if a willingness-to-pay threshold of CHF 50 000 per QALY gained is assumed.
Basing non-cardiovascular death from Swiss life tables, observations from the PARADIGM-HF trial were used for cardiovascular (CV) mortality. Towards this end, CV mortality parametric survival model was used where an effect of sacubitril/valsartan on CV mortality was considered.
Sacubitril/valsartan showed a small positive effect on European Quality of Life-5 Dimensions (EQ-5D) score (0.011, p = 0.001). To test the impact of this assumption, in the scenario analysis this effect was set to zero.
The treatment effect of sacubitril/valsartan was applied to heart failure (HF) hospitalisations only, whereas the base-case analyses modelled the observed impact of sacubitril/valsartan treatment on all cause hospitalisations.
Hospitalisation-related utility decrements were set to zero whereas in the base-case analyses, utility decrements for hospitalisation in the previous 30 days and in the previous 30-90 days were incorporated.
The median follow-up time in the PARADIGM-HF trial was 27 months. In the absence of long term follow up data, the base-case analyses assumed that the treatment effect of sacubitril/valsartan on mortality, hospitalisations and health-related quality of life (HRQoL) would continue over a lifetime horizon. In scenario analyses, all sacubitril/valsartan treatment effects were assumed to cease after 5 or 10 years (but the accrual of sacubitril/valsartan treatment costs was assumed to continue).
While the base-case analyses included discontinuation as seen in PARADIGM-HF, scenario analysis assumed an exponential survival model of treatment discontinuation, implying a constant rate of discontinuation. Upon discontinuation, costs and efficacy for sacubitril/valsartan patients were assumed to revert to that of angiotensin converting-enzyme inhibitors (ACEIs). This change in efficacy was assumed for all treatment effects, i.e. mortality, hospitalisations, HRQoL and adverse event occurrence. Costs for discontinued ACEI patients were based on angiotensin receptor blocker (ARB) costs, with efficacy assumed to be the same for ACEI and ARBs. (ARBs were shown to have comparable efficacy to ACEI [40].) Another scenario assumed there would be no discontinuation after 3 years.
Given geographical proximity, we additionally applied utility estimates based on the French and German EQ-5D value sets. The former was based on a French time trade-off study by Chevalier et al. [41]. This study recruited a total of 452 respondents aged over 18 years who were representative of the French population with regard to age, gender, and socio-professional group [41]. Secondly, Greiner et al. provided a German value set for the EQ-5D [42] based on the stated preferences of the German general public. A sample of 339 individuals in northern Germany valued 15 different health states from a sample of 36 states. Similarly as described for the base-case model, mixed-effects regression models based on patient-level EQ-5D utility values were estimated to predict EQ-5D utility as a function of baseline characteristics (including baseline EQ-5D), hospitalisations, adverse events, treatment arm and time since randomisation [42].
Another scenario analysis assumed that N-terminal pro-brain natriuretic peptide tests would be routinely performed in heart failure patients.
A last scenario analysis, assumed 4.6 times HF outpatient visits per year, instead of 12 times per year as per base case analysis. 4.6 times HF outpatient visits per year as per Agvall et al. 2005 [43].
Table S1 Baseline characteristics of the PARADIGM-HF trial population (full analysis set).
| Variable | Enalapril 10 mg twice daily | Sacubitril/valsartan 200 mg twice daily | p-value |
|---|---|---|---|
| No. | 4212 | 4187 | |
| Age (years), mean (SD) | 63.8 (11.3) | 63.8 (11.5) | 0.93 |
| Female, n (%) | 953 (22.6%) | 879 (21.0%) | 0.070 |
| Race, n (%) | |||
| White | 2781 (66.0%) | 2763 (66.0%) | 0.97 |
| Black | 215 (5.1%) | 213 (5.1%) | |
| Asian | 750 (17.8%) | 759 (18.1%) | |
| Other | 466 (11.1%) | 452 (10.8%) | |
| Region, n (%) | |||
| North America | 292 (6.9%) | 310 (7.4%) | 0.90 |
| Latin America | 720 (17.1%) | 713 (17.0%) | |
| Western Europe and other | 1025 (24.3%) | 1026 (24.5%) | |
| Central Europe | 1433 (34.0%) | 1393 (33.3%) | |
| Asia-Pacific | 742 (17.6%) | 745 (17.8%) | |
| Systolic blood pressure (mm Hg), mean (SD) | 121.2 (15.4) | 121.6 (15.2) | 0.31 |
| Heart rate (bpm), mean (SD) | 72.5 (12.1) | 72.2 (12.0) | 0.26 |
| Body mass index (kg/m2), mean (SD) | 28.2 (5.5) | 28.1 (5.5) | 0.65 |
| Serum creatinine (mg/l), mean (SD) | 1.1 (0.3) | 1.1 (0.3) | 0.39 |
| Ischaemic aetiology, n (% | 2530 (60.1%) | 2506 (59.9%) | 0.84 |
| Ejection fraction (%), mean (SD) | 29.4 (6.3) | 29.6 (6.1) | 0.30 |
| NT-proBNP (pg/ml), median (IQR) | 188.4 (104.8–390.8) | 192.8 (104.7–373.0) | 0.94 |
| BNP (pg/ml), median (IQR) | 72.4 (44.4–134.1) | 73.6 (44.6–136.6) | 0.57 |
| NYHA class, n (%) | |||
| I | 209 (5.0%) | 180 (4.3%) | 0.077 |
| II | 2921 (69.3%) | 2998 (71.6%) | |
| III | 1049 (24.9%) | 969 (23.1%) | |
| IV | 27 (0.6%) | 33 (0.8%) | |
| Missing | 6 (0.1%) | 7 (0.2%) | |
| Hypertension status, n (%) | 2971 (70.5%) | 2969 (70.9%) | 0.71 |
| Diabetic status, n (%) | 1450 (34.4%) | 1446 (34.5%) | 0.92 |
| Atrial fibrillation based on history, n (%) | 1574 (37.4%) | 1517 (36.2%) | 0.28 |
| Prior HF hospitalisation, n (%) | 2667 (63.3%) | 2607 (62.3%) | 0.32 |
| Prior myocardial infarction, n (%) | 1816 (43.1%) | 1818 (43.4%) | 0.78 |
| Prior stroke, n (%) | 370 (8.8%) | 355 (8.5%) | 0.62 |
| Prior use of ACEI, n (%) | 3266 (77.5%) | 3266 (78.0%) | 0.61 |
| Prior use of ARB, n (%) | 963 (22.9%) | 929 (22.2%) | 0.46 |
| Diuretic use, n (%) | 3375 (80.1%) | 3363 (80.3%) | 0.83 |
| Beta-blocker use, n (%) | 3912 (92.9%) | 3899 (93.1%) | 0.66 |
| Digoxin use, n (%) | 1316 (31.2%) | 1223 (29.2%) | 0.042 |
| Use of mineralocorticoid receptor antagonist, n (%) | 2400 (57.0%) | 2271 (54.2%) | 0.011 |
| Cardioverter-defibrillator implanted, n (%) | 620 (14.7%) | 623 (14.9%) | 0.84 |
| Use of cardiac resynchronisation therapy, n (%) | 282 (6.7%) | 292 (7.0%) | 0.61 |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin-receptor blocker; BNP = brain natriuretic peptide; HF = heart failure; IQR = interquartile range; NT-pro-BNP = N-terminal pro-brain natriuretic peptide
Table S2 Gompertz regression model for all-mortality (n = 8399).
| Coefficient | SE | z | P>z | 95% LCI | 95% UCI | |
|---|---|---|---|---|---|---|
| Sacubitril/valsartan | −0.161 | 0.051 | –3.150 | 0.002 | –0.261 | –0.061 |
| Age | –0.101 | 0.016 | –6.220 | 0.000 | –0.133 | –0.069 |
| Age squared | 0.001 | 0.000 | 6.780 | 0.000 | 0.001 | 0.001 |
| Female | –0.389 | 0.070 | –5.600 | 0.000 | –0.525 | –0.253 |
| Region - Latin America (vs North America) | 0.527 | 0.127 | 4.150 | 0.000 | 0.278 | 0.776 |
| Region - Western Europe (vs North America) | 0.128 | 0.112 | 1.140 | 0.254 | –0.091 | 0.346 |
| Region - Central Europe (vs North America) | 0.348 | 0.115 | 3.030 | 0.002 | 0.123 | 0.573 |
| Region - Other (vs North America) | –0.211 | 0.298 | –0.710 | 0.479 | –0.796 | 0.373 |
| Race - Black (vs Caucasian) | 0.285 | 0.130 | 2.190 | 0.029 | 0.030 | 0.540 |
| Race - Asian (vs Caucasian) | 0.709 | 0.283 | 2.500 | 0.012 | 0.154 | 1.265 |
| Race - Other (vs Caucasian) | 0.083 | 0.110 | 0.760 | 0.449 | –0.132 | 0.298 |
| NYHA class III/IV (vs I/II) | 0.202 | 0.061 | 3.300 | 0.001 | 0.082 | 0.322 |
| LVEF | –0.014 | 0.004 | –3.300 | 0.001 | –0.022 | –0.006 |
| Heart rate | 0.005 | 0.002 | 2.540 | 0.011 | 0.001 | 0.010 |
| log(eGFR) | –0.236 | 0.095 | –2.470 | 0.013 | –0.422 | –0.049 |
| log(NT-proBNP) | 0.387 | 0.027 | 14.140 | 0.000 | 0.333 | 0.440 |
| Sodium | –0.031 | 0.009 | –3.430 | 0.001 | –0.048 | –0.013 |
| QRS duration | 0.002 | 0.001 | 3.080 | 0.002 | 0.001 | 0.003 |
| Diabetes | 0.215 | 0.054 | 3.950 | 0.000 | 0.108 | 0.321 |
| Beta-blocker use | –0.287 | 0.088 | –3.260 | 0.001 | –0.460 | –0.115 |
| Lipid lowering medication use | –0.086 | 0.057 | –1.520 | 0.129 | –0.197 | 0.025 |
| 1–5 years since HF diagnosis (vs ≤1 year) | 0.205 | 0.067 | 3.040 | 0.002 | 0.073 | 0.337 |
| >5 years since HF diagnosis (vs ≤1 year) | 0.290 | 0.072 | 4.010 | 0.000 | 0.148 | 0.432 |
| Ischaemic aetiology | 0.186 | 0.059 | 3.140 | 0.002 | 0.070 | 0.302 |
| Prior stroke | 0.171 | 0.083 | 2.070 | 0.039 | 0.009 | 0.333 |
| Previously hospitalised for HF | 0.152 | 0.055 | 2.750 | 0.006 | 0.044 | 0.261 |
| EQ-5D | –0.541 | 0.115 | –4.700 | 0.000 | –0.767 | –0.315 |
| Constant | –12.760 | 0.583 | –21.890 | 0.000 | –13.902 | –11.617 |
| Gamma | 0.000 | 0.000 | 4.560 | 0.000 | 0.000 | 0.001 |
eGFR = estimated glomerular filtration rate; EQ-5D, European Quality of Life-5 Dimensions; HF = heart failure; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro-brain natriuretic peptide; NYHA II–IV = New York Heart Association class II–IV; SE = standard error
Table S3 Gompertz regression model for CV mortality (n = 8399).
| Mortality | Hazard ratio | Coefficient | SE | z | P>z | 95% CI | |
|---|---|---|---|---|---|---|---|
| Sacubitril/valsartan | 0.81 | –0.216 | 0.0570 | –3.79 | 0.000 | –0.328 | –0.104 |
| Age† | 0.91 | –0.092 | 0.0180 | –5.13 | 0.000 | –0.128 | –0.057 |
| Age squared | 1.00 | 0.001 | 0.0001 | 5.35 | 0.000 | 0.000 | 0.001 |
| Female | 0.70 | –0.357 | 0.0766 | –4.67 | 0.000 | –0.508 | –0.207 |
| Region | |||||||
| Latin America | 1.87 | 0.625 | 0.1455 | 4.3 | 0.000 | 0.340 | 0.910 |
| Western Europe | 1.18 | 0.168 | 0.1307 | 1.28 | 0.200 | –0.089 | 0.424 |
| Central Europe | 1.70 | 0.529 | 0.1319 | 4.01 | 0.000 | 0.270 | 0.787 |
| Asia-Pacific | 0.83 | –0.187 | 0.3172 | –0.59 | 0.556 | –0.809 | 0.435 |
| Race | |||||||
| Black | 1.50 | 0.409 | 0.1440 | 2.84 | 0.005 | 0.126 | 0.691 |
| Asian | 2.62 | 0.962 | 0.2989 | 3.22 | 0.001 | 0.377 | 1.548 |
| Other | 1.18 | 0.168 | 0.1226 | 1.37 | 0.169 | –0.072 | 0.409 |
| NYHA III/IV | 1.34 | 0.296 | 0.0669 | 4.42 | 0.000 | 0.165 | 0.427 |
| Ejection fraction† | 0.98 | –0.017 | 0.0046 | –3.6 | 0.000 | –0.026 | –0.008 |
| Log(eGFR)† | 0.79 | –0.238 | 0.1054 | –2.26 | 0.024 | –0.444 | –0.031 |
| Log(NT-proBNP)† | 1.56 | 0.443 | 0.0299 | 14.84 | 0.000 | 0.385 | 0.502 |
| Sodium† | 0.97 | –0.027 | 0.0099 | –2.69 | 0.007 | –0.046 | –0.007 |
| QRS duration | 1.00 | 0.002 | 0.0007 | 3.04 | 0.002 | 0.001 | 0.003 |
| Diabetes | 1.26 | 0.229 | 0.0599 | 3.82 | 0.000 | 0.111 | 0.346 |
| Beta-blocker use | 0.73 | –0.320 | 0.0964 | –3.32 | 0.001 | –0.509 | –0.131 |
| Time since diagnosis of HF | |||||||
| 1–5 years | 1.23 | 0.210 | 0.0748 | 2.8 | 0.005 | 0.063 | 0.356 |
| >5 years | 1.41 | 0.344 | 0.0805 | 4.28 | 0.000 | 0.186 | 0.502 |
| Ischaemic disease | 1.17 | 0.156 | 0.0626 | 2.48 | 0.013 | 0.033 | 0.278 |
| Prior HF hospitalisation | 1.17 | 0.159 | 0.0617 | 2.57 | 0.010 | 0.038 | 0.280 |
| Baseline EQ-5D | 0.57 | –0.563 | 0.1275 | –4.42 | 0.000 | –0.813 | –0.313 |
| Constant‡ | 0.00 | –12.665 | 0.6477 | –19.55 | 0.000 | –13.934 | –11.395 |
| Gamma§ | 1.00 | 0.000 | 0.0001 | 2.56 | 0.010 | 0.000 | 0.000 |
eGFR = estimated glomerular filtration rate; EQ-5D, European Quality of Life-5 Dimensions; HF = heart failure; NT-proBNP = N-terminal pro-brain natriuretic peptide; NYHA II–IV = New York Heart Association class II–IV; SE = standard error † Variable centred on mean ‡ Constant term in Gompertz regression § The ancillary parameter that controls the shape of the baseline hazard
Table S4 All-cause mortality of the Swiss population in 2012.
| Age group (years) | Population | Deaths males | Death rate males | Annual probability males | Death females | Death rates females | Annual probability females | |
|---|---|---|---|---|---|---|---|---|
| Males | Females | |||||||
| <1 | 41 914 | 39 495 | 156 | 0.00372 | 0.003715 | 140 | 0.00354 | 0.003538 |
| 1–4 | 169 732 | 160 469 | 26 | 0.00015 | 0.000153 | 15 | 0.00009 | 0.000093 |
| 5–9 | 204 230 | 193 511 | 11 | 0.00005 | 0.000054 | 17 | 0.00009 | 0.000087 |
| 10–14 | 206 846 | 196 080 | 20 | 0.00009 | 0.000097 | 11 | 0.00005 | 0.000056 |
| 15–19 | 226 301 | 214 933 | 85 | 0.00038 | 0.000376 | 26 | 0.00012 | 0.000121 |
| 20–24 | 253 574 | 245 387 | 112 | 0.00044 | 0.000442 | 40 | 0.00016 | 0.000163 |
| 25–29 | 274 522 | 268 685 | 118 | 0.00043 | 0.000429 | 65 | 0.00024 | 0.000242 |
| 30–34 | 288 145 | 282 589 | 152 | 0.00053 | 0.000527 | 71 | 0.00025 | 0.000251 |
| 35–39 | 281 336 | 278 235 | 204 | 0.00073 | 0.000724 | 106 | 0.00038 | 0.000381 |
| 40–44 | 304 469 | 300 842 | 368 | 0.00121 | 0.001207 | 08 | 0.00069 | 0.000691 |
| 45–49 | 338 087 | 330 167 | 590 | 0.00175 | 0.001744 | 388 | 0.00118 | 0.001174 |
| 50–54 | 314 108 | 307 365 | 958 | 0.00305 | 0.003045 | 569 | 0.00185 | 0.001849 |
| 55–59 | 266 125 | 261 023 | 1306 | 0.00491 | 0.004895 | 803 | 0.00308 | 0.003071 |
| 60–64 | 226 250 | 232 464 | 1960 | 0.00866 | 0.008626 | 1107 | 0.00476 | 0.004751 |
| 65–69 | 207 158 | 220 268 | 2638 | 0.01273 | 0.012654 | 1604 | 0.00728 | 0.007256 |
| 70–74 | 159 179 | 181 893 | 3012 | 0.01892 | 0.018744 | 2020 | 0.01111 | 0.011044 |
| 75–79 | 116 891 | 148 637 | 4124 | 0.03528 | 0.034666 | 3078 | 0.02071 | 0.020495 |
| 80–84 | 81 364 | 123 189 | 5146 | 0.06325 | 0.061288 | 5459 | 0.04431 | 0.043347 |
| 85+ | 61 860 | 132 308 | 9711 | 0.15698 | 0.145282 | 17 749 | 0.13415 | 0.125540 |
Table S5 Cardiovascular mortality of the Swiss population in 2012.
| Age group (years) | Population | Deaths males | Death rate males | Annual probability males | Death females | Death rates females | Annual probability females | |
|---|---|---|---|---|---|---|---|---|
| Males | Females | |||||||
| <1 | 41 914 | 39 495 | 1 | 0.00002 | 0.000024 | 1 | 0.00003 | 0.000025 |
| 1–4 | 169 732 | 160 469 | 1 | 0.00001 | 0.000006 | 1 | 0.00001 | 0.000006 |
| 5–9 | 204 230 | 193 511 | 0 | 0 | 0 | 1 | 0.00001 | 0.000005 |
| 10–14 | 206 846 | 196 080 | 2 | 0.00001 | 0.000009 | 0 | 0 | 0 |
| 15–19 | 226 301 | 214 933 | 2 | 0.00001 | 0.000009 | 2 | 0.00001 | 0.000009 |
| 20–24 | 253 574 | 245 387 | 4 | 0.00002 | 0.000016 | 3 | 0.00001 | 0.000012 |
| 25–29 | 274 522 | 268 685 | 6 | 0.00002 | 0.000022 | 5 | 0.00002 | 0.000019 |
| 30–34 | 288 145 | 282,589 | 11 | 0.00004 | 0.000038 | 7 | 0.00002 | 0.000025 |
| 35–39 | 281 336 | 278 235 | 25 | 0.00009 | 0.000089 | 9 | 0.00003 | 0.000032 |
| 40–44 | 304 469 | 300 842 | 70 | 0.00023 | 0.000229 | 26 | 0.00009 | 0.000086 |
| 45–49 | 338 087 | 330 167 | 115 | 0.00034 | 0.000340 | 48 | 0.00015 | 0.000145 |
| 50–54 | 314 108 | 307 365 | 211 | 0.00067 | 0.000672 | 57 | 0.00019 | 0.000185 |
| 55–59 | 266 125 | 261 023 | 306 | 0.00115 | 0.001149 | 89 | 0.00034 | 0.000341 |
| 60–64 | 226 250 | 232 464 | 456 | 0.00202 | 0.002013 | 139 | 0.00060 | 0.000598 |
| 65–69 | 207 158 | 220 268 | 641 | 0.00309 | 0.003089 | 242 | 0.00110 | 0.001098 |
| 70–74 | 159 179 | 181 893 | 769 | 0.00483 | 0.004819 | 448 | 0.00246 | 0.002459 |
| 75–79 | 116 891 | 148 637 | 1265 | 0.01082 | 0.010764 | 882 | 0.00593 | 0.005916 |
| 80–84 | 81 364 | 123 189 | 1793 | 0.02204 | 0.021796 | 1931 | 0.01568 | 0.015553 |
| 85+ | 61 860 | 132 308 | 4067 | 0.06575 | 0.063631 | 8038 | 0.06075 | 0.058944 |
Table S6 Non-cardiovascular mortality of the Swiss population in 2012.
| Age group (years) | All-cause mortality males (%) | All-cause mortality females (%) | CV mortality males (%) | CV mortality females (%) | Non-CV mortality males (%) | Non-CV mortality females (%) |
|---|---|---|---|---|---|---|
| <1 | 0.3715 | 0.3538 | 0.0024 | 0.0025 | 0.3691 | 0.3513 |
| 1–4 | 0.0153 | 0.0093 | 0.0006 | 0.0006 | 0.0147 | 0.0087 |
| 5–9 | 0.0054 | 0.0087 | 0 | 0.0005 | 0.0054 | 0.0082 |
| 10–14 | 0.0097 | 0.0056 | 0.0009 | 0 | 0.0088 | 0.0056 |
| 15–19 | 0.0376 | 0.0121 | 0.0009 | 0.0009 | 0.0367 | 0.0112 |
| 20–24 | 0.0442 | 0.0163 | 0.0016 | 0.0012 | 0.0426 | 0.0151 |
| 25–29 | 0.0429 | 0.0242 | 0.0022 | 0.0019 | 0.0407 | 0.0223 |
| 30–34 | 0.0527 | 0.0251 | 0.0038 | 0.0025 | 0.0489 | 0.0226 |
| 35–39 | 0.0724 | 0.0381 | 0.0089 | 0.0032 | 0.0635 | 0.0349 |
| 40–44 | 0.1207 | 0.0691 | 0.0229 | 0.0086 | 0.0978 | 0.0605 |
| 45–49 | 0.1744 | 0.1174 | 0.0340 | 0.0145 | 0.1404 | 0.1029 |
| 50–54 | 0.3045 | 0.1849 | 0.0672 | 0.0185 | 0.2373 | 0.1664 |
| 55–59 | 0.4895 | 0.3071 | 0.1149 | 0.0341 | 0.3746 | 0.2730 |
| 60–64 | 0.8626 | 0.4751 | 0.2013 | 0.0598 | 0.6613 | 0.4153 |
| 65–69 | 1.2654 | 0.7256 | 0.3089 | 0.1098 | 0.9565 | 0.6158 |
| 70–74 | 1.8744 | 1.1044 | 0.4819 | 0.2459 | 1.3925 | 0.8585 |
| 75–79 | 3.4666 | 2.0495 | 1.0764 | 0.5916 | 2.3902 | 1.4579 |
| 80–84 | 6.1288 | 4.3347 | 2.1796 | 1.5553 | 3.9492 | 2.7794 |
| 85+ | 14.5282 | 12.5540 | 6.3631 | 5.8944 | 8.1651 | 6.6596 |
Table S7 Negative binomial regression model for all-cause hospitalisation.
| IRR | Coefficient | SE | z | P>z | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Sacubitril/valsartan | 0.84 | –0.173 | 0.038 | –4.540 | 0.000 | –0.248 | –0.098 |
| Age† | 0.95 | –0.055 | 0.013 | –4.130 | 0.000 | –0.082 | –0.029 |
| Age squared | 1.00 | 0.000 | 0.000 | 4.350 | 0.000 | 0.000 | 0.001 |
| Female | 0.74 | –0.299 | 0.049 | –6.050 | 0.000 | –0.396 | –0.202 |
| Region | |||||||
| Latin America | 0.70 | –0.364 | 0.085 | –4.300 | 0.000 | –0.530 | –0.198 |
| Western Europe | 1.02 | 0.016 | 0.074 | 0.220 | 0.828 | –0.129 | 0.162 |
| Central Europe | 0.72 | –0.323 | 0.076 | –4.270 | 0.000 | –0.471 | –0.175 |
| Asia-Pacific | 0.70 | –0.352 | 0.085 | –4.130 | 0.000 | –0.519 | –0.185 |
| Heart rate† | 1.01 | 0.007 | 0.002 | 4.320 | 0.000 | 0.004 | 0.010 |
| Log (eGFR)† | 0.62 | –0.479 | 0.072 | –6.610 | 0.000 | –0.621 | –0.337 |
| Log (NT-proBNP)† | 1.26 | 0.229 | 0.020 | 11.260 | 0.000 | 0.189 | 0.269 |
| Sodium† | 0.98 | –0.021 | 0.007 | –3.220 | 0.001 | –0.035 | –0.008 |
| QRS duration† | 1.00 | 0.003 | 0.001 | 5.370 | 0.000 | 0.002 | 0.004 |
| Diabetes | 1.40 | 0.334 | 0.040 | 8.250 | 0.000 | 0.255 | 0.413 |
| Prior ACEi use | 0.90 | –0.104 | 0.047 | –2.220 | 0.026 | –0.196 | –0.012 |
| Beta-blocker use | 0.72 | –0.332 | 0.073 | –4.560 | 0.000 | –0.475 | –0.189 |
| Lipid lowering medication use | 1.07 | 0.072 | 0.043 | 1.680 | 0.094 | –0.012 | 0.157 |
| Time since diagnosis of HF | |||||||
| 1–5 years | 1.30 | 0.265 | 0.049 | 5.390 | 0.000 | 0.169 | 0.362 |
| >5 years | 1.50 | 0.404 | 0.052 | 7.740 | 0.000 | 0.302 | 0.506 |
| Ischaemic disease | 1.09 | 0.086 | 0.044 | 1.940 | 0.052 | –0.001 | 0.173 |
| Prior stroke | 1.16 | 0.147 | 0.065 | 2.250 | 0.024 | 0.019 | 0.275 |
| Atrial fibrillation | 1.10 | 0.094 | 0.042 | 2.250 | 0.024 | 0.012 | 0.176 |
| Prior cancer | 1.18 | 0.163 | 0.088 | 1.850 | 0.064 | –0.010 | 0.335 |
| Current smoker | 1.24 | 0.212 | 0.054 | 3.920 | 0.000 | 0.106 | 0.318 |
| Prior HF hospitalisation | 1.40 | 0.334 | 0.041 | 8.230 | 0.000 | 0.255 | 0.414 |
| Baseline EQ-5D† | 0.62 | –0.485 | 0.090 | –5.410 | 0.000 | –0.661 | –0.309 |
| Constant | 0.06 | –2.891 | 0.475 | –6.090 | 0.000 | –3.821 | –1.960 |
| ln(exposure) | 0.84 | –0.173 | 0.038 | –4.540 | 0.000 | –0.248 | –0.098 |
ACEi = angiotensin converting-enzyme inhibitor; GFR = estimated glomerular filtration rate; EQ-5D, European Quality of Life-5 Dimensions; HF = heart failure; IRR = incidence rate ratio; NT-proBNP = N-terminal pro-brain natriuretic peptide; NYHA II–IV = New York Heart Association class II–IV; SE = standard error † Variable centred on mean
Table S8 Occurrence of less serious adverse events in the PARADIGM-HF trial.
| Event | Sacubitril/valsartan (n = 4187) |
Angiotensin converting-enzyme inhibitor
(n = 4212) |
p-value | ||||
|---|---|---|---|---|---|---|---|
| Number† | Mean annual rate | Mean monthly probability | Number† | Mean annual rate | Mean monthly probability | ||
| Hypotension | 588 | 0.063 | 0.0052 | 388 | 0.042 | 0.0035 | <0.001 |
| Elevated serum creatinine | 139 | 0.015 | 0.0012 | 188 | 0.020 | 0.0017 | 0.007 |
| Elevated serum potassium | 674 | 0.073 | 0.0061 | 727 | 0.079 | 0.0066 | 0.15 |
| Cough | 474 | 0.051 | 0.0042 | 601 | 0.065 | 0.0054 | <0.001 |
| Angio-oedema | 19 | 0.002 | 0.0002 | 10 | 0.001 | 0.0001 | 0.19‡
0.52§ 0.31¶ |
† Absolute number of each adverse event taken from McMurray et al (10) ‡ No treatment or use of antihistamines § Use of catecholamines or glucocorticoids without hospitalisation ¶ Hospitalisation without airway compromise
Table S9 Mixed model for EQ-5D-based utility values.
| Coefficient | SE | z | P>z | 95% CI | ||
|---|---|---|---|---|---|---|
| Sacubitril/valsartan | 0.011 | 0.003 | 3.35 | 0.001 | 0.004 | 0.017 |
| Age† | –0.001 | 0.000 | –4.96 | 0.000 | –0.001 | 0.000 |
| Female | –0.031 | 0.004 | –7.8 | 0.000 | –0.039 | –0.023 |
| Region | ||||||
| Latin America | 0.041 | 0.007 | 5.72 | 0.000 | 0.027 | 0.055 |
| Western Europe | 0.013 | 0.007 | 1.86 | 0.063 | –0.001 | 0.026 |
| Central Europe | 0.000 | 0.007 | –0.04 | 0.969 | –0.014 | 0.013 |
| Asia-Pacific | 0.041 | 0.008 | 5.37 | 0.000 | 0.026 | 0.056 |
| NYHA | ||||||
| II (vs I) | –0.009 | 0.008 | –1.22 | 0.224 | –0.024 | 0.006 |
| III (vs I) | –0.051 | 0.008 | –6.05 | 0.000 | –0.067 | –0.034 |
| IV (vs I) | –0.092 | 0.021 | –4.46 | 0.000 | –0.132 | –0.051 |
| Heart rate† | 0.000 | 0.000 | –1.97 | 0.049 | –0.001 | 0.000 |
| Log(NT-proBNP)† | –0.009 | 0.002 | –5.35 | 0.000 | –0.013 | –0.006 |
| Sodium† | 0.001 | 0.001 | 1.8 | 0.071 | 0.000 | 0.002 |
| BMI | –0.002 | 0.000 | –6 | 0.000 | –0.003 | –0.001 |
| Diabetes | –0.014 | 0.003 | –4.02 | 0.000 | –0.021 | –0.007 |
| Time since diagnosis of HF | ||||||
| 1–5 years | –0.017 | 0.004 | –4.21 | 0.000 | –0.024 | –0.009 |
| >5 years | –0.023 | 0.004 | –5.34 | 0.000 | –0.031 | –0.014 |
| Ischaemic aetiology | –0.007 | 0.003 | –2.13 | 0.033 | –0.014 | –0.001 |
| Prior stroke | –0.012 | 0.006 | –2.06 | 0.039 | –0.023 | –0.001 |
| Current smoker | –0.013 | 0.005 | –2.8 | 0.005 | –0.022 | –0.004 |
| Baseline EQ-5D† | 0.488 | 0.008 | 61.39 | 0.000 | 0.473 | 0.504 |
| Hosp 0–30 days | –0.105 | 0.006 | –18.31 | 0.000 | –0.116 | –0.094 |
| Hosp 30–90 days | –0.054 | 0.004 | –12.43 | 0.000 | –0.062 | –0.045 |
| AE – cough | –0.028 | 0.007 | –4.33 | 0.000 | –0.041 | –0.015 |
| AE – hypotension | –0.029 | 0.006 | –4.63 | 0.000 | –0.042 | –0.017 |
| Time (years) | –0.008 | 0.001 | –8.56 | 0.000 | –0.010 | –0.006 |
| _cons | 0.822 | 0.010 | 79.67 | 0.000 | 0.802 | 0.843 |
AE = adverse event; BMI = body mass index; CI = confidence interval; eGFR = estimated glomerular filtration rate; EQ-5D, European Quality of Life-5 Dimensions; HF = heart failure; NT-proBNP = N-terminal pro-brain natriuretic peptide; NYHA II–IV = New York Heart Association class II–IV; SE = standard error † Variable centred on mean
Table S10 Swiss drug costs of primary and background therapy for heart failure.
| Tab strength (mg) | Cost/pack (CHF) | Tabs/pack | Cost/tab (CHF) | Cost/mg (CHF) | Daily dose | Daily cost (CHF) | Monthly cost (CHF) |
|---|---|---|---|---|---|---|---|
| Primary therapy | |||||||
| Angiotensin converting-enzyme inhibitor – enalapril | |||||||
| 5 | 7.30 | 30 | 0.244 | 0.05 | 18.9 mg | 0.78 | 23.72 |
| 10 | 9.40 | 28 | 0.337 | 0.03 | |||
| 20 | 17.80 | 28 | 0.636 | 0.03 | |||
| Angiotensin converting enzyme inhibitor – ramipril | |||||||
| 2.5 | 11.80 | 20 | 0.59 | 0.24 | |||
| 5 | 14.40 | 20 | 0.72 | 0.14 | 2 × 5 mg | 1.44 | 43.75 |
| 10 | 15.10 | 20 | 0.75 | 0.08 | |||
| Angiotensin converting enzyme inhibitor – perindopril | |||||||
| 2 | 12.73 | 30 | 0.42 | 0.21 | |||
| 4 | 18.27 | 30 | 0.61 | 0.15 | 8 mg | 0.79 | 23.91 |
| 8 | 23.57 | 30 | 0.79 | 0.10 | |||
| Angiotensin converting-enzyme inhibitor – lisinopril | |||||||
| 5 | 7.07 | 30 | 0.24 | 0.05 | |||
| 10 | 9.42 | 30 | 0.31 | 0.03 | 1 × 20 mg 1 × 10 mg |
0.86 | 26.21 |
| 20 | 16.42 | 30 | 0.55 | 0.03 | |||
| Angiotensin receptor blocker – losartan | |||||||
| 25 | 14.00 | 28 | 0.50 | 0.02 | |||
| 50 | 17.00 | 28 | 0.61 | 0.01 | 1 × 100 mg 1 × 50 mg |
1.21 | 36.96 |
| 100 | 17.00 | 28 | 0.61 | 0.01 | |||
| Angiotensin receptor blocker – candesartan | |||||||
| 4 | 5.85 | 7 | 0.84 | 0.21 | |||
| 8 | 16.00 | 30 | 0.53 | 0.07 | |||
| 16 | 17.60 | 30 | 0.59 | 0.04 | |||
| 32 | 26.50 | 30 | 0.88 | 0.03 | 1 × 32 mg | 0.88 | 26.89 |
| Angiotensin receptor blocker – valsartan | |||||||
| 80 | 20.20 | 28 | 0.72 | 0.01 | |||
| 160 | 26.75 | 28 | 0.96 | 0.01 | 2 × 160 mg | 1.96 | 58.16 |
| Background therapy | |||||||
| Beta-blocker – carvedilol | |||||||
| 3.125 | 6.95 | 30 | 0.23 | 0.07 | |||
| 6.25 | 7.45 | 22 | 0.34 | 0.05 | |||
| 12.5 | 17.80 | 30 | 0.59 | 0.05 | |||
| 25 | 26.63 | 30 | 0.89 | 0.04 | 2 × 25 mg | 1.78 | 54.03 |
| Beta-blocker – bisoprolol | |||||||
| 5 | 15.90 | 30 | 0.53 | 0.11 | |||
| 10 | 26.05 | 30 | 0.87 | 0.09 | 1 × 10 mg | 0.87 | 26.43 |
| Aldosterone antagonist – spironolactone | |||||||
| 25 | 7.85 | 20 | 0.39 | 0.02 | 1 × 25 mg 1 × 50 mg |
0.58 | 17.77 |
| 50 | 15.50 | 20 | 0.78 | 0.02 | |||
| 100 | 35.85 | 30 | 1.20 | 0.01 | |||
| Digoxin | |||||||
| 125µg | 7.10 | 100 | 0.07 | 0.001 | 1 × 125 µg | 0.08 | 2.42 |
| 250µg | 8.80 | 100 | 0.09 | 0.0004 | |||
| Lipid lowering medication – atorvastatin | |||||||
| 10 | 28.20 | 30 | 0.94 | 0.09 | 1 × 10 mg 1 × 20mg |
0.94 | 28.61 |
| 20 | 28.20 | 30 | 0.94 | 0.05 | |||
| 40 | 28.20 | 30 | 0.94 | 0.02 | |||
| 80 | 28.20 | 30 | 0.94 | 0.01 | |||
| Lipid lowering medication – simvastatin | |||||||
| 20 | 37.35 | 28 | 1.33 | 0.07 | |||
| 40 | 37.35 | 28 | 1.33 | 0.03 | 1 × 40 mg 1 × 80 mg |
1.33 | 40.60 |
| 80 | 37.35 | 28 | 1.33 | 0.02 | |||
| Loop diuretics – furosemide | |||||||
| 40 | 4.85 | 12 | 0.40 | 0.01 | 1 × 20 mg 1 × 40 mg |
0.40 | 12.30 |
| Aspirin | |||||||
| 100 | 6.60 | 28 | 0.24 | 0.0024 | 1 × 100 mg | 0.24 | 7.17 |
| Marcoumar | |||||||
| 3 | 7.65 | 25 | 0.31 | 0.1 | 1 × 3 mg | 0.25 | 7.61 |
| 3 | 20.80 | 100 | 0.21 | 0.07 | |||
| Clopidogrel | |||||||
| 75 | 44.98 | 28 | 1.58 | 0.02 | 1 × 75 mg | 1.61 | 48.90 |
Table S11 Swiss DRG codes for hospitalisation costs (description of surgical procedures).
| Hospitalisations and related DRG codes | PARADIGM-HF frequency | Activity | Unit cost | |||||
|---|---|---|---|---|---|---|---|---|
| Hospitalisations involving a surgical procedure (4% of total hospitalisations) | ||||||||
| Coronary artery bypass grafting | ||||||||
| F05Z | 16.0% | 88 | CHF 51 950 | |||||
| F06A | 28 | CHF 90 987 | ||||||
| F06B | 42 | CHF 59 328 | ||||||
| F06C | 119 | CHF 46 807 | ||||||
| F06D | 160 | CHF 40 015 | ||||||
| F06E | CHF 33 424 | |||||||
| Mitral valve repair/replacement and other valve surgery | ||||||||
| F03A | 28.0% | 93 | CHF 71 993 | |||||
| F03B | 100 | CHF 51 165 | ||||||
| F03C | 175 | CHF 49 118 | ||||||
| F03D | 442 | CHF 42 270 | ||||||
| F07Z | 217 | CHF 49 795 | ||||||
| F98Z | 258 | CHF 61 777 | ||||||
| F69Z | 218 | CHF 11 203 | ||||||
| Other cardiac surgeries | ||||||||
| F08Z | 39.0% | 58 | CHF 61 110 | |||||
| F09Z | 24 | CHF 38 057 | ||||||
| F13A | 166 | CHF 42 882 | ||||||
| F13B | 87 | CHF 21 845 | ||||||
| F13C | 436 | CHF 14 250 | ||||||
| F14A | 73 | CHF 33 106 | ||||||
| F14B | 213 | CHF 22 372 | ||||||
| F20Z | 65 | CHF 7 844 | ||||||
| F28A | 118 | CHF 57 969 | ||||||
| F28B | 120 | CHF 34 680 | ||||||
| F28C | 56 | CHF 20 726 | ||||||
| F30Z | 36 | CHF 48 685 | ||||||
| F31Z | 128 | CHF 34 456 | ||||||
| F33A | 127 | CHF 43 072 | ||||||
| F33B | 243 | CHF 25 924 | ||||||
| F34A | 272 | CHF 37 873 | ||||||
| F34B | 821 | CHF 20 207 | ||||||
| F35A | 86 | CHF 27 309 | ||||||
| F35B | 110 | CHF 15 845 | ||||||
| F38Z | 63 | CHF 17 622 | ||||||
| F39A | 1965 | CHF 6397 | ||||||
| F39B | 2873 | CHF 5097 | ||||||
| F54Z | 1731 | CHF 12 408 | ||||||
| F59A | 813 | CHF 20 232 | ||||||
| F59B | 2240 | CHF 7912 | ||||||
| F51A | 41 | CHF 35 034 | ||||||
| F51B | 55 | CHF 38 129 | ||||||
| F51C | 203 | CHF 29 486 | ||||||
| F61A | 30 | CHF 34 597 | ||||||
| F61B | 140 | CHF 26 897 | ||||||
| Ventricular assist device (VAD) | ||||||||
| ZE-2015-04.04 | 16.0% | 2 | 28 967.45 | |||||
| ZE-2015-04.05 | 1 | 57 934.90 | ||||||
| ZE-2015-04.08 | 1 | 36 439.15 | ||||||
| ZE-2015-04.08 | 1 | 36,439.15 | ||||||
| ZE-2015-04.09 | - | 71 839.55 | ||||||
| ZE-2015-04.11 | 12 | 115 918.95 | ||||||
| ZE-2015-04.11 | 13 | 115 918.95 | ||||||
| ZE-2015-04.12 | 2 | 182 347.20 | ||||||
| Heart transplantation | ||||||||
| A05B | 1.0% | 51 | CHF 147 414 | |||||
| A06Z | 35 | CHF 545 196 | ||||||
| Hospitalisations involving an interventional procedure (8% of total hospitalisations) | ||||||||
| Implantable cardioverter/defibrillator | ||||||||
| F01A | 36.0% | 31 | CHF 94 070 | |||||
| F01B | 130 | CHF 55 862 | ||||||
| F01C | 63 | CHF 72 725 | ||||||
| F01D | 198 | CHF 48 903 | ||||||
| F02Z | 54 | CHF 49 105 | ||||||
| F10Z | 22 | CHF 41 700 | ||||||
| Cardiac pacemaker (biventricular, defibrillating CRT-D), conventional | ||||||||
| F12A | 53.0% | 64 | CHF 36 719 | |||||
| F12B | 46 | CHF 41 076 | ||||||
| F12C | 147 | CHF 33 472 | ||||||
| F12D | 902 | CHF 21 423 | ||||||
| F12E | 581 | CHF 20 250 | ||||||
| F17A | 227 | CHF 17 182 | ||||||
| F17B | 101 | CHF 13 006 | ||||||
| F18A | 49 | CHF 25 039 | ||||||
| F18B | 170 | CHF 11 025 | ||||||
| Coronary angioplasty, percutaneous coronary intervention single / percutaneous coronary intervention (multiple) | ||||||||
| F52A | 11.0% | 189 | CHF 23 547 | |||||
| F52B | 1322 | CHF 14 285 | ||||||
| F56A | 187 | CHF 22 494 | ||||||
| F56B | 1632 | CHF 14 139 | ||||||
| F57A | 62 | CHF 14 470 | ||||||
| F57B | 1351 | CHF 9703 | ||||||
| F58Z | 178 | CHF 9730 | ||||||
| F24A | 180 | CHF 34 746 | ||||||
| F24B | 1338 | CHF 19 645 | ||||||
| F15Z | 101 | CHF 39 039 | ||||||
| Hospitalisations involving medical management procedures (88.0% of total hospitalisations) | ||||||||
| Cardiac failure / pneumonia / chronic obstructive pulmonary disease | ||||||||
| F62A | 65.0% | 364 | CHF 19 068 | |||||
| F62B | 1532 | CHF 14 350 | ||||||
| F62C | 7112 | CHF 8656 | ||||||
| Ventricular tachycardia / atrial fibrillation | ||||||||
| F50A | 11.0% | 310 | CHF 18 850 | |||||
| F50B | 30 | CHF 20 239 | ||||||
| F50C | 528 | CHF 11 921 | ||||||
| F50D | 210 | CHF 10 889 | ||||||
| F71B | 772 | CHF 7606 | ||||||
| Cerebrovascular accident | ||||||||
| B04B | 2.0% | 32 | CHF 30 082 | |||||
| B39A | 41 | CHF 63 370 | ||||||
| B39B | 68 | CHF 37 476 | ||||||
| B39C | 40 | CHF 27 626 | ||||||
| B70A | 350 | CHF 25 839 | ||||||
| B70B | 255 | CHF 19 414 | ||||||
| B70C | 945 | CHF 15 791 | ||||||
| B70D | 650 | CHF 14 717 | ||||||
| B70E | 4093 | CHF 11 456 | ||||||
| B70G | 126 | CHF 6122 | ||||||
| B70H | 433 | CHF 3721 | ||||||
| Angina pectoris | ||||||||
| F71A | 2.0% | 374 | CHF 13 020 | |||||
| F72A | 144 | CHF 7495 | ||||||
| F72B | 3584 | CHF 4444 | ||||||
| Acute myocardial infarction | ||||||||
| F41A | 2.0% | 39 | CHF 26 184 | |||||
| F41B | 411 | CHF 10 447 | ||||||
| F60A | 472 | CHF 14 707 | ||||||
| F60B | 2301 | CHF 7635 | ||||||
| Syncope | ||||||||
| F73Z | 3.0% | 4765 | CHF 4829 | |||||
| Non-cardiac chest pain | ||||||||
| F74Z | 5.0% | 2462 | CHF 3400 | |||||
| E65C | – | – | ||||||
| Renal failure acute | ||||||||
| L60B | 3.0% | 128 | CHF 26 224 | |||||
| Dyspnoea | ||||||||
| F43B | 3.0% | 208 | CHF 30 377 | |||||
| Transient ischaemic attack | ||||||||
| B69A | 1.0% | – | – | |||||
| B69B | 22 | CHF 10 926 | ||||||
| B69C | 1591 | CHF 8643 | ||||||
| B69D | 174 | CHF 9866 | ||||||
| Urinary tract infection | ||||||||
| L63D | 1.0% | 560 | CHF 5669 | |||||
| Anaemia | ||||||||
| Q61D | 2.0% | 256 | CHF 13 030 | |||||
Proportion of hospitalisations per procedure were derived from the Western European population of the PARADIGM-HF trial, including patients from Belgium, Denmark, Finland, France, Germany, Iceland, Italy, Netherlands, Portugal, Spain, Sweden, UK, Israel and South Africa.
Table S12 Parameters used in univariate and probabilistic sensitivity analyses. Cost parameters are in CHF.
| Parameter | Mean value | Lower value for univariate SA | Upper value for univariate SA | Reference for uncertainty | Distribution used in PSA |
|---|---|---|---|---|---|
| CV mortality (coef.): sacubitril/valsartan | –0.2159 | –0.3275 | –0.1042 | 95% CI | Multivariate normal |
| CV mortality (coef.): Age* | –0.0924 | –0.1277 | –0.0571 | 95% CI | Multivariate normal |
| CV mortality (coef.): Age squared | 0.0008 | 0.0005 | 0.0011 | 95% CI | Multivariate normal |
| CV mortality (coef.): Female | –0.3575 | –0.5076 | –0.2073 | 95% CI | Multivariate normal |
| CV mortality (coef.): Region – Latin America (vs North America) | 0.6252 | 0.3401 | 0.9103 | 95% CI | Multivariate normal |
| CV mortality (coef.): Region – Western Europe (vs North America) | 0.1675 | –0.0886 | 0.4237 | 95% CI | Multivariate normal |
| CV mortality (coef.): Region – Central Europe (vs North America) | 0.5286 | 0.2701 | 0.7871 | 95% CI | Multivariate normal |
| CV mortality (coef.): Region – Other (vs North America) | –0.1869 | –0.8086 | 0.4348 | 95% CI | Multivariate normal |
| CV mortality (coef.): Race – Black (vs Caucasian) | 0.4086 | 0.1264 | 0.6908 | 95% CI | Multivariate normal |
| CV mortality (coef.): Race – Asian (vs Caucasian) | 0.9624 | 0.3766 | 1.5482 | 95% CI | Multivariate normal |
| CV mortality (coef.): Race – Other (vs Caucasian) | 0.1685 | –0.0717 | 0.4087 | 95% CI | Multivariate normal |
| CV mortality (coef.): NYHA class III/IV (vs I/II) | 0.2959 | 0.1648 | 0.4270 | 95% CI | Multivariate normal |
| CV mortality (coef.): LVEF* | –0.0167 | –0.0257 | –0.0076 | 95% CI | Multivariate normal |
| CV mortality (coef.): log(eGFR)* | –0.2377 | –0.4442 | –0.0312 | 95% CI | Multivariate normal |
| CV mortality (coef.): log(NT–proBNP)* | 0.4432 | 0.3846 | 0.5017 | 95% CI | Multivariate normal |
| CV mortality (coef.): Sodium* | –0.0267 | –0.0462 | –0.0072 | 95% CI | Multivariate normal |
| CV mortality (coef.): QRS duration* | 0.0020 | 0.0007 | 0.0033 | 95% CI | Multivariate normal |
| CV mortality (coef.): Diabetes | 0.2289 | 0.1114 | 0.3464 | 95% CI | Multivariate normal |
| CV mortality (coef.): Beta blocker use | –0.3202 | –0.5092 | –0.1312 | 95% CI | Multivariate normal |
| CV mortality (coef.): 1–5 years since HF diagnosis (vs ≤1 year) | 0.2096 | 0.0630 | 0.3562 | 95% CI | Multivariate normal |
| CV mortality (coef.): >5 years since HF diagnosis (vs ≤1 year) | 0.3441 | 0.1864 | 0.5018 | 95% CI | Multivariate normal |
| CV mortality (coef.): Ischaemic aetiology | 0.1555 | 0.0328 | 0.2783 | 95% CI | Multivariate normal |
| CV mortality (coef.): Previously hospitalised for HF | 0.1588 | 0.0379 | 0.2797 | 95% CI | Multivariate normal |
| CV mortality (coef.): EQ–5D* | –0.5631 | –0.8129 | –0.3132 | 95% CI | Multivariate normal |
| CV mortality (coef.): Constant | –12.6648 | –13.9344 | –11.3953 | 95% CI | Multivariate normal |
| CV mortality (coef.): Gamma | 0.0002 | 0.0001 | 0.0004 | 95% CI | Multivariate normal |
| All-cause mortality: sacubitril/valsartan | –0.1608 | –0.2610 | –0.0606 | 95% CI | Multivariate normal |
| All-cause mortality: Age* | –0.1011 | –0.1329 | –0.0692 | 95% CI | Multivariate normal |
| All-cause mortality: Age squared | 0.0009 | 0.0006 | 0.0011 | 95% CI | Multivariate normal |
| All-cause mortality: Female | –0.3891 | –0.5253 | –0.2528 | 95% CI | Multivariate normal |
| All-cause mortality: Region - Latin America (vs North America) | 0.5271 | 0.2779 | 0.7763 | 95% CI | Multivariate normal |
| All-cause mortality: Region - Western Europe (vs North America) | 0.1275 | –0.0914 | 0.3464 | 95% CI | Multivariate normal |
| All-cause mortality: Region - Central Europe (vs North America) | 0.3482 | 0.1232 | 0.5732 | 95% CI | Multivariate normal |
| All-cause mortality: Region - Other (vs North America) | –0.2111 | –0.7956 | 0.3734 | 95% CI | Multivariate normal |
| All-cause mortality: Race - Black (vs Caucasian) | 0.2848 | 0.0296 | 0.5400 | 95% CI | Multivariate normal |
| All-cause mortality: Race - Asian (vs Caucasian) | 0.7093 | 0.1539 | 1.2648 | 95% CI | Multivariate normal |
| All-cause mortality: Race - Other (vs Caucasian) | 0.0831 | –0.1322 | 0.2984 | 95% CI | Multivariate normal |
| All-cause mortality: NYHA class III/IV (vs I/II) | 0.2021 | 0.0821 | 0.3221 | 95% CI | Multivariate normal |
| All-cause mortality: LVEF* | –0.0138 | –0.0220 | –0.0056 | 95% CI | Multivariate normal |
| All-cause mortality: Heart rate* | 0.0055 | 0.0012 | 0.0097 | 95% CI | Multivariate normal |
| All-cause mortality: log(eGFR)* | –0.2356 | –0.4225 | –0.0487 | 95% CI | Multivariate normal |
| All-cause mortality: log(NT-proBNP)* | 0.3866 | 0.3330 | 0.4402 | 95% CI | Multivariate normal |
| All-cause mortality: Sodium* | –0.0306 | –0.0480 | –0.0131 | 95% CI | Multivariate normal |
| All-cause mortality: QRS duration* | 0.0019 | 0.0007 | 0.0030 | 95% CI | Multivariate normal |
| All-cause mortality: Diabetes | 0.2149 | 0.1084 | 0.3214 | 95% CI | Multivariate normal |
| All-cause mortality: Beta blocker use | –0.2873 | –0.4598 | –0.1147 | 95% CI | Multivariate normal |
| All-cause mortality: Lipid lowering medication use | –0.0860 | –0.1970 | 0.0249 | 95% CI | Multivariate normal |
| All-cause mortality: 1-5 years since HF diagnosis (vs ≤1 year) | 0.2049 | 0.0729 | 0.3368 | 95% CI | Multivariate normal |
| All-cause mortality: >5 years since HF diagnosis (vs ≤1 year) | 0.2902 | 0.1482 | 0.4323 | 95% CI | Multivariate normal |
| All-cause mortality: Ischaemic aetiology | 0.1857 | 0.0696 | 0.3017 | 95% CI | Multivariate normal |
| All-cause mortality: Prior stroke | 0.1711 | 0.0088 | 0.3335 | 95% CI | Multivariate normal |
| All-cause mortality: Previously hospitalised for HF | 0.1522 | 0.0438 | 0.2606 | 95% CI | Multivariate normal |
| All-cause mortality: EQ-5D* | –0.5413 | –0.7672 | –0.3154 | 95% CI | Multivariate normal |
| All-cause mortality: Constant | –12.7596 | –13.9020 | –11.6172 | 95% CI | Multivariate normal |
| All-cause mortality: Gamma | 0.0004 | 0.0002 | 0.0005 | 95% CI | Multivariate normal |
| % of deaths with CV cause (Sacubitril/valsartan) | 0.7848 | 0.7527 | 0.8145 | 95% CI | Beta |
| % of deaths with CV cause (ACEi) | 0.8299 | 0.8027 | 0.8548 | 95% CI | Beta |
| Discontinuation: Sacubitril/valsartan | –0.1115 | –0.2104 | –0.0127 | 95% CI | Multivariate normal |
| Discontinuation: Region – Latin America (vs North America) | –0.2855 | –0.4783 | –0.0927 | 95% CI | Multivariate normal |
| Discontinuation: Region – Western Europe (vs North America) | –0.1076 | –0.2798 | 0.0646 | 95% CI | Multivariate normal |
| Discontinuation: Region – Central Europe (vs North America) | –0.4092 | –0.5880 | –0.2305 | 95% CI | Multivariate normal |
| Discontinuation: Region – Other (vs North America) | –0.8739 | –1.0988 | –0.6491 | 95% CI | Multivariate normal |
| Discontinuation: Heart rate* | 0.0065 | 0.0024 | 0.0107 | 95% CI | Multivariate normal |
| Discontinuation: log(eGFR)* | –0.5315 | –0.7069 | –0.3561 | 95% CI | Multivariate normal |
| Discontinuation: log(NT–proBNP)* | 0.2045 | 0.1517 | 0.2572 | 95% CI | Multivariate normal |
| Discontinuation: Sodium* | –0.0164 | –0.0338 | 0.0009 | 95% CI | Multivariate normal |
| Discontinuation: Diabetes | 0.1546 | 0.0500 | 0.2592 | 95% CI | Multivariate normal |
| Discontinuation: Beta blocker use | –0.1750 | –0.3624 | 0.0125 | 95% CI | Multivariate normal |
| Discontinuation: Lipid lowering medication use | –0.1914 | –0.3008 | –0.0819 | 95% CI | Multivariate normal |
| Discontinuation: 1–5 years since HF diagnosis (vs ≤1 year) | 0.1020 | –0.0299 | 0.2340 | 95% CI | Multivariate normal |
| Discontinuation: >5 years since HF diagnosis (vs ≤1 year) | 0.2879 | 0.1536 | 0.4222 | 95% CI | Multivariate normal |
| Discontinuation: Ischaemic aetiology | 0.1311 | 0.0186 | 0.2435 | 95% CI | Multivariate normal |
| Discontinuation: EQ–5D* | –0.4726 | –0.6869 | –0.2583 | 95% CI | Multivariate normal |
| Discontinuation: Constant | –7.9937 | –8.2645 | –7.7228 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Sacubitril/valsartan | –0.1729 | –0.2476 | –0.0983 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Age* | –0.0553 | –0.0816 | –0.0291 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Age^2 | 0.0005 | 0.0003 | 0.0007 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Female | –0.2989 | –0.3957 | –0.2022 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Region – Latin America (vs North America) | –0.3638 | –0.5296 | –0.1980 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Region – Western Europe (vs North America) | 0.0161 | –0.1294 | 0.1616 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Region – Central Europe (vs North America) | –0.3230 | –0.4714 | –0.1746 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Region – Other (vs North America) | –0.3520 | –0.5190 | –0.1850 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Heart rate* | 0.0070 | 0.0038 | 0.0102 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): log(eGFR)* | –0.4791 | –0.6211 | –0.3371 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): log(NT–proBNP)* | 0.2290 | 0.1891 | 0.2688 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Sodium* | –0.0215 | –0.0346 | –0.0084 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): QRS duration* | 0.0031 | 0.0019 | 0.0042 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Diabetes | 0.3340 | 0.2547 | 0.4134 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Prior use of ACEi | –0.1043 | –0.1962 | –0.0124 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Beta blocker use | –0.3320 | –0.4747 | –0.1893 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Lipid lowering medication use | 0.0722 | –0.0122 | 0.1567 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): 1–5 years since HF diagnosis (vs ≤1 year) | 0.2651 | 0.1687 | 0.3616 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): >5 years since HF diagnosis (vs ≤1 year) | 0.4038 | 0.3016 | 0.5061 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Ischaemic aetiology | 0.0862 | –0.0009 | 0.1734 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Prior stroke | 0.1469 | 0.0191 | 0.2746 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Prior atrial fibrillation/ flutter | 0.0942 | 0.0123 | 0.1761 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Prior cancer | 0.1629 | –0.0095 | 0.3353 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Current smoker | 0.2119 | 0.1060 | 0.3178 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Previously hospitalised for HF | 0.3345 | 0.2548 | 0.4142 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): EQ–5D* | –0.4855 | –0.6615 | –0.3095 | 95% CI | Multivariate normal |
| Hospitalisation (coef.): Constant | –2.8905 | –3.8207 | –1.9603 | 95% CI | Multivariate normal |
| Utility (coef.): Sacubitril/valsartan | 0.0106 | 0.0044 | 0.0168 | 95% CI | Multivariate normal |
| Utility (coef.): Age* | –0.0008 | –0.0011 | –0.0005 | 95% CI | Multivariate normal |
| Utility (coef.): Female | –0.0309 | –0.0387 | –0.0231 | 95% CI | Multivariate normal |
| Utility (coef.): Region – Latin America (vs North America) | 0.0412 | 0.0271 | 0.0553 | 95% CI | Multivariate normal |
| Utility (coef.): Region – Western Europe (vs North America) | 0.0126 | –0.0007 | 0.0259 | 95% CI | Multivariate normal |
| Utility (coef.): Region – Central Europe (vs North America) | –0.0003 | –0.0135 | 0.0130 | 95% CI | Multivariate normal |
| Utility (coef.): Region – Other (vs North America) | 0.0410 | 0.0261 | 0.0560 | 95% CI | Multivariate normal |
| Utility (coef.): NYHA class II (vs I) | –0.0093 | –0.0242 | 0.0057 | 95% CI | Multivariate normal |
| Utility (coef.): NYHA class III (vs I) | –0.0509 | –0.0674 | –0.0344 | 95% CI | Multivariate normal |
| Utility (coef.): NYHA class IV (vs I) | –0.0917 | –0.1319 | –0.0514 | 95% CI | Multivariate normal |
| Utility (coef.): Heart rate* | –0.0003 | –0.0005 | 0.0000 | 95% CI | Multivariate normal |
| Utility (coef.): log(NT–proBNP)* | –0.0093 | –0.0127 | –0.0059 | 95% CI | Multivariate normal |
| Utility (coef.): Sodium* | 0.0010 | –0.0001 | 0.0022 | 95% CI | Multivariate normal |
| Utility (coef.): BMI | –0.0020 | –0.0026 | –0.0013 | 95% CI | Multivariate normal |
| Utility (coef.): Diabetes | –0.0140 | –0.0208 | –0.0072 | 95% CI | Multivariate normal |
| Utility (coef.): 1–5 years since HF diagnosis (vs ≤1 year) | –0.0165 | –0.0242 | –0.0088 | 95% CI | Multivariate normal |
| Utility (coef.): >5 years since HF diagnosis (vs ≤1 year) | –0.0226 | –0.0309 | –0.0143 | 95% CI | Multivariate normal |
| Utility (coef.): Ischaemic aetiology | –0.0073 | –0.0140 | –0.0006 | 95% CI | Multivariate normal |
| Utility (coef.): Prior stroke | –0.0118 | –0.0230 | –0.0006 | 95% CI | Multivariate normal |
| Utility (coef.): Current smoker | –0.0130 | –0.0220 | –0.0039 | 95% CI | Multivariate normal |
| Utility (coef.): EQ–5D* | 0.4885 | 0.4729 | 0.5041 | 95% CI | Multivariate normal |
| Utility (coef.): Hospitalised within previous 30 days | –0.1047 | –0.1159 | –0.0935 | 95% CI | Multivariate normal |
| Utility (coef.): Hospitalised 30–90 days previously | –0.0539 | –0.0624 | –0.0454 | 95% CI | Multivariate normal |
| Utility (coef.): Adverse event – cough | –0.0282 | –0.0410 | –0.0154 | 95% CI | Multivariate normal |
| Utility (coef.): Adverse event – hypotension | –0.0292 | –0.0415 | –0.0168 | 95% CI | Multivariate normal |
| Utility (coef.): Annual change | –0.0079 | –0.0097 | –0.0061 | 95% CI | Multivariate normal |
| Utility (coef.): Constant | 0.8224 | 0.8022 | 0.8426 | 95% CI | Multivariate normal |
| Adverse events: hypotension, annual rate, Sacubitril/valsartan | 0.0630 | 0.0580 | 0.0680 | 95% CI | None |
| Adverse events: hypotension, annual rate, ACEi | 0.0420 | 0.0380 | 0.0460 | 95% CI | None |
| Adverse events: hypotension, mean duration (days) | 64.8721 | 58.8900 | 70.9000 | ± 25% | Log |
| Adverse events: cough, annual rate, Sacubitril/valsartan | 0.0510 | 0.0460 | 0.0560 | 95% CI | Log |
| Adverse events: cough, annual rate, ACEi | 0.0650 | 0.0600 | 0.0700 | 95% CI | Log |
| Adverse events: cough, mean duration (days) | 73.3328 | 66.0200 | 80.6500 | ± 25% | Log |
| Adverse events: angio-oedema, annual rate, Sacubitril/valsartan | 0.0020 | 0.0010 | 0.0030 | 95% CI | None |
| Adverse events: angio-oedema, annual rate, ACEi | 0.0010 | 0.0000 | 0.0020 | 95% CI | None |
| Adverse events: elevated serum creatinine, annual rate, Sacubitril/valsartan | 0.0150 | 0.0120 | 0.0170 | 95% CI | Log |
| Adverse events: elevated serum creatinine, annual rate, ACEi | 0.0200 | 0.0170 | 0.0230 | 95% CI | Log |
| Adverse events: elevated serum potassium, annual rate, Sacubitril/valsartan | 0.0730 | 0.0670 | 0.0780 | 95% CI | Log |
| Adverse events: elevated serum potassium, annual rate, ACEi | 0.0790 | 0.0730 | 0.0850 | 95% CI | Log |
| Costs, primary therapy, enalapril, cost per pack: 5 | 7.32 | 5.49 | 9.15 | ± 25% | None |
| Costs, primary therapy, enalapril, cost per pack: 10 | 9.43 | 7.07 | 11.79 | ± 25% | None |
| Costs, background therapy, carvedilol, cost per pack: 3.125 | 6.95 | 5.21 | 8.69 | ± 25% | None |
| Costs, background therapy, carvedilol, cost per pack: 6.25 | 7.45 | 5.59 | 9.31 | ± 25% | None |
| Costs, background therapy, carvedilol, cost per pack: 12.5 | 17.80 | 13.35 | 22.25 | ± 25% | None |
| Costs, background therapy, carvedilol, cost per pack: 25 | 26.63 | 19.97 | 33.28 | ± 25% | None |
| Costs, background therapy, bisoprolol, cost per pack: 5 | 15.90 | 11.93 | 19.88 | ± 25% | None |
| Costs, background therapy, bisoprolol, cost per pack: 10 | 26.05 | 19.54 | 32.56 | ± 25% | None |
| Costs, background therapy, spironolactone, cost per pack: 25 | 7.85 | 5.89 | 9.81 | ± 25% | None |
| Costs, background therapy, spironolactone, cost per pack: 50 | 15.50 | 11.63 | 19.38 | ± 25% | None |
| Costs, background therapy, spironolactone, cost per pack: 100 | 35.85 | 26.89 | 44.81 | ± 25% | None |
| Costs, background therapy, digoxin, cost per pack: 125 | 7.10 | 5.33 | 8.88 | ± 25% | None |
| Costs, background therapy, digoxin, cost per pack: 250 | 8.80 | 6.60 | 11.00 | ± 25% | None |
| Costs, background therapy, atorvastatin, cost per pack: 10 | 28.20 | 21.15 | 35.25 | ± 25% | None |
| Costs, background therapy, atorvastatin, cost per pack: 20 | 28.20 | 21.15 | 35.25 | ± 25% | None |
| Costs, background therapy, atorvastatin, cost per pack: 40 | 28.20 | 21.15 | 35.25 | ± 25% | None |
| Costs, background therapy, atorvastatin, cost per pack: 80 | 28.29 | 21.22 | 35.36 | ± 25% | None |
| Costs, background therapy, simvastatin, cost per pack: 20 | 37.35 | 28.01 | 46.69 | ± 25% | None |
| Costs, background therapy, simvastatin, cost per pack: 40 | 37.35 | 28.01 | 46.69 | ± 25% | None |
| Costs, background therapy, simvastatin, cost per pack: 80 | 37.35 | 28.01 | 46.69 | ± 25% | None |
| Costs, background therapy, furosemide, cost per pack: 40 | 4.85 | 3.64 | 6.06 | ± 25% | None |
| Costs, background therapy, aspirin, cost per pack: 100 | 6.60 | 4.95 | 8.25 | ± 25% | None |
| Costs, background therapy, warfarin, cost per pack: 3 | 7.65 | 5.74 | 9.56 | ± 25% | None |
| Costs, background therapy, clopidogrel, cost per pack: 75 | 44.98 | 33.74 | 56.23 | ± 25% | None |
| Beta blockers, % of patients | 0.9300 | 0.9200 | 0.9400 | 95% CI | Beta |
| Mineralocorticoid receptor antagonist, % of patients | 0.5561 | 0.5500 | 0.5700 | 95% CI | Beta |
| Digoxin, % of patients | 0.3023 | 0.2900 | 0.3100 | 95% CI | Beta |
| Lipid lowering medications, % of patients | 0.5630 | 0.5500 | 0.5700 | 95% CI | Beta |
| Diuretics, % of patients | 0.8022 | 0.7900 | 0.8100 | 95% CI | Beta |
| Aspirin, % of patients | 0.5178 | 0.5100 | 0.5300 | 95% CI | Beta |
| Anticoagulants, % of patients | 0.3197 | 0.3100 | 0.3300 | 95% CI | Beta |
| ADP antagonists, % of patients | 0.1500 | 0.1400 | 0.1600 | 95% CI | Beta |
| Costs, monthly cost of HF management | 110.30 | 82.73 | 137.88 | ± 25% | Log |
| Costs, adverse events – hypotension, cost per PCP visit | 110.30 | 82.73 | 137.88 | ± 25% | Log |
| Costs, adverse events – hypotension, number of PCP visits required | 2.00 | 1.50 | 2.50 | ± 25% | Log |
| Costs, adverse events – elevated serum creatinine, cost per PCP visit | 110.30 | 82.73 | 137.88 | ± 25% | Log |
| Costs, adverse events – elevated serum creatinine, number of PCP visits required | 2.00 | 1.50 | 2.50 | ± 25% | Log |
| Costs, adverse events – elevated serum creatinine, cost per lab test | 8.00 | 6.00 | 10.00 | ± 25% | Log |
| Costs, adverse events – elevated serum potassium, cost per PCP visit | 110.30 | 82.73 | 137.88 | ± 25% | Log |
| Costs, adverse events – elevated serum potassium, number of PCP visits required | 2.00 | 1.50 | 2.50 | ± 25% | Log |
| Costs, adverse events – elevated serum potassium, cost per lab test | 8.00 | 6.00 | 10.00 | ± 25% | Log |
| Costs, adverse events – cough, cost per PCP visit | 110.30 | 82.73 | 137.88 | ± 25% | Log |
| Costs, adverse events – cough, number of PCP visits required | 2.00 | 1.50 | 2.50 | ± 25% | Log |
| Costs, adverse events – cough, cost per lab test | 8.00 | 6.00 | 10.00 | ± 25% | Log |
| Costs, adverse events – angio-oedema, % with milder angio-oedema | 0.60 | 60% | 60% | ± 25% | Beta |
| Costs, adverse events – angio-oedema, cost per outpatient contact | 110.30 | 82.73 | 137.88 | ± 25% | Log |
| Costs, adverse events – angio-oedema, no. of outpatient visits required | 2.00 | 1.50 | 2.50 | not varied | Log |
| Costs, adverse events – angio-oedema, daily cost of antihistamines | 0.77 | 0.77 | 0.77 | ± 25% | None |
| Costs, adverse events – angio-oedema, no. of days on antihistamines | 14.00 | 10.50 | 17.50 | ± 25% | Log |
| Costs, adverse events – angio-oedema, cost per ER visit | 492.00 | 369.00 | 615.00 | ± 25% | Log |
| Costs, adverse events – angio-oedema, cost per PCP visit | 110.30 | 82.73 | 137.88 | ± 25% | Log |
| Costs, adverse events – angio-oedema,no. of PCP visits required | 1.00 | 1.00 | 1.00 | not varied | Log |
| Costs, adverse events – angio-oedema, daily cost of glucocorticoids | 1.42 | 1.42 | 1.42 | ± 25% | None |
| Costs, adverse events – angio-oedema, no. of days on glucocorticoids | 5.00 | 3.75 | 6.25 | ± 25% | Log |
| Costs, titration, cost per PCP visit | 110.30 | 82.73 | 137.88 | ± 25% | Log |
| Costs, titration, number of PCP visits required (titration) | 2.00 | 1.50 | 2.50 | ± 25% | Log |
| Costs, titration, NT–proBNP testing | 70.00 | 52.50 | 87.50 | ± 25% | Log |
| Costs, titration, number of outpatient visits required (NT–proBNP testing) | 1.00 | 0.75 | 1.25 | ± 25% | Log |
| Costs, titration, cost per outpatient contact | 132.02 | 99.02 | 165.03 | ± 25% | Log |
Table S13 Results of subgroup analyses.
| Subgroup | ∆ Costs | ∆ QALYs | ICER | % change from base-case |
|---|---|---|---|---|
| Full analysis set | CHF 10 926 | 0.425 | CHF 25 684 | 0% |
| Baseline age <65 years | CHF 11 707 | 0.444 | CHF 26 375 | 3% |
| Baseline age ≥65 years | CHF 10 115 | 0.406 | CHF 24 900 | –3% |
| Baseline age <75 years | CHF 11 396 | 0.437 | CHF 26 089 | 2% |
| Baseline age ≥75 years | CHF 8872 | 0.376 | CHF 23 624 | –8% |
| Region - North America | CHF 9697 | 0.418 | CHF 23 194 | –10% |
| Region - Latin America | CHF 10 766 | 0.428 | CHF 25 164 | –2% |
| Region - Western Europe | CHF 10 771 | 0.445 | CHF 24 229 | –6% |
| Region - Central Europe | CHF 11 153 | 0.396 | CHF 28 132 | 10% |
| Region - Other | CHF 11 359 | 0.455 | CHF 24 990 | –3% |
| Baseline NYHA class I/II | CHF 11 409 | 0.451 | CHF 25 317 | –1% |
| Baseline NYHA III/IV | CHF 9456 | 0.349 | CHF 27 128 | 6% |
| Baseline LVEF ≤ median | CHF 10 377 | 0.420 | CHF 24,698 | –4% |
| Baseline LVEF > median | CHF 11 565 | 0.432 | CHF 26 801 | 4% |
| Baseline SBP ≤ median | CHF 10 792 | 0.429 | CHF 25 127 | –2% |
| Baseline SBP > median | CHF 11 088 | 0.420 | CHF 26 373 | 3% |
| Baseline eGFR <60 | CHF 9394 | 0.397 | CHF 23 642 | –8% |
| Baseline eGFR ≥60 | CHF 11 805 | 0.441 | CHF 26 738 | 4% |
| Baseline NT-proBNP ≤ median | CHF 12 841 | 0.465 | CHF 27 589 | 7% |
| Baseline NT-proBNP > median | CHF 8863 | 0.382 | CHF 23 186 | –10% |
| Diabetes at baseline | CHF 9477 | 0.399 | CHF 23 762 | –7% |
| No diabetes at baseline | CHF 11 689 | 0.439 | CHF 26 602 | 4% |
| Hypertension at baseline | CHF 10 744 | 0.416 | CHF 25 801 | 0% |
| No hypertension at baseline | CHF 11 366 | 0.447 | CHF 25 421 | –1% |
| Prior use of ACEI | CHF 11 005 | 0.426 | CHF 25 855 | 1% |
| Prior use of ARB | CHF 10 642 | 0.425 | CHF 25 067 | –2% |
| Use of beta-blocker at baseline | CHF 11 075 | 0.428 | CHF 25 879 | 1% |
| No use of beta-blocker at baseline | CHF 8944 | 0.391 | CHF 22 856 | –11% |
| Use of aldosterone antagonist at baseline | CHF 10 845 | 0.422 | CHF 25 720 | 0% |
| No use of aldosterone antagonist at baseline | CHF 11 027 | 0.430 | CHF 25 640 | 0% |
| ≤1 year since diagnosis of HF | CHF 12 887 | 0.466 | CHF 27 674 | 8% |
| 1–5 years since diagnosis of HF | CHF 10 536 | 0.414 | CHF 25 464 | –1% |
| >5 years since diagnosis of HF | CHF 9532 | 0.401 | CHF 23 758 | –8% |
| Ischaemic aetiology | CHF 10 501 | 0.413 | CHF 25 434 | –1% |
| Non-ischaemic aetiology | CHF 11 563 | 0.444 | CHF 26 033 | 1% |
| Prior atrial fibrillation at baseline | CHF 10 089 | 0.404 | CHF 24 990 | –3% |
| No prior atrial fibrillation at baseline | CHF 11 414 | 0.438 | CHF 26 057 | 1% |
| Prior HF hospitalisation | CHF 10 306 | 0.416 | CHF 24 786 | –3% |
| No prior HF hospitalisation | CHF 11 973 | 0.442 | CHF 27 111 | 6% |
MRA = mineralocorticoid receptor antagonist; AF = atrial fibrillation; BB = beta-blocker; QALYs = quality-adjusted life years; ICER = incremental cost-effectiveness ratio; NYHA = New York Heart Association; LVEF = left ventricular ejection fraction; SBP = systolic blood pressure; eGFR = estimated glomerular filtration rate; NT-proBNP = N terminal pro-brain natriuretic peptide; ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; HF = heart failure.
This analysis and the PARADIGM-HF study were funded by Novartis AG. Elizabeth Hancock and David Trueman acted as paid consultants for Novartis Pharma AG. Céline Deschaseaux was employee at Novartis Pharma AG at study time. For the work under consideration, Matthias Schwenglenks has received research funding (via employment institution) from Novartis Pharma Schweiz AG.
1 Roger VL . Epidemiology of heart failure. Circ Res. 2013;113(6):646–59. doi:.https://doi.org/10.1161/CIRCRESAHA.113.300268
2 Heidenreich PA , Trogdon JG , Khavjou OA , Butler J , Dracup K , Ezekowitz MD , et al.; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44. doi:.https://doi.org/10.1161/CIR.0b013e31820a55f5
3 Bui AL , Horwich TB , Fonarow GC . Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30–41. doi:.https://doi.org/10.1038/nrcardio.2010.165
4 Edelmann F . Epidemiologie und Prognose der Herzinsuffizienz [Epidemiology and prognosis of heart failure]. Herz. 2015;40(2):176–84. Article in German. doi:.https://doi.org/10.1007/s00059-015-4215-5
5 Owens AT , Jessup M . The year in heart failure. J Am Coll Cardiol. 2012;60(5):359–68. doi:.https://doi.org/10.1016/j.jacc.2012.01.064
6 Hoekstra T , Jaarsma T , van Veldhuisen DJ , Hillege HL , Sanderman R , Lesman-Leegte I . Quality of life and survival in patients with heart failure. Eur J Heart Fail. 2013;15(1):94–102. doi:.https://doi.org/10.1093/eurjhf/hfs148
7 Rustøen T , Stubhaug A , Eidsmo I , Westheim A , Paul SM , Miaskowski C . Pain and quality of life in hospitalized patients with heart failure. J Pain Symptom Manage. 2008;36(5):497–504. doi:.https://doi.org/10.1016/j.jpainsymman.2007.11.014
8 McMurray JJ , Adamopoulos S , Anker SD , Auricchio A , Böhm M , Dickstein K , et al.; Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14(8):803–69. doi:.https://doi.org/10.1093/eurjhf/hfs105
9 Ponikowski P , Voors AA , Anker SD , Bueno H , Cleland JGF , Coats AJS , et al.; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi:.https://doi.org/10.1093/eurheartj/ehw128
10 McMurray JJ , Packer M , Desai AS , Gong J , Lefkowitz MP , Rizkala AR , et al.; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi:.https://doi.org/10.1056/NEJMoa1409077
11 McMurray JJ , Cowie MR , Cohen AA , Briggs A , de Pouvourville G , Taylor M , et al. A New Cost-Effectiveness Modelling Approach In Chronic Heart Failure With Reduced Ejection Fraction. Value Health. 2015;18(7):A394. doi:.https://doi.org/10.1016/j.jval.2015.09.887
12 Solomon SD , Claggett B , Desai AS , Packer M , Zile M , Swedberg K , et al. Influence of Ejection Fraction on Outcomes and Efficacy of Sacubitril/Valsartan (LCZ696) in Heart Failure with Reduced Ejection Fraction: The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) Trial. Circ Heart Fail. 2016;9(3):e002744. doi:.https://doi.org/10.1161/CIRCHEARTFAILURE.115.002744
13 McMurray JJ , Packer M , Desai AS , Gong J , Lefkowitz MP , Rizkala AR , et al.; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2013;15(9):1062–73. doi:.https://doi.org/10.1093/eurjhf/hft052
14Swiss Federal Statistical Office S. Ständige Wohnbevölkerung nach Alter, Geschlecht 2013 [cited 2015 March 11]. Available from: http://www.bfs.admin.ch/bfs/portal/de/index/themen/01/02/blank/key/alter/gesamt.html.
15Swiss Federal Statistical Office S. Sterbefälle und Sterbeziffern wichtiger Todesursachen, nach Alter, Geschlecht 2012 [cited 2015 March 11]. Available from: http://www.bfs.admin.ch/bfs/portal/de/index/themen/14/02/04/key/01.html.
16 Dolan P , Roberts J . Modelling valuations for Eq-5d health states: an alternative model using differences in valuations. Med Care. 2002;40(5):442–6. doi:.https://doi.org/10.1097/00005650-200205000-00009
17Swiss Heart Failure Working Group of the Swiss Society of Cardiology S. Diagnose und Behandlung der chronischen Herzinsuffizienz. www.heartfailure.ch2013.
18 Muntwyler J , Follath F . [Medical treatment of heart failure: an analysis of actual treatment practices in outpatients in Switzerland. The Swiss “IMPROVEMENT of HF” Group]. Schweiz Med Wochenschr. 2000;130(34):1192–8. Article in German.
19Federal Office of Public Health F. Spezialitätenliste (SL) 2015 [cited 2015 March 11]. Available from: www.spezialitaetenliste.ch.
20Swiss Federal Statistical Office S. Fallkostenstatistik 2012 - Kosten pro Fall nach SwissDRG 2012 [cited 2015 March 22]. Available from: http://www.bfs.admin.ch/bfs/portal/de/index/themen/14/04/01/data/01/05.html#C.
21Swiss Federal Statistical Office S. Landesindex der Konsumentenpreise (LIK) - Gesundheitspflege 2014 [cited 2015 March 24]. Available from: http://www.bfs.admin.ch/bfs/portal/de/index/themen/05/01/100.html.
22Federal Office of Public Health F. Analysenliste (AL) 2015 [cited 2015 March 11]. Available from: http://www.bag.admin.ch/themen/krankenversicherung/00263/00264/04185/index.html?lang=de.
23 Brändle M , Goodall G , Erny-Albrecht KM , Erdmann E , Valentine WJ . Cost-effectiveness of pioglitazone in patients with type 2 diabetes and a history of macrovascular disease in a Swiss setting. Swiss Med Wkly. 2009;139(11-12):173–84.
24 Hirth RA , Chernew ME , Miller E , Fendrick AM , Weissert WG . Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20(3):332–42. doi:.https://doi.org/10.1177/0272989X0002000310
25 McCabe C , Claxton K , Culyer AJ . The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. 2008;26(9):733–44. doi:.https://doi.org/10.2165/00019053-200826090-00004
26Swiss Federal Court decision 9C 334/2010, dated November 23rd, 2010.
27 Gaziano TA , Fonarow GC , Claggett B , Chan WW , Deschaseaux-Voinet C , Turner SJ , et al. Cost-effectiveness Analysis of Sacubitril/Valsartan vs Enalapril in Patients With Heart Failure and Reduced Ejection Fraction. JAMA Cardiol. 2016;1(6):666–72. doi:.https://doi.org/10.1001/jamacardio.2016.1747
28 King JB , Shah RU , Bress AP , Nelson RE , Bellows BK . Cost-Effectiveness of Sacubitril-Valsartan Combination Therapy Compared With Enalapril for the Treatment of Heart Failure With Reduced Ejection Fraction. JACC Heart Fail. 2016;4(5):392–402. doi:.https://doi.org/10.1016/j.jchf.2016.02.007
29 Sandhu AT , Ollendorf DA , Chapman RH , Pearson SD , Heidenreich PA . Cost-Effectiveness of Sacubitril-Valsartan in Patients With Heart Failure With Reduced Ejection Fraction. Ann Intern Med. 2016;165(10):681–9. doi:.https://doi.org/10.7326/M16-0057
30 van der Pol S , Degener F , Postma MJ , Vemer P . An Economic Evaluation of Sacubitril/Valsartan for Heart Failure Patients in the Netherlands. Value Health. 2017;20(3):388–96. doi:.https://doi.org/10.1016/j.jval.2016.10.015
31 Borowiec L , Faluta T , Filipiak KJ , Niewada M , Wrona W . Cost-effectiveness analysis of ivabradine in chronic heart failure in the polish setting. Value Health. 2013;16(7):A528–9. doi:.https://doi.org/10.1016/j.jval.2013.08.1296
32 Soto H , Pizarro M , Botello BS , Almeida E . Cost effectiveness and cost utility analyses of ivabradine (procoralan) in the treatment of patients with chronic heart failure in mexico. Value Health. 2013;16(3):A286. doi:.https://doi.org/10.1016/j.jval.2013.03.1486
33 Kourlaba G , Parissis J , Karavidas A , Beletsi A , Milonas C , Maniadakis N . Economic evaluation of ivabradine in chronic heart failure in Greece. Value Health. 2013;16(7):A524. doi:.https://doi.org/10.1016/j.jval.2013.08.1271
34 Ergene O , Oto A , Cavusoglu Y , Aydogdu S , Ozdemir O , Tan M . A Cost-Effectiveness Analysis of Coralan (Ivabradine) Plus Standard Care Versus Standard Care Alone in Chronic Heart Failure. Value Health. 2012;15(7):A372. doi:.https://doi.org/10.1016/j.jval.2012.08.998
35 Lacey L , McAuliffe A , Poisson M . Economic evaluation of ivabradine for chronic heart failure NYHA II to IV class with systolic dysfunction in Ireland. Value Health. 2013;16(7):A527. doi:.https://doi.org/10.1016/j.jval.2013.08.1290
36 Mihajlovic J , Brkic D , Seferovic P , Sakac D , Zivkov-Saponja D , Ponjevic I . Cost effectiveness of ivabradine in heart failure patients-an analysis from Serbian perspective. Eur J Heart Fail Suppl. 2012;11:S153.
37 Chang CJ , Chu PH , Fann CSJ . Cost Effectiveness Of Ivabradine In Chronic Heart Failure Patients With Heart Rate Above Bpm In Taiwan. Value Health. 2014;17(7):A488. doi:.https://doi.org/10.1016/j.jval.2014.08.1436
38 Griffiths A , Paracha N , Davies A , Branscombe N , Cowie MR , Sculpher M . The cost effectiveness of ivabradine in the treatment of chronic heart failure from the U.K. National Health Service perspective. Heart. 2014;100(13):1031–6. doi:.https://doi.org/10.1136/heartjnl-2013-304598
39 Jhund PS , Fu M , Bayram E , Chen CH , Negrusz-Kawecka M , Rosenthal A , et al.; PARADIGM-HF Investigators and Committees. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015;36(38):2576–84. doi:.https://doi.org/10.1093/eurheartj/ehv330
40 Heran BS , Musini VM , Bassett K , Taylor RS , Wright JM . Angiotensin receptor blockers for heart failure. Cochrane Database Syst Rev. 2012;4(4):CD003040.
41 Chevalier J , de Pouvourville G . Valuing EQ-5D using time trade-off in France. Eur J Health Econ. 2013;14(1):57–66. doi:.https://doi.org/10.1007/s10198-011-0351-x
42 Greiner W , Claes C , Busschbach JJV , von der Schulenburg JM . Validating the EQ-5D with time trade off for the German population. Eur J Health Econ. 2005;6(2):124–30. doi:.https://doi.org/10.1007/s10198-004-0264-z
43 Agvall B , Borgquist L , Foldevi M , Dahlström U . Cost of heart failure in Swedish primary healthcare. Scand J Prim Health Care. 2005;23(4):227–32. doi:.https://doi.org/10.1080/02813430500197647
This analysis and the PARADIGM-HF study were funded by Novartis AG. Elizabeth Hancock and David Trueman acted as paid consultants for Novartis Pharma AG. Céline Deschaseaux was employee at Novartis Pharma AG at study time. For the work under consideration, Matthias Schwenglenks has received research funding (via employment institution) from Novartis Pharma Schweiz AG.