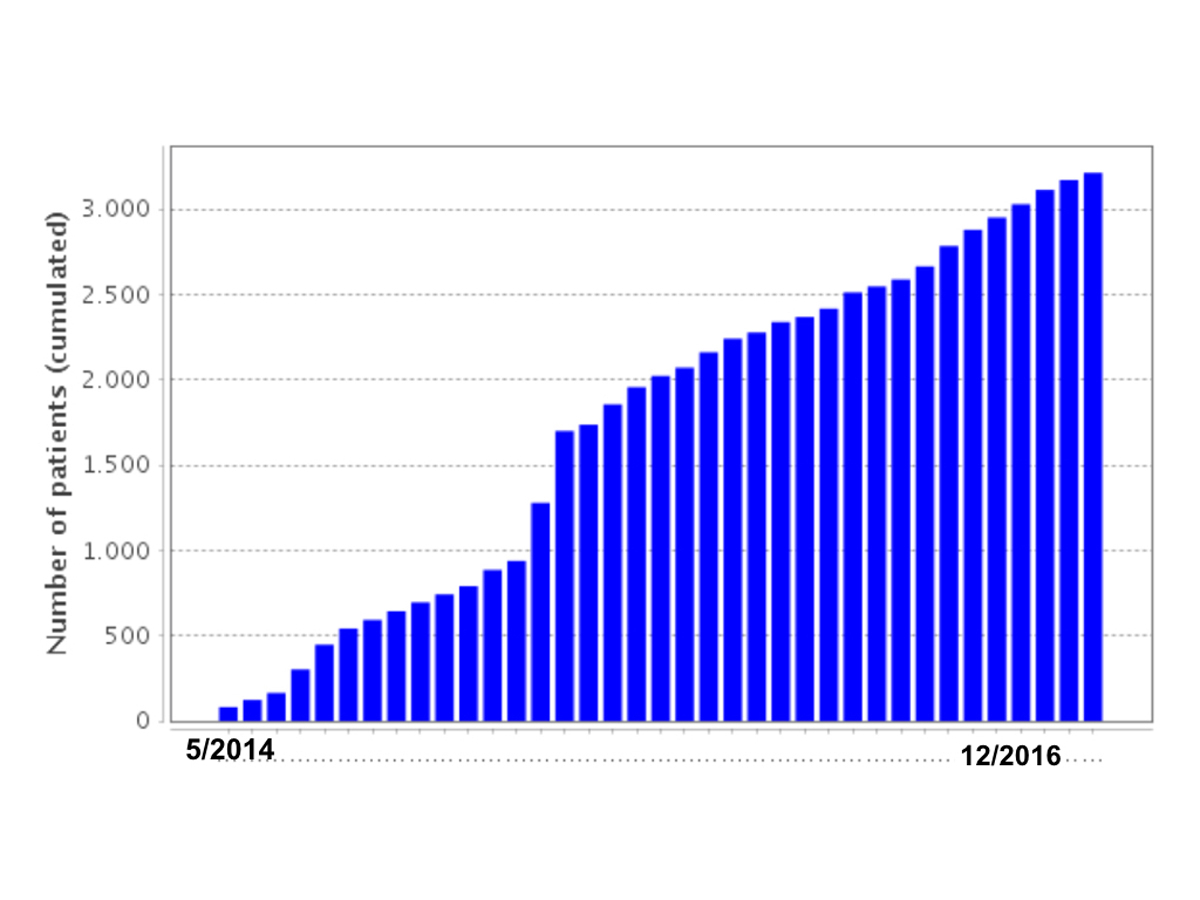
Figure 1 Monthly increase of patients enrolled into SACHER.
DOI: https://doi.org/10.4414/smw.2017.14519
Congenital heart disease (CHD) is the most common congenital defect, affecting about 1 in 100 newborns [1]. Without treatment, most defects of moderate or great complexity have a bleak prognosis [2]. With the invention of surgical repair techniques this has changed dramatically and CHD has become a treatable condition with survival into adulthood now expected for the majority of affected patients [3]. Over the last few decades, this has led to an ever-growing novel cohort of adult survivors with CHD [4, 5]. Although surgical repair and interventions are very effective and enable most patients to live active and productive lives, these patients are by no means “cured”. The vast majority remains at substantially increased risk for adverse events as young and middle-aged adults and many will have a markedly reduced life expectancy [6]. A better understanding of risk factors for adverse outcomes and measures that may prevent such adverse outcomes are urgently needed.
Therefore, in 2013 an initiative was started by several stakeholders in the Swiss adult CHD community to develop a national Swiss Adult Congenital HEart disease Registry (SACHER). The objectives of this registry are: (1) to define the number of adults with CHD in Switzerland followed up at specialised centres; and (2) to prospectively collect data on long-term outcomes. The registry will serve as the basis of future studies and allows national and international collaborations. We describe registry design and structure and present baseline patient characteristics.
The SACHER platform is a web-based electronic database (secuTRial®); secuTrial® is a complete web based data management system. The required application software is implemented on a central server at the University Hospital Zurich and maintained by the clinical trial unit of the University Hospital Zurich. Access can be obtained from any personal computer using one of the popular browsers (e.g., Microsoft Internet Explorer, Mozilla Firefox, Apple Safari). The secuTrial® system complies with all regulatory requirements regarding data safety. Each participant will be pseudonymised (depersonalised) and participants’ data will be entered by centre representatives or by trained study nurses.
The registry has three different types of CRF form – a baseline form, a visit form and an outcome form. The baseline form includes detailed patient characteristics, summarised in table 1 . The primary congenital heart defect (main diagnosis), associated congenital cardiac lesions, palliative and repair procedures are coded according to the International Paediatric and Congenital Cardiac Code (IPCCC). The main diagnosis is defined as the one congenital cardiac lesion with the highest lesion complexity according the Bethesda classification [7]. In patients with more than one main diagnosis of the same complexity (e.g., tetralogy of Fallot and atrioventricular septal defect) the treating cardiologist decides which diagnosis is clinically more relevant to the patient, thus defining the main diagnosis. In cases in which the main diagnosis remains contentious, the steering committee decides on a case-by-case basis, weighing all individual patient factors. All concomitant congenital defects (e.g. atrial septal defect in a patient with tetralogy of Fallot) are labelled as “secondary diagnoses”.
Table 1 Case reporting form for baseline characteristics.
| Variable | Characteristics recorded |
|---|---|
| Demographic data | |
| Gender | Male/female |
| Ethnic origin | Caucasian African Asian Hispanic Other |
| Diagnosis | |
| Working diagnosis | Diagnosis group Working diagnosis |
| Main diagnosis | IPCC Code Description |
| Additional lesions | IPCC Code Description |
| Syndromes | Yes/no Marfan syndrome Down’s syndrome (trisomy 21) Turner’s syndrome Noonan syndrome Williams-Beuren syndrome 22q11.2 deletion syndrome Other |
| Interventions | |
| Main intervention (intracardiac repair) | IPCC Code Description Date of main intervention |
| Palliative interventions (prior to main intervention) | IPCC Code Description Date of palliation |
| Subsequent interventions (after main intervention) | IPCC Code Description Date of subsequent interventions |
| Prior valve implantation (except RV-PA-conduit) | Yes/no Mechanical valve prosthesis Bioprosthetic valve Valve reconstruction Date of most recent replacement |
| Prior RV-PA conduit | Yes/no date of most recent conduit |
| Prior device implantation | Yes/no Pacemaker AICD CRT Date of most device implantation |
| Prior cardiac complications | |
| Myocardial infarction | Yes/no, date of first occurrence |
| Heart failure | Yes/no, date of first occurrence |
| Stroke/TIA | Yes/no, date of first occurrence |
| Endocarditis | Yes/no, date of first occurrence |
| Arrhythmias | Yes/no, date of first occurrence |
| AVNRT/AVRT | Yes/no, date of first occurrence |
| Atrial flutter/IART | Yes/no, date of first occurrence |
| Atrial fibrillation | Yes/no, date of first occurrence |
| VT/VF | Yes/no, date of first occurrence |
| AV Block >1st degree | Yes/no, date of first occurrence |
| Pulmonary hypertension | |
| Other (description) | |
AICD = automatic implantable cardioverter defibrillator; AV block = high degree atrioventricular block; AVNRT = atrioventricular nodal re-entrant tachycardia; AVRT = atrioventricular re-entrant tachycardia; CRT = cardiac resynchronisation therapy; IART = intra-atrial re-entrant tachycardia; RV-PA = right ventricular to pulmonary artery; TIA = transient ischaemic attack; VF = ventricular fibrillation; VT = ventricular tachycardia
The main intervention is defined as the clinically most definite repair operation (typically intracardiac repair procedure). Each procedure following the main intervention (surgery or percutaneous interventions) is defined as subsequent intervention. Each procedure (surgery or percutaneous intervention) before the main intervention is defined as palliative intervention. Interventions are coded according to the IPCCC.
In addition, for each patient a working diagnosis is defined. The aim of the working diagnosis is to best capture the patient’s current characteristics in one diagnosis, which is based on the main intervention and the type of the underlying defect (i.e., the working diagnosis differs for transposition of the great arteries with an atrial switch or with an arterial switch repair). The list of working diagnosis as defined by the steering committee is listed in table 2. If a patient cannot be labelled within one of the defined working diagnoses, the patient will be classified within the unclassified CHD group.
Table 2 Number of patients for each of the working diagnoses.
| Type of diagnosis | Number |
|---|---|
| Unrepaired valve lesion (n = 385, 14%) | |
| Isolated aortic valve disease without aortic dilatation | 185 |
| Bicuspid aortic valve with aortic dilatation ≥4.0cm | 102 |
| Isolated mitral valve disease | 37 |
| Isolated tricuspid valve disease (not Ebstein) | 14 |
| Pulmonary valve disease (including sub- or suprapulmonary obstruction) | 47 |
| Repaired valve lesions (n = 331, 12%) | |
| Replaced or repaired aortic valve | 119 |
| Replaced or repaired mitral valve | 15 |
| Replaced or repaired pulmonary valve | 73 |
| Replaced or repaired tricuspid valve (excluding Ebstein's repair anomaly) | 7 |
| RVOTO relief (excluding pulmonary valve disease) | 4 |
| Multiple valves repaired/replaced (excluding Ross procedure) | 16 |
| Composite graft (Bentall procedure) | 11 |
| Ross procedure | 75 |
| David procedure | 11 |
| Shunt lesions excluding Eisenmenger physiology (n = 624, 22%) | |
| ASD II (repaired or unrepaired) | 164 |
| VSD (repaired or unrepaired)) | 227 |
| PDA (repaired or unrepaired)) | 24 |
| Sinus venosus defect (repaired or unrepaired) | 36 |
| Partial anomalous pulmonary venous drainage (repaired or unrepaired) | 31 |
| Repaired total anomalous pulmonary venous drainage | 11 |
| Partial AVSD/ASD I (repaired or unrepaired) | 67 |
| Complete AVSD (repaired or unrepaired)) | 64 |
| Complex left ventricular outflow tract lesions (n = 377, 13%) | |
| Subvalvular aortic stenosis (repaired or unrepaired) | 44 |
| Supravalvular aortic stenosis (repaired or unrepaired) | 10 |
| Shone complex repaired | 23 |
| Coarctation of the aorta (repaired or unrepaired)) | 282 |
| Repaired interrupted aortic arch | 13 |
| Other aortic arch anomalies | 5 |
| Hereditary aortopathies (n = 93, 3%) | |
| Marfan syndrome | 62 |
| Other aortopathies | 31 |
| Right heart lesions (n = 415, 15%) | |
| Repaired double chambered right ventricle (repaired and unrepaired) | 7 |
| Ebstein anomaly (repaired and unrepaired) | 61 |
| Repaired tetralogy of Fallot - without conduit | 186 |
| Repaired tetralogy of Fallot - with conduit | 120 |
| Repaired tetralogy of Fallot - Absent pulmonary valve syndrome or pulmonary atresia | 29 |
| Repaired pulmonary atresia with intact ventricular septum (biventricular repair) | 12 |
| Cyanotic and other complex lesions (n = 460, 16%) | |
| Cyanotic heart disease – all forms, no Eisenmenger physiology | 27 |
| Eisenmenger syndrome | 53 |
| Fontan procedure | 78 |
| Atrial switch operation (Mustard or Senning) | 159 |
| Arterial switch operation (Jatene) | 74 |
| Rastelli repair | 15 |
| Congenitally corrected transposition of the great arteries | 45 |
| Repaired Common arterial trunk | 9 |
| Non-CHD or unclassified CHD lesions (n = 151, 5%) | |
| Coronary anomalies | 13 |
| Hypertrophic cardiomyopathy (including storage disease) | 69 |
| Cardiomyopathy - neuromuscular disease | 11 |
| Left ventricular non-compaction | 4 |
| Other non-congenital heart disease | 21 |
| Other congenital heart defect | 33 |
ASD = atrial septal defect; AVSD = atrioventricular septal defect; BAV = bicuspid aortic valve; CHD = congenital heart disease; RVOTO = right ventricular outflow tract obstruction; VSD = ventricular septal defect
Visit forms consist of the basic visit form (visit at the time of enrolment) and follow-up visit forms for each subsequent clinic visit. Visit forms are divided into several sub-forms (clinical parameters, data from cardiovascular imaging, blood test results, exercise testing, etc.) and additional sub-forms can be added at any time. Visit sub-forms will serve for collection of specific data required for a more detailed analysis or for prospective studies within the registry in the future. With the aim of synchronisation of follow-up visits and frequency of follow-up procedures/investigations, minimal follow-up intervals for major CHD subgroups and standardisation of cardiovascular tests (e.g., cardiovascular magnetic resonance imaging, cardio-pulmonary exercise testing and echocardiography) have been proposed by the Swiss working group for adults with CHD [8, 9].
The outcome form is intended to collect reoperations/reinterventions and adverse events, including hospital admissions. All subsequent interventions after inclusion will be recorded within the outcome form, but the original main intervention within the baseline form will not be changed once the patient is included in the registry. Types of adverse events to be collected are summarised in table 3.
Table 3 Case report form for cardiac outcomes.
| Death | |
| Death | Yes/no |
| Date of death | |
| Cause of death | Sudden cardiac death Heart failure death Other cardiac death Perioperative death Noncardiac death Unknown cause |
| Place of death | Intensive care unit Regular ward Home Unknown |
| Hospitalisation | |
| Admission | Yes/no |
| Date of admission | |
| Initial stay during admission | Regular ward Intensive care unit |
| Type of admission | Elective Emergency |
| Date of discharge | |
| State at discharge | Home Rehabilitation facility Other acute care hospital Death |
| Reason for admission | Heart failure Arrhythmia Other cardiac complication Elective intervention Emergent intervention Delivery Noncardiac intervention Noncardiac reason |
| Interventions/operations | |
| Operation | IPCCC Code Date of operation |
| Intervention | IPCCC Code Date of intervention |
| Cardiac complications | |
| Bleeding | Date of bleeding Type of bleeding: intracranial, gastrointestinal, pulmonary, other |
| Endocarditis | Date of endocarditis Type of endocarditis: native/prosthetic Type of valve: aortic, mitral, pulmonic, tricuspid |
| Systemic hypertension | Date of first diagnosis |
| Pulmonary hypertension | Date of first diagnosis Diagnosis made by: echo/invasive |
| Arrhythmia | Date of arrhythmia Type of arrhythmia: atrial flutter/intra-atrial re-entrant tachycardia, atrial fibrillation, other SVT, sustained VT, non-sustained VT, high grade AV-block, sinus node dysfunction, other Therapy: electric conversion, medication, other, unknown |
| Myocardial infarction | Date of myocardial infarction Type: STEMI, NSTEMI, other Therapy: PCI, thrombolysis, medical, unknown |
| Stroke | Date of first occurrence |
| Congestive heart failure | Date of first occurrence |
| Aortic dissection | Date of first occurrence Type A, type B |
| Other complications | Date of other cardiac complications Description |
AV = atrioventricular; NSTEMI = non-ST elevation myocardial infarction; PCI = percutaneous coronary intervention, STEMI = ST elevation myocardial infarction; SVT = supraventricular tachycardia, VT = ventricular tachycardia
SACHER has been initiated by board members of the national working group for adults and teenagers with congenital heart disease (WATCH). National programmes with a structured service for adults with CHD (regional and supra-regional adult CHD centres) have been invited to contribute to the registry. Staff and infrastructural requirements for regional and supra-regional centres for adult CHD in Switzerland have been defined previously [8]. All University hospitals (Basel, Bern, Geneva, Lausanne and Zurich) and three regional adult CHD clinics (Kantonsspital Lucerne, Kantonsspital St. Gallen and Klinik im Park) are currently enrolling patients. Since July 2016, the University hospital in Vienna is also participating in the registry. The registry remains open to other centres that will evolve in the future and fulfil requirements as defined by the working-group [8].
SACHER is conducted by a steering committee and projects partners. The steering committee includes the heads of the adult CHD programmes of the University hospital Zurich (M.G.), the University hospital Berne (M.S.) and the University hospital Basel (D.T.). Project partners include representatives from each contributing centre and their associates. The steering committee maintains and supervises the registry. Projects partners are responsible for consenting new patients, data entering and correctness of data assurance.
See appendix 1 for a complete list of participating centres and investigators.
Ethics review boards of all participating centres approved SACHER.
All patients with congenital heart defects or hereditary connective tissue disorders associated with aortopathies followed at one of the participating centres (either exclusively or in a shared-care model with a local hospital or a nonspecialist cardiologist) are eligible to participate.
Patients are approached and asked for participation by their CHD cardiologists during clinic visits or hospital admissions. A patient information form is given to the patient and written informed consent is obtained from each participant.
Each contributing centre has access to its own data and is allowed to analyse their data. The steering committee has access to all pseudonymised data. Proposals for data analysis with specific questions can be submitted to the steering committee. The steering committee evaluates the research proposals for scientific validity and for competing interests with previously launched proposals. The steering committee decides jointly whether the project will be supported.
In this data presentation, continuous data are reported as medians with ranges or means and standard deviations as appropriate. Categorical data are presented as numbers with percentages. For between-groups comparisons chi-square test, Fisher’s exact test, Student’s t-test or Mann-Whitney-test were used, as appropriate. Two-sided p values <0.05 were considered significant. SPSS software (version 23.0, SPSS Inc., Chicago, Illinois) was used for data analysis.
Active participant enrolment started in May 2014. Currently (as of 1 January 2017), 2836 patients (2703 patients from Swiss centres and 133 patients from Vienna) have been included into the registry, of whom 1560 patients (54.9%) were male. The increase in enrolled patients over time is illustrated in figure 1. There was a male predominance in cyanotic and other complex lesions (62% male) and valve lesions (62% male), and a female predominance in shunt lesions (59% female). Mean age at inclusion into the registry was 34.1 ± 14.3 years (range: 15–88 years), 71.4% of patients were younger than 40 years, and only 129 patients (4.5%) were older than 65 years. A total of 337 patients (11.9%) had CHD associated with a syndrome; 109 patients (3.8%) with Down’s syndrome, 23 patients (0.8%) with Turner’s syndrome; 21 patients (0.7%) with Noonan syndrome, 18 patients (0.6%) with known 22q11-microdeletion syndrome, 15 patients (0.5%) with Williams-Beuren syndrome, and 75 patients (2.6%) with Marfan syndrome. A total of 76 patients (2.7%) had rare or unclassified syndromes.

Figure 1 Monthly increase of patients enrolled into SACHER.
The most prevalent main diagnoses (anatomical diagnoses) were isolated aortic valve disease, defined as bicuspid aortic valve or congenital aortic stenosis (n = 446, 15.7%) followed by aortic coarctation (n = 286, 10.1%), tetralogy of Fallot (n = 283, 10%) and transposition of the great arteries (n = 242, 8.5%). The majority of patients had concomitant defects in addition to their main anatomical diagnoses (n = 1753, 62%).
The distribution of groups of working diagnoses is listed in table 2 and illustrated in figure 2.
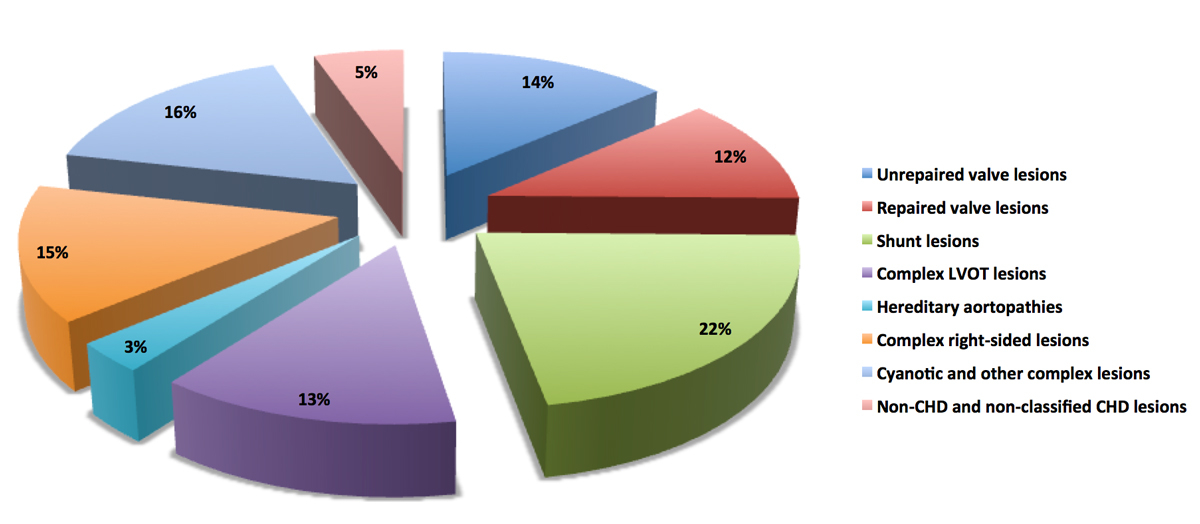
Figure 2 Distribution of lesions groups within SACHER. CHD = congenital heart disease; LVOT = left ventricular outflow tract
Table 4 illustrates how baseline characteristics differ between patients with the same anatomical diagnosis, but different surgical repair techniques and thus different working diagnosis (e.g., patients with complete transposition of the great arteries are grouped according to the type of operation, either as atrial switch patients, arterial switch patients or patients with Rastelli-type repairs).
Table 4 Stratification of baseline characteristics for transposition of the great arteries, according to working diagnosis.
| All Patients
(n = 2840) |
Main diagnosis: d-TGA*
(n = 252, 8.8%) |
Working diagnosis: arterial switch
(n = 74, 2.6%) |
Working diagnosis: atrial switch
(n = 159, 5.6%) |
Working diagnosis: Rastelli repair
(n = 15, 0.5%) |
p-value (within d-TGA) | |
|---|---|---|---|---|---|---|
| Male | 1560 (55%) | 176 (69%) | 56 (76%) | 108 (68%) | 11 (73%) | 0.5 |
| Age, mean | 34±14 | 35±16 | 21±3 | 34±8 | 27±7 | <0.001 |
| Additional lesions | 1753 (62%) | 130 (52%) | 44 (60%) | 67 (42%) | 15 (100%) | <0.0001 |
| Prior cardiac surgery | 2002 (72%) | 251 (99.6%) | ||||
| Palliative interventions | 519 (18%) | 195 (52%) | 50 (68%) | 130 (82%) | 212 (80%) | 0.053 |
| Subsequent interventions | 934 (33%) | 111 (44%) | 38 (51%) | 55 (35%) | 14 (93%) | <0.0001 |
| Prior valve implantation | 440 (15%) | 12 (6%) | 7 (9%) | 2 (1%) | 5 (33%) | <0.0001 |
| mechanical | 155 (6%) | 3 (1%) | 3 (4%) | 0 | 0 | |
| bioprosthesis | 196 (7%) | 9 (4%) | 4 (5%) | 1 (1%) | 4 (29%) | |
| reconstruction | 89 (3%) | 2 (1%) | 0 | 1 (1%) | 1 (7%) | |
| RV or LV to PA conduit | 296 (11%) | 17 (7%) | 2 (3%) | 0 | 15 (100%) | <0.0001 |
| Prior device implantation | 220 (8%) | 25 (10%) | 1 (1%) | 20 (10%) | 2 (13%) | <0.0001 |
| Pacemaker | 150 (5%) | 19 (8%) | 1 (1%) | 16 (10%) | 0 | |
| AICD/CRT | 70 (2%) | 6 (2%) | 0 | 4 (3%) | 2 (13%) |
AICD = automatic implantable cardioverter defibrillator; CRT = cardiac resynchronisation therapy; d-TGA = d-transposition of the great arteries; LV = left ventricle; PA = pulmonary artery; RV = right ventricle * Additional working diagnoses: Fontan palliation n = 3; cyanotic heart disease (unoperated) n = 1
Prior to inclusion into SACHER, the majority of patients had undergone intracardiac repair (2002 patients, 71%); of these, 934 patients (47%) had one or more reinterventions after the main repair. A substantial number of patients had undergone palliative procedures prior to intracardiac repair (519 patients, 18%). Palliative procedures were uncommon in patients with isolated valve or shunt lesions but were common in patients with cyanotic and other complex defects (279, 61%). Right heart lesions (58%) and cyanotic and other complex lesions (43%) had more subsequent interventions compared to valve lesions (21%) and shunt lesions (20%), p <0.0001.
Since May 2014, nine national regional or supraregional adult CHD centres and the largest centre in Austria are enrolling patients into SACHER. Herein we report the structure of the registry and the baseline characteristics of included participants.
Historically, evidence for adverse events and its risk factors in CHD patients is derived from retrospective analysis of patient cohorts, often followed up at single centres. These analyses have provided important insights into the outcome of CHD, but the validity of these data with regards to patient management is limited because of inherent selection bias. Randomised controlled trials comparing different treatment strategies would be ideal, but, for several reasons, to date only very few randomised controlled trials in CHD have been performed, and these are mostly underpowered and thus with limited ability to draw firm conclusions. Among the most important obstacles and limitations regarding the conduct of randomised controlled trials in CHD are: (1) diversity and complexity of CHD, (2) variations in disease complexity even within an individual disease entity, (3) different types and different timing of repair techniques, and (4) limited funding. Although many CHD patients will have a reduced life expectancy, the majority is expected to survive for many decades into adulthood. As a consequence, studies allowing follow-up durations of decades rather than years are important.
Given these considerations, one way to overcome many of the limitations of retrospective data analysis and randomised trials with limited follow-up periods are prospective multicentre registries such as SACHER. The aim of SACHER is to establish a prospective database of detailed clinical baseline and, ultimately, long-term follow-up information. This registry will serve as the basis for future analysis of lesion-specific outcomes and predictors of outcomes, as well as for future interventional studies. It also serves as a valuable database in which to identify available number of patients with a specific defect or type of repair who could potentially be recruited into a randomised clinical trial.
Although substantial efforts were and are made to provide specialist care to adults with CHD in Switzerland, many CHD patients are probably not followed up in centres with structured adult CHD services, either because they were lost to follow-up after paediatric care or because care is provided by regional hospital or community-based cardiologists. Thus our registry cannot yet provide meaningful data on the overall prevalence of specific congenital heart defects in Switzerland. As previously published national recommendations of care for adults with CHD encourage a model of shared care between dedicated community-based cardiologists and adult CHD centres, a more comprehensive inclusion of affected patients into this registry may be achieved over time [8]. Currently, the distribution of main diagnoses in SACHER is comparable to other national registries of CHD, with aortic valve disease, tetralogy of Fallot and coarctation of the aorta the most frequent lesions seen in adult CHD clinics [10].
The SACHER represents predominantly patients followed up at dedicated adult CHD centres. Although European guidelines recommend that all adults with CHD should be seen at least once at such a dedicated adult CHD centre [11], there may be a selection bias of patients with more complex disease. These patients may be overrepresented in SACHER, whereas simple defects, such as atrial septal defects, small ventricular septal defects or patients with bicuspid aortic valves may be followed at non-specialist centres.
Coding of the main CHD diagnosis in patient’s baseline characteristics follows the reported anatomical diagnosis at birth. However, adults with CHD, particularly those with anatomically complex lesions, usually had intracardiac repair operations in childhood that completely changed haemodynamic profiles and, possibly, the long-term burden of the underlying lesion. Furthermore, for many congenital heart defects (e.g., complete transposition of the great arteries) surgical strategies substantially differ as a result of the underlying anatomic variation or progress in surgical techniques over time. Therefore labelling adults by their main anatomical diagnosis may be a poor reflection of their cardiac condition as adults and may give a very imprecise appreciation of potential long-term complications during adulthood. For example, patients born with complete transposition of the great arteries may have undergone repair in an atrial switch operation (Mustard or Senning operation), had an arterial switch operation (Jatene operation) or had repair by Rastelli-type operation (in the setting of concomitant pulmonary stenosis and ventricular septal defect). Very rarely, patients with complete transposition may even survive into adulthood without surgical repair and present in adulthood as unrepaired cyanotic defects. It is obvious that risks of adverse outcomes and complications will differ substantially between these patient groups and thus the anatomical diagnosis “transposition of the great arteries” does not appropriately reflect the haemodynamic complexity of these patients.
Another illustrative example is isolated ventricular septal defects. If large and unrestricted, patients may develop irreversible pulmonary hypertension with Eisenmenger physiology before entering adulthood. Eisenmenger patients are among the highest risk patients among adults with CHD. This is in sharp contrast to patients with small restrictive ventricular septal defects or ventricular septal defects surgically closed in early childhood. These patients have a very low risk for adverse events in adulthood and are expected to have a normal life expectancy.
These shortcomings of classification of disease complexity according to anatomical diagnoses will be overcome with the introduction of the “working diagnosis” in SACHER. The intention of working diagnoses is to allow easy identification of clinically important patient groups not only by the main underlying anatomical defect but also by whether or not these defects have been repaired and with what type of repair.
The number of SACHER participants is lower than other European registries in adults with CHD because of the small population size of Switzerland and the short collection period [10, 12]. However, given its prospective nature, harmonisation process for follow-up protocols and close collaboration among the participating centres, SACHER will provide high quality data facilitating multicentre research among the participating centres. The specific structure of SACHER allows new electronic case reporting forms to be added at any time and additional data to be collected prospectively. The structure of SACHER is intended to support analysis lifelong outcomes of adult CHD patients. Ideally, in the future it would also include newborns or children with CHD. Currently, paediatric cardiologists in Switzerland are developing a dedicated paediatric CHD database also based on the secuTrial® platform. With the possibility to merge the evolving paediatric database with SACHER, this will allow assessment and research of outcome and treatment from the newborn to the adult patient with CHD. Future research proposals, including collaboration with researchers maintaining other registries within or outside Switzerland, will be carefully evaluated by the steering committee of SACHER. As one of the main goals of SACHER is to increase the knowledge base in adult CHD, such collaborations are generally welcome.
SACHER, a prospective multicentre registry, was successfully launched in 2014 more than 2800 patients have been enrolled as of the end of 2016. SACHER will facilitate multicentre outcome research. Its structure enables prospective data analysis to assess detailed, lesion-specific outcomes with the aim to improve long-term outcomes.
Daniel Tobler, Matthias Greutmann, Markus Schwerzmann
Participating centers: (Switzerland) University Hospital Basel, Inselspital Bern, University Hospital Geneva, University Hospital Lausanne, Kantonsspital Luzern,, Kantonsspital St. Gallen, Klinik im Park, Zurich, University Hospital Zurich (Austria) University Hospital Vienna,
University Hospital Basel: Daniel Tobler, Kerstin Buetler, Lukas Notz; University Hospital Inselspital Bern: Markus Schwerzmann, Fabienne Schwitz, Kerstin Wustmann, Corina Thomet; University Hospital Geneva: Judith Bouchardy, Corelie Blanche; University Hospital Lausanne: Judith Bouchardy, Tobias Rutz; Kantonsspital St. Gallen: Reto Engel, Dominik Stambach, Niklas Ehl; University Hospital Zurich: Matthias Greutmann, Francesca Bonassin, Angela Oxenius, Christine Attenhofer Jost, Theresa Seeliger, Bruno Santos Lopes; Kantonsspital Luzern: Hans Peter Kuen, Christoph Auf der Maur; Klinik im Park, Zürich: Christine Attenhofer Jost, Jolanda Vögele; University Hospital Vienna: Harald Gabriel, Christine Groiss
No financial support and no other potential conflict of interest relevant to this article was reported.
1 van der Linde D , Konings EE , Slager MA , Witsenburg M , Helbing WA , Takkenberg JJ , et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241–7. doi:.https://doi.org/10.1016/j.jacc.2011.08.025
2 Warnes CA , Liberthson R , Danielson GK, Jr , Dore A , Harris L , Hoffman JI , et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37(5):1170–5. doi:.https://doi.org/10.1016/S0735-1097(01)01272-4
3 Moons P , Bovijn L , Budts W , Belmans A , Gewillig M . Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation. 2010;122(22):2264–72. doi:.https://doi.org/10.1161/CIRCULATIONAHA.110.946343
4 Marelli AJ , Ionescu-Ittu R , Mackie AS , Guo L , Dendukuri N , Kaouache M . Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130(9):749–56. doi:.https://doi.org/10.1161/CIRCULATIONAHA.113.008396
5 Padrutt M , Bracher I , Bonassin F , Santos Lopes B , Gruner C , Stämpfli SF , et al. Impact of growing cohorts of adults with con-genital heart disease on clinical workload: a 20-year experience at a tertiary care centre. Swiss Med Wkly. 2017;147:w14443. Available at: https://smw.ch/en/article/doi/smw.2017.14443/.
6 Greutmann M , Tobler D , Kovacs AH , Greutmann-Yantiri M , Haile SR , Held L , et al. Increasing mortality burden among adults with complex congenital heart disease. Congenit Heart Dis. 2015;10(2):117–27. doi:.https://doi.org/10.1111/chd.12201
7 Warnes CA , Williams RG , Bashore TM , Child JS , Connolly HM , Dearani JA , et al.; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease); American Society of Echocardiography; Heart Rhythm Society; International Society for Adult Congenital Heart Disease; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52(23):e143–263. doi:.https://doi.org/10.1016/j.jacc.2008.10.001
8 Bouchardy J , Greutmann M , Schwerzmann M , Attenhofer Jost CH , De Pasquale G , Oxenius A , et al. Grown-up congenital heart disease: recommendations for standards of care. Cardiovascular Medicine. 2015;18(04):144–5. doi:.https://doi.org/10.4414/cvm.2015.00317
9 Rutz T , Wustmann K , Prsa M , Vallée J , Donner B , Bremerich J , et al. Cardiac magnetic resonance imaging in congenital heart disease. Cardiovascular Medicine. 2016;19(06):176–84. doi:.https://doi.org/10.4414/cvm.2016.00411
10 van der Velde ET , Vriend JWJ , Mannens MMAM , Uiterwaal CSPM , Brand R , Mulder BJM . CONCOR, an initiative towards a national registry and DNA-bank of patients with congenital heart disease in the Netherlands: rationale, design, and first results. Eur J Epidemiol. 2005;20(6):549–57. doi:.https://doi.org/10.1007/s10654-005-4264-9
11 Baumgartner H , Bonhoeffer P , De Groot NM , de Haan F , Deanfield JE , Galie N , et al.; Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC); ESC Committee for Practice Guidelines (CPG). ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. 2010;31(23):2915–57. doi:.https://doi.org/10.1093/eurheartj/ehq249
12 Bauer U , Niggemeyer E , Lange PE . Das Kompetenznetz Angeborene Herzfehler (KN AHF): Vernetzung statt Insellösungen für optimierte Forschung und Versorgung [The Competence Network for Congenital Heart Defects. Networking Instead of Isolated Efforts for Optimized Research and Care]. Med Klin (Munich). 2006;101(9):753–8. Article in German. doi:.https://doi.org/10.1007/s00063-006-1104-4
See appendix 1 for a complete list of participating centres and investigators.
DT, MS and MG contributed in the conception and design of the registry, acquisition of data, analysis and interpretation of data, drafting the article and final approval of the version to be published. JB, RE, DS, CAJ, KW, FS, TR, HG, HPK, CadM, TS, AO, BSL, and FB participated in acquisition of data, in critical revision of the manuscript for important intellectual content, and final approval of the version to be published.
No financial support and no other potential conflict of interest relevant to this article was reported.