Primary polydipsia in the medical and psychiatric patient: characteristics, complications and therapy
DOI: https://doi.org/10.4414/smw.2017.14514
Clara O.
Sailerab, Bettina
Winzelerab, Mirjam
Christ-Crainab
aEndocrinology, Diabetology and Metabolism, University Hospital Basel, Switzerland
bDepartment of Clinical Research, University Hospital Basel, Switzerland
Summary
Primary polydipsia (PP) has been defined as excessive intake of fluids. However, the pathogenesis of PP remains unexplored. Different theories include a dysfunction in the thirst mechanism, involvement of the hippocampus, stress-reducing behaviour and lesion occurrences in specific areas of the brain. Most studies have been performed in the psychiatric setting, indicating that PP coincides with schizophrenia, anxiety disorder and depression. However, an increasing number of case reports emphasise the incidence of PP in non-psychiatric patients. As often recommended by healthcare professions and in life-style programmes, the phenomenon of excessive fluid intake appears to be growing, especially in health-conscious and active people. PP is part of the polyuria-polydipsia syndrome, so the differential diagnosis diabetes insipidus (central or nephrogenic) must be excluded. The gold standard when differentiating between these disorders has been the water deprivation test. However, new options for distinguishing between these entities have been proposed e.g., measurement of copeptin, a reliable surrogate marker of the hormone arginine vasopressin (AVP). The major risk of excessive drinking is the development of hyponatraemia and the ensuing complications. In patients with PP, factors reducing the renal excretory capacity of the kidney such as acute illness, medications or low solute intake may accumulate in hyponatraemia. Treatment options for PP remain scarce. Different medication and behavioural therapy have been investigated, but never on a large scale and rarely in non-psychiatric patients. This review provides an overview of the pathophysiology, characteristics, complications, and outcomes of patients with PP in the medical and psychiatric patient.
Introduction
Primary polydipsia (PP) is characterised by an increased fluid intake and consistent excretion of profound quantities of dilute urine exceeding 40–50 ml/kg body weight (e.g., 3000 ml/day for a person of 60 kg) over an extended period, excluding reasons for secondary polydipsia [1–3]. It has most commonly been described in patients with schizophrenia spectrum disorder with an incidence of 11 to 20%, and has therefore been named psychogenic polydipsia [1–4]. With the increasing popularity of lifestyle programmes and the common conception that consuming several litres of fluid per day is healthy, the prevalence of this phenomenon is increasing, particularly outside of the psychiatric setting. However, the prevalence in the overall population is unknown and has yet to be studied. Presumably, a lack of knowledge regarding the burden, consequences and treatment options for this disorder has limited studies in this field until now.
A comparable disorder is beer potomania, which is also characterised by increased intake of beer, but in this condition urine output may be below the above-mentioned polyuria definition [5–8].
The most common and potentially severe complication of excessive fluid intake is the occurrence of hyponatraemia [1, 4, 9, 10]. Hyponatraemia is associated with increased morbidity and mortality and should therefore be prevented [11–14]. Different risk factors are thought to be associated with the development of hyponatraemia in PP such as medication, physical or psychological stress, and acute consumption of copious quantities of fluids [1, 4, 9].
Several treatment options for PP have been investigated, ranging from different medication to behavioural therapy [15–20]. Unfortunately, most studies were using explorative study designs, small sample sizes with limited success, and low generalisability.
This review is intended to give an overview of the current stage of research in the field of PP. We will describe causes, associated comorbidities, complications, and will discuss the diagnostic and therapeutic issues of this syndrome.
Pathophysiology
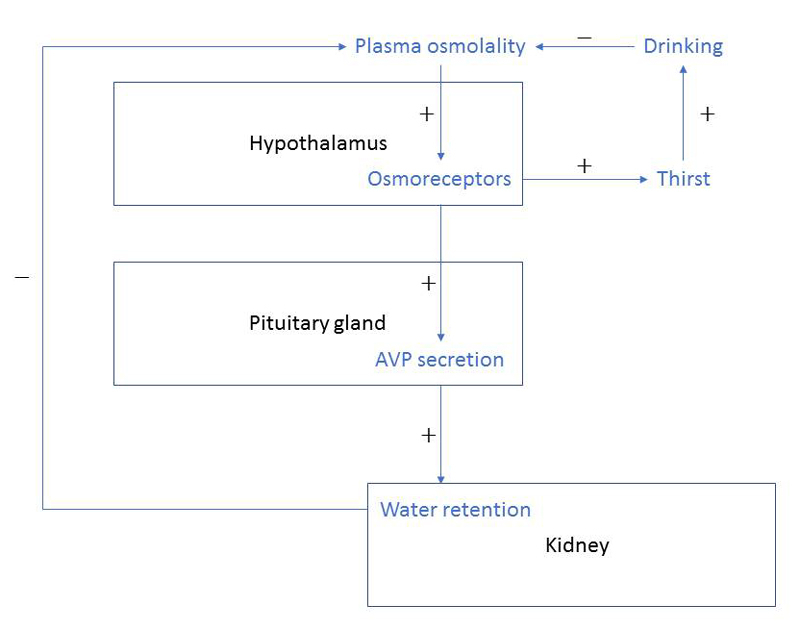
Maintaining a stable fluid level is a primary human need [21, 22]. Water balance is an incessant equilibrium of water intake and excretion through the kidneys, the lungs, the bowels and the skin. This balance, in order to keep plasma osmolality within a close range, is primarily regulated by the interplay of thirst and the hormone arginine vasopressin (AVP) [23]. AVP, promoting water retention in the kidney, is released upon two main stimuli namely high serum osmolality and low arterial blood volume [21, 23, 24] (fig. 1).
In healthy people, drinking leads to a pleasant feeling in response to thirst with an activation of the prefrontal cortex, the pleasure and reward centre of the brain, as shown in functional magnet resonance imaging (fMRI) experiments. In contrast, increased drinking after thirst has been satisfied results in an unpleasant or even aversive sensation, which then stops the healthy participant from further fluid intake [26, 27].
The pathogenesis of insatiable thirst and excessive fluid intake as seen in PP remains largely unknown. According to the underlying or associated conditions, PP may be classified in two main categories, whereby different causal mechanisms are discussed: psychogenic polydipsia and dipsogenic polydipsia [2, 24, 28, 29] (table 1). A related disorder is beer potomania, which is characterised by the chronic or acute consumption of large amounts of beer [7, 30].
Table 1 Causes of primary polydipsia.<su>Table-Small</su>
| Primary polydipsia (excessive water intake) |
|
Psychogenic polydipsia (e.g., in patients with acute psychosis, chronic schizophrenia spectrum disorder, anxiety disorder, depression, anorexia nervosa and dependency disorder) |
|
Dipsogenic polydipsia |
|
Habitual polydipsia |
|
Health-conscious men and women |
|
Sporty people |
|
Somatic (damage of thirst centre) |
|
Cerebral lesion |
|
Granulomatous (sarcoidosis) |
|
Infectious (tuberculous meningitis) |
|
Vascular (vasculitis) |
Most research has been done in patients with schizophrenia spectrum disorders and psychogenic polydipsia. In both, a central defect of thirst and a dysfunction in AVP regulation has been suggested [31, 32]. During acute psychotic episodes, worsening of polydiptic behaviour and increased levels of AVP have been observed [9]. It is speculated that during acute psychosis, the activation of the hypothalamic-pituitary-adrenal axis and AVP secretion influences behavioural traits and vice versa – probably through hippocampal involvement [9, 23, 33–37]. Interestingly, in cranial MRI, the hippocampus was found to have a diminished volume in patients with schizophrenia spectrum disorder and PP compared to those without PP [35].
Furthermore, a stress-induced increase in dopamine levels may also play a role in acute psychotic patients. This hypothesis has been tested in animal studies, which showed that exogenous dopamine initiated drinking and increased AVP levels [38–41].
Other psychiatric conditions such as affective and dependency disorder (e.g., smoking, alcoholism) and anorexia nervosa also appear frequently in PP [29]. In these diseases, drinking might be perceived as a coping strategy to deal with psychological stress or, especially in patients with anorexia nervosa, increased liquid consumption may compensate for low food intake and to decrease the sensation of hunger [32, 42, 43].
Dipsogenic polydipsia includes patients with an increased sensation of thirst due to hypothalamic lesions and subjects with habitual polydipsia, which is typically seen in life-style conscious men and women, which is the use of water to detox the body. Abnormally high water consumption is also seen in people who perform excessive amounts of sport [44–48]. Alongside hypothalamic affection after traumatic brain injuries, vascular or infiltrative diseases (e.g., sarcoidosis) may lead to dipsogenic polydipsia [24, 49–52]. In habitual polydipsia, social conditioning with constant motivation to drink may modify drinking behaviour relative to actual water deficit, thus resulting in a downward resetting of the thirst threshold [26, 27].
Finally, while in beer potomania the underlying disorder is alcohol dependency, the motivation to drink is the effect of alcohol and thus different from other forms of primary polydipsia [5–8]. Psychogenic and dipsogenic polydipsia seem to occur preferentially in women, whereas beer potomania is more often seen in men [1, 4, 11, 29].
Diagnosis and differential diagnosis of PP
The differential diagnoses of primary polydipsia (PP) are central and nephrogenic diabetes insipidus (DI). While PP is primarily characterised by increased fluid intake, DI is determined by polyuria due to impaired AVP secretion (central DI) or AVP resistance in the kidneys (nephrogenic DI) [24]. Central DI may be acquired after e.g., pituitary trauma (surgery), infections, auto-immune disease or congenital factors [2, 3, 24, 53]. Nephrogenic DI can be due to inherited mutations in the AVP-receptor-2 and aquaporine-2 gene, or acquired (e.g., chronic lithium use or metabolic/vascular kidney injuries) [3, 53].
The first step in the diagnosis and differential diagnosis of PP is a thorough history, including medical and psychiatric comorbidities and medication. Compared to patients with DI, polydiptic patients typically report a less acute onset and often deny nocturia and drinking during the night [24].
The next diagnostic step is to exclude other forms of polyuria (e.g., diabetes mellitus) and to measure plasma, urine osmolality and electrolytes. Low normal plasma sodium in the presence of a low urine osmolality is indicative of PP. The widely accepted gold standard for the differential diagnosis of PP is the indirect water deprivation test, introduced in 1964 [54]. The test indirectly assesses AVP activity by measurement of urinary osmolality, and thus the concentration capacity of the kidneys, during a prolonged dehydration period, and finishes by assessing the response (increase in urinary osmolality in %) to the administration of exogenous vasopressin (desmopressin) [55–57].
However, this procedure is limited by poor diagnostic accuracy of 70% overall and an especially poor diagnostic performance of only 41% for PP [2, 58]. Therefore, other methods have been studied. Direct measurement of AVP was used with initially promising results [59]. However, due to measuring difficulties and the instability of AVP, direct measurement of AVP has never entered everyday practice [60–62].
Copeptin, the c-terminal part of the AVP precursor peptide, was found to be a stable, sensitive and easily measured surrogate marker for AVP [61, 63, 64]. Copeptin has been shown to accurately discriminate between PP and DI [28, 64]. Without prior thirsting, a single basal copeptin value >21.4 pmol/l differentiates nephrogenic DI from other aetiologies with a high sensitivity and specificity. If the basal copeptin value is below this cut-off, an osmotic stimulation (plasma sodium >147 mmol/l) either by fluid restriction or the administration of a hypertonic saline infusion is necessary for differentiation between central DI and PP [28]. An osmotically stimulated copeptin value ≥4.9 pmol/l differentiates between patients with PP and central DI with high diagnostic accuracy (fig. 2).
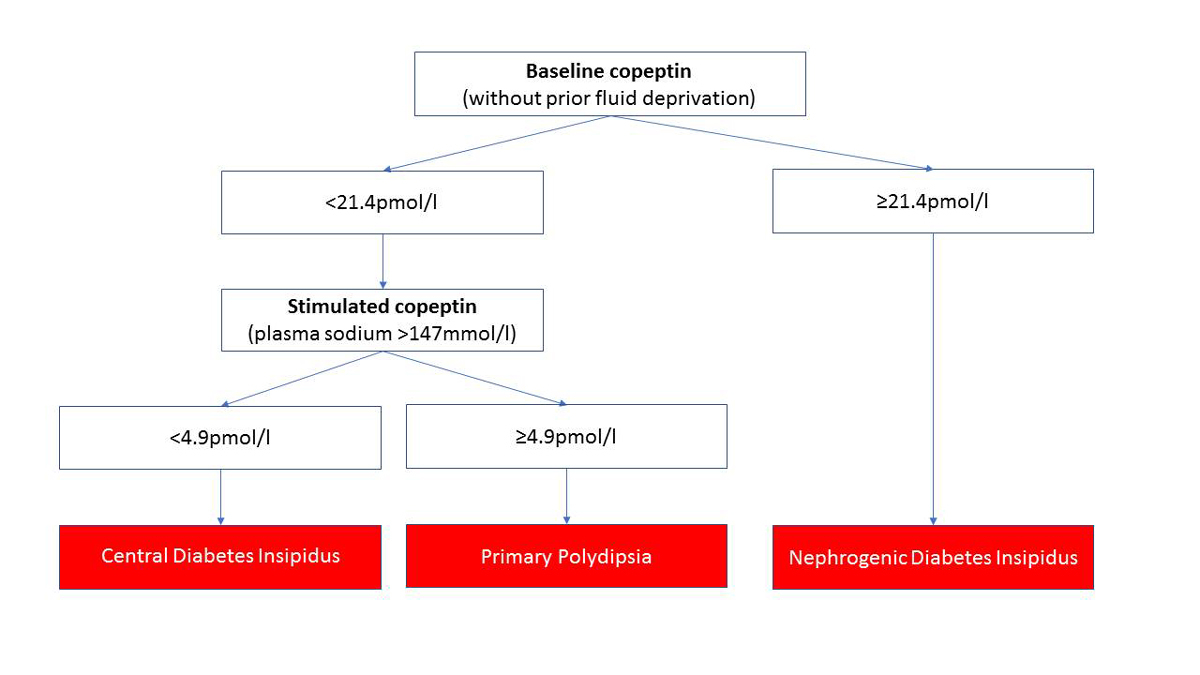
Figure 2
Diagnostic algorithm of copeptin in the differential diagnosis of patients with PP, central and nephrogenic DI. A basal copeptin value of ≥21.4 pmol/l confirms the diagnosis of nephrogenic DI. A basal copeptin value of <21.4 pmol/l needs an osmotic stimulation (water deprivation or hypertonic saline infusion) to differentiate between central DI and PP. An osmotically stimulated copeptin value ≥4.9 pmol/l differentiates between patients with central DI and PP with a high diagnostic accuracy [28].
Adapted from Christ-Crain and Fenske (2016) [53].
Complications of PP
The most common and severe acute complication of PP is the development of hyponatraemia [10, 11, 30, 65]. Hyponatraemia in PP occurs when free fluid intake exceeds free fluid excretion [31, 60, 66]. The normal excretory capacity of the kidneys can compensate for liquid intake up to 15-18l/day (considering a maximum urine diluting capacity of 50mmol/l and an excretion of 900mmol/24h), but this system may be altered by several factors [66]. Beside chronic and acute ingestion of excessive quantities of fluids, conditions impairing urine dilution capacity predispose hyponatraemia - primarily increased AVP release [1, 10, 11, 31, 65–71]. Risk factors for hyponatraemia are displayed in table 2.
Table 2 Factors predisposing to hyponatraemia.
| Acute fluid intake of high amount |
| Impaired water excretion |
|
Age |
|
Renal failure |
|
Low solute intake |
|
Malnutrition |
|
Anorexia nervosa |
|
Beer potomania |
|
Concomitant stimulus |
|
Medication (e.g., antidepressants, antipsychotics, diuretics) |
|
Acute infection (e.g., pneumonia, urogenital tract infection) or other acute diseases (e.g., stroke, myocardial infarction) |
|
Psychological stress (e.g., acute psychosis) |
About 20% of patients with schizophrenia and PP develop hyponatraemia [4, 9, 11]. As AVP is known to be a stress hormone, an acute psychotic episode increases the activity of AVP, leading to water retention and potentially hyponatraemia, especially if polydiptic behaviour persists [9, 60, 72]. Similarly, somatic stress (e.g., acute diseases, pain) stimulates AVP secretion [73]. Recent case reports have shown an association between acute infection and hyponatraemia. Particularly in urinary tract infections when both the infection per se and stress-induced AVP release reduce the renal excretory capacity, a combined with medical advice to increase fluid intake, patients in general and especially with PP are at risk of the development of hyponatraemia [74–77].
Several medications may promote hyponatraemia by stimulating AVP release or increasing the sensitivity of the kidneys to AVP: thiazide diuretics, antipsychotic drugs, antidepressant drugs, antiepileptic drugs, and lithium [68, 70, 71, 78–81]. Furthermore, the anticholinergic side effects of antidepressant drugs may result in an elevated sensation of thirst and hence lead to increased drinking.
In patients with beer potomania, patients with PP and malnutrition or anorexia nervosa, low solute intake plays a major role in the development of hyponatraemia [30]. The amount of solute intake defines the maximum dilution capacity of the kidneys as free fluid without solutes cannot be excreted. Thus, if solute intake decreases, the kidneys’ excretory capacity of water may decrease from around 15 l/d to 4 l/d, a threshold that is quickly passed in patients with chronic polydipsia and beer potomania [66].
Hyponatraemia may lead to several serious consequences. In the acute setting, if hyponatraemia treatment is delayed or inadequate, complications include brain oedema, seizures, falls and fractures as well as rhabdomyolysis and central pontine myelinolysis [11, 82–86]. In the long-term, hyponatraemia is associated with increased rehospitalisation rates, morbidity and mortality [12, 87].
Importantly, the risk of hyponatraemia seems to increase with duration of the underlying disease of PP [4]. It is speculated that excessive fluid intake over a long period may modulate drinking behaviour relative to actual water deficit and lead to a disturbance in the osmoregulation. Hence, over time, subjects declare thirst and keep drinking even in the presence of reduced serum osmolality [60, 88, 89]. Similarly, a downward resetting of the osmostat results in delayed or incomplete suppression of AVP and hence impairs water excretion.
Beside hyponatraemia, other complications of chronic excessive fluid consumption exist. Renal concentration capacity may diminish through a washout mechanism and downregulation of aquaporine-2 water channels, as shown in rodents [90]. Furthermore, malnutrition, gastrointestinal distress, bladder dilatation, hydronephrosis, renal failure, congestive heart failure, osteopenia and central nervous system dysfunction have been discussed [13, 16, 91, 92]. These data, however, mostly derive from case reports and retrospective studies and therefore provide low evidence.
Treating primary polydipsia and its complications
Treatment options for PP are scarce. Voluntary reduction of water intake would be the ideal therapy for PP, however, it often fails due to non-compliance of the polydiptic patient who suffers from thirst and compulsive drinking behaviour [88, 93–95]. Supportive measures to avoid hyponatraemia are the following: ingestion of a balanced diet, avoidance of drugs that may cause a dry mouth, hence increasing drinking, and frequent weighing to detect water retention. Studies have investigated behavioural therapy such as disease education, relaxation training using biofeedback, conditioning of desired behaviour, group therapy and others [15, 16, 92, 96] and have shown variable results. However, the feasibility of behavioural treatments, requiring substantial time and manpower, are limited in an outpatient setting [16].
Different medications have been suggested to improve polydiptic behaviour and prevent hyponatraemia. As PP has mainly been studied in acutely psychotic patients, it is not surprising that most drugs studied are antipsychotic drugs and mood stabilisers such as olanzapine, lithium, risperidone, aripiprazole and clozapine [15, 17, 19, 97–101]. The question however remains whether these drugs are treating the urge to drink, or if they are simply reducing acute psychosis and thus treat PP that might be a symptom of acute psychosis. Other medications that have been found to reduce polydiptic behaviour are phenytoin, bupropion, and propranolol [102–104]. All therapeutic options studied are considered low evidence, as these are descriptions of case reports, small case series or small case-control group studies. In conclusion, the wide spread of different medication used underlines the difficulty in treating this disorder and the need for better options.
Acute treatment of hyponatraemia in PP primarily consists of fluid restriction. In cases of profound and symptomatic hyponatraemia, a 3% saline infusion may be used. Overcorrection of hyponatraemia (increase of serum sodium >12 mmol/24h) has been described in patients with PP, fortunately without neurological complications [29, 84, 105]. Nevertheless, treating physicians should be aware of the risk of overcorrection and consequently pontine myelinolysis.
Conclusion
In conclusion, the pathophysiology of PP is complex and poorly understood. PP is associated with a wide spectrum of psychiatric comorbidities beyond schizophrenia. Moreover, habitual polydipsia appears to be increasing in prevalence in lifestyle conscious healthy people. Several factors impairing water excretion exist and may promote hyponatraemia in PP, a condition linked to substantial morbidity and mortality.
Fluid restriction is a successful measure to correct the complication of acute hyponatraemia, however, in the long run treatment options for this typically chronic condition are scarce. Studies elaborating novel therapeutic approaches would be desirable. But most importantly, educational measures in the general population might be needed to rationalise the prevalent advice to “drink enough”.
Acknowledgement
We thank Jennifer Küster and Dr Anke Scheel-Sailer for providing critical feedback and input, and Daniel Apap for English correction.
Author contributions
CS, BW and MCC wrote the manuscript and approved the final version of this manuscript. CS and BW are equally contributing first authors.
References:
1
Mercier-Guidez
E
,
Loas
G
. Polydipsia and water intoxication in 353 psychiatric inpatients: an epidemiological and psychopathological study. Eur Psychiatry. 2000;15(5):306–11 .https://doi.org/10.1016/S0924-9338(00)00399-0
2
Robertson
GL
. Differential diagnosis of polyuria. Annu Rev Med. 1988;39(1):425–42 .https://doi.org/10.1146/annurev.me.39.020188.002233
3
Robertson
GL
. Diabetes insipidus: Differential diagnosis and management. Best Pract Res Clin Endocrinol Metab. 2016;30(2):205–18 .https://doi.org/10.1016/j.beem.2016.02.007
4
de Leon
J
,
Verghese
C
,
Tracy
JI
,
Josiassen
RC
,
Simpson
GM
. Polydipsia and water intoxication in psychiatric patients: a review of the epidemiological literature. Biol Psychiatry. 1994;35(6):408–19 .https://doi.org/10.1016/0006-3223(94)90008-6
5
Pallavi
R
. An unsuspected cause of hyponatremia: beer potomania. J Am Geriatr Soc. 2015;63(8):1714–5 .https://doi.org/10.1111/jgs.13582
6
Harrow
AS
. Beer potomania syndrome in an alcoholic. Va Med. 1989;116(6):270–1.
7
McGraw
M
. Beer potomania: drink in this atypical cause of hyponatremia. Nursing. 2012;42(7):24–30, quiz 30–1 .https://doi.org/10.1097/01.NURSE.0000415301.20409.4e
8
Thaler
SM
,
Teitelbaum
I
,
Berl
T
. “Beer potomania” in non-beer drinkers: effect of low dietary solute intake. Am J Kidney Dis. 1998;31(6):1028–31 .https://doi.org/10.1053/ajkd.1998.v31.pm9631849
9
Goldman
MB
,
Robertson
GL
,
Luchins
DJ
,
Hedeker
D
,
Pandey
GN
. Psychotic exacerbations and enhanced vasopressin secretion in schizophrenic patients with hyponatremia and polydipsia. Arch Gen Psychiatry. 1997;54(5):443–9 .https://doi.org/10.1001/archpsyc.1997.01830170069010
10
Illowsky
BP
,
Kirch
DG
. Polydipsia and hyponatremia in psychiatric patients. Am J Psychiatry. 1988;145(6):675–83 .https://doi.org/10.1176/ajp.145.6.675
11
de Leon
J
,
Dadvand
M
,
Canuso
C
,
Odom-White
A
,
Stanilla
J
,
Simpson
GM
. Polydipsia and water intoxication in a long-term psychiatric hospital. Biol Psychiatry. 1996;40(1):28–34 .https://doi.org/10.1016/0006-3223(95)00353-3
12
Winzeler
B
,
Jeanloz
N
,
Nigro
N
,
Suter-Widmer
I
,
Schuetz
P
,
Arici
B
, et al.
Long-term outcome of profound hyponatremia: a prospective 12 months follow-up study. Eur J Endocrinol. 2016;175(6):499–507 .https://doi.org/10.1530/EJE-16-0500
13
Hawken
ER
,
Crookall
JM
,
Reddick
D
,
Millson
RC
,
Milev
R
,
Delva
N
. Mortality over a 20-year period in patients with primary polydipsia associated with schizophrenia: a retrospective study. Schizophr Res. 2009;107(2-3):128–33 .https://doi.org/10.1016/j.schres.2008.09.029
14
de Leon
J
. Polydipsia--a study in a long-term psychiatric unit. Eur Arch Psychiatry Clin Neurosci. 2003;253(1):37–9 .https://doi.org/10.1007/s00406-003-0403-z
15
Bowen
L
,
Glynn
SM
,
Marshall
BD, Jr
,
Kurth
CL
,
Hayden
JL
. Successful behavioral treatment of polydipsia in a schizophrenic patient. J Behav Ther Exp Psychiatry. 1990;21(1):53–61 .https://doi.org/10.1016/0005-7916(90)90049-Q
16
Thoma
JL
,
Howe
J
,
Gaudet
A
,
Brantley
PJ
. Behavioral treatment of chronic psychogenic polydipsia with hyponatremia: a unique case of polydipsia in a primary care patient with intractable hiccups. J Behav Ther Exp Psychiatry. 2001;32(4):241–50 .https://doi.org/10.1016/S0005-7916(02)00007-1
17
Verghese
C
,
de Leon
J
,
Josiassen
RC
. Problems and progress in the diagnosis and treatment of polydipsia and hyponatremia. Schizophr Bull. 1996;22(3):455–64 .https://doi.org/10.1093/schbul/22.3.455
18
Edoute
Y
,
Davids
MR
,
Johnston
C
,
Halperin
ML
. An integrative physiological approach to polyuria and hyponatraemia: a ‘double-take’ on the diagnosis and therapy in a patient with schizophrenia. QJM. 2003;96(7):531–40 .https://doi.org/10.1093/qjmed/hcg089
19
Brookes
G
,
Ahmed
AG
. Pharmacological treatments for psychosis-related polydipsia. Cochrane Database Syst Rev. 2006;(4):CD003544.
20
Fuller
MA
,
Jurjus
G
,
Kwon
K
,
Konicki
PE
,
Jaskiw
GE
. Clozapine reduces water-drinking behavior in schizophrenic patients with polydipsia. J Clin Psychopharmacol. 1996;16(4):329–32 .https://doi.org/10.1097/00004714-199608000-00010
21
Fliers
E
,
Korbonits
M
,
Romijn
J
. Disorders of water metabolism: diabetes insipidus and the syndrome of inappropriate antidiuretic hormone secretion. Clinical Neuroendocrinology. 2014;37.
22
Verghese
C
,
De Leon
J
,
Simpson
GM
. Neuroendocrine factors influencing polydipsia in psychiatric patients: an hypothesis. Neuropsychopharmacology. 1993;9(2):157–66 .https://doi.org/10.1038/npp.1993.54
23
Frank
E
,
Landgraf
R
. The vasopressin system--from antidiuresis to psychopathology. Eur J Pharmacol. 2008;583(2-3):226–42 .https://doi.org/10.1016/j.ejphar.2007.11.063
24
Fenske
W
,
Allolio
B
. Clinical review: Current state and future perspectives in the diagnosis of diabetes insipidus: a clinical review. J Clin Endocrinol Metab. 2012;97(10):3426–37 .https://doi.org/10.1210/jc.2012-1981
25
Knepper
MA
,
Kwon
T-H
,
Nielsen
S
. Molecular physiology of water balance. N Engl J Med. 2015;372(14):1349–58 .https://doi.org/10.1056/NEJMra1404726
26
Saker
P
,
Farrell
MJ
,
Adib
FR
,
Egan
GF
,
McKinley
MJ
,
Denton
DA
. Regional brain responses associated with drinking water during thirst and after its satiation. Proc Natl Acad Sci USA. 2014;111(14):5379–84 .https://doi.org/10.1073/pnas.1403382111
27
Saker
P
,
Farrell
MJ
,
Egan
GF
,
McKinley
MJ
,
Denton
DA
. Overdrinking, swallowing inhibition, and regional brain responses prior to swallowing. Proc Natl Acad Sci USA. 2016;113(43):12274–9 .https://doi.org/10.1073/pnas.1613929113
28
Timper
K
,
Fenske
W
,
Kühn
F
,
Frech
N
,
Arici
B
,
Rutishauser
J
, et al.
Diagnostic accuracy of copeptin in the differential diagnosis of the polyuria-polydipsia syndrome: a prospective multicenter study. J Clin Endocrinol Metab. 2015;100(6):2268–74 .https://doi.org/10.1210/jc.2014-4507
29
Sailer
CO
,
Winzeler
B
,
Nigro
N
,
Suter-Widmer
I
,
Arici
B
,
Bally
M
, et al.
Characteristics and outcomes of patients with profound hyponatraemia due to primary polydipsia. Clin Endocrinol (Oxf). 2017; [Epub ahead of print]. Available at: , .https://doi.org/10.1111/cen.13384
30
Musch
W
,
Xhaet
O
,
Decaux
G
. Solute loss plays a major role in polydipsia-related hyponatraemia of both water drinkers and beer drinkers. QJM. 2003;96(6):421–6 .https://doi.org/10.1093/qjmed/hcg078
31
Goldman
MB
. The mechanism of life-threatening water imbalance in schizophrenia and its relationship to the underlying psychiatric illness. Brain Res Brain Res Rev. 2009;61(2):210–20 .https://doi.org/10.1016/j.brainresrev.2009.06.004
32
Goldman
MB
. Brain circuit dysfunction in a distinct subset of chronic psychotic patients. Schizophr Res. 2014;157(1-3):204–13 .https://doi.org/10.1016/j.schres.2014.06.001
33
de Wied
D
,
Diamant
M
,
Fodor
M
. Central nervous system effects of the neurohypophyseal hormones and related peptides. Front Neuroendocrinol. 1993;14(4):251–302 .https://doi.org/10.1006/frne.1993.1009
34
Luchins
DJ
. A possible role of hippocampal dysfunction in schizophrenic symptomatology. Biol Psychiatry. 1990;28(2):87–91 .https://doi.org/10.1016/0006-3223(90)90625-C
35
Goldman
MB
,
Torres
IJ
,
Keedy
S
,
Marlow-O’Connor
M
,
Beenken
B
,
Pilla
R
. Reduced anterior hippocampal formation volume in hyponatremic schizophrenic patients. Hippocampus. 2007;17(7):554–62 .https://doi.org/10.1002/hipo.20292
36
Umbricht
D
. Polydipsia and hippocampal pathology. Biol Psychiatry. 1994;36(10):709–10 .https://doi.org/10.1016/0006-3223(94)91183-5
37
Luchins
DJ
,
Nettles
KW
,
Goldman
MB
. Anterior medial temporal lobe volumes in polydipsic schizophrenic patients with and without hypo-osmolemia: a pilot study. Biol Psychiatry. 1997;42(9):767–70 .https://doi.org/10.1016/S0006-3223(96)00491-X
38
Badiani
A
,
Vaccaro
R
,
Burdino
R
,
Casini
A
,
Valeri
P
,
Renda
TG
, et al.
Dissociation in the effects of the D2/D3 dopaminergic agonist quinpirole on drinking and on vasopressin levels in the rat. Neurosci Lett. 2002;325(2):79–82 .https://doi.org/10.1016/S0304-3940(02)00261-6
39
Amato
D
,
Milella
MS
,
Badiani
A
,
Nencini
P
. Compulsive-like effects of repeated administration of quinpirole on drinking behavior in rats. Behav Brain Res. 2006;172(1):1–13 .https://doi.org/10.1016/j.bbr.2006.03.038
40
Amato
D
,
Müller
CP
,
Badiani
A
. Increased drinking after intra-striatal injection of the dopamine D2/D3 receptor agonist quinpirole in the rat. Psychopharmacology (Berl). 2012;223(4):457–63 .https://doi.org/10.1007/s00213-012-2735-8
41
Zabik
JE
,
Sprague
JE
,
Odio
M
. Interactive dopaminergic and noradrenergic systems in the regulation of thirst in the rat. Physiol Behav. 1993;54(1):29–33 .https://doi.org/10.1016/0031-9384(93)90039-I
42
Bahia
A
,
Chu
ES
,
Mehler
PS
. Polydipsia and hyponatremia in a woman with anorexia nervosa. Int J Eat Disord. 2011;44(2):186–8.
43
Caregaro
L
,
Di Pascoli
L
,
Favaro
A
,
Nardi
M
,
Santonastaso
P
. Sodium depletion and hemoconcentration: overlooked complications in patients with anorexia nervosa?
Nutrition. 2005;21(4):438–45 .https://doi.org/10.1016/j.nut.2004.08.022
44
Almond
CS
,
Shin
AY
,
Fortescue
EB
,
Mannix
RC
,
Wypij
D
,
Binstadt
BA
, et al.
Hyponatremia among runners in the Boston Marathon. N Engl J Med. 2005;352(15):1550–6 .https://doi.org/10.1056/NEJMoa043901
45
Hew
TD
,
Chorley
JN
,
Cianca
JC
,
Divine
JG
. The incidence, risk factors, and clinical manifestations of hyponatremia in marathon runners. Clin J Sport Med. 2003;13(1):41–7 .https://doi.org/10.1097/00042752-200301000-00008
46
Ayus
JC
,
Varon
J
,
Arieff
AI
. Hyponatremia, cerebral edema, and noncardiogenic pulmonary edema in marathon runners. Ann Intern Med. 2000;132(9):711–4 .https://doi.org/10.7326/0003-4819-132-9-200005020-00005
47
Siegel
AJ
,
Verbalis
JG
,
Clement
S
,
Mendelson
JH
,
Mello
NK
,
Adner
M
, et al.
Hyponatremia in marathon runners due to inappropriate arginine vasopressin secretion. Am J Med. 2007;120(5):461.e11–7 .https://doi.org/10.1016/j.amjmed.2006.10.027
48
Davis
DP
,
Videen
JS
,
Marino
A
,
Vilke
GM
,
Dunford
JV
,
Van Camp
SP
, et al.
Exercise-associated hyponatremia in marathon runners: a two-year experience. J Emerg Med. 2001;21(1):47–57 .https://doi.org/10.1016/S0736-4679(01)00320-1
49
Bell
NH
. Endocrine complications of sarcoidosis. Endocrinol Metab Clin North Am. 1991;20(3):645–54.
50
Stuart
CA
,
Neelon
FA
,
Lebovitz
HE
. Disordered control of thirst in hypothalamic-pituitary sarcoidosis. N Engl J Med. 1980;303(19):1078–82 .https://doi.org/10.1056/NEJM198011063031902
51
Williamson
JE
,
Maddock
S
,
Castillo
V
,
Carbajal
R
. Hyponatremia as a result of posttraumatic primary polydipsia. Am J Med. 2015;128(6):e3–4 .https://doi.org/10.1016/j.amjmed.2014.12.013
52
Zafonte
RD
,
Watanabe
TK
,
Mann
NR
,
Ko
DH
. Psychogenic polydipsia after traumatic brain injury. A case report. Am J Phys Med Rehabil. 1997;76(3):246–8 .https://doi.org/10.1097/00002060-199705000-00018
53
Christ-Crain
M
,
Fenske
W
. Copeptin in the diagnosis of vasopressin-dependent disorders of fluid homeostasis. Nat Rev Endocrinol. 2016;12(3):168–76 .https://doi.org/10.1038/nrendo.2015.224
54
Dashe
AM
,
Cramm
RE
,
Crist
CA
,
Habener
JF
,
Solomon
DH
. A water deprivation test for the differential diagnosis of polyuria. JAMA. 1963;185(9):699–703 .https://doi.org/10.1001/jama.1963.03060090031011
55
Robertson
GL
. Diabetes insipidus. Endocrinol Metab Clin North Am. 1995;24(3):549–72.
56
Verbalis
JG
. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17(4):471–503 .https://doi.org/10.1016/S1521-690X(03)00049-6
57
Miller
M
,
Dalakos
T
,
Moses
AM
,
Fellerman
H
,
Streeten
DH
. Recognition of partial defects in antidiuretic hormone secretion. Ann Intern Med. 1970;73(5):721–9 .https://doi.org/10.7326/0003-4819-73-5-721
58
Thomas
WC, Jr
. Teaching Clinic—Review: DIABETES INSIPIDUS. J Clin Endocrinol Metab. 1957;17(4):565–82 .https://doi.org/10.1210/jcem-17-4-565
59
Zerbe
RL
,
Robertson
GL
. A comparison of plasma vasopressin measurements with a standard indirect test in the differential diagnosis of polyuria. N Engl J Med. 1981;305(26):1539–46 .https://doi.org/10.1056/NEJM198112243052601
60
Goldman
MB
,
Luchins
DJ
,
Robertson
GL
. Mechanisms of altered water metabolism in psychotic patients with polydipsia and hyponatremia. N Engl J Med. 1988;318(7):397–403 .https://doi.org/10.1056/NEJM198802183180702
61
Morgenthaler
NG
,
Struck
J
,
Alonso
C
,
Bergmann
A
. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112–9 .https://doi.org/10.1373/clinchem.2005.060038
62
Fenske
WK
,
Christ-Crain
M
,
Hörning
A
,
Simet
J
,
Szinnai
G
,
Fassnacht
M
, et al.
A copeptin-based classification of the osmoregulatory defects in the syndrome of inappropriate antidiuresis. J Am Soc Nephrol. 2014;25(10):2376–83 .https://doi.org/10.1681/ASN.2013080895
63
Balanescu
S
,
Kopp
P
,
Gaskill
MB
,
Morgenthaler
NG
,
Schindler
C
,
Rutishauser
J
. Correlation of plasma copeptin and vasopressin concentrations in hypo-, iso-, and hyperosmolar States. J Clin Endocrinol Metab. 2011;96(4):1046–52 .https://doi.org/10.1210/jc.2010-2499
64
Fenske
W
,
Quinkler
M
,
Lorenz
D
,
Zopf
K
,
Haagen
U
,
Papassotiriou
J
, et al.
Copeptin in the differential diagnosis of the polydipsia-polyuria syndrome--revisiting the direct and indirect water deprivation tests. J Clin Endocrinol Metab. 2011;96(5):1506–15 .https://doi.org/10.1210/jc.2010-2345
65
Siegler
EL
,
Tamres
D
,
Berlin
JA
,
Allen-Taylor
L
,
Strom
BL
. Risk factors for the development of hyponatremia in psychiatric inpatients. Arch Intern Med. 1995;155(9):953–7 .https://doi.org/10.1001/archinte.1995.00430090099011
66
Berl
T
. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol. 2008;19(6):1076–8 .https://doi.org/10.1681/ASN.2007091042
67
Adrogué
HJ
,
Madias
NE
. Hyponatremia. N Engl J Med. 2000;342(21):1581–9 .https://doi.org/10.1056/NEJM200005253422107
68
Kuz
GM
,
Manssourian
A
. Carbamazepine-induced hyponatremia: assessment of risk factors. Ann Pharmacother. 2005;39(11):1943–6 .https://doi.org/10.1345/aph.1G209
69
Kelly
BD
,
Hillery
J
. Hyponatremia during carbamazepine therapy in patients with intellectual disability. J Intellect Disabil Res. 2001;45(Pt 2):152–6 .https://doi.org/10.1046/j.1365-2788.2001.00338.x
70
Kennedy
RM
,
Earley
LE
. Profound hyponatremia resulting from a thiazide-induced decrease in urinary diluting capacity in a patient with primary polydipsia. N Engl J Med. 1970;282(21):1185–6 .https://doi.org/10.1056/NEJM197005212822107
71
Gandhi
S
,
Shariff
SZ
,
Al-Jaishi
A
,
Reiss
JP
,
Mamdani
MM
,
Hackam
DG
, et al.
Second-Generation Antidepressants and Hyponatremia Risk: A Population-Based Cohort Study of Older Adults. Am J Kidney Dis. 2017;69(1):87–96 .https://doi.org/10.1053/j.ajkd.2016.08.020
72
Katan
M
,
Morgenthaler
N
,
Widmer
I
,
Puder
JJ
,
König
C
,
Müller
B
, et al.
Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuroendocrinol Lett. 2008;29(3):341–6.
73
Katan
M
,
Christ-Crain
M
. The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly. 2010;140:w13101.
74
Lee
LC
,
Noronha
M
. When plenty is too much: water intoxication in a patient with a simple urinary tract infection. BMJ Case Rep. 2016;2016:bcr2016216882 .https://doi.org/10.1136/bcr-2016-216882
75
Olapade-Olaopa
EO
,
Morley
RN
,
Ahiaku
EK
,
Bramble
FJ
. Iatrogenic polydipsia: a rare cause of water intoxication in urology. Br J Urol. 1997;79(3):488 .https://doi.org/10.1046/j.1464-410X.1997.11538.x
76
Berry
EM
,
Halon
D
,
Fainaru
M
. Iatrogenic polydipsia. Lancet. 1977;2(8044):937–8 .https://doi.org/10.1016/S0140-6736(77)90880-7
77
Gross
R
. “Iatrogenic polydipsia”. J Gen Intern Med. 1994;9(12):713–4 .https://doi.org/10.1007/BF02599022
78
Correll
CU
,
Detraux
J
,
De Lepeleire
J
,
De Hert
M
. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015;14(2):119–36 .https://doi.org/10.1002/wps.20204
79
Goldman
MB
. Moderate hyponatremia and death in a polydipsic schizophrenic on lithium. Biol Psychiatry. 1994;36(7):485–6 .https://doi.org/10.1016/0006-3223(94)90646-7
80
Gandhi
S
,
McArthur
E
,
Mamdani
MM
,
Hackam
DG
,
McLachlan
RS
,
Weir
MA
, et al.
Antiepileptic drugs and hyponatremia in older adults: Two population-based cohort studies. Epilepsia. 2016;57(12):2067–79 .https://doi.org/10.1111/epi.13593
81
Gandhi
S
,
McArthur
E
,
Reiss
JP
,
Mamdani
MM
,
Hackam
DG
,
Weir
MA
, et al.
Atypical antipsychotic medications and hyponatremia in older adults: a population-based cohort study. Can J Kidney Health Dis. 2016;3(1):21.
82
Aguiar
D
. Recurrent rhabdomyolysis secondary to hyponatraemia: case report. Reactions.
2017;1636:105–28.
83
Akkaya
C
,
Sarandol
A
,
Sivrioglu
EY
,
Kotan
Z
,
Kirli
S
. A patient using ziprasidone with polydipsia, seizure, hyponatremia and rhabdomyolysis. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(8):1535–8 .https://doi.org/10.1016/j.pnpbp.2006.05.020
84
Ulstrup
A
,
Ugleholdt
R
,
Rasmussen
JV
. Fulminant crural compartment syndrome preceded by psychogenic polydipsia. BMJ Case Rep. 2015;2015(may14 1):bcr2014208603 .https://doi.org/10.1136/bcr-2014-208603
85
Secombe
P
,
Milne
C
. Hyponatraemia-induced rhabdomyolysis complicated by anuric acute kidney injury: a renal replacement conundrum. BMJ Case Rep. 2016;2016:bcr2016218198 .https://doi.org/10.1136/bcr-2016-218198
86
Bennett
M
,
Fitzpatrick
G
,
Donnelly
M
. Rhabdomyolysis associated with polydipsia induced hyponatraemia. BMJ Case Rep. 2011;2011(oct03 1):bcr0820114659 .https://doi.org/10.1136/bcr.08.2011.4659
87
Verbalis
JG
,
Goldsmith
SR
,
Greenberg
A
,
Korzelius
C
,
Schrier
RW
,
Sterns
RH
, et al.
Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10, Suppl 1):S1–42 .https://doi.org/10.1016/j.amjmed.2013.07.006
88
Vieweg
WV
,
Rowe
WT
,
David
JJ
,
Sutker
LH
,
Spradlin
WW
. Evaluation of patients with self-induced water intoxication and schizophrenic disorders (SIWIS). J Nerv Ment Dis. 1984;172(9):552–5 .https://doi.org/10.1097/00005053-198409000-00008
89
Delva
NJ
,
Chang
A
,
Hawken
ER
,
Lawson
JS
,
Owen
JA
. Effects of clonidine in schizophrenic patients with primary polydipsia: three single case studies. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(2):387–92 .https://doi.org/10.1016/S0278-5846(01)00246-9
90
Cadnapaphornchai
MA
,
Rogachev
B
,
Summer
SN
,
Chen
Y-C
,
Gera
L
,
Stewart
JM
, et al.
Evidence for bradykinin as a stimulator of thirst. Am J Physiol Renal Physiol. 2004;286(5):F875–80 .https://doi.org/10.1152/ajprenal.00243.2003
91
Blum
A
,
Friedland
GW
. Urinary tract abnormalities due to chronic psychogenic polydipsia. Am J Psychiatry. 1983;140(7):915–6 .https://doi.org/10.1176/ajp.140.7.915
92
Pavalonis
D
,
Shutty
M
,
Hundley
P
,
Leadbetter
R
,
Vieweg
V
,
Downs
M
. Behavioral intervention to reduce water intake in the syndrome of psychosis, intermittent hyponatremia, and polydipsia. J Behav Ther Exp Psychiatry. 1992;23(1):51–7 .https://doi.org/10.1016/0005-7916(92)90025-E
93
Bremner
AJ
,
Regan
A
. Intoxicated by water. Polydipsia and water intoxication in a mental handicap hospital. Br J Psychiatry. 1991;158(2):244–50 .https://doi.org/10.1192/bjp.158.2.244
94
Perkins
RM
,
Yuan
CM
,
Welch
PG
. Dipsogenic diabetes insipidus: report of a novel treatment strategy and literature review. Clin Exp Nephrol. 2006;10(1):63–7 .https://doi.org/10.1007/s10157-005-0397-0
95
Vieweg
WVR
. Treatment strategies in the polydipsia-hyponatremia syndrome. J Clin Psychiatry. 1994;55(4):154–60.
96
McNally
RJ
,
Calamari
JE
,
Hansen
PM
,
Kaliher
C
. Behavioral treatment of psychogenic polydipsia. J Behav Ther Exp Psychiatry. 1988;19(1):57–61 .https://doi.org/10.1016/0005-7916(88)90011-0
97
Alexander
RC
,
Karp
BI
,
Thompson
S
,
Khot
V
,
Kirch
DG
. A double blind, placebo-controlled trial of demeclocycline treatment of polydipsia-hyponatremia in chronically psychotic patients. Biol Psychiatry. 1991;30(4):417–20 .https://doi.org/10.1016/0006-3223(91)90300-B
98
Spears
NM
,
Leadbetter
RA
,
Shutty
MS, Jr
. Clozapine treatment in polydipsia and intermittent hyponatremia. J Clin Psychiatry. 1996;57(3):123–8.
99
de Leon
J
,
Verghese
C
,
Stanilla
JK
,
Lawrence
T
,
Simpson
GM
. Treatment of polydipsia and hyponatremia in psychiatric patients. Can clozapine be a new option?
Neuropsychopharmacology. 1995;12(2):133–8 .https://doi.org/10.1016/0893-133X(94)00069-C
100
Nishikawa
T
,
Tsuda
A
,
Tanaka
M
,
Nishikawa
M
,
Koga
I
,
Uchida
Y
. Involvement of the endogenous opioid system in the drinking behavior of schizophrenic patients displaying self-induced water intoxication: a double-blind controlled study with naloxone. Clin Neuropharmacol. 1996;19(3):252–8 .https://doi.org/10.1097/00002826-199619030-00007
101
Lee
HS
,
Kwon
KY
,
Alphs
LD
,
Meltzer
HY
. Effect of clozapine on psychogenic polydipsia in chronic schizophrenia. J Clin Psychopharmacol. 1991;11(3):222–3 .https://doi.org/10.1097/00004714-199106000-00022
102
Arora
G
,
Singh
M
,
Mudassar
T
. Psychogenic polydipsia and bupropion. J Neuropsychiatry Clin Neurosci. 2012;24(4):E3–4 .https://doi.org/10.1176/appi.neuropsych.11090213
103
Kishi
Y
,
Kurosawa
H
,
Endo
S
. Is propranolol effective in primary polydipsia?
Int J Psychiatry Med. 1998;28(3):315–25 .https://doi.org/10.2190/QPWL-14H7-HPGG-A29D
104
Bersani
G
,
Pesaresi
L
,
Orlandi
V
,
Gherardelli
S
,
Pancheri
P
. Atypical antipsychotics and polydipsia: a cause or a treatment?
Hum Psychopharmacol. 2007;22(2):103–7 .https://doi.org/10.1002/hup.825
105
Primavera
A
,
Fonti
A
,
Giberti
L
,
Cocito
L
. Recurrent absence status epilepticus and hyponatremia in a patient with polydipsia. Biol Psychiatry. 1995;38(3):189–91 .https://doi.org/10.1016/0006-3223(95)00145-7