Prevention and therapy of JC polyomavirus-mediated progressive multifocal leukoencephalopathy – a realistic possibility?
DOI: https://doi.org/10.4414/smw.2017.14520
Ivan
Jelcica, Benoit
Combaluzierb, Ilijas
Jelcica, Mireia
Sospedraa, Jan
Grimmb, Roland
Martina
aNeuroimmunology and Multiple Sclerosis Research Section, Department of Neurology, University of Zurich, University Hospital Zurich, Switzerland
bNeurimmune Holding AG, Schlieren, Switzerland
Summary
Progressive multifocal leukoencephalopathy (PML) is a serious opportunistic infection of the brain caused by the JC polyomavirus (JCPyV). PML occurs when immune control of persistent infection with JCPyV fails, the virus mutates and changes its cellular tropism, enters the brain and infects astrocytes, oligodendrocytes and, in particular cases, also neurones. Currently, there is no therapy for this often fatal disease. A number of approaches have failed, and only the restoration of protective immunity, if possible, can lead to clearance of the virus once PML has occurred. During the last two decades, investigators have attempted to understand the factors contributing to the development of PML, which immune mechanisms are involved in immune surveillance, and which in clearing JCPyV from the brain once PML has occurred. Recent data suggest that both CD4+ and CD8+ T cells of the cellular immune system, and also JCPyV-specific antibodies, are involved in protection against PML and in resolving the opportunistic infection. Based on the current immunological data, prophylactic and therapeutic vaccination strategies have been proposed, and first treatment attempts in PML patients have provided promising results that indicate therapeutic vaccination may be feasible.
Introduction
The clinical disease entity progressive multifocal leukoencephalopathy (PML) was first described in 1959 [1], and in 1971 the polyomavirus JC (JCPyV) was identified as the pathogen that causes it [2]. Characteristics of PML include an underlying hereditary or acquired compromise of immune function, particularly following treatment with immunomodulatory/immunosuppressive agents or human immunodeficiency virus (HIV) infection [3, 4]. A wide range of conditions have been described as potential reasons for the immunocompromise that may lead to PML. These include: immunodeficiencies such as CD4+ lymphopenia and hyper-immunoglobulin E (hyper-IgE) syndrome; broad-spectrum immunosuppressive treatment; highly specific immunomodulatory drugs such as anti-CD20 (rituximab) and anti-VLA4 (natalizumab) specific monoclonal antibodies; infections (HIV); organ transplantation; and autoimmune/inflammatory diseases such as sarcoidosis, rheumatoid arthritis or systemic lupus erythematosus. The breadth of these conditions indicates that immune control of JCPyV probably involves several aspects of adaptive immunity. Infection with JCPyV is widespread, and approximately 60 to 70% of the world's population is infected. In the immunologically healthy individual, the infection remains persistent throughout life in the kidney and urinary tract [5]. Whether JCPyV can also establish persistent infection in other organs and tissues, including the brain, is currently not clear.
Compromised immune control may lead to mutations of the archetypic or wild type (wt) strain of JCPyV to types with rearranged regulatory regions and mutations in the major capsid protein JCPyV VP1. Both are probably relevant for the switch of the cell tropism to glial cells and neurones [6–9]. In the immunocompromised host, PML causes a lytic infection of oligodendrocytes and morphological changes of astrocytes in the absence of an inflammatory response. Once immune function is restored, e.g., following antiretroviral therapy in the case of HIV, adaptive immune mechanisms lead to an inflammation in the area of the PML lesion, which is referred to as immune reconstitution inflammatory syndrome (IRIS) [3, 4, 10–12]. Although the immune mechanisms underlying IRIS mediate the elimination of JCPyV from the brain, the resulting inflammation can cause additional brain damage and may lead to the death of the patient [11, 12]. Nevertheless, restoration of immunity is, to date, the only way to eliminate the virus from the brain and to thereby halt PML. Several potential alternative treatments of PML that looked promising, e.g. with respect to preventing viral entry into glial cells by receptor-blocking drugs, failed [13]. Both cellular and humoral immune components were found to be important players during IRIS in PML lesions [14]. These findings provided the rationale for vaccination approaches against PML [15]. In parallel to a decline of PML frequency in HIV-infected individuals, there has been an increase associated with therapy with certain biologics, particularly with anti-CD20 treatment in haematopoietic malignancies and autoimmune diseases, and anti-VLA4 treatment in multiple sclerosis (MS) [4, 16].
In this review, we will summarise the types of immunodeficiencies leading to PML and give our outlook on future strategies towards the development of prophylactic and therapeutic approaches for PML.
Development of PML
JCPyV is a small ubiquitous DNA virus with six open reading frames under the control of a regulatory non-coding region [2]. The major capsid protein VP1 can self-assemble into virus-like particles (VLPs) consisting of 72 pentamers of VP1 [17]. The high anti-JCPyV antibody seroprevalence of up to 60% in healthy individuals indicates widespread infection with JCPyV [5]. In the healthy host, JCPyV persists in urothelial cells and is shed into the urine in 50% of individuals. The infection remains clinically invisible throughout life [4]. JCPyV can also establish latency in haematopoietic progenitor cells, which may mobilise under conditions of immunocompromise or immunomodulation from the bone marrow niche into the peripheral circulation [18, 19]. How JCPyV finally enters the central nervous system (CNS) is currently not entirely clear. B cells have been suggested as viral carriers into the CNS [18–20], but the fact that empty VLPs can enter the CNS compartment indicates that free virus may be able to cross the blood brain barrier [21]. JCPyV DNA fragments and proteins have been detected repeatedly in 13 to 50% of non-PML brain biopsies [22–24], which led to speculation that JCPyV may reach the brain and may persist at this site in a latent state without causing PML. In order to avoid potential contamination of brain biopsies with B cells from the peripheral circulation, some research groups used laser capture microdissection and detected fragments of JCPyV DNA in oligodendrocytes, astrocytes and cerebellar granular cell neurones of non-PML patients [25–27]. Because of the paucity of data and lack of definitive proof, the hypothesis that reactivation of latent JCPyV infection of the brain may lead to PML has been intensely debated since.
In PML, JCPyV variants enter the CNS and infect glial cells. This leads to lysis of oligodendrocytes and swelling of astrocytes in PML and of neurones in granule cell neuronopathy. In the vast majority of cases, glio-/neurotropic JCPyV strains isolated from PML patients display both rearrangements in the regulatory noncoding control region and mutations in specific areas of VP1 [7, 8, 28]. Interestingly, VP1 mutations were mainly identified in the three exterior loops of VP1 (fig. 1). In addition to classical PML, which presents with large multifocal, partly confluent white matter lesions, JCPyV infection may in some cases manifest in other regions of the CNS and may thereby cause distinct JCPyV-associated diseases, including GCN, JCPyV encephalopathy and possibly also JCPyV meningitis [6]. In contrast to PML, GCN is characterised by lytic infection of cerebellar granule cell neurones with JCPyV, which results in cerebellar atrophy [29]. Although reports of GCN cases are less frequent, a histopathological survey reported that JCPyV-infected neurones may also be found in classical PML [30]. In GCN, the isolated infection of cerebellar neurones has been linked to mutations in the C-terminal region of VP1, which are probably involved in the altered tropism for neurones [9]. The glycan receptor LSTc (sialylneolacto-N-tetraose c) was identified as functional host receptor of a prototypical JCPyV strain (MAD1) [31]; however, this binding is abrogated in PML-associated JCPyV variants [32]. Therefore, the cellular entry receptor of JCPyV in glial cells and neurones remains unknown.
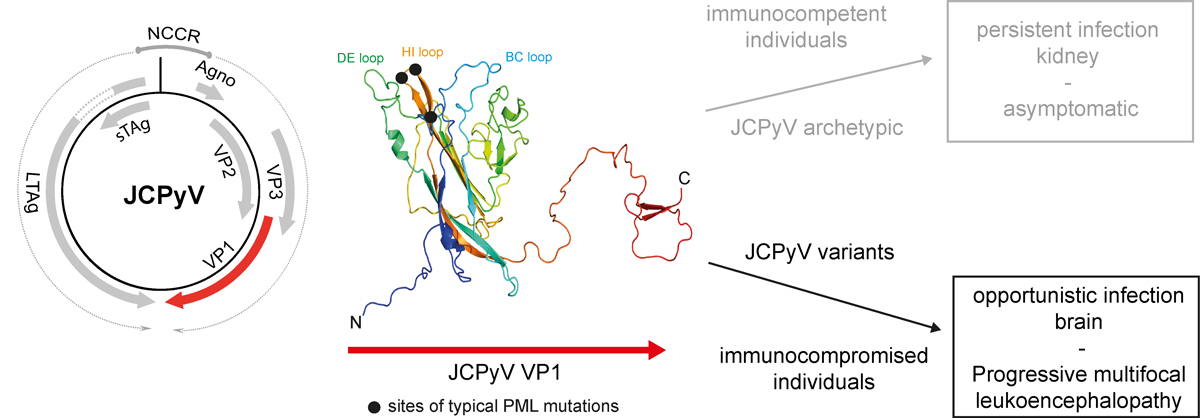
Figure 1
JCPyV and the development of PML. JCPyV encodes for six open reading frames under the control of the regulatory noncoding control region (NCCR) element. Mutations in the three exterior loops of the major capsid protein JCPyV VP1 may in situations of immunocompromise lead to an opportunistic infection in the brain, known as PML.
Protection from viral infections and elimination of virus-infected cells during acute infection involve cellular immunity, which is mediated by virus-specific CD4+ helper T cells and CD8+ cytotoxic T cells, as well as virus-specific antibodies from B cells. Their relative importance depends on the respective virus, on its tissue and/or cell tropism, on the structure of its envelope, on the composition of virus-encoded immunogenic epitopes and peptides, on the type of release from the infected cell (i.e. budding from the cell membrane or lytic infection), and other factors. Several lines of evidence suggest an important role of the adaptive immune system to protect the infected host during an asymptomatic persistent infection with archetypic JCPyV, as well as in clinically manifest PML, with infection of the brain with JCPyVPML variants. These pieces of evidence include PML occurrence in states of immunodeficiency of one or several arms of the adaptive immunity, such as hereditary antibody and/or cellular or acquired cellular immunodeficiencies, including HIV infection, or immunodeficiencies induced by chemotherapy, immunomodulatory or -suppressive therapies, allotransplantation or certain autoimmune diseases [14] (table 1). As patients with haematological malignancies are frequently treated with chemotherapy and immunosuppressive therapies, it remains difficult to discern if the primary disorder, the secondary immunosuppression, or both are critical for PML development.
Table 1 Hereditary and acquired diseases and immunomodulatory/- treatments associated with a risk of PML or other JCPyV-induced CNS infectious diseases.
|
Category of disease
|
Diseases
|
Hereditary immune deficiencies
[33] |
– Adenosine deaminase deficiency
– CD40 ligand deficiency
– Combined immune deficiency
– Common variable immune deficiency
– DOCK8 (dedicator of cytokinesis 8 protein) deficiency
– Gamma heavy chain disease
– Hyper-IgM syndrome
– Immunodeficiency-centromeric instability-facial dysmorphism syndrome syndrome
– Purine nucleoside phosphorylase deficiency
– Severe combined immune deficiency
– Signal transducer and activator of transcription 1 gain-of-function immune deficiency
– Wiskott-Aldrich syndrome
– X-linked agammaglobulinaemia
– Idiopathic CD4+ lymphopenia*
|
Acquired immune deficiencies
[18, 34] |
– Human immunodefiency infection or acquired immune deficiency syndrome
– Haematopietic stem cell transplantation
– Immunosuppressive therapy in organ transplant recipients
– Haematological malignancies (e.g. lymphomas and leukaemias)
– Immunosuppression during chemotherapy of solid cancers
– Systemic lupus erythematosus
– Sarcoidosis
– Immunosuppressive or -modulatory therapy in autoimmune diseases (rheumatoid arthritis, psoriatic arthritis, psoriasis, juvenile idiopathic arthritis, inflammatory bowel disease, ankylosing spondylitis, multiple sclerosis) |
Immunomodulatory treatments
[4, 18, 35, 36] |
– Natalizumab
– Efalizumab
– Belimumab
– Rituximab
– Fingolimod
– Dimethylfumarate
– Alemtuzumab
– Tumour necrosis factor-alpha inhibitors (infliximab, adalimumab, etanercept)
– Ofatumumab
– Mycophenolate mofetil
– Betalacept
– Brentuximab
– Fludarabine
– Ruxolitinib
– Leflunomide |
Types of immunodeficiencies leading to PML
After its first description in 1959 [1], PML was considered a rare disease that was mainly diagnosed in immunodeficient patients in the era before the emergence of acquired immunodeficiency syndrome (AIDS) [38]. The number of PML cases drastically increased with the AIDS pandemic, and PML became one of the main opportunistic infections in HIV+ patients and a major cause of death [39] (table 1). With the introduction of combined highly active antiretroviral therapy (HAART), the incidence of PML decreased, but remains a severe and life-threatening complication for AIDS patients [40–42].
The use of immunomodulatory therapies for various autoimmune diseases has led to an alarming increase of PML cases among patients with conditions that were previously not linked to any substantial PML risk. Immunotherapy-associated PML received broad attention with natalizumab in patients with MS or Crohn’s disease [16, 43–45]. Natalizumab had been approved for the treatment of relapsing-remitting MS (RRMS) in 2004, since it effectively reduced CNS inflammation via its inhibition of lymphocyte migration through the blood-brain barrier [46, 47]. It was briefly taken off the market after report of the first PML cases and then reintroduced with a strict safety and risk monitoring programme. Natalizumab is the immunotherapy with the highest number (698 as of December 2016) and highest rate (4.18 per 1000 cases treated) of confirmed PML cases [48]. Efalizumab is a humanised monoclonal antibody that was developed for the treatment of psoriasis by targeting CD11a in order to inhibit lymphocyte activation and migration [49]. It was voluntarily withdrawn from the market by the manufacturer after reports of four efalizumab-associated PML cases [4, 50, 51]. The B cell depleting monoclonal antibody rituximab is widely used to treat haematological cancers and autoimmune diseases, and to avoid graft rejection [52, 53]; although it was approved as long ago as 1997, rituximab has been associated with numerous PML cases only in the last 10 years [54]. The direct role of rituximab in PML development is still debated, since most of the patients suffered from immunosuppressed states either because of their primary haematological disorder or because of additional immunosuppressants, which made them more vulnerable to developing PML [54, 55]. Alemtuzumab, a monoclonal antibody against CD52 used as second-line therapy in MS to deplete B and T cells, was clearly linked to PML in some other diseases, such as leukaemia. So far, no PML cases have been observed in alemtuzumab-treated MS patients [56–58], and the same holds true for rituximab-treated MS patients, in whom there is so far no reported PML case. Further PML cases were reported with belimumab, a monoclonal antibody targeting the B-cell activating and survival factor BAFF, used for the treatment of systemic lupus erythematosus (SLE) [59]. Furthermore, with several tumour necrosis-alpha (TNFα) inhibitors such as adalimumab, infliximab and etanercept, which are currently used to treat psoriasis, rheumatoid arthritis and other autoimmune diseases, PML was observed in predisposed patients [60, 61].
For other immunomodulatory drugs such as fingolimod and dimethyl fumarate used for the treatment of MS or other autoimmune diseases, only a few PML cases have been reported [62]. In the fingolimod-treated MS patients, it is difficult to clearly link this drug with PML since most of these patients had been treated with natalizumab prior to fingolimod therapy [63, 64]. The first cases of PML under dimethyl fumarate were reported in psoriasis patients, but there are now publications of PML also in the context of MS [65–68]. Multiple PML cases occurred under immunosuppressive treatments to avoid allograft rejection or to treat cancer or autoimmune diseases; however, it is difficult to discern whether the underlying conditions of immunocompromise or the treatment-induced immunosuppression are the main cause for PML [69].
Hereditary immunodeficiencies may exclusively or preferentially compromise one specific immune cell type, e.g., in idiopathic CD4+ lymphopenia (table 1). Similarly, treatments that have been linked to PML may affect specific immune cells more profoundly than others. As an example, natalizumab blocks the entry of CD4+ T cells into the CNS much more efficiently than that of CD8+ T cells [70]. In the case of HIV infection, the virus specifically targets the CD4 receptor and leads to quantitative and qualitative alterations of CD4+ T cells, but each of these conditions also indirectly affects the function of CD8+ T cells and antibody responses. The occurrence of IRIS, such as in AIDS patients starting antiretroviral therapy or in natalizumab-treated MS patients after washout of natalizumab by plasmapheresis, indicates restoration of immune functions that leads to an inflammatory response within the area of the PML lesion [11, 12]. In depth studies of the cellular components of this inflammatory response revealed a central role of CD4+ T cells in orchestrating an efficient CD8+ effector T cell response in order to clear the virus [71–73].
Risk stratification and therapeutic approaches for PML
Natalizumab has been reapproved with the stipulation that factors contributing to PML risk must be identified. Studies of potential risk factors for PML identified prior immunosuppression, duration of natalizumab treatment and presence of anti-JCV antibodies as predisposing factors and, based on these, a risk classification scheme was proposed [74, 75]. The latter stratification involved monitoring of serum and plasma JCPyV VP1-specific antibodies during natalizumab treatment [75]. Interestingly, when the JCPyV antibody indices of natalizumab-treated MS patients were correlated with the incidence rate of PML in the different strata, patients with a higher antibody index had a substantially increased risk for developing PML. The risk stratification protocol ensured a reduction of PML incidence, but also limited the use of this very efficient drug in MS. Moreover, recent data showed that intrathecal production of JCPyV VP1-specific antibodies was enhanced in patients at time of PML [76]. As another potential risk marker, Schwab et al. showed that patients had low percentages of CD62L+ T cells before they developed PML [77, 78]. However, these findings are still debated, since an independent study did not confirm this observation [79]. Furthermore, MS patients with cerebrospinal fluid (CSF) lipid-specific IgM oligoclonal bands have a lower risk of developing natalizumab-associated PML than patients without such bands [80]. It is currently not clear how the above findings translate into increased risk with respect to cellular or humoral immune compromise.
Reconstituting the protective immunity or reversing the immunosuppression is so far the best way to eliminate JCPyV infection from the CNS and to overcome PML. Immune reconstitution in HIV+ patients can be achieved with HAART, which in turn will lead to the recovery of CD4+ lymphocytes. HAART has greatly reduced the number of HIV+ PML cases and significantly improved the survival of HIV+patients developing PML [3, 40, 41, 81–83]. However, immune reconstitution in PML can lead to IRIS in 10 to 30% of HIV+ patients, resulting in subacute onset of sometimes severe neurological deficits, which often persist after recovery from PML [3, 40, 83, 84]. The same applies to nearly all natalizumab-associated PML patients in whom natalizumab is discontinued and removed by plasma exchange after PML diagnosis [12, 85]. IRIS may be carefully treated with corticosteroids to ameliorate CNS tissue destruction by exuberant JCPyV-specific immune response [86, 87]. Corticosteroid treatment should be tapered slowly to avoid abrogation of the antiviral immune reaction. Alternatively, the C-C motif chemokine receptor (CCR5) antagonist maraviroc approved for the treatment of HIV infection, has been discussed to be useful in preventing overshooting IRIS [88–90]. Since maraviroc was not helpful in other PML cases [91], its therapeutic role for PML-IRIS prevention or treatment has to be defined in larger trials. However, it is not always possible to stop immune suppression, for example in organ transplant patients, in whom it could lead to graft rejection. Furthermore, some PML patients may not develop IRIS at all because of persisting severe immunosuppression related to haematological malignancy, or treatment with rituximab or alemtuzumab. In these cases, other therapies targeting JCPyV have to be considered.
Several antiviral drugs intended to target JCPyV have been investigated in vitro and tested in individual medical treatments or in clinical trials. These drugs target the virus at different stages of infection by blocking cell entry or viral replication. JCPyV uses sialylated receptors [31, 92] and the serotonin receptor 2 (5-HT2) [93, 94] to enter the cells. Several serotonin receptor antagonists are currently marketed for the treatment of mental disorders and have been tested as potential therapeutics for PML. Chlorpromazine efficiently blocked the viral infection in cell-based assays [94–96], but has never been tested clinically, probably because of its poor selectivity and the potential side effects. Mirtazapine was empirically reported to be beneficial in several independent PML case studies, but available data suggest that it is not effective [97–101]. The in vitro inhibitory effects of ziprasidone could not be translated into two PML patients [102], and another study [103] reported a PML case who recovered after risperidone treatment despite the fact that this drug could not block the attachment and internalisation of JCPyV into primary human fetal glial cells [104].
Another approach to interfering with the life cycle of JCPyV would be to block viral DNA replication, for example, by inhibiting viral DNA polymerases or topoisomerases. Cidofovir, a drug approved for the treatment of human cytomegalovirus (HCMV) infection, and its prodrug brincidofovir suppressed JCPyV replication in a cell-based assay even at a low, non-toxic concentration [105, 106], but neither improved survival nor neurological status of PML patients [107, 108]. Cytarabine reduced JCPyV replication in vitro [109], but failed to demonstrate an improvement of patients’ prognosis upon treatment [110]. Topotecan, a chemotherapeutic agent for certain ovarian cancers, inhibited JCPyV DNA replication in glial cells [111]. However, the results of a clinical study addressing its efficacy were not convincing, since only a few patients responded to the therapy and several enrolled patients experienced severe adverse events [112]. Mefloquine, a medication used to prevent or treat malaria, was selected as a potential JCPyV inhibitor in a high-throughput screening of approved drugs and was shown to block DNA replication after viral entry [113]. Given the availability and safety of this medicine, mefloquine was tested in a clinical trial that was prematurely terminated after interim data analyses suggested that the study was unlikely to succeed [114]. The lack of signs of efficacy for all these antiviral drugs has been disappointing, in particular after often promising in vitro results. The difficult translation into patients could be explained by stability issues, low brain penetration, toxicity issues, lack of specificity for JCPyV or JCPyV variants, and a cell type-dependent effect.
With the recent breakthrough in genome editing using the CRISPR/Cas9 system, two independent groups targeted functional regions in the JCPyV genome to inhibit viral replication in infected cells. The first approach aimed to introduce mutations in the early expressed JCPyV large T antigen in order to suppress viral replication [115], and another study focused on the modification of the regulatory noncoding control region element and the major capsid protein VP1 [116]. In both cases, the infection and spread of JCPyV could be inhibited in vitro in the JCPyV-permissive glial SVG-A cell line. However, this technique is still far away from potential clinical use in PML patients, and its efficacy has to be validated for JCPyV variants and different cell types. Furthermore, it is unclear whether those constructs can be delivered successfully to the infected cell types in the CNS and whether such an approach would have safety issues or even be more hazardous in combination of rearranged and mutated JCPyV variants.
Immune modulators, such as cytokines, are also used to treat viral diseases because of their potential antiviral capacity or ability to augment the immune response against the pathogen. Type 1 interferons, such as IFN-α, are known for their antiviral capacity, but failed in an open-label study in HIV+ PML patients [117], despite a retrospective analysis claiming increased survival times [118]. Interestingly, the use of lymphoproliferative cytokines, such as interleukin (IL)-2 of the common gamma-chain cytokine family, revealed beneficial effects on the response and outcome in single case studies with PML patients [119–122]. Another cytokine of the common gamma-chain family, IL-7, was also successfully tested in compassionate use alone or in combination with antiviral drugs [123–125].
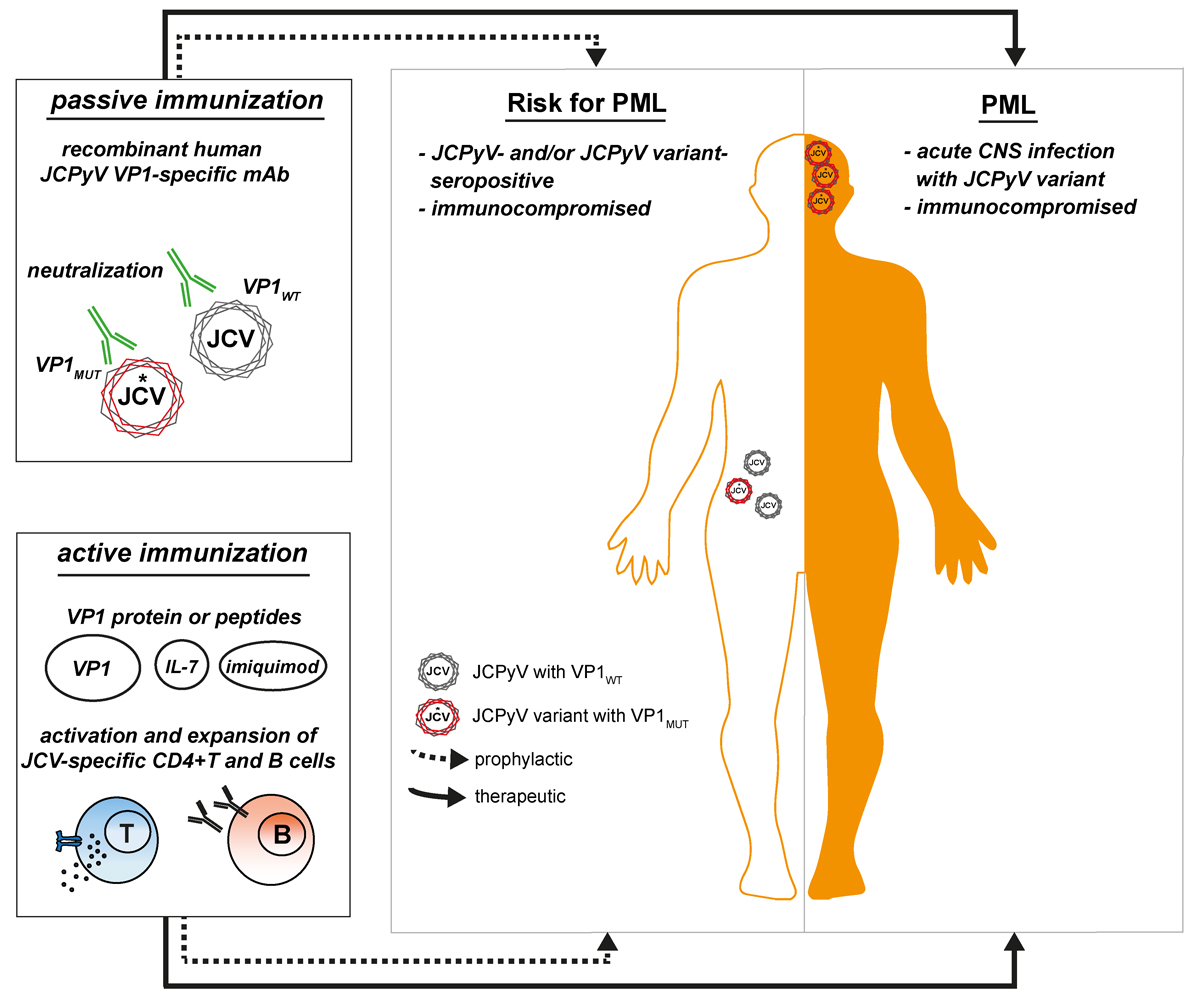
On the basis of current immunological data [14], approaches to boost specific immunity against JCPyV have also been explored. Recent studies suggested two novel promising immunotherapeutic approaches for the treatment or prevention of PML that are based on active or passive immunisation specifically targeting JCPyV. The development of prophylactic vaccines based on virus-like particles (VLPs) has been successful in the case of HPV [126], a nonenveloped DNA virus that shares similar structural elements with JCPyV. The use of VLPs formed by the reassembly of JCPyV VP1 proteins appears as a good approach for an effective vaccine since VLPs represent a combination of multiple epitopes and structural arrangement that should be optimal for uptake by antigen-presenting cells, in particular JCPyV-specific B cells. Furthermore, the choice of the right adjuvant is essential in order to efficiently boost the virus-specific immune response. A vaccine consisting of JCPyV VP1 VLPs in combination with IL-7 and imiquimod, a toll-like receptor 7 (TLR7) agonist, has been tested in two PML patients, one suffering from idiopathic CD4+ lymphopenia, the other from breast cancer, polychemotherapy, autologous and allogeneic bone marrow transplantation and subsequent graft-versus-host disease [15]. The treatment was well tolerated and safe, and several lines of evidence suggested that the immunisation caused a “therapeutic” immune response: (1) The CSF JCPyV viral load disappeared, (2) a pronounced proliferation of VP1-specific CD4+ T cells was observed, (3) magnetic resonance imaging studies showed contrast enhancement in the PML lesions as a clear sign of local immune response and IRIS, and (4) an amelioration of the neurological deficits was seen [15]. Although immune reconstitution with IL-7 alone has already shown promise in the management of PML, a combination with VLPs ensured a specific and robust activation and expansion of VP1-specific T cells. However, it is at present not clear whether IL-7 is needed or not in the above vaccination scheme. The same active vaccination approach was also successfully administered to a third PML patient with idiopathic CD4+ lymphopenia [127]. Vaccination substantially reduced the CSF viral load and the progression of PML. The patient also showed a strong rise in neutralising antibodies against her own mutant JCPyV strain, showing that this vaccine broadened the humoral response even against JCPyV variants. The active therapeutic immunisation would be most suitable for patients with residual and restorable immune function. We further assume that active prophylactic immunisation of healthy individuals or patients under treatments with PML risk has the potential to prevent PML or, in seronegative individuals, even the establishment of latent/persistent infection altogether.
With respect to possible passive vaccination against PML, antibody therapy has been successfully applied for various diseases; broadly neutralising antibodies are promising candidates for the treatment of viral infection and are currently in development for numerous pathogens, such as influenza, respiratory syncytial, Ebola and Zika viruses [128–132]. The above data indicate that an effective humoral response is important for controlling JCPyV infection and/or eliminating virus from the brain in PML. Evidence includes reports that HIV+ PML survivors showed higher IgG titres compared with controls [133], that B cells and plasma cells infiltrate the brain during IRIS [71, 134], that intrathecal production of JCPyV-specific antibodies increases with onset of IRIS [76, 135], that hereditary immunodeficiencies affecting B cells or antibodies can cause PML [136, 137] and that immunomodulatory drugs targeting B cells may lead to PML [4, 138]. The level of JCPyV VP1-specific antibodies is usually measured to stratify the patients at risk of developing PML, but this test does not discriminate between archetype JCPyV and PML-associated mutants. Further, there seems to be no correlation between the level of anti-JCPyV antibodies and their neutralising activity in the sera of MS patients [139]. Why higher anti-JCPyV antibody titres in natalizumab-treated MS patients are related to higher risk is at present not clear. We demonstrated that the humoral immune response against JCPyV mutants was compromised in natalizumab-associated PML patients, and that immune reconstitution led to a broadened antibody response against the PML-associated JCPyV variants [135]. We generated highly potent neutralising JCPyV VP1-specific antibodies by recombinant cloning from a PML patient who successfully controlled the viral infection after IRIS and recovered from PML [135]. These human-derived antibodies exhibited exquisite specificity and high affinity towards JCPyV, neutralised the JCPyV infection in vitro and showed cross-reactivity against the most common PML-causing JCPyV variants, and represent promising candidates for the development of a passive immunisation of PML patients. Similar work was also successfully carried out in Ebola survivors [128, 129]. Adequate brain exposure to antibodies would be expected, as observed for other human monoclonal antibodies in development for CNS indications [140]. Passive immunisation appears to be a promising approach, in particular for PML related to HIV, transplantation or immunosuppressive drug therapy patients, where immediate immune reconstitution is not possible.
Active and passive immunisation approaches for the prophylactic and therapeutic treatment of PML are summarised in figure 2.
Conclusion
PML remains an untreatable and often fatal opportunistic infection of the brain. Its resurgence with the increasing use of novel potent immunomodulatory treatments for a variety of diseases makes the development of prophylactic and therapeutic strategies for PML a high unmet medical need. Current data suggest that all three arms of the adaptive immune system, – virus-specific CD4+ and CD8+ T cells and virus-specific antibodies – are important for controlling infection with JCPyV and also for resolving PML once it has developed. Active therapeutic vaccination, as well as high affinity, cross-neutralising, anti-JCPyV VP1-specific human monoclonal antibodies are promising approaches to the development of an effective treatment and prophylaxis of PML.
Author contributions
Ivan Jelcic, Benoit Combaluzier and Ilijas Jelcic contributed equally to this work.
References
1
Cavanagh
JB
,
Greenbaum
D
,
Marshall
AH
,
Rubinstein
LJ
. Cerebral demyelination associated with disorders of the reticuloendothelial system. Lancet. 1959;274(7102):524–9. doi:.https://doi.org/10.1016/S0140-6736(59)91774-X
2
Padgett
BL
,
Walker
DL
,
ZuRhein
GM
,
Eckroade
RJ
,
Dessel
BH
. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;297(7712):1257–60. doi:.https://doi.org/10.1016/S0140-6736(71)91777-6
3
Cinque
P
,
Koralnik
IJ
,
Gerevini
S
,
Miro
JM
,
Price
RW
. Progressive multifocal leukoencephalopathy in HIV-1 infection. Lancet Infect Dis. 2009;9(10):625–36. doi:.https://doi.org/10.1016/S1473-3099(09)70226-9
4
Major
EO
. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61(1):35–47. doi:.https://doi.org/10.1146/annurev.med.080708.082655
5
Egli
A
,
Infanti
L
,
Dumoulin
A
,
Buser
A
,
Samaridis
J
,
Stebler
C
, et al.
Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199(6):837–46. doi:.https://doi.org/10.1086/597126
6
Gheuens
S
,
Wüthrich
C
,
Koralnik
IJ
. Progressive multifocal leukoencephalopathy: why gray and white matter. Annu Rev Pathol. 2013;8(1):189–215. doi:.https://doi.org/10.1146/annurev-pathol-020712-164018
7
Gorelik
L
,
Reid
C
,
Testa
M
,
Brickelmaier
M
,
Bossolasco
S
,
Pazzi
A
, et al.
Progressive multifocal leukoencephalopathy (PML) development is associated with mutations in JC virus capsid protein VP1 that change its receptor specificity. J Infect Dis. 2011;204(1):103–14. doi:.https://doi.org/10.1093/infdis/jir198
8
Reid
CE
,
Li
H
,
Sur
G
,
Carmillo
P
,
Bushnell
S
,
Tizard
R
, et al.
Sequencing and analysis of JC virus DNA from natalizumab-treated PML patients. J Infect Dis. 2011;204(2):237–44. doi:.https://doi.org/10.1093/infdis/jir256
9
Dang
X
,
Vidal
JE
,
Penalva de Oliveira
AC
,
Simpson
DM
,
Morgello
S
,
Hecht
JH
, et al.
JC virus granule cell neuronopathy is associated with VP1 C terminus mutants. J Gen Virol. 2012;93(Pt 1):175–83. doi:.https://doi.org/10.1099/vir.0.037440-0
10
Du Pasquier
RA
,
Koralnik
IJ
. Inflammatory reaction in progressive multifocal leukoencephalopathy: harmful or beneficial?
J Neurovirol. 2003;9(s1, Suppl 1):25–31. doi:.https://doi.org/10.1080/13550280390195315
11
Tan
K
,
Roda
R
,
Ostrow
L
,
McArthur
J
,
Nath
A
. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology. 2009;72(17):1458–64. doi:.https://doi.org/10.1212/01.wnl.0000343510.08643.74
12
Tan
IL
,
McArthur
JC
,
Clifford
DB
,
Major
EO
,
Nath
A
. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology. 2011;77(11):1061–7. doi:.https://doi.org/10.1212/WNL.0b013e31822e55e7
13
Pavlovic
D
,
Patera
AC
,
Nyberg
F
,
Gerber
M
,
Liu
M
; Progressive Multifocal Leukeoncephalopathy Consortium. Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disorder. 2015;8(6):255–73. doi:.https://doi.org/10.1177/1756285615602832
14
Jelcic
I
,
Jelcic
I
,
Faigle
W
,
Sospedra
M
,
Martin
R
. Immunology of progressive multifocal leukoencephalopathy. J Neurovirol. 2015;21(6):614–22. doi:.https://doi.org/10.1007/s13365-014-0294-y
15
Sospedra
M
,
Schippling
S
,
Yousef
S
,
Jelcic
I
,
Bofill-Mas
S
,
Planas
R
, et al.
Treating progressive multifocal leukoencephalopathy with interleukin 7 and vaccination with JC virus capsid protein VP1. Clin Infect Dis. 2014;59(11):1588–92. doi:.https://doi.org/10.1093/cid/ciu682
16
Kleinschmidt-DeMasters
BK
,
Tyler
KL
. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353(4):369–74. doi:.https://doi.org/10.1056/NEJMoa051782
17
Ou
WC
,
Wang
M
,
Fung
CY
,
Tsai
RT
,
Chao
PC
,
Hseu
TH
, et al.
The major capsid protein, VP1, of human JC virus expressed in Escherichia coli is able to self-assemble into a capsid-like particle and deliver exogenous DNA into human kidney cells. J Gen Virol. 1999;80(Pt 1):39–46. doi:.https://doi.org/10.1099/0022-1317-80-1-39
18
Brew
BJ
,
Davies
NW
,
Cinque
P
,
Clifford
DB
,
Nath
A
. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol. 2010;6(12):667–79. doi:.https://doi.org/10.1038/nrneurol.2010.164
19
Frohman
EM
,
Monaco
MC
,
Remington
G
,
Ryschkewitsch
C
,
Jensen
PN
,
Johnson
K
, et al.
JC virus in CD34+ and CD19+ cells in patients with multiple sclerosis treated with natalizumab. JAMA Neurol. 2014;71(5):596–602. doi:.https://doi.org/10.1001/jamaneurol.2014.63
20
Chapagain
ML
,
Nerurkar
VR
. Human polyomavirus JC (JCV) infection of human B lymphocytes: a possible mechanism for JCV transmigration across the blood-brain barrier. J Infect Dis. 2010;202(2):184–91. doi:.https://doi.org/10.1086/653823
21
Goldmann
C
,
Petry
H
,
Frye
S
,
Ast
O
,
Ebitsch
S
,
Jentsch
KD
, et al.
Molecular cloning and expression of major structural protein VP1 of the human polyomavirus JC virus: formation of virus-like particles useful for immunological and therapeutic studies. J Virol. 1999;73(5):4465–9.
22
White
FA, 3rd
,
Ishaq
M
,
Stoner
GL
,
Frisque
RJ
. JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. J Virol. 1992;66(10):5726–34.
23
Delbue
S
,
Branchetti
E
,
Boldorini
R
,
Vago
L
,
Zerbi
P
,
Veggiani
C
, et al.
Presence and expression of JCV early gene large T Antigen in the brains of immunocompromised and immunocompetent individuals. J Med Virol. 2008;80(12):2147–52. doi:.https://doi.org/10.1002/jmv.21313
24
Tan
CS
,
Ellis
LC
,
Wüthrich
C
,
Ngo
L
,
Broge
TA, Jr
,
Saint-Aubyn
J
, et al.
JC virus latency in the brain and extraneural organs of patients with and without progressive multifocal leukoencephalopathy. J Virol. 2010;84(18):9200–9. doi:.https://doi.org/10.1128/JVI.00609-10
25
Piña-Oviedo
S
,
De León-Bojorge
B
,
Cuesta-Mejías
T
,
White
MK
,
Ortiz-Hidalgo
C
,
Khalili
K
, et al.
Glioblastoma multiforme with small cell neuronal-like component: association with human neurotropic JC virus. Acta Neuropathol. 2006;111(4):388–96. doi:.https://doi.org/10.1007/s00401-006-0050-3
26
Perez-Liz
G
,
Del Valle
L
,
Gentilella
A
,
Croul
S
,
Khalili
K
. Detection of JC virus DNA fragments but not proteins in normal brain tissue. Ann Neurol. 2008;64(4):379–87. doi:.https://doi.org/10.1002/ana.21443
27
Bayliss
J
,
Karasoulos
T
,
McLean
CA
. Frequency and large T (LT) sequence of JC polyomavirus DNA in oligodendrocytes, astrocytes and granular cells in non-PML brain. Brain Pathol. 2012;22(3):329–36. doi:.https://doi.org/10.1111/j.1750-3639.2011.00538.x
28
Sunyaev
SR
,
Lugovskoy
A
,
Simon
K
,
Gorelik
L
. Adaptive mutations in the JC virus protein capsid are associated with progressive multifocal leukoencephalopathy (PML). PLoS Genet. 2009;5(2):e1000368. doi:.https://doi.org/10.1371/journal.pgen.1000368
29
Du Pasquier
RA
,
Corey
S
,
Margolin
DH
,
Williams
K
,
Pfister
LA
,
De Girolami
U
, et al.
Productive infection of cerebellar granule cell neurons by JC virus in an HIV+ individual. Neurology. 2003;61(6):775–82. doi:.https://doi.org/10.1212/01.WNL.0000081306.86961.33
30
Wüthrich
C
,
Cheng
YM
,
Joseph
JT
,
Kesari
S
,
Beckwith
C
,
Stopa
E
, et al.
Frequent infection of cerebellar granule cell neurons by polyomavirus JC in progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2009;68(1):15–25. doi:.https://doi.org/10.1097/NEN.0b013e3181912570
31
Neu
U
,
Maginnis
MS
,
Palma
AS
,
Ströh
LJ
,
Nelson
CD
,
Feizi
T
, et al.
Structure-function analysis of the human JC polyomavirus establishes the LSTc pentasaccharide as a functional receptor motif. Cell Host Microbe. 2010;8(4):309–19. doi:.https://doi.org/10.1016/j.chom.2010.09.004
32
Maginnis
MS
,
Ströh
LJ
,
Gee
GV
,
O’Hara
BA
,
Derdowski
A
,
Stehle
T
, et al.
Progressive multifocal leukoencephalopathy-associated mutations in the JC polyomavirus capsid disrupt lactoseries tetrasaccharide c binding. MBio. 2013;4(3):e00247–13. doi:.https://doi.org/10.1128/mBio.00247-13
33
Zerbe
CS
,
Marciano
BE
,
Katial
RK
,
Santos
CB
,
Adamo
N
,
Hsu
AP
, et al.
Progressive Multifocal Leukoencephalopathy in Primary Immune Deficiencies: Stat1 Gain of Function and Review of the Literature. Clin Infect Dis. 2016;62(8):986–94. doi:.https://doi.org/10.1093/cid/civ1220
34
Amend
KL
,
Turnbull
B
,
Foskett
N
,
Napalkov
P
,
Kurth
T
,
Seeger
J
. Incidence of progressive multifocal leukoencephalopathy in patients without HIV. Neurology. 2010;75(15):1326–32. doi:.https://doi.org/10.1212/WNL.0b013e3181f73600
35
Zaheer
F
,
Berger
JR
. Treatment-related progressive multifocal leukoencephalopathy: current understanding and future steps. Ther Adv Drug Saf. 2012;3(5):227–39. doi:.https://doi.org/10.1177/2042098612453849
36
Berger
JR
. Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord. 2017;12:59–63. doi:.https://doi.org/10.1016/j.msard.2017.01.006
37
Zonios
D
,
Sheikh
V
,
Sereti
I
. Idiopathic CD4 lymphocytopenia: a case of missing, wandering or ineffective T cells. Arthritis Res Ther. 2012;14(4):222. doi:.https://doi.org/10.1186/ar4027
38
Brooks
BR
,
Walker
DL
. Progressive multifocal leukoencephalopathy. Neurol Clin. 1984;2(2):299–313.
39
Berger
JR
,
Houff
S
. Progressive multifocal leukoencephalopathy: lessons from AIDS and natalizumab. Neurol Res. 2006;28(3):299–305. doi:.https://doi.org/10.1179/016164106X98198
40
Gasnault
J
,
Taoufik
Y
,
Goujard
C
,
Kousignian
P
,
Abbed
K
,
Boue
F
, et al.
Prolonged survival without neurological improvement in patients with AIDS-related progressive multifocal leukoencephalopathy on potent combined antiretroviral therapy. J Neurovirol. 1999;5(4):421–9. doi:.https://doi.org/10.3109/13550289909029483
41
Engsig
FN
,
Hansen
AB
,
Omland
LH
,
Kronborg
G
,
Gerstoft
J
,
Laursen
AL
, et al.
Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: a nationwide cohort study. J Infect Dis. 2009;199(1):77–83. doi:.https://doi.org/10.1086/595299
42
Khanna
N
,
Elzi
L
,
Mueller
NJ
,
Garzoni
C
,
Cavassini
M
,
Fux
CA
, et al.; Swiss HIV Cohort Study. Incidence and outcome of progressive multifocal leukoencephalopathy over 20 years of the Swiss HIV Cohort Study. Clin Infect Dis. 2009;48(10):1459–66. doi:.https://doi.org/10.1086/598335
43
Langer-Gould
A
,
Atlas
SW
,
Green
AJ
,
Bollen
AW
,
Pelletier
D
. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353(4):375–81. doi:.https://doi.org/10.1056/NEJMoa051847
44
Van Assche
G
,
Van Ranst
M
,
Sciot
R
,
Dubois
B
,
Vermeire
S
,
Noman
M
, et al.
Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353(4):362–8. doi:.https://doi.org/10.1056/NEJMoa051586
45
Berger
JR
,
Koralnik
IJ
. Progressive multifocal leukoencephalopathy and natalizumab--unforeseen consequences. N Engl J Med. 2005;353(4):414–6. doi:.https://doi.org/10.1056/NEJMe058122
46
Rice
GP
,
Hartung
HP
,
Calabresi
PA
. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64(8):1336–42. doi:.https://doi.org/10.1212/01.WNL.0000158329.30470.D0
47
Polman
CH
,
O’Connor
PW
,
Havrdova
E
,
Hutchinson
M
,
Kappos
L
,
Miller
DH
, et al.; AFFIRM Investigators. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi:.https://doi.org/10.1056/NEJMoa044397
48Biogen. TYSABRI® (natalizumab): PML in Patients Receiving TYSABRI. Biogen: Medical Information. 2016. Available from: https://medinfo.biogen.com/
49
Gordon
KB
,
Papp
KA
,
Hamilton
TK
,
Walicke
PA
,
Dummer
W
,
Li
N
, et al.; Efalizumab Study Group. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA. 2003;290(23):3073–80
. [doi:.].https://doi.org/10.1001/jama.290.23.3073
50
Molloy
ES
,
Calabrese
LH
. Therapy: Targeted but not trouble-free: efalizumab and PML. Nat Rev Rheumatol. 2009;5(8):418–9. doi:.https://doi.org/10.1038/nrrheum.2009.142
51
Schwab
N
,
Ulzheimer
JC
,
Fox
RJ
,
Schneider-Hohendorf
T
,
Kieseier
BC
,
Monoranu
CM
, et al.
Fatal PML associated with efalizumab therapy: insights into integrin αLβ2 in JC virus control. Neurology. 2012;78(7):458–67, discussion 465. doi:.https://doi.org/10.1212/WNL.0b013e3182478d4b
52
Gürcan
HM
,
Keskin
DB
,
Stern
JN
,
Nitzberg
MA
,
Shekhani
H
,
Ahmed
AR
. A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol. 2009;9(1):10–25. doi:.https://doi.org/10.1016/j.intimp.2008.10.004
53
Hauser
SL
,
Waubant
E
,
Arnold
DL
,
Vollmer
T
,
Antel
J
,
Fox
RJ
, et al.; HERMES Trial Group. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–88. doi:.https://doi.org/10.1056/NEJMoa0706383
54
Vermeer
NS
,
Straus
SM
,
Mantel-Teeuwisse
AK
,
Hidalgo-Simon
A
,
Egberts
AC
,
Leufkens
HG
, et al.
Drug-induced progressive multifocal leukoencephalopathy: Lessons learned from contrasting natalizumab and rituximab. Clin Pharmacol Ther. 2015;98(5):542–50. doi:.https://doi.org/10.1002/cpt.207
55
Carson
KR
,
Evens
AM
,
Richey
EA
,
Habermann
TM
,
Focosi
D
,
Seymour
JF
, et al.
Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113(20):4834–40. doi:.https://doi.org/10.1182/blood-2008-10-186999
56
Isidoro
L
,
Pires
P
,
Rito
L
,
Cordeiro
G
. Progressive multifocal leukoencephalopathy in a patient with chronic lymphocytic leukaemia treated with alemtuzumab. BMJ Case Rep. 2014;2014(jan08 1):bcr2013201781. doi:.https://doi.org/10.1136/bcr-2013-201781
57
D’Souza
A
,
Wilson
J
,
Mukherjee
S
,
Jaiyesimi
I
. Progressive multifocal leukoencephalopathy in chronic lymphocytic leukemia: a report of three cases and review of the literature. Clin Lymphoma Myeloma Leuk. 2010;10(1):E1–9. doi:.https://doi.org/10.3816/CLML.2010.n.009
58
Waggoner
J
,
Martinu
T
,
Palmer
SM
. Progressive multifocal leukoencephalopathy following heightened immunosuppression after lung transplant. J Heart Lung Transplant. 2009;28(4):395–8. doi:.https://doi.org/10.1016/j.healun.2008.12.010
59
Molloy
ES
,
Calabrese
CM
,
Calabrese
LH
. The Risk of Progressive Multifocal Leukoencephalopathy in the Biologic Era: Prevention and Management. Rheum Dis Clin North Am. 2017;43(1):95–109. doi:.https://doi.org/10.1016/j.rdc.2016.09.009
60
Kothary
N
,
Diak
IL
,
Brinker
A
,
Bezabeh
S
,
Avigan
M
,
Dal Pan
G
. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol. 2011;65(3):546–51. doi:.https://doi.org/10.1016/j.jaad.2010.05.033
61
Kumar
D
,
Bouldin
TW
,
Berger
RG
. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis Rheum. 2010;62(11):3191–5. doi:.https://doi.org/10.1002/art.27687
62
Faulkner
M
. Risk of progressive multifocal leukoencephalopathy in patients with multiple sclerosis. Expert Opin Drug Saf. 2015;14(11):1737–48. doi:.https://doi.org/10.1517/14740338.2015.1093620
63
Killestein
J
,
Vennegoor
A
,
van Golde
AE
,
Bourez
RL
,
Wijlens
ML
,
Wattjes
MP
. PML-IRIS during Fingolimod Diagnosed after Natalizumab Discontinuation. Case Rep Neurol Med. 2014;2014:307872. doi:.https://doi.org/10.1155/2014/307872
64
Fitzgerald
S
. Third PML Case Associated with Fingolimod: How to Distinguish PML from MS Relapse. Neurol Today. 2015;15(19):10–1. doi:.https://doi.org/10.1097/01.NT.0000472957.95553.c4
65
van Oosten
BW
,
Killestein
J
,
Wattjes
MP
. Case reports of PML in patients treated for psoriasis. N Engl J Med. 2013;369(11):1081–2.
66
Nieuwkamp
DJ
,
Murk
JL
,
van Oosten
BW
. PML in Patients Treated with Dimethyl Fumarate. N Engl J Med. 2015;373(6):584.
67
Rosenkranz
T
,
Novas
M
,
Terborg
C
. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372(15):1476–8. doi:.https://doi.org/10.1056/NEJMc1415408
68
Lehmann-Horn
K
,
Penkert
H
,
Grein
P
,
Leppmeier
U
,
Teuber-Hanselmann
S
,
Hemmer
B
, et al.
PML during dimethyl fumarate treatment of multiple sclerosis: How does lymphopenia matter?
Neurology. 2016;87(4):440–1. doi:.https://doi.org/10.1212/WNL.0000000000002900
69
Weber
T
. Progressive multifocal leukoencephalopathy. Neurol Clin. 2008;26(3):833–54, x–xi. doi:.https://doi.org/10.1016/j.ncl.2008.03.007
70
Stüve
O
,
Marra
CM
,
Bar-Or
A
,
Niino
M
,
Cravens
PD
,
Cepok
S
, et al.
Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol. 2006;63(10):1383–7. doi:.https://doi.org/10.1001/archneur.63.10.1383
71
Aly
L
,
Yousef
S
,
Schippling
S
,
Jelcic
I
,
Breiden
P
,
Matschke
J
, et al.
Central role of JC virus-specific CD4+ lymphocytes in progressive multi-focal leucoencephalopathy-immune reconstitution inflammatory syndrome. Brain. 2011;134(Pt 9):2687–702. doi:.https://doi.org/10.1093/brain/awr206
72
Yousef
S
,
Planas
R
,
Chakroun
K
,
Hoffmeister-Ullerich
S
,
Binder
TM
,
Eiermann
TH
, et al.
TCR bias and HLA cross-restriction are strategies of human brain-infiltrating JC virus-specific CD4+ T cells during viral infection. J Immunol. 2012;189(7):3618–30. doi:.https://doi.org/10.4049/jimmunol.1201612
73
Jelcic
I
,
Jelcic
I
,
Kempf
C
,
Largey
F
,
Planas
R
,
Schippling
S
, et al.
Mechanisms of immune escape in central nervous system infection with neurotropic JC virus variant. Ann Neurol. 2016;79(3):404–18. doi:.https://doi.org/10.1002/ana.24574
74
Bloomgren
G
,
Richman
S
,
Hotermans
C
,
Subramanyam
M
,
Goelz
S
,
Natarajan
A
, et al.
Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366(20):1870–80. doi:.https://doi.org/10.1056/NEJMoa1107829
75
Plavina
T
,
Subramanyam
M
,
Bloomgren
G
,
Richman
S
,
Pace
A
,
Lee
S
, et al.
Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76(6):802–12. doi:.https://doi.org/10.1002/ana.24286
76
Warnke
C
,
von Geldern
G
,
Markwerth
P
,
Dehmel
T
,
Hoepner
R
,
Gold
R
, et al.
Cerebrospinal fluid JC virus antibody index for diagnosis of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76(6):792–801. doi:.https://doi.org/10.1002/ana.24153
77
Schwab
N
,
Schneider-Hohendorf
T
,
Posevitz
V
,
Breuer
J
,
Göbel
K
,
Windhagen
S
, et al.
L-selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patients. Neurology. 2013;81(10):865–71. doi:.https://doi.org/10.1212/WNL.0b013e3182a351fb
78
Schwab
N
,
Schneider-Hohendorf
T
,
Pignolet
B
,
Spadaro
M
,
Görlich
D
,
Meinl
I
, et al.
PML risk stratification using anti-JCV antibody index and L-selectin. Mult Scler. 2016;22(8):1048–60. doi:.https://doi.org/10.1177/1352458515607651
79
Lieberman
LA
,
Zeng
W
,
Singh
C
,
Wang
W
,
Otipoby
KL
,
Loh
C
, et al.
CD62L is not a reliable biomarker for predicting PML risk in natalizumab-treated R-MS patients. Neurology. 2016;86(4):375–81. doi:.https://doi.org/10.1212/WNL.0000000000002314
80
Villar
LM
,
Costa-Frossard
L
,
Masterman
T
,
Fernandez
O
,
Montalban
X
,
Casanova
B
, et al.
Lipid-specific immunoglobulin M bands in cerebrospinal fluid are associated with a reduced risk of developing progressive multifocal leukoencephalopathy during treatment with natalizumab. Ann Neurol. 2015;77(3):447–57. doi:.https://doi.org/10.1002/ana.24345
81
Clifford
DB
,
Yiannoutsos
C
,
Glicksman
M
,
Simpson
DM
,
Singer
EJ
,
Piliero
PJ
, et al.
HAART improves prognosis in HIV-associated progressive multifocal leukoencephalopathy. Neurology. 1999;52(3):623–5. doi:.https://doi.org/10.1212/WNL.52.3.623
82
De Luca
A
,
Giancola
ML
,
Ammassari
A
,
Grisetti
S
,
Paglia
MG
,
Gentile
M
, et al.
The effect of potent antiretroviral therapy and JC virus load in cerebrospinal fluid on clinical outcome of patients with AIDS-associated progressive multifocal leukoencephalopathy. J Infect Dis. 2000;182(4):1077–83. doi:.https://doi.org/10.1086/315817
83
Berenguer
J
,
Miralles
P
,
Arrizabalaga
J
,
Ribera
E
,
Dronda
F
,
Baraia-Etxaburu
J
, et al.; GESIDA 11/99 Study Group. Clinical course and prognostic factors of progressive multifocal leukoencephalopathy in patients treated with highly active antiretroviral therapy. Clin Infect Dis. 2003;36(8):1047–52. doi:.https://doi.org/10.1086/374048
84
Sainz-de-la-Maza
S
,
Casado
JL
,
Pérez-Elías
MJ
,
Moreno
A
,
Quereda
C
,
Moreno
S
, et al.
Incidence and prognosis of immune reconstitution inflammatory syndrome in HIV-associated progressive multifocal leucoencephalopathy. Eur J Neurol. 2016;23(5):919–25. doi:.https://doi.org/10.1111/ene.12963
85
Clifford
DB
,
De Luca
A
,
Simpson
DM
,
Arendt
G
,
Giovannoni
G
,
Nath
A
. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9(4):438–46. doi:.https://doi.org/10.1016/S1474-4422(10)70028-4
86
Antoniol
C
,
Jilek
S
,
Schluep
M
,
Mercier
N
,
Canales
M
,
Le Goff
G
, et al.
Impairment of JCV-specific T-cell response by corticotherapy: effect on PML-IRIS management?
Neurology. 2012;79(23):2258–64. doi:.https://doi.org/10.1212/WNL.0b013e3182768983
87
Clifford
DB
. Progressive multifocal leukoencephalopathy therapy. J Neurovirol. 2015;21(6):632–6. doi:.https://doi.org/10.1007/s13365-014-0289-8
88
Giacomini
PS
,
Rozenberg
A
,
Metz
I
,
Araujo
D
,
Arbour
N
,
Bar-Or
A
; Maraviroc in Multiple Sclerosis–Associated PML–IRIS (MIMSAPI) Group. Maraviroc and JC virus-associated immune reconstitution inflammatory syndrome. N Engl J Med. 2014;370(5):486–8. doi:.https://doi.org/10.1056/NEJMc1304828
89
Martin-Blondel
G
,
Cuzin
L
,
Delobel
P
,
Cuvinciuc
V
,
Dumas
H
,
Alvarez
M
, et al.
Is maraviroc beneficial in paradoxical progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome management?
AIDS. 2009;23(18):2545–6. doi:.https://doi.org/10.1097/QAD.0b013e32833365f4
90
Shahani
L
,
Shah
M
,
Tavakoli-Tabasi
S
. Immune reconstitution inflammatory syndrome in a patient with progressive multifocal leukoencephalopathy. BMJ Case Rep. 2015;2015(jun10 1):bcr2014207325. doi:.https://doi.org/10.1136/bcr-2014-207325
91
Rodríguez
M
,
Silva-Sánchez
FA
,
Luna-Rivero
C
,
Vega-Barrientos
R
,
Alvarado-de la Barrera
C
,
Reyes-Terán
G
. Maraviroc Failed to Control Progressive Multifocal Leukoencephalopathy-Associated IRIS in a Patient with Advanced HIV Infection. Case Rep Med. 2014;2014:381480. doi:.https://doi.org/10.1155/2014/381480
92
Ströh
LJ
,
Maginnis
MS
,
Blaum
BS
,
Nelson
CD
,
Neu
U
,
Gee
GV
, et al.
The Greater Affinity of JC Polyomavirus Capsid for α2,6-Linked Lactoseries Tetrasaccharide c than for Other Sialylated Glycans Is a Major Determinant of Infectivity. J Virol. 2015;89(12):6364–75. doi:.https://doi.org/10.1128/JVI.00489-15
93
Assetta
B
,
Maginnis
MS
,
Gracia Ahufinger
I
,
Haley
SA
,
Gee
GV
,
Nelson
CD
, et al.
5-HT2 receptors facilitate JC polyomavirus entry. J Virol. 2013;87(24):13490–8. doi:.https://doi.org/10.1128/JVI.02252-13
94
Elphick
GF
,
Querbes
W
,
Jordan
JA
,
Gee
GV
,
Eash
S
,
Manley
K
, et al.
The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306(5700):1380–3. doi:.https://doi.org/10.1126/science.1103492
95
Pho
MT
,
Ashok
A
,
Atwood
WJ
. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J Virol. 2000;74(5):2288–92. doi:.https://doi.org/10.1128/JVI.74.5.2288-2292.2000
96
Atwood
WJ
. A combination of low-dose chlorpromazine and neutralizing antibodies inhibits the spread of JC virus (JCV) in a tissue culture model: implications for prophylactic and therapeutic treatment of progressive multifocal leukencephalopathy. J Neurovirol. 2001;7(4):307–10. doi:.https://doi.org/10.1080/13550280152537157
97
Park
JH
,
Ryoo
S
,
Noh
HJ
,
Seo
JM
,
Kang
HH
,
Shin
JS
, et al.
Dual therapy with cidofovir and mirtazapine for progressive multifocal leukoencephalopathy in a sarcoidosis patient. Case Rep Neurol. 2011;3(3):258–62. doi:.https://doi.org/10.1159/000333780
98
Verma
S
,
Cikurel
K
,
Koralnik
IJ
,
Morgello
S
,
Cunningham-Rundles
C
,
Weinstein
ZR
, et al.
Mirtazapine in progressive multifocal leukoencephalopathy associated with polycythemia vera. J Infect Dis. 2007;196(5):709–11. doi:.https://doi.org/10.1086/520514
99
Cettomai
D
,
McArthur
JC
. Mirtazapine use in human immunodeficiency virus-infected patients with progressive multifocal leukoencephalopathy. Arch Neurol. 2009;66(2):255–8. doi:.https://doi.org/10.1001/archneurol.2008.557
100
Epperla
N
,
Medina-Flores
R
,
Mazza
JJ
,
Yale
SH
. Mirtazapine and mefloquine therapy for non-AIDS-related progressive multifocal leukoencephalopathy. WMJ. 2014;113(6):242–5.
101
Jamilloux
Y
,
Kerever
S
,
Ferry
T
,
Broussolle
C
,
Honnorat
J
,
Sève
P
. Treatment of Progressive Multifocal Leukoencephalopathy With Mirtazapine. Clin Drug Investig. 2016;36(10):783–9. doi:.https://doi.org/10.1007/s40261-016-0433-8
102
Kharfan-Dabaja
MA
,
Ayala
E
,
Greene
J
,
Rojiani
A
,
Murtagh
FR
,
Anasetti
C
. Two cases of progressive multifocal leukoencephalopathy after allogeneic hematopoietic cell transplantation and a review of the literature. Bone Marrow Transplant. 2007;39(2):101–7. doi:.https://doi.org/10.1038/sj.bmt.1705548
103
Kast
RE
,
Focosi
D
,
Petrini
M
,
Altschuler
EL
. Treatment schedules for 5-HT2A blocking in progressive multifocal leukoencephalopathy using risperidone or ziprasidone. Bone Marrow Transplant. 2007;39(12):811–2. doi:.https://doi.org/10.1038/sj.bmt.1705682
104
Chapagain
ML
,
Sumibcay
L
,
Gurjav
U
,
Kaufusi
PH
,
Kast
RE
,
Nerurkar
VR
. Serotonin receptor 2A blocker (risperidone) has no effect on human polyomavirus JC infection of primary human fetal glial cells. J Neurovirol. 2008;14(5):448–54. doi:.https://doi.org/10.1080/13550280802235916
105
Jiang
ZG
,
Cohen
J
,
Marshall
LJ
,
Major
EO
. Hexadecyloxypropyl-cidofovir (CMX001) suppresses JC virus replication in human fetal brain SVG cell cultures. Antimicrob Agents Chemother. 2010;54(11):4723–32. doi:.https://doi.org/10.1128/AAC.00837-10
106
Gosert
R
,
Rinaldo
CH
,
Wernli
M
,
Major
EO
,
Hirsch
HH
. CMX001 (1-O-hexadecyloxypropyl-cidofovir) inhibits polyomavirus JC replication in human brain progenitor-derived astrocytes. Antimicrob Agents Chemother. 2011;55(5):2129–36. doi:.https://doi.org/10.1128/AAC.00046-11
107
Marra
CM
,
Rajicic
N
,
Barker
DE
,
Cohen
BA
,
Clifford
D
,
Donovan Post
MJ
, et al.; Adult AIDS Clinical Trials Group 363 Team. A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. AIDS. 2002;16(13):1791–7. doi:.https://doi.org/10.1097/00002030-200209060-00012
108
De Luca
A
,
Ammassari
A
,
Pezzotti
P
,
Cinque
P
,
Gasnault
J
,
Berenguer
J
, et al.; Gesida 9/99, IRINA, ACTG 363 Study Groups. Cidofovir in addition to antiretroviral treatment is not effective for AIDS-associated progressive multifocal leukoencephalopathy: a multicohort analysis. AIDS. 2008;22(14):1759–67. doi:.https://doi.org/10.1097/QAD.0b013e32830a5043
109
Hou
J
,
Major
EO
. The efficacy of nucleoside analogs against JC virus multiplication in a persistently infected human fetal brain cell line. J Neurovirol. 1998;4(4):451–6. doi:.https://doi.org/10.3109/13550289809114545
110
Hall
CD
,
Dafni
U
,
Simpson
D
,
Clifford
D
,
Wetherill
PE
,
Cohen
B
, et al.
Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. N Engl J Med. 1998;338(19):1345–51. doi:.https://doi.org/10.1056/NEJM199805073381903
111
Kerr
DA
,
Chang
CF
,
Gordon
J
,
Bjornsti
MA
,
Khalili
K
. Inhibition of human neurotropic virus (JCV) DNA replication in glial cells by camptothecin. Virology. 1993;196(2):612–8. doi:.https://doi.org/10.1006/viro.1993.1517
112
Royal
W, 3rd
,
Dupont
B
,
McGuire
D
,
Chang
L
,
Goodkin
K
,
Ernst
T
, et al.
Topotecan in the treatment of acquired immunodeficiency syndrome-related progressive multifocal leukoencephalopathy. J Neurovirol. 2003;9(3):411–9. doi:.https://doi.org/10.1080/713831540
113
Brickelmaier
M
,
Lugovskoy
A
,
Kartikeyan
R
,
Reviriego-Mendoza
MM
,
Allaire
N
,
Simon
K
, et al.
Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother. 2009;53(5):1840–9. doi:.https://doi.org/10.1128/AAC.01614-08
114
Clifford
DB
,
Nath
A
,
Cinque
P
,
Brew
BJ
,
Zivadinov
R
,
Gorelik
L
, et al.
A study of mefloquine treatment for progressive multifocal leukoencephalopathy: results and exploration of predictors of PML outcomes. J Neurovirol. 2013;19(4):351–8. doi:.https://doi.org/10.1007/s13365-013-0173-y
115
Wollebo
HS
,
Bellizzi
A
,
Kaminski
R
,
Hu
W
,
White
MK
,
Khalili
K
. CRISPR/Cas9 System as an Agent for Eliminating Polyomavirus JC Infection. PLoS One. 2015;10(9):e0136046. doi:.https://doi.org/10.1371/journal.pone.0136046
116
Chou
YY
,
Krupp
A
,
Kaynor
C
,
Gaudin
R
,
Ma
M
,
Cahir-McFarland
E
, et al.
Inhibition of JCPyV infection mediated by targeted viral genome editing using CRISPR/Cas9. Sci Rep. 2016;6(1):36921. doi:.https://doi.org/10.1038/srep36921
117
Berger
JR
, et al.
A pilot study of recombinant alpha 2a interferon in the treatment of AIDS-related progressive multifocal leukoencephalopathy. Neurology. 1992;42:257.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1734312&dopt=Abstract
118
Huang
SS
,
Skolasky
RL
,
Dal Pan
GJ
,
Royal
W, 3rd
,
McArthur
JC
. Survival prolongation in HIV-associated progressive multifocal leukoencephalopathy treated with alpha-interferon: an observational study. J Neurovirol. 1998;4(3):324–32. doi:.https://doi.org/10.3109/13550289809114533
119
Kunschner
L
,
Scott
TF
. Sustained recovery of progressive multifocal leukoencephalopathy after treatment with IL-2. Neurology. 2005;65(9):1510. doi:.https://doi.org/10.1212/01.wnl.0000183064.10227.b5
120
Buckanovich
RJ
,
Liu
G
,
Stricker
C
,
Luger
SM
,
Stadtmauer
EA
,
Schuster
SJ
, et al.
Nonmyeloablative allogeneic stem cell transplantation for refractory Hodgkin’s lymphoma complicated by interleukin-2 responsive progressive multifocal leukoencephalopathy. Ann Hematol. 2002;81(7):410–3. doi:.https://doi.org/10.1007/s00277-002-0481-4
121
Re
D
,
Bamborschke
S
,
Feiden
W
,
Schröder
R
,
Lehrke
R
,
Diehl
V
, et al.
Progressive multifocal leukoencephalopathy after autologous bone marrow transplantation and alpha-interferon immunotherapy. Bone Marrow Transplant. 1999;23(3):295–8. doi:.https://doi.org/10.1038/sj.bmt.1701568
122
Przepiorka
D
,
Jaeckle
KA
,
Birdwell
RR
,
Fuller
GN
,
Kumar
AJ
,
Huh
YO
, et al.
Successful treatment of progressive multifocal leukoencephalopathy with low-dose interleukin-2. Bone Marrow Transplant. 1997;20(11):983–7. doi:.https://doi.org/10.1038/sj.bmt.1701010
123
Patel
A
,
Patel
J
,
Ikwuagwu
J
. A case of progressive multifocal leukoencephalopathy and idiopathic CD4+ lymphocytopenia. J Antimicrob Chemother. 2010;65(12):2697–8. doi:.https://doi.org/10.1093/jac/dkq359
124
Gasnault
J
,
de Goër de Herve
MG
,
Michot
JM
,
Hendel-Chavez
H
,
Seta
V
,
Mazet
AA
, et al.
Efficacy of recombinant human interleukin 7 in a patient with severe lymphopenia-related progressive multifocal leukoencephalopathy. Open Forum Infect Dis. 2014;1(2):ofu074. doi:.https://doi.org/10.1093/ofid/ofu074
125
Alstadhaug
KB
,
Croughs
T
,
Henriksen
S
,
Leboeuf
C
,
Sereti
I
,
Hirsch
HH
, et al.
Treatment of progressive multifocal leukoencephalopathy with interleukin 7. JAMA Neurol. 2014;71(8):1030–5. doi:.https://doi.org/10.1001/jamaneurol.2014.825
126
Schiller
JT
,
Lowy
DR
. Raising expectations for subunit vaccine. J Infect Dis. 2015;211(9):1373–5. doi:.https://doi.org/10.1093/infdis/jiu648
127
Ray
U
,
Cinque
P
,
Gerevini
S
,
Longo
V
,
Lazzarin
A
,
Schippling
S
, et al.
JC polyomavirus mutants escape antibody-mediated neutralization. Sci Transl Med. 2015;7(306):306ra151. doi:.https://doi.org/10.1126/scitranslmed.aab1720
128
Bornholdt
ZA
,
Turner
HL
,
Murin
CD
,
Li
W
,
Sok
D
,
Souders
CA
, et al.
Isolation of potent neutralizing antibodies from a survivor of the 2014 Ebola virus outbreak. Science. 2016;351(6277):1078–83. doi:.https://doi.org/10.1126/science.aad5788
129
Corti
D
,
Misasi
J
,
Mulangu
S
,
Stanley
DA
,
Kanekiyo
M
,
Wollen
S
, et al.
Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science. 2016;351(6279):1339–42. doi:.https://doi.org/10.1126/science.aad5224
130
Sapparapu
G
,
Fernandez
E
,
Kose
N
,
Bin Cao
,
Fox
JM
,
Bombardi
RG
, et al.
Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016;540(7633):443–7. doi:.https://doi.org/10.1038/nature20564
131
Corti
D
,
Bianchi
S
,
Vanzetta
F
,
Minola
A
,
Perez
L
,
Agatic
G
, et al.
Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature. 2013;501(7467):439–43. doi:.https://doi.org/10.1038/nature12442
132
Corti
D
,
Lanzavecchia
A
. Broadly neutralizing antiviral antibodies. Annu Rev Immunol. 2013;31(1):705–42. doi:.https://doi.org/10.1146/annurev-immunol-032712-095916
133
Khanna
N
,
Wolbers
M
,
Mueller
NJ
,
Garzoni
C
,
Du Pasquier
RA
,
Fux
CA
, et al.; Swiss HIV Cohort Study. JC virus-specific immune responses in human immunodeficiency virus type 1 patients with progressive multifocal leukoencephalopathy. J Virol. 2009;83(9):4404–11. doi:.https://doi.org/10.1128/JVI.02657-08
134
Metz
I
,
Radue
EW
,
Oterino
A
,
Kümpfel
T
,
Wiendl
H
,
Schippling
S
, et al.
Pathology of immune reconstitution inflammatory syndrome in multiple sclerosis with natalizumab-associated progressive multifocal leukoencephalopathy. Acta Neuropathol. 2012;123(2):235–45. doi:.https://doi.org/10.1007/s00401-011-0900-5
135
Jelcic
I
,
Combaluzier
B
,
Jelcic
I
,
Faigle
W
,
Senn
L
,
Reinhart
BJ
, et al.
Broadly neutralizing human monoclonal JC polyomavirus VP1-specific antibodies as candidate therapeutics for progressive multifocal leukoencephalopathy. Sci Transl Med. 2015;7(306):306ra150. doi:.https://doi.org/10.1126/scitranslmed.aac8691
136
Tasca
G
,
Iorio
R
,
Basile
U
,
Lauriola
L
,
Tartaglione
R
,
Mirabella
M
, et al.
Progressive multifocal leukoencephalopathy in a patient with Franklin disease and hypogammaglobulinemia. J Neurol Sci. 2009;284(1-2):203–4. doi:.https://doi.org/10.1016/j.jns.2009.04.035
137
Squintani
G
,
Ferrari
S
,
Bazzoli
E
,
Eleopra
R
,
La Monaca
C
,
Cagliari
E
, et al.
Progressive multifocal leukoencephalopathy in a patient with Good’s syndrome. Int J Infect Dis. 2010;14(5):e444–7. doi:.https://doi.org/10.1016/j.ijid.2009.06.005
138
Fredericks
CA
,
Kvam
KA
,
Bear
J
,
Crabtree
GS
,
Josephson
SA
. A case of progressive multifocal leukoencephalopathy in a lupus patient treated with belimumab. Lupus. 2014;23(7):711–3. doi:.https://doi.org/10.1177/0961203314524292
139
Diotti
RA
,
Mancini
N
,
Clementi
N
,
Sautto
G
,
Moreno
GJ
,
Criscuolo
E
, et al.
Cloning of the first human anti-JCPyV/VP1 neutralizing monoclonal antibody: epitope definition and implications in risk stratification of patients under natalizumab therapy. Antiviral Res. 2014;108:94–103. doi:.https://doi.org/10.1016/j.antiviral.2014.05.017
140
Sevigny
J
,
Chiao
P
,
Bussière
T
,
Weinreb
PH
,
Williams
L
,
Maier
M
, et al.
The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–6. doi:.https://doi.org/10.1038/nature19323