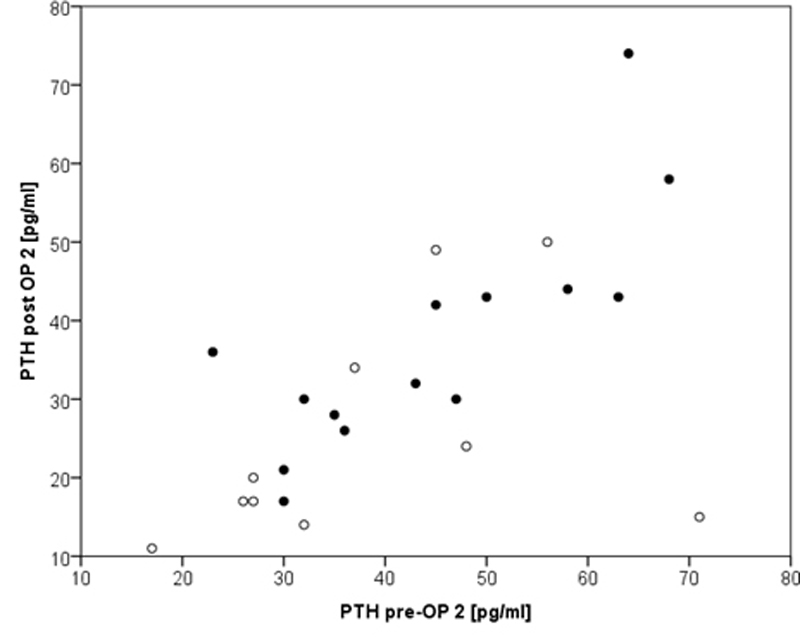
Figure 1 Correlation between pre- and postoperative parathyroid hormone (PTH) showing strong correlation (r = 0.802, p = 0.001) only in normocalcaemic group (group 2, filled circles).
DOI: https://doi.org/10.4414/smw.2017.14513
The incidence of differentiated thyroid cancer is estimated to be 1.2–3.8 per 100 000 individuals per year (66% papillary and 27% follicular), which represents 1–2% of all human malignancies [1]. The annual mortality rate is 0.5/100 000 both in men and women [1]. About 650 cases per year are diagnosed in Switzerland [2]. Temporary postoperative hypocalcaemia (HC) is the most common complication after total thyroidectomy (TE) with a rate of up to 70% whereas the 0.9% incidence of permanent HC is much lower [3]. After completion of TE one might assume a lower risk for temporary HC since the remaining parathyroid glands should have recovered their function in between the two surgeries. Indeed, a rate of temporary HC of 33% has been published recently [4]. While risk factors for HC after primary total TE have been well investigated and described in the literature (such as thyroid cancer, nodal dissection of level VI, female gender [3] and steep decline of parathyroid hormone (PTH) levels [5]), only few studies have been published concerning HC and HC risk factors after completion TE [6, 7]. Completion of thyroidectomy is necessary once a malignancy is detected on final histology exceeding the size of a microcarcinoma in one thyroid lobe according to the guidelines of Swiss society of head and neck surgery [8]. Subsequent irradiation using radioactive iodine ablation is only possible following total thyroidectomy.
Clinical symptoms of HC can vary from mild paraesthesia along the fingers up to severe hypocalcaemic tetany [9] accompanied by the typical “obstetrician’s hand” or even laryngospasm [10]. Postoperative management of HC (serum calcium and PTH measurements, calcium substitution oral or IV) is important in order to prevent or treat the patient’s symptoms and shorten the hospital stay [11].
Our aim was to investigate patients undergoing completion of TE with respect to their HC rate and to identify potential risk factors for HC to improve perioperative management. Additionally, a comparison of perioperative PTH and calcium course values as well as HC rates after primary total thyroidectomy from our department [12] was performed.
A retrospective study was undertaken including all patients from our ENT statistics database (www.innoforce.ch) undergoing completion of thyroidectomy (TE) between 2002 and 2013 in our tertiary care centre. The indication for completion hemithyroidectomy of the contralateral side was the unexpected histological finding of a differentiated thyroid cancer (pT1b and higher classification) after first surgery according to the guidelines of Swiss society of head and neck surgery [8]. All surgeries were performed by the same (senior) surgeon (W.M.). The patients’ data extracted were allocated to two groups: Group 1 developed a hypocalcaemia after second surgery while group 2 was normocalcaemic at any time. These two groups were compared to each other with respect to gender, age, type of thyroid cancer, time interval between the two surgeries, pre- and postoperative PTH and calcium levels along with clinical hypocalcaemia signs and calcium substitution treatment. Based on the thresholds of our hospital laboratory [13], hypocalcaemia was defined as <2.10 mmol/l on the first postoperative day, and hypoparathyroidism as <15pg/ml. Predictive Analytics Software (PASW) version 18.0 was used for statistical analysis performing Mann-Whitney U-Test for nonparametric data and the Chi-Square test for frequency data, both for group comparison where appropriate. In addition, binary logistic regression analysis was used to investigate the effects of time-interval from first to second surgery, as well as PTH and calcium level preoperative on the development of HC. Finally, Pearson correlation analysis was done to test for relationships between pre- and postoperative PTH level in both groups. Statistical significance was set at p <0.05.
As shown in table 1, no significant difference was found between the two groups with respect to gender, age and type of thyroid cancer (papillary/follicular cancer). Comparing the time between the two surgeries, no significant differences were found, although the time gap in group 2 was on average 6 days longer. Significant differences between groups were found regarding the postoperative PTH (p = 0.031) and calcium levels (p <0.001) after the second surgery. The values for PTH and serum calcium were on average 33 and 31% lower in the hypocalcaemia group than in the normocalcaemia group. The average postoperative PTH decline after second surgery was 32.6% in the HC group and therefore higher than the 14.4% in the normocalcaemia group. There was a higher rate of steep PTH decline defined as ≥50% of the preoperative value in the HC group (p-value not applicable). In both groups, there was only one patient with clinical HC signs. Oral calcium substitution was necessary in four cases and IV substitution in two cases in the HC group, whereas there was only one patient with oral substitution and no IV substitution in the NC group. The remaining six patients from the HC group were asymptomatic with borderline serum calcium levels and/or normal PTH levels, and consequently did not receive calcium substitution.
Table 1 Comparison of the cohort showing a significantly higher rate of hypoparathyroidism (<15 pg/ml), lower PTH levels and higher rate of steep PTH decline after second surgery (OP2). Also significantly more calcium substitution either orally or intravenously was necessary in the hypocalcaemia group.
|
Hypocalcaemia
(n = 12)* |
Normocalcaemia (n = 22) | p-value‡ | |
|---|---|---|---|
| Hypoparathyroidism (<15 pg/ml) | 3 (25%) | 0 (0%) | NA |
| Female | 8 (66%) | 17 (77%) | 0.061 |
| Male | 4 (34%) | 5 (23%) | |
| Age (y) | 46.5 ± 16.9 | 50.7 ± 18.8 | 0.589 |
| Papillary carcinoma | 7 (58%) | 11 (50%) | 0.728 |
| Follicular carcinoma | 5 (42%) | 11 (50%) | |
| Operation interval (d) | 10.9 ± 13.3 | 16.9 ± 19.1 | 0.243 |
| PTH post-OP2 (pg/ml) | 25.1 ±14.3 | 37.6 ± 14.0 | 0.031 |
| Ø PTH decline pre/post-OP2 | 32.6% | 14.4% | 0.174 |
| Steep PTH decline (≥50%) | 3 (25%) | 0 (0%) | NA |
| Calcium post-OP2 (mmol/l) | 1.87 ±0.3 | 2.27 ±0.1 | <0.001 |
| Symptomatic after OP2 | 1 (8%) | 1 (5%) | NA |
| Calcium substitution | |||
| Oral | 4. (4%) | 1. (5%) | NA |
| Intravenous | 2 (16%) | 0 (0%) | |
| Neck dissection level II–IV, VI | 2 (16%) | 2 (9%) | NA |
PTH = parathyroid hormone; Ca = calcium; HC = hypocalcaemia; Ø PTH decline pre/post OP2 = average PTH decline after second surgery compared to preoperative value; NA = statistical analysis in subgroup not applicable due small numbers Results are presented as frequency (%) or mean ± SD and the corresponding two-sided p-value of the group comparison ‡ p-value <0.05 is considered as a significant group difference * 1 patient in HC-group had 1 parathyroid autoimplantation. 2 patients in HC-group had 1 parathyroid-gland resection during first surgery due to tumour-infiltration and parathyroid-adenoma respectively.
In both groups, there were two patients undergoing a selective neck dissection (ND) level II–IV and VI (p-value not applicable).
One patient with hypocalcaemia (HC group) underwent an autoimplantation of one parathyroid gland, which had to be removed owing to extensive scar formation following the initial surgery eight days before. This patient had normal preoperative PTH and calcium levels. No postoperative hypoparathyroidism, steep PTH decline or clinical HC signs occurred although his calcium values had dropped to 1.82 mmol/l 24 hours after surgery. We therefore assume that the remaining parathyroid glands were able to maintain his PTH level. Another patient from the HC group had resection of one parathyroid gland during first surgery due to carcinoma infiltration. This patient also developed a hypoparathyroidism (without steep decline) and oral calcium substitution was necessary. The time interval between the surgeries was two days.
One other patient underwent removal of one parathyroid gland due to simultaneous parathyroid adenoma during initial surgery. Although the second surgery was performed 52 days later, the patient presented a normal preoperative PTH level and revealed a temporary postoperative hypoparathyroidism with steep PTH decline. The remaining 3 parathyroid glands were preserved at the two surgeries.
There is no significant difference between hypocalcaemia rate (33% vs. 43%, p-value 0.381) comparing our cohort (n = 34) with patients from our own department undergoing primary total thyroidectomy (n = 353) due to thyroid cancer or goitre [12].
Logistic regression analysis (table 2) shows that hypocalcaemic patients (<2.10 mmol/l) before second surgery were at a significantly higher risk of postoperative hypocalcaemia (p <0.05). All other parameters (time interval between the two surgeries, PTH and calcium level before second surgery) could not be identified as significant risk factors for hypocalcaemia after second surgery.
Table 2 logistic-regression analysis indicating preoperative hypocalcaemia as a risk factor for postoperative hypocalcaemia after second surgery (p <0.05). Time-interval between surgeries and preoperative PTH level could not be identified as risk factors for postoperative hypocalcaemia after second surgery (p >0.05).
| Odds ratio | p-value | 95% confidence interval | |
|---|---|---|---|
| Operation interval | 0.935 | 0.205 | 0.842–1.038 |
| PTH pre-OP2 | 0.979 | 0.570 | 0.908–1.054 |
| Calcium pre-OP2 | 0.000 | 0.014 | 0.000–0.016 |
PTH pre-OP2 = parathyroid hormone preoperative second surgery; Calcium pre-OP2 = calcium preoperative second surgery Although there was a good correlation in the normocalcaemia cohort (group 2) in respect of PTH (r = 0.802, p = 0.001), this finding could unfortunately not be confirmed (r = 0.409, p = 0.240) in the hypocalcaemia cohort (group 1), as shown in figure 1. For the correlation between the pre- and postoperative calcium level, neither group showed any correlation (r = 0.048, p = 0.882 group 1, r = 0.236, p = 0.291 group 2).

Figure 1 Correlation between pre- and postoperative parathyroid hormone (PTH) showing strong correlation (r = 0.802, p = 0.001) only in normocalcaemic group (group 2, filled circles).
Thyroid surgeries belong to the most frequently performed surgeries by both ENT surgeons and general or endocrine surgeons in Switzerland (3022 surgeries in 2013 [14]) and worldwide. The most common indications for thyroidectomy are goitre (uni/bilateral) [15], Grave’s disease [16], toxic nodules [17] and differentiated thyroid cancer (DTC) [18]. Total thyroidectomy is the choice for treatment of DTC for carcinoma larger than 1cm (pT1b and higher classification) [18]. Although patients undergoing revision surgery are at higher risk for hypoparathyroidism [19], completion thyroidectomy is indicated even in small thyroid cancer with multifocality since this histological finding is a predictive factor for the presence of additional cancer on the contralateral side [20]. Thanks to improvement of operation techniques over recent decades such as implementation of intraoperative neuromonitoring with an excellent specificity and negative predictive value for early laryngeal nerve (RLN) palsy [21], thyroid surgery complications such as RLN palsy and major bleeding could be minimised [22]. The use of energised vessel sealing systems (Harmonic®, Ligasure™) reveal a reduction of operation time and blood loss but do not have any impact on postoperative hypocalcaemia or RLN palsy [23]. In our centre, the Harmonic® ultrasound scalpel has been routinely used in all thyroid surgeries since 2009. Minimally invasive video-assisted thyroidectomy (MIVAT) as an alternative approach may be beneficial regarding the location and size of the scar and causes less postoperative pain, but has no impact on postoperative hypocalcaemia [24]. Robotic thyroidectomy with transaxillary/axillo- breast approach is controversial and there are no benefits concerning postoperative hypocalcaemia and RLN palsy. The operation time is also significantly longer [25].
Despite many existing studies about risk factors and management of postoperative hypocalcaemia after total thyroidectomy, no standardised and universally accepted algorithm could be established so far. It should be mentioned that hypocalcaemia in clinical studies is not homogenously defined from clinical HC symptoms [3] up to the laboratory definition of ≤2.0 mmol/l [26], which makes a comparison between studies quite difficult. Recent studies on postoperative hypocalcaemia pinpoint multifactorial causes and only a combination of factors may roughly predict hypocalcaemia occurrence [27]. Temporary hypocalcaemia after total thyroidectomy still remains the most common complication [3, 27]. Well-established risk factors for postoperative HC after total thyroidectomy are thyroid cancer, nodal dissection level VI, female gender [3] and steep decline of PTH level [5].
Our results show a significant difference between the two groups with respect to postoperative PTH and calcium levels as well as postoperative calcium substitution (both oral and IV). This means that the cut-offs (PTH <15 pg/ml, calcium <2.10 mmol/l) based on the thresholds from our laboratory [13] are well chosen, allowing good comparison between both groups.
The overall hypocalcaemia rate was 33% (12/34) and the hypoparathyroidism rate 9% (3/34). No hypoparathyroidism occurred in the normocalcaemic group. A noticeable fact is that, in the HC group, one patient underwent a parathyroid autoimplantation and two patients had one parathyroid gland removed at initial surgery for different reasons (tumour infiltration, parathyroid adenoma). Both factors are predictors for transient hypocalcaemia [28, 29].
Although there is a significant correlation between pre- and postoperative PTH levels in the normocalcaemia group, this finding could not be confirmed in the hypocalcaemia group. Therefore, routine measurement of preoperative PTH level appears not to be helpful, especially considering the laboratory costs (approximately 30 Swiss francs in our hospital).
Investigating pre- and postoperative calcium levels, there were no correlations in either group. Our logistic regression analysis could identify preoperative existence of hypocalcaemia (calcium level <2.10 mmol/l) as a significant risk factor for postoperative hypocalcaemia after second surgery. No other risk factors (time interval between surgery, PTH level preoperative) could be identified. A surgeon-dependent outcome can be ruled out since all surgeries were performed by a single high-volume surgeon (senior author W.M.)
Gulcelik et al. [4] describe a comparable transient hypocalcaemia rate of 20.7% and a permanent hypocalcaemia rate of 4.4% after completion of thyroidectomy (n = 159). Erdem et al. [6] found no significant difference between permanent hypoparathyroidism after completion (n = 141) and primary total thyroidectomy (n = 92) with a rate of only 4.2% and 4.3% respectively. Vaiman et al. [30] found a permanent hypocalcaemia rate of 5.9% after completion of thyroidectomy (n = 2238) compared to 3.5% after primary total thyroidectomy. However, the authors did not mention why patients underwent staged surgeries after near-total thyroidectomy for multinodular goitre. It appears that their study did not involve completion of thyroidectomy for malignancy as is the case in our study. There is no significant difference between HC rate after primary total thyroidectomy (n = 353) in our own department (Bähler et al. 2016) [12] compared to completion of thyroidectomy (43% vs 33%). Edafe et al. [31] describe in their meta-analysis including 115 studies a transient hypocalcaemia rate after total thyroidectomy of 27% and a permanent hypocalcaemia rate of 1% (table 3).
Table 3 Comparison of studies investigating completion thyroidectomy (CT) and primary total thyroidectomy (TT) referred to transient and permanent hypocalcaemia-rates (trans. HC, perm. HC) as well as hypoparathyroidism (HPT). Note that there is no internationally accepted definition for trans. and perm. HC and also for HPT.
| Trans. HC n (%) | Perm. HC n (%) | HPT n (%) | ||||
|---|---|---|---|---|---|---|
| CT | TT | CT | TT | CT | TT | |
| Present study | 12 (33) | – | – | – | 3 (9.0) | – |
| Gulcelik et al. (2012) [4] | 33 (20.7) | 27 (10.1) | 7 (4.4) | 10 (4.6) | – | – |
| Erdem et al. (2003) [6] | – | – | – | – | 6 (4.2) | 4 (4.3) |
| Rafferty et al. (2007) [7] | – | – | – | – | 5 (2.5) | 5 (3.3) |
| Vaiman et al. (2010) [30] | 564 (25.2) | 920 (24.0) | 132 (5.9) | 134 (3.5) | – | – |
| Bähler et al. (2016) [12] | – | 120 (42.2) | – | – | – | |
| Edafe et al. (2014) [31] | – | (27.0) | – | (1.0) | – | – |
Completion of thyroidectomy seems to have the same risk profile as for primary total thyroidectomy concerning hypocalcaemia. We consequently apply the same postoperative algorithm for prevention of HC in patients with primary total as well as completion thyroidectomy. Oral calcium substitution (3 ×1000 mg/d) is indicated with serum calcium of <2.10 mmol/l one day after surgery or, in rare cases of low preoperative values (starting substitution preoperatively) and additional prescription of calcitriol (2 × 0.25 µg/d) in cases with serum PTH of <6 pg/ml, serum calcium levels are measured on the first postoperative day. Oral calcium substitution is also indicated in the presence of clinical hypocalcaemia signs such as paraesthesia in the fingers and mouth despite normocalcaemia in blood samples. Intravenous calcium substitution is only given in severe hypocalcaemia signs such as tetany or in patients with inadequate elevation of serum calcium values after oral substitution. Routine preoperative calcium measurements are helpful, whereas preoperative PTH values do not add valuable information in completion thyroidectomy.
Alhefdhi et al. recommend in their meta-analysis routine calcium supplementation after total thyroidectomy for 1–2 weeks with addition of vitamin D for high-risk patients showing a significant decrease in postoperative hypocalcaemia [32]. Since routine calcium and calcitriol supplementation and the role of vitamin D on postoperative hypocalcaemia after primary total thyroidectomy are controversial [33–36], no such recommendations for completion thyroidectomy can be given at the present time.
The exact prediction of hypocalcaemia still remains difficult since it has multifactorial causes [27]. To the best of our knowledge, the present study is the first investigating not only perioperative calcium levels but also PTH level courses after completion of thyroidectomy. Considering the fact that all surgeries were performed by a single high-volume surgeon, our findings after completion of thyroidectomy show a similar hypocalcaemia and hypoparathyroidism rate of 33 and 9%, respectively, as described in the literature, and comparable hypocalcaemia rates after primary total thyroidectomy. Our study may be limited by the sample size, which consequently has an impact on its statistical significance. Nevertheless, patients undergoing completion of hemithyroidectomy are in a relatively special constellation with rather surprising histological findings after first surgery. This is also why only 34 patients could be identified between 2002 and 2013 in our tertiary care centre that performs over 300 thyroid surgeries per year. Considering these limitations, further multicentre (and therefore multi-surgeon) studies with higher sample sizes need to be done to investigate hypocalcaemia after completion of thyroidectomy. It is self-evident that a prospective study is not feasible when investigating this group of patients.
Our findings after completion of thyroidectomy show a similar hypocalcaemia and hypoparathyroidism rate as described in the literature, and comparable hypocalcaemia rates after primary total thyroidectomy. Thus completion of thyroidectomy is a safe procedure for the management of differentiated thyroid cancer. The identification of patients at risk remains difficult, since only a preoperative low calcium level could be identified as a specific risk factor for postoperative hypocalcaemia. Prophylactic and therapeutic management may be quite similar to the recommendations in primary total thyroidectomies. Still, further studies are needed to investigate hypocalcaemia and its risk factors after completion of thyroidectomy.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Agate L , Lorusso L , Elisei R . New and old knowledge on differentiated thyroid cancer epidemiology and risk factors. J Endocrinol Invest. 2012;35(6, Suppl):3–9.
2krebsliga.ch. [internet]. Bern: Schweizer Krebsliga (swiss cancer league) [updated 2015 October 9]. Available from: https://www.krebsliga.ch
3 Puzziello A , Rosato L , Innaro N , Orlando G , Avenia N , Perigli G , et al. Hypocalcemia following thyroid surgery: incidence and risk factors. A longitudinal multicenter study comprising 2,631 patients. Endocrine. 2014;47(2):537–42; epub ahead of print. doi:.https://doi.org/10.1007/s12020-014-0209-y
4 Gulcelik MA , Kuru B , Dincer H , Camlibel M , Yuksel UM , Yenidogan E , et al. Complications of completion versus total thyroidectomy. Asian Pac J Cancer Prev. 2012;13(10):5225–8. doi:.https://doi.org/10.7314/APJCP.2012.13.10.5225
5 Alia P , Moreno P , Rigo R , Francos JM , Navarro MA . Postresection Parathyroid Hormone and Parathyroid Hormone Decline Accurately Predict Hypocalcemia After Thyroidectomy. Am J Clin Pathol. 2007;127(4):592–7. doi:.https://doi.org/10.1309/J357LMD66E9X2505
6 Erdem E , Gülçelik MA , Kuru B , Alagöl H . Comparison of completion thyroidectomy and primary surgery for differentiated thyroid carcinoma. Eur J Surg Oncol. 2003;29(9):747–9. doi:.https://doi.org/10.1016/j.ejso.2003.08.006
7 Rafferty MA , Goldstein DP , Rotstein L , Asa SL , Panzarella T , Gullane P , et al. Completion thyroidectomy versus total thyroidectomy: is there a difference in complication rates? An analysis of 350 patients. J Am Coll Surg. 2007;205(4):602–7. doi:.https://doi.org/10.1016/j.jamcollsurg.2007.05.030
8Swiss society of otolaryngology, head- and neck-surgery. Guidelines for management and therapy of head- and neck-cancer. 2011. pp 62–6
9 Tartaglia F , Giuliani A , Sgueglia M , Biancari F , Juvonen T , Campana FP . Randomized study on oral administration of calcitriol to prevent symptomatic hypocalcemia after total thyroidectomy. Am J Surg. 2005;190(3):424–9. doi:.https://doi.org/10.1016/j.amjsurg.2005.04.017
10 Joosen DA , van de Laar RJ , Koopmans RP , Stassen PM . Acute dyspnea caused by hypocalcemia-related laryngospasm. J Emerg Med. 2015;48(1):29–30. doi:.https://doi.org/10.1016/j.jemermed.2014.09.034
11 Chow TL , Choi CY , Chiu AN . Postoperative PTH monitoring of hypocalcemia expedites discharge after thyroidectomy. Am J Otolaryngol. 2014;35(6):736–40. doi:.https://doi.org/10.1016/j.amjoto.2014.07.006
12 Bähler S , Müller W , Linder T , Frotzler A , Aqtashi B , Elmas F , et al. [Hypocalcemia after total thyroidectomy- A analysis of riskfactors]. Hypocalcämie nach totaler Thyroidektomie - Eine Analyse von Risikofaktoren. Submitted 2016 August. German
13Tietz N. Clinical guide to laboratory tests. 4th ed. Wu AHB, editor. St. Louis (MO): Sanders Elsevier; 2006. 202–7
14Swiss Federal Statistical Office (SFSO). Neuchâtel (Switzerland). 2015 March 17. https://www.bfs.admin.ch/bfs/en/home.html
15 Agarwal G , Aggarwal V . Is total thyroidectomy the surgical procedure of choice for benign multinodular goiter? An evidence-based review. World J Surg. 2008;32(7):1313–24. doi:.https://doi.org/10.1007/s00268-008-9579-8
16 Feroci F , Rettori M , Borrelli A , Coppola A , Castagnoli A , Perigli G , et al. A systematic review and meta-analysis of total thyroidectomy versus bilateral subtotal thyroidectomy for Graves’ disease. Surgery. 2014;155(3):529–40. doi:.https://doi.org/10.1016/j.surg.2013.10.017
17 Yano Y , Sugino K , Akaishi J , Uruno T , Okuwa K , Shibuya H , et al. Treatment of autonomously functioning thyroid nodules at a single institution: radioiodine therapy, surgery, and ethanol injection therapy. Ann Nucl Med. 2011;25(10):749–54. doi:.https://doi.org/10.1007/s12149-011-0526-7
18 Liao S , Shindo M . Management of well-differentiated thyroid cancer. Otolaryngol Clin North Am. 2012;45(5):1163–79. doi:.https://doi.org/10.1016/j.otc.2012.06.015
19 Erbil Y , Barbaros U , Işsever H , Borucu I , Salmaslioğlu A , Mete O , et al. Predictive factors for recurrent laryngeal nerve palsy and hypoparathyroidism after thyroid surgery. Clin Otolaryngol. 2007;32(1):32–7. doi:.https://doi.org/10.1111/j.1365-2273.2007.01383.x
20 Kim ES , Kim TY , Koh JM , Kim YI , Hong SJ , Kim WB , et al. Completion thyroidectomy in patients with thyroid cancer who initially underwent unilateral operation. Clin Endocrinol (Oxf). 2004;61(1):145–8. doi:.https://doi.org/10.1111/j.1365-2265.2004.02065.x
21 Dequanter D , Charara F , Shahla M , Lothaire P . Usefulness of neuromonitoring in thyroid surgery. Eur Arch Otorhinolaryngol. 2015;272(10):3039–43. doi:.https://doi.org/10.1007/s00405-014-3293-y
22 Contin P , Gooßen K , Grummich K , Jensen K , Schmitz-Winnenthal H , Büchler MW , et al. ENERgized vessel sealing systems versus CONventional hemostasis techniques in thyroid surgery--the ENERCON systematic review and network meta-analysis. Langenbecks Arch Surg. 2013;398(8):1039–56. doi:.https://doi.org/10.1007/s00423-013-1137-7
23 Zanghì A , Cavallaro A , Di Vita M , Cardì F , Di Mattia P , Piccolo G , et al. The safety of the Harmonic® FOCUS in open thyroidectomy: a prospective, randomized study comparing the Harmonic® FOCUS and traditional suture ligation (knot and tie) technique. Int J Surg. 2014;12(Suppl 1):S132–5. doi:.https://doi.org/10.1016/j.ijsu.2014.05.028
24 Pisanu A , Podda M , Reccia I , Porceddu G , Uccheddu A . Systematic review with meta-analysis of prospective randomized trials comparing minimally invasive video-assisted thyroidectomy (MIVAT) and conventional thyroidectomy (CT). Langenbecks Arch Surg. 2013;398(8):1057–68. doi:.https://doi.org/10.1007/s00423-013-1125-y
25 Sun GH , Peress L , Pynnonen MA . Systematic review and meta-analysis of robotic vs conventional thyroidectomy approaches for thyroid disease. Otolaryngol Head Neck Surg. 2014;150(4):520–32. doi:.https://doi.org/10.1177/0194599814521779
26 Noureldine SI , Genther DJ , Lopez M , Agrawal N , Tufano RP . Early predictors of hypocalcemia after total thyroidectomy: an analysis of 304 patients using a short-stay monitoring protocol. JAMA Otolaryngol Head Neck Surg. 2014;140(11):1006–13. doi:.https://doi.org/10.1001/jamaoto.2014.2435
27 Pradeep PV , Ramalingam K , Jayashree B . Post total thyroidectomy hypocalcemia: a novel multi-factorial scoring system to enable its predictions to facilitate an early discharge. J Postgrad Med. 2013;59(1):4–8.
28 Lo CY . Parathyroid autotransplantation during thyroidectomy. ANZ J Surg. 2002;72(12):902–7. doi:.https://doi.org/10.1046/j.1445-2197.2002.02580.x
29 Song CM , Jung JH , Ji YB , Min HJ , Ahn YH , Tae K . Relationship between hypoparathyroidism and the number of parathyroid glands preserved during thyroidectomy. World J Surg Oncol. 2014;12(1):200. doi:.https://doi.org/10.1186/1477-7819-12-200
30 Vaiman M , Nagibin A , Olevson J . Complications in primary and completed thyroidectomy. Surg Today. 2010;40(2):114–8. doi:.https://doi.org/10.1007/s00595-008-4027-9
31 Edafe O , Antakia R , Laskar N , Uttley L , Balasubramanian SP . Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg. 2014;101(4):307–20. doi:.https://doi.org/10.1002/bjs.9384
32 Alhefdhi A , Mazeh H , Chen H . Role of postoperative vitamin D and/or calcium routine supplementation in preventing hypocalcemia after thyroidectomy: a systematic review and meta-analysis. Oncologist. 2013;18(5):533–42. doi:.https://doi.org/10.1634/theoncologist.2012-0283
33 Lin Y , Ross HL , Raeburn CD , DeWitt PE , Albuja-Cruz M , Jones EL , et al. Vitamin D deficiency does not increase the rate of postoperative hypocalcemia after thyroidectomy. Am J Surg. 2012;204(6):888–93, discussion 893–4. doi:.https://doi.org/10.1016/j.amjsurg.2012.10.001
34 Wang TS , Cheung K , Roman SA , Sosa JA . To supplement or not to supplement: a cost-utility analysis of calcium and vitamin D repletion in patients after thyroidectomy. Ann Surg Oncol. 2011;18(5):1293–9. doi:.https://doi.org/10.1245/s10434-010-1437-x
35 Huang SM . Do we overtreat post-thyroidectomy hypocalcemia? World J Surg. 2012;36(7):1503–8. doi:.https://doi.org/10.1007/s00268-012-1580-6
36 Griffin TP , Murphy MS , Sheahan P . Vitamin D and risk of postoperative hypocalcemia after total thyroidectomy. JAMA Otolaryngol Head Neck Surg. 2014;140(4):346–51. doi:.https://doi.org/10.1001/jamaoto.2014.25
No financial support and no other potential conflict of interest relevant to this article was reported.