High participation rate among 25 721 patients with broad age range in a hospital-based research project involving whole-genome sequencing – the Lausanne Institutional Biobank
DOI: https://doi.org/10.4414/smw.2017.14528
Murielle
Bochuda, Christine
Curratb, Laurence
Chapatteb, Cindy
Rothc, Vincent
Moosercd
aInstitute for Social and Preventive Medicine, Centre Hospitalier Universitaire Vaudois and University of Lausanne, Switzerland
bSwiss Biobanking Platform, Lausanne, Switzerland
cValuation of Clinical Data and Biological Samples Unit, Centre Hospitalier Universitaire Vaudois and University of Lausanne, Switzerland
dService of Clinical Chemistry, Centre Hospitalier Universitaire Vaudois, Lausanne Switzerland
Summary
AIMS
We aimed to evaluate the interest of adult inpatients and selected outpatients in engaging in a large, real-life, hospital-based, genomic medicine research project and in receiving clinically actionable incidental findings.
METHODS
Within the framework of the cross-sectional Institutional Biobank of Lausanne, Switzerland, a total of 25 721 patients of the CHUV University Hospital were systematically invited to grant researchers access to their biomedical data and to donate blood for future analyses, including whole-genome sequencing. Multivariable logistic regression analysis was used to identify personal factors, including age, gender, religion, ethnicity, citizenship, education level and mode of admission, associated with willingness to participate in this genomic research project and with interest in receiving clinically actionable incidental findings.
RESULTS
The overall participation rate was 79% (20 343/25 721). Participation rate declined progressively with age, averaging 83%, 75%, 67% and 62% in patients aged <64 years (n = 13 108), ≥64 years (n = 12 613), ≥80 years (n = 4557) and ≥90 years (n = 1050), respectively. Factors associated with participation substantially differed between age strata. Patients less likely to participate included women (odds ratio 0.86, [95% confidence interval 0.79–0.95] and 0.78 [0.71–0.85] before and after age 64, respectively), non-Swiss (0.81 [0.74–0.90] and 0.58 [0.52–0.65]) and those admitted through the emergency ward (0.88 [0.79–0.98] and 0.66 [0.60–0.73]). Religion and marital status were associated with participation among patients aged <64 years. A total of 19 018 (93%) participants were willing to be re-contacted for incidental findings. A high education level was associated with higher participation rate, but not with higher willingness to receive incidental findings within the population who had agreed to participate.
CONCLUSION
A large proportion of adult patients, even among the elderly, are willing to actively participate and receive incidental findings in this systematic hospital-based precision and genomic medicine research program with broad consent.
Introduction
Breakthroughs in genomics, in other technologies designed to characterise and quantify pools of biological molecules such as proteomics or metabolomics (collectively referred to as *omic sciences) and in IT technologies open up unprecedented opportunities to tailor health maintenance and the diagnosis and treatment of diseases according to the particular biological profile of an individual, i.e., personalised or precision medicine [1]. A particular aspect of precision medicine pertains to the use of individual genomic information to optimise disease prevention and treatment (genomic medicine). The success of genomic medicine will largely depend, initially, on the ability to link high-quality clinical data with genomic data from large number of individuals. To that end, population-based cohorts have already been assembled, including the UK Biobank [2] or the Estonian Biobank [3], or are in the process of being assembled, such as the Precision Medicine Initiative in the USA [4, 5]. In parallel, major efforts are being devoted to linking genomic data with medical data retrieved from electronic medical records [6]. Returning information on variants involved in drug metabolism or toxicity (pharmacogenetics [7]), or on highly penetrant, clinically actionable variants discovered incidentally may turn into immediate benefits of genomic medicine. The American College of Medical Genetics has made recommendations for returning information pertaining to such incidental findings [8]. Such variants predispose mostly to potentially deadly cardiovascular diseases (including prolonged QT, cardiomyopathies, vascular abnormalities such as Marfan syndrome or premature atherosclerosis such as familial hypercholesterolaemia) or familial forms of cancer, including breast, kidney or endocrine neoplasms. Recent analysis from more than 50 000 sequenced exomes shows that, in a largely Caucasian population, approximately 3.5% of individuals are carriers of such variants and could benefit from genomic medicine [9]. Finally, for genomic medicine to fully deliver on its promises will require the demonstration of the clinical utility of the newly generated knowledge, through appropriate trials; one opportunity is to re-contact cohort participants and enrol them into genetically enriched intervention clinical trials [10] or observation studies for detailed phenotyping [11]. Hospitals with a cohort linked to a biobank and a dedicated clinical research centre represent a unique opportunity to address these needs.
Surveys in various countries indicate a relatively high interest in genetics from the population [12–19] and from patients suffering from selected diseases [20, 21]. In addition, patients engaged in genetic research are generally interested in signing a broad general consent [16] and in being informed about incidental findings [22–26]. A limited number of studies point to certain interethnic differences in engaging with genetic research [27]. However, the size of most studies is limited, ranging from a dozen to a few hundred participants, the majority of these studies are based on questionnaires or surveys, rather than real engagement into genetic research, and only a minority of studies relate to hospitalised patients. Accordingly, even though a hospital represents a privileged place to enrol participants in genomic research, to what extent hospitalised patients actually sign a broad consent including the future possibility for researchers to access their medical records and perform whole genome sequencing, and are interested in being re-contacted for incidental findings, remains poorly documented.
The CHUV University Hospital Institutional Biobank (BIL) in Lausanne, Switzerland [28] is a highly versatile hospital-based cross-sectional initiative launched in 2013. Briefly, adult patients admitted to selected wards at CHUV are systematically contacted individually and informed about research and the BIL project. Patients are invited to sign a broad consent, granting researchers access to their biological samples (leftover and additional material being collected if no risks and constraints are generated) and to their electronic medical records, including the possibility to address future Institutional Review Board-approved questions which were not necessarily anticipated at the time consent is obtained, without the need for re-consent. By signing this consent, patients agree to donate a 9-ml blood sample for DNA and plasma isolation, for extensive analyses, including whole genome sequencing and other *omic analyses. Patients who agree to participate in this project are offered the option to be re-contacted in the future, if findings requiring clinical intervention were incidentally made during these analyses. The project was approved by the local Ethics Committee (approval number 144/12), and is fully aligned with the Swiss Human Research Act and its Ordinances, which were enacted on 1 January 2014. A recent analysis of the prevalence of mutations causing familial hypercholesterolaemia among BIL participants with elevated plasma levels of low density lipoprotein (LDL)-cholesterol [29] provided a proof of concept that all the steps ranging from data and sample collection to genetic analyses are in place for future genomic medicine studies.
In the present study, we examined the participation rate and the personal factors associated with participation and future re-contact for incidental findings among patients who were contacted during the first 3 years of activity of the BIL.
Material and methods
Study population
The CHUV is a 1463-bed university hospital that serves as a primary and secondary care centre for the Lausanne area, and as tertiary care centre for a population of approximately 1 million people in western Switzerland. Since January 2013, patients hospitalised in selected wards at CHUV and outpatients from a limited number of clinics are systematically contacted and informed about the BIL project and the general consent, without any prespecified disease areas. Patients are interviewed about the project by a dedicated team of research assistants who have been specifically trained for this task. These face-to-face interviews are conducted at the bedside or during an outpatient visit. Patients are admitted through the emergency department, or have a planned, elective admission. In the latter situation, patients requiring surgery usually attend a prehospitalisation outpatient visit, during which the project is presented and they are invited to sign the consent. Patients with elective admission who do not require such a visit are contacted either via a specific mailing (since July 2014) or during the hospitalisation. Patients admitted through the emergency department are contacted once their medical situation has stabilised and after they have been transferred from the emergency room or intensive care unit into a regular ward. Patients are given the necessary time to consider and discuss their participation in the project and have the possibility to revoke their consent at any time. Between 7 January 2013 and 31 December 2015, 29 174 persons were individually contacted and informed about the BIL project. After exclusion of 1072 patients who had pending consent status at the time of database freeze, 1873 women from the maternity ward (who were not considered patients as they did not have any medical or surgical disease conditions), and 508 patients for whom the dataset was not complete, 25 721 patients were included in the present analyses.
Data collection
The consent and status of biospecimen collection, as well as a minimal data set, were registered in a dedicated secured web-based database (Labvantage Biobanking Solution, Somerset NJ). The minimal data set included the basic demographics (age, sex, origin, ethnicity, marital status, religion and, since 2014, education level), the way the patient had been admitted (through emergency, outpatient or elective visit) and the department where the project was presented and the patient hospitalised. Patients had the possibility to participate in the project by signing the broad consent and have their genetic data and samples used in a coded/pseudo-anonymised way. Pseudo-anonymisation was performed in accordance with HIPAA act. In that way, investigators cannot have access to patients’ personal data, and all sensitive information is removed in order to protect patients’ identities. It is possible to come back to the patient if necessary, in the case of research results disclosure for example, by using a specific key that is held by duly authorised people within the hospital. Patients who agreed to participate in the project with their genetic data and samples coded were further asked if they were interested in being re-contacted in case clinically actionable findings were to be discovered in future analyses done on their biological samples.
Statistical analyses
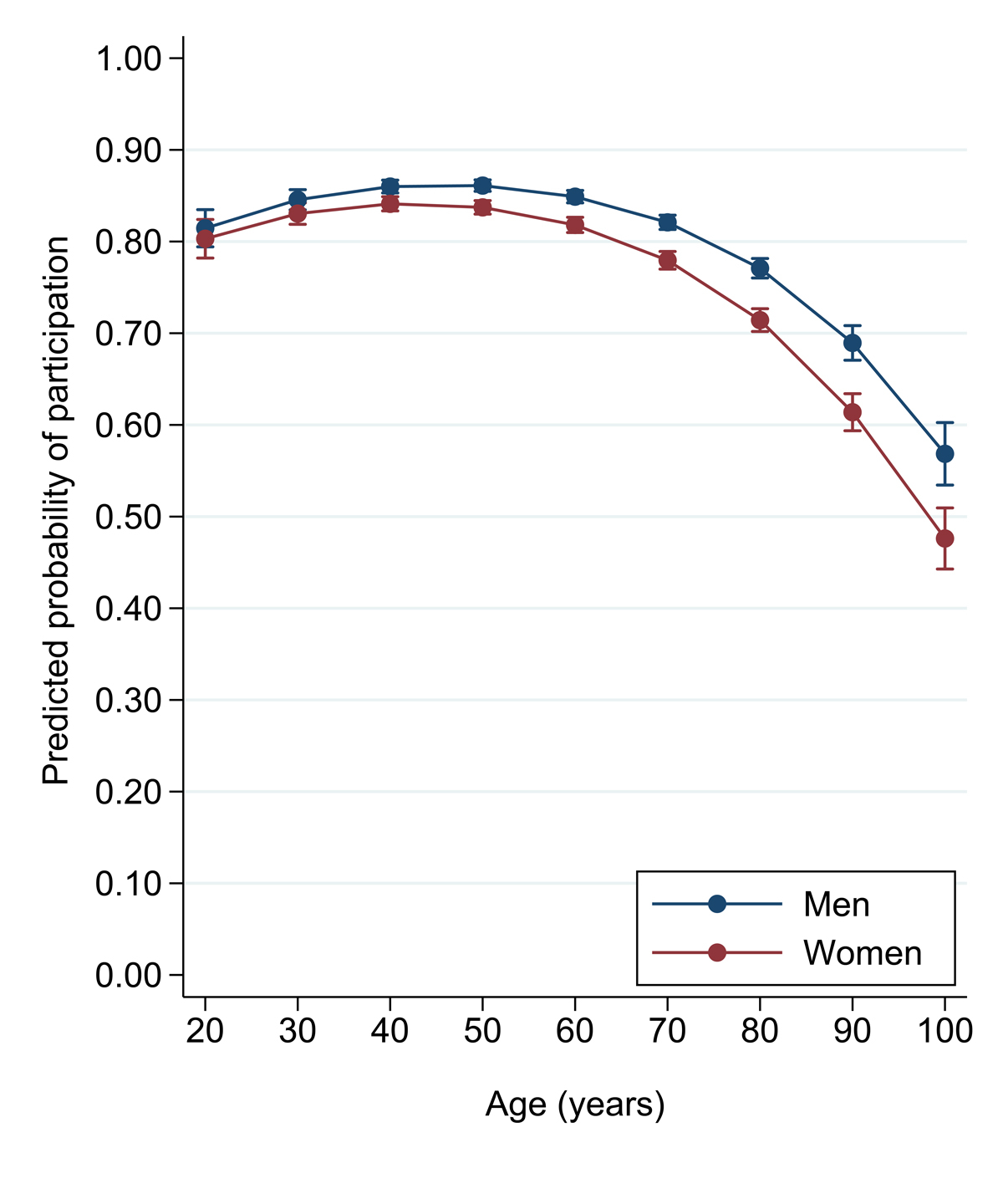
Data were extracted from the database with Access version 2007 and analysed with Stata 14·0 (Stata Corp., College Station TX). Categorical variables were expressed as number of people and percentages. Continuous variables were expressed as mean ± standard deviation (SD). Multiple logistic regression analyses were used to explore individual characteristics associated with participation in the BIL and, among participants, with interest in being re-contacted for incidental findings. We originally run analyses on 25 721 patients without using education as a covariate and, subsequently, on 17 518 patients for whom education level was available (these data were collected from 2014) and was used as a covariate. The predicted probability of participation in the BIL by age and sex (men coded as 0 and women as 1) was generated from a multiple logistic regression model including admission mode (elective [reference], outpatient or emergency), citizenship (Swiss coded as 0 and non-Swiss as 1), marital status (married [reference], single or other), religion (Catholic [reference], Protestant or other), and education (low [reference], middle, high or unknown) for selected analyses, as covariates coded as dummy variables. We used the “margin” function in Stata, which generates the predicted probability at specific age levels (here we arbitrarily chose each decade of age), separately for men and women, while setting each confounder to its mean value. Because characteristics associated with willingness to participate or to receive incidental findings differed by age groups (as assessed by formal statistical interaction testing), we conducted stratified analyses using 64 years of age as a cut-off, as it was close to the median age (i.e., 63 years) and happened to closely match the retirement age in Switzerland (i.e., 65 years). We conducted interaction testing by adding a multiplication term between the categorical covariate of interest (e.g., religion, nationality, marital status, sex, etc.) and dichotomised age (coded as 0 below 64 years and 1 otherwise). We then used a likelihood ratio test to assess the overall statistical significance of the interaction by comparing a model with and a model without the interaction terms.
Results
Study population
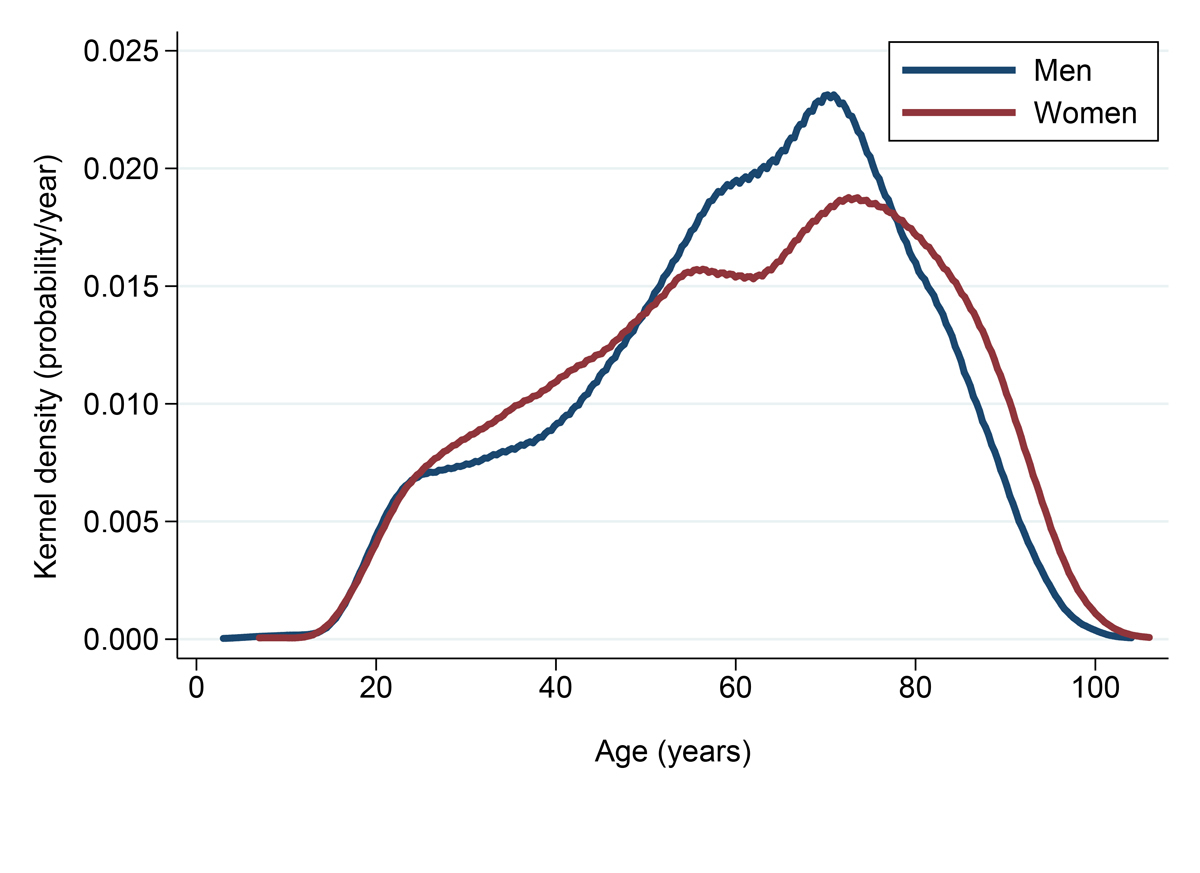
The majority of the 25 721 patients invited to participate in the BIL project during its 3 initial years of recruitment were men (13 912, 54%), Swiss citizens (18 531, 72%) and of European descent (21 333, 83%) (table1). Approximately one third of these patients were reported to be Catholic (9466, 37%) and one third Protestant (8693, 34%), whereas the remaining patients (7562, 29%) reported another or no religion. Age averaged 60.7 ± 19.0 (SD) years, with 4557 patients ≥80 years old and 1050 ≥90 years old (supplementary fig. S1 in appendix 1). More than one third of participants had been admitted through the emergency department (9635, 38%), whereas another third consisted of inpatients admitted for planned, elective admission with consent mailed and/or presented during the hospitalisation (8090, 31%). The remaining third (7996, 31%) were patients to whom the project was presented during an outpatient visit. The distribution according to the ward these patients were met, as well as their willingness to participate or receive incidental findings, are presented in supplementary table S1 (appendix 1). Education level was available for 17 518 patients, with 3197 (18%), 6180 (35%), 3216 (18%) and 4925 (28%) patients with low (no validated education or mandatory school certificate), middle (apprenticeship, secondary education, college), high (university degree or equivalent) and unknown level, respectively. Participants’ characteristics across four selected age groups are presented in table S2.
Table 1 Patient characteristics, overall and by gender.
|
|
All
|
Men
|
Women
|
| Number |
|
25 721 |
|
13 912 |
|
11 809 |
|
| Age (in years), mean (SD of mean) |
|
60.7 |
(19) |
60.4 |
(18) |
61.1 |
(20) |
| Citizenship, n (%) |
Swiss |
18 531 |
(72) |
9759 |
(70) |
8772 |
(74) |
| Non-Swiss |
7190 |
(28) |
4153 |
(30) |
3037 |
(26) |
| Origin, n (%) |
European |
21 333 |
(83) |
11 768 |
(85) |
9565 |
(81) |
| Other |
1422 |
(5) |
727 |
(5) |
695 |
(6) |
| Unknown |
2966 |
(12) |
1417 |
(10) |
1549 |
(13) |
| Civil status, n (%) |
Married |
12 909 |
(50) |
7798 |
(56) |
5111 |
(43) |
| Single |
5463 |
(21) |
3161 |
(23) |
2302 |
(19) |
| Other* |
7349 |
(29) |
2953 |
(21) |
4396 |
(37) |
| Religion, n (%) |
Catholic |
9466 |
(37) |
5049 |
(36) |
4417 |
(37) |
| Protestant |
8693 |
(34) |
4609 |
(33) |
4084 |
(35) |
| Other |
7562 |
(29) |
4254 |
(31) |
3308 |
(28) |
| Admission mode, n (%) |
Elective |
8090 |
(31) |
4667 |
(34) |
3423 |
(29) |
| Emergency |
9635 |
(38) |
5073 |
(36) |
4562 |
(39) |
| Outpatients |
7996 |
(31) |
4172 |
(30) |
3824 |
(32) |
| Participation in BIL |
Yes |
20 343 |
(79) |
11 241 |
(81) |
9102 |
(77) |
| No |
5378 |
(21) |
2671 |
(19) |
2707 |
(23) |
|
Among participants
|
|
|
|
|
|
|
|
| Interest in incidental findings, n (%) |
Yes |
19 018 |
(93) |
10 535 |
(94) |
8483 |
(93) |
| No |
1325 |
(7) |
706 |
(6) |
619 |
(7) |
Participation in the BIL genomic and precision medicine research project
Out of the 25 721 patients included in the analysis, 20 343 (79%) agreed to sign the broad consent form, with the possibility for researchers to link their clinical data (including electronic medical records) with future coded genomic data (table 1). This group constitutes the “participants” group. A total of 3941 patients refused to participate in the project, 30 revoked their consent, and 1407 patients agreed to participate, but with the request that their data and samples be anonymised, thus precluding future genetic association studies. Because ability to link medical data to genomic data, and to re-contact participants is critical for the type of genomic medicine studies envisioned here, these latter three groups were merged into the “nonparticipants” group (5378, 21%).
Whereas the probability of participation was very similar, across age groups up to 60 years of age, it declined in the older age groups, both in men and in women (fig. 1). Willingness to participate averaged 83% among the 13 108 patients aged <64 years, 75% among those aged >64 years (n = 12 613), 67% in patients aged ≥80 years (n = 4557) and 62% in patients aged ≥90 years (n = 1050). The proportion of patients who agreed to participate in the project was slightly lower among the 11 809 women than among the 13 912 men (77% vs 81%, p <0.001) (table 1).
Personal factors associated with willingness to participate differed between age strata, with significant interactions (p values <0.05) of age strata with sex, religion, citizenship, marital status and admission mode, taken one at a time (fig. 2). Among patients aged <64 years, women (odds ratio [OR] 0.86, 95% confidence interval [CI] 0.79–0.95), individuals reporting a religion other than Catholic or Protestant (OR 0.67, 95% CI 0.6–-0.74) as well as non-Swiss citizens (OR 0.81, 95% CI 0.74–0.90) were less likely to participate, whereas those with a civil status other than married or single (OR 1.21, 95% CI 1.06–1.37) were more likely to participate. The personal factor most strongly associated with participation was admission mode: people with emergency admission were less likely to participate (OR 0.88, 95% CI 0.79–0.98) and those contacted during an outpatient visit (OR 1.82, 95% CI 1.61–2.05) were more likely to participate than those with elective hospitalisation contacted within the hospital setting or via specific mailing (inpatients). Among patients aged 64 years or older, women (OR 0.78, 95% CI 0.71–0.85), non-Swiss citizen (OR 0.58, 95% CI 0.52–0.65) and those with emergency admission (OR 0.66, 95% CI 0.60–0.73) were less willing to participate. As was observed in the younger age strata, the factor most strongly associated with participation was admission mode. Sensitivity analyses conducted in the subset of 17 518 patients with information on education level led to results similar to those for the entire dataset (supplementary fig. S2 in appendix 1). We observed a greater willingness to participate among people of middle educational level (age <64 years: OR 1.49, 95% CI 1.26–1.76; age ≥64 years: OR 1.64, 95% CI 1.39–1.93 among) or high education level (age <64 years: OR 1.42, 95% CI 1.17–1.71; age ≥64 years: OR 2.17, 95% CI 1.77–2.66) as compared with those of a low or unknown education level.
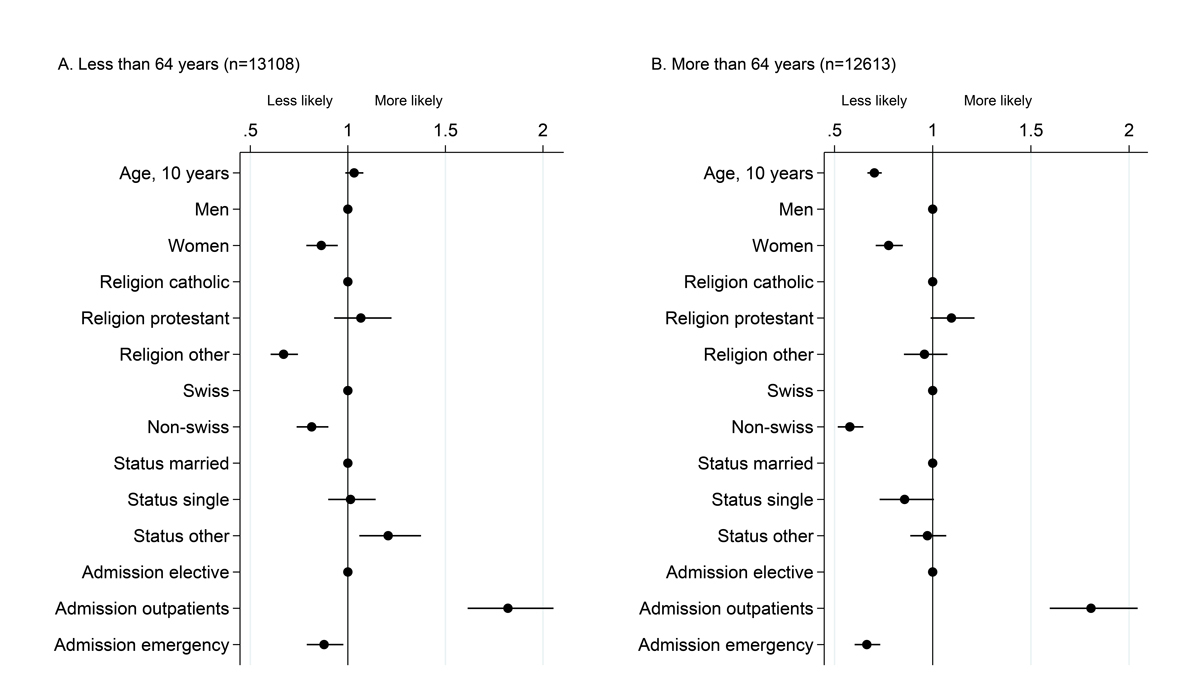
Figure 2
Factors associated with willingness to participate among 25 721 patients. Dots represent odds ratios and bars 95% confidence intervals from a multivariable logistic model including willingness to participate as the dependent variable, run separately in the two age strata. The age cut off at 64 years was chosen as it is close to the median age and happens to closely match the retirement age in Switzerland. Patients aged <64 years are depicted in Panel A, and those aged 64 or older are shown in Panel B.
Interest in being re-contacted for incidental findings
Out of 20 343 participants to the BIL project, 19 018 (93%) individuals were willing to be re-contacted for incidental findings (table 1). We observed significant interactions (p-values <0·05) of age strata with admission mode, sex, marital status and religion, taken one at a time (fig. 3). In both groups of participants (aged <64 years or >64 years), older age and admission through the emergency ward was associated with less interest in receiving this information, whereas, admission through the outpatient clinic was associated with a higher interest. Religion other than Catholic or Protestant was associated with less interest in the younger age group, whereas among the older age group being single was associated with less interest. Sensitivity analyses on the subset of patients with available information on education level led to similar results (supplementary fig. S3 in appendix 1). In contrast to willingness to participate, education level was not a strong predictor of interest in receiving incidental findings.
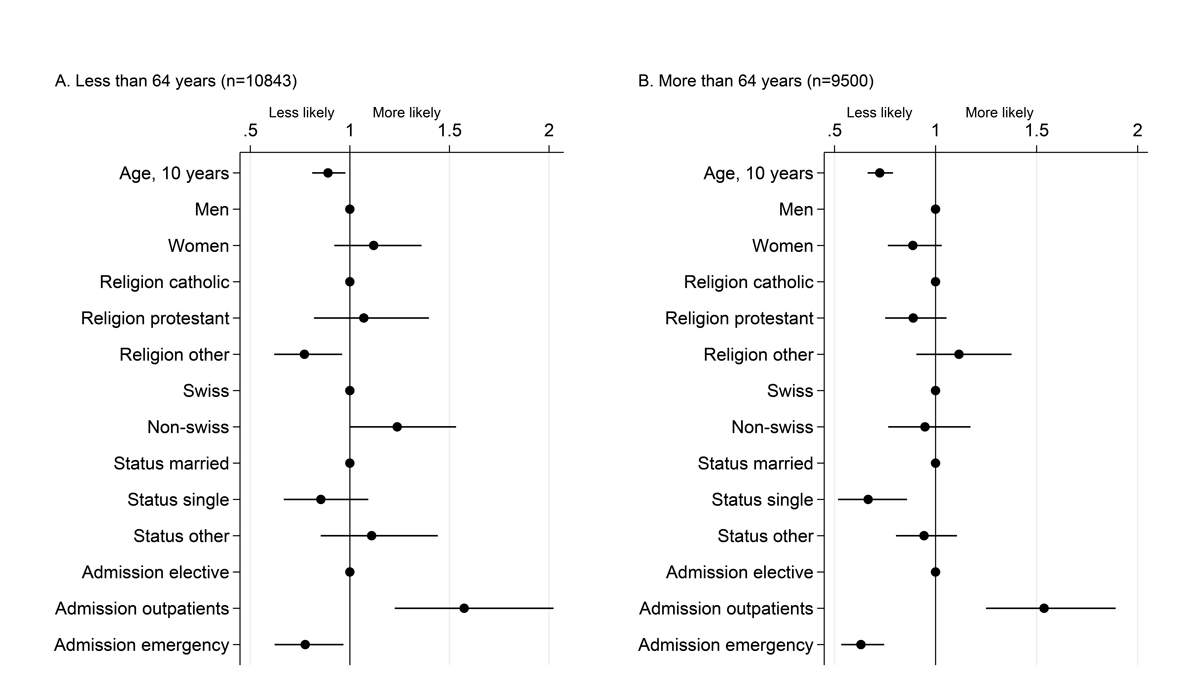
Figure 3
Factors associated with willingness to receive incidental findings among the 20 343 participants. Dots represent odds ratios and bars 95% confidence intervals from a multivariable logistic model including willingness to receive incidental findings, among patients having accepted to participate, as the dependent variable, run separately in the two age strata.
Discussion
Described here is, to the best of our knowledge, the largest analysis performed to evaluate the factors associated with participation in a real-life genomic research projects among hospitalised patients. We found a high participation rate in this particular systematic hospital-based genomic medicine research project, with a very large proportion of these participants interested in being re-contacted for potential clinically actionable incidental findings. Although willingness to participate declined from 60 years of age onwards, it still remained remarkably high even among the 1050 patients aged 90 years and over. Willingness to participate was associated with different patient characteristics depending on age.
The overall participation rate (79%) was higher than that reported in various population surveys [12–19]. A similarly high participation rate was observed across wards with very different source populations, such as orthopaedics, plastic surgery, cardiology and oncology. Several factors may account for this high participation rate. Building trust and educating the general population about research are important to encourage participation in research projects. In the present BIL project, each patient was contacted individually in a face-to-face interview with a research assistant dedicated to this task and specifically trained to inform patients, respond to their questions and collect their consent. The BIL project has also been highly visible, with broad media coverage and strong interest from the population.
A notable strength of this project was the inclusion of a sizeable number of patients aged ≥80 years (n = 4557) or ≥90 years (n = 1050). Older adults are the fastest growing part of the population in high and middle income countries, and the very old represent an increasing proportion of patients needing care, yet data are scarce in the medical literature. In Switzerland, life expectancy is high both at birth (80.8 years for men and 84.9 years for women in 2015, www.bfs.admin.ch) and at 80 years of age (8.5 years for men and 10.1 years for women). One may question the interest of using genomic medicine in very old people, but these people are frequently prescribed multiple drugs and usually have reduced renal function. Clear applications can be envisioned, such as maximising drug response and minimising adverse drug reactions on the basis of genomic background [7, 30], even though clinical utility for most of such applications has not yet been fully established.
Unsurprisingly, the willingness to provide a broad consent for research and the interest in receiving incidental findings was lower in people older than 80 years than in younger age group. The reduced participation rate in the elderly may be due to a limited interest in taking part in research, a higher prevalence of cognitive decline, a larger proportion of patients with multi-morbidity and hence more stressful and precarious living conditions [31].
A concern was that the participation rate in the BIL was inflated by the situation of the patients, who are hospitalised and thus in a position of weakness and dependence. The fact that patients with planned admission who were recruited either via specific mailing or during their pre-hospitalisation visit had a similarly high participation rate is reassuring. Patients who received specific mailing prior to their admission were found to be better informed and ready for a deeper discussion on research when they met the recruiters.
In this particular programme, the vast majority of patients wanted to be informed about incidental findings that might require clinical intervention. This observation is aligned with prior reviews, surveys, qualitative studies, and with findings from other hospital-based biobanks [22, 23, 26]. It emphasises the importance of keeping track of the participants and of putting in place an infrastructure to support disclosure, which adds substantial burden, including financial costs, on investigators and biobank curators [32]. Although a clear distinction needs to be made between incidental findings occurring during routine medical care and those made in a clinical research setting [33], there is growing consensus that researchers should be prepared to return incidental findings to participants who have expressed such an interest [34, 35]. Whether and how to disclose genomic results is a matter of intense discussion, as genomic results affect not only research participants, but also their biological relatives, and may induce discrimination and have other important societal implications.
The observations made here were based on a relatively large, yet limited number of adult patients recruited within a single Swiss academic hospital setting. Although extrapolation to other populations or other hospitals needs to be cautious, this study shows a strong engagement for research from adult patients, and illustrates that hospitals represent an efficient opportunity for recruiting in- and out-patients from a large panel of wards and disease areas as participants in genomic medicine initiatives and big data research.
Appendix 1 Supplementary tables and figures
Table S1 Distribution of the 25 721 patients, according to the ward where they were contacted.
|
All
|
Willingness to participate
|
Willingness to receive incidental findings†
|
|
n
|
%*
|
%
|
%
|
| Visceral surgery |
3355 |
13 |
83 |
94 |
| Internal medicine |
2858 |
11 |
66 |
89 |
| Cardiology |
2701 |
10 |
81 |
93 |
| Traumatology |
2506 |
10 |
77 |
92 |
| Other wards |
2195 |
9 |
82 |
94 |
| ENT |
2020 |
8 |
79 |
95 |
| Orthopaedics |
1983 |
8 |
86 |
94 |
| Neurosurgery |
1463 |
6 |
80 |
94 |
| Neurology |
1304 |
5 |
72 |
94 |
| Urology |
1238 |
5 |
82 |
93 |
| Plastic surgery |
1090 |
4 |
82 |
95 |
| Vascular surgery |
924 |
4 |
80 |
93 |
| Thoracic surgery |
877 |
3 |
82 |
94 |
| Cardiovascular surgery |
672 |
3 |
75 |
92 |
| Medical oncology |
535 |
2 |
82 |
95 |
Table S2 Patients characteristics across selected age groups.
|
|
Age groups (years)
|
|
<64
|
≥64 & <80
|
≥80 & <90
|
≥90
|
| Number |
|
13108 |
|
8056 |
|
3507 |
|
1050 |
|
| Age (in years), mean (SD of mean) |
|
45.4 |
(13.6) |
71.4 |
(4.4) |
84.0 |
(2.8) |
92.8 |
(2.7) |
| Sex, n (%) women |
|
5979 |
(45.6) |
3392 |
(42.1) |
1781 |
(50.8) |
657 |
(62.6) |
| Citizenship, n (%) |
Swiss |
8110 |
(62) |
6511 |
(81) |
2971 |
(85) |
939 |
(89) |
| Non-Swiss |
4998 |
(38) |
1545 |
(19) |
536 |
(15) |
111 |
(11) |
| Origin, n (%) |
European |
10246 |
(78) |
6912 |
(86) |
3173 |
(90) |
1002 |
(95) |
| Other |
1224 |
(9) |
161 |
(2) |
31 |
(1) |
6 |
(1) |
| Unknown |
1638 |
(13) |
983 |
(12) |
303 |
(9) |
42 |
(4) |
| Civil status, n (%) |
Married |
6057 |
(46) |
4802 |
(60) |
1,751 |
(50) |
299 |
(28) |
| Single |
4515 |
(34) |
646 |
(8) |
243 |
(7) |
59 |
(6) |
| Other*
|
2536 |
(19) |
2608 |
(32) |
1,513 |
(43) |
692 |
(66) |
| Religion, n (%) |
Catholic |
5000 |
(38) |
3007 |
(34) |
1187 |
(34) |
272 |
(26) |
| Protestant |
3099 |
(24) |
3342 |
(48) |
1680 |
(48) |
572 |
(54) |
| Other |
5009 |
(38) |
1707 |
(18) |
640 |
(18) |
206 |
(20) |
| Admission mode, n (%) |
Elective |
4197 |
(32) |
2734 |
(34) |
977 |
(28) |
182 |
(17) |
| Emergency |
4407 |
(34) |
2693 |
(33) |
1786 |
(51) |
749 |
(71) |
| Outpatients |
4504 |
(34) |
2629 |
(33) |
744 |
(21) |
119 |
(11) |
| Participation in BIL |
Yes |
10843 |
(83) |
6443 |
(80) |
2410 |
(69) |
647 |
(62) |
| No |
2265 |
(17) |
1613 |
(20) |
1097 |
(31) |
403 |
(38) |
|
Among participants
|
|
|
|
|
|
|
|
|
|
| Interest in incidental findings, n (%) |
Yes |
10393 |
(96) |
5948 |
(92) |
2153 |
(89) |
524 |
(81) |
| No |
450 |
(4) |
495 |
(8) |
257 |
(11) |
123 |
(19) |
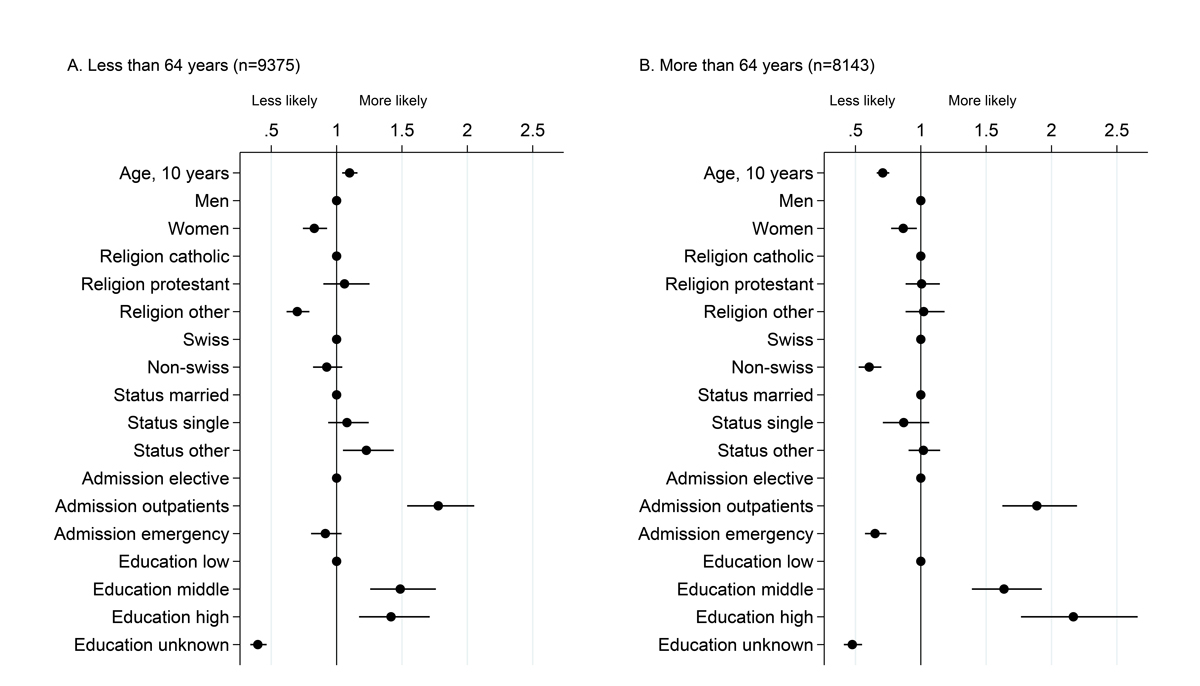
Figure S2
Factors associated with willingness to participate, among patients with information available on education level. This analysis is restricted to the 17 518 patients for whom information on education level is available (this information was collected from 2014). Dots represent odds ratios and bars 95% confidence intervals from a multivariable logistic model including willingness to participate as the dependent variable, run separately in the two age strata.
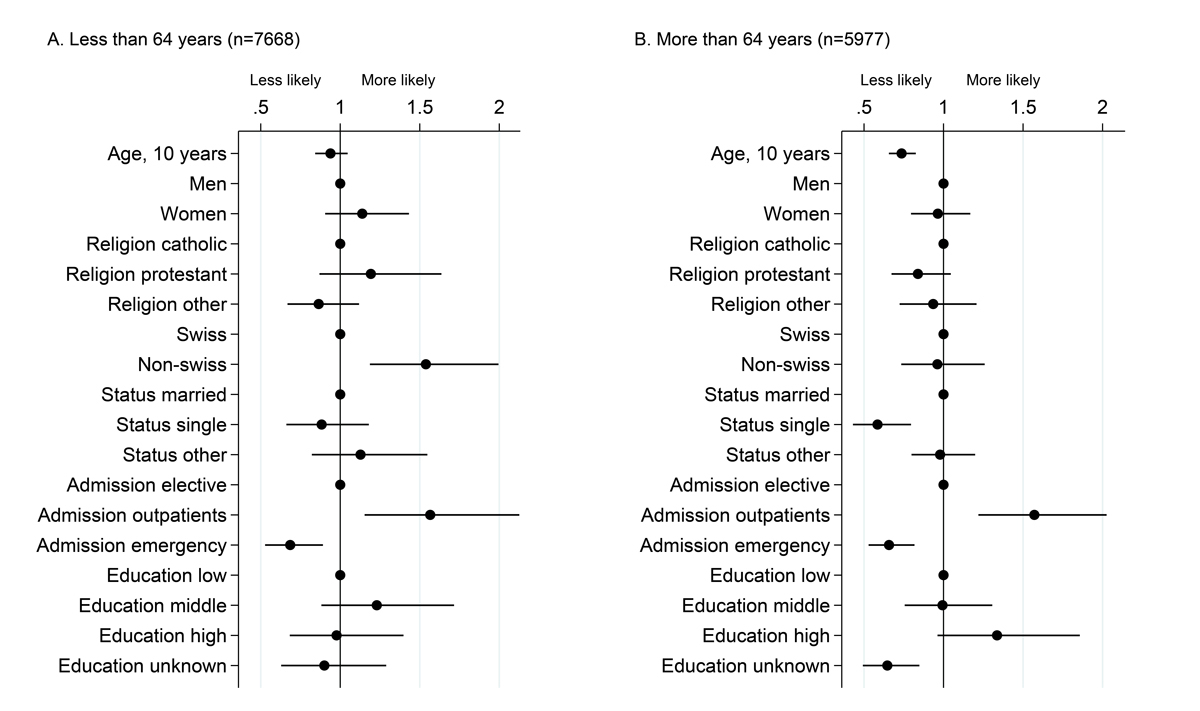
Figure S3
Factors associated with interest in being re-contacted for incidental findings among patients with information available on education level. This analysis is restricted to the 17 518 patients for whom information on education level is available. Dots represent odds ratios and bars 95% confidence intervals from a multivariable logistic model including willingness to receive incidental findings, among patients having accepted to participate, as the dependent variable, run separately in the two age strata.
Acknowledgments
The authors thank the General Management of the CHUV and the Rectorate of the Lausanne UNIL University for funding this project. They also want to express their gratitude to the following collaborators who are part of the recruitment team, the laboratory and the data management groups: Annick Antille, Anne-Lise Bastian, Pasqualina Corthésy, Ludovic Dey, Réjane Dietrich, Elise Dubois-Couture, Annick Ducraux Savoy, Vanessa Jaquet, Didier Foretay, Aurélie Fortin, Laurence Gander, Amédée Kibalabala, Naomi Kramer, Chiyama Mathivathanasekaram, Carole Morel, Anne-Laure Nicoulaz, Dominique Niksch, Julia Parafita, Clarisse Piccand, Karin Pierre Ryembault, Maria de Assunçao Ramos Varela, Claudia Rochat, Audrey Roth and Catherine Saner Zilian. In addition, they thank all the collaborators of the institution who contributed to the success of the BIL. The authors thank Jacques Fellay and Brent Richards for comments and their careful reading of an earlier version of the manuscript.
References
1
Collins
FS
,
Varmus
H
. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–5. doi:.https://doi.org/10.1056/NEJMp1500523
2
Muñoz
M
,
Pong-Wong
R
,
Canela-Xandri
O
,
Rawlik
K
,
Haley
CS
,
Tenesa
A
. Evaluating the contribution of genetics and familial shared environment to common disease using the UK Biobank. Nat Genet. 2016;48(9):980–3. doi:.https://doi.org/10.1038/ng.3618
3
Leitsalu
L
,
Alavere
H
,
Tammesoo
ML
,
Leego
E
,
Metspalu
A
. Linking a population biobank with national health registries-the estonian experience. J Pers Med. 2015;5(2):96–106. doi:.https://doi.org/10.3390/jpm5020096
4
Ashley
EA
. The precision medicine initiative: a new national effort. JAMA. 2015;313(21):2119–20. doi:.https://doi.org/10.1001/jama.2015.3595
5
Kaiser
J
. NIH plots million-person megastudy. Science. 2015;347(6224):817. doi:.https://doi.org/10.1126/science.347.6224.817
6
Mosley
JD
,
Van Driest
SL
,
Weeke
PE
,
Delaney
JT
,
Wells
QS
,
Bastarache
L
, et al.
Integrating EMR-linked and in vivo functional genetic data to identify new genotype-phenotype associations. PLoS One. 2014;9(6):e100322. doi:.https://doi.org/10.1371/journal.pone.0100322
7
Relling
MV
,
Evans
WE
. Pharmacogenomics in the clinic. Nature. 2015;526(7573):343–50. doi:.https://doi.org/10.1038/nature15817
8
Green
RC
,
Berg
JS
,
Grody
WW
,
Kalia
SS
,
Korf
BR
,
Martin
CL
, et al.; American College of Medical Genetics and Genomics. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15(7):565–74. doi:.https://doi.org/10.1038/gim.2013.73
9
Dewey
FE
,
Murray
MF
,
Overton
JD
,
Habegger
L
,
Leader
JB
,
Fetterolf
SN
, et al.
Distribution and clinical impact of functional variants in 50,726 whole-exome sequences from the DiscovEHR study. Science. 2016;354(6319):aaf6814. doi:.https://doi.org/10.1126/science.aaf6814
10
Hu
Y
,
Li
L
,
Ehm
MG
,
Bing
N
,
Song
K
,
Nelson
MR
, et al.
The benefits of using genetic information to design prevention trials. Am J Hum Genet. 2013;92(4):547–57. doi:.https://doi.org/10.1016/j.ajhg.2013.03.003
11
Bush
WS
,
Oetjens
MT
,
Crawford
DC
. Unravelling the human genome-phenome relationship using phenome-wide association studies. Nat Rev Genet. 2016;17(3):129–45. doi:.https://doi.org/10.1038/nrg.2015.36
12
Storr
CL
,
Or
F
,
Eaton
WW
,
Ialongo
N
. Genetic research participation in a young adult community sample. J Community Genet. 2014;5(4):363–75. doi:.https://doi.org/10.1007/s12687-014-0191-3
13
McVeigh
TP
,
Sweeney
KJ
,
Kerin
MJ
,
Gallagher
DJ
. A qualitative analysis of the attitudes of Irish patients towards participation in genetic-based research. Ir J Med Sci. 2016;185(4):825–31. doi:.https://doi.org/10.1007/s11845-015-1373-7
14
Groth
SW
,
Dozier
A
,
Demment
M
,
Li
D
,
Fernandez
ID
,
Chang
J
, et al.
Participation in Genetic Research: Amazon’s Mechanical Turk Workforce in the United States and India. Public Health Genomics. 2016;19(6):325–35. doi:.https://doi.org/10.1159/000452094
15
Critchley
C
,
Nicol
D
,
Otlowski
M
. The impact of commercialisation and genetic data sharing arrangements on public trust and the intention to participate in biobank research. Public Health Genomics. 2015;18(3):160–72. doi:.https://doi.org/10.1159/000375441
16
Porteri
C
,
Pasqualetti
P
,
Togni
E
,
Parker
M
. Public’s attitudes on participation in a biobank for research: an Italian survey. BMC Med Ethics. 2014;15(1):81. doi:.https://doi.org/10.1186/1472-6939-15-81
17
Platt
T
,
Platt
J
,
Thiel
DB
,
Fisher
N
,
Kardia
SL
. ‘Cool! and creepy’: engaging with college student stakeholders in Michigan’s biobank. J Community Genet. 2014;5(4):349–62. doi:.https://doi.org/10.1007/s12687-014-0190-4
18
Murphy Bollinger
J
,
Bridges
JF
,
Mohamed
A
,
Kaufman
D
. Public preferences for the return of research results in genetic research: a conjoint analysis. Genet Med. 2014;16(12):932–9. doi:.https://doi.org/10.1038/gim.2014.50
19
Mählmann
L
,
Röcke
C
,
Brand
A
,
Hafen
E
,
Vayena
E
. Attitudes towards personal genomics among older Swiss adults: An exploratory study. Appl Transl Genomics. 2016;8:9–15. doi:.https://doi.org/10.1016/j.atg.2016.01.009
20
Amiri
L
,
Cassidy-Bushrow
AE
,
Dakki
H
,
Li
J
,
Wells
K
,
Oliveria
SA
, et al.
Patient characteristics and participation in a genetic study: a type 2 diabetes cohort. J Investig Med. 2014;62(1):26–32. doi:.https://doi.org/10.2310/JIM.0000000000000022
21
Freeman
BD
,
Butler
K
,
Bolcic-Jankovic
D
,
Clarridge
BR
,
Kennedy
CR
,
LeBlanc
J
, et al.
Surrogate receptivity to participation in critical illness genetic research: aligning research oversight and stakeholder concerns. Chest. 2015;147(4):979–88. doi:.https://doi.org/10.1378/chest.14-0797
22
Richards
JE
,
Bane
E
,
Fullerton
SM
,
Ludman
EJ
,
Jarvik
G
. Allocation of Resources to Communication of Research Result Summaries: Biobank Participant Perspectives. J Empir Res Hum Res Ethics. 2016;11(4):364–9. doi:.https://doi.org/10.1177/1556264616667126
23
Middleton
A
,
Morley
KI
,
Bragin
E
,
Firth
HV
,
Hurles
ME
,
Wright
CF
, et al.; DDD study. Attitudes of nearly 7000 health professionals, genomic researchers and publics toward the return of incidental results from sequencing research. Eur J Hum Genet. 2016;24(1):21–9. doi:.https://doi.org/10.1038/ejhg.2015.58
24
Allen
NL
,
Karlson
EW
,
Malspeis
S
,
Lu
B
,
Seidman
CE
,
Lehmann
LS
. Biobank participants’ preferences for disclosure of genetic research results: perspectives from the OurGenes, OurHealth, OurCommunity project. Mayo Clin Proc. 2014;89(6):738–46. doi:.https://doi.org/10.1016/j.mayocp.2014.03.015
25
Ahram
M
,
Othman
A
,
Shahrouri
M
,
Mustafa
E
. Factors influencing public participation in biobanking. Eur J Hum Genet. 2014;22(4):445–51. doi:.https://doi.org/10.1038/ejhg.2013.174
26
Bollinger
JM
,
Scott
J
,
Dvoskin
R
,
Kaufman
D
. Public preferences regarding the return of individual genetic research results: findings from a qualitative focus group study. Genet Med. 2012;14(4):451–7. doi:.https://doi.org/10.1038/gim.2011.66
27
Dye
T
,
Li
D
,
Demment
M
,
Groth
S
,
Fernandez
D
,
Dozier
A
, et al.
Sociocultural variation in attitudes toward use of genetic information and participation in genetic research by race in the United States: implications for precision medicine. J Am Med Inform Assoc. 2016;23(4):782–6. doi:.https://doi.org/10.1093/jamia/ocv214
28
Mooser
V
,
Currat
C
. The Lausanne Institutional Biobank: a new resource to catalyse research in personalised medicine and pharmaceutical sciences. Swiss Med Wkly. 2014;144:w14033. https://smw.ch/en/article/doi/smw.2014.14033/
29
Maurer
F
,
Pradervand
S
,
Guilleret
I
,
Nanchen
D
,
Maghraoui
A
,
Chapatte
L
, et al.
Identification and molecular characterisation of Lausanne Institutional Biobank participants with familial hypercholesterolaemia - a proof-of-concept study. Swiss Med Wkly. 2016;146:w14326. https://smw.ch/en/article/doi/smw.2016.14326/
30
Maggo
SD
,
Savage
RL
,
Kennedy
MA
. Impact of New Genomic Technologies on Understanding Adverse Drug Reactions. Clin Pharmacokinet. 2016;55(4):419–36. doi:. Correction in: Clin Pharmacokinet. 2016;55(4):437. https://doi.org/10.1007/s40262-015-0324-9
31
Trevisan
C
,
Veronese
N
,
Maggi
S
,
Baggio
G
,
Toffanello
ED
,
Zambon
S
, et al.
Factors Influencing Transitions Between Frailty States in Elderly Adults: The Progetto Veneto Anziani Longitudinal Study. J Am Geriatr Soc. 2017;65(1):179–84. doi:.https://doi.org/10.1111/jgs.14515
32
Bledsoe
MJ
,
Clayton
EW
,
McGuire
AL
,
Grizzle
WE
,
O’Rourke
PP
,
Zeps
N
. Return of research results from genomic biobanks: cost matters. Genet Med. 2013;15(2):103–5. doi:.https://doi.org/10.1038/gim.2012.105
33
Burke
W
,
Evans
BJ
,
Jarvik
GP
. Return of results: ethical and legal distinctions between research and clinical care. Am J Med Genet C Semin Med Genet. 2014;166(1):105–11. doi:.https://doi.org/10.1002/ajmg.c.31393
34
Jarvik
GP
,
Amendola
LM
,
Berg
JS
,
Brothers
K
,
Clayton
EW
,
Chung
W
, et al.; eMERGE Act-ROR Committee and CERC Committee; CSER Act-ROR Working Group. Return of genomic results to research participants: the floor, the ceiling, and the choices in between. Am J Hum Genet. 2014;94(6):818–26. doi:.https://doi.org/10.1016/j.ajhg.2014.04.009
35
Shkedi-Rafid
S
,
Dheensa
S
,
Crawford
G
,
Fenwick
A
,
Lucassen
A
. Defining and managing incidental findings in genetic and genomic practice. J Med Genet. 2014;51(11):715–23. doi:.https://doi.org/10.1136/jmedgenet-2014-102435