Figure 1 The In-HospiTOOL.
DOI: https://doi.org/10.4414/smw.2017.14515
Industrialised healthcare systems are transforming as they seek to improve health outcomes of patient populations and individuals, while reducing the rise in healthcare costs. In Switzerland, one major focus is to identify the most appropriate management setting for the diagnosis and treatment of acute medical conditions in mostly multimorbid inpatients – particularly as it relates to hospital-based care, which accounts for 36% of total Swiss healthcare costs in 2014 [1]. So far, however, most team care interventions have shown ambiguous effects [2]. In regard to communication between patients and physicians, the Swiss healthcare system has reported some promising results [3]. Swiss patients are mostly sufficiently informed about main diagnostic and therapeutic goals, and about treatment alternatives, and receive specific instructions about when and how to seek further care [3, 4]. A mostly evidence-based usage of medication also increases trust between patients and physicians [5, 6]. Nevertheless, there is still room for improvement, particularly in terms of accountability for quality, appropriateness and costs of healthcare services in the complex context of older, multimorbid medical inpatients [7]. To advance this effort, federal, cantonal, and private organisations all require benchmark data from hospitals. Changing policy responsibility for planning and delivery of healthcare services, partial financing of hospitals, and provision of subsidies for insurance premiums, however, makes a national assessment and a consistent steering strategy challenging.
Developing comprehensive in-hospital patient management tools with adequate human resource allocation is a major challenge for healthcare systems, governments and societies worldwide [8].
Improved diagnostic and therapeutic measures have increased life expectancy, yet contribute to a growing number of multimorbid, elderly patients. Chronic disease burden and frailty are key risk factors for emergency hospitalisation. For a multimorbid patient, the trigger for hospitalisation might be a minor acute disease (e.g., infection of the respiratory tract), which disrupts the fragile bio-psycho-social homeostasis. Elderly, frail, medically complex patients and those who have low health literacy, are less well educated, or are cognitively impaired [9] are at risk for adverse outcomes when interfacing with the healthcare system. Frequently, postacute discharge to a nursing care facility is inevitable because of deconditioning during prolonged hospitalisation. High demands for medical, nursing and social care put a strain on our health care resources [10, 11].
Thus, the need for improved interprofessional coordination in this intricate inpatient setting is obvious.
Nevertheless, defining outcome measures on which to base the performance of patient management interventions is challenging because there is no consensus as to the metrics that best reflect the quality of general medical ward care. Quality mostly relates to structures, processes, or outcomes [12]. Interpretation of outcome parameters might be debatable; however, they are directly meaningful for patients and potentially influenced by interdisciplinary interventions [13]. We therefore also describe aspects of objective patient outcome measures used in studies of the general medical ward setting, and evaluate the performance of patient management interventions assessed with use of them.
To address this lack of evidence, we are performing a prospective multicentre trial to study the effect of an interprofessional inpatient management tool (“In-HospiTOOL”) on length of stay and other patient outcomes and quality measures. We aim to improve the transition process in multimorbid medical inpatients without negatively influencing their outcome. Improved resource allocation and interprofessional coordination are key elements of this trial..
For this narrative review, we searched for relevant studies independent of study design using the PubMed database. We used the terms “quality of care”, “transition”, “health care”, “care interventions”, “inter-professional”, “discharge planning”, “multimorbidity”, “medical inpatient”, “patient-centered outcome”, “readmission”, and “benchmark”. The terms “outpatient”, “surgical”, and “children” were excluded. We included articles in English.
In-hospital transition usually refers to transition of patients from the emergency department to the hospital ward and thereafter to postacute-care institutions or back home. Especially in elderly, multimorbid patients, this process requires preparation, with periodic assessment of progress toward transition readiness. Assessment of readiness involves the evaluation of indicators related to self-management of disease including knowledge of illness, medications and physician appointments after discharge, among others. In addition, parameters such as satisfaction with transition and patient-centred outcomes, such as readmission and mortality, might reflect important quality indicators. Nevertheless, development of meaningful measurement standards that permit accurate evaluations of healthcare performance in this complex patient population is challenging [14]. There are several reasons why this field is not more advanced, including cultural stigmata, low evidence regarding working procedures and few financial incentives. Some healthcare institutions have started to measure and communicate patient outcomes related to an episode of acute illness. Since acute-care episodes often involve multiple sites (e.g., clinics, emergency departments and skilled nursing facilities), situation-specific quality and outcome parameters are hard to assess. Therefore, improvement in quality or costs requires a better understanding of resources needed to deliver care during an acute illness and of the outcomes that are meaningful to patients. A recent paper propagated different measures of acute care such as timeliness as well as outcome metrics such as symptom relief and functional recovery, and costs related to episodes of care [14]. Length of hospital stay, 30-day readmission rate, and 30-day or in-hospital mortality rate were used to evaluate interdisciplinary team care interventions on a medical ward in a recent systematic review [2]. Complications of care, requirements for institutional care and functional status were further patient outcome measures [2].
However, to conclusively evaluate these different outcome measures, large scale validation and integration into routine care is needed [15]. Identification of outcome measures by a Delphi process with an interprofessional group of participants seems to be mandatory [16]. Use of outcomes predicted from population-level data will facilitate fulfilling patient expectations. Such a strategy may help physicians to identify areas where they need improvement, eliminate procedures with less favourable outcomes and avoid interventions in patients unlikely to benefit from them. It also enhances patient satisfaction with care by helping physicians set appropriate expectations regarding a patient’s return to daily routine [17].
Novel electronic health record-based transition tools are required to reduce resource misallocation and to better align healthcare needs in hospitalised medical patients.
In national healthcare discussions, a major priority is to reduce costs of care without impairing quality of care. Main areas to target are resource allocation defined as “overuse”, “unnecessary healthcare”, or “low value care”, as well as interprofessional communication to improve care-team coordination tightly involving patients. Early application of reasonable and justified care and reduction of unnecessary waiting time due to tardy recognition of postacute care needs are main goals to improve in-hospital transition process. Minimising overuse not only accounts for substantial health care savings, but also may prevent harm. Many factors contribute to overuse, including expansion of technology, physician payment schemes that encourage utilisation, indication creep, patient expectations and concerns about litigation [18].
As a platform for addressing resource optimisation, electronic health record-based patient management tools enable transparency and simultaneous interventions by different healthcare personnel to adapt transition planning. Research suggests that multifaceted interventions are required to change practice [19, 20]. Moreover, success in changing procedures seems more likely if tools and interventions adhere to principles around decision support, as in other disciplines such as behavioural economics [21].
To overcome the unintended negative clinical consequences by resource misallocation [22], the Swiss Federal Council has formulated a strategy to consecutively implement electronic health records in all Swiss hospitals and postacute-care institutions, in order to provide a basis for implementation of an improved transition management and discharge planning [23]. The electronic health record has the potential to be a powerful vehicle for measurements to guide interventions around low-value care and patient transition [18]. Current estimates of overuse, however, are still imprecise, and the methodology is prone to error; researchers are concerned about inconsistencies in definitions, datasets and denominators. In the following paragraphs, we illustrate two examples of how an electronic tool may help to optimise patient care by improving interprofessional team communication.
Because communication in acute-care settings involving challenging multimorbid inpatients is fragmented and involves the use of different electronic modalities, providers are not consistently informed about the planning of care and transition. In the US, Dalal et al., by performing an institutional review board-approved study, developed a secure, patient-centred “microblog” messaging platform that identifies care-team members by synchronising with the electronic health record and directs providers to a single forum where they can communicate about the plan of care in an acute-care setting [24]. Major themes in messages included care coordination (49%), clinical summarisation (29%), and care-team collaboration (27%). Message transparency and persistence were considered useful features by the majority, mirroring much potential to improve communication across settings once barriers are addressed.
A further effort to improve communication among healthcare personnel was done by Mueller et al. [25]. They reported the effect of implementing a web-based handoff tool on an US general medical ward. Using a prospective quasi-experimental design, they found that implementation of this web-based handoff tool led to reductions in rates of medical errors related to communication failures during end-of-shift handoffs (3.56 medical errors per 100 patient-days (95% confidence interval [CI] 1.70–7.44) before intervention vs 1.76 (95% CI 0.93–3.31) after intervention, p <0.001). Although the findings are noteworthy, the results of this study may be confounded. Physicians participated in handoff and communication skills workshops during the implementation period, and these workshops – rather than the handoff tool alone – may have contributed to the decrease in rates of errors. As hospital care is increasingly shift based, a clear and efficient handoff process is vital. The study by Mueller et al. shows how web-based handoff tools may improve hospital workflow and patient safety, but only if they are carefully built and integrated into existing systems [25].
Web- and electronic health record-based tools represent a key opportunity to begin addressing suboptimal patient transition, patient care and the high costs of care; and to realise benefit from a major healthcare investment by the government and policy makers.
As recently documented in a systematic review [2], interdisciplinary and interprofessional care-team interventions involving medical inpatients revealed heterogeneous impacts on length of stay.
Whereas the pooled analysis of all studies did not reveal a significant effect on length of stay, some individual studies reported reduced length of stay. Interestingly, most of the effective studies incorporated subspecialist input to the treating team in the general ward [26–28]. For example, one retrospective US study investigated the effect of involving a specialised house staff service (SHS) model for patients with hepatic disease supervised by a multidisciplinary hepatology team. The implementation of the SHS model was associated with a 5.4 day decrease in length of stay (mean 18.3 days, standard deviation [SD] 35.3 days) vs 12.9 days, SD 18.5; p = 0.05) [26]. Another US quasi-experimental trial compared lengths of stay on a specific internal medicine unit by using a proactive consultation model involving attending psychiatrists. The goal of the study was to ascertain any active psychiatric problems and potential barriers to discharge. Mean length of stay was shorter in the intervention group, 2.90 days (SD 2.12 days) compared with the pre- and post-control groups: 3.81 days (SD 3.01 days) and 3.66 days (SD 3.92 days), respectively, p <0.05 [27]. Finally, patients treated by a multidisciplinary antimicrobial use team at a large university-affiliated public hospital in the US had a shorter median length of stay compared with patients treated by the control group (7 days, range 1–50 vs 8 days, range 2–86, respectively; p = 0.03) [28]. Another systematic review and meta-analysis from Canada investigated the effectiveness of early discharge planning in acutely ill hospitalised older adults [29]. As in other studies, the authors did not find a difference in index length of hospital stay (mean difference −0.41, 95% CI −1.19–0.36), but lower readmission lengths of hospital stay (mean difference −2.47, 95% CI −4.13 – −0.81) within 3 to 12 months of index hospital discharge. Similar, a large Chinese meta-analysis involving ten randomised controlled trials demonstrated that, compared with standard care, early discharge planning programmes were not effective in reducing hospital length of stay of the index admission (mean difference 0.03, 95% CI −0.06–0.12), but decreased length of stay for those patients readmitted (mean difference −2.08, 95% CI −3.76 – −0.39) [30, 31]. However, a Canadian health technology assessment showed that individualised discharge planning in 1765 chronically ill patients was effective in reducing hospital length of stay (mean difference −0.91, 95% CI −1.55 – 0.27) [32].
Since early discharge planning with acutely admitted medical patients appears to improve different system level outcomes after index hospital discharge more than index length of stay, service providers may use these findings to adapt discharge tools by integrating into an accurate risk stratification scheme [33]. This finding will potentially improve resource allocation on the index admission itself and prevent functional disability associated with prolonged subsequent hospitalisation.
Thus, the impact of interdisciplinary care-team interventions with medical inpatients remains unclear, although these interventions affect different levels of patient outcomes. Differences in study designs and interventions may partly explain the non-significant results in prior research.
Hospital readmissions are common and costly [34], and have become a major focus of healthcare quality for clinical leaders and policymakers [35]. There are substantial differences in the rate of readmission within 30 days ranging, for example, from 7% in Switzerland to a minimum of 15% in the United States [36].
The US federal government has made significant efforts to shift toward value-based payments after passage of the “Affordable Care Act” in 2010. One key programme under this act is the Hospital Readmissions Reduction Program (HRRP) implemented in 2011, which penalises US hospitals with higher-than-expected readmission rates up to 3% of their base Medicare payments [37]. Early evidence from a US retrospective analysis shows that the introduction of HRRP is associated with larger improvements in readmission rates over time (21.5 to 17.8% vs 15.3 to 13.1% in controls) for patients with acute myocardial infarction, heart failure and pneumonia [38]. As shown in a recent pre-post analysis, the effect was greatest in hospitals with the lowest performance before introducing HRRP [39]. Nevertheless, the number of readmissions is still different between the US and Switzerland, which raises the question of transferability from one healthcare setting to another. Although reasons for these differences remain unclear, one may speculate that a less comprehensive distribution of primary care physicians, educational factors and differences in length of the index hospital stay may explain some of this variation [40].
To further standardise in-hospital transition and hospital discharge procedures, several studies have been performed. Some of these randomised controlled interventions have shown a reduction in hospital readmission rates and cost [41–44] and emergency department visits, whereas some have shown little or no effect [45–48].
A US reengineered discharge programme decreased hospital utilisation by implementing a nurse discharge advocate and a clinical pharmacist working together to coordinate the hospital discharge, educate patients and reconcile medications [44]. Similarly, an integrated care model was found to be helpful in reducing readmission without compromising patient outcome [49]. In an open-label, assessor-blinded, randomised controlled trial on patients with one or more unscheduled readmissions in the prior 90 days, patients in the intervention group had a significant reduction in the rate of 30-day readmissions (incidence rate ratio 0.67, 95% CI 0.52–0.86) and 30-day emergency department visits (incidence rate ratio 0.60, 95% CI 0.46–0.79) compared with those receiving standard hospital care. Importantly, the effectiveness was sustained at 90 and 180 days, with 1164 fewer (6292 vs 5128 beds) hospital bed-days used at 90 days after discharge in the intervention arm.
The systematic review by Pannick et al. revealed that discharge interdisciplinary interventions confined to the inpatient setting are unlikely to reduce readmissions [2]. Only 3 of 15 interventions (20%) reduced readmissions. A French randomised controlled trial involving a postprescription review by an infectious disease physician reduced 60-day readmissions related to relapsing infection (3.4 vs 7.9%, p = 0.01) [50]; a Canadian multicentre, quasi-randomised, controlled clinical trial investigating the effect of a proactive clinical pharmacist service reduced admissions at 3 months (36.2 vs 45.5%; adjusted odds ratio [OR] 0.63, 95% CI 0.42–0.94), although the effect had dissipated by 6 months (50.7 vs 56.3%; adjusted OR 0.78, 95% CI 0.53–1.15) [51]; a US prospective controlled intervention study suggested reduced readmissions after localising teams and introducing service census limits (18.1 vs 15.4%, p <0.001) [52]. Twelve of 15 interventions did not change the number of readmissions. Team composition interventions even tended to increase early readmissions, albeit with important confounding factors in the included studies: weighted risk ratio 1.34 (95%CI 1.12–1.61) [2]. In contrast, a US quasi-experimental evaluation in an urban academic medical centre found a 9.3% relative reduction (21.5 to 19.5%) in readmission among more than 10 000 high risk discharge patients by providing personalised transitional care, including education, medication reconciliation and follow-up telephone calls (target vs control population: OR 0.90, 95% CI 0.83–0.99) [53]. Similarly, Buurman et al. implemented a comprehensive geriatric assessment followed by a transitional care bridge programme [54]. However, the study did not observe a reduction in unplanned readmission rates, although the readmission rates in both arms were lower than in many other studies. Finally, to reduce readmissions, some studies found evidence regarding the use of teach-back methodology by checking the patient’s understanding of their medical and medication history [55]. One US study reported a significantly lower risk of readmission compared with the control group (hazard ratio [HR] 0.56, 95% CI 0.32–0.96) by a targeted education of patients with heart failure [56].
On the basis of this missing evidence, design of new (electronic) readmission reduction tools is important and the correct comprehension of patient-centred readmission factors is critical. A recent multisite study reported high understanding of discharge plans in readmitted patients but low perceived anticipatory guidance for resolving common barriers to recovery after discharge [57].
Therefore, a comprehensive discharge instruction programme, including patient education and teach-back methodology [58, 59], about relevant diagnoses and medication, instruction about follow-up procedure with coordination of appointments (physicians, nursing home) and clarification of logistic details (transport, location) is of high importance for establishing novel interventions to improve quality of care.
Loss of essential activities of daily living (ADLs) is a major challenge in hospitalised older patients [60].
Only few recent studies have focused on in-hospital interventions to avoid decline in ADLs. In 2011, a meta-analysis of randomised controlled trials evaluated the effectiveness of a comprehensive geriatric assessment (CGA) in hospitalised older adults, and found that patients who underwent CGA were more likely to be alive and in their own homes at 1 year compared with patients who did not receive CGA (OR 1.16, 95% CI 1.05–1.28) [61]. A subsequent double-blind, multicentre, randomised clinical trial conducted at three hospitals with affiliated home care organisations in the Netherlands investigated whether an intervention of systematic CGA followed by a transitional care bridge programme [62] improved ADLs compared with systematic CGA alone. In contrast, no improvement of ADLs could be achieved [63]. Also, an earlier German multicentre randomised trial by Kircher et al. did not achieve any differences in ADLs, functional status or nursing home placement by providing inpatient geriatric consultations [64].
Several notable findings arose from these studies. First, benefits were mostly observed in studies involving medical wards for comprehensive geriatric assessment [61]. Second, most trials included in the meta-analysis by Ellis dated from around the millennium or even earlier, with different standards of usual care in the control groups compared with more recent study settings [64–66]. Third, to some extent, CGA consultation exposure was incomplete as only 78% of 2-week and 66% of 6-week visits were completed [63]. In addition, intervention teams were missing aspects of interdisciplinary collaboration that are important in healthcare interventions involving complex multimorbid patients [67].
Effects of transitional care on mortality have been inconsistent [2, 41, 68, 69].
In the systematic review by Pannick et al., only one Australian randomised controlled trial of 15 studies that reported mortality rate showed a significant effect [2, 69]. In-hospital mortality was reduced (from 6.4 to 3.9%, p = 0.03) by enhanced assessment, communication, care and discharge planning by restructuring consistent, patient-centred multidisciplinary teams in a general medicine service. Although there was an effect on mortality, the observed reduction did not persist at 6 months. In an earlier randomised controlled trial with a two-by-two factorial design, patients received either care in an inpatient geriatric unit or usual inpatient care, followed by either care at an outpatient geriatric clinic or usual outpatient care; there was no significant effect on survival, however, but there were significant reductions in functional decline associated with inpatient geriatric evaluation [70]. In contrast, another randomised controlled trial of a CGA followed by a transitional care bridge programme, regression analysis adjusted for study site and cognitive functioning showed a significant protective intervention effect for 1-month (HR 0.63, 95% CI 0.39–0.99) and 6-month (HR 0.75, 95% CI 0.56–0.99) mortality [54]. The number needed to treat to prevent one death was 16.
As summarised by Pannick et al., team practice interventions tended to reduce early mortality, whereas interdisciplinary team composition interventions did not significantly reduce early mortality [11].
Innovative interdisciplinary and interprofessional healthcare interventions on general medical wards most commonly choose length of stay, readmission, mortality rates, or functional status as their primary outcome measures. Effects of most interprofessional interventions on these outcomes are inconsistent. Nevertheless, there is some evidence suggesting that improvements in interprofessional collaboration may reduce complications of care. Significant contemporaneous secular reductions in length of stay are reported [71], which most of these interventions did not reduce in addition. Most interventions confined to the inpatient setting were unlikely to reduce readmissions, to decrease need for institutional care after discharge, or to reduce mortality rates. However, generalisability of results remains unclear owing to differences between healthcare systems and standards of care nationally and internationally.
Many interventions to mirror and improve healthcare quality may not reflect true resource need and use, especially in elderly, multimorbid patients. Also, there are only a few validated interventions for optimisation of patient flow and discharge processes. Some hospitals have developed internal instruments with more or less sophistication and practicability. Safety, effectiveness, cost-efficiency, transferability and external validity of these interventions are, however, understudied [72, 73]. Healthcare authorities and hospital executives lack scientific evidence to promote, enforce and sanction changes, or to guide the flow of multimorbid patients. There is, therefore, a need to further validate benchmarks and interprofessional interventions to improve patient management, flow and length of stay, without compromising patient outcome and functional independence, despite multimorbidity in a pragmatic multicentre setting [74].
In Switzerland, there is an ongoing discussion about which performance data best reflect high quality of care and which intervention would best address current challenges in this complex multimorbid patient population. This lack of consensus regarding performance benchmarking data and needed interventions in Switzerland is a major obstacle for quality improvements [75–78]. To force translation from data collection to clinical impact, disease-specific standard sets will help to better understand how to intervene by adapting clinical processes. The Patient-Reported Outcomes Measurement Information System (PROMIS) – a system that was designed to develop, validate, and standardise item banks that measure key patient-reported outcomes, including symptoms of chronic conditions, functioning and health-related quality of life [79] – might be able to assess the effect of commonly performed diagnostic and therapeutic steps on patient outcome over the course of an episode of care [80]. Prospective time-series analyses, and crossover or cluster randomised controlled trials might be valuable study designs to investigate the effect of transition-changing interventions.
Availability of anonymised patient-level data from clinical trials can also permit verification of original results, enhance public trust and accountability, facilitate other critical research (e.g., evaluation of adverse event rates according to compound class or subpopulation or identification of surrogate end points) and avoid duplicate trials [81]. “Data dumpsters” must be prevented – that is, simply making more data openly available without linking them to relevant documentation and analyses that are applied to improve health [82]. Finally, novel interventions to improve transition will lead to new costs and a deviation of resources – at least at the beginning. Thus, a careful evaluation of costs and benefits is fundamental taking these additional costs into account.
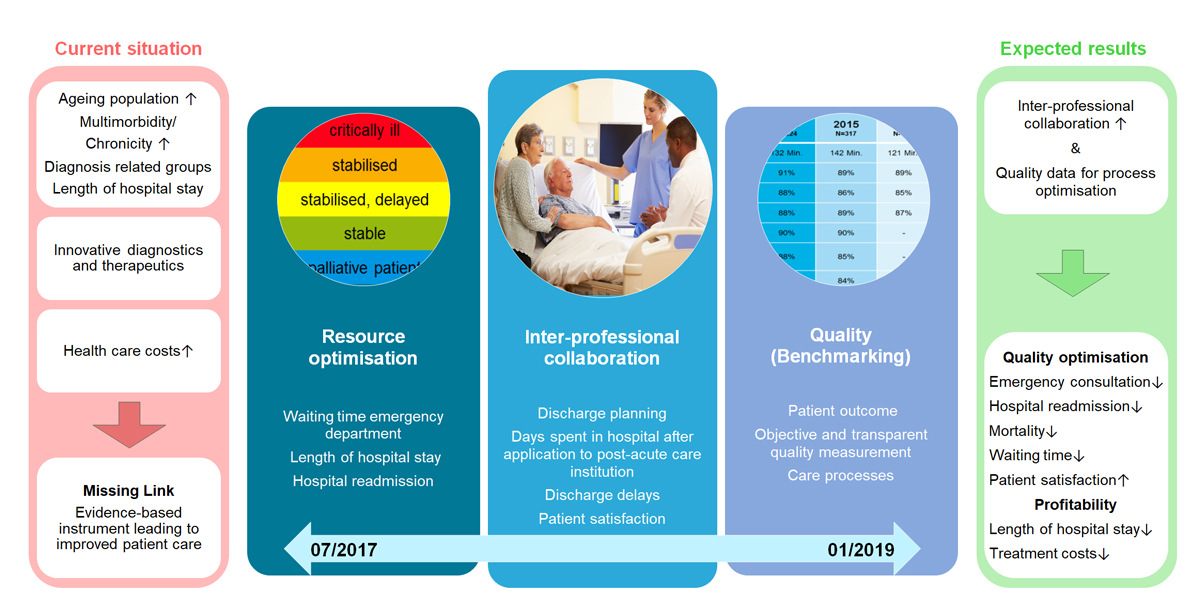
To address issues of national resource allocation, inter-professional collaboration, and benchmarking, the Swiss National Science Foundation has created the “National Research Programme” (NRP) 74 [83]. Within this programme, we are currently studying the effect of an interprofessional inpatient management tool (“In-HospiTOOL”) on patient outcomes in a pragmatic “quasi-experimental” multicentre trial (fig. 1). This tool combines several patient discharge measures and was developed at the Medical University Clinic of the Cantonal Hospital Aarau in an intensive multiprofessional collaboration over the past ten years. In our hospital, we found a decrease in length of hospital stay of pneumonia severity index-adjusted pneumonia patients without negatively influencing outcome compared with other secondary and tertiary hospitals in Switzerland (approximately −3 days, results from unpublished secondary analysis) [84]. To prove external validity and to exclude other local influencing factors, we will prospectively study consecutive multimorbid medical patients upon admission to the medical ward in eight Swiss hospitals. Because patient-level randomisation is not feasible for an intervention that focuses on the process of care, we will use a quasi-experimental approach and compare outcomes before and after hospital-wide implementation of the management tool. We will use time-trend analysis to compare length of stay before and after tool implementation. Data for other Swiss hospitals from the Swiss Federal Statistical Office serve as a control population. We target the inclusion of 45 000 patients over an 18-month period. The trial will inform us whether the “In-HospiTOOL” improves inter-professional team work and thereby reduces length of stay without negatively impacting subjective and objective markers of patient outcomes. The large amount of patient data collected within this trial will enable comparison of transition processes within different hospitals and establish a benchmarking for patient care quality. Our trial synergises funds, national networks and, thus, is likely to become a milestone in the current public healthcare discussions.

Figure 1 The In-HospiTOOL.
No financial support and no other potential conflict of interest relevant to this article was reported.
1Bundesamt für Statistik. Kosten und Finanzierung des Gesundheitswesens 2014: Definitive Daten. 2016. Available from: https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit.gnpdetail.2016-0423.html. Accessed 20.Dec.2016.
2 Pannick S , Davis R , Ashrafian H , Byrne BE , Beveridge I , Athanasiou T , et al. Effects of Interdisciplinary Team Care Interventions on General Medical Wards: A Systematic Review. JAMA Intern Med. 2015;175(8):1288–98. doi:.https://doi.org/10.1001/jamainternmed.2015.2421
3 Selby K , Edwards S . Time-Based Billing: What Primary Care in the United States Can Learn From Switzerland. JAMA Intern Med. 2016;176(7):881–2. doi:.https://doi.org/10.1001/jamainternmed.2016.2230
4Davis KSK, Squires D, Schoen C. Mirror, Mirror, On the Wall. New York: The Commonwealth Fund. 2014.
5 Filippini M , Masiero G , Moschetti K . Socioeconomic determinants of regional differences in outpatient antibiotic consumption: evidence from Switzerland. Health Policy. 2006;78(1):77–92. doi:.https://doi.org/10.1016/j.healthpol.2005.09.009
6 Blendon RJ , Benson JM , Hero JO . Public trust in physicians--U.S. medicine in international perspective. N Engl J Med. 2014;371(17):1570–2. doi:.https://doi.org/10.1056/NEJMp1407373
7 Biller-Andorno N , Zeltner T . Individual Responsibility and Community Solidarity--The Swiss Health Care System. N Engl J Med. 2015;373(23):2193–7. doi:.https://doi.org/10.1056/NEJMp1508256
8 Barnett K , Mercer SW , Norbury M , Watt G , Wyke S , Guthrie B . Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi:.https://doi.org/10.1016/S0140-6736(12)60240-2
9 Mercer SW , Guthrie B , Furler J , Watt GC , Hart JT . Multimorbidity and the inverse care law in primary care. BMJ. 2012;344(jun19 2):e4152. doi:.https://doi.org/10.1136/bmj.e4152
10 Bähler C , Huber CA , Brüngger B , Reich O . Multimorbidity, health care utilization and costs in an elderly community-dwelling population: a claims data based observational study. BMC Health Serv Res. 2015;15(1):23. doi:.https://doi.org/10.1186/s12913-015-0698-2
11 Kadam UT , Uttley J , Jones PW , Iqbal Z . Chronic disease multimorbidity transitions across healthcare interfaces and associated costs: a clinical-linkage database study. BMJ Open. 2013;3(7):e003109. doi:.https://doi.org/10.1136/bmjopen-2013-003109
12 Donabedian A . The quality of care. How can it be assessed? JAMA. 1988;260(12):1743–8. doi:.https://doi.org/10.1001/jama.1988.03410120089033
13 Lilford R , Mohammed MA , Spiegelhalter D , Thomson R . Use and misuse of process and outcome data in managing performance of acute medical care: avoiding institutional stigma. Lancet. 2004;363(9415):1147–54. doi:.https://doi.org/10.1016/S0140-6736(04)15901-1
14 Kocher KE , Ayanian JZ . Flipping the Script - A Patient-Centered Approach to Fixing Acute Care. N Engl J Med. 2016;375(10):915–7. doi:.https://doi.org/10.1056/NEJMp1601899
15 Parikh RB , Kakad M , Bates DW . Integrating Predictive Analytics Into High-Value Care: The Dawn of Precision Delivery. JAMA. 2016;315(7):651–2. doi:.https://doi.org/10.1001/jama.2015.19417
16 Fair C , Cuttance J , Sharma N , Maslow G , Wiener L , Betz C , et al.; International and Interdisciplinary Health Care Transition Research Consortium. International and Interdisciplinary Identification of Health Care Transition Outcomes. JAMA Pediatr. 2016;170(3):205–11. doi:.https://doi.org/10.1001/jamapediatrics.2015.3168
17 Baumhauer JF . Patient-Reported Outcomes - Are They Living Up to Their Potential? N Engl J Med. 2017;377(1):6–9. doi:.https://doi.org/10.1056/NEJMp1702978
18 Rumball-Smith J , Shekelle PG , Bates DW . Using the Electronic Health Record to Understand and Minimize Overuse. JAMA. 2017;317(3):257–8. doi:.https://doi.org/10.1001/jama.2016.18609
19 Colla CH , Mainor AJ , Hargreaves C , Sequist T , Morden N . Interventions Aimed at Reducing Use of Low-Value Health Services: A Systematic Review. Med Care Res Rev. 2016;1077558716656970. [Epub ahead of print]
20 Colla CH . Swimming against the current--what might work to reduce low-value care? N Engl J Med. 2014;371(14):1280–3. doi:.https://doi.org/10.1056/NEJMp1404503
21 Bates DW , Gawande AA . Improving safety with information technology. N Engl J Med. 2003;348(25):2526–34. doi:.https://doi.org/10.1056/NEJMsa020847
22 Willson A . The problem with eliminating ‘low-value care’. BMJ Qual Saf. 2015;24(10):611–4. doi:.https://doi.org/10.1136/bmjqs-2015-004518
23Suisse e: Das elektronische Patientendossier. Accessed 15 February 2017.
24 Dalal AK , Schnipper J , Massaro A , Hanna J , Mlaver E , McNally K , et al. A web-based and mobile patient-centered “microblog” messaging platform to improve care team communication in acute care. J Am Med Inform Assoc. 2017;24(e1):e178–84. doi:.https://doi.org/10.1093/jamia/ocw110
25 Mueller SK , Yoon C , Schnipper JL . Association of a Web-Based Handoff Tool With Rates of Medical Errors. JAMA Intern Med. 2016;176(9):1400–2. doi:.https://doi.org/10.1001/jamainternmed.2016.4258
26 Lai JC , Montero A , Lebwohl B , Brown RS, Jr . A novel housestaff educational model for quaternary-care patients at an academic health center. Acad Med. 2009;84(2):206–11. doi:.https://doi.org/10.1097/ACM.0b013e31819382d3
27 Desan PH , Zimbrean PC , Weinstein AJ , Bozzo JE , Sledge WH . Proactive psychiatric consultation services reduce length of stay for admissions to an inpatient medical team. Psychosomatics. 2011;52(6):513–20. doi:.https://doi.org/10.1016/j.psym.2011.06.002
28 Camins BC , King MD , Wells JB , Googe HL , Patel M , Kourbatova EV , et al. Impact of an antimicrobial utilization program on antimicrobial use at a large teaching hospital: a randomized controlled trial. Infect Control Hosp Epidemiol. 2009;30(10):931–8. doi:.https://doi.org/10.1086/605924
29 Fox MT , Persaud M , Maimets I , Brooks D , O’Brien K , Tregunno D . Effectiveness of early discharge planning in acutely ill or injured hospitalized older adults: a systematic review and meta-analysis. BMC Geriatr. 2013;13(1):70. doi:.https://doi.org/10.1186/1471-2318-13-70
30 Zhu QM , Liu J , Hu HY , Wang S . Effectiveness of nurse-led early discharge planning programmes for hospital inpatients with chronic disease or rehabilitation needs: a systematic review and meta-analysis. J Clin Nurs. 2015;24(19-20):2993–3005. doi:.https://doi.org/10.1111/jocn.12895
31 Fox M . Nurse-led early discharge planning for chronic disease reduces hospital readmission rates and all-cause mortality. Evid Based Nurs. 2016;19(2):62. doi:.https://doi.org/10.1136/eb-2015-102197
32 McMartin K . Discharge planning in chronic conditions: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(4):1–72.
33 Louis Simonet M , Kossovsky MP , Chopard P , Sigaud P , Perneger TV , Gaspoz JM . A predictive score to identify hospitalized patients’ risk of discharge to a post-acute care facility. BMC Health Serv Res. 2008;8(1):154. doi:.https://doi.org/10.1186/1472-6963-8-154
34 Jencks SF , Williams MV , Coleman EA . Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–28. doi:.https://doi.org/10.1056/NEJMsa0803563
35 Burwell SM . Setting value-based payment goals--HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–9. doi:.https://doi.org/10.1056/NEJMp1500445
36 Tsugawa Y , Jena AB , Figueroa JF , Orav EJ , Blumenthal DM , Jha AK . Comparison of Hospital Mortality and Readmission Rates for Medicare Patients Treated by Male vs Female Physicians. JAMA Intern Med. 2017;177(2):206–13. doi:.https://doi.org/10.1001/jamainternmed.2016.7875
37 Figueroa JF , Joynt KE , Zhou X , Orav EJ , Jha AK . Safety-net Hospitals Face More Barriers Yet Use Fewer Strategies to Reduce Readmissions. Med Care. 2017;55(3):229–35. doi:.https://doi.org/10.1097/MLR.0000000000000687
38 Zuckerman RB , Sheingold SH , Orav EJ , Ruhter J , Epstein AM . Readmissions, Observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543–51. doi:.https://doi.org/10.1056/NEJMsa1513024
39 Wasfy JH , Zigler CM , Choirat C , Wang Y , Dominici F , Yeh RW . Readmission Rates After Passage of the Hospital Readmissions Reduction Program: A Pre-Post Analysis. Ann Intern Med. 2017;166(5):324–31. doi:.https://doi.org/10.7326/M16-0185
40 Auerbach AD , Kripalani S , Vasilevskis EE , Sehgal N , Lindenauer PK , Metlay JP , et al. Preventability and Causes of Readmissions in a National Cohort of General Medicine Patients. JAMA Intern Med. 2016;176(4):484–93. doi:.https://doi.org/10.1001/jamainternmed.2015.7863
41 Coleman EA , Parry C , Chalmers S , Min SJ . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–8. doi:.https://doi.org/10.1001/archinte.166.17.1822
42 Naylor MD , Brooten D , Campbell R , Jacobsen BS , Mezey MD , Pauly MV , et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–20. doi:.https://doi.org/10.1001/jama.281.7.613
43 Anderson C , Deepak BV , Amoateng-Adjepong Y , Zarich S . Benefits of comprehensive inpatient education and discharge planning combined with outpatient support in elderly patients with congestive heart failure. Congest Heart Fail. 2005;11(6):315–21. doi:.https://doi.org/10.1111/j.1527-5299.2005.04458.x
44 Jack BW , Chetty VK , Anthony D , Greenwald JL , Sanchez GM , Johnson AE , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–87. doi:.https://doi.org/10.7326/0003-4819-150-3-200902030-00007
45 Siu AL , Kravitz RL , Keeler E , Hemmerling K , Kington R , Davis JW , et al. Postdischarge geriatric assessment of hospitalized frail elderly patients. Arch Intern Med. 1996;156(1):76–81. doi:.https://doi.org/10.1001/archinte.1996.00440010094012
46 Holland R , Lenaghan E , Harvey I , Smith R , Shepstone L , Lipp A , et al. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. BMJ. 2005;330(7486):293. doi:.https://doi.org/10.1136/bmj.38338.674583.AE
47 Naylor M , Brooten D , Jones R , Lavizzo-Mourey R , Mezey M , Pauly M . Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Ann Intern Med. 1994;120(12):999–1006. doi:.https://doi.org/10.7326/0003-4819-120-12-199406150-00005
48 Shepperd S , Parkes J , McClaren J , Phillips C . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2004;(1):CD000313.
49 Low LL , Tan SY , Ng MJ , Tay WY , Ng LB , Balasubramaniam K , et al. Applying the Integrated Practice Unit Concept to a Modified Virtual Ward Model of Care for Patients at Highest Risk of Readmission: A Randomized Controlled Trial. PLoS One. 2017;12(1):e0168757. doi:.https://doi.org/10.1371/journal.pone.0168757
50 Lesprit P , Landelle C , Brun-Buisson C . Clinical impact of unsolicited post-prescription antibiotic review in surgical and medical wards: a randomized controlled trial. Clin Microbiol Infect. 2013;19(2):E91–7. doi:.https://doi.org/10.1111/1469-0691.12062
51 Makowsky MJ , Koshman SL , Midodzi WK , Tsuyuki RT . Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study [NCT00351676] [NCT00351676]. Med Care. 2009;47(6):642–50. doi:.https://doi.org/10.1097/MLR.0b013e3181926032
52 Thanarajasingam U , McDonald FS , Halvorsen AJ , Naessens JM , Cabanela RL , Johnson MG , et al. Service census caps and unit-based admissions: resident workload, conference attendance, duty hour compliance, and patient safety. Mayo Clin Proc. 2012;87(4):320–7. doi:.https://doi.org/10.1016/j.mayocp.2011.12.012
53 Jenq GY , Doyle MM , Belton BM , Herrin J , Horwitz LI . Quasi-Experimental Evaluation of the Effectiveness of a Large-Scale Readmission Reduction Program. JAMA Intern Med. 2016;176(5):681–90. doi:.https://doi.org/10.1001/jamainternmed.2016.0833
54 Buurman BM , Parlevliet JL , Allore HG , Blok W , van Deelen BA , Moll van Charante EP , et al. Comprehensive Geriatric Assessment and Transitional Care in Acutely Hospitalized Patients: The Transitional Care Bridge Randomized Clinical Trial. JAMA Intern Med. 2016;176(3):302–9. doi:.https://doi.org/10.1001/jamainternmed.2015.8042
55 Ha Dinh TT , Bonner A , Clark R , Ramsbotham J , Hines S . The effectiveness of the teach-back method on adherence and self-management in health education for people with chronic disease: a systematic review. JBI Database Syst Rev Implement Reports. 2016;14(1):210–47. doi:.https://doi.org/10.11124/jbisrir-2016-2296
56 Krumholz HM , Amatruda J , Smith GL , Mattera JA , Roumanis SA , Radford MJ , et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39(1):83–9. doi:.https://doi.org/10.1016/S0735-1097(01)01699-0
57 Greysen SR , Harrison JD , Kripalani S , Vasilevskis E , Robinson E , Metlay J , et al. Understanding patient-centred readmission factors: a multi-site, mixed-methods study. BMJ Qual Saf. 2017;26(1):33–41. doi:.https://doi.org/10.1136/bmjqs-2015-004570
58 Paasche-Orlow MK , Riekert KA , Bilderback A , Chanmugam A , Hill P , Rand CS , et al. Tailored education may reduce health literacy disparities in asthma self-management. Am J Respir Crit Care Med. 2005;172(8):980–6. doi:.https://doi.org/10.1164/rccm.200409-1291OC
59 Peter D , Robinson P , Jordan M , Lawrence S , Casey K , Salas-Lopez D . Reducing readmissions using teach-back: enhancing patient and family education. J Nurs Adm. 2015;45(1):35–42. doi:.https://doi.org/10.1097/NNA.0000000000000155
60 Gill TM , Allore HG , Gahbauer EA , Murphy TE . Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304(17):1919–28. doi:.https://doi.org/10.1001/jama.2010.1568
61 Ellis G , Whitehead MA , Robinson D , O’Neill D , Langhorne P . Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ. 2011;343(oct27 1):d6553. doi:.https://doi.org/10.1136/bmj.d6553
62 Buurman BM , Parlevliet JL , van Deelen BA , de Haan RJ , de Rooij SE . A randomised clinical trial on a comprehensive geriatric assessment and intensive home follow-up after hospital discharge: the Transitional Care Bridge. BMC Health Serv Res. 2010;10(1):296. doi:.https://doi.org/10.1186/1472-6963-10-296
63 Pannill FC . In older hospitalized patients, adding transitional care to in-hospital geriatric assessment did not improve ADL. Ann Intern Med. 2016;164(12):JC63. doi:.https://doi.org/10.7326/ACPJC-2016-164-12-063
64 Kircher TT , Wormstall H , Müller PH , Schwärzler F , Buchkremer G , Wild K , et al. A randomised trial of a geriatric evaluation and management consultation services in frail hospitalised patients. Age Ageing. 2007;36(1):36–42. doi:.https://doi.org/10.1093/ageing/afl102
65 Stuck AE , Siu AL , Wieland GD , Rubenstein LZ , Adams J . Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993;342(8878):1032–6. doi:.https://doi.org/10.1016/0140-6736(93)92884-V
66 Reuben DB , Borok GM , Wolde-Tsadik G , Ershoff DH , Fishman LK , Ambrosini VL , et al. A randomized trial of comprehensive geriatric assessment in the care of hospitalized patients. N Engl J Med. 1995;332(20):1345–50. doi:.https://doi.org/10.1056/NEJM199505183322007
67Institute of Medicine, Board on Global Health. Measuring the Impact on Interprofessional Education on Collaborative Practice and Patient Outcomes. Washington: The National Academies Press; 2015.
68 Naylor MD , Brooten DA , Campbell RL , Maislin G , McCauley KM , Schwartz JS . Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675–84. doi:.https://doi.org/10.1111/j.1532-5415.2004.52202.x
69 Mudge A , Laracy S , Richter K , Denaro C . Controlled trial of multidisciplinary care teams for acutely ill medical inpatients: enhanced multidisciplinary care. Intern Med J. 2006;36(9):558–63. doi:.https://doi.org/10.1111/j.1445-5994.2006.01135.x
70 Cohen HJ , Feussner JR , Weinberger M , Carnes M , Hamdy RC , Hsieh F , et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002;346(12):905–12. doi:.https://doi.org/10.1056/NEJMsa010285
71 Saint S , Fowler KE , Krein SL , Flanders SA , Bodnar TW , Young E , et al. An academic hospitalist model to improve healthcare worker communication and learner education: results from a quasi-experimental study at a Veterans Affairs medical center. J Hosp Med. 2013;8(12):702–10. doi:.https://doi.org/10.1002/jhm.2105
72 Boyd CM , Darer J , Boult C , Fried LP , Boult L , Wu AW . Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–24. doi:.https://doi.org/10.1001/jama.294.6.716
73 Hughes LD , McMurdo ME , Guthrie B . Guidelines for people not for diseases: the challenges of applying UK clinical guidelines to people with multimorbidity. Age Ageing. 2013;42(1):62–9. doi:.https://doi.org/10.1093/ageing/afs100
74 Roland M , Paddison C . Better management of patients with multimorbidity. BMJ. 2013;346(may02 1):f2510. doi:.https://doi.org/10.1136/bmj.f2510
75 Higgins A , Veselovskiy G , McKown L . Provider performance measures in private and public programs: achieving meaningful alignment with flexibility to innovate. Health Aff (Millwood). 2013;32(8):1453–61. doi:.https://doi.org/10.1377/hlthaff.2013.0007
76 Meyer GS , Nelson EC , Pryor DB , James B , Swensen SJ , Kaplan GS , et al. More quality measures versus measuring what matters: a call for balance and parsimony. BMJ Qual Saf. 2012;21(11):964–8. doi:.https://doi.org/10.1136/bmjqs-2012-001081
77 Jha AK , Orav EJ , Epstein AM . Public reporting of discharge planning and rates of readmissions. N Engl J Med. 2009;361(27):2637–45. doi:.https://doi.org/10.1056/NEJMsa0904859
78 Moore L , Lauzier F , Stelfox HT , Kortbeek J , Simons R , Berthelot S , et al. Derivation and Validation of a Quality Indicator to Benchmark In-Hospital Complications Among Injury Admissions. JAMA Surg. 2016;151(7):622–30. doi:.https://doi.org/10.1001/jamasurg.2015.5484
79 Cella D , Riley W , Stone A , Rothrock N , Reeve B , Yount S , et al.; PROMIS Cooperative Group. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–94. doi:.https://doi.org/10.1016/j.jclinepi.2010.04.011
80 Gruber-Baldini AL , Velozo C , Romero S , Shulman LM . Validation of the PROMIS(®) measures of self-efficacy for managing chronic conditions. Qual Life Res. 2017;26(7):1915–24. doi:.https://doi.org/10.1007/s11136-017-1527-3
81 Bierer BE , Li R , Barnes M , Sim I . A Global, Neutral Platform for Sharing Trial Data. N Engl J Med. 2016;374(25):2411–3. doi:.https://doi.org/10.1056/NEJMp1605348
82 Merson L , Gaye O , Guerin PJ . Avoiding Data Dumpsters--Toward Equitable and Useful Data Sharing. N Engl J Med. 2016;374(25):2414–5. doi:.https://doi.org/10.1056/NEJMp1605148
83Gesundheitsversorgung, Nationales Forschungsprogram 74. Available from: http://www.nfp74.ch/de. Accessed 23 February 2017.
84 Blum CA , Nigro N , Briel M , Schuetz P , Ullmer E , Suter-Widmer I , et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015;385(9977):1511–8. doi:.https://doi.org/10.1016/S0140-6736(14)62447-8
No financial support and no other potential conflict of interest relevant to this article was reported.