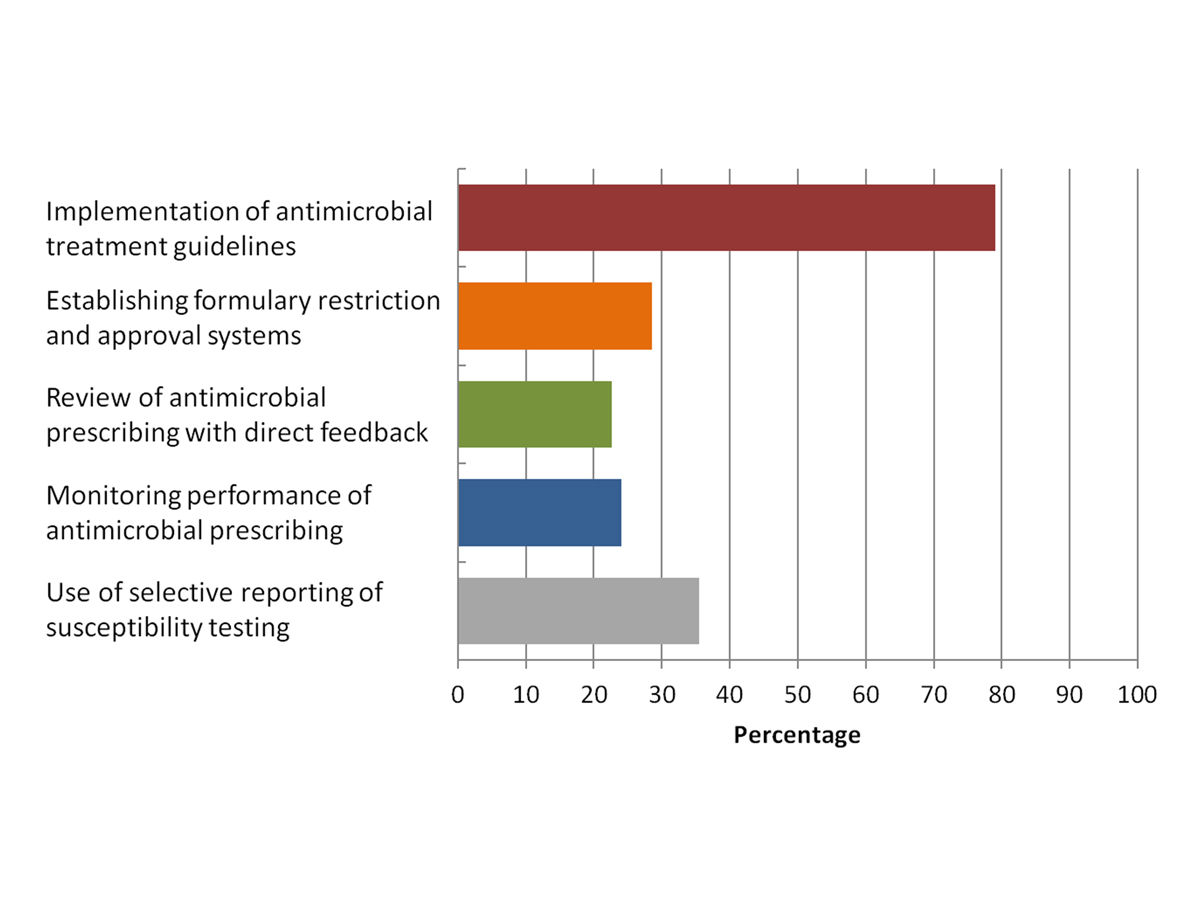
Figure 1 Presence of the five essential antimicrobial stewardship strategies (as defined by the Australian Commission on Safety and Quality in Health Care) in Swiss hospitals surveyed (n = 63).
DOI: https://doi.org/10.4414/smw.2017.14512
Antimicrobial resistance is a growing global health problem that threatens public health throughout the world as acknowledged by the 2014 World Health Organization (WHO) report on antimicrobial resistance [1]. This is particularly true for infections with multi-drug-resistant gram-negative bacteria, which are associated with a significantly elevated mortality risk [2, 3] and economic burden [4]. In addition, the World Bank considers antimicrobial resistance to cause global economic damage on a par with the 2008 financial crisis [5]. According to the Swiss surveillance system (www.anresis.ch), resistance of Escherichia coli and Klebsiella pneumoniae to third or fourth generation cephalosporins has increased continuously during the last ten years. Strategies to address this problem include establishing a comprehensive surveillance system for antimicrobial resistance and usage of an effective antimicrobial stewardship (AMS) system; improving infection prevention and control measures; investing in the development of new antibiotics; and increasing awareness and understanding of antimicrobial resistance.
AMS is a collection of strategies and tools to improve clinical outcomes and the use of antimicrobials by promoting the selection of the optimal antimicrobial regimen, dose, duration and route of administration. It is considered a key strategy in local and national programmes to prevent the emergence of antimicrobial resistance. Successful AMS programmes have demonstrated a significant reduction in overall and inappropriate antimicrobial use and may also impact on antimicrobial resistance and clinical outcomes [6–8]. However, comprehensive future studies are required to judge the impact of AMS programmes on cost effectiveness [9] and major health outcomes. A recent meta-analysis documented a significant association of AMS programmes with a reduced incidence of Clostridium difficile infection, the most common nosocomial pathogens in patients with diarrhoea [10]. Many hospitals around the world still lack AMS programmes, primarily because of a lack of personnel and funding [11], which may be true for Switzerland, too.
In 2013, the heads of the Swiss Federal Department of Home Affairs and the Federal Department of Economic Affairs, Education and Research commissioned the Federal Offices for Health (FOPH), Food Safety and Veterinary Affairs (FSVO), Agriculture (FOAG) and the Environment (FOEN) to draw up a comprehensive antibiotic strategy for Switzerland (StAR). Appropriate use of antibiotics has been defined as one of eight strategic objectives directed towards achieving the primary objective of ensuring the long-term efficacy of antibiotics in preserving human and animal health. This also includes developing national AMS guidelines and toolkits for use in hospitals and outpatient services. However, data on the prevalence of existing AMS programmes or local strategies to improve antimicrobial prescription in Switzerland are lacking.
The aim of the current survey was to perform an in-depth review describing AMS activities currently being undertaken in Swiss hospitals to elucidate specific gaps and to identify potential future aspects of AMS strategies in Switzerland.
The survey was developed by the authors following a review of the literature and previous AMS surveys [11, 12]. A web-based survey tool (SurveyMonkey®) was used. In December 2016, a letter of invitation stating the purpose of the survey was sent to qualified senior staff members at Swiss hospitals including infectious diseases physicians and members of Swissnoso (primary choice, whenever possible), internal medicine physicians, or other senior staff members. A total of 134 Swiss hospitals offering overnight stays to patients were included, with mental health facilities being excluded due to low levels of antimicrobial prescription. The original three-week collection period was extended to two months to maximise the response rate and included direct contact of the respective senior staff member via telephone. The survey (appendix 1, available in a separate file for downloading) consisted of 53 questions, which were mainly closed questions with the option of a free-text response. Only one respondent per hospital was allowed, but the survey could be accessed again to complete all required information. Only entries with 50% or more of the questions answered were included in the analysis. Non-respondents were reminded by telephone or email at least twice.
Sixty-nine of the134 eligible hospitals survey responses were returned. Six entries had to be excluded (>50% missing data). Hence, a total of 63/134 (47%) hospitals or hospital networks (n = 3 with a total of 11 hospitals) completed the questionnaire. University and cantonal hospitals accounted for 26/63 (41%) of the returns (response rate 74%), regional hospitals for 23/63 (37%) (response rate 51%), and private or rehabilitation hospitals for 14/63 (22%) (response rate 26%). The survey was mainly answered by infectious diseases (65%) or internal medicine physicians (25%). Hospitals with up to 200 beds accounted for 35/63 (55%), those with 201 to 500 beds for 18/63 (29%) and those with over 500 beds for 10/63 (16%) of the total. Intensive or intermediate care unit beds were available in all but 5 hospitals (median 10 (range 2–100) beds).
Infectious diseases physicians, clinical microbiologists, and ward pharmacists were available on site in 49/63 (79%), 27/63 (43%), and 25/63 (40%) of hospitals with none of these resources available in 10/63 (16%) of hospitals. Whereas the majority of hospitals reported the existence of a drug and therapeutics committee (67%), AMS committees were only present in 21% of hospitals. Similarly, a published AMS strategy and policy were only reported by 21/63 (33%) and14/63 (22%) of hospitals respectively. Infectious diseases physicians were responsible for improving and evaluating antimicrobial use in many hospitals (71% and 60%, respectively), whereas pharmacists were involved less frequently (13% and 24%, respectively). Overall, an official AMS programme was implemented in only 18/63 (29%) of hospitals (3 university, 6 cantonal, 5 regional and 4 private hospitals) for a median duration of 6 years (range 1–15), and a median of 3 hours (range 1–20) of dedicated resources per week were available for this programme.
Hospital-specific antimicrobial treatment recommendations were available in the majority of hospitals (79%) including the use of guidelines from tertiary hospitals (6%). Most treatment guidelines had been updated within the previous 2 years (87% of all guidelines). Topics covered in the majority (>50%) of guidelines were avoidance of broad-spectrum antimicrobials (70%), specimen collection (62%), duration of treatment (74%), intravenous to oral switch (60%), treatment recommendation for specific indications (76%), surgical antibiotic prophylaxis (74%), dosing for special populations (e.g., renal impairment, 50%), and alternative antimicrobial choices (e.g., if allergic, 64%). In contrast, guidelines less frequently (<50%) contained information regarding antibiotic formulary (including restricted antibiotics), review of antimicrobial therapy after 48–72 hours, therapeutic drug monitoring, antifungal/antiviral treatment, side effects, outpatient parenteral antimicrobial therapy, and emphasis on documenting the indications of antimicrobial treatment (table 1). 48/63 (76%) and 37/63 (59%) of respondents respectively reported that antimicrobial resistance and rates of infection with Clostridium difficile were monitored at their institution.
Table 1 Topics covered in antimicrobial treatment guidelines or stewardship policy according to the respondents surveyed (n = 50; guidelines were not available in 13 hospitals).
| Topic | Percentage |
|---|---|
| Avoidance of broad-spectrum antimicrobials | 70% |
| Documentation of indications and severity of illness | 30% |
| Specimen collection before antimicrobial treatment initiation | 62% |
| Treatment duration | 74% |
| Antibiotic formulary | 36% |
| Review of antibiotic therapy at 48 to 72 hours | 42% |
| Intravenous to oral switch | 60% |
| Therapeutic drug monitoring | 38% |
| Treatment of specific diseases | 76% |
| Situations when antimicrobial therapy is NOT required | 48% |
| Antifungal treatment | 46% |
| Antiviral treatment | 32% |
| Surgical antibiotic prophylaxis | 74% |
| Alternative antimicrobial choices (e.g., if allergic) | 64% |
| Dosage for special populations (e.g., renal impairment) | 50% |
| Side effects | 16% |
| Outpatient parenteral antibiotic therapy | 12% |
With regards to the three key objectives of a current or planned AMS programme, reduction of antimicrobial resistance (39/63, 62%), reduction of the use of antimicrobial drugs (34/63, 54%) and improvement of antimicrobial prescription (27/63, 43%) were cited most frequently. In contrast, reduction of C. difficile infections and costs were perceived as the least important goals (5/63, 8% and 9/63, 14%, respectively).
Barriers to successful implementation of an effective AMS programme were identified in 47/63 (75%) of hospitals, in particular with regards to lack of personnel or funding (44/63, 70% and 27/63, 43%, respectively), which was most commonly described in university/cantonal and regional hospitals. A further 20/63 (32%) perceived prescriber opposition as a relevant barrier to a comprehensive AMS, particularly in private hospitals (6/14 (43%). Appropriate resources (financial, manpower, IT support), the establishment of a multidisciplinary AMS teams, and support from hospital administration services were identified as the most important requirements for an effective AMS programme (51/63 (84%), 41/63 (67%), and 40/63 (66%), respectively) followed by the availability of hospital-specific antimicrobial resistance and antimicrobial usage data and of an antimicrobial prescription policy. The availability of electronic prescription and the implementation of national reporting and benchmarking of antimicrobial use and prescription quality were perceived as less important (<33%).
Hospitals were asked about core and optional elements of future Swiss AMS programmes (table 2). Implementation of national or in-house clinical treatment guidelines, education of prescribers about good antimicrobial prescription practice, monitoring of hospital-specific antimicrobial resistance, and active surveillance of positive blood cultures with direct feedback were perceived as core elements by the majority of hospitals (>75%). Additionally, implementation of national or in-house diagnostic evaluation guidelines, review of antimicrobial prescription with immediate feedback, and hospital-specific antibiograms were regarded as core elements by at least 50% of respondents. The most frequent optional elements were use of rapid diagnostic tests with or without stewardship advice, the use of procalcitonin to decrease antibiotic treatment duration, point prevalence surveys of the quality of antimicrobial prescription, selective reporting of susceptibility testing, therapeutic drug monitoring with stewardship advice, establishment of diagnostic pathways for patients with reported β-lactam antibiotic allergy, and the use of information technology such as clinical decision support or online approval systems (all >50%). With regard to local limitations, only the use of procalcitonin to decrease antibiotic treatment duration and of antimicrobial formulary restriction and approval systems were most commonly cited as not feasible by the surveyed hospitals (12/63, 19%, and 11/63, 17%).
Table 2 Core and optional elements that an antimicrobial stewardship programme in Swiss hospitals should consist of according to the respondents surveyed (n = 63).
| Antimicrobial stewardship element | Core/compulsory | Optional | Not feasible |
|---|---|---|---|
| Antimicrobial treatment guidelines | 90.5% | 7.9% | 1.6% |
| Diagnostic evaluation guidelines | 54.0% | 44.4% | 1.6% |
| Antimicrobial formulary restriction and approval systems | 38.1% | 46.0% | 15.9% |
| AMS ward rounds with intervention | 52.4% | 38.1% | 9.5% |
| Monitoring of prescription performance with feedback to prescribers | 31.8% | 55.6% | 12.7% |
| Monitoring of hospital-specific antimicrobial resistance | 84.1% | 14.3% | 1.6% |
| Selective reporting of susceptibility testing | 46.0% | 50.8% | 3.2% |
| Education of prescribers about good antimicrobial prescribing practice and resistance | 87.3% | 12.7% | 0% |
| Clinical decision-support systems | 19.1% | 63.5% | 17.5% |
| Annual publication of hospital-specific antibiograms | 58.7% | 36.5% | 3.2% |
| Therapeutic drug monitoring with stewardship advice | 27.0% | 61.9% | 11.1% |
| Use of rapid diagnostic tests with/without stewardship advice | 27.0% | 65.1% | 7.9% |
| Active surveillance of positive blood cultures | 74.6% | 23.8% | 1.6% |
| Diagnostic pathway for patients with reported beta-lactam allergy | 38.1% | 57.1% | 4.8% |
| Use of procalcitonin to decrease treatment duration | 14.3% | 66.7% | 19.1% |
Almost all respondents agreed that their patients would benefit from an AMS programme (60/63, 95%) and that a national AMS strategy including compulsory and optional stewardship elements would be desirable (53/63, 84%).
Overall, the majority of respondents indicated that they provide education to hospital staff on antimicrobial resistance and on improving antimicrobial prescription (90%).This included senior and junior medical staff (78% and 84%, respectively), but rarely nurses or pharmacists (19% and 2%, respectively). Face-to-face training was the mode primarily used in the majority of hospitals (76%), with short education courses or written information provided less frequently (49% and 27%, respectively).
Various strategies were employed by the hospitals surveyed. Whereas most hospitals had a defined formulary of antimicrobial agents (43/63 (68%)), restrictions on the use of selected and/or broad-spectrum antimicrobials were infrequently implemented (18/63 (29%)), which mainly included carbapenems (56%), daptomycin and/or linezolid (44%), antifungal drugs excluding fluconazole (56%) and rarely vancomycin, quinolones and cephalosporins (17%, 17% and 11%, respectively). Infectious diseases physicians were mainly involved in the approval process (72%).
AMS ward rounds existed in a minority of surveyed hospitals (9/63 (14%)) and were mainly conducted in the intensive care unit. Similarly, review of antimicrobial prescriptions with point-of-care intervention and direct feedback was employed infrequently (14/63 (23%)) with a median of 2 hours spent per week and almost exclusively performed by infectious diseases specialists (92%). Respondents indicated that these sessions mainly included the review of indications and compliance with policy/guidelines, appropriateness of dose, route and duration, treatment according to culture results, and de-escalation possibilities (all 100%), whereas IV to oral switch and therapeutic drug monitoring were evaluated less frequently (79%).
Additional AMS interventions and strategies were employed infrequently (table 3) in the majority of hospitals with the exception of routine follow-up of patients with positive blood cultures and the monitoring of Staphylococcus aureus or Candida spp. bloodstream infection. In particular, interventions to reduce the duration of antibiotic treatment including procalcitonin testing, automatic stop/review orders including a recommendation to review the appropriateness of all antimicrobials within 48 to 72 hours, alerts or criteria for IV to oral switch, and alerts for dose adjustment in case of organ dysfunction were utilised rarely.
Table 3 Antimicrobial stewardship interventions implemented in the hospitals surveyed (n = 62; answers from one single hospital missing).
| Antimicrobial stewardship intervention | In all areas | In some areas | Not implemented |
|---|---|---|---|
| Selective reporting of antimicrobial susceptibilities | 38.7% | 14.5% | 46.8% |
| Automatic stop/review orders | 8.1% | 3.2% | 88.7% |
| Dose adjustment alerts in case of organ dysfunction | 6.5% | 8.1% | 85.5% |
| Intravenous to oral switch alerts or criteria | 9.7% | 6.5% | 83.9% |
| Therapeutic drug monitoring alerts | 14.5% | 8.1% | 77.4% |
| Surveillance of positive blood cultures with feedback | 58.1% | 8.1% | 33.9% |
| Surveillance of S. aureus and/or Candida spp. Bloodstream infection with active involvement | 45.2% | 3.2% | 51.6% |
| Policy to review all antibiotics after 48 to 72 hours | 21.0% | 8.1% | 71.0% |
| Review of selected antimicrobial agents with feedback | 14.5% | 4.8% | 80.6% |
| Procalcitonin testing to prevent initiation of antimicrobials | 12.9% | 22.6% | 64.5% |
| Procalcitonin testing to stop antimicrobials | 8.1% | 21.0% | 71.0% |
| Clinical decision-support systems | 3.2% | 0% | 96.8% |
| Interventions for specific infections | 30.7% | 12.9% | 56.5% |
| Interventions to reduce duration of antimicrobial treatment | 25.8% | 9.7% | 64.5% |
| Use of procalcitonin to decrease treatment duration | 14.3% | 66.7% | 19.1% |
Cumulative antibiograms provided by internal or external microbiological laboratories were available in the majority of hospitals surveyed (38/63 (60%)). However, access to these antibiograms was limited to infectious disease physicians or infection-control nurses in a considerable number of hospitals (27%). Some physicians indicated that their microbiological laboratory would use selective reporting of susceptibility data for all areas (24/62 (39%)).
Antimicrobial consumption at the hospital level was monitored by 15/63 (24%) of hospitals and even less so at ward or department level (5/63, 8% and 7/63, 11%). Antimicrobial costs and data on consumption of specific antimicrobials were assessed by only 7/63 (11%) of hospitals.
Formal antimicrobial audits were conducted in 33/63 (52%) of hospitals (table 4). The most frequently undertaken audits were compliance with surgical antibiotic prophylaxis (27/33, 82%), microbiological cultures taken before start of antimicrobial treatment (9/33, 27%), and therapeutic drug monitoring for vancomycin and/or aminoglycosides (5/33, 15%). Documentation of indication, time to first dose in sepsis, adherence to treatment guidelines, duration of antimicrobial therapy, IV to oral switch, de-escalation/adaption of antimicrobial therapy after 48–72 hours or audits of specific clinical conditions (e.g., community-acquired pneumonia) were carried out infrequently (<10%) or not at all. Many respondents quoted that practice related to antimicrobial prescription and management was never audited (30/63, 48%), and even in hospitals with formal antimicrobial audits, only a minority of audits suggested in the literature were performed. More than two different audits were undertaken in only a single surveyed hospital. The results of the respective audits were reported to prescribers in a minority of hospitals conducting formal antimicrobial audits (15/33, 45%).
Table 4 Antimicrobial audits conducted at the hospitals surveyed (n = 63).
| Topic | Percentage |
|---|---|
| Time to first dose in sepsis | 3.2% |
| Surgical antibiotic prophylaxis | 43.6% |
| Microbiological specimen taken before starting antimicrobial therapy | 14.5% |
| Adherence to treatment guidelines | 4.8% |
| De-escalation of antimicrobial therapy within 48–72 hours | 0% |
| Duration of antimicrobial therapy | 3.2% |
| Intravenous to oral switch | 4.8% |
| Use of therapeutic drug monitoring for vancomycin and/or aminoglycosides | 8.1% |
| Audit of specific antimicrobials | 3.2% |
| Audit of restricted antimicrobials | 3.2% |
| Audit of specific units/wards | 6.5% |
| None | 48.3% |
The present survey is the first to describe existing AMS strategies and activities in Switzerland, including the desired aims of a future AMS programme and barriers to its implementation. Overall, the presence of a comprehensive AMS programme, including the presence of an AMS committee, policy, audits and guidelines, is unusual in Switzerland. Instead, individual standalone AMS strategies are being implemented in the majority of hospitals. These include the use of treatment guidelines and monitoring of positive blood cultures with direct feedback.
The most obvious differences as compared to results from a worldwide survey of 660 hospitals [11] and a survey from Victoria/Australia [12] are the following:
AMS activities are less structured in Swiss hospitals compared with other settings. Whereas AMS programmes were reported in 56% of worldwide hospitals, this was only true in 29% of the Swiss hospitals surveyed. With the exception of implementation of treatment guidelines and monitoring of bloodstream infections, the majority of recognised AMS strategies were considerably under-utilised in Swiss hospitals. For example, restrictions on the use of broad-spectrum antimicrobial drugs and AMS ward rounds with direct feedback to the prescriber were only present in 29% and 14% of hospitals surveyed respectively, in contrast to 84% and 64% of worldwide and 30% and 52% of hospitals in Victoria. Interventions to reduce treatment duration and automatic stop/review orders were rarely employed, as were selective reporting of susceptibility data (39% vs 93% in hospitals in Victoria). Similarly, evaluation of antimicrobial use is conducted less frequently in the Swiss hospitals surveyed (52% vs 80% of worldwide hospitals and 47% of hospitals in Victoria) with the exception of compliance with surgical antibiotic prophylaxis. In particular, documentation of indications, adherence to treatment guidelines, and duration of antimicrobial treatment were routinely evaluated in less than 10% of Swiss hospitals surveyed. Overall, antimicrobial consumption was monitored in 22% of Swiss hospitals compared to 85% of worldwide hospitals.
In Australia, the Australian Commission on Safety and Quality in Health Care has defined five essential strategies for an effective AMS programme [13]. When assessing the results of the present survey against these strategies (fig. 1), it is clear that considerable gaps exist in most hospitals with regard to the implementation of an AMS programme (with the exception of the implementation of treatment guidelines). The same result is evident when using the Core Elements of Hospital Antibiotic Stewardship Programmes of the Centers for Disease Control and Prevention as the comparator [14]. As expected, AMS strategies were most frequently implemented in university hospitals, whereas the opposite was true for private hospitals. This was particularly the case for the establishment of formulary restrictions and approval systems (57% and 8% in university and private hospitals respectively) and the implementation of antimicrobial treatment guidelines (100% and 69% respectively).

Figure 1 Presence of the five essential antimicrobial stewardship strategies (as defined by the Australian Commission on Safety and Quality in Health Care) in Swiss hospitals surveyed (n = 63).
In line with hospitals around the globe, education on AMS was provided in the majority of Swiss hospitals, mainly in a face-to-face manner. In addition, lack of personnel or funding and prescriber opposition were perceived as the main barriers by survey respondents.
Respondents were also queried about their vision for elements and aims for a future Swiss AMS programme. Although lack of funding and personnel followed by prescriber opposition, in other words potentially major administrative/managerial challenges, were perceived as the main barriers to AMS implementation, almost all respondents thought that a national AMS strategy including compulsory and optional stewardship elements would be desirable (53/63, 84%) and that AMS programmes are of benefit for patients (95%). The Joint Commission for Accreditation of hospitals, an independent, not-for-profit organisation of the United States, has set requirements for AMS that, to our knowledge, have not been met in a single Swiss hospital [15]. The most frequently cited goal of any future AMS programme was reduction of antimicrobial resistance followed by reduction in the overall use of antimicrobials, even when limited data are available to support the view of the respondents. Implementation of treatment guidelines, active surveillance of positive blood cultures, monitoring of hospital-specific antimicrobial resistance and education of prescribers about good antimicrobial prescription practice were perceived as core elements of a future AMS programme in Switzerland, which is not surprising as these elements are already established in the majority of hospitals surveyed. Other important AMS strategies such as point prevalence survey of quality of antimicrobial prescription, selective reporting of susceptibility testing, and the use of clinical decision or online approval systems were mainly regarded as optional elements. Interestingly, restriction of certain antimicrobials combined with approval systems was most commonly cited as not feasible. This is a strategy that is regarded as essential in some countries.
Our survey has several limitations. First, the response rate of only 47% was low, which limits the validity of our survey results. Whereas all university hospitals and the majority of cantonal hospitals responded, many private, regional and rehabilitation hospitals, which may have limited resources for AMS, did not participate. Hence, our survey provides insight into current and future AMS activities and requirements of mainly larger hospitals. Second, a comprehensive validation of data entry was not feasible. Although our intent was to select respondents with the most comprehensive overview of AMS strategies employed in a hospital and who are familiar with the AMS terminology, the interpretation of definitions used in the survey may still differ significantly. Third, our results regarding AMS programmes, strategies and activities may represent an overestimation of the true prevalence owing to significant non-response bias (mainly of private, regional and rehabilitation hospitals) and the inclusion of mainly infectious diseases physicians who may overestimate their AMS activities. On the other hand, this survey may also underestimate currently implemented AMS strategies, as pharmacists, who play a crucial role in delivering AMS activities in many countries and maybe also in Switzerland, were not surveyed. On May 9, 2017, Swissnoso organised a kick-off meeting for a Swiss national AMS with stakeholders including hospital pharmacists and insurance representatives, the Swiss medical association (FMH), the Swiss hospital association (H+) and others to gain as much input as possible with a view to launching AMS in Switzerland. For 2017 several activities are planned including on-site visits to different hospitals, development of monitoring strategies (antibiotic consumption and resistance) and identification of AMS strategies, and interventions with a significant impact and a realistic chance of successful (in terms of resources required) implementation in different Swiss hospitals.
In conclusion, Swiss hospitals are at different stages of implementing AMS strategies. Comprehensive AMS programmes are unusual, which may be related to a lack of funding and personnel, given that the overwhelming majority of respondents favour AMS programmes to reduce antimicrobial resistance and the use of antimicrobial drugs. The establishment of modular national AMS standards or guidelines may aid in advancing current AMS strategies and introduce AMS programmes in Swiss hospitals, while at the same time offering flexibility to account for local healthcare structure and resources.
The questionnaire is available as a separate file at: https://smw.ch/en/article/doi/smw.2017.14512/
Members of Swissnoso: Carlo Balmelli, Marie-Christine Eisenring, Stephan Harbarth, Stefan Kuster, Jonas Marschall, Virginie Masserey Spicher, Didier Pittet, Christian Ruef, Hugo Sax, Matthias Schlegel, Alexander Schweiger, Nicolas Troillet, Andreas Widmer, and Giorgio Zanetti.
Supported by the Office of Public Health, Bern, Switzerland
1World Health Organization. Antimicrobial resistance: global report on surveillance 2014. 2014. Available from: http://www.who.int/drugresistance/documents/surveillancereport/en/.
2 Nelson RE , Slayton RB , Stevens VW , Jones MM , Khader K , Rubin MA , et al. Attributable Mortality of Healthcare-Associated Infections Due to Multidrug-Resistant Gram-Negative Bacteria and Methicillin-Resistant Staphylococcus Aureus . Infect Control Hosp Epidemiol. 2017;38(7):848–56. doi:.https://doi.org/10.1017/ice.2017.83
3 Lim C , Takahashi E , Hongsuwan M , Wuthiekanun V , Thamlikitkul V , Hinjoy S , et al. Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. eLife. 2016;5:5. doi:.https://doi.org/10.7554/eLife.18082
4 Evans HL , Lefrak SN , Lyman J , Smith RL , Chong TW , McElearney ST , et al. Cost of Gram-negative resistance. Crit Care Med. 2007;35(1):89–95. doi:.https://doi.org/10.1097/01.CCM.0000251496.61520.75
5The World Bank. Drug-resistant infections: a threat to our economic future. 2017. Available from: http://documents.worldbank.org/curated/en/323311493396993758/final-report.
6 Feazel LM , Malhotra A , Perencevich EN , Kaboli P , Diekema DJ , Schweizer ML . Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69(7):1748–54. doi:.https://doi.org/10.1093/jac/dku046
7 Brink AJ , Messina AP , Feldman C , Richards GA , Becker PJ , Goff DA , et al.; Netcare Antimicrobial Stewardship Study Alliance. Antimicrobial stewardship across 47 South African hospitals: an implementation study. Lancet Infect Dis. 2016;16(9):1017–25. doi:.https://doi.org/10.1016/S1473-3099(16)30012-3
8 Boel J , Andreasen V , Jarløv JO , Østergaard C , Gjørup I , Bøggild N , et al. Impact of antibiotic restriction on resistance levels of Escherichia coli: a controlled interrupted time series study of a hospital-wide antibiotic stewardship programme. J Antimicrob Chemother. 2016;71(7):2047–51. doi:.https://doi.org/10.1093/jac/dkw055
9 Naylor NR , Zhu N , Hulscher M , Holmes A , Ahmad R , Robotham JV . Is antimicrobial stewardship cost-effective? A narrative review of the evidence. Clin Microbiol Infect. 2017;S1198-743X(17)30330-0. [Epub ahead of print.]
10 Baur D , Gladstone BP , Burkert F , Carrara E , Foschi F , Döbele S , et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(9):990–1001. doi:.https://doi.org/10.1016/S1473-3099(17)30325-0
11 Howard P , Pulcini C , Levy Hara G , West RM , Gould IM , Harbarth S , et al.; ESCMID Study Group for Antimicrobial Policies (ESGAP); ISC Group on Antimicrobial Stewardship. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J Antimicrob Chemother. 2015;70(4):1245–55.
12 James RS , McIntosh KA , Luu SB , Cotta MO , Marshall C , Thursky KA , et al. Antimicrobial stewardship in Victorian hospitals: a statewide survey to identify current gaps. Med J Aust. 2013;199(10):692–5. doi:.https://doi.org/10.5694/mja13.10422
13Duguid M, Cruickshank M. Antimicrobial stewardship in Australian hospitals 2011. Available from: http://www.safetyandquality.gov.au/wp-content/uploads/2011/01/Antimicrobial-stewardship-in-Australian-Hospitals-2011.pdf.
14CDC. Core elements of hospital antibiotic stewardship programs 2014. Available from: http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html.
15The Joint Commission. Proposed Standard for Antimicrobial Stewardship in AHC, CAH, HAP, NCC, and OBS. 2015. Available from: https://jointcommission.az1.qualtrics.com/CP/File.php?F=F_5tDHGzIVDMHenDn.
Supported by the Office of Public Health, Bern, Switzerland