The subcutaneous implantable cardioverter defibrillator in daily clinical practice
DOI: https://doi.org/10.4414/smw.2017.14518
Tardu
Özkartala, Alexander
Breitensteina, Ardan M.
Sagunera, Devdas T.
Inderbitzinb, Markus J.
Wilhelmb, Stefano
Benussib, Francesco
Maisanob, Thomas F.
Lüschera, Frank
Ruschitzkaa, Jan
Steffela
aDepartment of Cardiology, Electrophysiology and Cardiac Devices, University Heart Centre Zurich, Switzerland
bDepartment of Cardiac Surgery, University Heart Centre Zurich, Switzerland
Summary
INTRODUCTION
In Switzerland, the first implantation of a subcutaneous implantable cardioverter-defibrillator (S-ICD) took place in November 2012. Up until the end of 2016, a total of 111 S-ICDs have been implanted. The aim of this study was to summarise the experience of a tertiary centre in Switzerland and to discuss the results in the context of international registries.
METHODS
All patients in whom an S-ICD was implanted between November 2012 and the end of December 2016 at the University Heart Centre Zurich were included in this study. The clinical records of all patients were reviewed for retrospective collection of baseline characteristics as well as implantation and follow-up data.
RESULTS
A total of 37 S-ICDs were implanted. The majority of patients (81%) were male, the mean age was 47 ± 15 years. The most common underlying cardiac condition was coronary artery disease (30%), followed by hypertrophic cardiomyopathy (24%), inherited channelopathies (19%) and nonischaemic cardiomyopathy (11%). The median left ventricular ejection fraction was 44% (interquartile range 28–61%). There were four peri-interventional complications, all of which were pocket site-related. There were no cases of systemic infection or perioperative death. During a median follow up of 3.7 months, there were three appropriate and successful ICD shocks (8.1%). Two patients (5.4%) experienced a total of three inappropriate shocks, all due to T-wave oversensing.
CONCLUSION
This first large Swiss experience demonstrates results consistent with available international data. The S-ICD may hence represent an attractive alternative to conventional transvenous ICDs for a variety of patients.
Introduction
Despite improvements in medical therapy, sudden cardiac death (SCD) remains a major cause of cardiovascular mortality. To date, implantable cardioverter-defibrillators (ICDs) represent the most effective treatment in the prevention of SCD related to ventricular arrhythmias, in the context of a previously survived event (secondary prevention) as well as in patients with an increased risk for malignant life-threatening arrhythmias (primary prevention) [1–6]. Since the first ICD implantations in the early 1980s, surgical techniques, detection and discrimination algorithms, as well as programming experience, have improved considerably [3, 7–10]. Despite the proven benefit on survival in a large proportion of patients, transvenous ICD therapy comes with the risk of significant morbidity [11], mainly due to the use of a transvenous lead with an elevated risk for acute and long-term lead complications. These include pneumothorax, cardiac perforation and electrode displacement/dysfunction, as well as infection and inappropriate therapies. Implantation-related complications were reported to be as high as 10% [12, 13], and long-term lead failures due to abrasions or fractures over the intra- and extravascular course of the lead are present in up to 20% over 10 years [14]. The risk for device related infection is continuously present with a reported prevalence of 0.5–7% [15]. Once infected, the entire system has to be extracted in almost all instances for a definitive cure, again putting the patient at risk of substantial morbidity and mortality depending on the electrode type and dwell time [16]. Additionally, since the risk for complications increases with each intervention such as generator replacement, the life-time risk of transvenous ICD complications is particularly pronounced in younger patients.
To address these shortcomings of transvenous ICDs, a totally subcutaneous ICD system (S-ICD) was developed, which was approved for use in Europe in June 2009 [17]. This device is typically placed in a pocket between the anterior and mid-axillary line at the level of the apex of the left ventricle, while the S-ICD electrode is tunnelled subcutaneously from the device pocket to ≈1 cm above the xiphoid process, and subsequently cephalad towards the substernal notch (fig. 1) [18]. In contrast to traditional transvenous ICDs, the S-ICD is located completely in the extravascular (and even extrathoracic) space. Therefore, the typical risks associated with transvenous leads such as pneumothorax, cardiac perforation and endocarditis are practically nonexistent or substantially reduced (such as for system infection). Furthermore, the S-ICD lead is more stable and therefore less prone to dysfunction, since its design is different from that of transvenous leads [19].

Figure 1 Chest X-ray of a male patient after S-ICD implantation. The generator of the S-ICD is shown on the left side of the patient together with the electrode, which is tunnelled subcutaneously from the generator to the xiphoid area and towards the suprasternal notch.
In Switzerland, the first S-ICD was implanted in November 2012 at the University Hospital Zurich. Up until the end of the year 2016, a total of 111 S-ICDs were implanted in our country, 37 of these at our institution. The aim of this study was to summarise the results of our Swiss tertiary care centre and to discuss them in the context of international data registries.
Methods
Patient population
All patients in whom an S-ICD was implanted between November 2012 and the end of December 2016 at the University Heart Centre Zurich were included in this retrospective analysis. The implantation and clinical records of all patients were reviewed for collection of baseline characteristics and follow-up data. Baseline variables included age, gender, body mass index, heart rate and blood pressure prior to implantation, underlying cardiomyopathy, comorbidities such as diabetes mellitus, history of stroke, peripheral arterial disease, chronic obstructive pulmonary disease and arterial hypertension, risk factors such as family history and smoking, New York Heart Association functional and Canadian Cardiovascular Society class, medication, laboratory results, such as kidney function, electrolytes, prothrombin time international normalised ratio (INR) and pro-B-type natriuretic peptide (pro-BNP), ECG parameters such as QRS, PR and corrected QT interval as well as presence of bundle-branch or fascicular block; echocardiography results including left ventricular ejection fraction, end-diastolic and endsystolic volumes as well as diameters of the left ventricle, endsystolic diameter and indexed volume of the left atrium, presence of mitral regurgitation, systolic pressure gradient between right ventricle and atrium, diameter of the right atrium, end-diastolic right ventricular area and parameters of right ventricular function such as tricuspid annular plane systolic excursion and fractional area change. Implantation data included indication, operator, positive screening vectors prior to and during implantation, shock-test performance, duration of implantation, use of surgical drain and discharge days after implantation. Follow-up data included complications, heart failure hospitalisations, cardiovascular and all-cause mortality, implantation of a circulatory assist device or heart transplantation, number and cause of appropriate and inappropriate shocks and finally duration of follow up.
The study was approved by the local ethics committee and did not receive any specific extramural funding.
Screening
All patients with an indication for ICD implantation without known episodes of sustained monomorphic ventricular tachycardia, or necessity of pacing for bradycardia or resynchronisation therapy, as well as all patients in whom a transvenous defibrillator had been explanted due to a device infection, were screened for eligibility of S-ICD therapy. For screening, the screening tool provided by the manufacturer was used at rest (supine and seated) and, in special cases such as Brugada syndrome, during exercise and/or flecainide challenge. Of note, the broader screening of Brugada patients became standard at our institution only after a case with inappropriate shocks due to T-wave oversensing because of dynamic changes in T-wave morphology that necessitated explantation of the S-ICD system (see Results for details).
Implantation procedure
The majority of S-ICD implantations were performed by a consultant electrophysiologist (n = 33), the remainder by a consultant cardiac surgeon (n = 4). All procedures were performed under general anaesthesia in an operating theatre or a catheterisation laboratory under standard sterile conditions. To ensure the correct positioning of the generator and the electrode, a dummy was placed on the skin surface under guidance of anatomical landmarks and the position confirmed via fluoroscopy prior to the actual intervention. The S-ICD implantation was performed following the standard operating procedure [17] with the device placed between the serratus anterior and latissimus dorsi muscles [20]. The majority of implantations were performed using the two-incision technique, tunnelling the electrode left to the sternum from the xiphoid area towards the substernal notch using an 11F peel-away sheath.
In the majority of cases (n = 34), a peri-procedural device defibrillation test was performed. Ventricular fibrillation was induced using a 50-Hz stimulation via the device. Successful testing was defined as correct recognition and successful termination of the ventricular arrhythmia via a 65 J defibrillation shock. If unsuccessful, a second attempt was made >5 minutes later with 70 J and reversed shock polarity. If this attempt was also ineffective, position of the lead and the generator were checked fluoroscopically, and, if necessary, they were repositioned and the defibrillation test repeated afterwards.
Follow-up after implantation
All patients underwent routine follow-up including device interrogation and a chest X-ray prior to hospital discharge. Patients were seen in the outpatient clinic 2 to 4 weeks later. An exercise treadmill test was routinely performed 1 month after the implantation.
Statistical analysis
Statistical analysis was performed using Microsoft® Excel for Mac (Version 15.29.1) and RStudio (Version 0.99.902). Normality was assessed visually with histograms and quantile-quantile-plots, as well as the Shapiro-Wilk test. Normally distributed continuous variables are shown as mean ± standard deviation, non-normally distributed continuous data as median (interquartile range [IQR], 1st quartile – 3rd quartile) and categorical data as n (%).
Results
Baseline characteristics
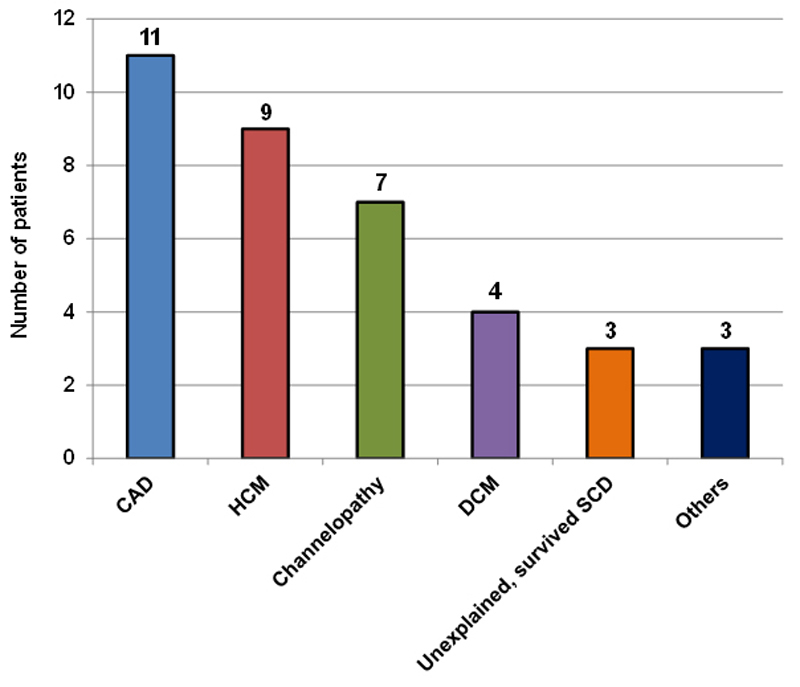
Patients´ baseline characteristics are summarised in table 1. In 38 patients, a total of 37 S-ICDs were successfully implanted. Most of the subjects (81%) were male with a mean age of 47 ± 15 years at implantation. Approximately 60% underwent S-ICD implantation for primary prevention, and three patients underwent the procedure for an unexplained survived sudden cardiac arrest (table 2). The most common cardiac condition (fig. 2) was coronary artery disease (30%), followed by hypertrophic cardiomyopathy (24%) and channelopathies (18%). Less common were valvular heart disease with reduced ejection fraction, left ventricular non-compaction and arrhythmogenic right ventricular cardiomyopathy with one case each (“Others” in fig. 2).
Table 1 Baseline characteristics of patients undergoing S-ICD implantation.
| Demographic |
|
| Age, years |
47 ± 15 |
| Male |
30 (81.1) |
| Female |
7 (18.9) |
| Height, cm |
176 ± 7 |
| Body mass index, kg/m2
|
26.4 ± 4.7 |
| Clinical |
|
| Arterial hypertension |
10 (27) |
| Diabetes |
5 (13.5) |
| Systolic blood pressure (mm Hg) |
114 ± 16 |
| Heart rate (bpm) |
63 ± 10 |
| Positive family history |
9 (25.7) |
| Smoker |
7 (22.6) |
| History of stroke |
4 (10.8) |
| NYHA functional class |
|
| I |
17 (56.7) |
| II |
11 (36.7) |
| III |
2 (6.7) |
| IV |
0 (0) |
| Laboratory results |
|
| Creatinine, µmol/l |
87.5 (71.5–104.8) |
| NT-proBNP, ng/l |
646 (260–1872) |
| Potassium, mmol/l |
4.1 ± 0.2 |
| INR |
1.2 (1.1–1.2) |
| 12-lead ECG parameters |
|
| Sinus rhythm |
36 (97.3) |
| QRS, ms |
100 (90–110) |
| 1st degree AV block |
5 (13.5) |
| Normal ventricular conduction |
31 (86.1) |
| QTc, ms |
420 (400–450) |
| Echocardiography parameters |
|
| EDVi (ml/m2) |
73.2 (64.3–102.3) |
| EF, % |
43.5 (28.3–60.5) |
| LAVi, ml/m2
|
46.0 (40.3–57.5) |
| Medication at implantation |
|
| Amiodarone |
6 (16.2) |
| Beta-blockers |
26 (70.3) |
| ACE inhibitors |
19 (51.4) |
| Oral anticoagulation |
8 (21.6) |
| Aspirin |
10 (27.0) |
| ADP antagonists |
2 (5.7) |
Table 2 Implantation data.
| Indication |
|
| Primary prevention |
22 (59.5) |
| Secondary prevention |
15 (40.5) |
| Post transvenous lead extraction |
7 (18.9) |
| Intraoperative positive vectors |
|
| 1 of 3 |
0 (0) |
| 2 of 3 |
15 (60) |
| 3 of 3 |
10 (40) |
| Defibrillation test |
|
| Successful |
34 (100) |
| 1st attempt |
29 (85.3) |
| Not performed |
3 (8.1) |
| Surgery and complications |
|
| Duration, minutes |
70 (60–80) |
| Haematoma |
2 (5.4) |
| Other pocket complications |
2 (5.4) |
Echocardiography, ECG and laboratory findings
The median left ventricular ejection fraction (LVEF) was 44% (IQR 28–61%). In nearly all patients (97%), the ECG prior to implantation demonstrated sinus rhythm with a narrow QRS complex (median 100 ms, IQR 90–110 ms) and a normal median corrected QT interval (using the Bazett’s formula) of 420 ms. Patients eligible for CRT implantation according to current guidelines were excluded from receiving an S-ICD. However, two patients in our cohort already had an epicardial CRT-P system in place and subsequently underwent S-ICD implantation.
S-ICD device implantation and complications
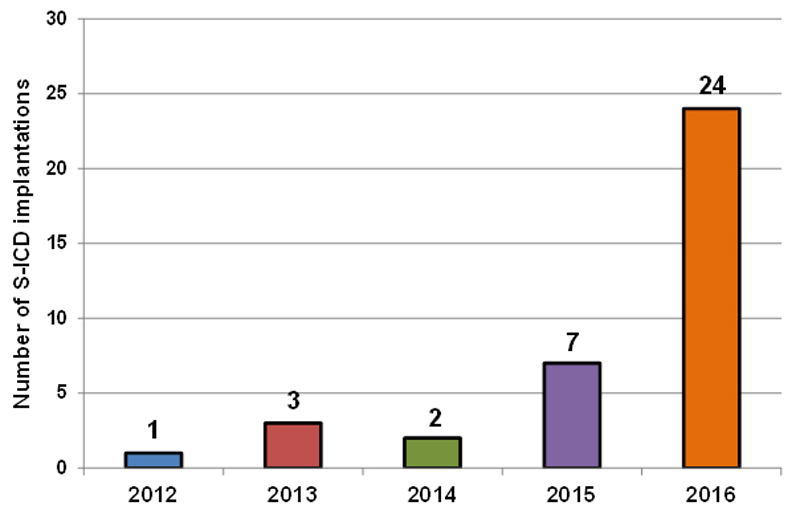
After the first procedure in 2012, 5 additional implantations were performed in the years 2013 and 2014. In 2015, the number of procedures increased to 7, and further to 24 in the year 2016 (fig. 3). In all except one subject the implantation was successful. In spite of a positive screening, the procedure failed in this latter patient with arrhythmogenic right ventricular cardiomyopathy because of insufficient R-wave sensing and concomitant P-wave oversensing in all vector configurations, which persisted even with different lead and generator positions. As a result, the decision was made to switch to a transvenous ICD system during the same intervention, which was successfully implanted (albeit equally with difficulties due to a severely enlarged right atrium and extensive areas of low voltage in the right ventricle).
Most of the initial procedures (70%) were performed in the cardiac surgery operating theatre; since August 2016, implantations were routinely performed in the electrophysiology laboratory. All procedures were done under general anaesthesia. The median duration of the procedures was 70 minutes (IQR 60–80 min).
A total of 34 patients underwent defibrillation testing (92%). In all cases the induced ventricular arrhythmia was detected correctly and in 85% of cases, the first defibrillation attempt with a shock energy of 65 J was effective. In three patients, a second defibrillation testing with reversed polarity was necessary to successfully terminate the induced arrhythmia. The generator and the defibrillation lead had to be repositioned in one patient each, resulting in a final successful defibrillation rate of 100%. In three patients, defibrillation testing was not performed owing to severe heart failure and a perceived risk of harm outweighing the benefit of testing.
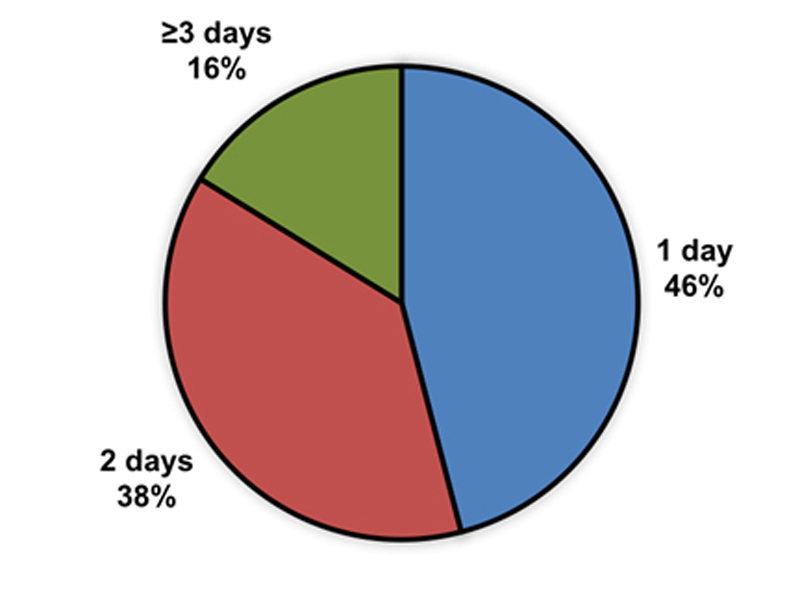
The majority of patients could be discharged from the hospital the day (46%) or 2 days after implantation (38%; fig. 4). The other subjects (16%) were in-patients for 3 or more days and were either survivors of a sudden cardiac arrest or were hospitalised for other reasons (e.g., acute heart failure). No implantations have been performed in an outpatient setting.
Four patients experienced perioperative complications (10.8%), which where exclusively related to the pocket site. There were two cases of haematoma without a significant drop in blood haemoglobin levels, which could be managed conservatively. One of the patients was under dual antiplatelet therapy with aspirin and ticagrelor, and the other patient was taking aspirin alone. In the other two patients, a revision of the pocket incision was necessary because of impaired wound healing. In both subjects the wound healed completely after local revision without the need of further interventions.
Follow-up
The median follow-up was 3.7 months (IQR 1.7–10.1 months). The results are summarised in table 3. During this period, three patients (8.1%) experienced a sustained ventricular arrhythmia, which was correctly detected and successfully terminated with the delivery of a single shock in all cases (100%).
Table 3 Outcome data of patients undergoing S-ICD implantation.
| Patients with ICD shocks |
|
| Appropriate and successful |
3 (8.1%) |
| Inappropriate |
2 (5.4%) |
| Follow up |
|
| Follow-up period, months |
3.2 (1.7 - 10.1) |
| Heart transplantation |
1 (2.7%) |
| Heart failure hospitalisation |
2 (5.4%) |
Two patients (5.4%) experienced a total of three inappropriate shocks (IAS) due to T-wave oversensing. In the first patient, with Brugada syndrome, the S-ICD had to be explanted since no sensing vector adjustment could avoid the T-wave oversensing occurring exclusively during exercise-induced QRS widening. Of note, S-ICD screening in this patient was performed at rest without flecainide/ajmaline testing. A conventional transvenous single-chamber ICD was implanted. In the other S-ICD recipient, changing of the sensing vector could avoid further IAS.
During the follow-up period, one patient (2.7%) underwent combined heart and lung transplantation 1.5 years after the S-ICD implantation. During the entire observation period, no device-related infection or lead dysfunction occurred.
Discussion
The current work represents the largest clinical experience with the S-ICD system from a tertiary care centre in Switzerland. In our cohort of 37 patients, we observed efficacy and safety in the peri-interventional setting and during short-term follow-up similar to reports based on international registries [21, 22].
Baseline characteristics of the patient population
The median age of our population was younger than the average of transvenous ICD recipients in recent trials [3, 23], and the median LVEF mildly reduced. These findings mirror those of the large S-ICD registries (EFFORTLESS and IDE) [21, 22]; indeed, a recent pooled analysis showed a mean LVEF of around 40% and an average age of 50.3 years [21]. It is not surprising that the S-ICD population differs from traditional ICD recipients in being younger and with less advanced heart failure, which is primarily due to patient selection. These candidates more frequently present with heart diseases other than “typical” heart failure, and underlying heart diseases differ from those of recipients of conventional transvenous ICDs [3, 23], [21, 22]. In contrast, subjects suffering from non-ischaemic diseases such as hypertrophic cardiomyopathy, inherited channelopathies, congenital heart disease and dilated cardiomyopathy are commonly considered candidates for an S-ICD [21, 22]. Nevertheless, especially over the last 2 years, primary prevention implantation in patients with underlying coronary artery disease has increased, similar to the picture observed in the aforementioned international registries.
Efficacy and safety of the S-ICD
During the follow-up period, three patients suffered from three ventricular arrhythmia episodes (8.1%). The conversion efficacy of the S-ICD was 100% with the first shock. This is in line with data from both previous transvenous and S-ICD analyses [22, 24–26]. A recent pooled analysis from the EFFORTLESS and IDE registry demonstrated a successful conversion rate of 98% [21]. This is of particular importance because the S-ICD population included a younger age group with a higher prevalence of nonischaemic cardiomyopathies [22], which are known to be more difficult to cardiovert.
Implantation of a conventional transvenous ICD device carries a risk of intervention-related complications of 2 to 6% [27–29]. Similarly, short-term complications after an S-ICD implantation in large registries occur at around 4 to 6%, and are primarily a result of pocket infection or device erosion [21, 22]. Others include patient discomfort, wound haematoma and suboptimal electrode position. We observed in our registry a slightly higher complication rate of 10.9%, which is most likely due to the lower total number of implanted devices and the associated “learning curve” during the first implantations. However, only two patients (5.5%) required a pocket revision, and no infection occurred. Indeed, all complications were exclusively related to the device pocket and were managed without need of a complete system revision. This highlights a relevant advantage of the S-ICD system: whereas the risk of complications in the perioperative period may be numerically similar to that observed with transvenous ICDs, the associated morbidity is much lower due to the absence of possibly life-threatening complications like cardiac tamponade or haemo-/pneumothorax.
In contrast to the short-term period, device-related complications over the long-term are significantly higher with transvenous ICDs and are mainly lead-related, with an even higher impact on morbidity and mortality [19, 30]. Indeed, the electrode of conventional ICDs has been described as the “Achilles’ heel” of the device system [14]. The S-ICD lead, in contrast, has a fundamentally different structure with no central lumen, resulting, above everything else, in a greater tensile strength. Additionally, the subcutaneous as opposed to intracardiac location exposes the lead to less environmental stress. Registry data from the Netherlands confirmed the very low risk of lead malfunctions, with only 0.8% over an observational period of 5 years (compared with 15% in the matched transvenous ICD cohort) [19]. For this reason, the necessity of future lead extraction is much lower with the S-ICD system, and the extraction procedure itself far less invasive than that of transvenous leads [16, 31]. An analysis of long-term results, however, was not part of our current study and will require further investigation.
A subcutaneous lead further eliminates the necessity for a vascular access, which can be of tremendous advantage in patients suffering from complex congenital heart disease or in patients on haemodialysis. The entire extravascular localisation makes the system less prone to hardware-associated systemic infection and essentially eliminates the risk of device-related endocarditis [32]. Indeed, the risk for device-associated infection in the pooled analysis of the EFFORTLESS and IDE registries was low at 1.7% [21]. Notably, all infections were exclusively local and no case of endovascular infection with endocarditis has been documented so far.
In two patients of our cohort, an S-ICD was implanted in the setting of a pre-existing epicardial pacemaker without relevant interaction between the two devices. In this particular setting, the main risk consists of undersensing of ventricular fibrillation by the pacemaker, resulting in “pacing” of the ventricle and subsequent oversensing of the pacemaker spike by the S-ICD with failure to deliver therapy. This is particularly problematic after shock delivery of the S-ICD, which may lead to a “return on reset” of the pacemaker, resulting in unipolar stimulation (which is even more prone to oversensing by the S-ICD). Therefore, prior to implanting such a combined system, the choice of device and pacing mode need to be carefully considered. After implantation, bi- and unipolar pacing at maximal output in all possible configurations should be performed to ensure the absence of device-device interaction as well as inappropriate under- and oversensing.
Since its introduction into medical practice in 2009, the S-ICD has undergone major relevant adjustments. Currently, the third generation of devices is being implanted. Large international registries have been published (EFFORTLESS and IDE), already summarising the benefit and safety of the S-ICD. Several prospective studies, such as PRAETORIAN, UNTOUCHED and MADIT-S-ICD, are ongoing and will need to confirm these positive results.
Current limitations of the S-ICD system
Since there is no direct contact between the S-ICD lead and myocardium, the S-ICD lacks functionality for bradycardia pacing (except for limited transthoracic post-shock pacing). Therefore, in patients with a requirement for demand pacing (e.g., atrioventricular block [AVB] or sinus bradycardia), a conventional transvenous ICD system is the preferred option. None of our patients presented with higher degree (>1st degree) AVB, and only 13.5% with 1st degree AVB. In this context, it is reassuring that only a minority (5 to 10%) of patients qualifying for an ICD will develop the need for pacing over time [1, 33, 34]. In turn, however, some patients may develop an indication for an ICD when a pacemaker has been implanted in the past. Limited data from case series show that the concurrent use of an S-ICD and a previously implanted pacemaker, transvenous, epicardial or even leadless, is possible [35–37]. Closely related to the lack of antibradycardia pacing is the inability of the S-ICD to offer cardiac resynchronisation therapy (CRT), and patients suffering from advanced systolic heart failure with a wide QRS complex are not suitable candidates for this system. Reflecting this limitation, the QRS duration in our cohort was short (median 100 ms).
Finally, the S-ICD is unable to deliver antitachycardia pacing; therefore, patients presenting with monomorphic ventricular arrhythmias, which are potentially treatable via overdrive pacing, are not ideal candidates for an S-ICD. Several studies have demonstrated the benefit and high success rate of antitachycardia pacing in patients with a transvenous-ICD [38, 39]. In a primary prevention setting, however, data from the SCD-HeFT trial demonstrated that the annual likelihood over 3 years to require antitachycardia pacing is only 1.8% [40], which is in line with the results from the MADIT-RIT study showing that delayed treatment for ventricular arrhythmias reduces the amount of “appropriate” antitachycardia pacing from 15 to 3% per year [3]. [22, 26].
Inappropriate shocks
Inappropriate shocks (IASs) are a rare, but serious problem in ICD recipients, resulting from delivery of ICD treatment in the absence of a life-threatening arrhythmia. Indeed, IASs have been shown to not only be associated with psychological distress and reduced quality of life [41], but also with an increase in mortality [42]. The IAS rate in conventional transvenous ICD registries and trials has varied between 4 and 18% in the past [43–47], but may be as low as 2.8 to 3.7% over 2 years with modern-type devices and programming [10]. During the initial experience with the S-ICD, the IAS rate was high, with a risk of 5 to 25% [17, 48–50]. IASs in S-ICDs typically result from oversensing of cardiac signals (e.g., T-wave oversensing), due to supraventricular tachycardias misinterpreted as ventricular arrhythmias, or due to noncardiac oversensing (e.g., myopotentials). Since the electrical cardiac activation used for the S-ICD analysis is not a true local bipolar but rather a “farfield” signal, the system is potentially more prone to oversensing of non-QRS signals such as the T-wave.
With technical advances such as introduction of the suture sleeve to avoid lead migration and algorithm improvements such as introduction of the INSIGHT discrimination algorithm, the risk of IAS has been reduced to an annual rate of 7 to 10% [21, 22]. Furthermore, current nominal device settings include a conditional zone with supraventricular tachycardia discrimination and a shock zone for high ventricular rates >220 bpm. The incidence of T-wave oversensing has been reduced with the introduction of pre-implant electrocardiographic screening [51]. Additionally, a software feature has been designed (SMART Pass Technology) to further minimise oversensing while maintaining appropriate detection of the ventricular arrhythmia. A modelled analysis of IAS episodes from the EFFORTLESS registry demonstrated that T-wave oversensing could thereby be reduced by 71% and the rate of IAS could be reduced to 3.8% (L Boersma et al., presented at HRS meeting 2015), which is within the range of most recent transvenous ICDs [3, 10, 23]. In line with these findings, two patients (5.4%) of our cohort experienced a total of three IASs, all of them due to T-wave oversensing.
Limitations of our current study
Limitations of our current work include its retrospective nature, potential selection bias due to inclusion of patients only from a large referral centre, and lack of long-term outcome data. Further limitations include the lack of data on all screened patients, number of rejected patients and reasons for rejection.
Conclusions and perspectives
Since its introduction in Switzerland in the year 2012, the number of S-ICD implantations has steadily increased. Our data, in line with large international registries, demonstrate that the S-ICD may represent an attractive alternative to conventional transvenous ICDs for a relevant number of ICD candidates, offering efficacy similar to transvenous-ICDs with similar or even fewer long-term complications. The only exception to this is a slightly higher rate of peri-operative complications in our cohort, probably resulting from individual operator learning curves.
Although the S-ICD system still has some shortcomings, simple measures can help to improve the care of S-ICD patients. For example, dual zone programming (with a high-rate and a conditional zone) should be the standard of care; in patients with Brugada syndrome, screening should be performed not only at rest but also during exercise or Class I antiarrhythmic drug challenge, to assure that dynamic changes in QRS- and/or T-wave morphology do not lead to inappropriate shocks [52]; and implantations should be concentrated in experienced centres with a high volume of procedures per operator to minimise perioperative complications [53]. Furthermore, experienced support with follow-up and trouble-shooting should be provided to office-based cardiologists to ensure optimal programming.
To summarise, with increasing experience, optimised programming as well as new device algorithms and filters, the S-ICD is becoming increasingly safe and may represent an attractive alternative to transvenous ICDs for a growing number of ICD candidates. Further studies are necessary (and largely ongoing) comparing the S-ICD to transvenous systems regarding hard clinical endpoints.
Author contributions
Tardu Özkartal and Alexander Breitenstein contributed equally to this work
References
1
Bardy
GH
,
Lee
KL
,
Mark
DB
,
Poole
JE
,
Packer
DL
,
Boineau
R
, et al.; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–37. doi:.https://doi.org/10.1056/NEJMoa043399
2
Kadish
A
,
Dyer
A
,
Daubert
JP
,
Quigg
R
,
Estes
NA
,
Anderson
KP
, et al.; Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151–8. doi:.https://doi.org/10.1056/NEJMoa033088
3
Moss
AJ
,
Schuger
C
,
Beck
CA
,
Brown
MW
,
Cannom
DS
,
Daubert
JP
, et al.; MADIT-RIT Trial Investigators. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367(24):2275–83. doi:.https://doi.org/10.1056/NEJMoa1211107
4
Priori
SG
,
Blomström-Lundqvist
C
,
Mazzanti
A
,
Blom
N
,
Borggrefe
M
,
Camm
J
, et al.
2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793–867. doi:.https://doi.org/10.1093/eurheartj/ehv316
5
Moss
AJ
,
Hall
WJ
,
Cannom
DS
,
Daubert
JP
,
Higgins
SL
,
Klein
H
, et al.; Multicenter Automatic Defibrillator Implantation Trial Investigators. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335(26):1933–40. doi:.https://doi.org/10.1056/NEJM199612263352601
6
Moss
AJ
,
Zareba
W
,
Hall
WJ
,
Klein
H
,
Wilber
DJ
,
Cannom
DS
, et al.; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–83. doi:.https://doi.org/10.1056/NEJMoa013474
7
Mirowski
M
,
Reid
PR
,
Mower
MM
,
Watkins
L
,
Gott
VL
,
Schauble
JF
, et al.
Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med. 1980;303(6):322–4. doi:.https://doi.org/10.1056/NEJM198008073030607
8
Brady
PA
,
Friedman
PA
,
Trusty
JM
,
Grice
S
,
Hammill
SC
,
Stanton
MS
. High failure rate for an epicardial implantable cardioverter-defibrillator lead: implications for long-term follow-up of patients with an implantable cardioverter-defibrillator. J Am Coll Cardiol. 1998;31(3):616–22. doi:.https://doi.org/10.1016/S0735-1097(97)00529-9
9
van Welsenes
GH
,
Borleffs
CJ
,
van Rees
JB
,
Atary
JZ
,
Thijssen
J
,
van der Wall
EE
, et al.
Improvements in 25 Years of Implantable Cardioverter Defibrillator Therapy. Neth Heart J. 2011;19(1):24–30. doi:.https://doi.org/10.1007/s12471-010-0047-3
10
Auricchio
A
,
Schloss
EJ
,
Kurita
T
,
Meijer
A
,
Gerritse
B
,
Zweibel
S
, et al.; PainFree SST Investigators. Low inappropriate shock rates in patients with single- and dual/triple-chamber implantable cardioverter-defibrillators using a novel suite of detection algorithms: PainFree SST trial primary results. Heart Rhythm. 2015;12(5):926–36. doi:.https://doi.org/10.1016/j.hrthm.2015.01.017
11
Tung
R
,
Zimetbaum
P
,
Josephson
ME
. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol. 2008;52(14):1111–21. doi:.https://doi.org/10.1016/j.jacc.2008.05.058
12
Borleffs
CJ
,
van Erven
L
,
van Bommel
RJ
,
van der Velde
ET
,
van der Wall
EE
,
Bax
JJ
, et al.
Risk of failure of transvenous implantable cardioverter-defibrillator leads. Circ Arrhythm Electrophysiol. 2009;2(4):411–6. doi:.https://doi.org/10.1161/CIRCEP.108.834093
13
Alter
P
,
Waldhans
S
,
Plachta
E
,
Moosdorf
R
,
Grimm
W
. Complications of implantable cardioverter defibrillator therapy in 440 consecutive patients. Pacing Clin Electrophysiol. 2005;28(9):926–32. doi:.https://doi.org/10.1111/j.1540-8159.2005.00195.x
14
Kleemann
T
,
Becker
T
,
Doenges
K
,
Vater
M
,
Senges
J
,
Schneider
S
, et al.
Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation. 2007;115(19):2474–80. doi:.https://doi.org/10.1161/CIRCULATIONAHA.106.663807
15
Edelstein
S
,
Yahalom
M
. Cardiac device-related endocarditis: Epidemiology, pathogenesis, diagnosis and treatment - a review. Int J Angiol. 2009;18(4):167–72. doi:.https://doi.org/10.1055/s-0031-1278347
16
Maytin
M
,
Jones
SO
,
Epstein
LM
. Long-term mortality after transvenous lead extraction. Circ Arrhythm Electrophysiol. 2012;5(2):252–7. doi:.https://doi.org/10.1161/CIRCEP.111.965277
17
Bardy
GH
,
Smith
WM
,
Hood
MA
,
Crozier
IG
,
Melton
IC
,
Jordaens
L
, et al.
An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363(1):36–44. doi:.https://doi.org/10.1056/NEJMoa0909545
18
Rowley
CP
,
Gold
MR
. Subcutaneous implantable cardioverter defibrillator. Circ Arrhythm Electrophysiol. 2012;5(3):587–93. doi:.https://doi.org/10.1161/CIRCEP.111.964676
19
Brouwer
TF
,
Yilmaz
D
,
Lindeboom
R
,
Buiten
MS
,
Olde Nordkamp
LR
,
Schalij
MJ
, et al.
Long-Term Clinical Outcomes of Subcutaneous Versus Transvenous Implantable Defibrillator Therapy. J Am Coll Cardiol. 2016;68(19):2047–55. doi:.https://doi.org/10.1016/j.jacc.2016.08.044
20
Brouwer
TF
,
Miller
MA
,
Quast
AB
,
Palaniswamy
C
,
Dukkipati
SR
,
Reddy
V
, et al.
Implantation of the Subcutaneous Implantable Cardioverter-Defibrillator: An Evaluation of 4 Implantation Techniques. Circ Arrhythm Electrophysiol. 2017;10(1):e004663. doi:.https://doi.org/10.1161/CIRCEP.116.004663
21
Burke
MC
,
Gold
MR
,
Knight
BP
,
Barr
CS
,
Theuns
DA
,
Boersma
LV
, et al.
Safety and Efficacy of the Totally Subcutaneous Implantable Defibrillator: 2-Year Results From a Pooled Analysis of the IDE Study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65(16):1605–15. doi:.https://doi.org/10.1016/j.jacc.2015.02.047
22
Lambiase
PD
,
Barr
C
,
Theuns
DA
,
Knops
R
,
Neuzil
P
,
Johansen
JB
, et al.; EFFORTLESS Investigators. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J. 2014;35(25):1657–65. doi:.https://doi.org/10.1093/eurheartj/ehu112
23
Gasparini
M
,
Proclemer
A
,
Klersy
C
,
Kloppe
A
,
Lunati
M
,
Ferrer
JB
, et al.
Effect of long-detection interval vs standard-detection interval for implantable cardioverter-defibrillators on antitachycardia pacing and shock delivery: the ADVANCE III randomized clinical trial. JAMA. 2013;309(18):1903–11. doi:.https://doi.org/10.1001/jama.2013.4598
24
Blatt
JA
,
Poole
JE
,
Johnson
GW
,
Callans
DJ
,
Raitt
MH
,
Reddy
RK
, et al.; SCD-HeFT Investigators. No benefit from defibrillation threshold testing in the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial). J Am Coll Cardiol. 2008;52(7):551–6. doi:.https://doi.org/10.1016/j.jacc.2008.04.051
25
Kutyifa
V
,
Huth Ruwald
AC
,
Aktas
MK
,
Jons
C
,
McNitt
S
,
Polonsky
B
, et al.
Clinical impact, safety, and efficacy of single- versus dual-coil ICD leads in MADIT-CRT. J Cardiovasc Electrophysiol. 2013;24(11):1246–52. doi:.https://doi.org/10.1111/jce.12219
26
Weiss
R
,
Knight
BP
,
Gold
MR
,
Leon
AR
,
Herre
JM
,
Hood
M
, et al.
Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013;128(9):944–53. doi:.https://doi.org/10.1161/CIRCULATIONAHA.113.003042
27
van Rees
JB
,
de Bie
MK
,
Thijssen
J
,
Borleffs
CJ
,
Schalij
MJ
,
van Erven
L
. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: a systematic review of randomized clinical trials. J Am Coll Cardiol. 2011;58(10):995–1000. doi:.https://doi.org/10.1016/j.jacc.2011.06.007
28
Kirkfeldt
RE
,
Johansen
JB
,
Nohr
EA
,
Moller
M
,
Arnsbo
P
,
Nielsen
JC
. Risk factors for lead complications in cardiac pacing: a population-based cohort study of 28,860 Danish patients. Heart Rhythm. 2011;8(10):1622–8. doi:.https://doi.org/10.1016/j.hrthm.2011.04.014
29
Hammill
SC
,
Kremers
MS
,
Stevenson
LW
,
Heidenreich
PA
,
Lang
CM
,
Curtis
JP
, et al.
Review of the registry’s fourth year, incorporating lead data and pediatric ICD procedures, and use as a national performance measure. Heart Rhythm. 2010;7(9):1340–5. doi:.https://doi.org/10.1016/j.hrthm.2010.07.015
30
Honarbakhsh
S
,
Providencia
R
,
Srinivasan
N
,
Ahsan
S
,
Lowe
M
,
Rowland
E
, et al.
A propensity matched case-control study comparing efficacy, safety and costs of the subcutaneous vs. transvenous implantable cardioverter defibrillator. Int J Cardiol. 2017;228:280–5. doi:.https://doi.org/10.1016/j.ijcard.2016.11.017
31
Maytin
M
,
Epstein
LM
. The challenges of transvenous lead extraction. Heart. 2011;97(5):425–34. doi:.https://doi.org/10.1136/hrt.2009.189910
32
Lewis
GF
,
Gold
MR
. Safety and Efficacy of the Subcutaneous Implantable Defibrillator. J Am Coll Cardiol. 2016;67(4):445–54. doi:.https://doi.org/10.1016/j.jacc.2015.11.026
33
Sweeney
MO
,
Ellenbogen
KA
,
Tang
AS
,
Whellan
D
,
Mortensen
PT
,
Giraldi
F
, et al.; Managed Ventricular Pacing Versus VVI 40 Pacing Trial Investigators. Atrial pacing or ventricular backup-only pacing in implantable cardioverter-defibrillator patients. Heart Rhythm. 2010;7(11):1552–60. doi:.https://doi.org/10.1016/j.hrthm.2010.05.038
34
Wilkoff
BL
,
Cook
JR
,
Epstein
AE
,
Greene
HL
,
Hallstrom
AP
,
Hsia
H
, et al.; Dual Chamber and VVI Implantable Defibrillator Trial Investigators. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288(24):3115–23. doi:.https://doi.org/10.1001/jama.288.24.3115
35
Huang
J
,
Patton
KK
,
Prutkin
JM
. Concomitant Use of the Subcutaneous Implantable Cardioverter Defibrillator and a Permanent Pacemaker. Pacing Clin Electrophysiol. 2016;39(11):1240–5. doi:.https://doi.org/10.1111/pace.12955
36
Erath
JW
,
Sirat
AS
,
Vamos
M
,
Hohnloser
SH
. Implantation eines epikardialen CRT-P- und S-ICD bei einem jungen Patienten mit persistierender linker oberer Hohlvene [Epicardial CRT-P- and S-ICD Implantation in a Young Patient with Persistent Left Superior Vena Cava]. Herzschrittmacherther Elektrophysiol. 2016;27(4):396–8. Article in English. doi:.https://doi.org/10.1007/s00399-016-0451-5
37
Tjong
FV
,
Brouwer
TF
,
Smeding
L
,
Kooiman
KM
,
de Groot
JR
,
Ligon
D
, et al.
Combined leadless pacemaker and subcutaneous implantable defibrillator therapy: feasibility, safety, and performance. Europace. 2016;18(11):1740–7. doi:.https://doi.org/10.1093/europace/euv457
38
Mont
L
,
Valentino
M
,
Sambola
A
,
Matas
M
,
Aguinaga
L
,
Brugada
J
. Arrhythmia recurrence in patients with a healed myocardial infarction who received an implantable defibrillator: analysis according to the clinical presentation. J Am Coll Cardiol. 1999;34(2):351–7. doi:.https://doi.org/10.1016/S0735-1097(99)00206-5
39
Goldenberg
I
,
Gillespie
J
,
Moss
AJ
,
Hall
WJ
,
Klein
H
,
McNitt
S
, et al.; Executive Committee of the Multicenter Automatic Defibrillator Implantation Trial II. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: an extended 8-year follow-up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation. 2010;122(13):1265–71. doi:.https://doi.org/10.1161/CIRCULATIONAHA.110.940148
40
Poole
JE
,
Johnson
GW
,
Hellkamp
AS
,
Anderson
J
,
Callans
DJ
,
Raitt
MH
, et al.
Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359(10):1009–17. doi:.https://doi.org/10.1056/NEJMoa071098
41
Humphreys
NK
,
Lowe
R
,
Rance
J
,
Bennett
PD
. Living with an implantable cardioverter defibrillator: The patients’ experience. Heart Lung. 2016;45(1):34–40. doi:.https://doi.org/10.1016/j.hrtlng.2015.10.001
42
Proietti
R
,
Labos
C
,
Davis
M
,
Thanassoulis
G
,
Santangeli
P
,
Russo
V
, et al.
A systematic review and meta-analysis of the association between implantable cardioverter-defibrillator shocks and long-term mortality. Can J Cardiol. 2015;31(3):270–7. doi:.https://doi.org/10.1016/j.cjca.2014.11.023
43
Gold
MR
,
Ahmad
S
,
Browne
K
,
Berg
KC
,
Thackeray
L
,
Berger
RD
. Prospective comparison of discrimination algorithms to prevent inappropriate ICD therapy: primary results of the Rhythm ID Going Head to Head Trial. Heart Rhythm. 2012;9(3):370–7. doi:.https://doi.org/10.1016/j.hrthm.2011.10.004
44
Wilkoff
BL
,
Williamson
BD
,
Stern
RS
,
Moore
SL
,
Lu
F
,
Lee
SW
, et al.; PREPARE Study Investigators. Strategic programming of detection and therapy parameters in implantable cardioverter-defibrillators reduces shocks in primary prevention patients: results from the PREPARE (Primary Prevention Parameters Evaluation) study. J Am Coll Cardiol. 2008;52(7):541–50. doi:.https://doi.org/10.1016/j.jacc.2008.05.011
45
Gilliam
FR
,
Hayes
DL
,
Boehmer
JP
,
Day
J
,
Heidenreich
PA
,
Seth
M
, et al.
Real world evaluation of dual-zone ICD and CRT-D programming compared to single-zone programming: the ALTITUDE REDUCES study. J Cardiovasc Electrophysiol. 2011;22(9):1023–9. doi:.https://doi.org/10.1111/j.1540-8167.2011.02086.x
46
van Rees
JB
,
Borleffs
CJ
,
de Bie
MK
,
Stijnen
T
,
van Erven
L
,
Bax
JJ
, et al.
Inappropriate implantable cardioverter-defibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol. 2011;57(5):556–62. doi:.https://doi.org/10.1016/j.jacc.2010.06.059
47
Daubert
JP
,
Zareba
W
,
Cannom
DS
,
McNitt
S
,
Rosero
SZ
,
Wang
P
, et al.; MADIT II Investigators. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51(14):1357–65. doi:.https://doi.org/10.1016/j.jacc.2007.09.073
48
Dabiri Abkenari
L
,
Theuns
DA
,
Valk
SD
,
Van Belle
Y
,
de Groot
NM
,
Haitsma
D
, et al.
Clinical experience with a novel subcutaneous implantable defibrillator system in a single center. Clin Res Cardiol. 2011;100(9):737–44. doi:.https://doi.org/10.1007/s00392-011-0303-6
49
Olde Nordkamp
LR
,
Dabiri Abkenari
L
,
Boersma
LV
,
Maass
AH
,
de Groot
JR
,
van Oostrom
AJ
, et al.
The entirely subcutaneous implantable cardioverter-defibrillator: initial clinical experience in a large Dutch cohort. J Am Coll Cardiol. 2012;60(19):1933–9. doi:.https://doi.org/10.1016/j.jacc.2012.06.053
50
Aydin
A
,
Hartel
F
,
Schlüter
M
,
Butter
C
,
Köbe
J
,
Seifert
M
, et al.
Shock efficacy of subcutaneous implantable cardioverter-defibrillator for prevention of sudden cardiac death: initial multicenter experience. Circ Arrhythm Electrophysiol. 2012;5(5):913–9. doi:.https://doi.org/10.1161/CIRCEP.112.973339
51
Groh
CA
,
Sharma
S
,
Pelchovitz
DJ
,
Bhave
PD
,
Rhyner
J
,
Verma
N
, et al.
Use of an electrocardiographic screening tool to determine candidacy for a subcutaneous implantable cardioverter-defibrillator. Heart Rhythm. 2014;11(8):1361–6. doi:.https://doi.org/10.1016/j.hrthm.2014.04.025
52
Conte
G
,
Regoli
F
,
Moccetti
T
,
Auricchio
A
. Subcutaneous implantable cardioverter-defibrillator and drug-induced Brugada syndrome: the importance of repeat morphology analysis during ajmaline challenge. Eur Heart J. 2016;37(19):1498. doi:.https://doi.org/10.1093/eurheartj/ehv572
53
Knops
RE
,
Brouwer
TF
,
Barr
CS
,
Theuns
DA
,
Boersma
L
,
Weiss
R
, et al.; IDE and EFFORTLESS investigators. The learning curve associated with the introduction of the subcutaneous implantable defibrillator. Europace. 2016;18(7):1010–5. doi:.https://doi.org/10.1093/europace/euv299