Ascending aortic remodelling in Fabry disease after long-term enzyme replacement therapy
DOI: https://doi.org/10.4414/smw.2017.14517
Pierre
Monneya, Salah D.
Qanadlib, Steven D.
Hajdub, Christel
Tranc, Juerg
Schwittera, Olivier
Dormondd, Frédéric
Barbeyc
aDepartment of Cardiology, Lausanne University Hospital, Switzerland
bDepartment of Diagnostic and Interventional Radiology, Lausanne University Hospital, Switzerland
cService of Genetic Medicine, Lausanne University Hospital, Switzerland
dDepartment of Visceral Surgery, Lausanne University Hospital, Switzerland
Summary
BACKGROUND
Previous cross-sectional studies reported a high prevalence of ascending aorta dilations/aneurysms in male adults with Fabry disease, independently of cardiovascular risk factors.
AIMS OF THE STUDY
To characterise the remodelling of the ascending aorta in classic Fabry disease under long-term enzyme replacement therapy.
METHODS
Diameter of the ascending aorta was measured with magnetic resonance imaging at the sino-tubular junction (STJ), and proximal (pAsAo), and distal ascending aorta (dAsAo) at baseline, and after 5 and 10 years of enzyme replacement therapy in 15 adult Fabry patients (10 males; 5 females).
RESULTS
Over a mean follow-up of 9.5 years, the annual expansion rates measured in 10 males with Fabry disease were 0.41 ± 0.16, 0.36 ± 0.25 and 0.41 ± 0.26 mm/y at the STJ, pAsAo and dAsAo, respectively. Expansion rate at the pAsAo level in male patients was significantly higher than the expected expansion projected from theoretical normal values: 0.36 ± 0.25 vs 0.13 ± 0.05, p = 0.017. In 5 females, the annual expansion rates at the STJ, pAsAo and dAsAo were 0.14 ± 0.11, 0.21 ± 0.18 and 0.26 ± 0.24 mm/y, respectively. There was no significant difference from the projected normal expansion rate at the level of the pAsAo: 0.21 ± 0.18 vs 0.13 ± 0.04, p = 0.39.
CONCLUSION
Our data suggest that the remodelling of the ascending aorta is more pronounced in male patients with Fabry disease under long-term enzyme replacement therapy compared with the progression observed in a large population study.
Introduction
Fabry disease is a rare X-linked disorder caused by deficiency of the lysosomal enzyme alpha-galactosidase A (α-Gal A). This defect leads to progressive accumulation of glycosphingolipids (GSL) in many tissues and organs, and in particular in endothelial and vascular smooth muscle cells (VSMC) of the arterial tree [1]. Males affected by the classic form of the disease, defined as a residual α-Gal A activity <1%, develop vascular abnormalities. Early vascular manifestations, appearing from childhood and adolescence, include angiokeratomas, conjunctival and retinal vessel tortuosity and dilation, hearing loss and priapism [2]. In adulthood, the majority of males suffer from chronic renal insufficiency, as well as cardiovascular and cerebrovascular complications responsible for premature death [3]. The clinical presentation of heterozygous females is more variable and ranges from a silent course to severe disease with multiorgan involvement similar to males [4].
The main vascular structural change observed in normotensive adults with Fabry disease is a marked and accelerated increase in the intima-media thickness (IMT) of the arterial wall, via a process distinct from atherosclerosis [5]. Arterial remodelling is further characterised by progressive vascular dilation and elongation of the conduits, resulting in a tortuous appearance, particularly in the basilar trunk and conjunctival and retinal vessels [6]. A high prevalence of asymptomatic dilation and aneurysms of the proximal ascending aorta (pAsAo), which occurred at a younger age compared with the general population, was also previously reported in male patients with the classic form of Fabry disease [7]. Female patients may also develop dilation of the pAsAo, but at a significantly lower rate compared to male patients and generally at an older age [7].
Since 2001, enzyme replacement therapy (ERT) has become commercially available and is recommended for patients with Fabry disease presenting early clinical signs of kidney, heart or brain involvement [8]. Whereas several studies have characterised the effect of long-term ERT on cardiac, renal and cerebral outcomes, no data are currently available regarding the evolution of the aortic remodelling process under long-term ERT [9, 10]. The aim of the present study was to compare the remodelling of the ascending aorta in adult patients with Fabry disease under long-term ERT with the normal aortic remodelling computed from large population data.
Materials and methods
Study population
Fifteen adult patients with classic Fabry disease, included in our prospective cohort (Service of Genetic Medicine of the Lausanne University Hospital) from 1 October 2000 to 31 December 2015, and for whom a minimal follow-up of 5 years under ERT was available, were enrolled in the study. In male patients, classic Fabry disease was confirmed on the basis of a residual α-Gal A activity in leucocytes <1%. In females, the diagnosis of classic Fabry disease was confirmed by means of genetic analysis. These patients presented initially with multisystem involvement of the disease and received ERT (agalsidase alfa, Replagal®, Shire Pharmaceutical Inc, 0.2 mg/kg body weight intravenously every other week) after confirmation of the diagnosis.
Exclusion criteria were: presence of a bicuspid aortic valve, valvular aortic stenosis or insufficiency considered at least mild, signs of heart failure (ejection fraction of the left ventricle <50%) at baseline, and pregnancy during follow-up. Written informed consent was obtained from all patients. The Institutional Review Boards approved the study.
All patients underwent a baseline assessment at T0, before the initiation of ERT, which included physical examination, blood pressure measurement, laboratory blood tests, transthoracic echocardiography and cardiac magnetic resonance imaging (CMR) of the thoracic aorta. The same assessment was repeated after 5 (T5) and 10 years (T10) of ERT.
Image acquisition
During the study, image acquisition at three time points (T0, T5 and T10) was performed with CMR (Philips Intera, Philips Medical Systems, Amsterdam, the Netherlands, and Magnetom Symphony and Aera, Siemens, Erlangen, Germany) using a phased-array receiver coil. At the beginning of the study (from 2000 to 2006), the images obtained in order to measure the diameters of the ascending aorta were acquired in the coronal, transverse and oblique sagittal planes using single-phase ECG-gated (end-diastolic phase) breath-hold images with a steady-state free precession (SSFP) sequence (6 mm slice thickness). From 2007 to the end of the study, a contrast-enhanced 3-D MR-angiography was additionally performed during breath-holds in all patients, with injection of either 0.2 mmol/kg Gadolinium DOTA (Dotarem, Guerbet, France) or Gadobutrol (Gadovist, Bayer, Switzerland).
CMR image analysis
Two experienced radiologists (S.D.H. and S.D.Q.) analysed the anonymised images on a commercially available DICOM workstation (Advantage Window, GE Healthcare, Milwaukee, USA), blinded to patient’s characteristics and date of the examination. For each patient, the maximal endoluminal diameters of the ascending aorta at the sino-tubular junction (STJ), the proximal ascending aorta at the level of the right pulmonary artery (pAsAo), and of the distal ascending aorta (dAsAo) before the origin of the brachiocephalic artery were measured (fig. 1). At each level, the average diameter obtained from two measurements in two orthogonal planes was used for analysis.
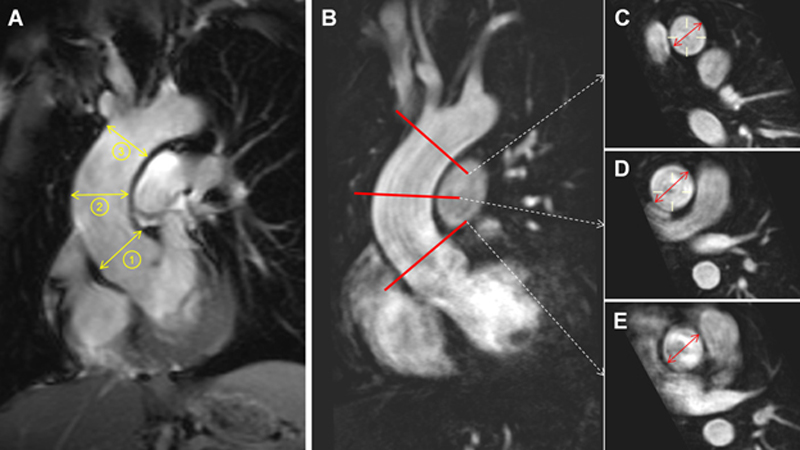
Figure 1 Measurement of the aortic diameters. A. With datasets where 3-D MR-angiography was not available, the luminal diameters, at end-diastole, were directly measured on a non-cine SSFP acquisition of the ascending aorta in the coronal plane at three different levels: (1) sino-tubular junction, (2) proximal ascending and (3) distal ascending aorta. B. In all cases where MR-angiography was available the maximal diameters were measured on the individual aortic cross-section planes reconstructed at the level of the distal ascending aorta (C), proximal ascending aorta (D) and sino-tubular junction (E)
The annual aortic expansion rate, at each of the three aforementioned levels of the ascending aorta, was calculated as the difference in aortic diameter between baseline and the last available follow-up divided by the time (in years) between baseline and the last available follow-up.
Statistical analysis
Results are presented as mean ± standard deviation (SD) for continuous variables and percentages for categorical variables. Paired t-tests were used for longitudinal comparisons between aortic diameters at T0, T5 and T10. Categorical variables were compared with Fisher’s exact test.
For each patient, a predicted normal pAsAo was calculated for each time-point by use of the regression formula reported in the Multi-Ethnic Study of Atherosclerosis (MESA, n = 3573 participants recruited in the general population): predicted pAsAo diameter = [14.99 + (0.12 × age) + 1.79 (if male) + (4.63 × body surface area)] [11]. A predicted annual aortic expansion rate was then calculated as the difference in predicted pAsAo diameter between baseline and the last available follow-up divided by the time (in years) between baseline and the last available follow-up. The measured vs predicted pAsAo annual expansion rates were compared with unpaired t-tests with unequal variance. All the statistical analyses were performed using STATA 14 (StataCorp LP, Texas, USA). A value of p <0.05 was considered statistically significant.
Results
Study cohort
Fabry patients’ clinical characteristics at baseline are summarized in table 1. Patients came from eight unrelated families, whose mutations were: c.337T>C (F113L); c.136C>T (H46Y); c.488G>T (G163V); c.658C>T (R220X); c.901C>T (R301X); c.1284-1287 del (L428Ffs*23); c.1046G>A (W349X); c.389insT (K130R). In four male patients, residual α-Gal A activity in leucocytes was undetectable; it was between 0.01 and 0.04 mE/mg protein (range: 0.41–0.76) in the others (including the patient with the F113L mutation). Alpha-Gal A activity was not measured in the females. During the follow-up, 15/15 patients had imaging at T5 (5.0 ± 1.5 years) and 12/15 at T10 (10.3 ± 1.6 years). Three male patients did not undergo the T10 examination: one patient died of cardiac arrest before T10, one had claustrophobia, and the last one severe renal failure with glomerular filtration rate <30 ml/min. The mean follow-up duration for the whole study was therefore 9.5 ± 2.3 years.
Table 1 Clinical characteristics of the male and female Fabry population at baseline.
|
Parameter
|
Units
|
Males
(n = 10)
|
Females
(n = 5)
|
| Age |
years |
36.5 ± 14.1 |
41.5 ± 15.1 |
| Height |
cm |
172.2 ± 4.9 |
168.2 ± 3.0 |
| Weight |
kg |
70.0 ± 10.1 |
64.6 ± 11.6 |
| Body mass index |
kg/m2
|
23.6 ± 3.3 |
22.9 ± 4.9 |
| Systolic blood pressure |
mm Hg |
122.3 ± 12.0 |
131.8 ± 16.6 |
| Diastolic blood pressure |
mm Hg |
71.9 ± 10.3 |
78.0 ± 14.9 |
| Serum total cholesterol |
mmol/l |
4.7 ± 1.1 |
5.5 ± 0.7 |
| Serum HDL cholesterol |
mmol/l |
1.5 ± 0.3 |
1.7 ± 0.7 |
| Serum LDL cholesterol |
mmol/l |
2.7 ± 0.8 |
3.5 ± 0.8 |
| Serum triglycerides |
mmol/l |
1.1 ± 0.4 |
0.9 ± 0.2 |
| Serum creatinine |
µmol/l |
94.8 ± 33.0 |
71.2 ± 7.8 |
| Proteinuria |
g/l |
1.62 ± 2.50 |
1.14 ± 2.44 |
| CRP |
mg/l |
1.8 ± 2.5 |
2.6 ± 1.8 |
| Current smokers |
n (%) |
2 (20%) |
0 (0%) |
| Antihypertensive treatment |
n (%) |
7 (70%) |
2 (40%) |
| Statin therapy |
n (%) |
4 (40%) |
2 (40%) |
| Diabetes |
n (%) |
0 (0%) |
0 (0%) |
At baseline, the male patients’ mean age was 36.6 ± 14.3 years (range: 15–60 years). Systolic and diastolic blood pressure remained within normal range throughout the study (5/10 patients received antihypertensive/antiproteinuric treatment), as did mean blood cholesterol (4/10 patients under statin treatment). Mean serum creatinine and urinary protein levels were increased because of chronic renal failure secondary to Fabry nephropathy in 6/10 patients. Two patients who reached end-stage renal disease received a kidney transplant during their follow-up and one patient started chronic haemodialysis.
At baseline, the female patients’ mean age was 41.6 ± 15.3 years (range: 22–59). Blood pressure was well controlled (2/5 patients received antihypertensive/antiproteinuric medication). Mean blood cholesterol was within the normal range (2/5 patients with statin therapy). One female patient developed nephrotic syndrome due to Fabry nephropathy. She received a kidney transplant from a living donor after reaching end-stage renal disease.
Ascending aortic diameters at baseline in Fabry patients
In males, mean aortic diameters at the STJ, pAsAo and dAsAo were 29.9 ± 5.9, 30.3 ± 6.6 and 29.0 ± 6.5 mm, respectively. Although 5/10 patients fulfilled the criteria for pAsAo dilation (diameter >19.5 mm/m2), the mean actual diameter at the pAsAo was not statistically different from the predicted diameter: 30.3 ± 6.6 vs 29.6 ± 1.7 mm, p = 0.77. Baseline aortic diameter at pAsAo depended strongly on patient’s age, since a dilation was observed in all patients older than 40 years but in only one patient under 40 years of age (p = 0.048).
In female patients, the mean diameters at the STJ, pAsAo and dAsAo were 27.4 ± 1.6, 29.7 ± 2.7 and 27.5 ± 1.1 mm, respectively. A single 55-year-old female patient fulfilled the criteria for pAsAo dilation. On average, the measured diameter at the pAsAo was comparable to the predicted diameter: 29.7 ± 2.7 vs 28.0 ± 1.8 mm, p = 0.28.
Expansion of the aortic diameters between T0 and T10 in Fabry patients
Mean pAsAo diameter was 30.3 ± 6.6 mm (16.6 ± 3.5 mm/m2 body surface area) in male patients at T0. Mean increase in pAsAo diameter was 1.83 ± 1.66 mm (95% confidence interval [CI] 0.64–3.02, p = 0.007) between T0 and T5, 1.91 ± 1.41 mm (95% CI 0.61–3.21, p = 0.01) between T5 and T10 and 3.46 ± 1.39 mm (95% CI 2.17–4.75, p = 0.0006) between T0 and T10 (fig. 2). Mean annual expansion rate of the pAsAo was significantly higher than the predicted value calculated based on the MESA formula: 0.36 ± 0.25 mm/y vs 0.13 ± 0.05 mm/y, corresponding to a difference of 0.23 mm/y (95% CI 0.05–0.41, p = 0.017) between actual and predicted annual expansion rate. A comparable significant increase in diameters was also observed at the level of the STJ and dAsAo (table 2).
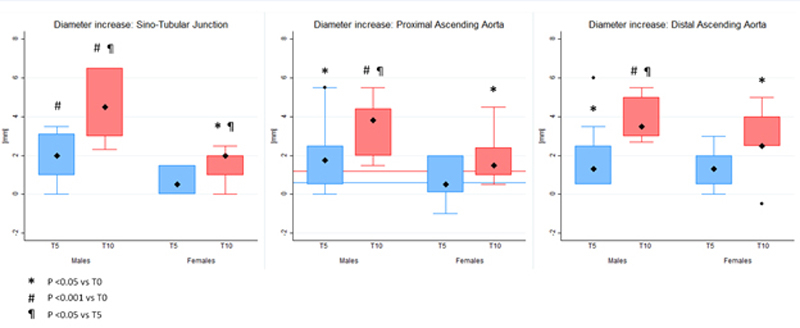
Figure 2 Progression of aortic diameters at the sino-tubular junction, proximal and distal ascending aorta between T0 and T5 and between T0 and T10 in males and females with Fabry disease. The two horizontal lines in the central panel indicate the expected progression in the general population according to MESA at 5 years (blue line) and at 10 years (red line). For each diameter progression, the box indicates the 25th and the 75th percentile and the large diamond indicates the mean value. Outliers are depicted as small diamonds.
Table 2 Increase in measured aortic diameters in mm.
|
T0 to T5
|
p-value
|
T5 to T10
|
p-value
|
T0 to T10
|
p-value
|
|
Male patients
|
n = 10 |
n = 7 |
n = 7 |
| Sino-tubular junction |
2.06 ± 1.20 |
0.0004 |
2.53 ± 1.21 |
0.002 |
4.54 ± 1.81 |
0.0006 |
| Ascending aorta |
1.83 ± 1.66 |
0.007 |
1.91 ± 1.41 |
0.01 |
3.46 ± 1.39 |
0.0006 |
| Distal ascending aorta |
1.91 ± 1.73 |
0.007 |
2.37 ± 1.23 |
0.002 |
3.96 ± 1.05 |
0.0001 |
|
Female patients
|
n = 5 |
n = 5 |
n = 5 |
| Sino-tubular junction |
0.70 ± 0.76 |
0.11 |
0.80 ± 0.57 |
0.03 |
1.50 ± 1.59 |
0.03 |
| Ascending aorta |
0.72 ± 1.29 |
0.28 |
1.26 ± 1.65 |
0.16 |
1.98 ± 1.57 |
0.048 |
| Distal ascending aorta |
1.36 ± 1.19 |
0.06 |
1.34 ± 1.85 |
0.18 |
2.7 ± 2.07 |
0.04 |
Mean pAsAo diameter was 29.7 ± 2.7 mm (17.2 ± 1.9 mm/m2 body surface area) in female patients at T0. We observed a significant increase in pAsAo diameter between T0 and T10: mean increase 2.0 ± 1.6 mm (95% CI 0.03–3.93, p = 0.048). Likewise, the diameter progressed significantly between T0 and T10 at the level of the STJ and dAsAo (table 2). At the level of the pAsAo, the mean annual expansion rate was 0.21 ± 0.18 mm/y. This value was not significantly different from the expected expansion of 0.13 ± 0.04 mm/y. The difference between actual and predicted annual expansion rate was 0.08 mm/y (95% CI 0.14–0.30, p = 0.39).
Comparison between ascending aortic expansion in Fabry patients and normal population
At T0, 5/10 male patients were diagnosed with an abnormally dilated pAsAo (fig. 3). These patients were significantly older: 47.6 ± 8.6 vs 25.5 ± 8.2 years, p = 0.003, compared with Fabry patients with a normal aortic diameter, and also showed a trend toward higher mean serum creatinine concentration (112.8 ± 39.3 vs 76.8 ± 10.1 µmol/l; p = 0.08) and higher diastolic blood pressure (77.6 ± 11.4 vs 66.2 ± 5.0 mm Hg; p = 0.08). Between T0 and T10, there was no significant difference in mean aortic expansion rate at pAsAo between patients with normal and dilated aorta at baseline: 0.27 ± 0.06 vs 0.44 ± 0.15 mm/year, p = 0.35.
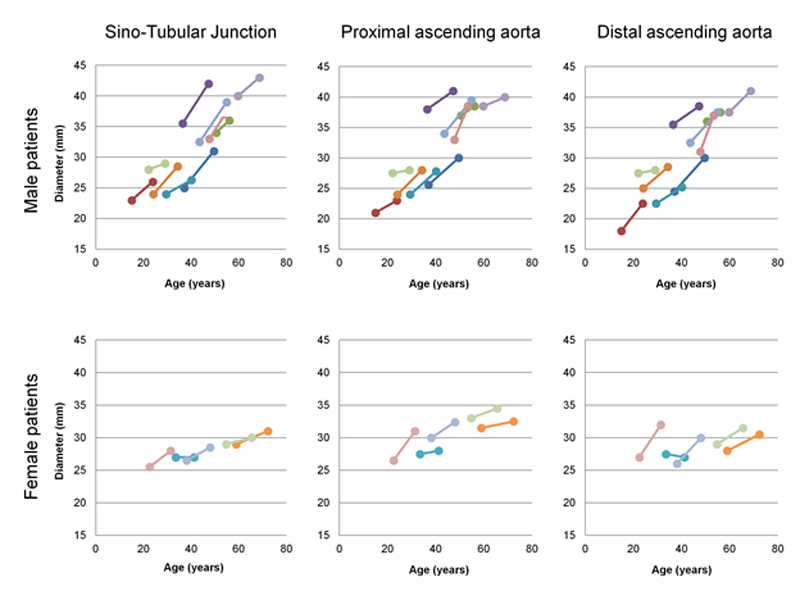
Figure 3 Individual profiles of aortic diameter increase in male and female patients with Fabry disease.
Discussion
This long-term longitudinal cohort study provides several important findings regarding aortic remodelling in patients with the classic form of Fabry disease: (1) the prevalence of dilation of the pAsAo is high among untreated patients, occurring in 50% of males and 20% of females, almost exclusively after 40 years of age; (2)in male patients under long-term ERT, the average annual expansion rate of the AsAo is still more than twice as high as the normal annual expansion rate projected from the transversal data of the MESA study [11] (0.36 ± 0.25 mm/y vs 0.13 ± 0.05 mm/y, p<0.017); (3) in male patients, remodelling of the ascending aorta is homogenous, with comparable baseline diameters and annual aortic expansion rates at the STJ (0.41 ± 0.16 mm/y), pAsAo (0.36 ± 0.25 mm/y) and dAsAo (0.41 ± 0.26 mm/y); and (4) accelerated aortic remodelling is not found in treated heterozygous females (average annual expansion rate at the pAsAo: 0.21 ± 0.18 mm/y vs 0.13 ± 0.04 mm/y, p = 0.43).
Ageing in healthy males is associated with a progressive increase in the ascending aortic diameter at the level of the pAsAo, with a normal expansion rate between 0.9 [12] and 1.2 mm [11] per decade, according to previous population studies. In our study, the longitudinal follow-up of male patients with classic Fabry disease treated with ERT showed an average expansion rate 2 to 3 times higher than that observed in general population. In the absence of a control group (untreated patients with classic Fabry disease), the efficacy of ERT in reducing the aortic expansion rate cannot be directly assessed. Additionally, such accelerated aortic remodelling was not found in heterozygous females. It is well known that heterozygous females have a greater phenotypic variation than males [13]. However, mean age of our female cohort was relatively low (41.5 ± 15.1 years) and a longer follow-up, beyond 60 years of age, is probably warranted to detect pathological dilation of the AsAo in females. To support this assumption, the only female patient with significant aortic dilation was among the oldest in our cohort.
ERT currently represents the standard treatment for classic Fabry disease and is most effective when started early in the course of the disease, prior to the development of significant kidney, heart and/or brain lesions [14, 15]. Indeed, several studies reported a limited efficacy of long-term ERT in both male and female patients with advanced disease at the time of the initiation of ERT [16, 17], especially if fibrosis had already developed in heart, kidney or in the central nervous system [18]. Conversely, early ERT initiation in younger male patients without significant kidney involvement is associated with favourable outcome at 10-year follow-up with most patients remaining alive [10]. Moreover, the absence of cardiac involvement at the time of ERT initiation, defined as an absence of left ventricular hypertrophy (due to hypertrophy/hyperplasia of cardiomyocytes) [9] or a normal ECG trace [19], is also associated in both male and female patients with an absence of increased left ventricular mass at long-term follow-up. In contrast, regarding the ascending aorta, we found that initiation of ERT before any dilation did not appear to provide any clear benefit in terms of expansion rate reduction in male patients. Indeed, in our cohort, the average annual aortic expansion rate under ERT in younger males (with non-dilated pAsAo at baseline, age: 25.5 ± 8.2 years) was not different from that of older patients (47.6 ± 8.6 years) with significant aortic dilatation before ERT: 0.27 ± 0.06 vs 0.44 ± 0.15 mm/y, p = 0.35. However, it is possible that the small size of the subgroups may limit the statistical power of this comparison.
In the most recent Fabry Outcome Survey analysis, the estimated median survival in males on long-term treatment is 77.5 years compared with 60 years in untreated males, suggesting an important benefit of ERT on life expectancy [20]. Hence, because of prolongation of life expectancy with only a partial or limited impact on aortic remodelling, patients on long-term ERT might have a greater lifetime risk of developing a dilation/aneurysm of the AsAo. Although, to date, no increased risk of AsAo dissection or rupture has been reported in Fabry disease, we believe that a periodic assessment of the ascending aorta is warranted to exclude the development of large and potentially harmful aneurysms principally in patients over 60 years of age.
Despite the fact that the cascade of events of Fabry disease-related vasculopathy has been recently characterised, the main underlying mechanism leading to accelerated aortic expansion remains unclear [6]. Pompen et al. have reported the case of a 41-year-old man in whom the wall of the aorta was thickened [21]. The main anomalies observed were the presence of hypertrophied VSMC and the accumulation of a protein-like substance among muscle fibres. They also reported normal appearing elastic fibres without any sign of parietal inflammation. In addition, Elleder observed predominant storage of globotriaosylceramide in VSMC in the media of arterial walls [22]. Cells distended with storage material were necrotic. Shrunken and densified dead cells were also observed, with the formation of hyaline, strongly PAS-positive structures, suggesting apoptosis. These structural anomalies may weaken the arterial wall and promote the development of aortic dilation. In contrast, in the main forms of familial thoracic aneurysm and dissection, the primary underlying mechanism leading to aneurysm is the breakdown of extracellular matrix proteins and the accumulation of mucoid material and loss of VSMC, in a process called cystic medial necrosis [23]. In these forms, the micro-architectural changes lead to vessel dilation, subsequent aneurysm formation and possible dissection/rupture with diameters typically smaller than 50 mm.
Interestingly, a protective role of increased intima-media thickness of the aorta has been reported. Among patients presenting a dilation of the AsAo of more than 45 mm, aortic dissection occurred at a significantly higher aortic diameter to wall thickness ratio and a thinner aortic media [24]. Hence, in classic Fabry disease, the abnormal increased intima-media thickness of the aorta could limit the risk of dissection.
Based on our observations, our local practice is to measure the root of the ascending aorta at baseline with echocardiography in both male and female patients. Upon suspicion of dilation, and/or presence of left ventricular hypertrophy, investigations are completed with thoracic MR (or with computed tomography angiography in the case of contraindications for MR). If a dilation/aneurysm is confirmed, monitoring of the ascending aorta is performed every 1 to 2 years with echocardiography (root of aorta) and 2 to 4 years with CMR. Associated risk factors for accelerated aortic dilation, such as a bicuspid aortic valve and/or arterial hypertension, should be detected and treated. Additionally, prophylactic treatment with beta-blockers has to be considered [25]. In the absence of any specific data concerning the risk of aortic dissection in Fabry disease patients, a surgical intervention should be evaluated for ascending aortic diameters ≥55 mm.
Limitations of this study first include the small size of our cohort. Although our data indicated that the progression of aortic diameters in Fabry disease was not significantly reduced when ERT was started at a younger age (i.e., before development of aortic dilatation), the cohort size might be insufficient to definitely exclude an additional benefit of early initiation of ERT on aortic diameter progression. A second limitation is the absence of a matched control group of untreated Fabry disease patients. Without such a control population, it is difficult to draw specific conclusions about the efficacy (or the lack of efficacy) of ERT in reducing the aortic diameter progression associated with Fabry disease. Such a control group would appear unethical as ERT is now widely accepted as an effective treatment for symptomatic Fabry patients. Considering these limitations, our data nevertheless indicate that despite ERT, aortic remodelling remains significantly more pronounced in Fabry patients than in the general population. Third, the predicted normal aortic diameters computed from the data of the MESA study are adjusted for age, gender and body surface area, which appeared to be the major determinants of aortic size. However, other potential minor determinants of aortic diameter, including blood pressure, renal failure or ethnicity, have not been taken into account in the computation and may potentially affect the accuracy of the diameter prediction in the control group.
In conclusion, male patients with the classic Fabry disease have a high prevalence of ascending aorta dilation, related to an accelerated aortic expansion rate whose pathological process remains unclear. The progression of this aortic remodelling appears to be more pronounced in male Fabry patients as compared with the progression expected in the normal population, even if ERT is initiated before any aortic dilation is present. Conversely, the aortic diameter appears to remain relatively stable in female patients treated with ERT. Further studies are needed to better characterise the pathological mechanism and the natural history of aortic remodelling in order to better define the follow-up and prognosis of aortic dilation in Fabry disease. In the meantime, Fabry disease patients with aortic dilation or aneurysm should be managed in accordance with the current European guidelines [25].
References
1
Dormond
O
,
Rotman
S
,
Barbey
F
. Vasculopathy of Fabry Disease: From Fetal Life to Adulthood. CML-Lysosomal Storage Diseases.
2014;12(1):1–12.
2
Germain
DP
. Fabry disease. Orphanet J Rare Dis. 2010;5(1):30. doi:.https://doi.org/10.1186/1750-1172-5-30
3
Waldek
S
,
Patel
MR
,
Banikazemi
M
,
Lemay
R
,
Lee
P
. Life expectancy and cause of death in males and females with Fabry disease: findings from the Fabry Registry. Genet Med. 2009;11(11):790–6. doi:.https://doi.org/10.1097/GIM.0b013e3181bb05bb
4
MacDermot
KD
,
Holmes
A
,
Miners
AH
. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet. 2001;38(11):769–75. doi:.https://doi.org/10.1136/jmg.38.11.769
5
Barbey
F
,
Brakch
N
,
Linhart
A
,
Jeanrenaud
X
,
Palecek
T
,
Bultas
J
, et al.
Increased carotid intima-media thickness in the absence of atherosclerotic plaques in an adult population with Fabry disease. Acta Paediatr Suppl. 2006;95(451):63–8. doi:.https://doi.org/10.1080/08035320600618924
6
Rombach
SM
,
Twickler
TB
,
Aerts
JM
,
Linthorst
GE
,
Wijburg
FA
,
Hollak
CE
. Vasculopathy in patients with Fabry disease: current controversies and research directions. Mol Genet Metab. 2010;99(2):99–108. doi:.https://doi.org/10.1016/j.ymgme.2009.10.004
7
Barbey
F
,
Qanadli
SD
,
Juli
C
,
Brakch
N
,
Palacek
T
,
Rizzo
E
, et al.
Aortic remodelling in Fabry disease. Eur Heart J. 2010;31(3):347–53. doi:.https://doi.org/10.1093/eurheartj/ehp426
8
Biegstraaten
M
,
Arngrímsson
R
,
Barbey
F
,
Boks
L
,
Cecchi
F
,
Deegan
PB
, et al.
Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: the European Fabry Working Group consensus document. Orphanet J Rare Dis. 2015;10(1):36. doi:.https://doi.org/10.1186/s13023-015-0253-6
9
Kampmann
C
,
Perrin
A
,
Beck
M
. Effectiveness of agalsidase alfa enzyme replacement in Fabry disease: cardiac outcomes after 10 years’ treatment. Orphanet J Rare Dis. 2015;10(1):125. doi:.. Correction published in: Orphanet J Rare Dis. 2016;11:95. https://doi.org/10.1186/s13023-015-0338-2
10
Germain
DP
,
Charrow
J
,
Desnick
RJ
,
Guffon
N
,
Kempf
J
,
Lachmann
RH
, et al.
Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet. 2015;52(5):353–8. doi:.https://doi.org/10.1136/jmedgenet-2014-102797
11
Turkbey
EB
,
Jain
A
,
Johnson
C
,
Redheuil
A
,
Arai
AE
,
Gomes
AS
, et al.
Determinants and normal values of ascending aortic diameter by age, gender, and race/ethnicity in the Multi-Ethnic Study of Atherosclerosis (MESA). J Magn Reson Imaging. 2014;39(2):360–8. doi:.https://doi.org/10.1002/jmri.24183
12
Burman
ED
,
Keegan
J
,
Kilner
PJ
. Aortic root measurement by cardiovascular magnetic resonance: specification of planes and lines of measurement and corresponding normal values. Circ Cardiovasc Imaging. 2008;1(2):104–13. doi:.https://doi.org/10.1161/CIRCIMAGING.108.768911
13
Wilcox
WR
,
Oliveira
JP
,
Hopkin
RJ
,
Ortiz
A
,
Banikazemi
M
,
Feldt-Rasmussen
U
, et al.; Fabry Registry. Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab. 2008;93(2):112–28. doi:.https://doi.org/10.1016/j.ymgme.2007.09.013
14
Eng
CM
,
Germain
DP
,
Banikazemi
M
,
Warnock
DG
,
Wanner
C
,
Hopkin
RJ
, et al.
Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med. 2006;8(9):539–48. doi:.https://doi.org/10.1097/01.gim.0000237866.70357.c6
15
Tøndel
C
,
Bostad
L
,
Larsen
KK
,
Hirth
A
,
Vikse
BE
,
Houge
G
, et al.
Agalsidase benefits renal histology in young patients with Fabry disease. J Am Soc Nephrol. 2013;24(1):137–48. doi:.https://doi.org/10.1681/ASN.2012030316
16
Weidemann
F
,
Niemann
M
,
Störk
S
,
Breunig
F
,
Beer
M
,
Sommer
C
, et al.
Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: evidence for disease progression towards serious complications. J Intern Med. 2013;274(4):331–41. doi:.https://doi.org/10.1111/joim.12077
17
Rombach
SM
,
Smid
BE
,
Bouwman
MG
,
Linthorst
GE
,
Dijkgraaf
MG
,
Hollak
CE
. Long term enzyme replacement therapy for Fabry disease: effectiveness on kidney, heart and brain. Orphanet J Rare Dis. 2013;8(1):47. doi:.https://doi.org/10.1186/1750-1172-8-47
18
Weidemann
F
,
Sanchez-Niño
MD
,
Politei
J
,
Oliveira
JP
,
Wanner
C
,
Warnock
DG
, et al.
Fibrosis: a key feature of Fabry disease with potential therapeutic implications. Orphanet J Rare Dis. 2013;8(1):116. doi:.https://doi.org/10.1186/1750-1172-8-116
19
Schmied
C
,
Nowak
A
,
Gruner
C
,
Olinger
E
,
Debaix
H
,
Brauchlin
A
, et al.
The value of ECG parameters as markers of treatment response in Fabry cardiomyopathy. Heart. 2016;102(16):1309–14. doi:.https://doi.org/10.1136/heartjnl-2015-308897
20
Beck
M
,
Hughes
D
,
Kampmann
C
,
Larroque
S
,
Mehta
A
,
Pintos-Morell
G
, et al.; Fabry Outcome Survey Study Group. Long-term effectiveness of agalsidase alfa enzyme replacement in Fabry disease: A Fabry Outcome Survey analysis. Mol Genet Metab Rep. 2015;3:21–7. doi:.https://doi.org/10.1016/j.ymgmr.2015.02.002
21
Pompen
AW
,
Ruiter
M
,
Wyers
HJ
. Angiokeratoma corporis diffusum (universale) Fabry, as a sign of an unknown internal disease; two autopsy reports. Acta Med Scand. 1947;128(3):234–55. doi:.https://doi.org/10.1111/j.0954-6820.1947.tb06596.x
22
Elleder
M
. Sequelae of storage in Fabry disease--pathology and comparison with other lysosomal storage diseases. Acta Paediatr Suppl. 2003;92(443):46–53, discussion 45.
23
Carlson
RG
,
Lillehei
CW
,
Edwards
JE
. Cystic medial necrosis of the ascending aorta in relation to age and hypertension. Am J Cardiol. 1970;25(4):411–5. doi:.https://doi.org/10.1016/0002-9149(70)90006-8
24
Van Puyvelde
J
,
Verbeken
E
,
Verbrugghe
P
,
Herijgers
P
,
Meuris
B
Aortic wall thickness in patients with ascending aortic aneurysm versus acute aortic dissection. Eur J Cardiothorac Surg. 2016;49(3):756–62.
25
Erbel
R
,
Aboyans
V
,
Boileau
C
,
Bossone
E
,
Bartolomeo
RD
,
Eggebrecht
H
, et al., The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur Heart J. 2014;35(41):2873–926. doi:.https://doi.org/10.1093/eurheartj/ehu281


