Humanised mouse models for haematopoiesis and infectious diseases
DOI: https://doi.org/10.4414/smw.2017.14516
Veronika
Lysenkoa, Donal
McHughb, Lena
Behrmannc, Mary-Aude
Rochatd, Christian M.
Wilka, Larisa
Kovtonyuka, Jean-Pierre
Bourquinc, Christian
Münzb, Markus G.
Manza, Roberto
Speckd, Alexandre P.A.
Theocharidesa
aHaematology, University Hospital Zurich and University of Zurich, Switzerland
bViral Immunobiology, Institute of Experimental Immunology, University of Zurich, Switzerland
cPaediatric Oncology, Children's Research Centre, University Children's Hospital, University of Zurich, Switzerland
dDivision of Infectious Diseases and Hospital Epidemiology, University Hospital of Zurich, University of Zurich, Switzerland
Summary
“Humanised” mouse models have emerged over past years as powerful tools for investigating human haematopoiesis and immunity. They allowed the identification of key factors for the maintenance and function of normal and leukaemic human haematopoietic stem cells. These findings have been widely used to dissect the pathogenesis of multiple myeloid and lymphoid neoplasms, such as acute myeloid leukaemia and acute lymphoblastic leukaemia. Furthermore, these models can serve as a stepping-stone to clinical trials by testing novel drugs that target leukaemic stem cells. The investigation of human immunity in vivo is also of great interest in both the context of understanding the innate and adaptive immune system and responses to viral infections with exclusive human tropism, such as Epstein-Barr virus and human immunodeficiency virus. This review focuses on recent advances in the study of human haematopoiesis and immunity in humanised mouse models, underlining their relevance and limitations.
Introduction
The need to understand normal or malignant human cell development and disease heterogeneity, and to investigate novel therapeutic strategies in order to accelerate their transition into clinical trials, strongly supported the development of “humanised” mouse models [1, 2]. Another important driving force for the development of these models is the ethical concerns raised by clinical trials in humans.
Three major advancements over past decades have significantly shaped the field (fig. 1). The first was the discovery of a spontaneous autosomal recessive mutation in the Prkdc gene, termed Prkdcscid (Prkdc: protein kinase, DNA activated, catalytic polypeptide; scid: severe combined immunodeficiency) in C.B-17 mice (called SCID mice). This permitted low engraftment of human fetal tissues, peripheral blood mononuclear cells and haematopoietic stem cells (HSCs) owing to a T and B cell deficiency [3–5]. Low human haematopoietic engraftment in C.B-17 mice is due to a tendency to develop functional mouse T and B cell immunity (termed “leakiness”) and normal natural killer (NK) cell activity [6]. Furthermore, mutations in the locus of one of the recombination-activating genes (RAG1 or RAG2) resulted in Rag1tm1Mom
or Rag2tm1.1Cgn
mice with a T- and B-cell deficiency similar to that seen in C.B-17 mice, but in addition rendered them resistant to radiation and leakiness [7].
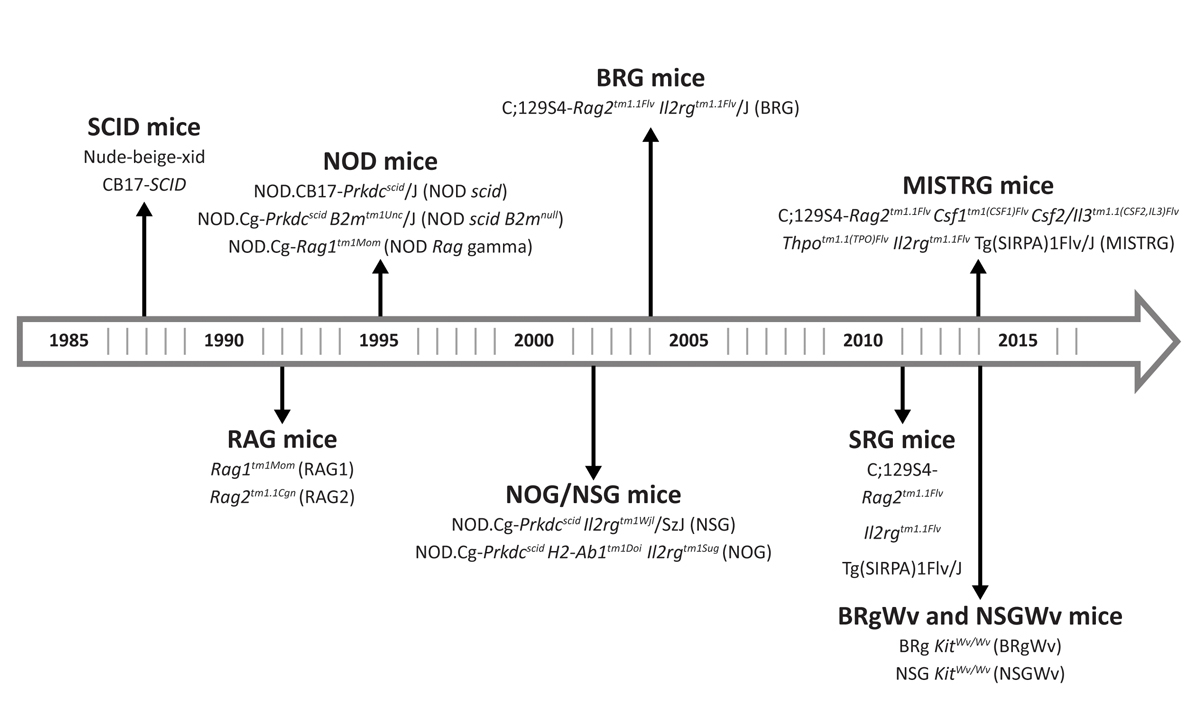
Figure 1
Historical development of humanised mouse strains. The first major advancement was the discovery of a mutation in the Prkdc gene that led to development of SCID mice. The second was the development of NOD.CB17-Prkdcscid
/J (NOD scid) mice, generated by backcrossing mice with the Prkdcscid
mutation to mice on the NOD (non-obese diabetic) background. The introduction of a targeted mutation in the locus of the interleukin-2 receptor (IL-2R) γ-chain led to the development of the NOD.Cg-Prkdcscid Il2rgtm1Wjl
/SzJ (NSG) mice, which represents the most widely used host strain so far. Further discoveries, took into account the importance of cytokines and growth factors in supporting HSC development, which led to the development of “next-generation” humanised mouse strains, such as MISTRG mice.
The second advancement was the development of NOD.CB17-Prkdcscid
/J mice, generated by backcrossing mice with the Prkdcscid
mutation to mice on the NOD (non-obese diabetic) background [8]. In comparison to the C.B-17 mice, these mice showed improved engraftment of HSCs as a result of lower NK cell activity and defects in their innate immunity, although they had short life-spans due to spontaneous development of thymic lymphomas [8].
The introduction of a targeted mutation in the locus of the interleukin-2 receptor (IL-2R) γ-chain, which leads to NK cell depletion, was the third key achievement that led to a substantially higher engraftment of functional human HSCs [9–12]. Today, the NOD.Cg-Prkdcscid Il2rgtm1Wjl
/SzJ (NSG) model that resulted represents the most commonly used host strain for xenograft experiments. Although the introduction of NSG mice has significantly advanced the field, NSG mice fail to support maturation of human B cells into memory and plasma cells. In addition, T cell development lacks thymic support and the development of innate immunity (i.e., NK cell and macrophage development) is limited [13].
A crucial immune determinant of human haematopoietic cell survival identified in xenograft models is macrophage tolerance [14, 15]. Binding of human CD47 on human haematopoietic cells to mouse signal regulatory protein-alpha (SIRPα) on resident host macrophages generates a “don’t eat me” signal that leads to inhibition of phagocytosis, protects the transplanted human cells and consecutively contributes to the human cell survival in vivo [15, 16]. The interaction between human CD47 and mouse SIRPα is present in mice on a NOD background since the mouse SIRPα IgV domain is very similar to the human homologue.
In addition, the recognition of the importance of cytokines and growth factors secreted by bone marrow stromal cells in supporting HSC development led to the development of “next-generation” humanised mouse strains, such as MISTRG mice [17]. These mice were genetically engineered to express human macrophage colony stimulating factor (M-CSF), interleukin-3 (IL-3), SIRPα, thrombopoietin and granulocyte-macrophage-CSF (GM-CSF) (abbreviated MISTRG) to allow efficient human haematopoietic development. In contrast to previous models, these support the development of functional human macrophages and NK cells [17]. Moreover, these mice favour myelomonocytic differentiation and the engraftment of mobilised peripheral blood mononuclear cells, which is limited in the NOD.CB17-Prkdcscid
/J and NSG mouse models [18]. Recently, an improved “next-generation” humanised mouse strain of MISTRG that harbours an additional knock-in of human interleukin-6 (IL-6) was developed. These mice support efficient engraftment of human plasma cell neoplasms, which particularly rely on the presence of IL-6 [19, 20]. Improved models that support multilineage engraftment of human HSCs while minimising the mouse HSC compartment by ablating mouse cells were also developed. These models were generated by the introduction of mutations in the Kit receptor, which is vital for HSC maintenance and function, in immunodeficient mouse strains on the BALB/c (BRg KitWv/Wv
) or NOD (NSG KitWv/Wv
) background [21].
Although the humanised mice field has advanced extensively, there are still obstacles that need to be overcome in order to generate a model that fully recapitulates the complexity of the human haematopoietic system. This review focuses on current advances in the development of humanised mouse models for human haematopoiesis and immunity, highlighting their relevance and limitations.
Haematopoiesis
Normal human haematopoiesis
The haematopoietic system is a hierarchically organised cellular system, in which HSCs reside at the apex and are characterised by their ability to self-renew and to differentiate into mature blood cells of the myeloid and the lymphoid lineage (fig. 2 and table 1). By definition, human HSCs are capable of repopulating immune-compromised mice and are enriched in the lineage-marker negative (Lin−), CD34+, CD38−, CD90+ (Thy1+) and CD45RA− population in the human fetal liver / fetal bone marrow, umbilical cord blood and adult bone marrow [24–30]. Later, CD49f integrin was identified as a marker that further enriches for human HSCs in umbilical cord blood samples. In combination with the high efflux of the mitochondrial dye rhodamin 123, 14 to 28% (i.e., 1 in 3.5–7 cells) of functional umbilical cord blood human HSCs that express the surface phenotype mentioned above are capable of engrafting NSG mice at the single cell level [22]. HSCs give rise to multipotent progenitors (MPPs), which in turn give rise to oligopotent multilymphoid progenitors (MLPs) and common myeloid progenitors (CMPs), which further differentiate into unipotent progenitors (fig. 2). For example, CMPs give rise to lineage-committed megakaryocyte/erythrocyte progenitors (MEPs) and granulocyte/monocyte progenitors (GMPs). MPPs are distinguished from HSCs by the lack of CD49f expression (table 1) [22]. Recently, the surface marker profile of CMPs, MEPs and MPPs was re-defined by the addition of CD71 and the thrombopoietin receptor, which allowed better purification from the rest of the populations in the haematopoietic tree [31]. Furthermore, it was shown that cells with megakaryocyte/erythroid potential branch out directly from HSCs, rather than from MEPs, a concept that resembles the mouse haematopoietic tree [32].
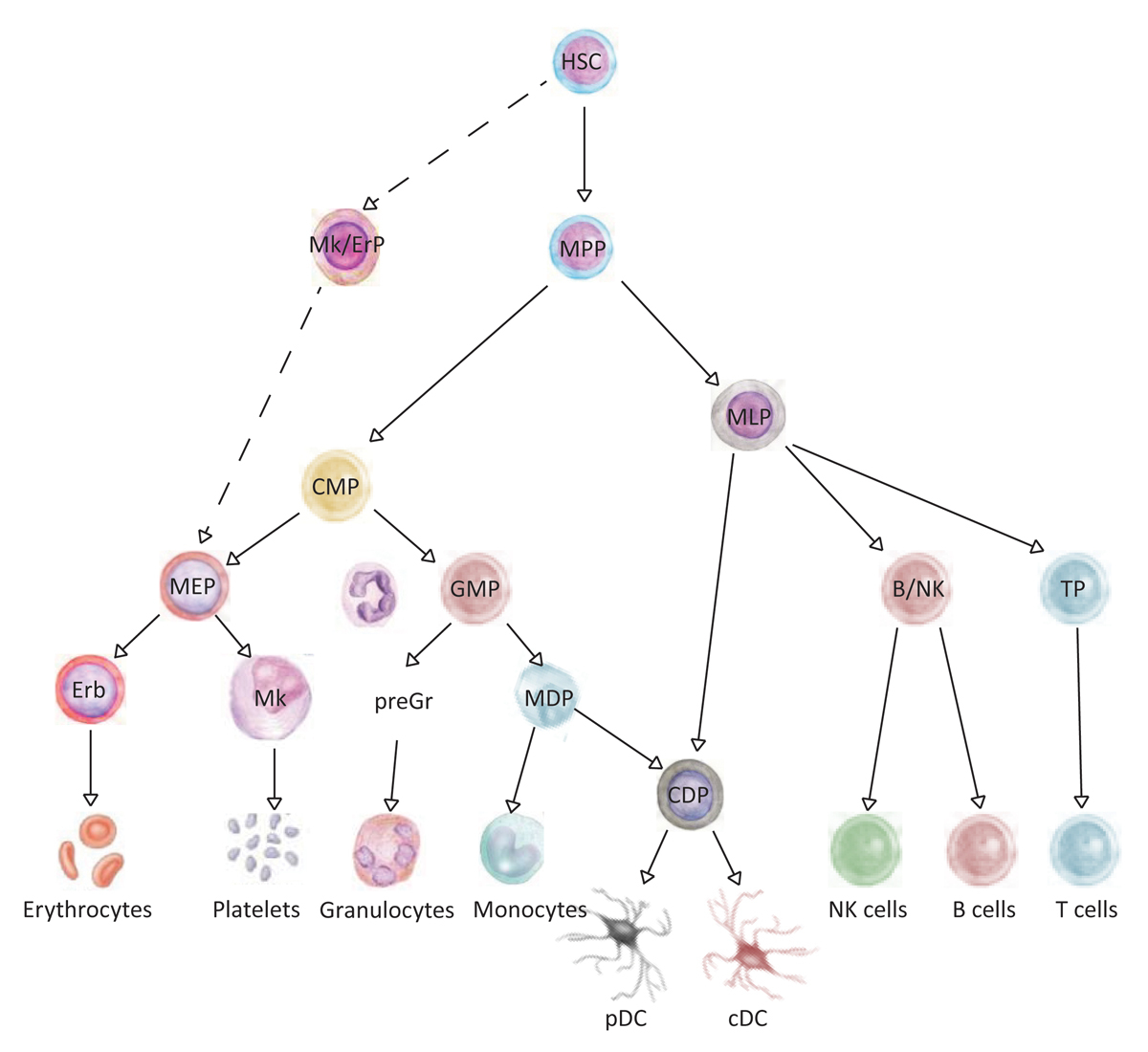
Figure 2
Hierarchy within human haematopoietic system. The tree-like organisation of the haematopoietic system is composed of haematopoietic stem cells (HSCs) with long-term self-renewal capacity that reside at the apex of the hierarchy and through multipotent and lineage-committed progenitors generate the differentiated mature cells with no or limited self-renewal capacity.
B/NK = B and NK cell progenitor; cDC = conventional dendritic cells; CDP = common dendritic cell progenitor; CMP = common myeloid progenitor; Erb = erythroblast; GMP = granulocyte monocyte progenitor; HSC = haematopoietic stem cells; MDP = monocyte and dendritic cell progenitor; MEP = megakaryocyte-erythrocyte progenitor; Mk = megakaryocytes; Mk/ErP = megakaryocyte/erythrocyte progenitor; MLP = multi-lymphoid progenitor; MPP = multi-potent progenitor; pDC = plasmocytoid dendritic cells; PreGr = pre granulocytes; TP = T cell progenitor
Table 1 Human haematopoietic stem and progenitor cell phenotypes.
|
Haematopoietic cell population
|
Cell surface marker combination
|
| HSPC |
Lin−CD34+CD38−
|
| HSC |
Lin−CD34+CD38−CD45RA-CD90+Rho−/lowCD49f+
|
| MPP |
Lin−CD34+CD38−CD45RA−CD90−CD49f−
|
| *MPP F1 |
Lin−CD34+CD38−CD45RA−CD90−CD49f−CD71−BAH1−
|
| *MPP F2/3 |
Lin−CD34+CD38−CD45RA−CD90−CD49f−CD71+BAH1−/+
|
| MLP |
Lin−CD34+CD38−CD45RA+CD90−/low
|
| CMP |
CD34+CD38+CD45RA−CD10−Flt3+
|
| *CMP F1 |
CD34+CD38+CD45RA−CD10−Flt3+CD71−BAH1−
|
| *CMP F2/3 |
CD34+CD38+CD45RA−CD10−Flt3+CD71+BAH1−/+
|
| MEP |
CD34+CD38+CD45RA−CD10−Flt3-
|
| *MEP F1 |
CD34+CD38+CD45RA−CD10−Flt3−CD71−BAH1−
|
| *MEP F2/3 |
CD34+CD38+CD45RA−CD10−Flt3−CD71+BAH1−/+
|
| GMP |
CD34+CD38+CD45RA+CD10−Flt3+
|
| B/NK |
CD34+CD38+CD45RA+CD10+
|
The regulation of HSC self-renewal and differentiation by cell-intrinsic and cell-extrinsic factors has been extensively investigated in the mouse system using knockout models, whereas their human analogues have not been explored because of the lack of suitable humanised model. Nonetheless, some key human HSCs factors have been cross-confirmed and/or newly identified. HSCs are a quiescent cell population that mostly reside in the G0 phase of the cell cycle [33]. Recently, it was demonstrated that cyclin-dependent kinase C (CDK6) levels regulate the kinetics of quiescence exit of human HSC subsets in xenograft models [34]. Further intrinsic factors that have been associated with the human HSC state include the transcription factors SOX8, SOX18, HoxB4, HES1 and BMI1. However, their functional role remains to be validated in xenograft studies. Extrinsic factors that support human HSC function and engraftment in xenograft models include the macrophage receptor SIRPα (see introduction) and thrombopoietin. Both human factors have been genetically introduced into immune-compromised mice in order to facilitate normal and malignant human HSC engraftment [35, 36]. Overall, there are still many gaps in the current knowledge of normal human HSC maintenance and function, which with the development of new humanized mouse models can be further elucidated.
Malignant human haematopoiesis
Myeloid neoplasms
Patient-derived xenograft (PDX) models have been extensively used to dissect the pathogenesis of acute myeloid leukaemia (AML) at the clonal level and for preclinical testing of novel therapeutic regimens. One major contribution of PDX models to the AML field is the identification of preleukaemic (pre-LSCs) and leukaemic stem cells (LSCs) (fig. 3). By definition, LSCs can repopulate immune-compromised mice and propagate disease when transplanted from the primary into secondary recipients. It is well accepted that LSCs initiate disease and their persistence after therapy causes relapse in patients [38, 39]. Thus, the identification and therapeutic targeting of LSCs is of high clinical relevance.
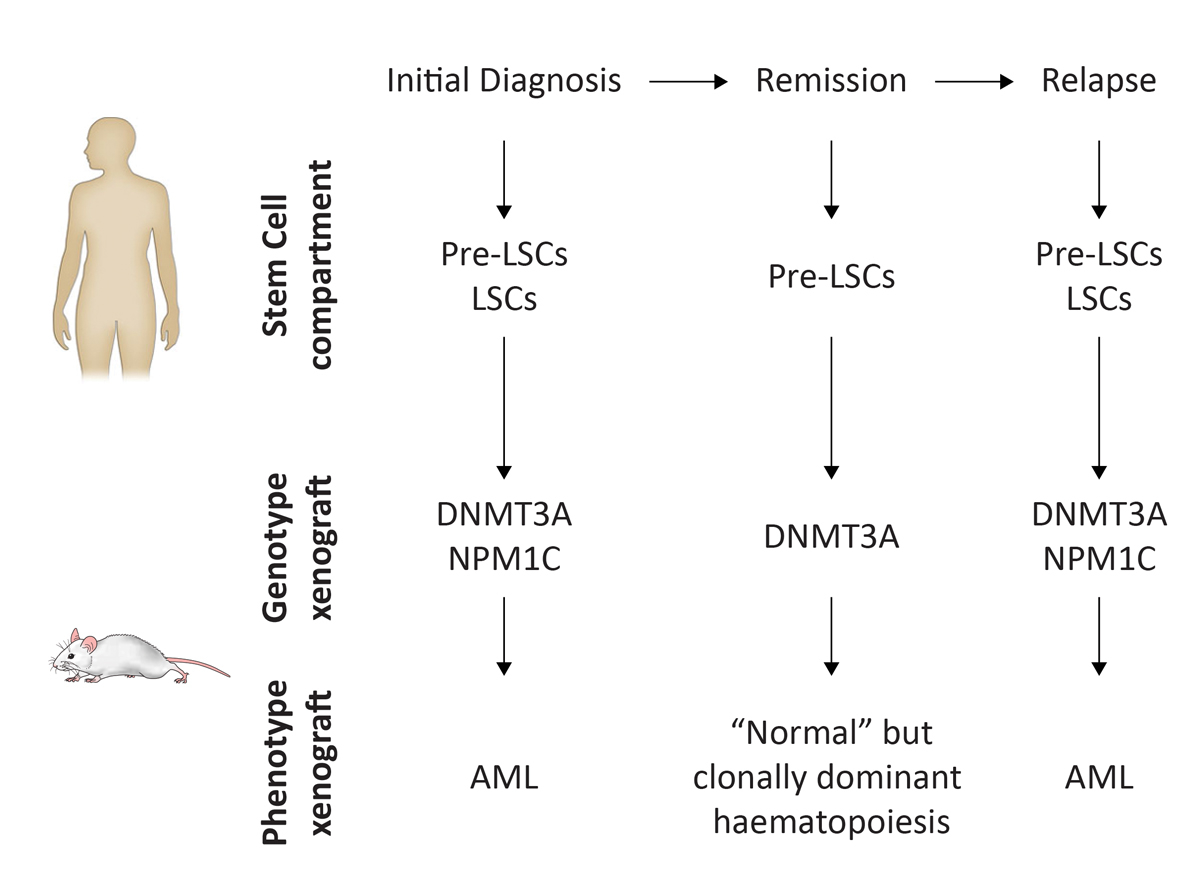
Figure 3
Concept of acute myeloid leukaemia (AML) xenograft models as an example for myeloid neoplasms. PDX models reflect phenotypic as well as genetic features of myeloid neoplasms. In AML, the HSC compartment consists of preleukaemic stem cells (pre-LSCs) and leukaemic stem cells (LSCs) that harbour somatic mutations. Transplantation of AML cells from the initial diagnosis into PDX models leads to AML development in vivo. Genetically aberrant pre-LSCs can persist in patient remission, but lead to multilineage (normal) repopulation in PDX models. Frequently, the persistence of pre-LSCs that acquire additional somatic mutations is at the origin of disease relapse, which can be reproduced in PDX models (adapted from [37]).
More recently, AML PDX models led to the identification of pre-LSCs. Pre-LSCs are HSCs that harbour recurrent somatic mutations and, with the acquisition of additional genetic hits, can lead to AML initiation and relapse [37, 40]. Mutations present in pre-LSCs are mostly found in genes involved in chromatin remodelling, whereas mutations that confer growth advantage to the AML clone occur later in AML development [41]. In contrast to LSCs, pre-LSCs lead to multilineage repopulation in PDX models, which makes it an excellent model to study the progression of preleukaemic lesions to overt AML.
Although PDX models significantly contributed to the AML field by enabling LSCs to be identified and characterised, the knowledge extracted from these models also answered more clinically related questions. In particular, potentially critical therapeutic factors for the survival of LSCs have been identified [42]. Among these, the immunoglobulin superfamily member CD47, which is highly expressed on LSCs, has been shown to protect LSCs from innate immune surveillance [43, 44]. Targeting CD47 in the AML PDX models demonstrated significant efficacy and constituted the basis for currently on-going clinical trials in AML, other haematopoietic neoplasms and solid tumours [45]. Furthermore, the gene expression profile of AML stem and progenitor fractions was correlated with AML development in the PDX model and led to the identification of an AML gene expression signature that correlates with clinical outcome and may be used for risk stratification in future clinical trials [46].
The relevance of AML PDX models has been questioned in the past since immune-compromised hosts such as NOD.CB17-Prkdcscid
/J and NSG mice support engraftment of only a fraction of all AML patient samples. In particular, AML with a favourable risk profile was shown to poorly engraft conventional models. In an attempt to extend the spectrum of transplantable AML, a defined subgroup of AML characterised by the presence of an inversion of chromosome 16, excellent response to conventional chemotherapy, but poor engraftment in NSG mice, was investigated in MISTRG mice (see introduction) [47]. In contrast to NSG mice, MISTRG mice support robust engraftment of this AML subtype. The same study identified M-CSF as a critical factor for AML engraftment, a finding that could potentially be exploited therapeutically. In another study, favourable risk AML and acute promyelocytic leukaemia were successfully transplanted into engineered human bone fragments, so called ossicles, implanted into immune-deficient mice [48].
Only a few studies have successfully demonstrated engraftment of less aggressive diseases such as myelodysplastic syndromes and myeloproliferative neoplasms in immune-compromised mice. Engraftment of certain human myelodysplastic syndrome subtypes was improved by transplantation into newborn animals and by direct intrafemoral transplantation into adults [49, 50]. Other approaches include the co-transplantation of myelodysplastic syndrome samples along with healthy mesenchymal stromal cells and transplantation into ossicles (see above) [48, 51]. Similar approaches have also been undertaken for the development of myeloproliferative neoplasm PDX models.
In conclusion, PDX models for myeloid neoplasms have significantly contributed to understanding the pathogenesis of AML in recent decades and today have become a standard in preclinical testing of novel therapeutic regimens. Future studies will show whether the knowledge gained from these models can be directly translated into clinical practice and help elucidate the pathogenesis of less aggressive diseases.
Lymphoid neoplasms
Lymphoid neoplasms include acute and chronic leukaemia as well as a wide range of lymphomas that are characterised by variable stages of B and T cell maturation [52]. Acute B-precursor lymphoblastic leukaemia is a malignant disease most commonly found in children; in adulthood chronic lymphoblastic leukaemia (CLL) is more common [53]. The first leukaemia xenograft model was established more than two decades ago with AML patient samples, and provided the first functional insights into the haematopoietic hierarchy of this disease [38]. Systematic studies for acute lymphoblastic leukaemia (ALL) later set the stage for comprehensive preclinical testing [54, 55].
The development of NSG mice (see introduction) substantially improved the engraftment capacities of human lymphoma, B and T cell leukaemia [56–58]. In contrast to AML, emerging evidence indicates that most ALL cells can propagate the disease in this xenotransplantation model [59–61]. Between 50 and 100 cells are sufficient to reconstitute the leukaemic phenotype observed in the diagnostic sample [60–63]. In fact, leukaemia can be reconstituted from bone marrow samples with minimal residual disease [64] (fig. 4).
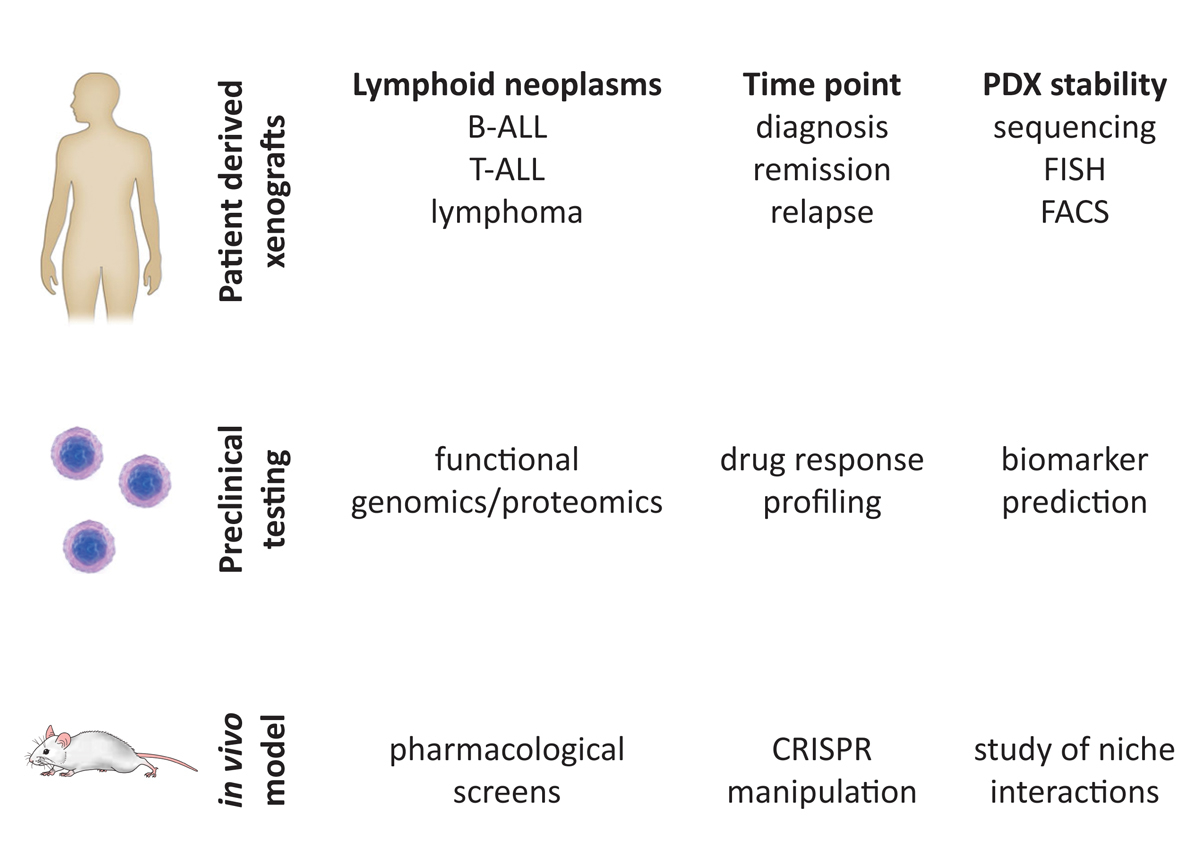
Figure 4
Generation of PDX models for preclinical studies. Patient-derived xenograft models of B-acute lymphocytic leukaemia (ALL), T-ALL and lymphoma can be generated from different disease time points like diagnosis, remission or relapse. PDX stability and complexity are highly conserved and can be proven by targeted sequencing, FISH (fluorescence in situ hybridisation) or FACS (fluorescence activated cell sort) analysis. PDX models can be used for a broad range of translation studies. Use of a co-culture system with mesenchymal stem cellss enables in vitro approaches for drug efficacy testing or biomarker identification. The high conservation of patient samples and xenografts enables the development of in vivo models for pharmacological screens, cell manipulations with CRISPR or the study of niche interactions in the microenvironment of the cancer cells.
These models also contributed to the understanding of leukaemic stem cell plasticity found in certain ALL subtypes, in particular mixed lineage leukaemia (MLL)-rearranged leukaemia [65]. They further clarified the role of the MLL-rearranged ALL lineage switch to escape immunotherapeutic approaches that target CD19 on ALL cells [66]. Residual subclones that retained CD19 expression and displayed a myeloid phenotype in patients could reconstitute both a myeloid and lymphoid leukaemia in xenografts. Thus, this model can be used to address essential functional questions with leukaemia cells that are directly derived from patients. In fact, several publications have shown that leukaemia xenografts can recapitulate the clonal complexity of ALL with reasonable fidelity [60, 63, 64, 67, 68].
Recent studies indicate that this approach is also effective for adult leukaemia samples, although conditioning using total body irradiation of NSG may improve the engraftment capacities [69]. Lymphomas represent one of the most complex and heterogeneous set of malignancies and although a high diversity of PDX models are available, they fall short in truly representing patient heterogeneity [70, 71]. Additionally, the injection of lymphoma cells into specific tissues might introduce potential bias. However, tumour injection models are useful for studying the impact of different mutations and drugs on tumour aggressiveness [72–74]. Furthermore, repositories of AML, ALL and lymphoma PDXs will be available that should facilitate translational research forward [42].
The use of xenograft ALL models for preclinical testing of predictive biomarkers and drug efficacies offers new possibilities for translational research and is a big step towards clinical trials [64, 68, 75–77]. Finally, given the high degree of phenotypic and genotypic conservation between patient samples and their corresponding xenografts, this model will enable us to study the interaction of leukaemic cells within their complex niche in the bone marrow, which may provide improved and more precise rationales for therapeutic targeting of resistant leukaemia clones in their microenvironment [61, 78] (fig. 4). Xenograft models for chronic lymphoid malignancies are also being developed using “next-generation” humanised mouse strains and will be extremely useful for preclinical testing of novel therapies [79].
Immune system and infectious diseases
Human innate and adaptive immune responses in humanised mice
The human leucocytes of mice with human immune system components reconstituted from transferred human haematopoietic progenitor cells (HIS mice) show similarities to cord blood immune cells. Most B cells have a transitional or naïve phenotype, NK cells have a low frequency of killer immunoglobulin-like receptor (KIR) expression and still contain a CD56 negative subpopulation, and most T cells are naïve [80–83]. In addition, lymph nodes and mucosal secondary lymphoid tissues are poorly developed as a result of blocked lymphoid tissue inducer (LTi) cell differentiation in the absence of the cytokine receptor common gamma chain (γc) in most mouse strains that support efficient human immune compartment reconstitution [84]. Accordingly, cell-mediated immune responses can be mounted, while humoral immune responses are compromised, in HIS mice. Dendritic cells (DCs), the antigen-presenting cells that coordinate both cell-mediated and humoral immune responses, are reconstituted at frequencies and with a subset representation similar to that of human secondary lymphoid tissues [85–87]. Furthermore, they react to adjuvant exposure in the form of defined toll-like receptor (TLR) ligands similar to human peripheral blood DCs, whereas mouse DCs within HIS mice react with a different hierarchy that is consistent with the dissimilar TLR distribution between mouse and man [85]. Antigen that is targeted to these human DCs with suitable adjuvants can elicit CD4+ T cell responses [85, 88]. These are HLA restricted and can recognise a diverse set of antigen-derived epitopes. In addition, primed CD4+ and CD8+ T cell clones can target EBV-transformed B cells and, in HLA transgenic HIS mice, specificities develop which are also found in human EBV carriers [80, 89–91]. These T cell responses restrict viral loads not only during EBV infection, but also after human immunodeficiency virus (HIV) and hepatic adenovirus infection of HIS mice [80, 89, 92–94]. Therefore, protective human T cell responses can be reproduced in the humanised mouse model.
In addition to priming T cells, DCs are also capable to activate innate lymphocytes. Indeed, human DC-activating adjuvants render NK cells of HIS mice more cytotoxic [81]. The early differentiated NK cell compartment of HIS mice can restrict lytic EBV infection [95] and recognises tumour cells with their distinct set of human NK cell-specific receptors [81, 96]. Furthermore, reconstituting two human immune systems in parallel from two donors that are mismatched in the HLA ligands of inhibitory NK cell receptors changes the education of these innate cytotoxic lymphocytes and allows the resulting NK cell population to more efficiently control EBV infection in the mixed B cell compartment [83]. This improved innate immune control most likely results from efficient targeting of EBV-infected B cells of one donor by the less inhibited NK cell compartment of the other donor. Thus, NK cell responses that are similar to those in small children can be modelled in HIS mice and manipulated for improved EBV-specific immune control.
In contrast to functional DC, T and NK cell responses, humoral immune responses are difficult to achieve. Usually only a minority of HIS mice develop specific isotype-switched antibody responses after viral infection [97]. Furthermore, the steady-state levels of human IgG in HIS mice are with below one microgram per millilitre more than a thousand-fold lower than in human serum [9, 82]. Accordingly, germinal centres are rarely observed, but even so the B and T cell zone is segregated in secondary lymphoid tissues of HIS mice (fig. 5) [85]. However, most B cells are of transitional and naïve phenotype, and follicular helper T cells that assist B cells in the germinal centre reaction are absent [82]. Interestingly, providing additional DCs either by adoptive transfer or possibly also by expansion of the reconstituted human DC populations might at least partially overcome these deficiencies and lead to B cell plasmablast differentiation, follicular helper T cell priming and antigen specific IgG responses [82]. Therefore, humoral immune responses are largely absent in HIS mice, but further manipulation of this model system might allow them to develop.
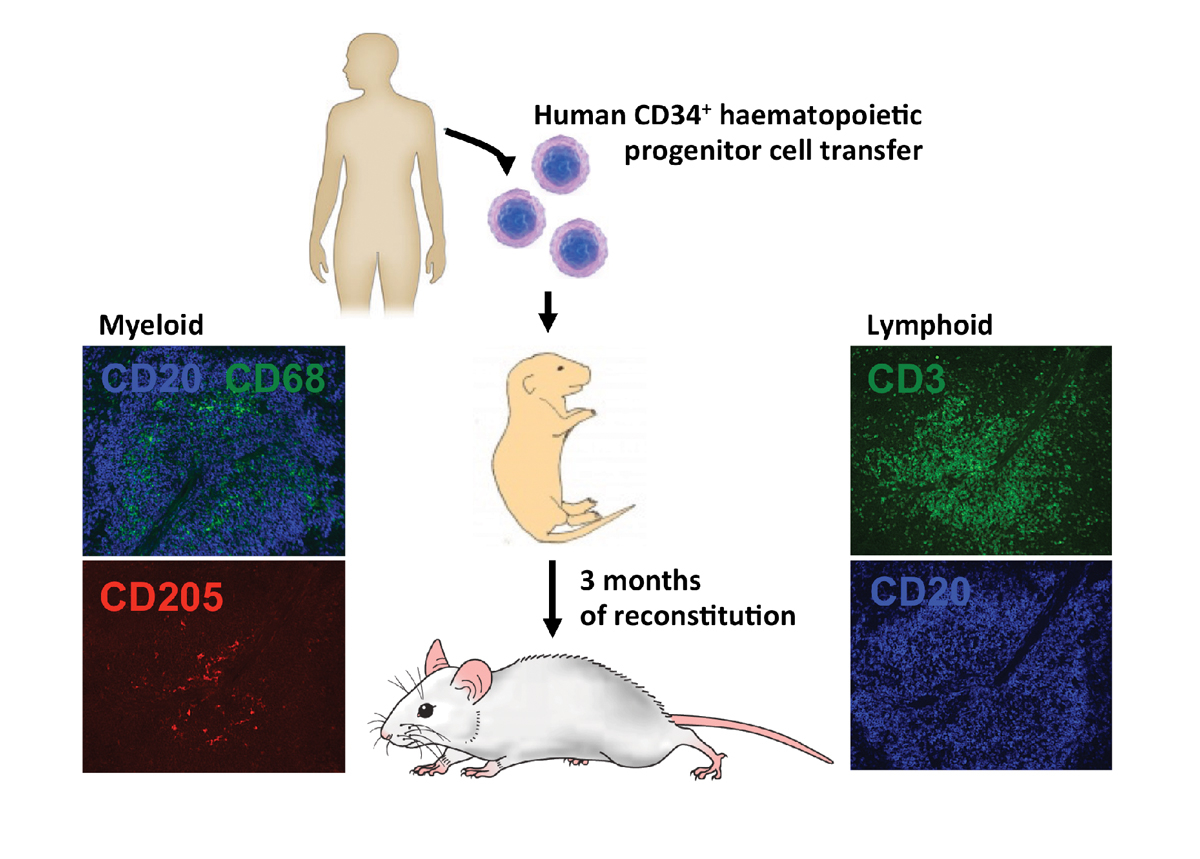
Figure 5
Human immune compartments of HIS mice. HIS mice reconstitute most human immune compartments within 3 months after neonatal intrahepatic human CD34+ haematopoietic progenitor cell transfer. In the spleen of NSG mice, macrophages (CD68), dendritic cells (CD205), T cells (CD3) and B cells (CD20) can be found in the white pulp.
In addition to the compromised B cell compartment, it remains unclear if there is sufficient human myeloid reconstitution in HIS mice. Although DCs are found at considerable frequencies, it has been argued that macrophage populations are poorly developed [98]. Along these lines, human alveolar macrophages were able to protect HIS mice from lung proteinosis only when they developed in a human IL-3 and GM-CSF transgenic mouse background [98]. However, some lung infections could be studied in the absence of this enhanced human myeloid cell reconstitution in HIS mice. Influenza A virus infection caused the induction of virus-specific T cell responses and Mycobacterium tuberculosis infection led to granuloma formation [99, 100]. Therefore, whether enhanced myeloid cell reconstitution is beneficial or not might depend on the particular application of HIS mice. With respect to immune responses, it has to be kept in mind that the immunoregulatory function of some myeloid cells might be detrimental for the induction of immune responses. It has been shown that stem cell factor, IL-3 and GM-CSF transgenic mice allow elevated myeloid cell reconstitution, although at the same time expanded FoxP3 positive regulatory T cells could dampen the induction of immune responses [101]. Thus, HIS mice reconstitute a wide variety of human immune system compartments. Some of these already demonstrate functionality in the currently available models, whereas others, especially B cells, might benefit from additional manipulations of the model system in order to obtain the ability to execute their full range of immune functions. The resulting novel HIS mice may allow for a more comprehensive investigation of human immune responses.
Espstein-Barr virus research with humanised mice
The ubiquitous gamma-herpes virus, Espstein-Barr virus (EBV), is the cause of infectious mononucleosis, and is associated with lymphomas predominantly of B cell origin and certain epithelial cell malignancies. Owing to its exclusive tropism for humans, studying this virus in vivo is difficult and was until recently restricted to related viruses in old-world monkeys [102]. However, EBV was not only the first human oncogenic virus to be discovered, fifty years ago [103], but it was also the first pathogenic agent to be studied in HIS mice [9]. EBV infection of B cells in HIS mice can result in asymptomatic infection or lymphoproliferative disease, depending on the infectious dose [80, 104]. Both latent and lytic EBV infection can be modelled in HIS mice (fig. 6). Depending on the viral strain, the majority of infected B cells either express all eight latent EBV proteins and only a minority initiates lytic replication (latency III predominates after B95-8 infection), or the frequency of lytically EBV replicating cells can be significantly increased (high lytic replication after M81 infection) [105]. The Hodgkin’s and Burkitt’s lymphoma-associated latencies I and II have been suggested to be present after HIS mouse infection with EBV [106–108], but they probably contribute to infection only at very low frequencies.
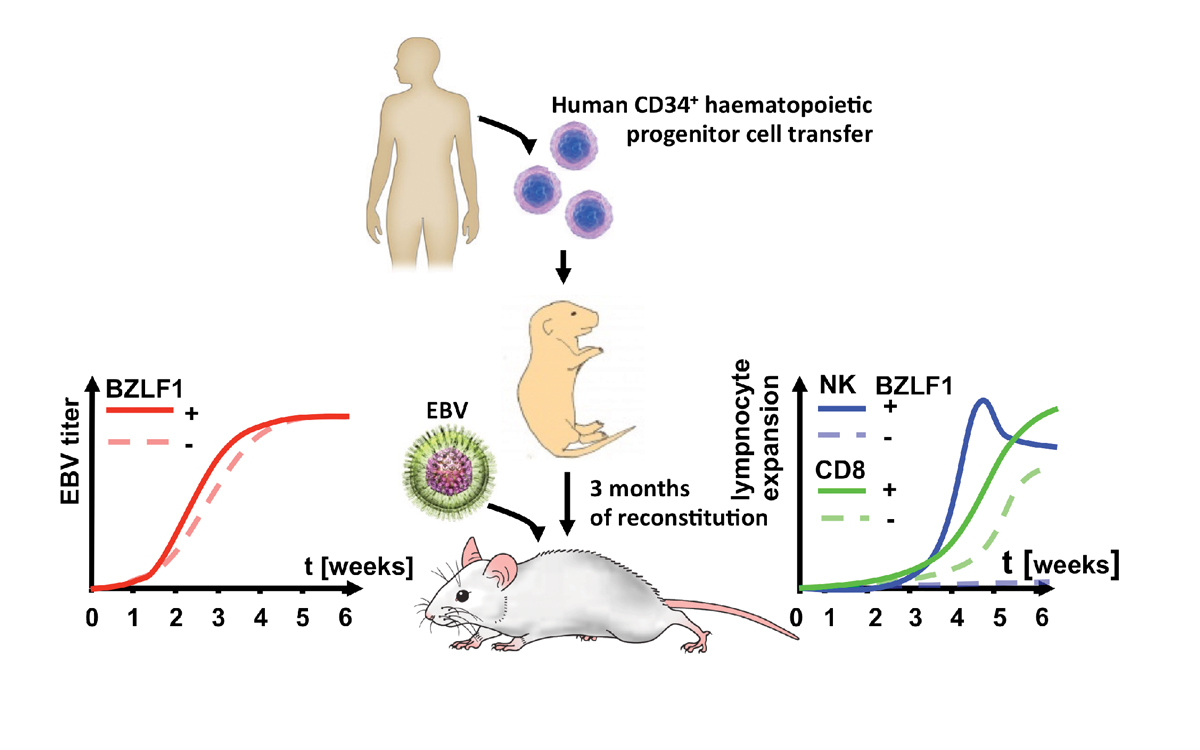
Figure 6
HIS mice can be persistently infected with Epstein-Barr virus (EBV) and develop immune responses to the infection. Mice that have been reconstituted by means of neonatal intrahepatic injection with human CD34+ haematopoietic progenitor cells develop viraemia upon intraperitoneal EBV infection. Lytic replication deficient virus (BZLF1-) has a lower viral titre at 3 weeks after infection. NK cells expand to lytic EBV infection at 4 weeks after infection and CD8+ T cells react to both lytic and latent infection with expansion by 5 weeks of infection.
Apart from these virological parameters, some hallmarks of EBV specific immune control can also be recapitulated in HIS mice (fig. 6). The importance of NK cells in protecting from EBV-associated diseases, especially in the innate restriction of primary infection, was first demonstrated in HIS mice. Depletion of NK cells prior to infection revealed a protective effect against lytic EBV replication [83, 95]. The importance of NK cells in recognising lytic replication during acute EBV infection was then also confirmed in longitudinal studies of paediatric patients with infectious mononucleosis [109], indicating the predictive nature of the humanised mouse model for EBV-associated diseases.
After the transient and protective NK cell proliferation, CD8+ T cells massively expand during EBV infection of HIS mice [80, 89]. Both CD4+ and CD8+ T cells control viral loads, whereas CD8+ T cells are more important in preventing EBV-associated lymphomas [80]. Among these expanding CD8+ T cells, both latent and lytic EBV antigen specificities can be detected in HLA transgenic HIS mice [80, 90, 91]. Furthermore, a protective effect of EBV-specific T cell clones, which target lytic antigens against high viremia and extra-lymphoid lymphoma genesis, could be documented [91], suggesting the importance of these specificities for future vaccine development against EBV. Indeed, targeting of EBV antigens to DC populations is under investigation in HIS mice [85, 110]. However, these distinct antigen specificities are so far still difficult to modulate and the role of their effector functions cannot be assessed. The generation of recombinant viral vectors to manipulate the expression of certain genes in the developing haematopoietic lineage would constitute a valuable tool for assessing the importance of specific human immune functions during EBV-associated lymphoma genesis. For the investigation of adaptive cellular immune responses to EBV, T cell receptor transgenes would allow the development of T cell populations that could not only be tracked during the course of EBV challenge, but also further investigated for their protective potential during viral infection. Together with recombinant manipulation of the EBV genome [91, 95, 107, 111], the aforementioned strategies might lead to predictive preclinical models for relevant human diseases, which would provide insight that can be more readily translated into targeted clinical research.
Human immunodeficiency virus infection in humanised mice
The human immunodeficiency virus (HIV) type-1 belongs to the lentivirus genus (family of retroviruses), comprising five serogroups that can infect a number of vertebrates. Clinical hallmarks of lentiviral infections are chronicity, intra-host evolution and cellular tropism [112]. Their basic genomic structure comprises the long-terminal repeat (LTR; transcription start), three genes encoding the structural proteins – gag, pol and env – and regulatory genes (Tat and Rev) [113]. Notably, accessory genes contribute to their virulence [114, 115].
HIV is a human-specific virus; the HIV pandemic counts currently 36.7 million of HIV-infected human (http://www.who.int/hiv/en/). HIV infects mainly CD4+ T-cells and macrophages, leading to their progressive depletion [116, 117] and exhaustion [118, 119]. Moreover, HIV can remain latent by integrating its genome into the host chromosome, allowing viral rebound upon interruption of antiretroviral treatment (ART). Many in vivo aspects of HIV infection remain an enigma, e.g., HIV transmission [120], HIV latency [121, 122], tissue target cells [123–125], role of the HIV accessory genes [126], immune activation [127] and co-infection pathogenesis [128–130]. Of course, a key in any research efforts is the identification of novel therapeutic approaches [131–136].
The lack of a small HIV animal model has hampered HIV research since its discovery. The feline immunodeficiency virus (FIV), the simian immunodeficiency virus (SIV) and hybrid viruses between SIV and HIV (SHIV) have been used as a substitute for HIV in domestic cats or nonhuman primates, respectively. Findings with these models must further be validated with HIV itself. Notably, the “first” generation of hu mice, already used in the 1980s i.e., the SCID hu thy/liv or hu PBL SCID [137–142], lacked de novo multilineage haematopoiesis and/or suffered from graft-versus-host disease (GvHD), limiting their use in HIV research.
The “new” generation of hu mice (see introduction) are highly sensitive to HIV infection (figs 7 and 8
) [97, 143–146] and recapitulate key features of HIV infection in human, e.g., dissemination [146], pathogenesis [147] and latency [148]. In the following, we will give a short update about the various humanized mouse models currently used in HIV research, including the pros and cons (figs 7 and 8
).
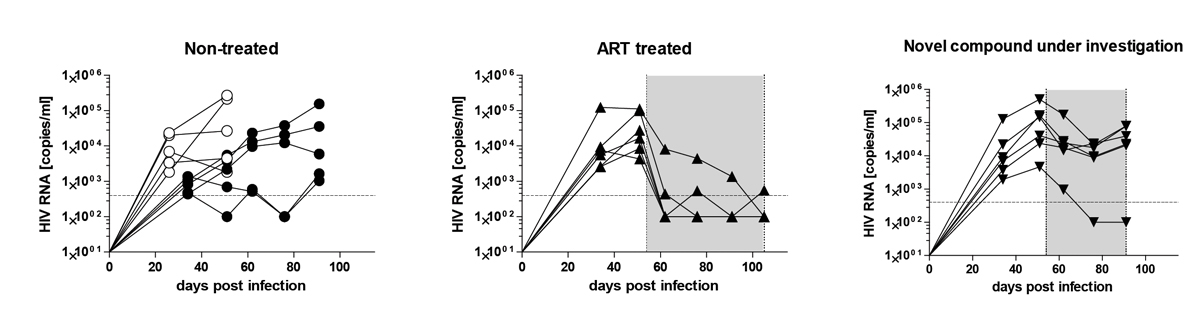
Figure 7
Humanised mice are highly susceptible to human immunodeficiency virus (HIV) and allow the examination investigational drugs. Humanised mice were infected with the CCR5-tropic HIV strain, YU-2, and viral load was monitored over time with quantitative real-time PCR. The left panel shows untreated mice. The middle panel shows mice treated with antiretroviral therapy (tenofovir, lamivudine and raltegravir). The right panel shows mice treated with an investigational compound. The grey shaded area indicates the treatment period. The filled and empty circles in the untreated group represent mice from two independent cohorts.
NSG mice intraperitoneally transplanted with adult human peripheral blood mononuclear cells s comprise a simple and direct lymphoid-based model that allows HIV infection over 10 weeks [149]. This model is similar to the hu PBL SCID mouse model [4] and has similar limitations, i.e., lack of multilineage haematopoiesis and risk of GvHD. Thus, we would like to highlight only one study, which showed the value of this model to assess the presence of residual HIV reservoir in immunosuppressed patients, employed as a murine viral outgrowth assay [150].
Hu mice based on the transplantation of human CD34+ HSPCs develop long-term de novo multilineage haematopoiesis and immunity [9] with low prevalence of GvHD. They are very valuable for long-term studies, studying HIV infection in deep tissues, HIV latency establishment [146, 148] and virus-specific CD8+ T-cell development [92].
These mice have been very useful for dissecting the pathogenic role of the HIV accessory proteins and for studying HIV-associated immune activation. For example, by use of these mice, the destructive potential of a distinct motif of the Nef gene, the secretory modification region (SMR) by promoting CD4 apoptosis and a cytokine storm was convincingly demonstrated [151]. As related to HIV-associated immune activation, a recent study reported how exosomes containing transactivation response (TAR) RNA-stimulated macrophages and plasmacytoid dendritic cells (pDC), thereby promoting interferon-beta (IFN-β) and IL-6 secretion [152].
Notably, NSG mice have been successfully used to investigate novel treatment approaches (fig. 7), pre-exposure prophylaxis (PreP) against vaginal transmission [153, 154], testing novel innovative compounds [155], optimised long-acting ART [156–158] or broadly neutralising antibodies (bNAb) [159]. Importantly, bNAb also reduced the latent reservoir size, either through FcүR engagement [160] or by additional treatment with latency reversing agents [161] – the latter was the first study that successfully targeted the latent reservoir for its eradication and thus a potential cure of HIV. Similarly, gene therapy-based approaches resulted in functional cure in NSG mice.
Bone marrow, liver, thymus mice (BLT) produced by concomitant transplantation of fetal liver/thymus tissues with CD34+ cells into adult immunodeficient mice are a widely used model. The thymic tissue resulted in an increase of T cell reconstitution, maturation and selection, enabling the generation of an MHC-restricted adaptive immune response from which HIV shows immune escape [162–164]. Notably, engraftment of human cells in the gastrointestinal tract and female reproductive tract is very prominent as compared with NSG mice, which permits study of HIV transmission and replication in mucosal tissues [165–169]. Viral replication was found preferentially in intestinal crypts, which released a pool of both immature and mature virions [170]. These findings were in contradiction to in vitro findings, where virion maturation occurred quickly, emphasising the value of in vivo investigations with the corresponding microenvironment [171].
Furthermore, BLT mice facilitated broad HIV transmission research; for example, ART reduced the amount of cell-free virus in the female reproductive tract and in the breast milk, overall decreasing the prevalence of HIV transmission [172, 173]. Another study showed that early ART initiation was able to preserve the immune system and functionally control productive HIV infection thanks to the development of a HIV-specific immune response [174, 175]. These results corroborated findings from the Visconti study in humans, where 14 patients treated during primary infection were able to constrain HIV infection for several years without treatment [174, 175].
Moreover, numerous prophylactic approaches have been validated using this model. For instance, lentiviral modification of HSPCs with a polymeric anti-HIV IgA construct or on adeno-associated virus (AAV) containing a bNAb (VRC07) hampered mucosal HIV transmission [176, 177]. Last, but not least, a study examining cross-species transmission events from chimpanzees to humans: Yuan et al. decoded critical viral envelop mutations required to allow HIV zoonosis [178]. Altogether, the BLT model is highly valuable, especially for mucosal tissues and adaptive immune response analysis.
More and more sophisticated humanised mice models are generated in order to recapitulate the human cell distribution and functionality in animals and which are examined for studying HIV. For example, MITRG and MISTRG mice demonstrated superior myeloid and NK cell development and function, in comparison to preceding models [17]. Deng et al., using MISKITRG mice demonstrated the potential of cytotoxic CD8+ T cells (CTL) obtained from chronically infected patients, to constrain HIV released from reactivated latent cells [179].
To address more precise questions, highly specific humanised mouse models are used, such as, the T cell only and macrophage only mouse model (TOM and MOM, respectively) that permits development of only a specific human lineage. TOM can be readily infected with HIV and establishment of latency occurred in absence of other cell types [180]. Similarly, MOM confirmed the capacity of some strains of HIV to replicate and infect macrophages in vivo [181].
The studies mentioned above represent only a small fraction of insights gained in HIV infection/pathogenesis and cure using humanised mice that underscores their value for studying HIV. They likely represent the long awaited small animal model for the HIV research community. Since various models are available, and more will be available soon, the HIV researcher will be faced with the challenge to identify the most suitable model to address their research question.
Conclusions and outlook
Our understanding of human haematopoiesis and immunity has extensively evolved with the help of humanised mouse models. Currently, there are an increasing number of models for more aggressive neoplasms, such as AML and ALL, and for infectious diseases, such as EBV and HIV. Experimental strategies used in order to improve these and create new models include the expression of supportive human factors by genetic modification of mice, the co-transplantation of human mesenchymal stem cells, and the xenotransplantation of humanised ossicles. One particularly interesting approach is the use of the CRISPR/Cas9 technology for fast genetic modification of humanised host strains to express specific human factors. Despite on-going efforts, modelling less aggressive neoplasms, reproducing disease heterogeneity seen in patients, and probing certain immune cell pathologies is still a challenge that researchers face. Future models will need to support engraftment of the majority of patient samples for any given neoplasm or infection to allow researchers to draw substantial conclusions and predict accurate therapy responses in humans.
References
1
Mestas
J
,
Hughes
CC
. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–8. doi:.https://doi.org/10.4049/jimmunol.172.5.2731
2
Rongvaux
A
,
Takizawa
H
,
Strowig
T
,
Willinger
T
,
Eynon
EE
,
Flavell
RA
, et al.
Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol. 2013;31(1):635–74. doi:.https://doi.org/10.1146/annurev-immunol-032712-095921
3
Bosma
GC
,
Custer
RP
,
Bosma
MJ
. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301(5900):527–30. doi:.https://doi.org/10.1038/301527a0
4
Mosier
DE
,
Gulizia
RJ
,
Baird
SM
,
Wilson
DB
. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335(6187):256–9. doi:.https://doi.org/10.1038/335256a0
5
Lapidot
T
,
Pflumio
F
,
Doedens
M
,
Murdoch
B
,
Williams
DE
,
Dick
JE
. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992;255(5048):1137–41. doi:.https://doi.org/10.1126/science.1372131
6
Nonoyama
S
,
Smith
FO
,
Bernstein
ID
,
Ochs
HD
. Strain-dependent leakiness of mice with severe combined immune deficiency. J Immunol. 1993;150(9):3817–24.
7
Mombaerts
P
,
Iacomini
J
,
Johnson
RS
,
Herrup
K
,
Tonegawa
S
,
Papaioannou
VE
. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68(5):869–77. doi:.https://doi.org/10.1016/0092-8674(92)90030-G
8
Shultz
LD
,
Schweitzer
PA
,
Christianson
SW
,
Gott
B
,
Schweitzer
IB
,
Tennent
B
, et al.
Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154(1):180–91.
9
Traggiai
E
,
Chicha
L
,
Mazzucchelli
L
,
Bronz
L
,
Piffaretti
JC
,
Lanzavecchia
A
, et al.
Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–7. doi:.https://doi.org/10.1126/science.1093933
10
Kerre
TC
,
De Smet
G
,
De Smedt
M
,
Zippelius
A
,
Pittet
MJ
,
Langerak
AW
, et al.
Adapted NOD/SCID model supports development of phenotypically and functionally mature T cells from human umbilical cord blood CD34(+) cells. Blood. 2002;99(5):1620–6. doi:.https://doi.org/10.1182/blood.V99.5.1620
11
Ito
M
,
Hiramatsu
H
,
Kobayashi
K
,
Suzue
K
,
Kawahata
M
,
Hioki
K
, et al.
NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100(9):3175–82. doi:.https://doi.org/10.1182/blood-2001-12-0207
12
Ishikawa
F
,
Yasukawa
M
,
Lyons
B
,
Yoshida
S
,
Miyamoto
T
,
Yoshimoto
G
, et al.
Development of functional human blood and immune systems in NOD/SCID/IL2 receptor gamma chain(null) mice. Blood. 2005;106(5):1565–73. doi:.https://doi.org/10.1182/blood-2005-02-0516
13
Theocharides
AP
,
Rongvaux
A
,
Fritsch
K
,
Flavell
RA
,
Manz
MG
. Humanized hemato-lymphoid system mice. Haematologica. 2016;101(1):5–19. doi:.https://doi.org/10.3324/haematol.2014.115212
14
Terpstra
W
,
Leenen
PJ
,
van den Bos
C
,
Prins
A
,
Loenen
WA
,
Verstegen
MM
, et al.
Facilitated engraftment of human hematopoietic cells in severe combined immunodeficient mice following a single injection of Cl2MDP liposomes. Leukemia. 1997;11(7):1049–54. doi:.https://doi.org/10.1038/sj.leu.2400694
15
Takenaka
K
,
Prasolava
TK
,
Wang
JC
,
Mortin-Toth
SM
,
Khalouei
S
,
Gan
OI
, et al.
Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8(12):1313–23. doi:.https://doi.org/10.1038/ni1527
16
Takizawa
H
,
Manz
MG
. Macrophage tolerance: CD47-SIRP-alpha-mediated signals matter. Nat Immunol. 2007;8(12):1287–9. doi:.https://doi.org/10.1038/ni1207-1287
17
Rongvaux
A
,
Willinger
T
,
Martinek
J
,
Strowig
T
,
Gearty
SV
,
Teichmann
LL
, et al.
Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014;32(4):364–72. doi:.https://doi.org/10.1038/nbt.2858
18
Saito
Y
,
Ellegast
JM
,
Rafiei
A
,
Song
Y
,
Kull
D
,
Heikenwalder
M
, et al.
Peripheral blood CD34(+) cells efficiently engraft human cytokine knock-in mice. Blood. 2016;128(14):1829–33. doi:.https://doi.org/10.1182/blood-2015-10-676452
19
Verma
R
,
Strowig
T
,
Das
R
,
Koduru
S
,
Hafemann
A
,
Hopf
S
, et al.
Humanized Mouse Model of Myeloma Reveals Clinically Occult Genomic Changes in Primary Tumor Cells. Blood. 2015;126(23).
20
Das
R
,
Strowig
T
,
Verma
R
,
Koduru
S
,
Hafemann
A
,
Hopf
S
, et al.
Microenvironment-dependent growth of preneoplastic and malignant plasma cells in humanized mice. Nat Med. 2016;22(11):1351–7. doi:.https://doi.org/10.1038/nm.4202
21
Cosgun
KN
,
Rahmig
S
,
Mende
N
,
Reinke
S
,
Hauber
I
,
Schäfer
C
, et al.
Kit regulates HSC engraftment across the human-mouse species barrier. Cell Stem Cell. 2014;15(2):227–38. doi:.https://doi.org/10.1016/j.stem.2014.06.001
22
Notta
F
,
Doulatov
S
,
Laurenti
E
,
Poeppl
A
,
Jurisica
I
,
Dick
JE
. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333(6039):218–21. doi:.https://doi.org/10.1126/science.1201219
23
Laurenti
E
,
Doulatov
S
,
Zandi
S
,
Plumb
I
,
Chen
J
,
April
C
, et al.
The transcriptional architecture of early human hematopoiesis identifies multilevel control of lymphoid commitment. Nat Immunol. 2013;14(7):756–63. doi:.https://doi.org/10.1038/ni.2615
24
Baum
CM
,
Weissman
IL
,
Tsukamoto
AS
,
Buckle
AM
,
Peault
B
. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA. 1992;89(7):2804–8. doi:.https://doi.org/10.1073/pnas.89.7.2804
25
Craig
W
,
Kay
R
,
Cutler
RL
,
Lansdorp
PM
. Expression of Thy-1 on human hematopoietic progenitor cells. J Exp Med. 1993;177(5):1331–42. doi:.https://doi.org/10.1084/jem.177.5.1331
26
Bhatia
M
,
Wang
JC
,
Kapp
U
,
Bonnet
D
,
Dick
JE
. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci USA. 1997;94(10):5320–5. doi:.https://doi.org/10.1073/pnas.94.10.5320
27
Larochelle
A
,
Vormoor
J
,
Hanenberg
H
,
Wang
JC
,
Bhatia
M
,
Lapidot
T
, et al.
Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med. 1996;2(12):1329–37. doi:.https://doi.org/10.1038/nm1296-1329
28
Kondo
M
,
Wagers
AJ
,
Manz
MG
,
Prohaska
SS
,
Scherer
DC
,
Beilhack
GF
, et al.
Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21(1):759–806. doi:.https://doi.org/10.1146/annurev.immunol.21.120601.141007
29
Majeti
R
,
Park
CY
,
Weissman
IL
. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1(6):635–45. doi:.https://doi.org/10.1016/j.stem.2007.10.001
30
McKenzie
JL
,
Takenaka
K
,
Gan
OI
,
Doedens
M
,
Dick
JE
. Low rhodamine 123 retention identifies long-term human hematopoietic stem cells within the Lin-CD34+CD38- population. Blood. 2007;109(2):543–5. doi:.https://doi.org/10.1182/blood-2006-06-030270
31
Notta
F
,
Zandi
S
,
Takayama
N
,
Dobson
S
,
Gan
OI
,
Wilson
G
, et al.
Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science. 2016;351(6269):aab2116. doi:.https://doi.org/10.1126/science.aab2116
32
Yamamoto
R
,
Morita
Y
,
Ooehara
J
,
Hamanaka
S
,
Onodera
M
,
Rudolph
KL
, et al.
Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154(5):1112–26. doi:.https://doi.org/10.1016/j.cell.2013.08.007
33
Catlin
SN
,
Busque
L
,
Gale
RE
,
Guttorp
P
,
Abkowitz
JL
. The replication rate of human hematopoietic stem cells in vivo. Blood. 2011;117(17):4460–6. doi:.https://doi.org/10.1182/blood-2010-08-303537
34
Laurenti
E
,
Frelin
C
,
Xie
S
,
Ferrari
R
,
Dunant
CF
,
Zandi
S
, et al.
CDK6 levels regulate quiescence exit in human hematopoietic stem cells. Cell Stem Cell. 2015;16(3):302–13. doi:.https://doi.org/10.1016/j.stem.2015.01.017
35
Rongvaux
A
,
Willinger
T
,
Takizawa
H
,
Rathinam
C
,
Auerbach
W
,
Murphy
AJ
, et al.
Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc Natl Acad Sci USA. 2011;108(6):2378–83. doi:.https://doi.org/10.1073/pnas.1019524108
36
Strowig
T
,
Rongvaux
A
,
Rathinam
C
,
Takizawa
H
,
Borsotti
C
,
Philbrick
W
, et al.
Transgenic expression of human signal regulatory protein alpha in Rag2-/-gamma(c)-/- mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci USA. 2011;108(32):13218–23. doi:.https://doi.org/10.1073/pnas.1109769108
37
Shlush
LI
,
Zandi
S
,
Mitchell
A
,
Chen
WC
,
Brandwein
JM
,
Gupta
V
, et al.; HALT Pan-Leukemia Gene Panel Consortium. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506(7488):328–33. doi:.https://doi.org/10.1038/nature13038
38
Lapidot
T
,
Sirard
C
,
Vormoor
J
,
Murdoch
B
,
Hoang
T
,
Caceres-Cortes
J
, et al.
A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–8. doi:.https://doi.org/10.1038/367645a0
39
Hope
KJ
,
Jin
L
,
Dick
JE
. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5(7):738–43. doi:.https://doi.org/10.1038/ni1080
40
Thomas
D
,
Majeti
R
. Biology and relevance of human acute myeloid leukemia stem cells. Blood. 2017;129(12):1577–85. doi:.https://doi.org/10.1182/blood-2016-10-696054
41
Corces-Zimmerman
MR
,
Hong
WJ
,
Weissman
IL
,
Medeiros
BC
,
Majeti
R
. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci USA. 2014;111(7):2548–53. doi:.https://doi.org/10.1073/pnas.1324297111
42
Townsend
EC
,
Murakami
MA
,
Christodoulou
A
,
Christie
AL
,
Köster
J
,
DeSouza
TA
, et al.
The Public Repository of Xenografts Enables Discovery and Randomized Phase II-like Trials in Mice. Cancer Cell. 2016;29(4):574–86. doi:.https://doi.org/10.1016/j.ccell.2016.03.008
43
Theocharides
AP
,
Jin
L
,
Cheng
PY
,
Prasolava
TK
,
Malko
AV
,
Ho
JM
, et al.
Disruption of SIRPα signaling in macrophages eliminates human acute myeloid leukemia stem cells in xenografts. J Exp Med. 2012;209(10):1883–99. doi:.https://doi.org/10.1084/jem.20120502
44
Majeti
R
,
Chao
MP
,
Alizadeh
AA
,
Pang
WW
,
Jaiswal
S
,
Gibbs
KD, Jr
, et al.
CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–99. doi:.https://doi.org/10.1016/j.cell.2009.05.045
45
Petrova
PS
,
Viller
NN
,
Wong
M
,
Pang
X
,
Lin
GH
,
Dodge
K
, et al.
TTI-621 (SIRPαFc): A CD47-Blocking Innate Immune Checkpoint Inhibitor with Broad Antitumor Activity and Minimal Erythrocyte Binding. Clin Cancer Res. 2017;23(4):1068–79. doi:.https://doi.org/10.1158/1078-0432.CCR-16-1700
46
Ng
SW
,
Mitchell
A
,
Kennedy
JA
,
Chen
WC
,
McLeod
J
,
Ibrahimova
N
, et al.
A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature. 2016;540(7633):433–7. doi:.https://doi.org/10.1038/nature20598
47
Ellegast
JM
,
Rauch
PJ
,
Kovtonyuk
LV
,
Müller
R
,
Wagner
U
,
Saito
Y
, et al.
inv(16) and NPM1mut AMLs engraft human cytokine knock-in mice. Blood. 2016;128(17):2130–4. doi:.https://doi.org/10.1182/blood-2015-12-689356
48
Reinisch
A
,
Thomas
D
,
Corces
MR
,
Zhang
X
,
Gratzinger
D
,
Hong
WJ
, et al.
A humanized bone marrow ossicle xenotransplantation model enables improved engraftment of healthy and leukemic human hematopoietic cells. Nat Med. 2016;22(7):812–21. doi:.https://doi.org/10.1038/nm.4103
49
Pang
WW
,
Pluvinage
JV
,
Price
EA
,
Sridhar
K
,
Arber
DA
,
Greenberg
PL
, et al.
Hematopoietic stem cell and progenitor cell mechanisms in myelodysplastic syndromes. Proc Natl Acad Sci USA. 2013;110(8):3011–6. doi:.https://doi.org/10.1073/pnas.1222861110
50
Woll
PS
,
Kjällquist
U
,
Chowdhury
O
,
Doolittle
H
,
Wedge
DC
,
Thongjuea
S
, et al.
Myelodysplastic syndromes are propagated by rare and distinct human cancer stem cells in vivo. Cancer Cell. 2014;25(6):794–808. doi:.. Correction in: Cancer Cell. 2015;27(4)603–5. http://www.cell.com/cancer-cell/fulltext/S1535-6108(15)00091-4
https://doi.org/10.1016/j.ccr.2014.03.036
51
Medyouf
H
,
Mossner
M
,
Jann
JC
,
Nolte
F
,
Raffel
S
,
Herrmann
C
, et al.
Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell. 2014;14(6):824–37. doi:.https://doi.org/10.1016/j.stem.2014.02.014
52
Campo
E
,
Swerdlow
SH
,
Harris
NL
,
Pileri
S
,
Stein
H
,
Jaffe
ES
. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–32. doi:.https://doi.org/10.1182/blood-2011-01-293050
53
Dores
GM
,
Devesa
SS
,
Curtis
RE
,
Linet
MS
,
Morton
LM
. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. 2012;119(1):34–43. doi:.https://doi.org/10.1182/blood-2011-04-347872
54
Liem
NL
,
Papa
RA
,
Milross
CG
,
Schmid
MA
,
Tajbakhsh
M
,
Choi
S
, et al.
Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103(10):3905–14. doi:.https://doi.org/10.1182/blood-2003-08-2911
55
Jones
L
,
Carol
H
,
Evans
K
,
Richmond
J
,
Houghton
PJ
,
Smith
MA
, et al.
A review of new agents evaluated against pediatric acute lymphoblastic leukemia by the Pediatric Preclinical Testing Program. Leukemia. 2016;30(11):2133–41. doi:.https://doi.org/10.1038/leu.2016.192
56
Imada
K
. Immunodeficient mouse models of lymphoid tumors. Int J Hematol. 2003;77(4):336–41. doi:.https://doi.org/10.1007/BF02982640
57
Shultz
LD
,
Lyons
BL
,
Burzenski
LM
,
Gott
B
,
Chen
X
,
Chaleff
S
, et al.
Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–89. doi:.https://doi.org/10.4049/jimmunol.174.10.6477
58
Agliano
A
,
Martin-Padura
I
,
Mancuso
P
,
Marighetti
P
,
Rabascio
C
,
Pruneri
G
, et al.
Human acute leukemia cells injected in NOD/LtSz-scid/IL-2Rgamma null mice generate a faster and more efficient disease compared to other NOD/scid-related strains. Int J Cancer. 2008;123(9):2222–7. doi:.https://doi.org/10.1002/ijc.23772
59
le Viseur
C
,
Hotfilder
M
,
Bomken
S
,
Wilson
K
,
Röttgers
S
,
Schrauder
A
, et al.
In childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell. 2008;14(1):47–58. doi:.https://doi.org/10.1016/j.ccr.2008.05.015
60
Schmitz
M
,
Breithaupt
P
,
Scheidegger
N
,
Cario
G
,
Bonapace
L
,
Meissner
B
, et al.
Xenografts of highly resistant leukemia recapitulate the clonal composition of the leukemogenic compartment. Blood. 2011;118(7):1854–64. doi:.https://doi.org/10.1182/blood-2010-11-320309
61
Ebinger
S
,
Özdemir
EZ
,
Ziegenhain
C
,
Tiedt
S
,
Castro Alves
C
,
Grunert
M
, et al.
Characterization of Rare, Dormant, and Therapy-Resistant Cells in Acute Lymphoblastic Leukemia. Cancer Cell. 2016;30(6):849–62. doi:.https://doi.org/10.1016/j.ccell.2016.11.002
62
Rehe
K
,
Wilson
K
,
Bomken
S
,
Williamson
D
,
Irving
J
,
den Boer
ML
, et al.
Acute B lymphoblastic leukaemia-propagating cells are present at high frequency in diverse lymphoblast populations. EMBO Mol Med. 2013;5(1):38–51. doi:.https://doi.org/10.1002/emmm.201201703
63
Clappier
E
,
Gerby
B
,
Sigaux
F
,
Delord
M
,
Touzri
F
,
Hernandez
L
, et al.
Clonal selection in xenografted human T cell acute lymphoblastic leukemia recapitulates gain of malignancy at relapse. J Exp Med. 2011;208(4):653–61. doi:.https://doi.org/10.1084/jem.20110105
64
Fischer
U
,
Forster
M
,
Rinaldi
A
,
Risch
T
,
Sungalee
S
,
Warnatz
HJ
, et al.
Genomics and drug profiling of fatal TCF3-HLF-positive acute lymphoblastic leukemia identifies recurrent mutation patterns and therapeutic options. Nat Genet. 2015;47(9):1020–9. doi:.https://doi.org/10.1038/ng.3362
65
Barabé
F
,
Kennedy
JA
,
Hope
KJ
,
Dick
JE
. Modeling the initiation and progression of human acute leukemia in mice. Science. 2007;316(5824):600–4. doi:.https://doi.org/10.1126/science.1139851
66
Lin
S
,
Luo
RT
,
Ptasinska
A
,
Kerry
J
,
Assi
SA
,
Wunderlich
M
, et al.
Instructive Role of MLL-Fusion Proteins Revealed by a Model of t(4;11) Pro-B Acute Lymphoblastic Leukemia. Cancer Cell. 2016;30(5):737–49. doi:.https://doi.org/10.1016/j.ccell.2016.10.008
67
Notta
F
,
Mullighan
CG
,
Wang
JC
,
Poeppl
A
,
Doulatov
S
,
Phillips
LA
, et al.
Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature. 2011;469(7330):362–7. doi:.https://doi.org/10.1038/nature09733
68
Frismantas
V
,
Dobay
MP
,
Rinaldi
A
,
Tchinda
J
,
Dunn
SH
,
Kunz
J
, et al.
Ex vivo drug response profiling detects recurrent sensitivity patterns in drug resistant ALL. Blood. 2017; 129(11):e26–37. doi:.https://doi.org/10.1182/blood-2016-09-738070
69
Patel
B
,
Dey
A
,
Castleton
AZ
,
Schwab
C
,
Samuel
E
,
Sivakumaran
J
, et al.
Mouse xenograft modeling of human adult acute lymphoblastic leukemia provides mechanistic insights into adult LIC biology. Blood. 2014;124(1):96–105. doi:.https://doi.org/10.1182/blood-2014-01-549352
70
Macor
P
,
Secco
E
,
Zorzet
S
,
Tripodo
C
,
Celeghini
C
,
Tedesco
F
. An update on the xenograft and mouse models suitable for investigating new therapeutic compounds for the treatment of B-cell malignancies. Curr Pharm Des. 2008;14(21):2023–39. doi:.https://doi.org/10.2174/138161208785294591
71
Donnou
S
,
Galand
C
,
Touitou
V
,
Sautès-Fridman
C
,
Fabry
Z
,
Fisson
S
. Murine models of B-cell lymphomas: promising tools for designing cancer therapies. Adv Hematol. 2012;2012:701704. doi:.https://doi.org/10.1155/2012/701704
72
Birkenmeier
K
,
Moll
K
,
Newrzela
S
,
Hartmann
S
,
Dröse
S
,
Hansmann
ML
. Basal autophagy is pivotal for Hodgkin and Reed-Sternberg cells’ survival and growth revealing a new strategy for Hodgkin lymphoma treatment. Oncotarget. 2016;7(29):46579–88. doi:.https://doi.org/10.18632/oncotarget.10300
73
Chu
Y
,
Hochberg
J
,
Yahr
A
,
Ayello
J
,
van de Ven
C
,
Barth
M
, et al.
Targeting CD20+ Aggressive B-cell Non-Hodgkin Lymphoma by Anti-CD20 CAR mRNA-Modified Expanded Natural Killer Cells In Vitro and in NSG Mice. Cancer Immunol Res. 2015;3(4):333–44. doi:.https://doi.org/10.1158/2326-6066.CIR-14-0114
74
Deutsch
AJA
,
Rinner
B
,
Pichler
M
,
Prochazka
K
,
Pansy
K
,
Bischof
M
, et al.
NR4A3 suppresses lymphomagenesis through induction of proapoptotic genes. Cancer Res. 2017;77(9):2375–86. doi:.https://doi.org/10.1158/0008-5472.CAN-16-2320
75
Suryani
S
,
Carol
H
,
Chonghaile
TN
,
Frismantas
V
,
Sarmah
C
,
High
L
, et al.
Cell and molecular determinants of in vivo efficacy of the BH3 mimetic ABT-263 against pediatric acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2014;20(17):4520–31. doi:.https://doi.org/10.1158/1078-0432.CCR-14-0259
76
Bonapace
L
,
Bornhauser
BC
,
Schmitz
M
,
Cario
G
,
Ziegler
U
,
Niggli
FK
, et al.
Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest. 2010;120(4):1310–23. doi:.https://doi.org/10.1172/JCI39987
77
McComb
S
,
Aguadé-Gorgorió
J
,
Harder
L
,
Marovca
B
,
Cario
G
,
Eckert
C
, et al.
Activation of concurrent apoptosis and necroptosis by SMAC mimetics for the treatment of refractory and relapsed ALL. Sci Transl Med. 2016;8(339):339ra70. doi:.https://doi.org/10.1126/scitranslmed.aad2986
78
Hawkins
ED
,
Duarte
D
,
Akinduro
O
,
Khorshed
RA
,
Passaro
D
,
Nowicka
M
, et al.
T-cell acute leukaemia exhibits dynamic interactions with bone marrow microenvironments. Nature. 2016;538(7626):518–22. doi:.https://doi.org/10.1038/nature19801
79
Herman
SE
,
Wiestner
A
. Preclinical modeling of novel therapeutics in chronic lymphocytic leukemia: the tools of the trade. Semin Oncol. 2016;43(2):222–32. doi:.https://doi.org/10.1053/j.seminoncol.2016.02.007
80
Strowig
T
,
Gurer
C
,
Ploss
A
,
Liu
YF
,
Arrey
F
,
Sashihara
J
, et al.
Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med. 2009;206(6):1423–34. doi:.https://doi.org/10.1084/jem.20081720
81
Strowig
T
,
Chijioke
O
,
Carrega
P
,
Arrey
F
,
Meixlsperger
S
,
Rämer
PC
, et al.
Human NK cells of mice with reconstituted human immune system components require preactivation to acquire functional competence. Blood. 2010;116(20):4158–67. doi:.https://doi.org/10.1182/blood-2010-02-270678
82
Salguero
G
,
Daenthanasanmak
A
,
Münz
C
,
Raykova
A
,
Guzmán
CA
,
Riese
P
, et al.
Dendritic cell-mediated immune humanization of mice: implications for allogeneic and xenogeneic stem cell transplantation. J Immunol. 2014;192(10):4636–47. doi:.https://doi.org/10.4049/jimmunol.1302887
83
Landtwing
V
,
Raykova
A
,
Pezzino
G
,
Béziat
V
,
Marcenaro
E
,
Graf
C
, et al.
Cognate HLA absence in trans diminishes human NK cell education. J Clin Invest. 2016;126(10):3772–82. doi:.https://doi.org/10.1172/JCI86923
84
Nochi
T
,
Denton
PW
,
Wahl
A
,
Garcia
JV
. Cryptopatches are essential for the development of human GALT. Cell Reports. 2013;3(6):1874–84. doi:.https://doi.org/10.1016/j.celrep.2013.05.037
85
Meixlsperger
S
,
Leung
CS
,
Rämer
PC
,
Pack
M
,
Vanoaica
LD
,
Breton
G
, et al.
CD141+ dendritic cells produce prominent amounts of IFN-α after dsRNA recognition and can be targeted via DEC-205 in humanized mice. Blood. 2013;121(25):5034–44. doi:.https://doi.org/10.1182/blood-2012-12-473413
86
Ishikawa
F
,
Niiro
H
,
Iino
T
,
Yoshida
S
,
Saito
N
,
Onohara
S
, et al.
The developmental program of human dendritic cells is operated independently of conventional myeloid and lymphoid pathways. Blood. 2007;110(10):3591–660. doi:.https://doi.org/10.1182/blood-2007-02-071613
87
Ding
Y
,
Wilkinson
A
,
Idris
A
,
Fancke
B
,
O’Keeffe
M
,
Khalil
D
, et al.
FLT3-ligand treatment of humanized mice results in the generation of large numbers of CD141+ and CD1c+ dendritic cells in vivo. J Immunol. 2014;192(4):1982–9. doi:.https://doi.org/10.4049/jimmunol.1302391
88
Gurer
C
,
Strowig
T
,
Brilot
F
,
Pack
M
,
Trumpfheller
C
,
Arrey
F
, et al.
Targeting the nuclear antigen 1 of Epstein-Barr virus to the human endocytic receptor DEC-205 stimulates protective T-cell responses. Blood. 2008;112(4):1231–9. doi:.https://doi.org/10.1182/blood-2008-03-148072
89
Yajima
M
,
Imadome
K
,
Nakagawa
A
,
Watanabe
S
,
Terashima
K
,
Nakamura
H
, et al.
T cell-mediated control of Epstein-Barr virus infection in humanized mice. J Infect Dis. 2009;200(10):1611–5. Published online October 17, 2009. doi:.https://doi.org/10.1086/644644
90
Shultz
LD
,
Saito
Y
,
Najima
Y
,
Tanaka
S
,
Ochi
T
,
Tomizawa
M
, et al.
Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci USA. 2010;107(29):13022–7. doi:.https://doi.org/10.1073/pnas.1000475107
91
Antsiferova
O
,
Müller
A
,
Rämer
PC
,
Chijioke
O
,
Chatterjee
B
,
Raykova
A
, et al.
Adoptive transfer of EBV specific CD8+ T cell clones can transiently control EBV infection in humanized mice. PLoS Pathog. 2014;10(8):e1004333. doi:.https://doi.org/10.1371/journal.ppat.1004333
92
Gorantla
S
,
Makarov
E
,
Finke-Dwyer
J
,
Gebhart
CL
,
Domm
W
,
Dewhurst
S
, et al.
CD8+ cell depletion accelerates HIV-1 immunopathology in humanized mice. J Immunol. 2010;184(12):7082–91. doi:.https://doi.org/10.4049/jimmunol.1000438
93
Billerbeck
E
,
Horwitz
JA
,
Labitt
RN
,
Donovan
BM
,
Vega
K
,
Budell
WC
, et al.
Characterization of human antiviral adaptive immune responses during hepatotropic virus infection in HLA-transgenic human immune system mice. J Immunol. 2013;191(4):1753–64. doi:.https://doi.org/10.4049/jimmunol.1201518
94
Chijioke
O
,
Marcenaro
E
,
Moretta
A
,
Capaul
R
,
Munz
C
. Role of the 2B4 Receptor in CD8+ T-Cell-Dependent Immune Control of Epstein-Barr Virus Infection in Mice With Reconstituted Human Immune System Components. J Infect Dis. 2015;212(5):803–7. doi:. https://doi.org/10.1093/infdis/jiv114
95
Chijioke
O
,
Müller
A
,
Feederle
R
,
Barros
MH
,
Krieg
C
,
Emmel
V
, et al.
Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Reports. 2013;5(6):1489–98. Published online December 24, 2013. doi:.. Correction published in: Cell Reports. 2015;12(5):901. https://doi.org/10.1016/j.celrep.2013.11.041
96
Jandus
C
,
Boligan
KF
,
Chijioke
O
,
Liu
H
,
Dahlhaus
M
,
Démoulins
T
, et al.
Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J Clin Invest. 2014;124(4):1810–20. doi:.https://doi.org/10.1172/JCI65899
97
Baenziger
S
,
Tussiwand
R
,
Schlaepfer
E
,
Mazzucchelli
L
,
Heikenwalder
M
,
Kurrer
MO
, et al.
Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2-/-gamma c-/- mice. Proc Natl Acad Sci USA. 2006;103(43):15951–6. doi:.https://doi.org/10.1073/pnas.0604493103
98
Willinger
T
,
Rongvaux
A
,
Takizawa
H
,
Yancopoulos
GD
,
Valenzuela
DM
,
Murphy
AJ
, et al.
Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci USA. 2011;108(6):2390–5. doi:.https://doi.org/10.1073/pnas.1019682108
99
Yu
CI
,
Becker
C
,
Wang
Y
,
Marches
F
,
Helft
J
,
Leboeuf
M
, et al.
Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-β. Immunity. 2013;38(4):818–30. doi:.https://doi.org/10.1016/j.immuni.2013.03.004
100
Heuts
F
,
Gavier-Widén
D
,
Carow
B
,
Juarez
J
,
Wigzell
H
,
Rottenberg
ME
. CD4+ cell-dependent granuloma formation in humanized mice infected with mycobacteria. Proc Natl Acad Sci USA. 2013;110(16):6482–7. doi:.https://doi.org/10.1073/pnas.1219985110
101
Billerbeck
E
,
Barry
WT
,
Mu
K
,
Dorner
M
,
Rice
CM
,
Ploss
A
. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rγ(null) humanized mice. Blood. 2011;117(11):3076–86. doi:.https://doi.org/10.1182/blood-2010-08-301507
102
Wang
F
. Nonhuman primate models for Epstein-Barr virus infection. Curr Opin Virol. 2013;3(3):233–7. doi:.https://doi.org/10.1016/j.coviro.2013.03.003
103
Epstein
MA
,
Achong
BG
,
Barr
YM
. Virus Particles in Cultured Lymphoblasts from Burkitt’s Lymphoma. Lancet. 1964;1(7335):702–3. doi:.https://doi.org/10.1016/S0140-6736(64)91524-7
104
Yajima
M
,
Imadome
K
,
Nakagawa
A
,
Watanabe
S
,
Terashima
K
,
Nakamura
H
, et al.
A new humanized mouse model of Epstein-Barr virus infection that reproduces persistent infection, lymphoproliferative disorder, and cell-mediated and humoral immune responses. J Infect Dis. 2008;198(5):673–82. doi:.https://doi.org/10.1086/590502
105
Tsai
MH
,
Raykova
A
,
Klinke
O
,
Bernhardt
K
,
Gärtner
K
,
Leung
CS
, et al.
Spontaneous lytic replication and epitheliotropism define an Epstein-Barr virus strain found in carcinomas. Cell Reports. 2013;5(2):458–70. doi:.https://doi.org/10.1016/j.celrep.2013.09.012
106
Cocco
M
,
Bellan
C
,
Tussiwand
R
,
Corti
D
,
Traggiai
E
,
Lazzi
S
, et al.
CD34+ cord blood cell-transplanted Rag2-/- gamma(c)-/- mice as a model for Epstein-Barr virus infection. Am J Pathol. 2008;173(5):1369–78. doi:.https://doi.org/10.2353/ajpath.2008.071186
107
Ma
SD
,
Hegde
S
,
Young
KH
,
Sullivan
R
,
Rajesh
D
,
Zhou
Y
, et al.
A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J Virol. 2011;85(1):165–77. doi:.https://doi.org/10.1128/JVI.01512-10
108
Heuts
F
,
Rottenberg
ME
,
Salamon
D
,
Rasul
E
,
Adori
M
,
Klein
G
, et al.
T cells modulate Epstein-Barr virus latency phenotypes during infection of humanized mice. J Virol. 2014;88(6):3235–45. doi:.https://doi.org/10.1128/JVI.02885-13
109
Azzi
T
,
Lünemann
A
,
Murer
A
,
Ueda
S
,
Béziat
V
,
Malmberg
KJ
, et al.
Role for early-differentiated natural killer cells in infectious mononucleosis. Blood. 2014;124(16):2533–43. doi:.https://doi.org/10.1182/blood-2014-01-553024
110
Gurer
C
,
Strowig
T
,
Brilot
F
,
Pack
M
,
Trumpfheller
C
,
Arrey
F
, et al.
Targeting the nuclear antigen 1 of Epstein-Barr virus to the human endocytic receptor DEC-205 stimulates protective T-cell responses. Blood. 2008;112(4):1231–9. doi:.https://doi.org/10.1182/blood-2008-03-148072
111
White
RE
,
Rämer
PC
,
Naresh
KN
,
Meixlsperger
S
,
Pinaud
L
,
Rooney
C
, et al.
EBNA3B-deficient EBV promotes B cell lymphomagenesis in humanized mice and is found in human tumors. J Clin Invest. 2012;122(4):1487–502. Published online March 13, 2012. doi:.https://doi.org/10.1172/JCI58092
112
Clapham
PR
,
McKnight
A
. Cell surface receptors, virus entry and tropism of primate lentiviruses. J Gen Virol. 2002;83(Pt 8):1809–29. doi:.https://doi.org/10.1099/0022-1317-83-8-1809
113
Gifford
RJ
. Viral evolution in deep time: lentiviruses and mammals. Trends Genet. 2012;28(2):89–100. doi:.https://doi.org/10.1016/j.tig.2011.11.003
114
Goff
SP
. Intracellular trafficking of retroviral genomes during the early phase of infection: viral exploitation of cellular pathways. J Gene Med. 2001;3(6):517–28. doi:.https://doi.org/10.1002/1521-2254(200111)3:6<517::AID-JGM234>3.0.CO;2-E
115Boeke JD, Stoye JP. Retrotransposons, Endogenous Retroviruses, and the Evolution of Retroelements. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor (NY): 1997.
116
Doitsh
G
,
Greene
WC
. Dissecting How CD4 T Cells Are Lost During HIV Infection. Cell Host Microbe. 2016;19(3):280–91. doi:.https://doi.org/10.1016/j.chom.2016.02.012
117
Nasi
M
,
Pinti
M
,
Mussini
C
,
Cossarizza
A
. Persistent inflammation in HIV infection: established concepts, new perspectives. Immunol Lett. 2014;161(2):184–8. doi:.https://doi.org/10.1016/j.imlet.2014.01.008
118
Moir
S
,
Fauci
AS
. B-cell exhaustion in HIV infection: the role of immune activation. Curr Opin HIV AIDS. 2014;9(5):472–7. doi:.https://doi.org/10.1097/COH.0000000000000092
119
Seddiki
N
,
Brezar
V
,
Draenert
R
. Cell exhaustion in HIV-1 infection: role of suppressor cells. Curr Opin HIV AIDS. 2014;9(5):452–8. doi:.https://doi.org/10.1097/COH.0000000000000087
120
Sagar
M
. Origin of the transmitted virus in HIV infection: infected cells versus cell-free virus. J Infect Dis. 2014;210(Suppl 3):S667–73. doi:.https://doi.org/10.1093/infdis/jiu369
121
Battistini
A
,
Sgarbanti
M
. HIV-1 latency: an update of molecular mechanisms and therapeutic strategies. Viruses. 2014;6(4):1715–58. doi:.https://doi.org/10.3390/v6041715
122
Mbonye
U
,
Karn
J
. Transcriptional control of HIV latency: cellular signaling pathways, epigenetics, happenstance and the hope for a cure. Virology. 2014;454-455:328–39. doi:.https://doi.org/10.1016/j.virol.2014.02.008
123
Churchill
MJ
,
Deeks
SG
,
Margolis
DM
,
Siliciano
RF
,
Swanstrom
R
. HIV reservoirs: what, where and how to target them. Nat Rev Microbiol. 2016;14(1):55–60. doi:.https://doi.org/10.1038/nrmicro.2015.5
124
Fois
AF
,
Brew
BJ
. The Potential of the CNS as a Reservoir for HIV-1 Infection: Implications for HIV Eradication. Curr HIV/AIDS Rep. 2015;12(2):299–303. doi:.https://doi.org/10.1007/s11904-015-0257-9
125
Kandathil
AJ
,
Sugawara
S
,
Balagopal
A
. Are T cells the only HIV-1 reservoir?
Retrovirology. 2016;13(1):86. doi:.. Correction published in: Retrovirology. 2017;14:11. https://doi.org/10.1186/s12977-016-0323-4
126
Giri
MS
,
Nebozhyn
M
,
Showe
L
,
Montaner
LJ
. Microarray data on gene modulation by HIV-1 in immune cells: 2000-2006. J Leukoc Biol. 2006;80(5):1031–43. doi:.https://doi.org/10.1189/jlb.0306157
127
Younas
M
,
Psomas
C
,
Reynes
J
,
Corbeau
P
. Immune activation in the course of HIV-1 infection: Causes, phenotypes and persistence under therapy. HIV Med. 2016;17(2):89–105. doi:.https://doi.org/10.1111/hiv.12310
128
Pawlowski
A
,
Jansson
M
,
Sköld
M
,
Rottenberg
ME
,
Källenius
G
. Tuberculosis and HIV co-infection. PLoS Pathog. 2012;8(2):e1002464. doi:.https://doi.org/10.1371/journal.ppat.1002464
129
Desai
DV
,
Kulkarni
SS
. Herpes Simplex Virus: The Interplay Between HSV, Host, and HIV-1. Viral Immunol. 2015;28(10):546–55. doi:.https://doi.org/10.1089/vim.2015.0012
130
Carbone
A
,
Gloghini
A
,
Caruso
A
,
De Paoli
P
,
Dolcetti
R
. The impact of EBV and HIV infection on the microenvironmental niche underlying Hodgkin lymphoma pathogenesis. Int J Cancer. 2017;140(6):1233–45. doi:.https://doi.org/10.1002/ijc.30473
131
Kuritzkes
DR
. Hematopoietic stem cell transplantation for HIV cure. J Clin Invest. 2016;126(2):432–7. doi:.https://doi.org/10.1172/JCI80563
132
Wang
HB
,
Mo
QH
,
Yang
Z
. HIV vaccine research: the challenge and the way forward. J Immunol Res. 2015;2015:503978. doi:.https://doi.org/10.1155/2015/503978
133
Brockman
MA
,
Jones
RB
,
Brumme
ZL
. Challenges and Opportunities for T-Cell-Mediated Strategies to Eliminate HIV Reservoirs. Front Immunol. 2015;6:506. doi:.https://doi.org/10.3389/fimmu.2015.00506
134
Shang
HT
,
Ding
JW
,
Yu
SY
,
Wu
T
,
Zhang
QL
,
Liang
FJ
. Progress and challenges in the use of latent HIV-1 reactivating agents. Acta Pharmacol Sin. 2015;36(8):908–16. doi:.https://doi.org/10.1038/aps.2015.22
135
Chun
TW
,
Stuyver
L
,
Mizell
SB
,
Ehler
LA
,
Mican
JA
,
Baseler
M
, et al.
Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94(24):13193–7. doi:.https://doi.org/10.1073/pnas.94.24.13193
136
Finzi
D
,
Blankson
J
,
Siliciano
JD
,
Margolick
JB
,
Chadwick
K
,
Pierson
T
, et al.
Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5(5):512–7. doi:.https://doi.org/10.1038/8394
137
McCune
JM
. The SCID-hu mouse: a small animal model for the analysis of human hematolymphoid differentiation and function. Bone Marrow Transplant. 1992;9(Suppl 1):74–6.
138
Namikawa
R
,
Weilbaecher
KN
,
Kaneshima
H
,
Yee
EJ
,
McCune
JM
. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172(4):1055–63. doi:.https://doi.org/10.1084/jem.172.4.1055
139
McCune
JM
,
Namikawa
R
,
Shih
CC
,
Rabin
L
,
Kaneshima
H
. Suppression of HIV infection in AZT-treated SCID-hu mice. Science. 1990;247(4942):564–6. doi:.https://doi.org/10.1126/science.2300816
140
Shih
CC
,
Kaneshima
H
,
Rabin
L
,
Namikawa
R
,
Sager
P
,
McGowan
J
, et al.
Postexposure prophylaxis with zidovudine suppresses human immunodeficiency virus type 1 infection in SCID-hu mice in a time-dependent manner. J Infect Dis. 1991;163(3):625–7. doi:.https://doi.org/10.1093/infdis/163.3.625
141
Stoddart
CA
,
Bales
CA
,
Bare
JC
,
Chkhenkeli
G
,
Galkina
SA
,
Kinkade
AN
, et al.
Validation of the SCID-hu Thy/Liv mouse model with four classes of licensed antiretrovirals. PLoS One. 2007;2(7):e655. doi:.https://doi.org/10.1371/journal.pone.0000655
142
Pettoello-Mantovani
M
,
Kollmann
TR
,
Raker
C
,
Kim
A
,
Yurasov
S
,
Tudor
R
, et al.
Saquinavir-mediated inhibition of human immunodeficiency virus (HIV) infection in SCID mice implanted with human fetal thymus and liver tissue: an in vivo model for evaluating the effect of drug therapy on HIV infection in lymphoid tissues. Antimicrob Agents Chemother. 1997;41(9):1880–7.
143
Berges
BK
,
Wheat
WH
,
Palmer
BE
,
Connick
E
,
Akkina
R
. HIV-1 infection and CD4 T cell depletion in the humanized Rag2-/-gamma c-/- (RAG-hu) mouse model. Retrovirology. 2006;3(1):76. doi:.https://doi.org/10.1186/1742-4690-3-76
144
Watanabe
S
,
Terashima
K
,
Ohta
S
,
Horibata
S
,
Yajima
M
,
Shiozawa
Y
, et al.
Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood. 2007;109(1):212–8. doi:.https://doi.org/10.1182/blood-2006-04-017681
145
Zhang
L
,
Kovalev
GI
,
Su
L
. HIV-1 infection and pathogenesis in a novel humanized mouse model. Blood. 2007;109(7):2978–81. doi:.https://doi.org/10.1182/blood-2006-07-033159
146
Araínga
M
,
Su
H
,
Poluektova
LY
,
Gorantla
S
,
Gendelman
HE
. HIV-1 cellular and tissue replication patterns in infected humanized mice. Sci Rep. 2016;6(1):23513. doi:.https://doi.org/10.1038/srep23513
147
Berges
BK
,
Rowan
MR
. The utility of the new generation of humanized mice to study HIV-1 infection: transmission, prevention, pathogenesis, and treatment. Retrovirology. 2011;8(1):65. doi:.https://doi.org/10.1186/1742-4690-8-65
148
Choudhary
SK
,
Archin
NM
,
Cheema
M
,
Dahl
NP
,
Garcia
JV
,
Margolis
DM
. Latent HIV-1 infection of resting CD4+ T cells in the humanized Rag2−/− γc−/− mouse. J Virol. 2012;86(1):114–20. doi:.https://doi.org/10.1128/JVI.05590-11
149
Kim
KC
,
Choi
BS
,
Kim
KC
,
Park
KH
,
Lee
HJ
,
Cho
YK
, et al.
A Simple Mouse Model for the Study of Human Immunodeficiency Virus. AIDS Res Hum Retroviruses. 2016;32(2):194–202. doi:.https://doi.org/10.1089/aid.2015.0211
150
Metcalf Pate
KA
,
Pohlmeyer
CW
,
Walker-Sperling
VE
,
Foote
JB
,
Najarro
KM
,
Cryer
CG
, et al.
A Murine Viral Outgrowth Assay to Detect Residual HIV Type 1 in Patients With Undetectable Viral Loads. J Infect Dis. 2015;212(9):1387–96. doi:.https://doi.org/10.1093/infdis/jiv230
151
Konadu
KA
,
Anderson
JS
,
Huang
MB
,
Ali
SA
,
Powell
MD
,
Villinger
F
, et al.
Hallmarks of HIV-1 pathogenesis are modulated by Nef’s Secretion Modification Region. J AIDS Clin Res. 2015;6(7):476. doi:.https://doi.org/10.4172/2155-6113.1000476
152
Sampey
GC
,
Saifuddin
M
,
Schwab
A
,
Barclay
R
,
Punya
S
,
Chung
MC
, et al.
Exosomes from HIV-1-infected Cells Stimulate Production of Pro-inflammatory Cytokines through Trans-activating Response (TAR) RNA. J Biol Chem. 2016;291(3):1251–66. doi:.https://doi.org/10.1074/jbc.M115.662171
153
Neff
CP
,
Ndolo
T
,
Tandon
A
,
Habu
Y
,
Akkina
R
. Oral pre-exposure prophylaxis by anti-retrovirals raltegravir and maraviroc protects against HIV-1 vaginal transmission in a humanized mouse model. PLoS One. 2010;5(12):e15257. doi:.https://doi.org/10.1371/journal.pone.0015257
154
Mandal
S
,
Prathipati
PK
,
Kang
G
,
Zhou
Y
,
Yuan
Z
,
Fan
W
, et al.
Tenofovir alafenamide and elvitegravir loaded nanoparticles for long-acting prevention of HIV-1 vaginal transmission. AIDS. 2017;31(4):469–76. doi:.https://doi.org/10.1097/QAD.0000000000001349
155
Campos
N
,
Myburgh
R
,
Garcel
A
,
Vautrin
A
,
Lapasset
L
,
Nadal
ES
, et al.
Long lasting control of viral rebound with a new drug ABX464 targeting Rev - mediated viral RNA biogenesis. Retrovirology. 2015;12(1):30. doi:.https://doi.org/10.1186/s12977-015-0159-3
156
Nischang
M
,
Sutmuller
R
,
Gers-Huber
G
,
Audigé
A
,
Li
D
,
Rochat
MA
, et al.
Humanized mice recapitulate key features of HIV-1 infection: a novel concept using long-acting anti-retroviral drugs for treating HIV-1. PLoS One. 2012;7(6):e38853. doi:.https://doi.org/10.1371/journal.pone.0038853
157
Dash
PK
,
Gendelman
HE
,
Roy
U
,
Balkundi
S
,
Alnouti
Y
,
Mosley
RL
, et al.
Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice. AIDS. 2012;26(17):2135–44. doi:.https://doi.org/10.1097/QAD.0b013e328357f5ad
158
Gnanadhas
DP
,
Dash
PK
,
Sillman
B
,
Bade
AN
,
Lin
Z
,
Palandri
DL
, et al.
Autophagy facilitates macrophage depots of sustained-release nanoformulated antiretroviral drugs. J Clin Invest. 2017;127(3):857–73. doi:.https://doi.org/10.1172/JCI90025
159
Pardi
N
,
Secreto
AJ
,
Shan
X
,
Debonera
F
,
Glover
J
,
Yi
Y
, et al.
Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat Commun. 2017;8:14630. doi:.https://doi.org/10.1038/ncomms14630
160
Lu
CL
,
Murakowski
DK
,
Bournazos
S
,
Schoofs
T
,
Sarkar
D
,
Halper-Stromberg
A
, et al.
Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016;352(6288):1001–4. doi:.https://doi.org/10.1126/science.aaf1279
161
Halper-Stromberg
A
,
Lu
CL
,
Klein
F
,
Horwitz
JA
,
Bournazos
S
,
Nogueira
L
, et al.
Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158(5):989–99. doi:.https://doi.org/10.1016/j.cell.2014.07.043
162
Lan
P
,
Tonomura
N
,
Shimizu
A
,
Wang
S
,
Yang
YG
. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108(2):487–92. doi:.https://doi.org/10.1182/blood-2005-11-4388
163
Brainard
DM
,
Seung
E
,
Frahm
N
,
Cariappa
A
,
Bailey
CC
,
Hart
WK
, et al.
Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol. 2009;83(14):7305–21. doi:.https://doi.org/10.1128/JVI.02207-08
164
Dudek
TE
,
No
DC
,
Seung
E
,
Vrbanac
VD
,
Fadda
L
,
Bhoumik
P
, et al.
Rapid evolution of HIV-1 to functional CD8+ T cell responses in humanized BLT mice. Sci Transl Med. 2012;4(143):143ra98. doi:.https://doi.org/10.1126/scitranslmed.3003984
165
Hofer
U
,
Baenziger
S
,
Heikenwalder
M
,
Schlaepfer
E
,
Gehre
N
,
Regenass
S
, et al.
RAG2-/- gamma(c)-/- mice transplanted with CD34+ cells from human cord blood show low levels of intestinal engraftment and are resistant to rectal transmission of human immunodeficiency virus. J Virol. 2008;82(24):12145–53. doi:.https://doi.org/10.1128/JVI.01105-08
166
Sun
Z
,
Denton
PW
,
Estes
JD
,
Othieno
FA
,
Wei
BL
,
Wege
AK
, et al.
Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med. 2007;204(4):705–14. doi:.https://doi.org/10.1084/jem.20062411
167
Denton
PW
,
Nochi
T
,
Lim
A
,
Krisko
JF
,
Martinez-Torres
F
,
Choudhary
SK
, et al.
IL-2 receptor γ-chain molecule is critical for intestinal T-cell reconstitution in humanized mice. Mucosal Immunol. 2012;5(5):555–66.
168
Deruaz
M
,
Luster
AD
. BLT humanized mice as model to study HIV vaginal transmission. J Infect Dis. 2013;208(Suppl 2):S131–6. doi:.https://doi.org/10.1093/infdis/jit318
169
Stoddart
CA
,
Maidji
E
,
Galkina
SA
,
Kosikova
G
,
Rivera
JM
,
Moreno
ME
, et al.
Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rγ(-/-) (NSG) BLT mice. Virology. 2011;417(1):154–60. doi:.https://doi.org/10.1016/j.virol.2011.05.013
170
Ladinsky
MS
,
Kieffer
C
,
Olson
G
,
Deruaz
M
,
Vrbanac
V
,
Tager
AM
, et al.
Electron tomography of HIV-1 infection in gut-associated lymphoid tissue. PLoS Pathog. 2014;10(1):e1003899. doi:.https://doi.org/10.1371/journal.ppat.1003899
171
de Marco
A
,
Müller
B
,
Glass
B
,
Riches
JD
,
Kräusslich
HG
,
Briggs
JA
. Structural analysis of HIV-1 maturation using cryo-electron tomography. PLoS Pathog. 2010;6(11):e1001215. doi:.https://doi.org/10.1371/journal.ppat.1001215
172
Olesen
R
,
Swanson
MD
,
Kovarova
M
,
Nochi
T
,
Chateau
M
,
Honeycutt
JB
, et al.
ART influences HIV persistence in the female reproductive tract and cervicovaginal secretions. J Clin Invest. 2016;126(3):892–904. doi:.https://doi.org/10.1172/JCI64212
173
Wahl
A
,
Swanson
MD
,
Nochi
T
,
Olesen
R
,
Denton
PW
,
Chateau
M
, et al.
Human breast milk and antiretrovirals dramatically reduce oral HIV-1 transmission in BLT humanized mice. PLoS Pathog. 2012;8(6):e1002732. doi:.https://doi.org/10.1371/journal.ppat.1002732
174
Li
Q
,
Tso
FY
,
Kang
G
,
Lu
W
,
Li
Y
,
Fan
W
, et al.
Early Initiation of Antiretroviral Therapy Can Functionally Control Productive HIV-1 Infection in Humanized-BLT Mice. J Acquir Immune Defic Syndr. 2015;69(5):519–27. doi:.https://doi.org/10.1097/QAI.0000000000000687
175
Sáez-Cirión
A
,
Bacchus
C
,
Hocqueloux
L
,
Avettand-Fenoel
V
,
Girault
I
,
Lecuroux
C
, et al.; ANRS VISCONTI Study Group. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3):e1003211. doi:.https://doi.org/10.1371/journal.ppat.1003211
176
Hur
EM
,
Patel
SN
,
Shimizu
S
,
Rao
DS
,
Gnanapragasam
PN
,
An
DS
, et al.
Inhibitory effect of HIV-specific neutralizing IgA on mucosal transmission of HIV in humanized mice. Blood. 2012;120(23):4571–82. doi:.https://doi.org/10.1182/blood-2012-04-422303
177
Balazs
AB
,
Ouyang
Y
,
Hong
CM
,
Chen
J
,
Nguyen
SM
,
Rao
DS
, et al.
Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat Med. 2014;20(3):296–300. doi:.https://doi.org/10.1038/nm.3471
178
Yuan
Z
,
Kang
G
,
Ma
F
,
Lu
W
,
Fan
W
,
Fennessey
CM
, et al.
Recapitulating Cross-Species Transmission of Simian Immunodeficiency Virus SIVcpz to Humans by Using Humanized BLT Mice. J Virol. 2016;90(17):7728–39. doi:.https://doi.org/10.1128/JVI.00860-16
179
Deng
K
,
Pertea
M
,
Rongvaux
A
,
Wang
L
,
Durand
CM
,
Ghiaur
G
, et al.
Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517(7534):381–5. doi:.https://doi.org/10.1038/nature14053
180
Honeycutt
JB
,
Wahl
A
,
Archin
N
,
Choudhary
S
,
Margolis
D
,
Garcia
JV
. HIV-1 infection, response to treatment and establishment of viral latency in a novel humanized T cell-only mouse (TOM) model. Retrovirology. 2013;10(1):121. doi:.https://doi.org/10.1186/1742-4690-10-121
181
Honeycutt
JB
,
Wahl
A
,
Baker
C
,
Spagnuolo
RA
,
Foster
J
,
Zakharova
O
, et al.
Macrophages sustain HIV replication in vivo independently of T cells. J Clin Invest. 2016;126(4):1353–66. doi:.https://doi.org/10.1172/JCI84456







