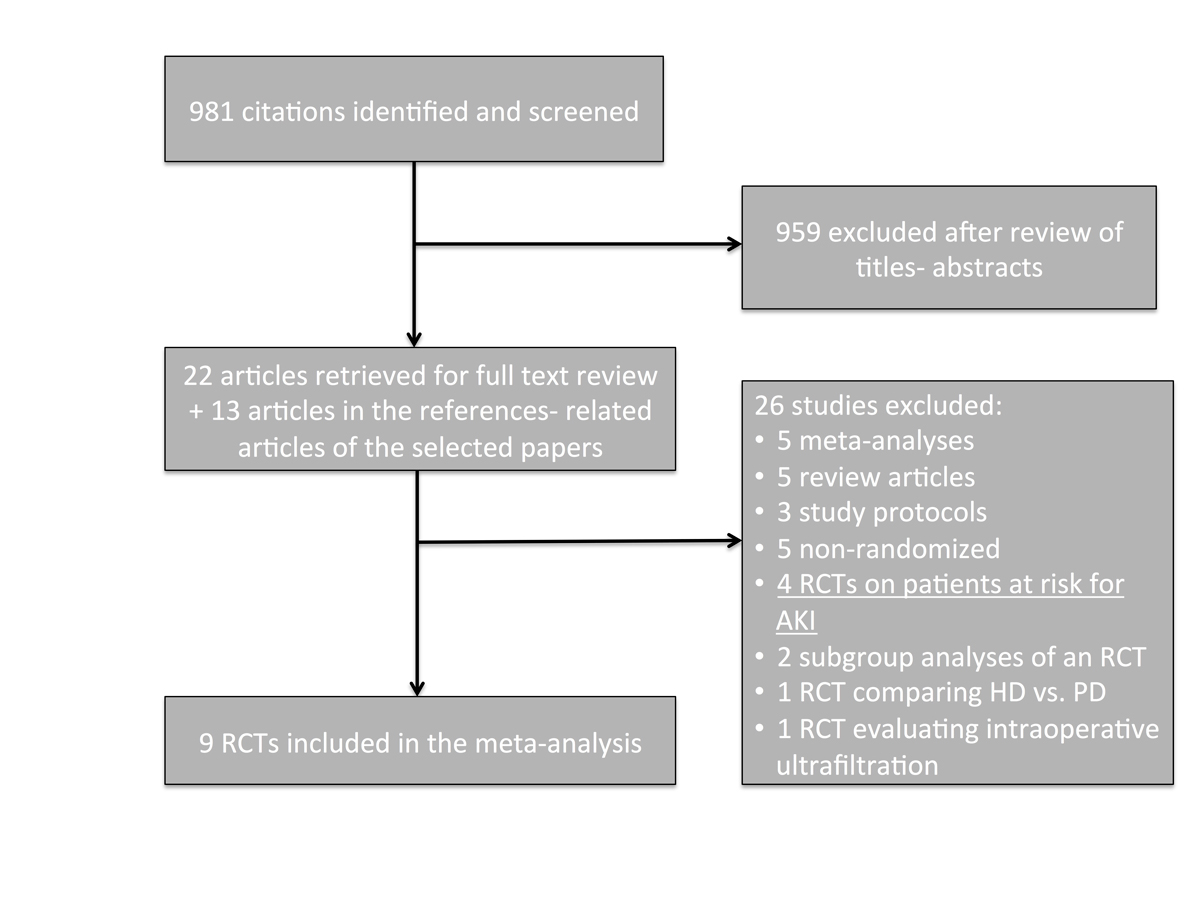
Figure 1 Study selection flow-chart.
AKI = acute kidney injury; RCT = randomised controlled trial
DOI: https://doi.org/10.4414/smw.2017.14507
The optimal timing of renal replacement therapy (RRT) initiation in acute kidney injury (AKI) remains a matter of debate [1]. Early removal of the uraemic toxins and prevention of acid-base, electrolyte and volume-related disorders with pre-emptive dialysis could theoretically improve outcomes in critically ill patients. However, unnecessary initiation of RRT in patients who could shortly recover enough renal function to stay off dialysis might expose them to dialysis-related complications and impede prompt recovery of renal function. Two relatively recent meta-analyses, derived mostly from observational cohort studies, suggested a survival benefit among patients with AKI who were offered “early” RRT [2, 3]. However, given the paucity of randomised data, clinical practice guidelines have suggested further research on this topic [4].
Four randomised controlled trials (RCTs) attempting to address this question have been published over the last 4 years [5–8]. Systematic review and meta-analysis of the larger pool of randomised data now available is needed to provide clarity on the potential benefits and risks of early compared with delayed initiation of RRT in AKI and to provide the information needed to most efficiently plan definitive trials. We therefore conducted a systematic review and meta-analysis of randomised trials to better estimate the effects of early initiation of RRT among patients with AKI compared with late initiation of RRT on short-term mortality as well as other relevant outcomes such as recovery from AKI and infectious complications. We also analysed the impact of preventive dialysis on the same outcomes.
The protocol for this meta-analysis was pre-specified and registered in the PROSPERO registry (http://www.crd.york.ac.uk/PROSPERO/ , CRD42016045603). The PRISMA 2009 checklist was used for results reporting [9]. Ethics approval was not required, this study being a meta-analysis of published RCTs.
A Medline literature research was conducted in PubMed from January 1960 to July 2016, inclusive. The reference lists of all selected studies and available meta-analyses were also reviewed. The following Mesh terms were used: Renal Dialysis or Peritoneal Dialysis or Kidneys, Artificial or Acute Kidney Injury/therapy and Acute Kidney Injury. The search was limited to Clinical Trials or Randomized Clinical Trials or Meta-Analyses, and concerned articles in the English or French language. The search was repeated for the same terms as free text rather than Mesh terms in the Title or the Abstract, using the same limits. An alternative strategy, using clinical trial-specific Mesh terms or text words, was also used (appendix 1).
All the following criteria were required for inclusion: (1) study population: adult patients with AKI; (2) intervention: timing of RRT (early vs late); (3) study design: RCTs published in the form of an article or an abstract (nonrandomised cohort trials were not included in this meta-analysis); (4) at least one of the relevant outcomes reported: mortality at the end of the study follow-up period or recovery from AKI.
All studies with patients at risk for but without established AKI who fulfilled criteria 2 to 4 were also identified. Those studies were separately analysed in a post-hoc analysis.
Two authors (T.M. and D.E.A.B.) independently reviewed the literature and selected the studies based on the aforementioned eligibility criteria. Any discrepancies were resolved in a conference with the participation of the third author (D.C.). Study investigators were contacted if more information was needed on the study design and population.
The primary outcome was in-hospital or ≤60 day mortality. The secondary outcome was renal recovery. Recovery of the renal function was defined as no need for RRT in patients who were assigned to or required dialysis. The Jadad score was used to assess the quality of each study [10]. Data extraction and quality assessment were independently performed by two authors (T.M. and D.E.A.B.).
The principal summary measure was the risk ratio (RR) with 95% confidence interval (CI). The pooled risk ratio for each outcome was estimated using a random-effects model. Results are presented in a forest plot. A sensitivity analysis was performed based on a Jadad score above or below 2.
The I2 index was used to quantify heterogeneity and assess inconsistency. Publication bias was assessed on the basis of the funnel plot. To explore heterogeneity, the following additional analyses were pre-specified: (1) year of the study: study period before or after 1996; (2) RRT modality: intermittent haemodialysis versus continuous RRT; (3) cause of AKI: surgical vs medical, sepsis vs not sepsis; (4) Definition of early vs late renal replacement therapy. A separate analysis for all outcomes was performed excluding studies with a Jadad score of 2 or less.
Analyses were performed using Review Manager 5.3.5 (The Cochrane Collaboration, Copenhagen). A p-value <0.05 was considered significant.
A total of 981 citations were identified and screened. Thirty-five articles were retrieved for full-text evaluation (fig. 1). After exclusion of ineligible manuscripts, 14 potentially eligible RCTs were identified. One was excluded because it compared patients with multiple myeloma who were treated with plasma exchange and early peritoneal dialysis versus late haemodialysis [11]. The other four were analysed separately because they enrolled patients at risk for AKI but did not require the diagnosis of AKI upon inclusion [12–15]. Thus, our main analysis included seven RCTs [5–8, 16–18] and two semi-randomised trials [19, 20]. One study randomised patients to receive early high-volume haemofiltration, early low-volume haemofiltration, or late low-volume haemofiltration [16]. For the purpose of this analysis, the two early groups were merged.

Figure 1 Study selection flow-chart.
AKI = acute kidney injury; RCT = randomised controlled trial
The characteristics of the nine selected studies are listed in tables 1 and 2 . The studies were conducted between 1970 and 2015 in seven different countries. All studies were open-label, with a Jadad score of 1 to 3. Patients randomised in the early initiation group received RRT within 6 to 12 hours of eligibility in four studies [5–7, 16]. In the remaining five studies, early group patients had also to fulfil certain criteria in order to receive RRT (table 2). Late RRT initiation was based on variable criteria that differed throughout the studies. RRT was provided in 91 to 100% of patients in the early group and 51 to 100% of patients in the late group. Three studies used only continuous RRT at least for the first week [6, 16, 17] and three studies only intermittent haemodialysis [8, 19, 20]. The follow-up period varied from 14 days to 6 months.
Table 1 Characteristics of the selected studies.
| Reference (Trial name) | [ 5 ] (AKIKI) | [ 6 ] (ELAIN) | [ 7 ] (STARRT-AKI) | [8] | [17] | [16] | [18] | [19] | [20] |
|---|---|---|---|---|---|---|---|---|---|
| Publication year | 2016 | 2016 | 2015 | 2013 | 2004 | 2002 | 1997 | 1975 | 1986 |
| Country | France | Germany | Canada | India | Japan | The Netherlands | India | United States | United States |
| Study period | 2013–2016 | 2013–2015 | 2012–2013 | 2011–2012 | 1995–1997 | 1998–2000 | NA | 1970 | NA |
| Jadad score | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 1 | 1 |
| Patients number (early/late arm) | 619 (311/308) | 231 (112/119) | 100 (48/52) | 208 (102/106) | 28 (14/14) | 106 (70/36) | 35 (18/17) | 18 (8/10) | 34 (17/17) |
| Males (%) | 409 (66) | 146 (63) | 72 (72) | 102 (68) | 18 (64) | 63 (59) | NA | 18 (100) | 29 (85) |
| Age (years) early vs late | 64.8 vs 67.4 | 65.7 vs 68.2 | 62.2 vs 63.9 | 42.8 vs 42.1 | 65 vs 64 | 69 vs 67 | NA | 21.4 vs 23.9 | 56.5 vs 56.5 |
| SOFA score (early/late) | 10.9/10.8 | 15.6/16 | 13.3/12.8 | 7.6/8.2 | NA | 10.2/10.6 | NA | NA | NA |
| APACHE II (early/late) | NA | 30.6/32.7 | NA | NA | 19/18 | 22.6/23.6 | NA | NA | NA |
| Sepsis (%) | 495 (80) | 74 (32) | 56 (56) | 44 (21) | NA | NA | 7 (20) | NA | 9 (26) |
| AKI definition | Severe (KDIGO stage 3) | KDIGO stage 2 | KDIGO stage 2 (both criteria or one and N-GAL ≥400 ng/ml) | Severe | UOP <30 ml/h & Δcreat >0.5 mg/dl/24h | UOP <30 ml/h for >6 h & CrCl <20 ml/min | NA | Severe (BUN ≥70 mg/dl or creat ≥5 mg/dl) | Creat ≥8 mg/dl |
| AKI cause | ATN | Excluded if GN, AIN, vasculitis, HUS, TTP, renal artery injury, postrenal, hepatorenal, CKD stage 4–5 | Excluded if RPGN, AIN, postrenal, AKI for >48 h, CKD stage 4–5 | Any (mostly infectious) Excluded if CKD stage 3–5 |
Post-CABG | Excluded if GN, AIN, vasculitis, postrenal, or renal artery occlusion | ATN | Trauma | Any |
| Other eligibility criteria | Mechanical ventilation and/or pressors | Sepsis or pressors or refractory volume overload or SOFA >1, N-GAL >150 ng/ml | K+ ≤5.5 mmol/l, HCO3 − ≥15 mmol/l, CVP ≥8 cm H20, clinical equipoise (intensivist-nephrologist) | Not requiring urgent dialysis or judged likely to recover | Proteinuria <2 g/d & baseline creat <1.4 mg/dl | Age 18–90, mechanical ventilation. Excluded if baseline CrCl <30 ml/min, cirrhosis |
Creat <7 mg/dl, urea<120 mg/dl |
Excluded if survival <24 h, septic shock, or AKI of cause other than trauma | Age >20, UNa+ >50 mmol/l, UOsm <400 mOsm/kg, urine/plasma creat <20, renal failure index >2 |
AIDS = acquired immunodeficiency syndrome; AIN = acute interstitial nephritis; AKI = acute kidney injury; APACHE II = Acute Physiology and Chronic Health Evaluation II score; ATN = acute tubular necrosis; BUN = blood urea nitrogen; CABG = coronary artery bypass grafting, CKD = chronic kidney disease; creat = creatinine; CrCl = creatinine clearance; CVP = central venous pressure; Δcreat = change in creatinine levels from baseline; GCS = Glasgow coma scale; GN = glomerulonephritis; HUS = haemolytic uraemic syndrome; KDIGO = Kidney Disease Improving Global Outcomes; NA = not available; N-GAL = neutrophil gelatinase-associated lipocalin; RPGN = rapidly progressive glomerulonephritis; SOFA = Sequential Organ Failure Assessment score; TTP = thrombotic thrombopenic purpura; UNa+ = urine sodium; UOP = urine output; UOsm, urine osmolality
Table 2 Definition of early renal replacement therapy strategy, indications for dialysis in the late strategy arm, and renal replacement modality in each treatment group.
| Reference (Trial name) | [ 5 ] (AKIKI) | [ 6 ] (ELAIN) | [ 7 ] (STARRT-AKI) | [8] | [17] | [16] | [18] | [19] | [20] |
|---|---|---|---|---|---|---|---|---|---|
| Early strategy definition | Within 6 h of AKI diagnosis | Within 8 h of AKI diagnosis | Within 12 h of eligibility | BUN >70 mg/dl or creat >7 mg/dl | UOP <30 ml/h for 3 h or <750 ml/24h | Within 12 h of eligibility | NA | BUN <70 mg/dl & creat <5 mg/dl | Target creat <5 mg/dl & BUN <60 mg/dl |
| Indications for RRT in the late strategy arm | BUN >112 mg/dl, K+ >6 mmol/l, pH <7.15, pulmonary oedema despite diuresis, oliguria-anuria >72 hours | K+ >6 mmol/l, urea >100 mg/dl, Mg++ >4 mmol/l, diuretic-resistant oedema, UOP <200 ml/12h or anuria, AKI stage 3 | K+ >6 mmol/l, HCO3 − <10 mmol/l, pulmonary oedema, other clinical indication | Refractory hyperkalaemia-acidosis-volume overload, uraemic, nausea-anorexia | UOP <20 ml/h for 2 h or <500 ml/24h | Urea >40 mmol/l, K+ >6.5 mmol/l, severe pulmonary oedema | NA | BUN = 150 mg/dl creat = 10 mg/dl refractory hyperkalaemia or volume overload, encephalopathy | Target creat <9 mg/dl & BUN <100 mg/dl |
| RRT in early group | 305/311 | 112/112 | 48/48 | 93/102 | 14/14 | 70/70 | NA | NA | 17/17 |
| RRT in late group | 157/308 | 108/119 | 33/52 | 88/106 | 14/14 | 30/36 | NA | NA | 17/17 |
| RRT modality in early group | IHD 142, CRRT 101, both 61 | All had CVVHDF; SLED-SCUF-IHD offered after day 7 | IHD (31%), SLED or CRRT (69%) | IHD | CVVHD | CVVH | NA | IHD | IHD |
| RRT modality in late group | IHD 73, CRRT 47, both 37, none 151 | All had CVVHDF; SLED-SCUF-IHD offered after day 7 | IHD (34%), SLED or CRRT (66%) | IHD | CVVHD | CVVH | NA | IHD | IHD |
| Follow-up | 60 days | 90 days | 90 days | 90 days | 14 days | In-hospital | In-hospital | 6 months | In-hospital |
AKI = acute kidney injury; BUN = blood urea nitrogen; creat = creatinine; CRRT = continuous renal replacement therapy; CVVH = continuous veno-venous haemofiltration; CVVHD = continuous veno-venous haemodialysis; CVVHDF = continuous veno-venous haemodiafiltration; IHD = intermittent haemodialysis; NA = not available; RRT = renal replacement therapy; SCUF = slow continuous ultrafiltration; SLED = sustained low efficiency dialysis; UOP = urine output
Four studies enrolled patients at risk for AKI [12–15]. Their characteristics are presented in table 3.
Table 3 Characteristics of the selected studies in patients at risk for acute kidney injury.
| Reference (Trial name) | [13] | [12] | [14] | [ 15 ] (HEROICS) |
|---|---|---|---|---|
| Publication year | 2003 | 2006 | 2009 | 2015 |
| Country | Turkey | Korea | France | France |
| Study period | 1999–2001 | NA | 1997–2000 | 2009–2012 |
| Jadad score | 1 | 2 | 3 | 3 |
| Patients number (early/late arm) | 21/23 | 43/59 | 37/39 | 112/112 |
| Males (%) | 80 | 61 | 71 | 79 |
| Age (years) early vs late | 58.1 vs 54.3 | 63 | 57.6 vs 58.6 | 61 vs 58 |
| SOFA score (early/late) | NA | NA | 11.6/10.4 | 11.5/12 |
| Sepsis (%) | NA | 100 | 100 | NA |
| Reason at risk for AKI | Patients undergoing CABG | Sepsis | Sepsis | Shock post-cardiac surgery |
| Other eligibility criteria | Creat >2.5 mg/dl, not requiring dialysis | NA | Severe sepsis with SAPS II score 35–63 | Non-ESRD, within 3–24 h from admission, requiring either high-dose pressors or ECMO, SAPS II score ≤90 |
| Early strategy definition | 2 sessions within 72 hours before surgery AND within 48 h after surgery if Δcreat >10% from baseline | After diagnosis of severe sepsis or septic shock | Within 24 hours after randomisation | As soon as possible after randomisation |
| Indications for RRT in the late strategy arm | Creat increase ≥50% from baseline or UOP <400 ml for 24 hours | Refractory volume overload, BUN >80 mg/dl, pH <7.2, K+ >6.5 mmol/l | NA | AKI stage 3, life-threatening hyperkalaemia, urea >36 mmol/l |
| RRT in early group | 21/21 | 43/43 | NA | 111/112 |
| RRT in late group | 8/23 | 29/59 | NA | 64/112 |
| RRT modality in early group | IHD | CVVH for at least 48 h | CVVH for at least 96 h | High-volume CVVH for 48 hours, then CVVHDF |
| RRT modality in late group | IHD | CVVH | CVVH | CVVHDF |
| Follow-up | 30 days | In-hospital | 14 days | In-hospital |
AKI = acute kidney injury; BUN = blood urea nitrogen; CABG = coronary artery bypass grafting; creat = creatinine; CVVH = continuous veno-venous haemofiltration; CVVHDF = continuous veno-venous haemodiafiltration; Δcreat = increase in creatinine levels; ECMO = extracorporeal membrane oxygenation; ESRD = end-stage renal disease; IHD = intermittent haemodialysis; NA = not available; RRT = renal replacement therapy; SAPS II = simplified acute physiology score II; SOFA = Sequential Organ Failure Assessment score; UOP = urine output
A funnel plot for all 13 studies (9 in patients with AKI and 4 in patients at risk for AKI) is depicted in supplementary figure S1 (appendix 2). No major asymmetry was identified.
The primary analysis included six studies that reported in-hospital mortality [7, 8, 16, 18–20], two studies reporting 60-day mortality [5, 6] and one study reporting 14-day mortality [17]. Early RRT was not associated with reduced mortality: RR 0.91, 95% CI 0.72–1.16, p = 0.46 (fig. 2a). The results were moderately heterogeneous (I2 = 49%, p = 0.05).
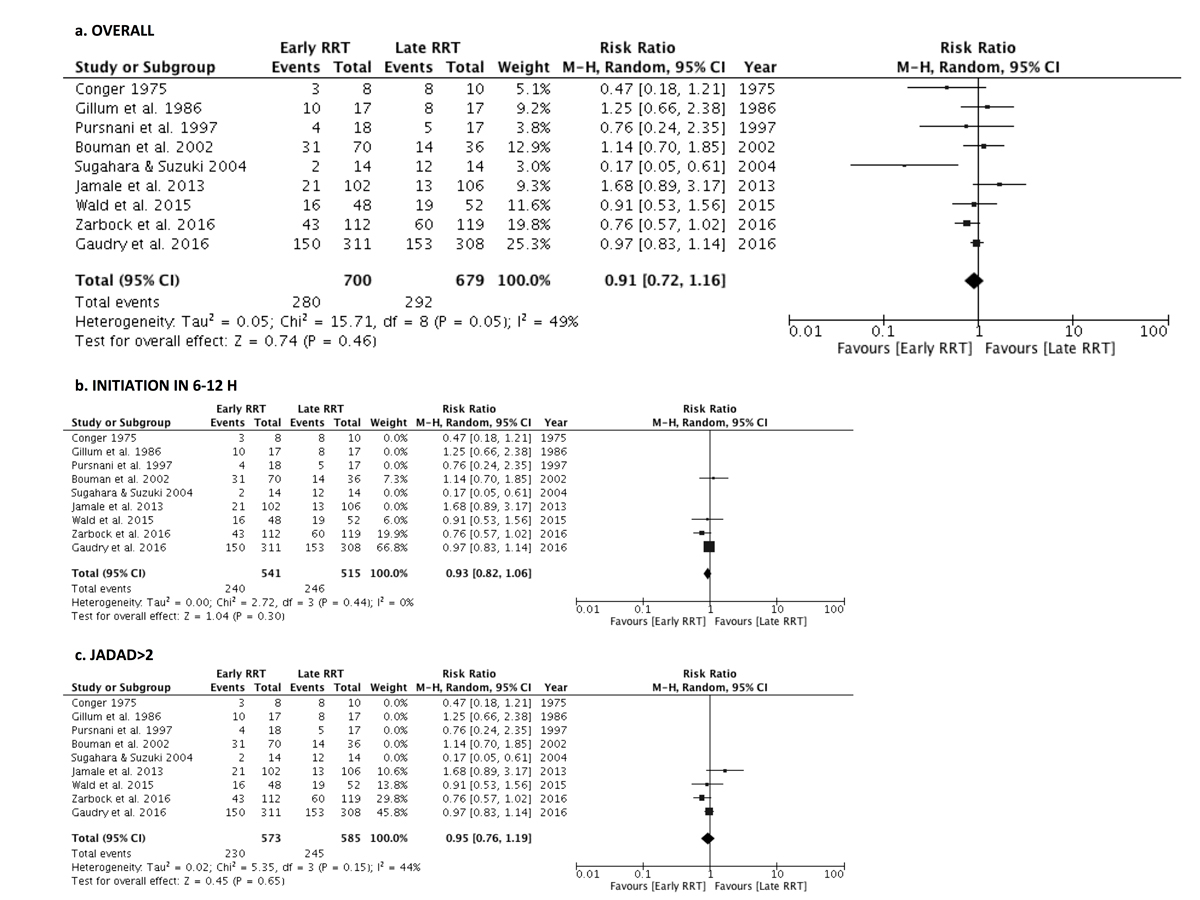
Figure 2 Forest plot demonstrating the impact of early renal replacement therapy on in-hospital or 60-day mortality compared with late renal replacement therapy. (a) All studies in patients with acute kidney injury (AKI) were included. (b) Only the trials that offered renal replacement therapy within 6 to 12 hours of eligibility were included. © Only studies with a Jadad score >2 were included. Data are presented as risk ratios with 95% confidence intervals. A random-effects model was used.
Study design accounted for the majority of the heterogeneity. When only the four RCTs that offered RRT in the early arm within 6 to 12 hours of eligibility were included in the analysis, the results were similar (RR 0.93, 95% CI 0.82–1.06, p = 0.30) but the I2 dropped to 0% (p = 0.44) (fig. 2b) [5–7, 16]. No significant survival benefit was identified in the four studies conducted before 1996 (RR 0.58, 95% CI 0.25–1.35, p = 0.20, I2 = 67%) [17–20] or the five studies conducted after 1996 (RR 0.97, 95% CI 0.80–1.17, p = 0.72, I2 = 32%) [5–8, 16]. The results were similar when the studies with a Jadad score of 1 or 2 were excluded (RR 0.95, 95% CI 0.76–1.19, p = 0.65, I2 = 44%) (fig. 2c). There was still moderate heterogeneity after those additional analyses.
The analysis of the studies in patients at risk for AKI included one study reporting mortality at 14 days [14], one at 30 days [13] and two studies reporting in-hospital mortality [12, 15]. No survival benefit was identified in patients undergoing preventive dialysis compared with patients in the conservative arm: RR 0.85 (95% CI 0.52–1.41, p = 0.54). The results were highly heterogeneous (I2 = 69%, p = 0.02). When all 13 studies were analysed together, no survival benefit was identified with early or preventive dialysis versus standard of care: RR 0.91 (95% CI 0.75–1.12, p = 0.38, I2 = 53%) (fig. 3).
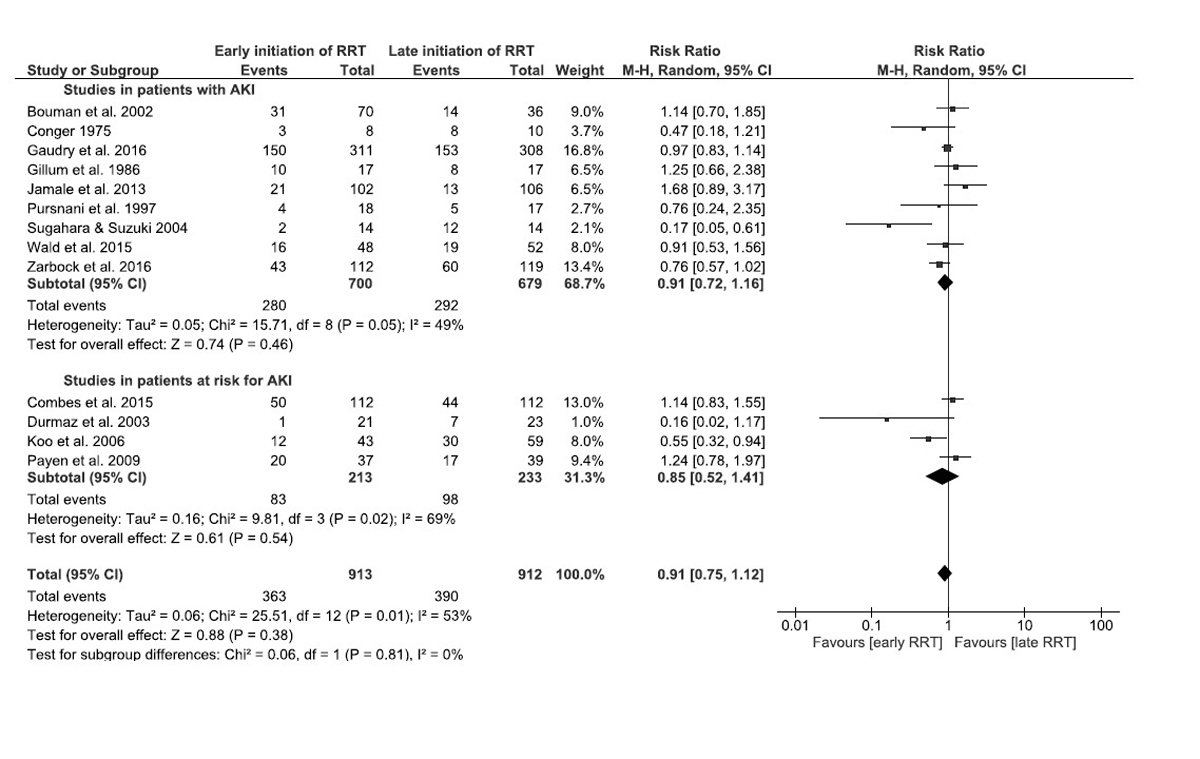
Figure 3 Forest plot demonstrating the impact of early renal replacement therapy on in-hospital or 60-day mortality compared with late renal replacement therapy in patients with acute kidney injury (AKI) (9 studies), at risk for AKI (4 studies), and in all 13 studies. Data are presented as risk ratios with 95% confidence intervals. A random-effects model was used.
Three studies reported mortality at 28 days [5, 6, 16], two in the intensive care unit [7, 16], and two at 90 days [6, 7] (supplementary figs S2, S3, S4 , appendix 2). Among the studies in patients at risk for AKI, two reported mortality at 28 to 30 days [13, 15], one in the intensive care unit [15], and one at 90 days [15] (figs S2, S3, S4 ). Results were qualitatively similar to the overall results at 60 days.
Seven studies provided data on renal recovery: three at 90 days [6–8], one at 60 days [5], one at 14 days [17], and two upon hospital discharge [13, 20]. The percentage of patients among survivors who recovered enough renal function to be off dialysis was similar with early RRT and with late RRT: RR 1.02, 95% CI 0.99–1.06, p = 0.16 ( fig. 4). No significant heterogeneity was detected (I2 = 0%, p = 0.87). Additional analyses including only studies with a Jadad score of 3, those conducted after 1996, those that used only continuous RRT at least during the first week, or those that offered RRT within 6 to 12 hours of eligibility provided similar results. One study in patients at risk for AKI provided data on renal recovery [15]. All patients recovered enough renal function to be off dialysis at 90 days. Inclusion of this study in the meta-analysis yielded similar results (fig. 4).
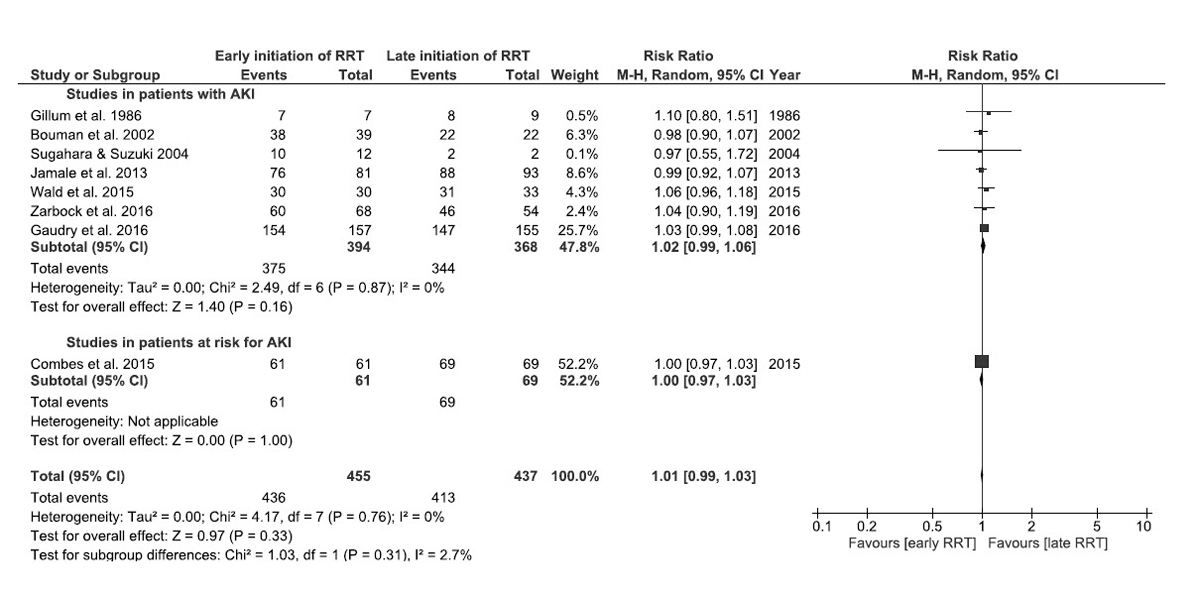
Figure 4 Forest plot demonstrating the impact of early or preventive renal replacement therapy on renal recovery at the end of the study follow-up compared with late renal replacement therapy in patients with acute kidney injury (AKI) (seven studies), at risk for AKI (one study), and in all eight studies. Data are presented as risk ratios with 95% confidence intervals. A random-effects model was used.
Seven studies provided data on the number of patients who received RRT in each group, as well as three studies in patients at risk for AKI. The proportion of patients who received RRT was higher in the early group compared with the late group: RR 1.41, 95% CI 1.13–1.77, p <0.01.
Four studies reported catheter-related infections [5, 7, 8, 16]. Early initiation of RRT was associated with higher incidence of catheter-related infections compared with late initiation of RRT: RR 1.82, 95% CI 1.03–3.21, p = 0.04. No significant heterogeneity was detected (I2 = 0%, p = 0.55).
Early RRT with prompt removal of the uraemic toxins and prevention of acid-base, electrolyte and volume- related disorders has been considered in an attempt to improve survival among critically ill patients. This was suggested by few small RCTs and several heterogeneous observational cohort studies. The KDIGO clinical practice guidelines suggest considering “the broader clinical context, the presence of conditions that can be modified with RRT, and trends of laboratory tests” to make the decision to start RRT (table S1, appendix 2) [4].
To better estimate the risks and benefits associated with early RRT, we performed a systematic review and meta-analysis including 1379 patients from nine different RCTs. We found that early initiation of RRT was not associated with a significant improvement in mortality, particularly in studies conducted in the context of contemporary critical care therapy (after 1996). The overall power of the available studies, even when combined, may be limited. Although the results were not significant, suggesting that the best interpretation is that there is no difference in survival, the point estimates did not rule out the possibility of a modest survival benefit in the range of 7 to 9%. Conversely, the data did not suggest any apparent impact on renal recovery with early dialysis and confirmed that early RRT is associated with an increased risk of serious infection.
We also examined in a post hoc analysis whether preventive RRT in patients at risk for AKI was associated with improved survival. The studies were highly heterogeneous and did not suggest any survival benefit. However, the point estimate was in the same range as in studies in AKI patients and a modest protective effect cannot be formally ruled out.
Our results are in agreement with two recently published meta-analyses, including most of the studies in this report [21, 22]. However, the authors of the first study did not review trials in patients at risk for AKI and did not include the older RCTs [21]. The second meta-analysis was not prospectively registered, did not include the older RCTs, and did not differentiate between studies in patients at risk for AKI and studies in patients with AKI [22].
The absence of a mortality benefit in our analysis contrasts with the results of previous meta-analyses that mainly included observational studies [2, 3, 23]. However, observational trials have clear limitations, as patients in the two treatment arms might have been fundamentally different. There is a substantial probability that these studies were impacted by indication bias in which the delayed use of dialysis in sicker or moribund patients accounted for the detected benefits rather than any treatment effect. Notably, there was significant heterogeneity among the included trials. Study design seems to be the most important factor underlying this heterogeneity. Older trials used different arbitrarily selected cut-offs in urea or creatinine levels to assign patients to the early RRT group, whereas most recent studies offered RRT in the next few hours post randomization in all patients in the early group. When only trials with this design were included in the analysis, the result was similar but the confidence interval was narrower, suggesting a potential trend towards modestly lower mortality with early RRT. The heterogeneity was significantly lower with I2 dropping from 53 to 0%.
The percentage of patients among survivors who recover enough renal function to be off dialysis tended to be higher, although not statistically significantly, with late RRT compared with early RRT. This result may be explained by haemodynamic factors in dialysis patients who are exposed to high ultrafiltration rates [24]. Notwithstanding, the choice of RRT modality (intermittent haemodialysis versus continuous RRT) did not affect the analysis results.
We also identified a higher incidence of catheter-related infections among early RRT patients. This result was mainly driven by one RCT [5], but raises concern about a potentially serious complication observed with a strategy that failed to show any clear clinical benefit. The cost associated with RRT and vascular access issues in patients who would otherwise recover enough renal function to stay off dialysis are other potential implications of the early strategy that have not been explored.
Our findings suggest that early RRT has no significant effect on mortality compared with late RRT among patients with AKI. One may suggest that watchful waiting can still identify patients who will benefit from RRT in a timely fashion while avoiding RRT-related complications in patients who will not ultimately need it. However, we cannot formally rule out a modest effect with a best estimate of 9%, suggesting a critical need for adequately powered RCTs to provide definitive data on this question. Nevertheless, if the true effect is close to what was seen in the main analysis, a future RCT should have a sample size of 3130 patients to identify this effect with a power of 80% and a two-sided alpha test set at 0.05, assuming a baseline mortality rate of 50% [5, 6]. However, if the true benefit is closer to that seen in higher quality studies, 12 548 patients would be needed.
Most importantly, this study points out the appalling lack of data on a critical question that has not been adequately addressed despite dialysis being available for almost 70 years. Only four RCTs with significant sample size have been conducted and only one study included more than 250 patients. Two clinical trials are currently recruiting participants: the definitive phase of the STARRT-AKI trial with an estimated enrolment of 2866 patients and the IDEAL-ICU study with an estimated enrolment of 864 patients [25, 26]. Their results are eagerly awaited, but our data suggest that they may be inadequately powered to detect the most likely effect size on their own. With no definite answer to this question, clinicians are probably justified to use clinical intuition when treating AKI in critically ill patients.
Several limitations of this meta-analysis need to be mentioned. Although we combined nine studies with 1379 patients, our results should be interpreted with some caution as we lacked power to rule out potentially small but real benefits with this sample size. Patients across different RCTs had variable causes and degrees of AKI. The decision of RRT modality was left to the investigators’ discretion. There was no information on the impact of volume overload at the time of RRT initiation. Delivered dose of RRT was not available and might have been different from the prescribed dose. Patients with acute glomerular or interstitial disorders were excluded from most trials. Severity of underlying disease, as depicted by the SOFA and APACHE II scores when available, was variable across the different studies. The definitions of the early and the late strategy were very different across the studies: for example, the early group in the AKIKI trial was similar to the late group in the ELAIN study. RRT weaning, used to assess for renal recovery, reflects heterogeneous clinical decision making and is an imperfect surrogate for physiological recovery, although clearly clinically relevant. Furthermore, six trials were single-centre, and the quality of most of them, as assessed by the Jadad score, was suboptimal. Searching was limited to one database (PubMed) and English and French languages. We did not systematically search for unpublished, meeting abstracts not cited in PubMed. Given the small number of published trials of any size in this area, it is unlikely than any trials of even small sample size would remain unpublished, particularly if positive. Thus, inclusion of any such studies would almost certainly have attenuated the mortality benefit even further and would have been extremely unlikely to have increased the apparent mortality benefits. In addition, we did not detect significant evidence of major publication bias.
In conclusion, early RRT in patients with AKI is not associated with significantly lower mortality rates compared with late RRT, and appears to be associated with more infectious complications. At the present time, therefore, the data suggest that the approach to AKI patients should remain individualised, with careful observation for infectious complications in those receiving dialysis. Our analysis also points out the absence of adequate data to address a clinical question that has been present for more than six decades and the need for a trial including more than 3000 randomised patients to answer this question definitively.
Two separate search strategies were used:
1) First Medline Search strategy:
((((((((("Renal Dialysis"[Mesh]) OR "Dialysis"[Mesh]) OR "Peritoneal Dialysis"[Mesh]) OR "Kidneys, Artificial"[Mesh]) OR "Acute Kidney Injury/therapy"[Mesh])) AND "Acute Kidney Injury"[Mesh]) AND ((Clinical Trial[ptyp] OR Meta-Analysis[ptyp] OR Randomized Controlled Trial[ptyp]) AND (English[lang] OR French[lang])))) OR ((((((((("Renal Dialysis"[Tiab]) OR "Dialysis"[Tiab]) OR "Peritoneal Dialysis"[Tiab]) OR "Kidneys, Artificial"[Tiab]) OR "Acute Kidney Injury/therapy"[Tiab])) AND "Acute Kidney Injury"[Tiab])) AND ((Clinical Trial[ptyp] OR Meta-Analysis[ptyp] OR Randomized Controlled Trial[ptyp]) AND (English[lang] OR French[lang])))
This search yielded 880 articles.
2) Second Medline Search strategy:
(((((((((("Renal Dialysis"[Mesh]) OR "Dialysis"[Mesh]) OR "Peritoneal Dialysis"[Mesh]) OR "Kidneys, Artificial"[Mesh]) OR "Acute Kidney Injury/therapy"[Mesh])) AND "Acute Kidney Injury"[Mesh]))) OR (((((((((("Renal Dialysis"[Tiab]) OR "Dialysis"[Tiab]) OR "Peritoneal Dialysis"[Tiab]) OR "Kidneys, Artificial"[Tiab]) OR "Acute Kidney Injury/therapy"[Tiab])) AND "Acute Kidney Injury"[Tiab]))))) AND ((((randomized controlled trial[pt] OR clinical trial, phase iii[pt] OR clinical trial, phase iv[pt] OR clinicaltrials.gov[si] OR isrctn[si] OR randomized controlled trials as topic[mh]) OR (clinical trial[pt] AND (((single[tw] OR double[tw] OR doubleblind[tw] OR doubleblinded[tw] OR treble[tw] OR triple[tw]) AND (blind[tw] OR blinded[tw] OR mask[tw] OR masked[tw] OR masks[tw] OR sham[tw] OR shams[tw] OR dummy[tw])) OR (random[tw] OR randomise[tw] OR randomize[tw] OR randomised[tw] OR randomized[tw] OR rct[tw] OR rcts[tw] OR single-blind method[mh] OR double-blind method[mh] OR random allocation[mh]))) AND ((comparative study[pt] OR compare[tw] OR compares[tw] OR compared[tw] OR comparing[tw] OR comparison[tw] OR comparative[tw] OR effective[tw] OR effectiveness[tw] OR versus[ti] OR vs[ti]) OR (activities of daily living[mh] OR benefit[tw] OR benefits[tw] OR budgets[mh] OR chronic disease[mh] OR clinical trials data monitoring committees[mh] OR cognitive function[tw] OR ec[sh] OR death[mh] OR diffusion of innovation[mh] OR discharge[tw] OR economics, pharmaceutical[mh] OR evidence based practice[mh] OR functional status[tw] OR guideline adherence[mh] OR harm[tw] OR harms[tw] OR health services research[mh] OR health status[mh] OR hospitalization[mh] OR interventions[tw] OR life expectancy[mh] OR longevity[mh] OR models, statistical[mh] OR models, theoretical[mh:noexp] OR morbidity[mh] OR mortality[mh] OR noninferior[tw] OR noninferiority[tw] OR outcome and process assessment[mh] OR outcome[tw] OR outcomes[tw] OR patient compliance[mh] OR postoperative care[mh] OR postoperative complications[mh] OR product surveillance, postmarketing[mh] OR propensity score[tw] OR quality-adjusted life years[mh] OR quality of life[mh] OR recovery of function[mh] OR recurrence[mh] OR relapse[tw] OR remission[tw] OR reoperation[mh] OR risk[tw] OR risk management[mh] OR survival analysis[mh] OR survival rate[mh] OR technology assessment, biomedical[mh] OR trial[ti] OR trials[ti]))) OR clinical effectiveness[tw]) NOT systematic[sb]) AND (English[lang])
This search yielded 577 articles.
3) When both strategies were combined and duplicates removed, a total of 981 potentially eligible articles was identified.
Table S1 Criteria for initiating acute renal replacement therapy with the current KDIGO recommendations.
| Indication | Comment |
|---|---|
| Severe hyperkalaemia | Life threatening indication |
| Severe acidosis | In association with other indications |
| Volume overload | Especially in patients with acute pulmonary edema |
| Uremic complications | For example, pericarditis, bleeding, encephalopathy, etc. |
| Poisoning, drug overdose | For example, salicylates, ethylene glycol, methanol, metformin |
| Solute control | With very high urea or creatinine levels, in anticipation of uremic complications |
| Nutrition | To facilitate adequate nutritional support in volume overloaded patients |
| Drug delivery | To facilitate large volume iv drug administration in fluid overloaded patients |
One or more criteria may be present for renal replacement therapy initiation. Consider the broader clinical context, risks related to the RRT procedure, and potential for spontaneous recovery [4].
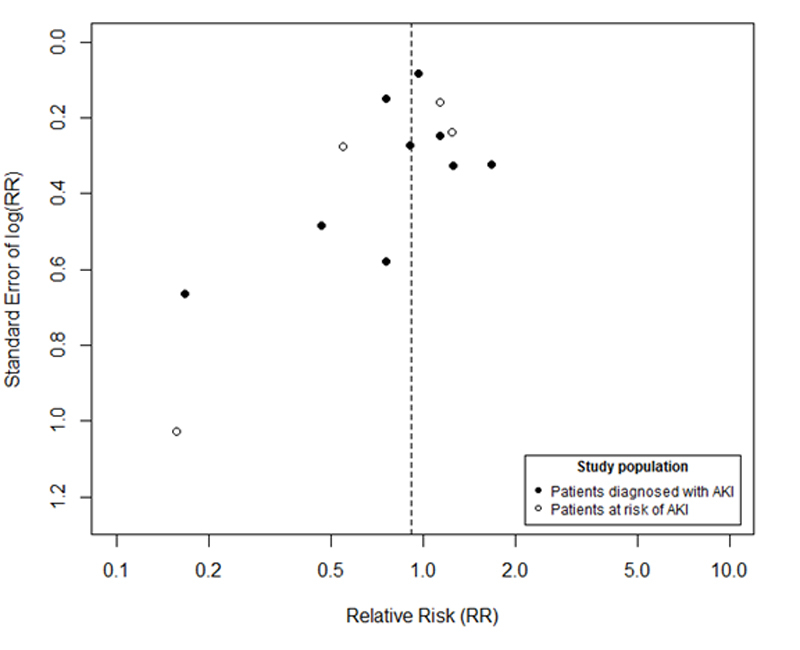
Figure S1 Funnel plot of the 9 randomised controlled trials in patients with acute kidney injury and the 4 trials in patients at risk for acute kidney injury. X axis is the risk ratio for mortality on a natural logarithm scale. Y axis is the standard error of the natural logarithm of risk ratio for mortality.
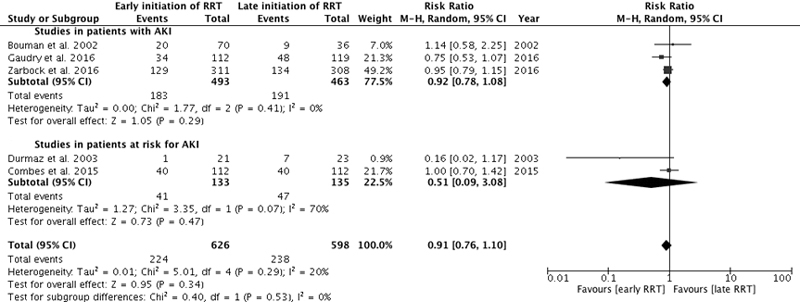
Figure S2 28-day mortality. Forest plot demonstrating the impact of early or preventive renal replacement therapy on 28-day mortality compared with late renal replacement therapy. Data are presented as risk ratios with 95% confidence intervals. A random-effects model was used.
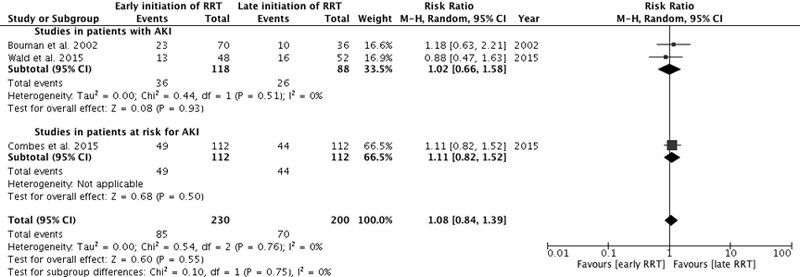
Figure S3 Intensive care unit mortality. Forest plot demonstrating the impact of early or preventive renal replacement therapy on intensive care unit mortality compared with late renal replacement therapy. Data are presented as risk ratios with 95% confidence intervals. A random-effects model was used.
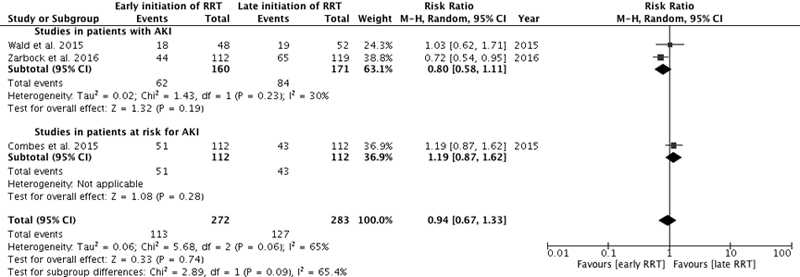
Figure S4 90-day mortality. Forest plot demonstrating the impact of early or preventive renal replacement therapy on 90-day mortality compared with late renal replacement therapy. Data are presented as risk ratios with 95% confidence intervals. A random-effects model was used.
Dr Mavrakanas was supported by a grant from the foundation G. & L. Hirsch (General Internal Medicine Division, Geneva University Hospitals). Dr Charytan has received research support from Sentien Biotechnologies and consulting fees for services on a clinical events committee from PLC Medical (Renal Guard Solutions).
1 Wald R , Bagshaw SM . The timing of renal replacement therapy initiation in acute kidney injury: is earlier truly better? Crit Care Med. 2014;42(8):1933–4 .https://doi.org/10.1097/CCM.0000000000000432
2 Karvellas CJ , Farhat MR , Sajjad I , Mogensen SS , Leung AA , Wald R , et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15(1):R72 .https://doi.org/10.1186/cc10061
3 Seabra VF , Balk EM , Liangos O , Sosa MA , Cendoroglo M , Jaber BL . Timing of renal replacement therapy initiation in acute renal failure: a meta-analysis. Am J Kidney Dis. 2008;52(2):272–84 .https://doi.org/10.1053/j.ajkd.2008.02.371
4 KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(Suppl 1):1–138. Available from: http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO%20AKI%20Guideline.pdf https://doi.org/10.1038/kisup.2012.1
5 Gaudry S , Hajage D , Schortgen F , Martin-Lefevre L , Pons B , Boulet E , et al.; AKIKI Study Group. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375(2):122–33 .https://doi.org/10.1056/NEJMoa1603017
6 Zarbock A , Kellum JA , Schmidt C , Van Aken H , Wempe C , Pavenstädt H , et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315(20):2190–9 .https://doi.org/10.1001/jama.2016.5828
7 Wald R , Adhikari NK , Smith OM , Weir MA , Pope K , Cohen A , et al.; Canadian Critical Care Trials Group. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int. 2015;88(4):897–904 .https://doi.org/10.1038/ki.2015.184
8 Jamale TE , Hase NK , Kulkarni M , Pradeep KJ , Keskar V , Jawale S , et al. Earlier-start versus usual-start dialysis in patients with community-acquired acute kidney injury: a randomized controlled trial. Am J Kidney Dis. 2013;62(6):1116–21 .https://doi.org/10.1053/j.ajkd.2013.06.012
9 Moher D , Liberati A , Tetzlaff J , Altman DG ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, W64 .https://doi.org/10.7326/0003-4819-151-4-200908180-00135
10 Jadad AR , Moore RA , Carroll D , Jenkinson C , Reynolds DJ , Gavaghan DJ , et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12 .https://doi.org/10.1016/0197-2456(95)00134-4
11 Zucchelli P , Pasquali S , Cagnoli L , Ferrari G . Controlled plasma exchange trial in acute renal failure due to multiple myeloma. Kidney Int. 1988;33(6):1175–80 .https://doi.org/10.1038/ki.1988.127
12 Koo JR , Yoon JW , Oh JE , et al. Prospective evaluation of early continuous venovenous hemofiltration on the outcome in patients with severe sepsis or septic shock [abstract F-FC062]. J Am Soc Nephrol. 2006;17:50A.
13 Durmaz I , Yagdi T , Calkavur T , Mahmudov R , Apaydin AZ , Posacioglu H , et al. Prophylactic dialysis in patients with renal dysfunction undergoing on-pump coronary artery bypass surgery. Ann Thorac Surg. 2003;75(3):859–64 .https://doi.org/10.1016/S0003-4975(02)04635-0
14 Payen D , Mateo J , Cavaillon JM , Fraisse F , Floriot C , Vicaut E ; Hemofiltration and Sepsis Group of the Collège National de Réanimation et de Médecine d’Urgence des Hôpitaux extra-Universitaires. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit Care Med. 2009;37(3):803–10 .https://doi.org/10.1097/CCM.0b013e3181962316
15 Combes A , Bréchot N , Amour J , Cozic N , Lebreton G , Guidon C , et al. Early high-volume hemofiltration versus standard care for post-cardiac surgery shock. The HEROICS study. Am J Respir Crit Care Med. 2015;192(10):1179–90 .https://doi.org/10.1164/rccm.201503-0516OC
16 Bouman CS , Oudemans-Van Straaten HM , Tijssen JG , Zandstra DF , Kesecioglu J . Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med. 2002;30(10):2205–11 .https://doi.org/10.1097/00003246-200210000-00005
17 Sugahara S , Suzuki H . Early start on continuous hemodialysis therapy improves survival rate in patients with acute renal failure following coronary bypass surgery. Hemodial Int. 2004;8(4):320–5 .https://doi.org/10.1111/j.1492-7535.2004.80404.x
18 Pursnani ML , Hazra DK , Singh B , Pandey DN . Early haemodialysis in acute tubular necrosis. J Assoc Physicians India. 1997;45(11):850–2.
19 Conger JD . A controlled evaluation of prophylactic dialysis in post-traumatic acute renal failure. J Trauma. 1975;15(12):1056–63 .https://doi.org/10.1097/00005373-197512000-00003
20 Gillum DM , Dixon BS , Yanover MJ , Kelleher SP , Shapiro MD , Benedetti RG , et al. The role of intensive dialysis in acute renal failure. Clin Nephrol. 1986;25(5):249–55.
21 Xu Y , Gao J , Zheng X , Zhong B , Na Y , Wei J . Timing of initiation of renal replacement therapy for acute kidney injury: a systematic review and meta-analysis of randomized-controlled trials. Clin Exp Nephrol. 2017;21(4):552–62.
22 Feng YM , Yang Y , Han XL , Zhang F , Wan D , Guo R . The effect of early versus late initiation of renal replacement therapy in patients with acute kidney injury: A meta-analysis with trial sequential analysis of randomized controlled trials. PLoS One. 2017;12(3):e0174158 .https://doi.org/10.1371/journal.pone.0174158
23 Wang C , Lv LS , Huang H , Guan J , Ye Z , Li S , et al. Initiation time of renal replacement therapy on patients with acute kidney injury: A systematic review and meta-analysis of 8179 participants. Nephrology (Carlton). 2017;22(1):7–18 .https://doi.org/10.1111/nep.12890
24 Bellomo R , Prowle JR , Echeverri JE , Ligabo V , Ronco C . Fluid management in septic acute kidney injury and cardiorenal syndromes. Contrib Nephrol. 2010;165:206–18 .https://doi.org/10.1159/000313760
25 Smith OM , Wald R , Adhikari NK , Pope K , Weir MA , Bagshaw SM ; Canadian Critical Care Trials Group. Standard versus accelerated initiation of renal replacement therapy in acute kidney injury (STARRT-AKI): study protocol for a randomized controlled trial. Trials. 2013;14(1):320 .https://doi.org/10.1186/1745-6215-14-320
26 Barbar SD , Binquet C , Monchi M , Bruyère R , Quenot JP . Impact on mortality of the timing of renal replacement therapy in patients with severe acute kidney injury in septic shock: the IDEAL-ICU study (initiation of dialysis early versus delayed in the intensive care unit): study protocol for a randomized controlled trial. Trials. 2014;15(1):270 .https://doi.org/10.1186/1745-6215-15-270
Dr Mavrakanas was supported by a grant from the foundation G. & L. Hirsch (General Internal Medicine Division, Geneva University Hospitals). Dr Charytan has received research support from Sentien Biotechnologies and consulting fees for services on a clinical events committee from PLC Medical (Renal Guard Solutions).