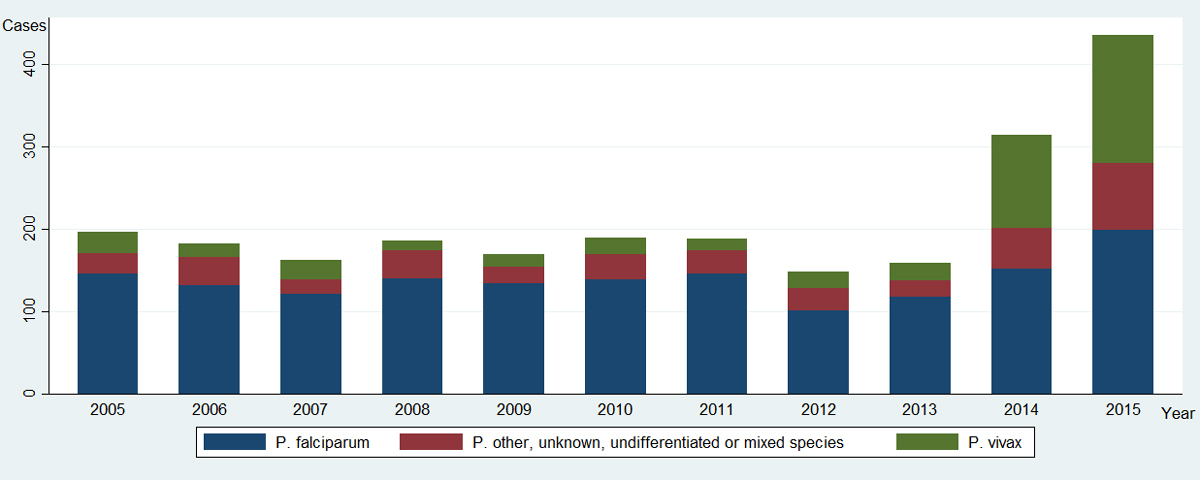
Figure 1 Number of malaria cases by Plasmodium species reported per year to the Federal Office of Public Health, Switzerland, 2005–2015 (n = 2336).
DOI: https://doi.org/10.4414/smw.2017.14510
Owing to huge global efforts, the worldwide burden of malaria has dropped impressively since 2000. Whereas the incidence rate fell by 37%, the number of deaths fell by 60% [1]. This reduction was seen in all affected regions of the world and is mainly due to multiple interventions: vector control (insecticide-treated mosquito nets, indoor residual spraying), preventive chemotherapy, and improved diagnosis (rapid diagnostic tests) and treatment (artemisinin-based combination therapies) [2]. However, much of this impressive result is due to the reduction of Plasmodium falciparum (Pf) malaria, which is the most frequent cause of malaria morbidity and mortality [1]. The incidence of Plasmodium vivax (Pv) malaria however seems not to follow the same decrease [3]. In 2015, there were an estimated 15 million cases of Pv per year (around 6.5% of all malaria cases), essentially occurring in Southern Asia, the Western Pacific and South America [1, 3], where Pv causes the majority of malaria cases. Controlling Pv malaria is challenging due to different parasitological characteristics. Several studies report an increase or re-emergence of autochthonous Pv malaria in the last few years [4–8].
In non-endemic countries, malaria can be “imported” by infected international travellers or immigrants. Switzerland, as a member of European Free Trade Association (EFTA), has signed the Dublin Regulation that determines the European Union (EU) Member State responsible for examining an application for asylum seekers [9] for international protection [10] under the Geneva Convention [11]. Since the Balkan Wars in the early 1990s, Switzerland has received many applications from asylum seekers. However, more recently the profile of asylum seekers in Switzerland has diverged from those in EU countries, where Syrians now represent the vast majority. Indeed, in 2015, 29% of asylum seekers in the EU were Syrians [12], while in the same year, among the 39 523 people who applied for asylum in Switzerland, 25% were Eritreans, 20% Afghans and only 12% Syrians [13].
In 2014, following the change in migration patterns, an increase of Pv cases was seen in Europe [14–16]. Since the number of imported malaria cases has doubled in the recent years in Switzerland, analysing characteristics and origins seemed essential in order to improve surveillance and to increase awareness among general practitioners for this forgotten but re-emerging disease.
“Immigration” as defined in this study refers to all individuals who come to a country to take up permanent residence, whatever the reason. Immigrants can be divided into refugees and migrants. Defined by the United Nations High Commissioner for Refugees (UNHCR) [9], a “refugee” is a person who is forced to leave his country of residence because of armed conflict or persecution, while “migrants” define people who move for economic, educational or other reasons, but who are not under direct threat of persecution or death [17]. “Asylum seekers” are people claiming to be refugees, but whose request for sanctuary has yet to be processed.
In this report, when used the word “refugee” refers to people who applied for asylum in Switzerland, whether or not they are actual asylum seekers, refugees, or rejected asylum seekers.
The Horn of Africa is defined in this article as the region comprising Eritrea, Ethiopia, Somalia, Sudan and South Sudan. The term '”multiple countries in the Horn of Africa'” represents two or more countries among this group.
Since 1974, reporting of malaria cases by physicians and diagnostic laboratories to the Federal Office of Public Health (FOPH) has been mandatory in Switzerland, as specified by the Epidemics Act and the Federal Ordinance on the notification of communicable diseases. Laboratories must declare a positive diagnostic result for Plasmodium. The reporting form lists the initials of the patients, test methods, test materials and results. The physician's report form supplies additional data with information on the patient, clinical (observed or reported fever; management, disease course) and infection (route of infection, a list of up to three visited countries prior to entry, and reason for travelling). Reason for travelling is subdivided into tourism, visiting friends and relatives (VFR), professional reasons, immigration (without distinction between refugees or migrants), and unknown. The notification delay is one week. The FOPH stores and processes all data collected from labs and physicians. All data on cases are aggregated, and cases are classified according to the European Centre for Disease Prevention and Control case definition. For this study, only the aggregated data including nationality, up to three visited countries prior to entry, reason for travelling, year of diagnosis and Plasmodium species of notified malaria cases from 2005 to 2015 were available for analysis. No individual data and, consequently, no individual characteristics like age, sex, clinical presentation or disease progression were available. Under Swiss law (art. 2 LRH) on anonymized data, no ethical clearance is required for the use of this aggregate data.
Because of this limited data granularity, only descriptive analyses (sum and proportion) of Pv cases according to nationality, visited countries and reason for travelling were performed by year, without further statistical tests.
As some patients might have stayed in or visited multiple endemic countries before reaching Switzerland, the variable “visited regions” was created according to the definition used by the United Nations Statistics Division [18]. The regions were defined as Northern Africa, Eastern Africa, Middle Africa, Southern Africa, Western Africa; Latin America and the Caribbean; Asia; Oceania; Europe and “unknown”. Only known malaria-endemic countries according to the World Health Organization (WHO) [1] were taken into account when converting national data to regional data. For example, a patient who had visited Cameroon then France before Switzerland was classified as coming from the Middle African region. Data from countries in the Horn of Africa were analysed separately. Following the observation that often multiple countries in the Horn of Africa were visited, an additional category “multiple countries in the Horn of Africa” was created. This allowed us to differentiate Pv cases from only one visited country to multiple countries in the Horn of Africa. Moreover, some “visited regions” were combined in continents. Finally, the “visited country/continent” variable was defined to differentiate Pv cases coming from Eritrea, Ethiopia, Somalia, Sudan and South Sudan, multiple countries in the Horn of Africa, Africa (except the Horn of Africa), America, Asia, Oceania, and “unknown”.
Plasmodium species were classified into three different categories: Pf, Pv, and other species. The variable 'other species' includes cases with unknown, undifferentiated (e.g., lack of distinction between Pv and P. ovale), or mixed infection with multiple Plasmodium species.
According to the results, yearly Pv malaria incidence by nationality was calculated, using Pv in immigrants (reason for travelling) as the numerator, and the annual number of new asylum seekers [13] as the denominator.
Whereas from 2003 to 2013, the incidence of reported malaria cases in Switzerland followed the worldwide epidemiological downward trend, a doubling in the number of cases was observed in each of the last two years (375 cases in 2014, and 435 in 2015, compared with a mean of 176 annual cases from 2005 to 2013). While all categories of malaria increased, this doubling was mainly due to a sharp increase in reported Pv malaria (fig. 1). Pv represented around 11% of all malaria cases in Switzerland from 2003 to 2013, then this proportion tripled to 36% in 2014 (113/315) and 2015 (156/435).

Figure 1 Number of malaria cases by Plasmodium species reported per year to the Federal Office of Public Health, Switzerland, 2005–2015 (n = 2336).
While 27% (45/168) of the patients with Pv malaria diagnosed before 2014 had visited the Horn of Africa (including Eritrea, Ethiopia, Somalia, Sudan and South Sudan), 87% (235/269) had visited this region for those diagnosed since 2014 (fig. 2). During the same period (2014-2015), Pf was diagnosed in 25 patients originating from this area.

Figure 2 Number of P. vivax malaria cases diagnosed in Switzerland by most probable country or continent of infection reported per year to the Federal Office of Public Health, 2005–2015 (n = 437).
“Multiple countries in the Horn of Africa” represents ≥ 2 countries among Eritrea, Ethiopia, Somalia, Sudan and South Sudan.
Prior to 2014, 34% (57/168) of Pv malaria cases in Switzerland were diagnosed among tourists but this proportion decreased to 1.5% from 2014 onwards. The proportion of Pv malaria cases detected among immigrant patients followed an opposite trend from 26% (43/168) to 90% (243/269) during the same period (fig. 3). Among these, excepting patients with unrecorded nationality (42/286), most of the immigrants diagnosed in Switzerland with Pv malaria were natives of the Horn of Africa (232/286), predominantly from Eritrea (77%: 220/286) then Ethiopia (9/286).
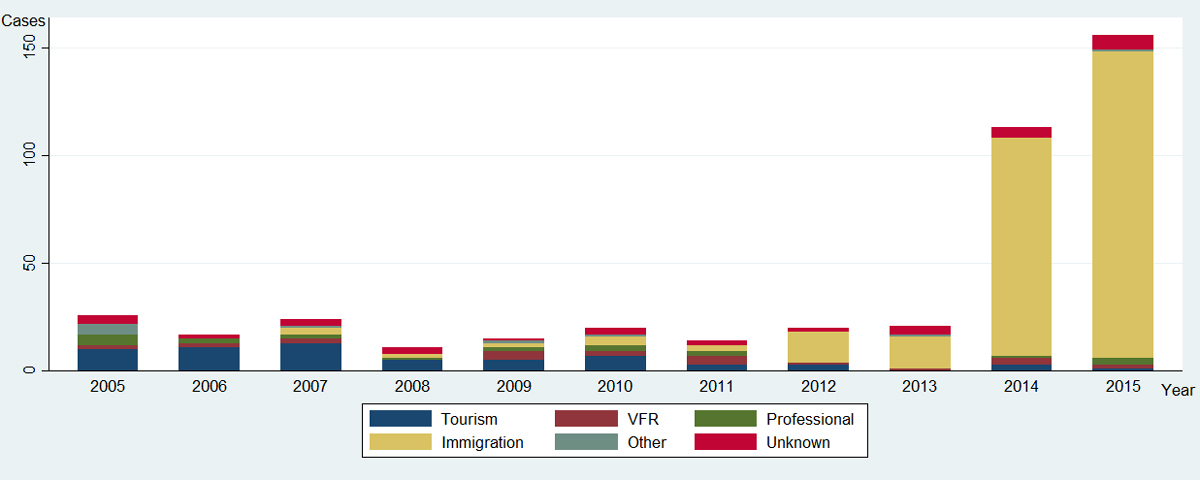
Figure 3 Reason for travel of patients with Pv malaria imported from endemic countries, among cases reported in Switzerland to the Federal Office of Public Health, 2005–2015 (n = 437).
Immigration: category without distinction between refugees, asylum seekers or migrants; VFR: visiting friends and relatives
The incidence of Pv malaria cases among refugees with an Eritrean nationality in Switzerland has dramatically increased by ten times from the earlier decade, and is estimated to be around 12‰ since 2014 (table 1).
Table 1 Estimated P. vivax malaria incidence rate per 1000 Eritrean refugees in Switzerland.
| Year | 2005–2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|
| Incidence rate (‰) (Pv/asylum seekers [13]) |
0.4 (7/12 777) |
2.0 (9/4407) |
2.7 (7/2563) |
12.0 (83/6923) |
11.4 (114/9966) |
Pv: includes only Pv cases among Eritreans with immigration as reason for travelling
While malaria has decreased worldwide since the turn of the Millennium, we found that the number of malaria cases reported in Switzerland has followed an opposite trend in the last few years. This rise in malaria cases since 2014, mostly Pv, is essentially the consequence of an increase in the proportion of refugees from the Horn of Africa, especially Eritrea, whose numbers in Switzerland have increased tenfold in the last decade [13]. Their number increased from around 200 per year prior to 2005, to around 2,000 since 2006, to reach nearly 10 000 in 2015 [13], a trend that sets Switzerland apart from other European countries. However, a similar rise of Pv cases has also been reported among Eritrean refugees [14–16].
In addition, our data demonstrate a clear increase in the estimated Pv malaria incidence rate in Eritrean refugees, reaching 12‰ in 2014 and 2015. According to the 2015 WHO report on malaria [1], cases of malaria in Eritrea have indeed increased in the last five years, but the present estimated Pv malaria incidence (around 2‰) is far below our observations. One explanation for the discrepancy is that the political deterioration has led both to a worsening of the malaria situation and the country's reporting capabilities. Moreover, immigrants represent a particularly vulnerable population, while hotspots of malaria transmission are also likely to be situated along multiple routes of migration. An increase or re-emergence of Pv malaria has been reported around the world [1] and according to the same WHO Report, a recent rise of Pv malaria incidence occurred in neighbouring Ethiopia, reaching 10‰ in 2014 [1, 19]. The number of patients with Pv malaria native of countries of the Horn of Africa other than Eritrea was too small in our database to detect a similar upward trend.
Pv has some particularities that might explain a possible local (and global) re-emergence and a smaller (or no) decrease of the worldwide incidence in comparison with Pf. First, the persistence of dormant – or latent – Pv parasite stages (hypnozoites) in the liver with malaria relapse occurring months or even years after the initial clinical episode. Second, the lack of diagnostic tools for liver-stage infections. Third, the paucity of specific treatments against hypnozoites. No antimalarial treatment except primaquine kills hypnozoites. Fourth, patients can transmit infectious gametocytes to mosquitoes even before symptoms arise. Fifth, the lack of sensitivity of the usual rapid diagnostic tests for the Pv erythrocytic (symptomatic) stage and, subsequently, the need for microscopy (thick/thin smears) for diagnosis. Sixth, Pv's propensity for transmission by several Anopheles mosquito species with different feeding and breeding behaviours, and requiring lower ambient temperatures than Pf. All these reasons might explain why current control measures (artemisinin-based combination therapies, rapid diagnostic test, insecticide-treated bed nets and indoor residual spraying) have not been as successful as for Pf [3, 20].
The changing epidemiology reported here highlights the necessity for primaquine, the recommended treatment for the latent phase of Pv malaria, to be registered and commercialized in Switzerland [20]. Primaquine must be ordered through an international pharmacy, causing a delay of two weeks. Moreover, standard medical insurance does not automatically refund the cost of the drug to the patient, which could be a barrier to access the treatment. This recommendation may benefit not only patients coming from endemic areas. Unlike Pf malaria, which is a strictly tropical disease, Pv is eminently adapted to climates in Europe, where it was widespread until the middle of the last century. Europe also still hosts a large number of Anopheles species that can transmit the disease. Re-emergence in Europe is now a reality, as autochthonous cases in Greece were reported during the summer of 2017 (personal communication).
The limitations of this study are related to the lack of detail in our dataset. First, data were anonymized and aggregated, and multiple relapses from the same patient might have been counted several times. In our institution (the University Hospitals of Geneva), we gave primaquine treatment to all patients with Pv malaria through the hospital pharmacy, and we did not observe subsequent relapses. However, we cannot be certain that the same therapeutic approach was followed in all Swiss centres. Moreover patients were not actively followed and some of them could have sought treatment in different centres in the case of relapse. Second, underreporting of malaria cases to the FOPH or changes in the notification system might have affected reporting. However, we believe it this to be limited due to redundant reporting by both physicians and laboratories. Furthermore, the reporting legislation and case definition have not significantly changed over 40 years. Third, most of the undifferentiated Plasmodium species (e.g., from a lack of distinction between Pv and P. ovale) in the 'other species' category were undiagnosed Pv malaria. This would, however, result in an underestimation of the incidence. Fourth, assigning the most probable country of infection was problematic. Aggregating multiple visited countries in regional or continental locations could solve this issue. Fifth, when calculating incidence rates, we counted the number of new asylum seekers as the denominator instead of all immigrants without distinction, as the exact number of immigrants is unknown. The vast majority of Eritreans who have moved recently to Switzerland were asylum seekers and very few are undocumented migrants. We therefore believe that the denominator used in our study is appropriate.
In conclusion, even if the bulk of international effort to control malaria is focused on Pf, Pv should not be forgotten. Its ability to relapse and to re-emerge steadily (e.g., South in Korea [4]) or sporadically in previously endemic regions like southern Europe (e.g. Greece [7]) should be kept in mind. Unlike Pf, Pv is transmissible worldwide. An increased incidence in the country of origin (Eritrea) might be the cause of the rise of Pv cases imported in Switzerland, but infections are also likely to occur along the harsh and long migration journey, often through Ethiopia and Sudan. General practitioners in Switzerland (and similar hosting countries) should be aware of Pv malaria in febrile patients originating from the Horn of Africa, even if fevers occur months after reaching Europe.
We would like to thank Dr W. Robert Taylor, for his help in the subject, and Rob Hooft van Huijsduijnen for critical reading of the manuscript.
No financial support and no other potential conflict of interest relevant to this article was reported.
1World Health Organization World Malaria Report 2015 [Internet]. [cited 2017 Jun 15]. Available from: http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/
2 Bhatt S , Weiss DJ , Cameron E , Bisanzio D , Mappin B , Dalrymple U , et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–11. doi:.https://doi.org/10.1038/nature15535
3World Health Organization. Global Malaria Programme, Control and elimination of plasmodium vivax malaria: a technical brief [Internet]. 2015 [cited 2017 Jun 15]. Available from: http://apps.who.int/iris/bitstream/10665/181162/1/9789241509244_eng.pdf?ua=1&ua=1
4 Jun G , Yeom J-S , Hong J-Y , Shin E-H , Chang K-S , Yu J-R , et al. Resurgence of Plasmodium vivax malaria in the Republic of Korea during 2006-2007. Am J Trop Med Hyg. 2009;81(4):605–10. doi:.https://doi.org/10.4269/ajtmh.2009.09-0111
5 Sattabongkot J , Tsuboi T , Zollner GE , Sirichaisinthop J , Cui L . Plasmodium vivax transmission: chances for control? Trends Parasitol. 2004;20(4):192–8. doi:.https://doi.org/10.1016/j.pt.2004.02.001
6 Santos-Ciminera PD , Roberts DR , Alecrim M , Costa MR , Quinnan GV, Jr . Malaria diagnosis and hospitalization trends, Brazil. Emerg Infect Dis. 2007;13(10):1597–600. doi:.https://doi.org/10.3201/eid1310.070052
7 Danis K , Lenglet A , Tseroni M , Baka A , Tsiodras S , Bonovas S . Malaria in Greece: historical and current reflections on a re-emerging vector borne disease. Travel Med Infect Dis. 2013;11(1):8–14. doi:.https://doi.org/10.1016/j.tmaid.2013.01.001
8 Petersen E , Severini C , Picot S . Plasmodium vivax malaria: a re-emerging threat for temperate climate zones? Travel Med Infect Dis. 2013;11(1):51–9. doi:.https://doi.org/10.1016/j.tmaid.2013.01.003
9Refugees UNHC for. Asylum-Seekers [Internet]. UNHCR. [cited 2017 Jun 15]. Available from: http://www.unhcr.org/asylum-seekers.html
10Right of asylum. In: Wikipedia [Internet]. 2017. Available from: https://en.wikipedia.org/w/index.php?title=Right_of_asylum&oldid=783386993
11Convention Relating to the Status of Refugees. In: Wikipedia [Internet]. 2017. Available from: https://en.wikipedia.org/w/index.php?title=Convention_Relating_to_the_Status_of_Refugees&oldid=783557299
12Eurostat. Asylum statistics/fr [Internet]. [cited 2017 Jun 15]. Available from: http://ec.europa.eu/eurostat/statistics-explained/pdfscache/17180.pdf
13Secrétariat d’état aux migrations, Confédération suisse. Statistiques en matière d’asile [Internet]. 2015 [cited 2017 Jun 15]. Available from: https://www.sem.admin.ch/sem/fr/home/publiservice/statistik/asylstatistik/uebersichten.html
14 Sondén K , Castro E , Törnnberg L , Stenström C , Tegnell A , Färnert A . High incidence of Plasmodium vivax malaria in newly arrived Eritrean refugees in Sweden since May 2014. Euro Surveill. 2014;19(35):20890. doi:.https://doi.org/10.2807/1560-7917.ES2014.19.35.20890
15De Gier B, Suryapranata FST, Croughs M, van Genderen PJJ, Keuter M, Visser LG, et al. Increase in imported malaria in the Netherlands in asylum seekers and VFR travellers. Malar J [Internet]. 2017 Dec [cited 2017 Mar 28];16(1). Available from: http://malariajournal.biomedcentral.com/articles/10.1186/s12936-017-1711-5
16Roggelin L, Tappe D, Noack B, Addo MM, Tannich E, Rothe C. Sharp increase of imported Plasmodium vivax malaria seen in migrants from Eritrea in Hamburg, Germany. Malar J [Internet]. 2016 Dec [cited 2017 Mar 28];15(1). Available from: http://malariajournal.biomedcentral.com/articles/10.1186/s12936-016-1366-7
17UNHCR viewpoint: ‘Refugee’ or ‘migrant’ - Which is right? [Internet]. UNHCR. [cited 2017 Jun 15]. Available from: http://www.unhcr.org/news/latest/2015/8/55df0e556/unhcr-viewpoint-refugee-migrant-right.html
18United Nations Statistics division. Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings [Internet]. 2013 [cited 2017 Jun 15]. Available from: http://unstats.un.org/unsd/methods/m49/m49regnf.htm
19Taylor WRJ. Personal communication. 2016.
20 Allgower A , Taylor WR , Chappuis F , Eperon G . [Plasmodium vivax, a parasite coming out of the shadows]. Rev Med Suisse. 2016;12(517):876 , 878–81.
No financial support and no other potential conflict of interest relevant to this article was reported.