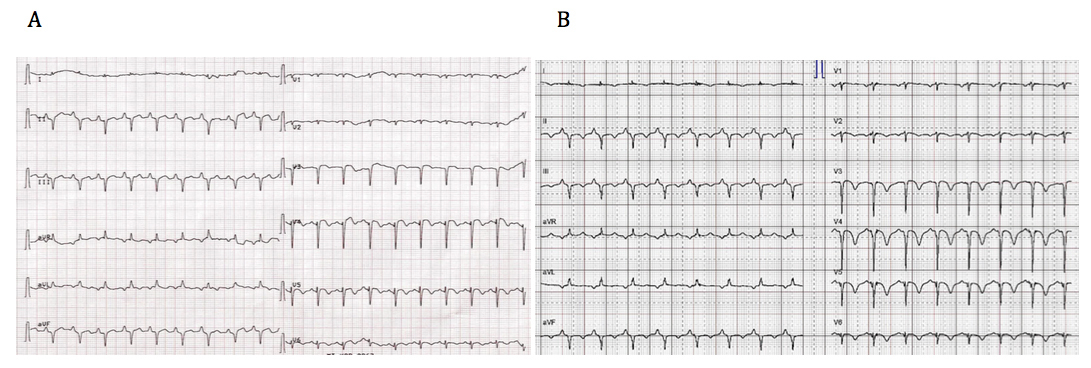
Figure 1 (A) ECG showing diffuse ST segment elevations. (B) Follow-up ECG after 10 hours demonstrates diffuse T wave inversions, particularly prominent in V3–V5.
DOI: https://doi.org/10.4414/smw.2017.14490
A 71-year-old female was hospitalised for acute exacerbation of chronic obstructive pulmonary disease after complaining of severe dyspnoea and chest pain. During hospitalisation she developed diffuse ST-segment elevation (fig. 1A). Computed tomography angiography excluded pulmonary embolism and revealed pulmonary oedema. Laboratory testing showed mildly increased troponin T and normal levels of creatine kinase. The patient underwent emergency coronary angiography, which demonstrated no culprit lesion. Transthoracic echocardiography (TTE) showed a moderately reduced left ventricular ejection fraction (LVEF) with akinesia of the apical segments and a hypercontractile base. Ten hours later a repeat electrocardiogram (ECG) demonstrated diffuse T wave inversion (fig. 1B). At follow-up echocardiography, left ventricular function and wall motion abnormalities had returned to normal.

Figure 1 (A) ECG showing diffuse ST segment elevations. (B) Follow-up ECG after 10 hours demonstrates diffuse T wave inversions, particularly prominent in V3–V5.
A 65-year-old man was admitted to a stroke unit after a cerebrovascular accident. A few days after hospital admission, the patient developed dyspnoea and episodes of complete heart block (fig. 2) necessitating cardiopulmonary resuscitation. After successful return of spontaneous circulation, continuous intravenous epinephrine was started. TTE showed severely reduced left ventricular systolic function (LVEF 25%), with typical apical ballooning. Furthermore, coronary angiography confirmed normal coronary arteries. During monitoring in the intensive care unit, the clinical course was complicated by ventricular tachycardia. Therefore, therapy with epinephrine was discontinued and replaced by levosimendan. No further tachyarrhythmias were observed.
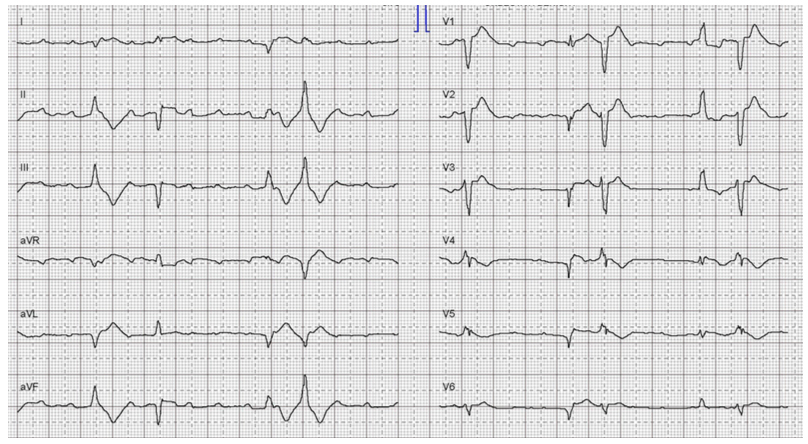
Figure 2 Complete heart block requiring cardiopulmonary resuscitation in a male patient with Takotsubo syndrome.
A 72-year-old female developed abdominal pain during her grandchildren’s birthday party. She was admitted to the hospital with ST-segment depression in the inferior-lateral leads. Troponin was mildly elevated. She was transferred to the cardiology department for suspected non-ST elevation myocardial infarction (NSTEMI). Coronary angiography excluded coronary artery disease, but left ventricular angiography showed midventricular hypokinesia and a moderately impaired LVEF (fig. 3).
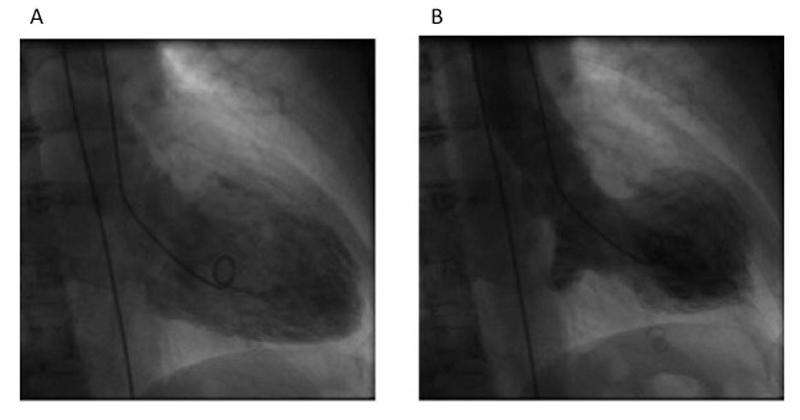
Figure 3 Left ventricular angiography showing a midventricular Takotsubo pattern.
A 23-year-old female with no past medical history underwent rhinoplasty. During the operation, lidocaine and epinephrine were administrated locally. The patient subsequently developed ventricular tachyarrhythmia. Emergency TTE revealed a mildly reduced LVEF. Cardiac magnetic resonance imaging (CMR) was performed without late gadolinium enhancement, but revealed a severely reduced LVEF, as well as basal hypokinesia and oedema indicative of atypical, basal Takotsubo syndrome. On follow-up 4 days later a TTE confirmed complete recovery of left ventricular function and a normal wall motion pattern.
Takotsubo syndrome (TTS) was first described more than 20 years ago by Dote et al. as an unusual systolic regional dysfunction of the left ventricle in Japanese patients [1, 2]. This form of acute reversible heart failure was given the name of Takotsubo disease, on the basis of the resemblance of the left ventricular systolic shape to a Japanese octopus trap [3, 4]. As most cases were first reported in Japan, this disease was thought to be peculiar to this country [5]; however, when reports of TTS also emerged in the USA and Europe, clinical studies and case reports began to be increasingly published in a variety of medical journals worldwide [6].
As a result of the clinical presentation of TTS, the disease originally also became widely known as “broken heart syndrome” or “apical ballooning syndrome” [7]. However, extensive recent research has improved our understanding of the disease definition, symptoms, triggers and outcome in TTS. Indeed, TTS was previously thought only to affect elderly, postmenopausal women who suffered an emotionally negative trigger, leading to acute chest pain with apical ballooning on a left ventricular angiogram with absence of clinically relevant coronary artery disease. However, we now know that TTS can occur at any age and affect both sexes. Moreover, it is not only a disease of the “broken-hearted”, but it can also occur after a joyful moment [8].
TTS can present variously on imaging, not only as apical ballooning, and includes midventricular, focal, and basal forms. Coronary artery disease can also be present in up to 15% of TTS cases, and obstructive stenosis on coronary angiography does not exclude for the diagnosis of TTS. Furthermore, new outcome data contradict the previous assumption that TTS is a benign disease. Instead, TTS carries a substantial risk of morbidity and mortality in the acute phase. The reported variations in the case presentations of TTS must be carefully studied and a certain expertise is needed to identify TTS correctly. This is especially important as the clinical presentation of TTS is quite similar to the more frequent thombo-occlusive (classic) type of acute coronary syndrome (ACS), which makes differential diagnosis quite challenging [1, 2, 9].
The number of patients diagnosed with TTS is increasing, especially in the last decade. For example, the Swedish registry SWEDEHEART reported a rise of TTS diagnoses from 0.16% in 2005 to 2.2% in 2012 [6]. Currently, the overall incidence of TTS is about 2%, which includes patients who are initially diagnosed with classic ACS [9, 10]. The incidence is even higher in females, ranging from 5.9% to 7.5% [11, 12]. And although the disease affects mostly females (89.9%) at postmenopausal age (66.4 years) [13], TTS cases at any age have been reported. Interestingly, 10 to 15% of TTS patients are male [14, 15].
TTS has been described in over 50 countries to date. It seems to affect individuals from any ethnic background, but for as yet unknown reasons, in Japan men seem to be more often affected [16]. There are only a few reports of cases in African-Americans and Hispanics [17]. Furthermore, family clustering suggests that TTS has a hereditary component, but, as yet, there is no firm evidence to support this claim [18, 19].
Despite a postulated microvascular disease component in TTS, it is supposed that TTS-specific risk factors exist. These risk factors include female gender, hormones, age, neurological and psychiatric disease, genetics and stressful life events.
In contrast to myocardial infarction, a chronobiological pattern with a peak incidence in the summer and in the evening has been reported for TTS [20].
The most common symptom of TTS is acute chest pain. Dyspnoea, palpitations, syncope and fatigue are other possible symptoms, although less common. Given the clinical presentation, differentiation of TTS from classic thrombo-oclusive ACS in the emergency setting is not easy. Intriguingly, patients often experience TTS in an in-hospital setting, while in an intensive care unit or perioperatively and then present with more severe disease as reflected by systemic hypotension, pulmonary oedema, or arrhythmic events [21–23]. Furthermore, what was once thought to be a major clue for the diagnosis of TTS, a preceding negative emotional trigger, had to be reconsidered in recent years. It is reported that 27.7% of patients present with a preceding emotional trigger, but TTS may, in contrast, be associated with a joyful emotional event, lately described as “happy heart syndrome” [1, 2, 8, 24, 25]. An even greater proportion of patients (36%) experience TTS following a physical trigger, for example, exacerbation of chronic obstructive pulmonary disease (COPD), cancer, stroke, bone fractures, or operations, among others [23, 26]. In 7.8% of cases, both emotional and physical triggers can be identified simultaneously, whereas in 28.5% of cases, no evident trigger precedes TTS [13].
Not only are the patients’ collective histories and clinical presentations of TTS more varied than initially thought, but so also are the pattern of regional wall motion disturbances. Although present in 81.7% of cases to date, the classic apical “ballooning”, characterised by apical akinesia and basal hyperdynamic contraction, is not the only manifestation of TTS. Three atypical variants without apical involvement exist, comprising the midventricular (14.6%), the basal (2.2%), and the focal (1.5%) presentation [26]. Furthermore, biventricular forms (42%) have also been described and are associated with a more serious clinical course [27]. A biventricular form, however, is often unrecognised because its identification requires transthoracic echocardiography, which is not always routinely performed. Isolated right ventricular presentations have rarely been reported as another atypical form, although their existence remains controversial [28].
Despite ongoing research on TTS, whether sympathetic hyperexcitation, a microcirculatory disturbance, or both are the underlying pathophysiological mechanism of this disease entity, remains unclear. As TTS is provoked most often by emotional or physical stress factors, and similar cardiac dysfunction has been observed in patients with phaeochromocytoma, it is likely that sympathetic hyperexcitation at least is co-involved in TTS pathogenesis. Indeed, plasma concentrations of noradrenaline and adrenaline are higher in patients with TTS than in those with myocardial infarction. Histopathological findings characteristic of contractile band necrosis further support the idea that excessive catecholamine surge leads finally to TTS [1]. Moreover, an imbalance of the distribution of β1 and β2 adrenergic receptors, with a higher concentration in the apical segments and a lower concentration in the basal segments (and the opposite gradient for sympathetic nerve endings) could explain the apical ballooning [29, 30].
A catecholamine-induced microcirculatory disturbance is currently the most attractive explanation for the underlying pathogenesis of TTS. Indeed, analysis of the coronary angiographic findings in patients with TTS showed impaired myocardial perfusion in 69% of cases [31], and assessment of the coronary flow reserve in the acute and recovery phase showed improvement with progressive resolution of the wall motion abnormality [32]. It seems likely that a combination of sympathetic overactivation and oxidative stress [33] might contribute to the microcirculatory disturbance in TTS, but more research is warranted.
Diagnostic criteria for TTS have been proposed by different authors, groups, and societies, but the Mayo Clinic Diagnostic Criteria from 2008 [9] are currently used most often. Contrary to popular belief, concomitant coronary artery disease is not an exclusion criteria [34], and at present there is no universally accepted definition for TTS. For instance, some diagnostic criteria for TTS exclude phaeochromocytoma whereas others do not. The lack of consistent diagnostic criteria is indicative of the fact that TTS is still not well understood.
ST-segment elevation, particularly in the anterior leads, is often observed in the acute phase of TTS (i.e., in 40–50% of cases) [26], making timely differentiation from ST elevation myocardial infarction (STEMI) even more challenging [35]. Less often, the initial ECG shows ST-segment depression [26], a left bundle-branch block pattern, anterior Q waves or diffuse T wave inversions. A completely normal ECG can also be present in ≈2% of cases [24].
ECG changes exhibit four distinct phases, though these are not universally present. Phase I includes typical ST-segment elevation at acute presentation; phase II (day 1–3) is characterised by T wave inversions; phase III (day 2–6) shows T wave reversions; and phase IV is characterised by giant negative T waves, which can appear up to 60 days after onset of symptoms [36]. Given this typical time course of ECG changes, patients presenting with T wave inversions on the initial ECG are thought to be already in the subacute TTS phase. In this subacute phase, a prolongation of the QTc interval is also observed [23]. This is of particular interest as it can be the basis for unusual arrhythmic events such as torsades de pointes tachycardia [37]. Therefore, it is important to avoid medications or certain disorders (for example hypokalaemia) that further prolong the QTc interval in these patients.
As the above-mentioned ECG changes may also occur in patients suffering from myocardial infarction, differential diagnosis based on the ECG alone and without urgent coronary angiography is not yet possible. Nevertheless, a recent algorithm for differentiation of TTS and acute myocardial infarction based on the ECG has been proposed, reporting a specificity of 95% [38].
Given the extensive wall motion abnormalities in patients with TTS, the often rather limited elevation of the cardiac biomarkers creatine kinase and troponin is surprising. Indeed, creatine kinase rarely exceeds values above 500 U/l [1, 2, 14, 15, 24]. However, as a result of left ventricular wall stretching, plasma concentrations of brain natriuretic peptide (BNP) are remarkably elevated in patients with TTS compared with those presenting with ACS [1, 5]. BNP values typically reach their maximum concentration 48 hours after the acute event, but may remain elevated up to 3 months thereafter [39]. Although different profiles of cardiac biomarkers are evident, no cut-off values for troponin, creatine kinase, and BNP have yet been defined to allow meaningful discrimination of TTS and ACS. Indeed, while the ratio of BNP to troponin T has been proposed as diagnostic tool for differentiation of TTS and ACS, further investigation on this issue is needed [40]. Interestingly, it was recently shown that a signature of four circulating microRNAs could serve as a novel diagnostic tool in the acute phase to differentiate TTS from STEMI. However, microRNA analyses have not yet entered clinical practice [41].
Transthoracic echocardiography (TTE) plays a major role in the diagnosis and identification of complications of TTS. Furthermore TTE is also used to evaluate the disease course. The mainstay of the TTS diagnosis is the assessment for typical left ventricular wall motion abnormalities, beyond the distribution of a single coronary artery, which allows the differentiation of the apical, midventricular and basal forms. In rare cases, a wall motion abnormality can be present in the territory of a single epicardial vessel, which is termed focal TTS and is particularly challenging to diagnose. TTE also permits identification of potential complications such as significant mitral valve regurgitation (MR), left ventricular outflow tract obstruction (LVOTO), ventricular thrombi in often atypical and multiple locations, right ventricular involvement, and cardiac rupture [42].
The underlying mechanism of mitral valve regurgitation in TTS is not completely understood. It has mostly been reported in patients presenting with the classic apical form, in the presence of hyperdynamic contraction of the basal segments and systolic anterior movement (SAM) of the anterior mitral valve leaflet (i.e., in 33–50% of cases) [43, 44]. Significant reversible mitral regurgitation has also been observed in patients with severe LVEF impairment in the absence of SAM. In a dilated left ventricle, symmetric tethering of the mitral valve leaflets, secondary to papillary muscle displacement, is another obvious mechanism of mitral regurgitation [44]. The dynamic and intermediate to severe LVOTO, caused by hyperdynamic contraction of the basal segments and SAM, may lead to life-threatening arrhythmias and fatal left ventricular rupture. Hence, identification of potential complications of TTS first of all by echocardiography is of major importance for risk stratification and clinical management.
Cardiac magnetic resonance imaging (CMR) has also gained increasing attention in the assessment of TTS. Recently, specific CMR criteria for the diagnosis of TTS have been published [9]. CMR allows analysis of the function of both ventricles, myocardial tissue characterisation and detection of the distribution of myocardial oedema, as well as exclusion of transmural or subendocardial scar by absence of late gadolinium enhancement [45]. This allows differentiation of TTS from myocardial infarction or myocarditis, and makes CMR particularly helpful in identifying the rare focal type of TTS [45]. CMR appears to be more sensitive for detecting right ventricular involvement and left ventricular thrombi [27]. However, given its limited availability (especially in the emergency setting) and low acceptance by some patients, CMR should not be considered an alternative to echocardiography, but instead a complementary technique [42].
Radionuclide imaging such 31I/123I-metaiodobenzylguanidine (MIBG) scintigraphy and positron emission tomography (PET) can provide insight into the myocardial metabolism in TTS [5], and may help further in clarifying the underlying pathogenic mechanism. However, to date, accurate differentiation of ACS and TTS is not possible by noninvasive tests, and urgent coronary angiography remains the diagnostic “gold standard”.
Recently it has been demonstrated that patients with TTS carry a considerable morbidity and mortality risk. Although spontaneous reversibility is a unique characteristic of TTS, this is only true for patients who survive the acute phase and, even so, the long-term prognosis is not entirely positive with a major adverse cardiac and cerebrovascular event (MACCE) rate of 9.9% per patient year [26]. In most cases, TTS can be effectively managed with follow-up only. However, severe complications may cause a life-threatening disease course in the acute phase, with a reported 30-day mortality comparable to STEMI and NSTEMI [6]. LVEF is often severely reduced in the acute phase of the disease, far more than in acute myocardial infarction. Therefore, it is not surprising that about half of TTS patients suffer from pulmonary congestion and about 10 to 20% from acute pulmonary oedema [23, 26] (fig. 4).
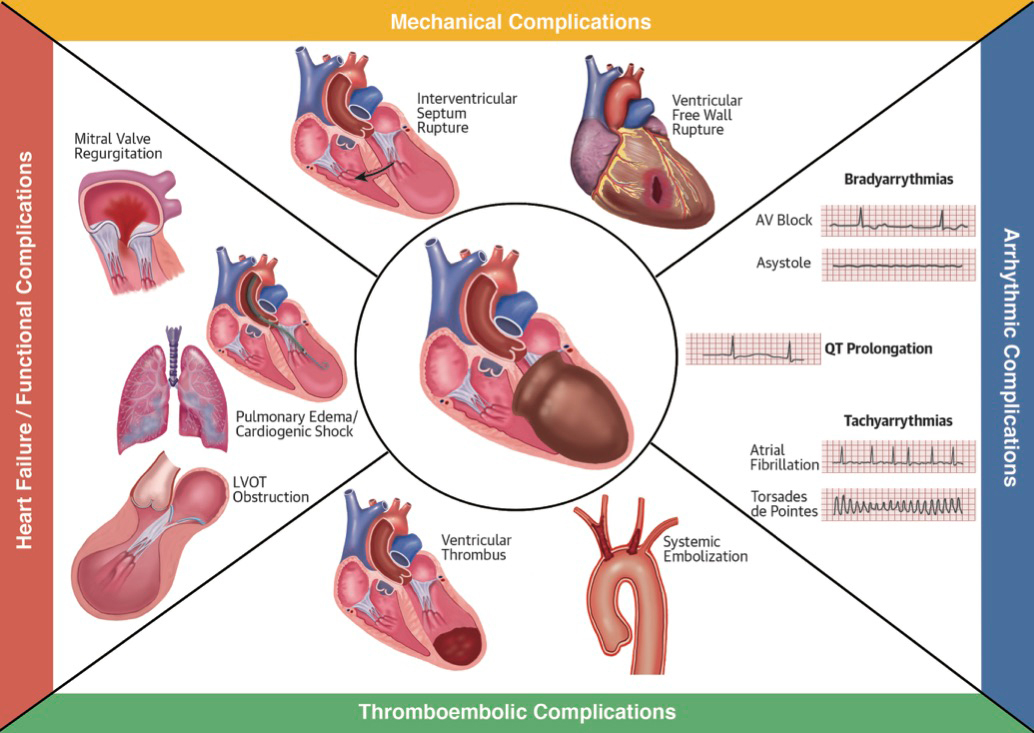
Figure 4 Different complications that can occur in Takotsubo syndrome. Complications are divided into heart failure and functional complications as well as mechanical, arrhythmic and thromboembolic.
Assessing for presence of LVOTO, mitral valve regurgitation and right ventricular involvement in patients with suspected TTS is crucial, as when these are present, patients often develop cardiogenic shock as a complication [46]. In these patients, special treatment is needed. Given the supposed important role of catecholamine excess in the pathogenesis of the disease, inotropic agents such as epinephrine, norepinephrine and dobutamine may worsen LVOTO. The calcium sensitiser levosimendan may be an effective pharmacological alternative [1]. In some cases, the use of a percutaneous cardiopulmonary support device (e.g., the micro-axial Impeller Pump) can be useful and preferable to catecholaminergic agents. In addition, as systemic embolisation of ventricular thrombi may further complicate the clinical course, anticoagulants for primary prevention may be useful for patients in whom ventricular thrombi are identified or LVEF is severely reduced [23] (fig. 4).
No specific long-term pharmacological treatment for TTS has been established thus far. As sympathetic hyperactivation probably contributes to the underlying pathogenic mechanism, the use of beta-blocking agents was suggested to be helpful both in the acute setting and for the prevention of recurrence [1, 5]. However, as yet there is no evidence that beta-blockers provide beneficial treatment for TTS. Indeed, as a prolongation of the QT interval is typically observed in TTS patients, particular attention must be paid to the use of beta-blockers in patients with potential risk of pause-dependent torsades de pointes, especially those with bradycardia and severe QTc prolongation [47]. Furthermore, in patients with TTS and a QT interval exceeding 500 ms, the risk of potential life-threating arrhythmias is substantially increased [48]. Other arrhythmic complications that must be taken into account in the acute phase include bradycardia, heart block and atrial fibrillation [49] (fig. 4).
Overall in-hospital mortality for TTS is 4.1% and most patients (95%) experience complete LVEF recovery within 4 to 8 weeks. Nevertheless, recurrence occurs in 5 to 10% of cases, and different types of TTS can occur in the same patient [50–52]. Somewhat surprisingly, recurrence of TTS manifests even in patients with chronic beta-blocker treatment, which makes the effectiveness of these agents in TTS secondary prevention questionable [1].
TTS was first described as a rare left ventricular systolic dysfunction resembling clinical presentation of classic ACS in a few Japanese patients at the beginning of the 1990s [3]. The worldwide incidence of TTS is now reported to be ≈2% in patients with suspected ACS [9, 10, 53]. This incidence is 8.8 times higher in women than in men, and 4.8 times higher in women older than 55 years compared with those younger than 55 [5]. However, the cases of 71-year old-lady suffering from acute exacerbation of chronic obstructive pulmonary disease, the 65-year-old man experiencing complete heart block and ventricular arrhythmia, the 72-year-old lady celebrating her grandchild’s birthday, and the 23-year-old woman undergoing rhinoplasty highlight that TTS can occur in both sexes, at any age, and does not always have a favourable clinical course. Furthermore, these cases show that TTS may necessitate intensive care treatment and advanced therapeutics.
TTS is not only provoked by negative emotional events, but also quite often by physical stressors, or even both. In rare cases, TTS even occurs without any evident preceding stressor [26]. Although TTS gained worldwide attention as “broken heart syndrome”, recently the term “happy heart syndrome” has also been used in connection with this disease [8]. Indeed, the case of the 71-year-old lady who was joyfully attending her grandson’s wedding described here [54], as well as others who presented with TTS after a joyful event (such as winning a jackpot, visiting the opera, or celebrating an anniversary), indicate that TTS can be preceded by a positive emotional trigger [8]. Classic cardiovascular risk factors such as diabetes mellitus, smoking, dyslipidaemia and arterial hypertension do not seem to play an important role in this disease. Instead, psychiatric and neurological disorders are more frequent in patients experiencing TTS, representing a possible link between the heart and the brain [26].
In summary, what is indisputably evident about TTS so far is that this syndrome is far more complex and heterogeneous than initially thought. We have a long way to go towards understanding the pathogenic mechanism of this disease. Such knowledge is required to establish noninvasive diagnostic tools with enough sensitivity and specificity to clearly differentiate TTS very early from ACS, as well as to determine a specific treatment approach for the acute event and prevention of recurrences. Growing attentiveness to the many diverse faces of TTS may lead to its improved diagnosis, which finally may increase our knowledge of this enigmatic condition.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Ghadri JR , Ruschitzka F , Lüscher TF , Templin C . Takotsubo cardiomyopathy: still much more to learn. Heart. 2014;100(22):1804–12. doi:.https://doi.org/10.1136/heartjnl-2013-304691
2 Kurisu S , Kihara Y . Tako-tsubo cardiomyopathy: clinical presentation and underlying mechanism. J Cardiol. 2012;60(6):429–37. doi:.https://doi.org/10.1016/j.jjcc.2012.06.015
3 Dote K , Sato H , Tateishi H , Uchida T , Ishihara M . [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases]. J Cardiol. 1991;21(2):203–14. Article in Japanese.
4 Kurisu S , Sato H , Kawagoe T , Ishihara M , Shimatani Y , Nishioka K , et al. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J. 2002;143(3):448–55. doi:.https://doi.org/10.1067/mhj.2002.120403
5 Yoshikawa T . Takotsubo cardiomyopathy, a new concept of cardiomyopathy: clinical features and pathophysiology. Int J Cardiol. 2015;182:297–303. doi:.https://doi.org/10.1016/j.ijcard.2014.12.116
6 Redfors B , Vedad R , Angerås O , Råmunddal T , Petursson P , Haraldsson I , et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction - A report from the SWEDEHEART registry. Int J Cardiol. 2015;185:282–9. doi:.https://doi.org/10.1016/j.ijcard.2015.03.162
7 Sharkey SW , Lesser JR , Maron MS , Maron BJ . Why not just call it tako-tsubo cardiomyopathy: a discussion of nomenclature. J Am Coll Cardiol. 2011;57(13):1496–7. doi:.https://doi.org/10.1016/j.jacc.2010.11.029
8 Ghadri JR , Sarcon A , Diekmann J , Bataiosu DR , Cammann VL , Jurisic S , et al.; InterTAK Co-investigators. Happy heart syndrome: role of positive emotional stress in takotsubo syndrome. Eur Heart J. 2016;37(37):2823–9. doi:.https://doi.org/10.1093/eurheartj/ehv757
9 Prasad A , Lerman A , Rihal CS . Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408–17. doi:.https://doi.org/10.1016/j.ahj.2007.11.008
10 Prasad A , Dangas G , Srinivasan M , Yu J , Gersh BJ , Mehran R , et al. Incidence and angiographic characteristics of patients with apical ballooning syndrome (takotsubo/stress cardiomyopathy) in the HORIZONS-AMI trial: an analysis from a multicenter, international study of ST-elevation myocardial infarction. Catheter Cardiovasc Interv. 2014;83(3):343–8. doi:.https://doi.org/10.1002/ccd.23441
11 Wedekind H , Möller K , Scholz KH . Tako-Tsubo-Kardiomyopathie [Tako-tsubo cardiomyopathy. Incidence in patients with acute coronary syndrome]. Herz. 2006;31(4):339–46. Article in German. doi:.https://doi.org/10.1007/s00059-006-2822-x
12 Sy F , Basraon J , Zheng H , Singh M , Richina J , Ambrose JA . Frequency of Takotsubo cardiomyopathy in postmenopausal women presenting with an acute coronary syndrome. Am J Cardiol. 2013;112(4):479–82. doi:.https://doi.org/10.1016/j.amjcard.2013.04.010
13 Templin C , Ghadri JR , Diekmann J , Napp LC , Bataiosu DR , Jaguszewski M , et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015;373(10):929–38. doi:.https://doi.org/10.1056/NEJMoa1406761
14 Bybee KA , Prasad A . Stress-related cardiomyopathy syndromes. Circulation. 2008;118(4):397–409. doi:.https://doi.org/10.1161/CIRCULATIONAHA.106.677625
15 Akashi YJ , Goldstein DS , Barbaro G , Ueyama T . Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118(25):2754–62. doi:.https://doi.org/10.1161/CIRCULATIONAHA.108.767012
16 Aizawa K , Suzuki T . Takotsubo cardiomyopathy: Japanese perspective. Heart Fail Clin. 2013;9(2):243–7, x. doi:.https://doi.org/10.1016/j.hfc.2012.12.001
17 Nascimento FO , Larrauri-Reyes MC , Santana O , Pérez-Caminero M , Lamas GA . Comparison of stress cardiomyopathy in Hispanic and non-Hispanic patients. Rev Esp Cardiol (Engl Ed). 2013;66(1):67–8. doi:.https://doi.org/10.1016/j.rec.2012.05.006
18 Kumar G , Holmes DR, Jr , Prasad A . “Familial” apical ballooning syndrome (Takotsubo cardiomyopathy). Int J Cardiol. 2010;144(3):444–5. doi:.https://doi.org/10.1016/j.ijcard.2009.03.078
19 Pison L , De Vusser P , Mullens W . Apical ballooning in relatives. Heart. 2004;90(12):e67. doi:.https://doi.org/10.1136/hrt.2004.046813
20 Sharkey SW , Lesser JR , Garberich RF , Pink VR , Maron MS , Maron BJ . Comparison of circadian rhythm patterns in Tako-tsubo cardiomyopathy versus ST-segment elevation myocardial infarction. Am J Cardiol. 2012;110(6):795–9. doi:.https://doi.org/10.1016/j.amjcard.2012.04.060
21 Haghi D , Fluechter S , Suselbeck T , Saur J , Bheleel O , Borggrefe M , et al. Takotsubo cardiomyopathy (acute left ventricular apical ballooning syndrome) occurring in the intensive care unit. Intensive Care Med. 2006;32(7):1069–74. doi:.https://doi.org/10.1007/s00134-006-0111-z
22 Park JH , Kang SJ , Song JK , Kim HK , Lim CM , Kang DH , et al. Left ventricular apical ballooning due to severe physical stress in patients admitted to the medical ICU. Chest. 2005;128(1):296–302. doi:.https://doi.org/10.1378/chest.128.1.296
23 Sharkey SW , Maron BJ . Epidemiology and clinical profile of Takotsubo cardiomyopathy. Circ J. 2014;78(9):2119–28. doi:.https://doi.org/10.1253/circj.CJ-14-0770
24 Sharkey SW , Windenburg DC , Lesser JR , Maron MS , Hauser RG , Lesser JN , et al. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55(4):333–41. doi:.https://doi.org/10.1016/j.jacc.2009.08.057
25 Summers MR , Prasad A . Takotsubo cardiomyopathy: definition and clinical profile. Heart Fail Clin. 2013;9(2):111–22, vii. doi:.https://doi.org/10.1016/j.hfc.2012.12.007
26 Templin C , Ghadri JR , Diekmann J , Napp LC , Bataiosu DR , Jaguszewski M , et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015;373(10):929–38. doi:.https://doi.org/10.1056/NEJMoa1406761
27 Eitel I , von Knobelsdorff-Brenkenhoff F , Bernhardt P , Carbone I , Muellerleile K , Aldrovandi A , et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306(3):277–86. doi:.https://doi.org/10.1001/jama.2011.992
28 Elesber AA , Prasad A , Bybee KA , Valeti U , Motiei A , Lerman A , et al. Transient cardiac apical ballooning syndrome: prevalence and clinical implications of right ventricular involvement. J Am Coll Cardiol. 2006;47(5):1082–3. doi:.https://doi.org/10.1016/j.jacc.2005.12.004
29 Lyon AR , Rees PS , Prasad S , Poole-Wilson PA , Harding SE . Stress (Takotsubo) cardiomyopathy--a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5(1):22–9. doi:.https://doi.org/10.1038/ncpcardio1066
30 Mori H , Ishikawa S , Kojima S , Hayashi J , Watanabe Y , Hoffman JI , et al. Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli. Cardiovasc Res. 1993;27(2):192–8. doi:.https://doi.org/10.1093/cvr/27.2.192
31 Elesber A , Lerman A , Bybee KA , Murphy JG , Barsness G , Singh M , et al. Myocardial perfusion in apical ballooning syndrome correlate of myocardial injury. Am Heart J. 2006;152(3):469.e9–13. doi:.https://doi.org/10.1016/j.ahj.2006.06.007
32 Kume T , Akasaka T , Kawamoto T , Yoshitani H , Watanabe N , Neishi Y , et al. Assessment of coronary microcirculation in patients with takotsubo-like left ventricular dysfunction. Circ J. 2005;69(8):934–9. doi:.https://doi.org/10.1253/circj.69.934
33 Willis BC , Salazar-Cantú A , Silva-Platas C , Fernández-Sada E , Villegas CA , Rios-Argaiz E , et al. Impaired oxidative metabolism and calcium mishandling underlie cardiac dysfunction in a rat model of post-acute isoproterenol-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2015;308(5):H467–77. doi:.https://doi.org/10.1152/ajpheart.00734.2013
34 Scantlebury DC , Prasad A . Diagnosis of Takotsubo cardiomyopathy. Circ J. 2014;78(9):2129–39. doi:.https://doi.org/10.1253/circj.CJ-14-0859
35 Kosuge M , Ebina T , Hibi K , Morita S , Okuda J , Iwahashi N , et al. Simple and accurate electrocardiographic criteria to differentiate takotsubo cardiomyopathy from anterior acute myocardial infarction. J Am Coll Cardiol. 2010;55(22):2514–6. doi:.https://doi.org/10.1016/j.jacc.2009.12.059
36 Mitsuma W , Kodama M , Ito M , Tanaka K , Yanagawa T , Ikarashi N , et al. Serial electrocardiographic findings in women with Takotsubo cardiomyopathy. Am J Cardiol. 2007;100(1):106–9. doi:.https://doi.org/10.1016/j.amjcard.2007.02.062
37 Syed FF , Asirvatham SJ , Francis J . Arrhythmia occurrence with takotsubo cardiomyopathy: a literature review. Europace. 2011;13(6):780–8. doi:.https://doi.org/10.1093/europace/euq435
38 Frangieh AH , Obeid S , Ghadri JR , Imori Y , D’Ascenzo F , Kovac M , et al.; InterTAK Collaborators. ECG Criteria to Differentiate Between Takotsubo (Stress) Cardiomyopathy and Myocardial Infarction. J Am Heart Assoc. 2016;5(6):e003418. doi:.https://doi.org/10.1161/JAHA.116.003418
39 Nguyen TH , Neil CJ , Sverdlov AL , Mahadavan G , Chirkov YY , Kucia AM , et al. N-terminal pro-brain natriuretic protein levels in takotsubo cardiomyopathy. Am J Cardiol. 2011;108(9):1316–21. doi:.https://doi.org/10.1016/j.amjcard.2011.06.047
40 Ahmed KA , Madhavan M , Prasad A . Brain natriuretic peptide in apical ballooning syndrome (Takotsubo/stress cardiomyopathy): comparison with acute myocardial infarction. Coron Artery Dis. 2012;23(4):259–64. doi:.https://doi.org/10.1097/MCA.0b013e3283526a57
41 Jaguszewski M , Osipova J , Ghadri JR , Napp LC , Widera C , Franke J , et al. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur Heart J. 2014;35(15):999–1006. doi:.https://doi.org/10.1093/eurheartj/eht392
42 Citro R , Lyon AR , Meimoun P , Omerovic E , Redfors B , Buck T , et al. Standard and advanced echocardiography in takotsubo (stress) cardiomyopathy: clinical and prognostic implications. J Am Soc Echocardiogr. 2015;28(1):57–74. doi:.https://doi.org/10.1016/j.echo.2014.08.020
43 Parodi G , Del Pace S , Salvadori C , Carrabba N , Olivotto I , Gensini GF ; Tuscany Registry of Tako-Tsubo Cardiomyopathy. Left ventricular apical ballooning syndrome as a novel cause of acute mitral regurgitation. J Am Coll Cardiol. 2007;50(7):647–9. doi:.https://doi.org/10.1016/j.jacc.2007.04.057
44 Izumo M , Nalawadi S , Shiota M , Das J , Dohad S , Kuwahara E , et al. Mechanisms of acute mitral regurgitation in patients with takotsubo cardiomyopathy: an echocardiographic study. Circ Cardiovasc Imaging. 2011;4(4):392–8. doi:.https://doi.org/10.1161/CIRCIMAGING.110.962845
45 Templin C , Ghadri JR , Napp LC . Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015;373(27):2689–91. doi:.https://doi.org/10.1056/NEJMc1512595
46 Citro R , Rigo F , D’Andrea A , Ciampi Q , Parodi G , Provenza G , et al.; Tako-Tsubo Italian Network Investigators. Echocardiographic correlates of acute heart failure, cardiogenic shock, and in-hospital mortality in tako-tsubo cardiomyopathy. JACC Cardiovasc Imaging. 2014;7(2):119–29. doi:.https://doi.org/10.1016/j.jcmg.2013.09.020
47 Migliore F , Zorzi A , Peruzza F , Perazzolo Marra M , Tarantini G , Iliceto S , et al. Incidence and management of life-threatening arrhythmias in Takotsubo syndrome. Int J Cardiol. 2013;166(1):261–3. doi:.https://doi.org/10.1016/j.ijcard.2012.09.107
48 Madias C , Fitzgibbons TP , Alsheikh-Ali AA , Bouchard JL , Kalsmith B , Garlitski AC , et al. Acquired long QT syndrome from stress cardiomyopathy is associated with ventricular arrhythmias and torsades de pointes. Heart Rhythm. 2011;8(4):555–61. doi:.https://doi.org/10.1016/j.hrthm.2010.12.012
49 Samuelov-Kinori L , Kinori M , Kogan Y , Swartzon M , Shalev H , Guy D , et al. Takotsubo cardiomyopathy and QT interval prolongation: who are the patients at risk for torsades de pointes? J Electrocardiol. 2009;42(4):353–357.e1. doi:.https://doi.org/10.1016/j.jelectrocard.2009.01.005
50 Ghadri JR , Jaguszewski M , Corti R , Lüscher TF , Templin C . Different wall motion patterns of three consecutive episodes of takotsubo cardiomyopathy in the same patient. Int J Cardiol. 2012;160(2):e25–7. doi:.https://doi.org/10.1016/j.ijcard.2012.01.021
51 Blessing E , Steen H , Rosenberg M , Katus H , Frey N . Recurrence of takotsubo cardiomyopathy with variant forms of left ventricular dysfunction. J Am Soc Echocardiogr. 2007;20(4):439.e11–2. doi:.https://doi.org/10.1016/j.echo.2006.10.021
52 Kaushik M , Alla VM , Madan R , Arouni AJ , Mohiuddin SM . Recurrent stress cardiomyopathy with variable regional involvement: insights into etiopathogenetic mechanisms. Circulation. 2011;124(22):e556–7. doi:.https://doi.org/10.1161/CIRCULATIONAHA.111.059329
53 Bybee KA , Prasad A , Barsness GW , Lerman A , Jaffe AS , Murphy JG , et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94(3):343–6. doi:.https://doi.org/10.1016/j.amjcard.2004.04.030
54 Qin D , Patel SM , Champion HC . “Happiness” and stress cardiomyopathy (apical ballooning syndrome/takotsubo syndrome). Int J Cardiol. 2014;172(1):e182–3. doi:.https://doi.org/10.1016/j.ijcard.2013.12.140
No financial support and no other potential conflict of interest relevant to this article was reported.