Quality of vitamin K antagonist oral anticoagulation in 322 patients with atrial fibrillation – real-life data from a survey in Eastern Switzerland
DOI: https://doi.org/10.4414/smw.2017.14503
Micha T.
Maedera, Tabea
Königa, Sanja
Bogdanovica, Irene
Schneidera, Werner
Eugsterbc, Peter
Ammanna, Marius
Könige, Jürg H.
Beerd, Hans
Ricklia
aCardiology Department, Kantonsspital St. Gallen, Switzerland
bHerzteam Wil, Switzerland
cAerztenetz “Xundart”, Wil, Switzerland
dInternal Medicine Department, Kantonsspital St. Gallen, Switzerland
eInternal Medicine Department, Kantonsspital Baden and Molecular Cardiology, University Hospital of Zurich, Switzerland
AIM OF THE STUDY: To better appreciate the role of the non-vitamin K oral anticoagulants (NOACs) for patients with non-valvular atrial fibrillation in Switzerland we aimed to assess the quality of vitamin K antagonist (VKA) anticoagulation in daily practice.
METHODS: In a cross-sectional study, clinically stable patients on VKA treatment for non-valvular atrial fibrillation for at least 6 months, documentation of international normalised ratio (INR) values for at least 3 months and with at least two INR values were included. The percentage of INR values within the therapeutic range of 2.0 to 3.0 and the time in therapeutic range (TTR; Rosendaal method) and predictors for these measures of VKA anticoagulation quality were assessed.
RESULTS: We studied 332 patients (62% male, mean age 74 ± 9 years) with median (interquartile range) CHA2DS2Vasc and HAS-BLED scores of 4 (3–5) and 3 (2–4) points. The median number of INR values per patient was 8 (5–14), and the average interval between INR measurements was 20 (13–27) days. The percentage of INR values between 2.0 and 3.0 was 67% (50–83%). The median TTR was 69% (51–89%), and TTR ≥65% was found in 202 (61%) patients. Independent predictors of ≥80% INR values between 2.0 and 3.0 included a longer interval between INR measurements and the non-use of spironolactone. The non-use of amiodarone and spironolactone and a longer interval between INR measurements were the only independent predictors of a TTR ≥65%.
CONCLUSIONS: The quality of VKA anticoagulation in Switzerland is highly variable. Importantly, only 60% of patients achieve a TTR ≥65%, which is currently considered to be the minimal acceptable TTR required for VKA therapy. There are few clinical predictors of a good VKA anticoagulation quality. These data may represent a novel basis for decision making regarding the choice of anticoagulation for atrial fibrillation in Switzerland.
Introduction
A key clinical question to address in every patient with atrial fibrillation (AF) is whether long-term oral anticoagulation (OAC) is required, and if yes, which type of OAC should be used. Current guidelines recommend the use of the CHA2DS2Vasc score to determine whether the thromboembolic risk reduction by OAC outweighs the concomitantly increased risk of bleeding [1]. In male AF patients with a CHA2DS2Vasc score of 2 or more and in female AF patients with a CHA2DS2Vasc score of 3 or more, OAC is clearly recommended (class I indication), and in males with a CHA2DS2Vasc score of 1 and in females with a CHA2DS2Vasc score of 2, OAC should be considered (class IIa indication), but decisions should be individualised [1]. A key assumption underlying these recommendations is that an effective and safe mode of OAC is used. This can be achieved by use of either vitamin K antagonist (VKA) OAC with a high time in the therapeutic range (TTR) (i.e., an INR of 2.0–3.0) or non-vitamin K antagonist oral anticoagulants (NOACs) [1]. In studies showing comparable thromboembolic risks in AF patients treated with VKA or NOACs, the mean TTR values were between 55 und 65% in the VKA-treated patients [2–5]. Thus, in practice a TTR in this range must be achieved to ensure that VKA therapy is likely to provide a similar thromboembolic risk reduction as NOACs. The Swedish national registry has revealed that a TTR of ≈75% is achievable [6], and that VKA therapy is very safe and effective under these circumstances [7].
However, several studies have shown that in clinical practice TTR is less optimal [8–11]. Current guidelines, therefore, give a preference to NOACs over VKAs for the initiation of OAC and suggest a switch from VKAs to NOACs if there is evidence of insufficient TTR [1]. Importantly, information on the quality of VKA anticoagulation is mainly based on data from OAC clinics and from patients treated with warfarin [8]. However, in many patients VKA therapy is not managed by OAC clinics but by general practitioners (GPs), and in Europe VKAs other than warfarin are commonly used. Little is known about the quality of VKA therapy in this situation, the main reason being the lack of larger international normalised ratio (INR) databases for patients managed outside of OAC clinics. There are arguments for both better (e.g., long-term contact with patients, knowledge of comorbidities and nutrition behaviours, etc.) and worse (e.g., less systematic approach to VKA prescription) VKA OAC management by GPs compared with OAC clinics. In addition, there are pharmacokinetic differences between warfarin and phenprocoumon (the most commonly used VKA in Europe) that may have an effect on OAC quality, i.e., a longer half-life for phenprocoumon than for warfarin [12]. To better appreciate the role of NOACs for patients with non-valvular AF in daily practice in Switzerland we performed a survey of the real-life quality of VKA OAC (percentage of INR values within the therapeutic range and TTR) in Eastern Switzerland. We hypothesised that VKA OAC quality in daily practice would be worse than in study settings.
Materials and methods
Patients
In a cross-sectional study, clinically stable patients either seen as outpatients or electively admitted as inpatients in the Cardiology Department of the Kantonsspital St. Gallen with VKA treatment for non-valvular AF for at least 6 months, documentation of INR values over a period of at least 3 months with at least two INR values, and available INR booklet (a pocket card consisting of a calendar where INR values and VKA dose prescriptions are entered by the GP or other physicians) at the time of hospital admission were included between January 2011 and December 2014. These criteria were applied to make sure that patients were in a chronic state of VKA anticoagulation. Available VKAs in Switzerland include phenprocoumon (Marcoumar®) and acenocoumarol (Sintrom®), whereas warfarin is not available. In Switzerland, there are no OAC clinics. Measurement of INR and VKA dose prescription is performed by GPs in the vast majority of patients. Inpatients were typically included when admitted for elective cardiac catheterisation or other procedures, including catheter ablation or pacemaker implantation. All these procedures are performed with uninterrupted OAC at the Cardiology Department of the Kantonsspital St. Gallen, and this is explicitly communicated to referring cardiologists, GPs and external hospitals. Apart from the presence of valvular AF (mechanical prosthesis or at least moderate mitral stenosis) or concomitant conditions requiring a different INR range (e.g., patients with chronic thromboembolic pulmonary hypertension), there were no exclusion criteria. All patients provided oral and written informed consent, and the local Ethics Committee approved the study.
Data extraction
A photocopy of the INR booklet was obtained. Clinical information including patient history, underlying cardiac disease, comorbidities and co-medication was obtained from the clinic information system or hard copies of patient histories. The CHA2DS2Vasc [1], HAS-BLED [1], and SAMe-TT2R2 [13] scores were calculated for all patients. The CHA2DS2Vasc score is used to quantify the thromboembolic risk. The following items contribute to the score: Congestive heart failure (1 point), Hypertension (1 point), Age ≥75 years (2 points), Diabetes (1 point), previous Stroke (2 points), Vascular disease (1 point), age 65-74 (1 point), and female gender (1 point). The HAS-BLED score is used to quantify the bleeding risk. The following items contribute to the score (1 point for each): Hypertension, Abnormal liver/renal function, Stroke, Bleeding history or predisposition, Labile INR, Elderly (>65 years), and Drugs/alcohol. The SAMe-TT2R2 score has been proposed as a tool to predict INR stability [13]. The following items contribute to the score: female Sex (1 point), Age <60 years (1 point), Medical history (1 point if more than two of the following: hypertension, diabetes, coronary artery disease/myocardial infarction, peripheral arterial disease, congestive heart failure, prior stroke, pulmonary disease, hepatic, or renal disease), Treatment (mainly amiodarone, 1 point), Tabacco use (2 points), and non-white Race (2 points).
Data analysis
All available INR data and intervals between INR measurements were manually extracted and included in the analysis. Descriptive statistics are expressed as mean ± standard deviation, or median (interquartile range [IQR]) for continuous variables, and as numbers and percentages for categorical variables. The quality of VKA OAC was assessed in two ways. First, the percentage of INR values within the therapeutic range of 2.0 to 3.0 was assessed. Second, the TTR was estimated according to the Rosendaal’s method of linear interpolation [14]. Linear regression analysis was performed to assess multivariate predictors of the percentage of INR values within the therapeutic range and TTR as continuous variables. Multivariate logistic regression was performed to assess predictors of a high (≥80%) and low (≤50%) percentage of INR values within the therapeutic range. These cut-offs are arbitrary, but plausible from a clinical point of view. Multivariate logistic regression was performed to predict a TTR ≥65%. This cut-off for dichotomised TTR was selected as it is considered to represent the minimal TTR value required for acceptable VKA OAC quality [12]. Selection of covariates for the multivariate analysis was based on the results of the univariate analysis. All available and reported patient characteristics were included in the univariate analysis. Stepwise backward models were used. The level of statistical significance was set at a 2-tailed probability value ≤0.05. Statistical analysis was performed using the IBM® SPSS® for Windows® software (version 20.0, SPSS® Inc., Chicago, Illinois, USA).
Results
Study population
A total of 401 patients with available INR booklets were screened for inclusion in the study. The final study population who fulfilled all inclusion criteria comprised 332 patients (62% male, mean age 74 ± 9 years). Patient characteristics and drug therapy are presented in table 1. The median CHA2DS2Vasc and HAS-BLED scores were 4 (IQR 3–5) and 3 (IQR 2–4) points. The median SAMe-TT2R2 score was 2 (IQR 1–2) points.
Table 1 Patient characteristics (n = 332).
|
Variable
|
Result
|
| Age (years) |
74 ± 9 |
| Gender (male/female) |
206 (62%) / 126(38%) |
| Body mass index (kg/m2) |
28.3 ± 4.9 |
| Cardiac disease |
|
| Hypertensive heart disease |
157 (48%) |
| Coronary artery disease |
61 (19%) |
| Valvular heart disease |
57 (17%) |
| Other cardiac disease |
53 (16%) |
| Left ventricular ejection fraction (%) |
55 (41–60) |
| Atrial fibrillation classification |
|
| Permanent |
103 (31%) |
| Non-permanent |
229 (69%) |
| European Heart Rhythm Association class |
2 (1–2) |
| Diabetes |
65 (19%) |
| Hypertension |
273 (82%) |
| Dyslipidaemia |
197 (59%) |
| Previous stroke / transient ischaemic attack |
63 (19%) |
| Previous cardiac surgery |
|
| Coronary artery bypass grafting |
31 (9%) |
| Valve surgery |
14 (4%) |
| Other |
23 (7%) |
| Smoking |
|
| Current |
31 (9%) |
| Previous |
110 (33%) |
| Alcohol consumption |
|
| Current |
149 (45%) |
| Previous |
11 (3%) |
| Type of Vitamin K antagonist (phenprocoumon/acenocoumarol) |
328 (99%) / 4 (1%) |
| Comedication |
|
| Aspirin |
42 (13%) |
| Clopidogrel |
13 (4%) |
| Statin |
156 (47%) |
| Angiotensin converting enzyme inhibitor / angiotensin receptor blocker |
224 (67%) |
| Beta-blocker |
246 (74%) |
| Verapamil, diltiazm |
63 (19%) |
| Amlodipine |
19 (6%) |
| Digoxin |
32 (10%) |
| Amiodarone |
65 (20%) |
| Dronedarone |
5 (2%) |
| Sotalol |
5 (2%) |
| Class I antiarrhythmic drug |
6 (2%) |
| Loop diuretic |
149 (45%) |
| Thiazide |
77 (23%) |
| Nitrate |
31 (9%) |
| Spironolactone |
56 (17%) |
| Thromboembolic and bleeding risk |
|
| CHA2DS2Vasc-Score (points) |
4 (3–5) |
| HASBLED score (points) |
3 (2–4) |
INR values
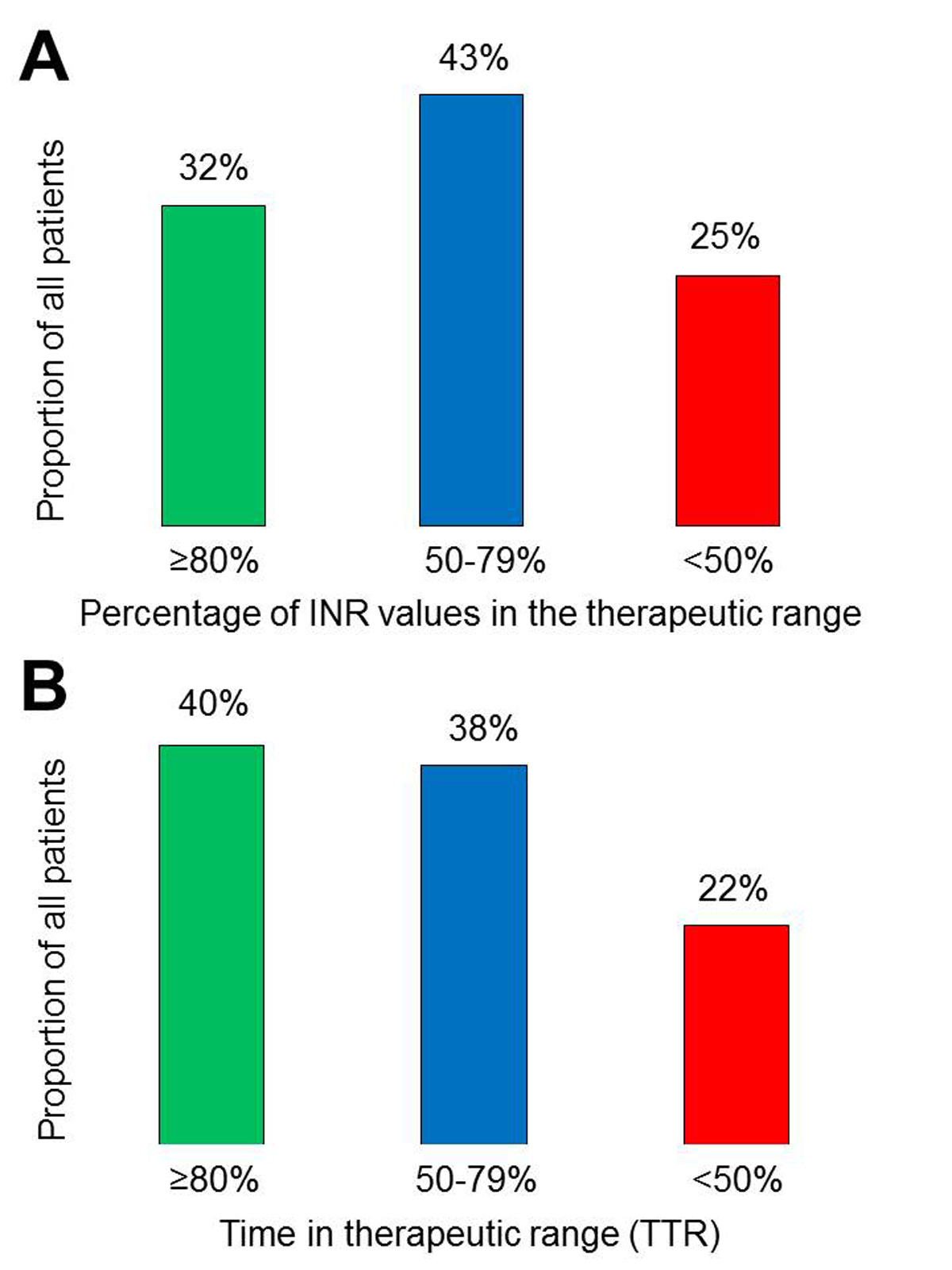
A total of 3672 INR values were available for the analysis. The median number of INR values per patient was 8 (IQR 5–14). The median observation period per patient was 158 (IQR 103–246) days with a median average interval between INR measurements of 20 (IQR 13–27) days. The median percentage of INR values between 2.0 and 3.0 was 67% (IQR 50–83%). The median percentages of subtherapeutic (<2.0) and supratherapeutic (>3.0) INR values were 16% (IQR 0–32%) and 10% (IQR 0–25%), respectively. In 105 (32%) patients, ≥80% INR values were in the range of 2.0 to 3.0, whereas 82 (25%) patients had <50% of values in this range (fig. 1A). The median TTR was 69% (IQR 51–89%). A TTR ≥80% was seen in 134 (40%) patients, whereas 74 (22%) patients had a TTR <50% (fig. 1B). A TTR ≥65% was found in 202 (61%) patients.
Predictors of a high and low percentage of INR values between 2.0 and 3.0
In table 2, univariate and multivariate predictors of the percentage of INR values in the therapeutic range expressed as a continuous and dichotomised variable are shown. The non-use of amiodarone and a longer interval between INR measurements were the only independent predictors of a higher percentage of INR values in the therapeutic range when expressed as a continuous variable. There was no significant correlation between percentage of INR values between 2.0 and 3.0 and the SAMe-TT2R2 score (Spearman r = −0.09; p = 0.09). Independent predictors of ≥80% INR values between 2.0 and 3.0 included a longer interval between INR measurements and the non-use of spironolactone (table 2). The only independent predictor of <50% of values in the therapeutic range was a shorter interval between INR measurements (table 2). The use of amiodarone was a predictor of <50% of values in the therapeutic range in the univariate but not in the multivariate analysis (p = 0.08). Other patient characteristics were not independently related to a high or low percentage of INR values in the therapeutic range.
Table 2 Predictors of high/low percentage of INR values between 2.0 and 3.0.
|
Univariate
|
p-value
|
Multivariate
|
p-value
|
|
Percentage of INR values between 2.0 and 3.0 as a continuous variable
|
| EHRA class |
−0.154 |
0.007 |
|
NS |
| Permanent AF |
0.149 |
0.007 |
|
NS |
| Amiodarone |
−0.140 |
0.01 |
−0.115 |
0.03 |
| Loop diuretic |
−.157 |
0.004 |
|
NS |
| Spironolactone use |
−0.127 |
0.02 |
|
NS |
| HASBLED score |
−0.103 |
0.063 |
|
NS |
| Interval between INR measurements |
0.273 |
<0.001 |
0.261 |
<0.001 |
|
Percentage of INR values between 2.0 and 3.0 ≥80%
|
| Permanent AF |
2.05 (1.26–3.35) |
0.004 |
NS |
|
| EHRA class |
0.68 (0.50–0.93) per class
|
0.02 |
NS |
|
| Spironolactone use |
0.41 (0.20–0.85) |
0.02 |
0.43 (0.20–0.93) |
p = 0.03 |
| Interval between INR measurements |
1.08 (1.05–1.10) per day |
<0.001 |
1.08 (1.05–1.10) per day |
p<0.001 |
|
Percentage of INR values between 2.0 and 3.0 <50%
|
| Permanent AF |
0.45 0.25–0.83) |
0.01 |
NS |
|
| EHRA class |
1.48 (1.07–2.03) per class |
0.02 |
NS |
|
| Amiodarone use |
2.15 (1.20–3.84) |
0.01 |
NS |
|
| Number of INR values |
1.04 (1.01–1.07) |
0.003 |
NS |
|
| Interval between INR measurements |
0.92 (0.89–0.95) per day |
<0.001 |
0.92 (0.89–0.95) per day |
p<0.001 |
Predictors of high and low TTR
Univariate and multivariate predictors of TTR expressed as a continuous and dichotomised variable are shown in table 3. Absence of dyslipidaemia, the non-use of amiodarone and spironolactone, and a longer interval between INR measurements were independently associated with a higher TTR expressed as a continuous variable. There was no significant correlation between TTR expressed as a continuous variable and the SAMe-TT2R2 score (Spearman r = −0.08; p = 0.13). The non-use of amiodarone and spironolactone and a longer interval between INR measurements were the only independent predictors of a TTR ≥65% (table 3).
Table 3 Predictors of high time in therapeutic range (TTR).
|
Univariate
|
p-value
|
Multivariate
|
p-value
|
|
TTR as a continuous variable
|
| Dyslipidaemia |
−0.104 |
0.06 |
−0.110 |
0.041 |
| Amiodarone |
−0.125 |
0.023 |
−0.110 |
0.041 |
| Loop diuretic |
−0.114 |
0.038 |
|
NS |
| Spironolactone use |
−0.133 |
0.015 |
−0.133 |
0.014 |
| HASBLED score |
−0.098 |
0.08 |
|
NS |
| Interval between INR measurements |
0.182 |
0.001 |
0.164 |
0.002 |
|
TTR ≥65%
|
| Amiodarone |
0.52 (0.30–0.89) |
0.02 |
0.57 (0.32–0.99) |
0.049 |
| Spironolactone use |
0.45 (0.25–0.81) |
0.008 |
0.47 (0.26–0.85) |
0.012 |
| HASBLED score |
0.84 (0.71–1.01) |
0.07 |
|
NS |
| Interval between INR measurements |
1.04 (1.02–1.07) per day |
<0.001 |
1.04 (1.02–1.06) per day |
0.001 |
Discussion
The present study showed that in a cross-sectional sample of VKA-treated AF patients with INR measurements and VKA (phenprocoumon in the vast majority of patients) dosing done by GPs in Eastern Switzerland, the OAC quality was highly variable. The median TTR was 69%, which is at least as good as the average OAC quality in clinical studies using warfarin [2–5]. In one third of patients, ≥80% of all INR values were within the therapeutic range of 2 to 3, 40% of patients had a TTR ≥80%, and 60% of patients had a TTR of at least 65%. On the other hand, one quarter of patients had less than 50% of INR values in the therapeutic range, and 22% had a TTR <50%, which represents a situation where patients are exposed to an increased risk of thromboembolic events and/or bleeding, and where the VKA OAC does not confer a benefit [15].
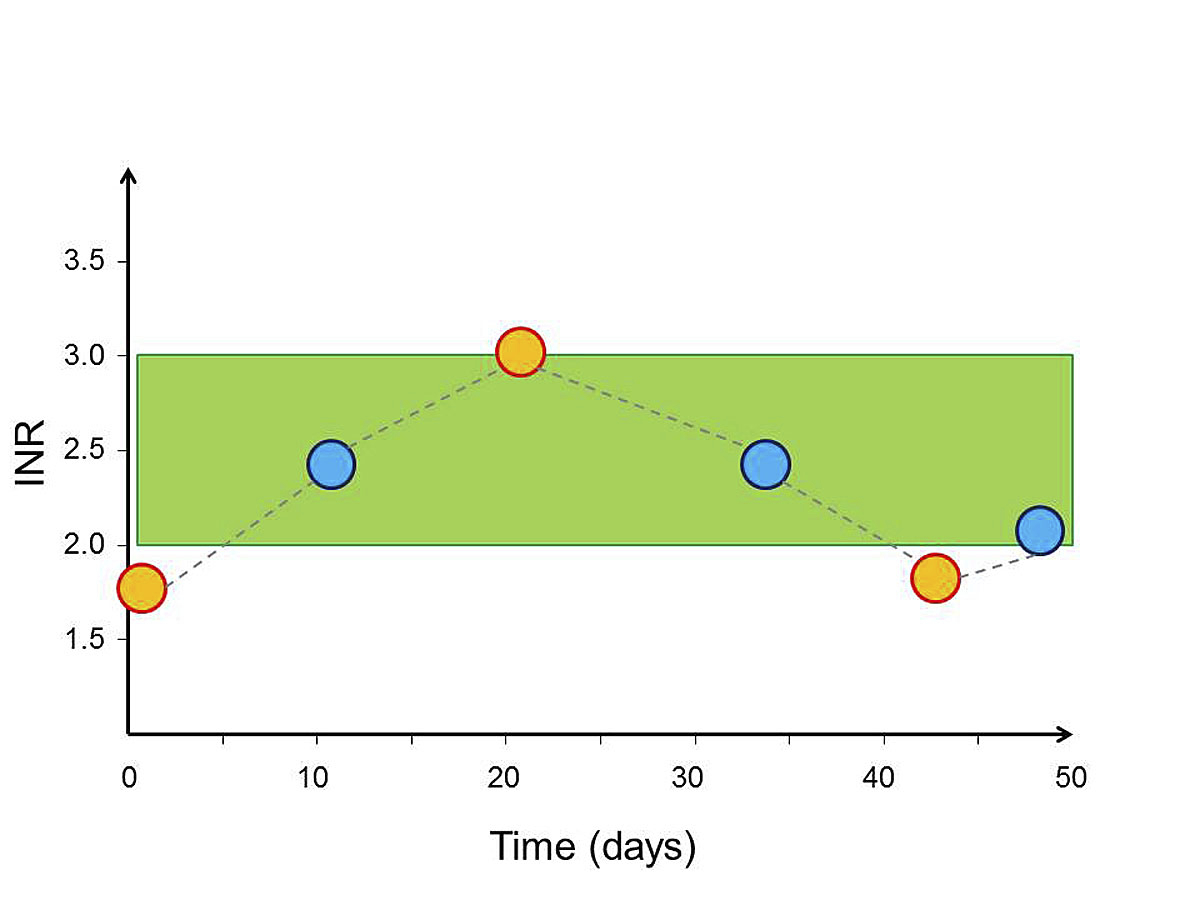
The present study included a limited number of patients. Still, the results represent novel and clinically relevant information. Although AF is very common, and a large number of patients in Switzerland have been on VKA therapy for years, the quality of this treatment has never been systematically assessed, most likely because of lack of alternatives to VKA and the idea that there is little room for improvement. However, this has changed a great deal in recent years. The NOACs are available, they are increasingly prescribed [16], and they have received a prominent role in guidelines [1]. In addition, cost-effectiveness analyses [17, 18], including a Swiss model [19], have suggested that NOACs may be cost-effective compared with VKAs. Furthermore, data on TTR from several countries and healthcare systems and from clinical trials and registries have become available [2–5, 8–10, 20]. Thus, an analysis of VKA quality in Switzerland was clearly needed. The gut feeling of many Swiss doctors about VKA quality in Switzerland, based on impressions from INR booklets, has been only moderately optimistic (personal communication, M.T. Maeder). The present analysis highlights, however, that simply counting INR values in the therapeutic range of 2 to 3 can be misleading and that calculating TTR is indeed helpful in appreciating VKA treatment quality (fig. 2).
Recently, the results of the German thrombEVAL cohort study were published, which included patients with phenprocoumon OAC for a variety of indications including AF, venous thrombosis and prosthetic heart valves (n = 2771). The TTR in this registry was 66% for patients in “regular medical care”, which is in line with the present findings [20]. In patients managed by a specialised coagulation service, an even higher TTR of 76% was achieved [20]. It has also been shown that patients performing INR self-testing and self-management achieve a high TTR. The present findings and those from the significantly larger German registry suggest that approximately 60% VKA-treated AF patients fulfil the minimal TTR requirement of 65%. The German registry [20] and the Swedish registry [6] also make clear that there is still room for improvement in INR control. On the other hand, there are a significant proportion of VKA-treated patients who are outside the TTR range for whom a benefit from VKA compared with no treatment can be expected [15]. In contrast, the data suggest that in some patients VKA therapy may even cause harm. Thus, these data may therefore represent a novel basis for decision-making regarding OAC type in Switzerland.
Attempts have been made to find ways to predict which patients will be able to achieve a good OAC stability when using a VKA. Recently, the SAMe-TT2R2 score has been developed and validated as a tool to identify patients with good INR stability during VKA therapy [13]. However, the score has been tested in warfarin-treated cohorts, and we found no significant correlation between the SAMe-TT2R2 score and TTR or the number of INR values in the therapeutic range. We found only scarce predictors of a high TTR and/or a high number of INR values in the therapeutic range, which is also in line with previous observations [21]. The analysis revealed an association between amiodarone use and poor OAC control. This interaction between amiodarone and VKA therapy is well known [21], and the replication of this finding in the present dataset increases the plausibility of the results. In addition, worse INR stability was found in spironolactone users compared with patients not on spironolactone. The mechanisms of this association are not entirely clear but may be related to the diuretic effect of spironolactone with subsequent haemoconcentration and an increase in the concentration of clotting factors [22], and also to unmeasured confounding factors such as heart failure symptoms [21] or poor hypertension control. The better quality of VKA anticoagulation in patients with less frequent INR measurements is most likely explained by the fact that patients with better anticoagulation quality have fewer INR measurements rather than the opposite.
The relatively high TTR in the present cross-sectional study compared with the TTR in clinical trials using warfarin is somewhat surprising. Pharmacokinetic differences between warfarin and phenprocoumon may have played a role, in particular the longer half-life of phenprocoumon compared with warfarin [12].
The present study has a number of limitations. First, because the number of patients was relatively small, and based on certain inclusion criteria (e.g., availability of the INR booklet) and limited resources for the recruitment of patients, a selection bias is possible. Second, the observation period was relatively short. A recent study has shown that patients with a stable INR in the therapeutic range over a certain period of time will not necessarily maintain this good INR control in the coming months [23]. Thus, TTR needs to be assessed repeatedly during long-term VKA therapy to make sure that treatment is effective and safe. Third, patient compliance would have been a key variable in the present study setting. Unfortunately, there was no information on compliance. And finally, our study was performed in one single institution in Eastern Switzerland, and thus findings cannot automatically be applied for entire Switzerland.
When putting this all together the data can be interpreted as follows: approximately 60% of patients on VKA for AF seem to fulfil the requirement for the minimal TTR of 65%. If this TTR can be achieved during a longer-term period, these patients can remain on VKA, depending on patient and GP preference, if we follow the guidelines. Importantly, these VKA-treated patients may also derive “collateral benefits” [24] from their visits in the GP’s office to check the INR, i.e., short be regular interactions with the GP or his or her assistant which will allow discovery of major changes in health status. On the other hand, there are approximately 40% of patients who are not achieving a sufficient INR control the reasons for which remain speculative but may belong to two main categories: a “difficult patient” (interactions and other biological factors precluding good INR stability) or a non-compliant patient. The proportion of non-compliant patients is very hard to estimate. Given that there were no patients with a constantly non-therapeutic INR value around of 1.0 (no VKA effect) but that both supratherapeutic (≈10%) and subtherapeutic (≈16%) values contributed to the INR values outside the therapeutic range, there were probably very few patients who did not take VKA therapy at all. Still, non-compliance with the prescribed VKA doses remains a possibility. We think that the majority of patients not achieving a sufficient INR were “difficult patients”, i.e., polymorbid and polymedicated patients. Thus, there seem to be up-to 40% of VKA-treated patients who are likely to benefit from a switch from VKA to NOAC.
Conclusions
The quality of VKA therapy for patients with AF in Switzerland is highly variable. Approximately 60% of patients on VKA for AF seem to fulfil the requirement for the minimal TTR of 65%. On the other hand, there are approximately 40% of patients who are not achieving a sufficient INR control and in whom the best method to prevent thromboembolism and bleeding needs to be reconsidered. These patients seem to be candidates for a switch from VKA to NOAC except for the patients in whom a poor compliance is suspected. Thus, the present data may therefore represent a novel basis for decision making regarding OAC type in Switzerland.
Acknowledgements
The excellent help of with the data collection by Johannes Rigger, Sebastian Kopp, Flurina Arquint, Johannes Blumer, Marc Häfliger, René Vollenbroich, and Sebastian Rogowski is greatly appreciated.
Author contributions
MTM and TK equal contribution and joint first authorship
References
1
Kirchhof
P
,
Benussi
S
,
Kotecha
D
,
Ahlsson
A
,
Atar
D
,
Casadei
B
, et al.
2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS.
Eur Heart J.
2016;37(38):2893-962. https://doi.org/10.1093/eurheartj/ehw210
2
Connolly
SJ
,
Ezekowitz
MD
,
Yusuf
S
,
Eikelboom
J
,
Oldgren
J
,
Parekh
A
, et al.
Dabigatran versus warfarin in patients with atrial fibrillation.
N Engl J Med.
2009;361(12):1139-51. https://doi.org/10.1056/NEJMoa0905561
3
Patel
MR
,
Mahaffey
KW
,
Garg
J
,
Pan
G
,
Singer
DE
,
Hacke
W
, et al.
Rivaroxaban versus warfarin in nonvalvular atrial fibrillation.
N Engl J Med.
2011;365(10):883-91. https://doi.org/10.1056/NEJMoa1009638
4
Granger
CB
,
Alexander
JH
,
McMurray
JJ
,
Lopes
RD
,
Hylek
EM
,
Hanna
M
, et al.
Apixaban versus warfarin in patients with atrial fibrillation.
N Engl J Med.
2011;365(11):981-92. https://doi.org/10.1056/NEJMoa1107039
5
Giugliano
RP
,
Ruff
CT
,
Braunwald
E
,
Murphy
SA
,
Wiviott
SD
,
Halperin
JL
, et al.
Edoxaban versus warfarin in patients with atrial fibrillation.
N Engl J Med.
2013;369(22):2093-104. https://doi.org/10.1056/NEJMoa1310907
6
Wieloch
M
,
Sjalander
A
,
Frykman
V
,
Rosenqvist
M
,
Eriksson
N
,
Svensson
PJ
. Anticoagulation control in Sweden: reports of time in therapeutic range, major bleeding, and thrombo-embolic complications from the national quality registry AuriculA.
Eur Heart J.
2011;32(18):2282-9. https://doi.org/10.1093/eurheartj/ehr134
7
Sjogren
V
,
Grzymala-Lubanski
B
,
Renlund
H
,
Friberg
L
,
Lip
GY
,
Svensson
PJ
, et al.
Safety and efficacy of well managed warfarin. A report from the Swedish quality register Auricula.
Thromb Haemost.
2015;113(6):1370-7. https://doi.org/10.1160/TH14-10-0859
8
Dlott
JS
,
George
RA
,
Huang
X
,
Odeh
M
,
Kaufman
HW
,
Ansell
J
, et al.
National assessment of warfarin anticoagulation therapy for stroke prevention in atrial fibrillation.
Circulation.
2014;129(13):1407-14. https://doi.org/10.1161/CIRCULATIONAHA.113.002601
9
Baker
WL
,
Cios
DA
,
Sander
SD
,
Coleman
CI
. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States.
J Manag Care Pharm.
2009;15(3):244-52. https://doi.org/10.18553/jmcp.2009.15.3.244
10
Ansell
J
,
Hollowell
J
,
Pengo
V
,
Martinez-Brotons
F
,
Caro
J
,
Drouet
L
. Descriptive analysis of the process and quality of oral anticoagulation management in real-life practice in patients with chronic non-valvular atrial fibrillation: the international study of anticoagulation management (ISAM).
J Thromb Thrombolysis.
2007;23(2):83-91. https://doi.org/10.1007/s11239-006-9022-7
11
Mearns
ES
,
Kohn
CG
,
Song
JS
,
Hawthorne
J
,
Meng
J
,
White
CM
, et al.
Meta-analysis to assess the quality of international normalized ratio control and associated outcomes in venous thromboembolism patients.
Thromb Res.
2014;134(2):310-9. https://doi.org/10.1016/j.thromres.2014.05.035
12Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G, et al. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-88S.
13
Apostolakis
S
,
Sullivan
RM
,
Olshansky
B
,
Lip
GY
. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe-TT(2)R(2) score.
Chest.
2013;144(5):1555-63. https://doi.org/10.1378/chest.13-0054
14
Rosendaal
FR
,
Cannegieter
SC
,
van der Meer
FJ
,
Briet
E
. A method to determine the optimal intensity of oral anticoagulant therapy.
Thromb and Haemost.
1993;69(3):236-9.
15
Gallagher
AM
,
Setakis
E
,
Plumb
JM
,
Clemens
A
,
van Staa
TP
. Risks of stroke and mortality associated with suboptimal anticoagulation in atrial fibrillation patients.
Thromb Haemost.
2011;106(5):968-77. https://doi.org/10.1160/TH11-05-0353
16
Weitz
JI
,
Semchuk
W
,
Turpie
AG
,
Fisher
WD
,
Kong
C
,
Ciaccia
A
, et al.
Trends in Prescribing Oral Anticoagulants in Canada, 2008-2014.
Clin Ther.
2015;37(11):2506. https://doi.org/10.1016/j.clinthera.2015.09.008
17
Freeman
JV
,
Zhu
RP
,
Owens
DK
,
Garber
AM
,
Hutton
DW
,
Go
AS
, et al.
Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation.
Ann Inter Med.
2011;154(1):1-11. https://doi.org/10.7326/0003-4819-154-1-201101040-00289
18
Harrington
AR
,
Armstrong
EP
,
Nolan
PE, Jr
.,
Malone
DC
. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation.
Stroke.
2013;44(6):1676-81. https://doi.org/10.1161/STROKEAHA.111.000402
19
Pletscher
M
,
Plessow
R
,
Eichler
K
,
Wieser
S
. Cost-effectiveness of dabigatran for stroke prevention in atrial fibrillation in Switzerland.
Swiss Med Wkly.
2013;143:w13732.
20
Prochaska
JH
,
Gobel
S
,
Keller
K
,
Coldewey
M
,
Ullmann
A
,
Lamparter
H
, et al.
Quality of oral anticoagulation with phenprocoumon in regular medical care and its potential for improvement in a telemedicine-based coagulation service--results from the prospective, multi-center, observational cohort study thrombEVAL.
BMC Med.
2015;13:14. https://doi.org/10.1186/s12916-015-0268-9
21
Schein
JR
,
White
CM
,
Nelson
WW
,
Kluger
J
,
Mearns
ES
,
Coleman
CI
. Vitamin K antagonist use: evidence of the difficulty of achieving and maintaining target INR range and subsequent consequences.
Thromb J.
2016;14:14. https://doi.org/10.1186/s12959-016-0088-y
22
O'Reilly
RA
. Spironolactone and warfarin interaction.
Clin Pharmacol Ther.
1980;27(2):198-201. https://doi.org/10.1038/clpt.1980.31
23
Pokorney
SD
,
Simon
DN
,
Thomas
L
,
Gersh
BJ
,
Hylek
EM
,
Piccini
JP
, et al.
Stability of International Normalized Ratios in Patients Taking Long-term Warfarin Therapy.
JAMA. 2016;316(6):661-3. https://doi.org/10.1001/jama.2016.9356
24
Beer
JH
. Vitamin K antagonists are hard to beat by the price - are they? Some answers, new questions and the GPs dilemma.
Swiss Med Wkly.
2013;143:w13739.