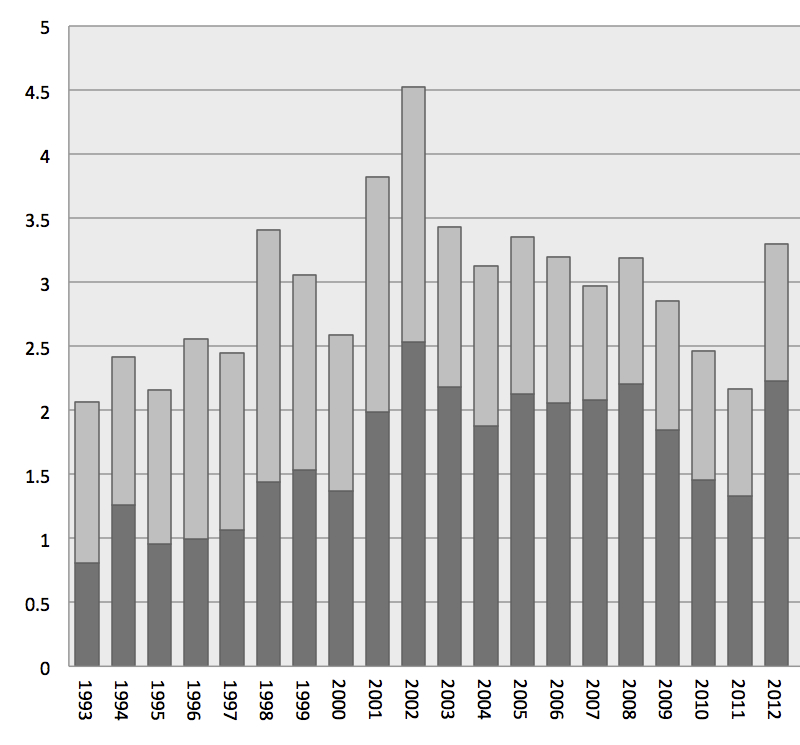
Figure 1 Incidence of MSSA bloodstream infection per 10 000 patient-days from 1993 to 2013.
CA = community acquired; MSSA = methicillin-sensitive Staphylococcus aureus; NO = nosocomial
DOI: https://doi.org/10.4414/smw.2017.14508
More than a century after its discovery, Staphylococcus aureus is still one of the most important causes of infections in both inpatient and outpatient settings worldwide [1–4]. The burden of disease, and especially the mortality of S. aureus bloodstream infection (SA BSI), remain high despite advances in supportive care and improved treatment options over the recent years [5, 6]. Age, presence of comorbidities, shock and the source of SA BSI are strong predictors of outcome. The impact of other host factors remains largely unclear [5]. Interestingly, even though hospital- and community-acquired cases differ in their characteristics [7–9], evidence does not indicate worse outcomes for either of the two groups [5, 10].
The incidence of SA BSI has been rising during the last decades [2, 11]. This is, at least partly, explained by the emergence of methicillin-resistant S. aureus (MRSA) [6, 12]. But the more frequent use of intravascular devices, larger numbers of immunocompromised patients and an increased number of surgical procedures are also responsible for this phenomenon [2, 4, 13]. There is evidence that the epidemiology of methicillin-susceptible S. aureus (MSSA) has also changed over recent decades. In particular, there seems to be a steady increase of community-acquired cases of SA BSI in the recent years, similar to that seen in MRSA [1, 6, 8]. MRSA has been thought to be associated with a much higher mortality than MSSA [14, 15]; however, this difference may possibly be biased, as prior studies may not have taken possible confounders into account [16].
Most studies evaluating mortality and risk factors for SA BSI include MSSA and MRSA cases [5]. The high prevalence of MRSA in many countries precludes analyses regarding MSSA BSI.
Our institution reports a very low prevalence of MRSA [17, 18], similarly to acute-care hospitals in Scandinavian countries and the Netherlands. Given this unique setting, we have the opportunity to report from an almost homogenous MSSA cohort. We performed a prospective cohort study to analyse incidence and mortality of MSSA BSI occurring at the University Hospital Basel, Switzerland, over the last 20 years.
The University Hospital Basel is an 855-bed primary- and tertiary-care centre for adult patients, with >34 000 admissions per year, located in the north-western region of Switzerland, a few kilometres from the borders with France and Germany. It follows the “search and destroy” policy for MRSA, and has reported a very low prevalence of MRSA [17].
We established a prospective cohort of SA BSI at the University Hospital Basel. Between January 1993 and December 2013, all consecutive patients with SA BSI were included in the study.
All patients older than 16 years with at least one positive blood culture for S. aureus were included. Positive blood cultures with isolation of S. aureus within 7 days were defined as one single episode of SA BSI. Patients with MRSA bloodstream infection were excluded.
A detailed description of methodology and data acquisition has been published elsewhere [17, 19, 20]. In brief, microbiology laboratory bloodstream infection reports were the key source of data: a daily report was sent to the Infection Control Department and data about the episodes were registered in a case report form and then transferred to a database (MS Excel). Additional sources of data were medical charts, microbiology reports and pathology reports available in the Patient Management IT program (IsMED) of University Hospital Basel, or original files if needed. Until 1999, when electronic patient records were implemented, paper charts were photocopied and stored.
We carried out an analysis of basic demographics, basic comorbidities and underlying immunocompromising conditions, predictors for severity of disease (time in hospital, intensive care unit [ICU] stay, central line, septic shock, etc.), origin of the SA BSI (nosocomial vs community-acquired), as well as source and mortality. We stratified the cohort into four equal 5-year periods (5 years being a common timeframe for analyses) and compared for trends.
MSSA BSI was defined as at least one positive blood culture with S. aureus and associated systemic inflammatory response syndrome [21, 22].
Nosocomial SA BSI was defined according to Centers for Disease Control and Prevention (CDC) criteria [23].
Secondary SA BSI was defined as infection originating from an established focus, identified by the treating clinician. Primary BSI was defined as BSI without a documented source of infection [23].
Blood cultures were performed by use of the BacT/ALERT blood culture system (bioMérieux, USA). S. aureus was identified with standard methods including evaluation of typical growth, Gram staining, catalase production and detection of clumping factor, protein A and capsular antigens (Pastorex Staph-Plus; Bio-Rad, France) [24]. Since 2012, matrix-assisted laser desorption / ionisation-time of flight (MALDI-TOF) mass spectrometry (MALDI Biotyper, Bruker Daltonik, Germany) was used for identification. If results were equivocal, additional analyses were performed, such as aurease (Rapidec Staph, bioMérieux, France) or sequencing of the 16S rRNA gene. Susceptibility testing was done according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS) and from June 2011 of the European Committee on Antimicrobial Susceptibility Testing (EUCAST). Phenotypic identification of MRSA or suspicious results were confirmed by detection of penicillin-binding protein 2a (MRSA-Screen, Denka Seiken, Japan), followed by PCR for mecA and femA genes.
The objectives of this study were to evaluate and demonstrate trends in incidence and mortality of MSSA BSI over the last 20 years in a setting with low MRSA incidence, as well as to establish risk factors for mortality.
The χ2 or Fisher’s exact test were used for analyses of categorical variables and the Kruskal-Wallis test was applied for analyses of continuous variables. Incidence was expressed per 10 000 patient-days and trends during the observation period were analysed using linear regression.
Uni- and multivariate Cox-regression models were performed to identify variables associated with mortality. Two separate models were used in order to avoid confounding by codependent variables. A third multivariate model was created solely to correct for confounders between year groups and mortality.
Kaplan-Meier survival-plots and the log-rank test were used to compare survival distributions between the different time periods. Mortality was expressed as in-hospital, all-cause mortality. A p-value of <0.05 was considered significant for all calculations.
All analyses were performed using SPSS version 22 software (SPSS, IBM, Inc., Chicago, IL).
During the 20-year study period, 540 669 blood samples were cultured. The number of blood cultures drawn increased annually from 17 201 in 1993 to 38 029 in 2013 in absolute numbers and from 865 to 1568 per 10 000 patient-days [17].
In total, we identified 1370 episodes of SA BSI in 1239 patients. MRSA accounted for 34 episodes in 30 patients and were excluded from the analysis.
Baseline characteristics were stratified in four 5-year periods (table 1). Overall, 41% of the cases were nosocomial, with a significant decrease over time. One fifth of the patients was immunocompromised, took intravenous drugs, or had diabetes mellitus.
Table 1 Baseline characteristics of 1328 patients with MSSA BSI over 20 years, overall and stratified.
| Variable | Overall | 1993–1997 | 1998–2002 | 2003–2007 | 2008–2013 | p for trend | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n/mean | %/SD/IQR | n/mean | %/SD/IQR | n/mean | %/SD/IQR | n/mean | %/SD/IQR | n/mean | %/SD/IQR | ||
| Mean age at onset (y) | 59 | SD: 18 | 59 | SD: 18 | 57 | SD: 19 | 59 | SD: 18 | 62 | SD: 18 | 0.02 |
| Male | 869 | 65% | 145 | 65% | 217 | 67% | 234 | 65% | 273 | 65% | 0.9 |
| Median time in hospital (d) | 24 | IQR: 14–38 | 28 | IQR: 17–46 | 26 | IQR: 13–40 | 22 | IQR: 11–34 | 22 | IQR: 15–36 | 0.001 |
| ICU stay | 394 | 30% | 85 | 38% | 58 | 18% | 134 | 37% | 117 | 28% | <0.001 |
| Median time in ICU (d) | 3 | IQR: 2–6 | 3 | IQR: 2–6 | 3 | IQR: 1–9 | 4 | 10B: 2–9 | 2 | IQR: 1–4 | |
| Intubation | 209 | 16% | 31 | 14% | 40 | 12% | 95 | 27% | 43 | 10% | <0.001 |
| Nosocomial BSI | 545 | 41% | 126 | 57% | 157 | 49% | 128 | 36% | 134 | 32% | <0.001 |
| Comorbidities | |||||||||||
| Diabetes mellitus | 278 | 21% | 49 | 22% | 52 | 16% | 82 | 23% | 95 | 23% | 0.1 |
| IVDU | 254 | 19% | 20 | 9% | 56 | 17% | 96 | 27% | 82 | 19% | <0.001 |
| Alcohol | 130 | 10% | 40 | 18% | 21 | 7% | 46 | 13% | 23 | 5% | <0.001 |
| Immunosuppression | 272 | 20% | 18 | 8% | 37 | 11% | 44 | 12% | 173 | 41% | <0.001 |
| HIV | 67 | 5% | 16 | 7% | 20 | 6% | 21 | 6% | 10 | 2% | 0.02 |
| Number of patients with: | |||||||||||
| Central line | 637 | 48% | 76 | 34% | 230 | 71% | 225 | 63% | 106 | 25% | <0.001 |
| Surgery in past 30 days | 332 | 25% | 74 | 33% | 73 | 23% | 92 | 26% | 93 | 22% | 0.03 |
| Median duration of antibiotic therapy (d) | 19 | IQR: 13–38 | 19 | IQR: 1242 | 16 | IQR: 11–31 | 19 | IQR: 12–34 | 28 | IQR: 1543 | <0.001 |
| Median number of pos. blood cultures | 4 | IQR: 2–5 | 4 | IQR: 2–6 | 4 | IQR: 2–5 | 4 | IQR: 2–6 | 4 | IQR: 2–5 | 0.1 |
| Focus | |||||||||||
| Primary (w/o catheter) | 234 | 18% | 53 | 24% | 77 | 24% | 67 | 19% | 37 | 9% | <0.001 |
| Catheter–associated | 268 | 20% | 61 | 27% | 72 | 22% | 71 | 20% | 64 | 15% | 0.002 |
| Surgical-site infection | 161 | 12% | 31 | 14% | 68 | 21% | 27 | 8% | 35 | 8% | <0.001 |
| Endocarditis | 150 | 11% | 24 | 11% | 35 | 11% | 47 | 13% | 44 | 10% | 0.6 |
| Skin & soft-tissue | 138 | 10% | NA | NA | 20 | 6% | 33 | 9% | 85 | 20% | <0.001 |
| Respiratory | 101 | 8% | 19 | 9% | 23 | 7% | 27 | 8% | 32 | 8% | 0.9 |
| Bones and joints | 94 | 7% | 16 | 7% | 11 | 3% | 29 | 8% | 38 | 9% | 0.02 |
| Urogenital | 29 | 2% | 6 | 3% | 7 | 2% | 5 | 1% | 11 | 3% | |
| Orthopaedic implant | 27 | 2% | NA | NA | 4 | 1% | 5 | 1% | 18 | 4% | |
| Vascular implant/device | 19 | 1% | NA | NA | 1 | <1% | 6 | 2% | 12 | 3% | |
| Other | 106 | 8% | 13 | 6% | 6 | 2% | 40 | 11% | 47 | 11% | |
| Septic shock | 65 | 5% | 14 | 6% | 17 | 5% | 18 | 5% | 18 | 4% | 0.7 |
| In-hospital mortality | 258 | 19% | 45 | 20% | 62 | 19% | 63 | 18% | 88 | 21% | 0.5 |
BSI = bloodstream infection; CI = confidence interval; IQR = interquartile range; ICU = intensive care unit; IVDU = intravenous drug use; HIV = human immunodeficiency virus; SD = standard deviation
The main sources for MSSA BSI were, in descending order: catheter-associated infections (20%), primary/unknown (18%) focus, surgical-site infections (12%), endocarditis (11%), and skin and soft-tissue infections (10%). Stratified comparison of infection sources over time showed a steady decline of surgical-site infections and primary infections with unknown source, and a slight decrease of catheter-associated infections, whereas endocarditis remained unchanged. Skin and soft-tissue infections, on the other hand, showed a steady increase over time.
The overall incidence of MSSA BSI per 10 000 patient-days per year varied between 2.1 and 4.5 and did not change significantly during the observation period (p = 0.2). However, there was a highly significant increase in the incidence of community-acquired MSSA BSI from 0.8 to 2.9 per 10 000 patient-days (p <0.001), with a similarly significant decrease in incidence of nosocomial MSSA BSI (p = 0.005; fig. 1).

Figure 1 Incidence of MSSA bloodstream infection per 10 000 patient-days from 1993 to 2013.
CA = community acquired; MSSA = methicillin-sensitive Staphylococcus aureus; NO = nosocomial
All-cause in-hospital mortality was 19%. In the multivariate analysis of demographic factors, we identified age, ICU referral, immunosuppression, alcohol and septic shock at presentation as risk factors for mortality, whereas nosocomial infection and surgery prior to onset of BSI were protective (table 2a). In the multivariate analysis of infection sources, we identified intravascular catheter-associated sources, surgical-site infections, bones and joints, and mainly respiratory focus as risk factors for mortality (Table 2b).
Table 2 a: Uni- and multivariate analysis of demographic factors for mortality.
| Variables | Survivors | Nonsurvivors | Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %/SD | n | %/SD | HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Mean age (y) | 58 | SD 19 | 66 | SD 16 | 1 | 1.01–1.03 | <0.001 | 1. | 1.02–1.04 | <3.001 | |
| Nosocomial | 440 | 42% | 103 | 40% | 0.7 | 0.5–0.9 | 0.004 | 0.7 | 0.5–0.9 | 0.005 | |
| ICU referral | 291 | 28% | 103 | 40% | 1. | 1.1–1.8 | 0.005 | 2 | 1.5–2.6 | <0.001 | |
| Surgery in past 30 days | 289 | 28% | 43 | 17% | 0.5 | 0.4–0.7 | <0.001 | 0.4 | 0.3–0.6 | <0.001 | |
| Comorbidities | IVDU | 220 | 21% | 28 | 11% | 0.6 | 0.4–0.9 | 0.01 | 1. | 0.7–1.9 | 0.7 |
| Diabetes | 204 | 19% | 69 | 27% | 1. | 1.0–1.7 | 0.05 | ||||
| Immunosuppression | 190 | 18% | 81 | 31% | 2. | 1.3–2.2 | <0.001 | 2. | 1.1–1.9 | 0.006 | |
| HIV | 59 | 6% | 8 | 3% | 0.7 | 0.3–1.3 | 0.26 | ||||
| Alcohol | 94 | 9% | 35 | 14% | 2. | 1.1–2.2 | 0.02 | 2. | 1.03–2.2 | 0.03 | |
| Septic shock | 31 | 3% | 36 | 14% | 4. | 2.7–5.5 | <0.001 | 3. | 1.9–3.9 | <0.001 | |
CI = confidence interval; ICU = intensive care unit; IVDU = Intravenous drug use; HIV = human immunodeficiency virus; HR = hazard ratio; SD = standard deviation Significance level p <0.05
Table 2b Uni- and multivariate analysis of infection foci for mortality.
| Variables | Survivors | Nonsurvivors | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | %/SD | n | %/SD | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Focus primary (w/o catheter) | 165 | 16% | 56 | 22% | 2. | 1.2–2.1 | 0.002 | 1. | 1.01–1.9 | 0.05 |
| Catheter-associated | 232 | 22% | 36 | 14% | 0.6 | 0.4–0.9 | 0.004 | 0.6 | 0.4–0.9 | 0.02 |
| Surgical site infection | 143 | 14% | 18 | 7% | 0.5 | 0.3–0.8 | 0.002 | 0.5 | 0.3–0.8 | 0.004 |
| Endocarditis | 115 | 11% | 32 | 12% | 0.9 | 0.6–1.3 | 0.6 | |||
| Skin & soft-tissue | 112 | 11% | 24 | 9% | 1 | 0.6–1.5 | 0.9 | |||
| Respiratory | 60 | 6% | 41 | 16% | 3. | 2.1–4.1 | <0.001 | 3. | 1.7–3.6 | <0.001 |
| Bones & joints | 83 | 8% | 11 | 4% | 0.5 | 0.3–0.9 | 0.02 | 0.5 | 0.3–0.9 | 0.03 |
| Urogenital | 23 | 2% | 6 | 2% | 1. | 0.6–2.8 | 0.6 | |||
| Orthopaedic implant | 25 | 2% | 1 | 0.4% | 0.2 | 0.03–1.4 | 0.1 | |||
| Vascular implant/device | 14 | 1% | 5 | 2% | 1. | 0.4–2.6 | 0.9 | |||
| Other | 78 | 7% | 28 | 11% | NA | NA | NA | |||
CI = confidence interval; HR = hazard ratio; SD = standard deviation Significance level p <0.05
Mortality rates did not change significantly during the observation period, when the 5-year intervals (p = 0.1, fig. 2) were compared, and remained nonsignificant, despite correction for possible confounders in a separate multivariate model.
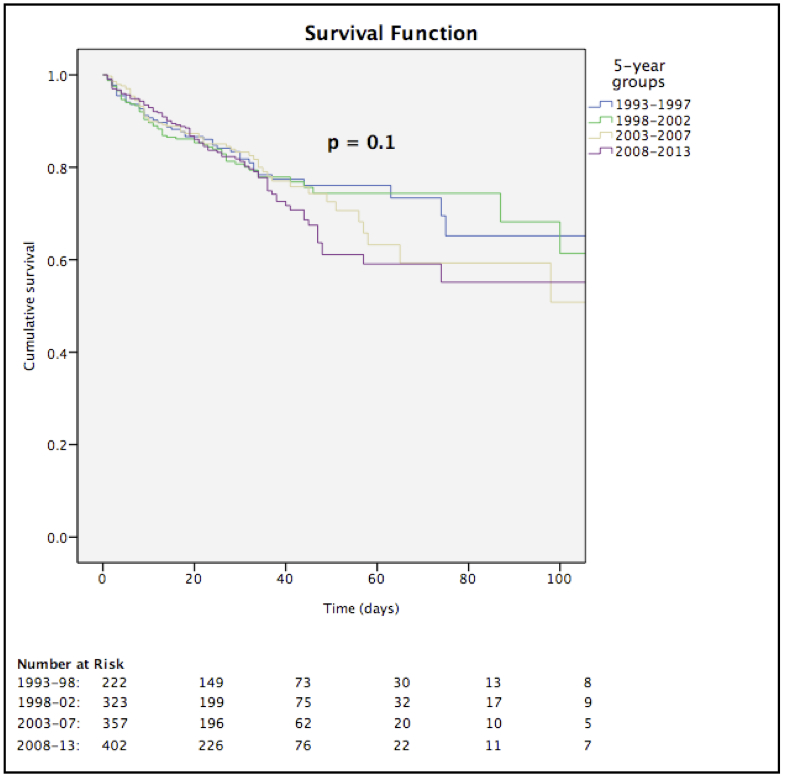
Figure 2 Five-year stratification of all-cause in-hospital mortality.
In this large observational study, mortality related to MSSA BSI remained high and unchanged over the last 20 years. Improvements of medical care during the last two decades did not result in lower mortality and, therefore, new approaches are required to lower mortality in MSSA BSI. The incidence of community-acquired cases increased significantly.
In our cohort, we observed a mean total incidence of three MSSA BSIs per 10 000 patient-days, with no significant change over the study period. This is in contrast to a report of the European Antimicrobial Resistance Surveillance Network examining BSIs in 22 European countries, which showed a highly significant increase in the incidence of SA BSI [11]. It has to be noted that this latter report involved many countries where MRSA is endemic, which most likely explains the increasing incidence, as a growing body of evidence points to MRSA adding to the burden of disease of MSSA, rather than replacing it [12]. Another large multinational study showed no increase in overall incidence of BSI [1], supporting our results in a low MRSA incidence setting.
Our finding of a significant increase in community-acquired MSSA BSI is mirrored by several other studies [1, 25–27]. The reasons for this epidemiological shift remain elusive. The increasing availability of outpatient treatment options and virulence factors associated with community-acquired strains may be possible explanations [28]. The formation of the Infection Control Department at our institution in 1993, with the resulting implementation and improvement of several infection control measures over the last two decades [17, 26] may possibly explain the concomitant decrease in nosocomial MSSA BSIs observed over the study period.
Overall, all-cause mortality was 19% in our cohort. This is in line with previous publications [5]. We identified age, ICU referral, immunosuppression, alcohol consumption, septic shock at presentation, catheters as underlying source, and skin and soft-tissue, bone and joint, and respiratory tract foci as risk factors for mortality. Most of these predictors of mortality are confirmed by other studies [5].
Strikingly, mortality did not improve over the last two decades of our study period, despite important advances being made in medical care during the same time. Several other studies have come to similar conclusions [27, 29, 30]. Since the pre-antibiotic era, mortality for BSI has improved dramatically, but for the last couple of decades this trend has come to a standstill.
The changing demographics of an ageing population with a larger number of comorbid conditions in combination with established risk factors could counterbalance the critical advances in medical care. However, after correcting for such confounders by multivariable analyses, mortality remained unchanged over time. Therefore, novel approaches for the management of SA BSI are needed. Several comprehensive reviews report on the lack of good quality evidence required for guidance of BSI management and point to several key questions, such as the role of echocardiography, duration of antibiotic therapy, or administration of combination therapy, that remain unanswered to date [21, 27].
Our study has several limitations. This is an observational study from a single centre, possibly limiting generalisability. However, the infectious diseases consultations (IDC) service did not change over time, the immediate reporting of the laboratory essentially remained the same as was the antimicrobial regimen with flucloxacillin. Reporting of positive blood cultures is changing from individual phone calls to automatic electronic reports, but not all hospitals can rely on such infrastructure. In addition, an observational study – in particular over a long-time period – cannot exclude confounding variables that we did not detect and control for. The laboratory methods essentially remained the same. The laboratory techniques did change from the US standards to the European standards, but these changes did not influence the results: the sensitivity to detect MRSA has increased by switching from oxacillin to cefoxitin. However, very few cases would have been missed with the low prevalence of MRSA at this institution.
As Laupland et al. have pointed out, it is important to establish the incidence of infectious diseases in order to define the respective burden. It is, however, equally important to exercise care in generalising results about the epidemiology of a selected population or single region, since there are a number of factors influencing the incidence of SA BSI in a given population [1]. As a result of the lack of follow-up data after discharge, we only were able to analyse in-hospital mortality and did not consider long-term outcomes. Further, we analysed only all-cause mortality, which may overestimate attributable mortality.
In summary, mortality related to MSSA BSI remained high and unchanged over the last 20 years. The incidence of community-acquired cases increased significantly. Improvements of medical care during the last 20 years did not result in lower mortality calling for reassessment of current treatment strategies to reduce mortality of MSSA BSI. As for the moment, rapid appropriate antibiotic treatment may be the most effective measure to improve survival, being standard of care for nowadays treatment of sepsis [31].
We acknowledge the contribution of the Infection Control team of University Hospital Basel to this work.
No financial support was provided relevant to this article.
On behalf of all authors, the corresponding author states that there is no conflict of interest.
1 Laupland KB , Lyytikäinen O , Søgaard M , Kennedy KJ , Knudsen JD , Ostergaard C , et al., International Bacteremia Surveillance Collaborative. The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect. 2013;19(5):465–71. doi:.https://doi.org/10.1111/j.1469-0691.2012.03903.x
2 Naber CK . Staphylococcus aureus bacteremia: epidemiology, pathophysiology, and management strategies. Clin Infect Dis. 2009;48(s4, Suppl 4):S231–7. doi:.https://doi.org/10.1086/598189
3 Biedenbach DJ , Moet GJ , Jones RN . Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY Antimicrobial Surveillance Program (1997-2002). Diagn Microbiol Infect Dis. 2004;50(1):59–69. doi:.https://doi.org/10.1016/j.diagmicrobio.2004.05.003
4 Wisplinghoff H , Bischoff T , Tallent SM , Seifert H , Wenzel RP , Edmond MB . Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–17. doi:.https://doi.org/10.1086/421946
5 van Hal SJ , Jensen SO , Vaska VL , Espedido BA , Paterson DL , Gosbell IB . Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev. 2012;25(2):362–86. doi:.https://doi.org/10.1128/CMR.05022-11
6 Filice GA , Nyman JA , Lexau C , Lees CH , Bockstedt LA , Como-Sabetti K , et al. Excess costs and utilization associated with methicillin resistance for patients with Staphylococcus aureus infection. Infect Control Hosp Epidemiol. 2010;31(4):365–73. doi:.https://doi.org/10.1086/651094
7 Lenz R , Leal JR , Church DL , Gregson DB , Ross T , Laupland KB . The distinct category of healthcare associated bloodstream infections. BMC Infect Dis. 2012;12(1):85. doi:.https://doi.org/10.1186/1471-2334-12-85
8 Wang JL , Chen SY , Wang JT , Wu GH , Chiang WC , Hsueh PR , et al. Comparison of both clinical features and mortality risk associated with bacteremia due to community-acquired methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus. Clin Infect Dis. 2008;46(6):799–806. doi:.https://doi.org/10.1086/527389
9 Cunney RJ , McNamara EB , alAnsari N , Smyth EG . Community and hospital acquired Staphylococcus aureus septicaemia: 115 cases from a Dublin teaching hospital. J Infect. 1996;33(1):11–3.https://doi.org/10.1016/S0163-4453(96)92643-2
10 Retamar P , López-Prieto MD , Nátera C , de Cueto M , Nuño E , Herrero M , et al.; Sociedad Andaluza de Enfermedades Infecciosas/Sociedad Andaluza de Microbiología y Parasitología Clínica and Red Española de Investigación en Enfermedades Infecciosas (SAEI/SAMPAC/REIPI) Bacteremia Group. Reappraisal of the outcome of healthcare-associated and community-acquired bacteramia: a prospective cohort study. BMC Infect Dis. 2013;13(1):344. doi:.https://doi.org/10.1186/1471-2334-13-344
11 Gagliotti C , Balode A , Baquero F , Degener J , Grundmann H , Gür D , et al.; EARS-Net Participants (Disease Specific Contact Points for AMR). Escherichia coli and Staphylococcus aureus: bad news and good news from the European Antimicrobial Resistance Surveillance Network (EARS-Net, formerly EARSS), 2002 to 2009. Euro Surveill. 2011;16(11):19819.
12 Mostofsky E , Lipsitch M , Regev-Yochay G . Is methicillin-resistant Staphylococcus aureus replacing methicillin-susceptible S. aureus? J Antimicrob Chemother. 2011;66(10):2199–214. doi:.https://doi.org/10.1093/jac/dkr278
13 Bassetti M , Trecarichi EM , Mesini A , Spanu T , Giacobbe DR , Rossi M , et al. Risk factors and mortality of healthcare-associated and community-acquired Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2012;18(9):862–9. doi:.https://doi.org/10.1111/j.1469-0691.2011.03679.x
14 Cosgrove SE , Sakoulas G , Perencevich EN , Schwaber MJ , Karchmer AW , Carmeli Y . Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36(1):53–9. doi:.https://doi.org/10.1086/345476
15 Wolkewitz M , Frank U , Philips G , Schumacher M , Davey P ; BURDEN Study Group. Mortality associated with in-hospital bacteraemia caused by Staphylococcus aureus: a multistate analysis with follow-up beyond hospital discharge. J Antimicrob Chemother. 2011;66(2):381–6. doi:.https://doi.org/10.1093/jac/dkq424
16 Yaw LK , Robinson JO , Ho KM . A comparison of long-term outcomes after meticillin-resistant and meticillin-sensitive Staphylococcus aureus bacteraemia: an observational cohort study. Lancet Infect Dis. 2014;14(10):967–75. doi:.https://doi.org/10.1016/S1473-3099(14)70876-X
17 Widmer AF , Lakatos B , Frei R . Strict infection control leads to low incidence of methicillin-resistant Staphylococcus aureus bloodstream infection over 20 years. Infect Control Hosp Epidemiol. 2015;36(6):702–9. doi:.https://doi.org/10.1017/ice.2015.28
18 Blanc DS , Pittet D , Ruef C , Widmer AF , Mühlemann K , Petignat C , et al. Epidemiology of methicillin-resistant Staphylococcus aureus: results of a nation-wide survey in Switzerland. Swiss Med Wkly. 2002;132(17-18):223–9.
19 Laffer RR , Frei R , Widmer AF . [Epidemiology of septicemias in a university hospital over 5 years]. Schweiz Med Wochenschr. 2000;130(41):1471–8. Article in German.
20 Kaech C , Elzi L , Sendi P , Frei R , Laifer G , Bassetti S , et al. Course and outcome of Staphylococcus aureus bacteraemia: a retrospective analysis of 308 episodes in a Swiss tertiary-care centre. Clin Microbiol Infect. 2006;12(4):345–52. doi:.https://doi.org/10.1111/j.1469-0691.2005.01359.x
21 Thwaites GE , Edgeworth JD , Gkrania-Klotsas E , Kirby A , Tilley R , Török ME , et al.; UK Clinical Infection Research Group. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis. 2011;11(3):208–22. doi:.https://doi.org/10.1016/S1473-3099(10)70285-1
22 Elzi L , Babouee B , Vögeli N , Laffer R , Dangel M , Frei R , et al. How to discriminate contamination from bloodstream infection due to coagulase-negative staphylococci: a prospective study with 654 patients. Clin Microbiol Infect. 2012;18(9):E355–61. doi:.https://doi.org/10.1111/j.1469-0691.2012.03964.x
23 Horan TC , Andrus M , Dudeck MA . CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–32. doi:.https://doi.org/10.1016/j.ajic.2008.03.002
24Versalovic J, American Society for Microbiology. Manual of clinical microbiology. 10th ed. Washington, DC: ASM Press; 2011.
25 Benfield T , Espersen F , Frimodt-Møller N , Jensen AG , Larsen AR , Pallesen LV , et al. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect. 2007;13(3):257–63. doi:.https://doi.org/10.1111/j.1469-0691.2006.01589.x
26 Asgeirsson H , Gudlaugsson O , Kristinsson KG , Heiddal S , Kristjansson M . Staphylococcus aureus bacteraemia in Iceland, 1995-2008: changing incidence and mortality. Clin Microbiol Infect. 2011;17(4):513–8. doi:.https://doi.org/10.1111/j.1469-0691.2010.03265.x
27 Tong SY , Davis JS , Eichenberger E , Holland TL , Fowler VG, Jr . Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603–61. doi:.https://doi.org/10.1128/CMR.00134-14
28 David MZ , Daum RS . Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616–87. doi:.https://doi.org/10.1128/CMR.00081-09
29 Paulsen J , Mehl A , Askim Å , Solligård E , Åsvold BO , Damås JK . Epidemiology and outcome of Staphylococcus aureus bloodstream infection and sepsis in a Norwegian county 1996-2011: an observational study. BMC Infect Dis. 2015;15(1):116. doi:.https://doi.org/10.1186/s12879-015-0849-4
30 Lyytikäinen O , Ruotsalainen E , Järvinen A , Valtonen V , Ruutu P . Trends and outcome of nosocomial and community-acquired bloodstream infections due to Staphylococcus aureus in Finland, 1995-2001. Eur J Clin Microbiol Infect Dis. 2005;24(6):399–404. doi:.https://doi.org/10.1007/s10096-005-1345-3
31 Seymour CW , Gesten F , Prescott HC , Friedrich ME , Iwashyna TJ , Phillips GS , et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235–44. doi:.https://doi.org/10.1056/NEJMoa1703058
All authors have contributed significantly to the work. Benedikt Wiggli and Andreas Widmer had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
No financial support was provided relevant to this article.
On behalf of all authors, the corresponding author states that there is no conflict of interest.