Challenges of congenital heart disease in grown-up patients
DOI: https://doi.org/10.4414/smw.2017.14495
Markus
Schwerzmanna, Fabienne
Schwitza, Corina
Thometa, Alexander
Kadnerb, Jean-Pierre
Pfammatterc, Kerstin
Wustmanna
aGrown-up Congenital Heart Disease, Centre for Congenital Heart Disease, University Hospital Inselspital, University of Bern, Switzerland
bCentre for Congenital Heart Disease, Department for Cardiovascular Surgery, University Hospital Inselspital, University of Bern, Switzerland
cPaediatric Cardiology, Centre for Congenital Heart Disease, University Hospital Inselspital, University of Bern, Switzerland
Summary
Nowadays, more than 90% of all children born with congenital heart disease (CHD) reach adult life. Although initially considered to be cured, the majority of them continue to need specialised follow-up because they require re-do interventions or are at increased risk of cardiovascular complications and premature death. Arrhythmias are the most common cause of unscheduled hospital visits for grown-up CHD (GUCH) patients, accounting for one third of emergency admissions in these patients. Some GUCH patients are also at increased risk for sudden cardiac death. The principles of arrhythmia management and the prevention of sudden cardiac death in GUCH patients are similar to those used in adults with acquired heart disease, but are not evidence based. Decompensated heart failure is the other leading cause of death. Conventional medical heart-failure therapy for left ventricular dysfunction is not effective in GUCH patients at highest risk of heart failure, i.e., those with right or single ventricular failure. Careful haemodynamic assessment and structural interventions are the first step to consider in GUCH patients presenting with heart failure symptoms. Adults with moderate or complex CHD and regular follow-up in specialised GUCH centres have a survival benefit compared with patients without such follow-up. Cardiac surgery in GUCH patients should be performed by surgeons trained in treatment of CHD, i.e., surgeons also operating on paediatric patients. A structured transition programme with a defined transfer of care from the paediatric to the adult care environment is important to avoid lapses of care in today’s adolescents with CHD. For GUCH patients with an intervention performed decades ago and no specific cardiac follow-up in later life, referral to a specialised GUCH centre is recommended and may save lives.
Introduction
Cardiac defects are the most common birth defect and affect approximately 0.8% of newborns [1]. In Switzerland, every year approximately 700 children are born with congenital heart disease (CHD). Half of these children have simple defects (e.g., a ventricular septal defect), the other half have moderately complex (e.g., coarctation of the aorta) or complex (e.g., transposition of the great arteries) defects. Many of the simple defects close spontaneously during the first years of life, but eventually 200 to 300 of the infants with CHD will require an intervention.
Nowadays, more than 90% of children with repaired CHD survive into adulthood [2]. The majority of them enter adult life with the need for a lifelong specialised follow-up. In the current era, the number of grown-up CHD (GUCH) patients has outgrown the paediatric CHD cohort: two out of three CHD patients are adults [3]. With further reductions in mortality resulting from advances in diagnostic methods, cardiac surgery, catheter interventions and electrophysiology, the prevalence of GUCH patients will continue to increase. It is estimated that currently 2.5 million adults with CHD are living in Europe, of whom 20 000 in Switzerland [4]. The following review article highlights historical milestones in CHD and the resulting challenges GUCH patients have to face during their lifetime.
Historical background
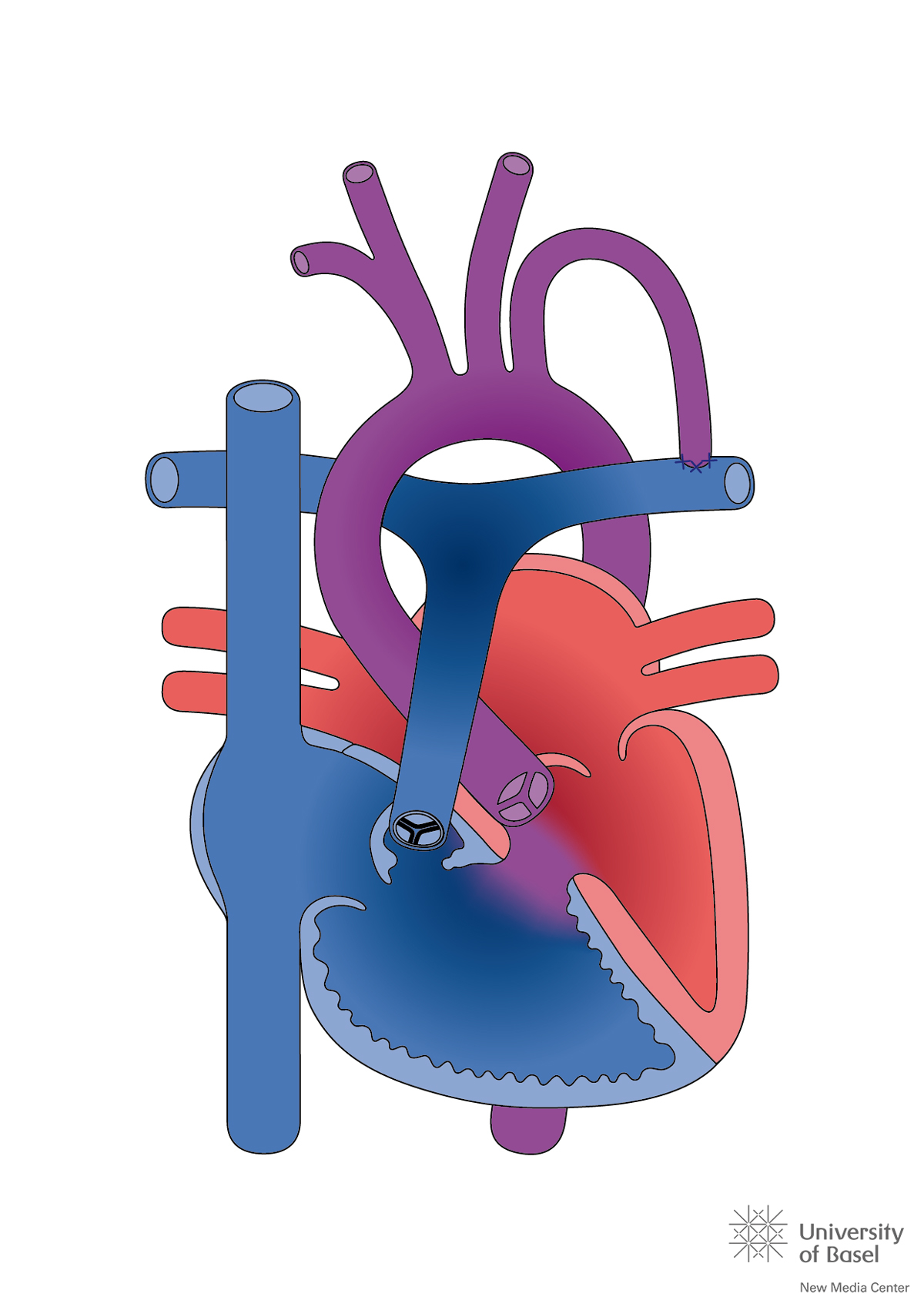
The medical success story of CHD was strongly related to pioneering work in cardiac surgery, and started with a palliation procedure. In 1945, the John Hopkins’ surgeon Alfred Blalock performed a shunt operation in a 15-month-old child with cyanotic heart disease and reduced pulmonary blood flow. He sutured the left subclavian artery end-to-side on the left pulmonary artery (fig. 1), with the aim to increase pulmonary blood flow without the risk of inducing shunt-related pulmonary hypertension. This procedure, later called the Blalock-Taussig shunt, reduced CHD mortality from 23 to 17% in older cyanotic children [5]. In 1952, palliative procedures were succeeded by intracardiac surgery. James Lewis closed an atrial septal defect in a 5-year-old girl, using a special clamp technique to avoid exsanguination of the beating heart. In 1955, John W. Kirklin at the Mayo Clinic in Rochester reported the successful use of a heart-lung machine and heralded the era of open-heart surgery with use of extracorporeal circulation. However, the reported mortality for intracardiac correction of tetralogy of Fallot, the most common cyanotic defect, was 39% with the heart-lung machine, substantially higher than the reported 17% mortality after palliation with a Blalock-Taussig shunt [6]. The policy was adopted to initially palliate complex defects by a shunt or banding procedure with the aim to gain time and let the infants and their cardiovascular structures grow, and thereby delay the repair of the cardiac defect into early childhood (secondary repair).
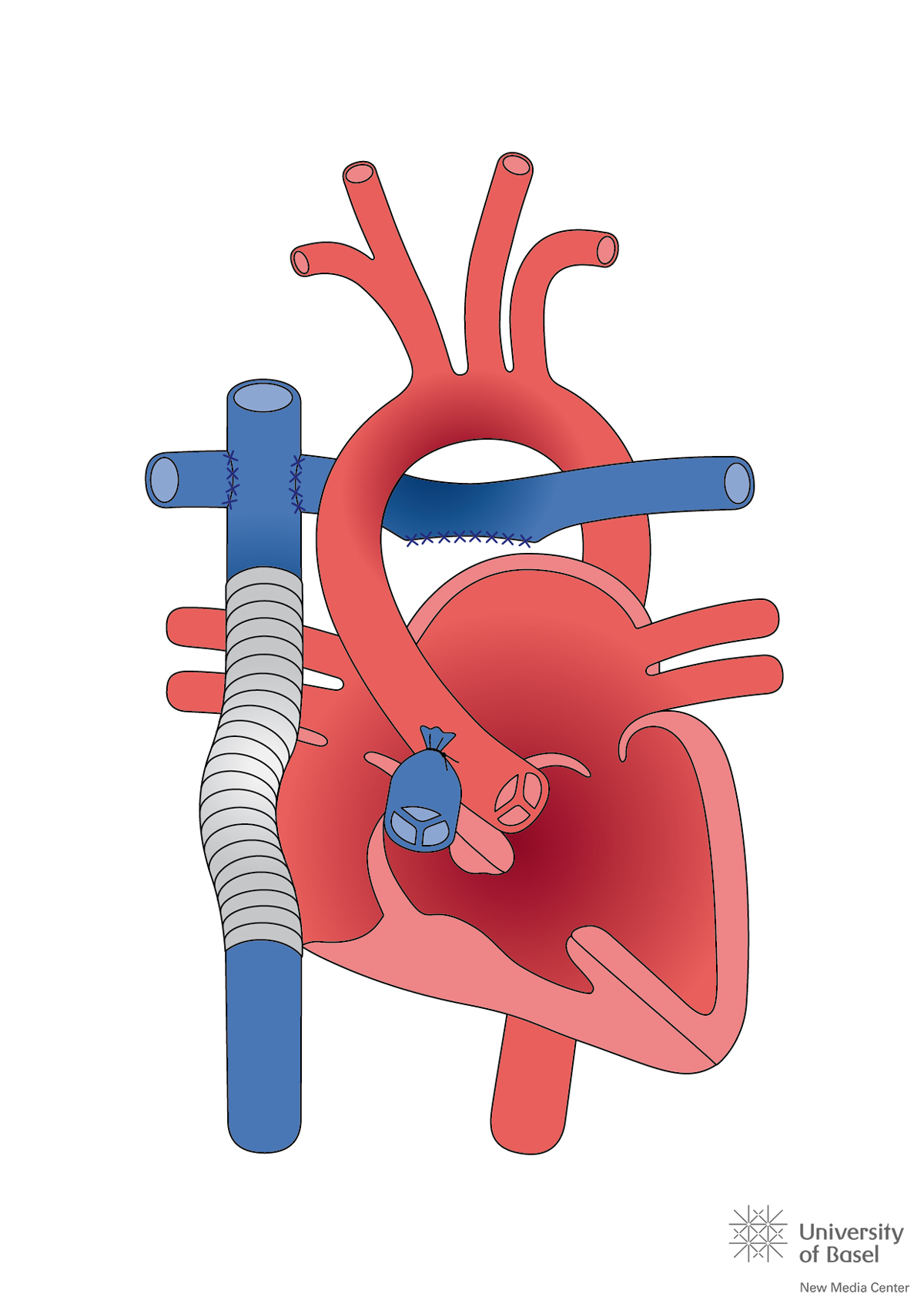
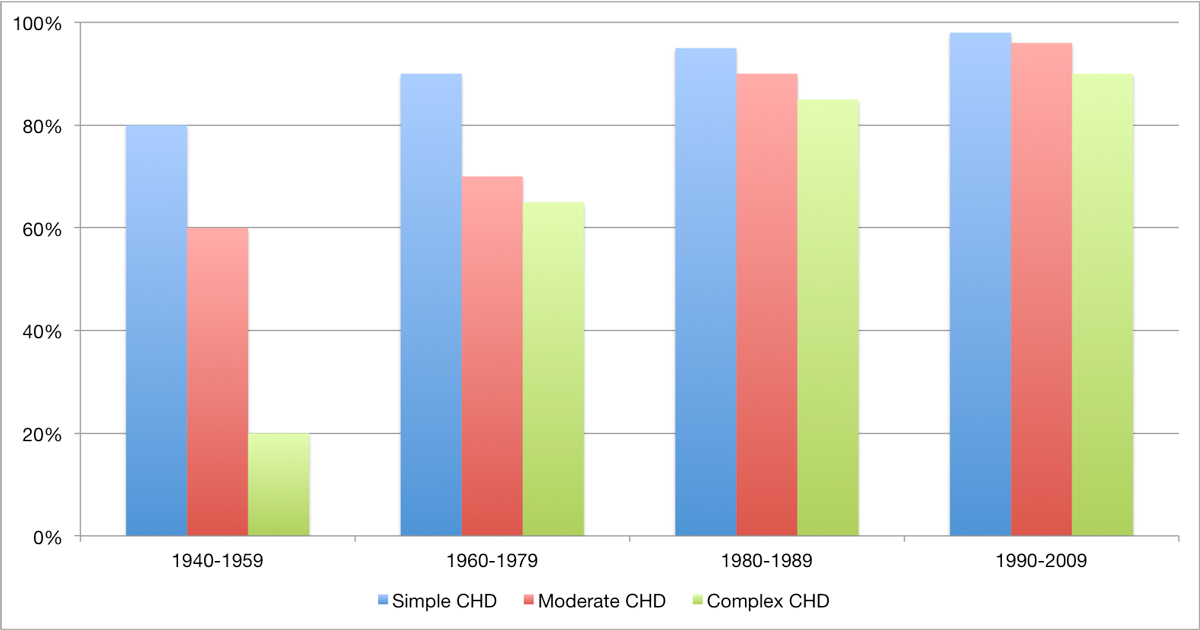
During the 1970s, a new intraoperative strategy was established, consisting of cooling the patient to about 20°C on the heart-lung machine, with subsequent cardiac arrest during the repair operation, followed by rewarming prior to weaning from the bypass. With this strategy, correction of almost all cardiac anomalies was progressively mastered. Further advances in surgical techniques and perioperative care allowed a stepwise abandonment of secondary repair strategies for primary repair, without the need for an initial palliation procedure. Even the most complex lesions, such as a heart with a single ventricle due to hypoplasia of one of the ventricles, can nowadays be approached (fig. 2) [7]. Contemporary mortality in the first year of life for children with non-severe CHD is now 2 to 4%, with an operative mortality of 0 to 2%. For children with severe CHD, the corresponding mortality is 8 to 13%, with an operative mortality of 4 to 6% [8]. Figure 3 depicts the remarkable increase in survival rates with CHD over the past 70 years.
Repaired does not mean cured
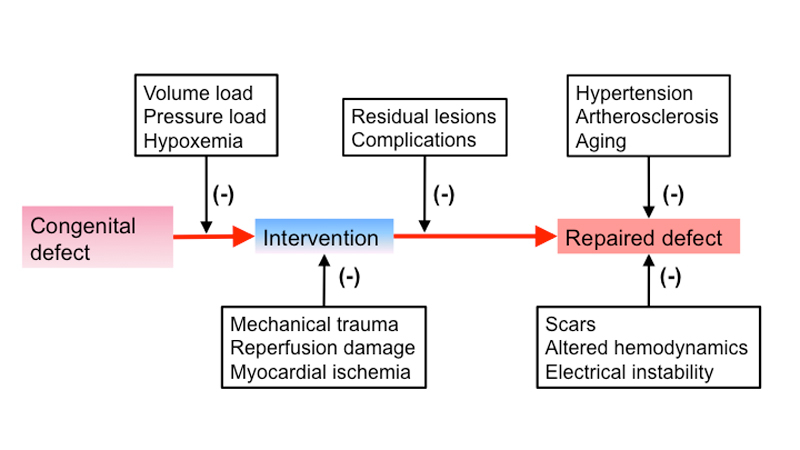
Although originally assumed to be curative, it was realised over time that most interventions to treat CHD allowed these children to grow up without severe physical limitations, but with an – initially unexpected – increased long-term morbidity and mortality, manifesting once these patients were in the midst of their adult life [10]. Figure 4 shows some of the factors contributing to the cardiovascular burden observed in GUCH patients. Table 1 lists common heart defects and the expected complications in adult life.
Table 1 Late complications in common congenital cardiac defects.
|
Congenital heart defect type
|
Possible complications / sequelae
|
|
1. Simple defects
|
| Isolated small or repaired ventricular septal defect |
Arrhythmia, endocarditis |
| Small or repaired secundum atrial septal defect |
Arrhythmia |
| Mild pulmonary stenosis |
Endocarditis |
| Bicuspid aortic valve |
Endocarditis, ascending aortic dilation, valvular dysfunction |
|
2. Moderately complex defects
|
| Atrioventricular septal defects (native or repaired) |
Arrhythmia, atrioventricular block, endocarditis, heart failure, pulmonary hypertension |
| Large unrepaired secundum atrial septal defect or sinus venosus defect |
Arrhythmia, pulmonary hypertension, heart failure |
| Aortic coarctation (native or repaired) |
Systemic hypertension, local pseudoaneurysm or re-stenosis, cerebral aneurysm |
| Unrepaired anomalous pulmonary venous drainage |
Arrhythmia, pulmonary hypertension, heart failure |
| Repaired sinus venosus defect |
Arrhythmia, pulmonary hypertension |
| Ebstein’s anomaly (native or repaired) |
Arrhythmia, heart failure, endocarditis |
| Repaired tetralogy of Fallot |
Arrhythmia, heart failure, endocarditis |
|
3. Complex defects
|
|
| Implanted conduits (e.g., RV-PA conduit) |
Arrhythmia, endocarditis, heart failure |
| Repaired truncus arteriosus |
Arrhythmia, endocarditis, heart failure, pulmonary hypertension |
| Transposition of the great arteries |
|
| – After atrial switch procedure |
Arrhythmia, sinus node dysfunction, heart failure, pulmonary hypertension |
| – After aterial switch procedure |
Ostial coronary occlusion, right ventricular outflow tract obstruction, ascending aorta dilatation, heart failure, |
| – Congenitally corrected transposition |
Arrhythmia, atrioventricular block, heart failure |
| Cyanotic heart condition, including Eisenmenger syndrome |
Arrhythmia, heart failure, endocarditis, paradoxical embolism / stroke, pulmonary hypertension, gout, renal failure |
| Single ventricle after Fontan procedure |
Heart failure, arrhythmia, liver disease, protein loosing enteropathy, varicosis |
The increased morbidity and mortality risk is reflected by a steadily growing number of re-hospitalisations within the adult CHD population, out of proportion to the pure increase in the population size over time [11]. Padrutt et al. recently presented numbers for GUCH patients followed up at the University Hospital Zurich: from 2005 to 2015, the number of adults under specialised care doubled, and the frequency of patients requiring hospital admission (e.g., for cardioversion) increased from 3.1 to 5.4% per year, a relative increase of 75% [12].
The main reasons for hospital admissions in adult CHD patients are supraventricular arrhythmias, lesion-specific surgery or percutaneous interventions, decompensated heart failure, ventricular tachycardias, the need for a pacemaker, an intracardiac defibrillator (ICD) or other electrophysiology procedures, pregnancy or delivery, evaluation and treatment for pulmonary hypertension, infective endocarditis and perioperative care for noncardiac surgery [13, 14]. In addition, within the aging CHD population, acquired morbidities (e.g., coronary artery disease, renal failure, diabetes, liver dysfunction, endocrinological and haematological disorders or cancer) are becoming important outcome factors in conjunction with the underlying congenital heart defect and add to the medical burden of these patients [15]. Some of today’s adult patients also face complications related to limited diagnostic possibilities in the past. Screening blood products for the presence of hepatitis C virus became routine only in 1992. Adults with congenital heart surgery who received blood transfusions before 1992 have a 5- to 20-fold increased prevalence of hepatitis C infection [16].
Specialised care saves lives
Based on experience and outcome data, specialised care by trained cardiologists of GUCH patients has been endorsed and recommended by different national and international societies within the last 20 years [4, 17, 18]. Minimal standards of care for GUCH patients in Switzerland are listed in table 2.
Table 2 Minimal standards of care for adolescents and adults with congenital heart disease.
|
Transition
|
– Transition process begins in teenage years
– Structured transfer from paediatric to adult care centre |
|
Regular follow-up
|
– Every non-simple repaired or unrepaired defect should be presented once in a GUCH centre
– Individualised but lifelong regular follow-up is mandatory for all non-simple lesions |
|
Pregnancy
|
– Prepregnancy counselling for all women with CHD
– High-risk pregnancy managed by a multidisciplinary team at GUCH centre |
|
Cardiac interventions
|
– Expertise of a congenital cardiac surgeon or congenital invasive cardiologist mandatory |
|
Non-cardiac surgery
|
– Cyanotic patients, patients with a systemic right ventricle or with single ventricle physiology need referral to specialised centre for even minor surgery |
| CHD = congenital heart disease; GUCH = grown-up CHD patients |
Mylotte et al. observed a decline in GUCH mortality in a province-wide database of Québec, Canada after the publication of the first Canadian GUCH guidelines in 1997, which emphasised the importance of specialised care for adults with moderate or complex cardiac defects [19]. In a case-control analysis, referral to a specialised GUCH centre was associated with lower risk of death (odds ratio [OR] 0.82, 95% confidence interval [CI] 0.68–0.97), independent of comorbidities, age and gender. Survival improvement was highest for patients with complex defects. Several explanations are provided: the level of training and experience of the healthcare providers in GUCH centres, a higher likelihood of use of practice guidelines and therefore less variability in care, the maximised use of medical advances, and the availability of congenital cardiac surgeons. There is growing evidence that substantially lower mortality rates (1.8 vs 4.8%) are achieved when a congenital heart surgery team is operating on GUCH patients, i.e., a cardiac surgeon who also operates on children, and not a “general” cardiac surgeon [20]. Currently, the University Hospitals of Lausanne/Geneva, Bern and Zurich provide the optimal surgical environment to operate on GUCH patients in Switzerland. Cardiac surgery in GUCH patients is characterised by a wide anatomical-clinical spectrum, frequent multiorgan involvement, and the absence of evidence-based data [21]. In contrast to cardiac surgery for acquired heart disease, there is no validated surgical risk score for GUCH patients [22]. All these factors enforce the need for meticulous preoperative planning and preparation of surgical interventions in GUCH patients, by a multidisciplinary team with experience in CHD.
Transition and patient education
CHD patients and their families need to be aware of the importance of uninterrupted specialised care. This requires education and a structured transition and transfer process in adolescence [23]. Studies on transfer destinations have shown that without a structured transition, up to 75% of adolescents with CHD are lost to follow-up once they reached adult life [24]. This has important implications: adults with CHD and a lapse of care are more likely to be symptomatic at the time they are readmitted to a hospital (OR 2.5, 95% CI 1–6), are more likely to have new haemodynamic problems or an additional cardiac diagnosis (OR 9.6, 95% CI 4–23), and have a 3-fold (95% CI 2–7) greater likelihood of requiring urgent cardiac interventions [25]. Multiple efforts are necessary to keep patients on board [26]. Protective and risk factors for care gaps in GUCH patients are presented in table 3. Organised transition programmes providing education on the heart defect, on healthy lifestyle, and coping and self-management strategies are emphasised, [27]. A transition coordinator is recommended to facilitate the transition process, and nurses are predestined for this position. Fifteen to 60 min educational sessions led by nurses have shown to increase patient’s CHD knowledge [28, 29].
Table 3 Predictors of care gaps in congenital heart disease patients.
|
Protective factors
|
Risk factors
|
– Higher family income
–
1 comorbid condition
– Having a written referral to a specialist GUCH centre
– Keeping first and second GUCH appointment
– Patient’s belief that follow-up should be performed at specialised clinic
– No substance use
– Compliance with antibiotic prophylaxis
– Greater independence in attending appointments |
– Living independently
– Male gender
– Lower family income
– Travel distance to GUCH care
– Fewer outpatient visits in paediatric care
– The last visit taking place outside a university hospital
– Childhood hospitalisations
– History of at least one missed appointment
– Mild disease severity
– No previous heart surgery |
| GUCH = grown-up congenital heart disease patients |
Common cardiovascular problems in GUCH patients
The need for lifelong medical follow-up is related to the common cardiovascular complications seen in in GUCH patients.
Arrhythmias and stroke
More than half of all GUCH patients with complex CHD will develop arrhythmias by the age 65 years [30]. Arrhythmias are the most common cause of unscheduled hospital visits in adults with CHD and account for one third of all emergency admissions in this population [31]. All types of cardiac arrhythmias are prevalent, ranging from clinically occult arrhythmias, such as silent atrial fibrillation, to potentially fatal ventricular tachycardia. Table 4 illustrates the frequency of arrhythmia disturbances in different congenital defects. Residual anatomic or haemodynamic lesions and surgical scars from atrio- or ventriculotomies form the substrate for arrhythmias or dyssynchrony [32]. GUCH patients are routinely advised to consult their primary physician in the event of persistent palpitations or a sudden decrease in exercise capacity, in order to rule out arrhythmias with a routine 12-lead ECG or Holter ECG.
Table 4 Frequency of rhythm problems in GUCH patients.
|
Cardiac defect
|
SVT
|
VT
|
SND
|
AVB
|
DYS
|
| Ventricular septal defect |
|
|
|
|
|
| Secundum type atrial septal defect |
|
|
|
|
|
| Primum type atrial septal defect |
|
|
|
|
|
| Aortic coarctation |
|
|
|
|
|
| Ebstein’s anomaly |
|
|
|
|
|
| Tetralogy of Fallot |
|
|
|
|
|
| TGA with an atrial switch procedure |
|
|
|
|
|
| Single ventricle after Fontan palliation |
|
|
|
|
|
A particular challenge in CHD patients with supraventricular arrhythmias is the decision when to start oral anticoagulation. Atrial fibrillation and intra-atrial re-entry tachycardia are the most common arrhythmias, and are associated with thrombosis and embolic events [33]. Among GUCH patients, the stroke incidence is 9 to 12 times higher than in the general population, particularly in younger adults (below age 55 years). Important predictors of ischaemic stroke, besides the absence of sinus rhythm, are the severity of the congenital defect, active infective endocarditis, the presence of endovenous pacemaker leads, heart failure, diabetes mellitus, and a recent history of myocardial infarction [33, 34]. The CHA2DS2-VASc score has proven benefits in identifying patients with nonvalvular atrial fibrillation at increased risk for stroke, but has a limited ability in GUCH patients [35]. Anticoagulation is recommended by experts in all GUCH patients with atrial fibrillation or intra-atrial re-entrant tachycardia and a history of previous intracardiac repair, cyanosis, after a Fontan palliation or in the presence of a systemic right ventricle, independent of the CHA2DS2 VASc score [36].
Sudden cardiac death accounts for 15 to 25% of fatalities in GUCH patients [12, 37]. The incidence of sudden cardiac death is in the range of 1 per 1000 patient-years, and similar to the incidence of sustained ventricular tachycardia in these adults [38]. Intracardiac defibrillators (ICDs) are indicated in CHD adults for secondary prevention after resuscitation from sudden cardiac death and in those with spontaneous sustained ventricular tachycardia, after a careful workup has failed to identify a clear reversible cause. Selecting candidates for primary prevention is challenging. In general, ICDs are recommended in patients with a systemic left ventricular ejection fraction ≤35%, biventricular physiology, and New York Heart Association (NYHA) class II or III symptoms [38]. They may also be considered in GUCH patients with a systemic right ventricular ejection fraction ≤35% and NYHA class III or IV, if additional risk factors are present, such as a history of syncope, or QRS duration >140 ms [39].
Heart failure
Beside sudden cardiac death, heart failure is the other leading cause of death in GUCH patients. Together, they account for nearly half of all late mortality in mixed cohorts of children and adults [40, 41]. Mechanisms for heart failure in CHD are often lesion specific and may involve different pathophysiological pathways (table 5). Recognition of heart failure symptoms can be delayed in GUCH patients because many have lived their entire life with the notion of a limited exercise capacity, do not perceive changes over time and underreport symptoms [42].
Table 5 Pathophysiology of heart failure in grown-up congenital heart disease patients.
|
Mechanism
|
Example
|
| Volume overload |
Long-sustained regurgitation of native or repaired/replaced valves, shunt lesions (residual shunt, late diagnosis) |
| Pressure overload |
Valve or conduit stenosis, coarctation, peripheral pulmonary artery stenosis, systemic or pulmonary hypertension |
| Ventricular anatomy |
Systemic right ventricle, functionally single ventricle |
| Myocardial injury |
Scars, cyanotic heart disease, limited myocardial protection during bypass, myocardial fibrosis |
| Coronary artery supply |
Coronary artery anomalies, coronary artery disease |
| Myocardial architecture |
Ventricular non-compaction |
| Ventricular dyssynchrony |
Complete bundle-branch block after surgery, intractable arrhythmias |
The Dutch CONCOR (CONgenital CORvitia Dutch national Registry) database reported an incidence of heart failure admission in GUCH patients of 1.2 per 1000 patient-years, which was 10 times higher than the incidence in the general population of adults of the same age [43]. GUCH patients admitted for heart failure had a 5-fold higher mortality than CHD patients without heart failure history, and the 1- and 3-year mortality after first heart failure admission were 24% and 35%, respectively. GUCH patients at high risk for heart failure are the ones with cardiac defects affecting the right ventricle, i.e. corrected tetralogy of Fallot, transposition of the great arteries with the right ventricle in systemic position, Ebstein’s anomaly, or with a single ventricle physiology.
Whereas medical heart failure treatment with beta-blockers, angiotensin converting-enzyme inhibitors or angiotensin receptor blockers, and aldosterone antagonists have proven benefits in left ventricular heart failure, the role of these agents has not been established in GUCH patients with right or single ventricular dysfunction [44]. Whereas neurohormonal modulation is the cornerstone of heart failure treatment in classic left heart failure patients and invasive haemodynamic assessment is reserved for patients with refractory heart failure, the reverse is true for most CHD diagnoses: careful haemodynamic assessment and structural interventions are the first step to consider in GUCH patients presenting with cardiac dysfunction [45].
Cardiac resynchronisation therapy has been used in a few CHD patients, but data from small studies in heterogeneous CHD populations could not clearly determine which GUCH patients will benefit, irrespective of anatomical difficulties for implantation [42]. Currently, the only established therapy for end-stage heart failure in CHD patients without pulmonary hypertension is heart transplantation [46]. Although early post-transplant mortality is increased in CHD patients, the 10-year survival is similar to non-CHD transplant recipients [47]. Ventricular assist devices can be used as a bridge to transplant [48]. Given the ubiquitous shortage of donor organs and the number of GUCH patients at risk, further medical options are currently being investigated in adults with a systemic right ventricle [49].
Infective endocarditis
CHD patients are at increased lifetime risk of infective endocarditis [50]. The risk varies with the underlying defect, but on average 1 in 1000 CHD patients suffers an episode of infective endocarditis per year [51]. This incidence is 20 to 30 times higher than the incidence observed in the general population (3–5 cases per 100 000 patients per year). In contemporary GUCH cohorts, the acute mortality of infective endocarditis is still 16%, and 1-year mortality is 19% [52]. Adults with repaired or palliated pulmonary atresia and adults with corrected congenital transposition of the great arteries have the highest incidence of infective endocarditis with 5.8 (95% CI 1.8–9.8) and 2.3 (95% CI 0.5–4.1) cases per 1000 patient-years, followed by adults with a single ventricle, a bicuspid aortic valve, and a ventricular septal defect. The risk is very low in adults with a pulmonary stenosis or an atrial septal defect, and almost non-existent in adults with a repaired or still patent arterial duct. Besides the underlying cardiac anatomy, the presence of prosthetic material also confers a high risk of infective endocarditis. Among 14 000 GUCH patients, biological or mechanical valves were independently associated with infective endocarditis, in the short and long term after implantation (0–6 months: hazard ratio [HR] 17.29, 95% CI 7.34–40.70; 6–12 months: HR 15.91, 95% CI 6.76–37.45; after 12 months: HR 5.26, 95% CI 3.52–7.86), whereas valve repair carried only an increased risk in the first 6 months following valve procedure (HR 3.34, 95% CI 1.33–8.41), but not thereafter [52].
Interestingly, infective endocarditis occurs less frequently in women (odds ratio [OR] 0.5, 95% CI 0.4–0.7), independent of the underlying defect or other risk factors [53]. Evidence supports a relationship between dental hygiene and the risk of developing endocarditis-associated bacteraemia after daily events such as tooth-brushing [54]. A large Swiss population study has shown that men are less likely to practice good dental hygiene than women [55]. It was shown that female gender and better disease knowledge are strongly related to good dental hygiene in GUCH patients [56], reinforcing the concept that patients’ education plays an important role in endocarditis prevention, as well as dental care. According to the current guidelines, optimal oral health is considered more important for infective endocarditis prevention than prophylactic antibiotics for dental procedures [57]. We discourage the empiric use of antibiotic treatments for suspected bacterial infections without evident focus, unless blood-cultures have been obtained prior to starting antibiotic therapy.
GUCH and pregnancy
Many women with CHD are in childbearing age and consider pregnancy [58]. From a cardiac point of view, pregnancy is a prolonged proarrhythmic and prothrombotic state with increased cardiac output and decreased peripheral resistance. The majority of women with CHD, normal biventricular function and no severe outflow tract obstruction tolerate pregnancy well. However, an impaired contractile function (e.g., a systemic right ventricle), a fixed obstruction (e.g., aortic coarctation, mitral stenosis, severe pulmonary hypertension), mechanical valves, aortic dilatation and residual cyanotic heart disease are conditions with an increased risk for maternal cardiovascular complications during pregnancy and delivery [59]. Extremely high risks of maternal mortality or morbidity are encountered in women with pulmonary arterial hypertension of any cause, severe systemic ventricular dysfunction with an ejection fraction <30% or in functional NYHA class III or IV, severe symptomatic mitral or aortic stenosis, native severe coarctation, Marfan syndrome with an ascending aortic diameter >45 mm or a bicuspid aortic valve with an ascending aortic diameter >50 mm, and in women with a history of peripartum cardiomyopathy and residual impairment of left ventricular function. If pregnancy occurs in these situations, termination should be discussed. A multidisciplinary team of cardiologists, obstetricians and anaesthesiologists should monitor CHD patients before and during pregnancy, delivery and the postpartum period. After delivery, cardiac output may take up to 6 months to return to prepregnancy levels. Individual preconception counselling of women with CHD concerning contraception, pregnancy risks and inheritance is prerequisite to an informed decision.
End-of-life discussions and advanced care planning
Death is not predictable in most CHD patients, and heart failure symptoms may manifest late in the disease trajectory, making early end-of-life discussions indispensable. This includes the completion of advanced directives, palliative care and resuscitation options, and identifying an attorney [60]. As in many other disciplines, advanced care planning (ACP) discussions are rarely held with GUCH patients. In a Canadian GUCH centre, out of 200 patients only 2 (1%) recalled having discussed ACP, whereas 24 of 48 (50%) healthcare providers indicated that they discussed life expectancy, ACP and resuscitation preferences regularly with their patients [61]. Patients’ preferences for ACP did not depend on the CHD complexity, but healthcare providers stated that they discussed ACP more often with patients with complex lesions. Similar results were found in a recent survey of an American GUCH centre [62]. Patients favour ACP discussions prior to a life-threatening illness, but the physicians’ focus on life, rather on death, hindered a timely initiation [63]. Adult CHD poses many challenges, not only to patients, but also to their treating physicians.
References
1
Dolk
H
,
Loane
M
,
Garne
E
; European Surveillance of Congenital Anomalies (EUROCAT) Working Group. Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation. 2011;123(8):841–9. doi:.https://doi.org/10.1161/CIRCULATIONAHA.110.958405
2
Moons
P
,
Bovijn
L
,
Budts
W
,
Belmans
A
,
Gewillig
M
. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation. 2010;122(22):2264–72. doi:.https://doi.org/10.1161/CIRCULATIONAHA.110.946343
3
Marelli
AJ
,
Ionescu-Ittu
R
,
Mackie
AS
,
Guo
L
,
Dendukuri
N
,
Kaouache
M
. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130(9):749–56. doi:.https://doi.org/10.1161/CIRCULATIONAHA.113.008396
4
Baumgartner
H
,
Budts
W
,
Chessa
M
,
Deanfield
J
,
Eicken
A
,
Holm
J
, et al.; Working Group on Grown-up Congenital Heart Disease of the European Society of Cardiology. Recommendations for organization of care for adults with congenital heart disease and for training in the subspecialty of ‘Grown-up Congenital Heart Disease’ in Europe: a position paper of the Working Group on Grown-up Congenital Heart Disease of the European Society of Cardiology. Eur Heart J. 2014;35(11):686–90. doi:.https://doi.org/10.1093/eurheartj/eht572
5
Baum
VC
. Pediatric cardiac surgery: an historical appreciation. Paediatr Anaesth. 2006;16(12):1213–25. doi:.https://doi.org/10.1111/j.1460-9592.2006.02029.x
6
The surgical treatment of tetralogy of Fallot. Dis Chest. 1958;34(1):103–9.
7
Fontan
F
,
Baudet
E
. Surgical repair of tricuspid atresia. Thorax. 1971;26(3):240–8. doi:.https://doi.org/10.1136/thx.26.3.240
8
Jortveit
J
,
Øyen
N
,
Leirgul
E
,
Fomina
T
,
Tell
GS
,
Vollset
SE
, et al.
Trends in Mortality of Congenital Heart Defects. Congenit Heart Dis. 2016;11(2):160–8. doi:.https://doi.org/10.1111/chd.12307
9
Warnes
CA
,
Liberthson
R
,
Danielson
GK, Jr
,
Dore
A
,
Harris
L
,
Hoffman
JIE
, et al.
Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37(5):1170–5. doi:.https://doi.org/10.1016/S0735-1097(01)01272-4
10
Warnes
CA
. Adult congenital heart disease: the challenges of a lifetime. Eur Heart J. 2016 Dec 23:ehw529. [Epub ahead of print] doi:.https://doi.org/10.1093/eurheartj/ehw529
11
Opotowsky
AR
,
Siddiqi
OK
,
Webb
GD
. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol. 2009;54(5):460–7. doi:.https://doi.org/10.1016/j.jacc.2009.04.037
12
Padrutt
M
,
Bracher
I
,
Bonassin
F
,
Santos Lopes
B
,
Gruner
C
,
Stämpfli
SF
, et al.
Impact of growing cohorts of adults with con-genital heart disease on clinical workload: a 20-year experience at a tertiary care centre. Swiss Med Wkly. 2017;147:w14443. doi:.https://doi.org/10.4414/smw.2017.14443
13
Cedars
AM
,
Burns
S
,
Novak
EL
,
Amin
AP
. Predictors of Rehospitalization Among Adults With Congenital Heart Disease Are Lesion Specific. Circ Cardiovasc Qual Outcomes. 2016;9(5):566–75. doi:.https://doi.org/10.1161/CIRCOUTCOMES.116.002733
14
Verheugt
CL
,
Uiterwaal
CS
,
van der Velde
ET
,
Meijboom
FJ
,
Pieper
PG
,
Sieswerda
GT
, et al.
The emerging burden of hospital admissions of adults with congenital heart disease. Heart. 2010;96(11):872–8. doi:.https://doi.org/10.1136/hrt.2009.185595
15
Tutarel
O
. Acquired heart conditions in adults with congenital heart disease: a growing problem. Heart. 2014;100(17):1317–21. doi:.https://doi.org/10.1136/heartjnl-2014-305575
16
Wang
A
,
Book
WM
,
McConnell
M
,
Lyle
T
,
Rodby
K
,
Mahle
WT
. Prevalence of hepatitis C infection in adult patients who underwent congenital heart surgery prior to screening in 1992. Am J Cardiol. 2007;100(8):1307–9. doi:.https://doi.org/10.1016/j.amjcard.2007.05.059
17
Warnes
CA
,
Williams
RG
,
Bashore
TM
,
Child
JS
,
Connolly
HM
,
Dearani
JA
, et al.
ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation. 2008;118(23):e714–833. doi:.https://doi.org/10.1161/CIRCULATIONAHA.108.190690
18
Bouchardy
J
,
Greutmann
M
,
Schwerzmann
M
,
Attenhofer Jost
CH
,
De Pasquale
G
,
Oxenius
A
, et al.
Grown-up Congenital Heart Disease (GUCH) Recommendations for Standards of Care. Cardiovasc Med.
2015;18(4):144–5. doi:.https://doi.org/10.4414/cvm.2015.00317
19
Mylotte
D
,
Pilote
L
,
Ionescu-Ittu
R
,
Abrahamowicz
M
,
Khairy
P
,
Therrien
J
, et al.
Specialized adult congenital heart disease care: the impact of policy on mortality. Circulation. 2014;129(18):1804–12. doi:.https://doi.org/10.1161/CIRCULATIONAHA.113.005817
20
Karamlou
T
,
Diggs
BS
,
Person
T
,
Ungerleider
RM
,
Welke
KF
. National practice patterns for management of adult congenital heart disease: operation by pediatric heart surgeons decreases in-hospital death. Circulation. 2008;118(23):2345–52. doi:.https://doi.org/10.1161/CIRCULATIONAHA.108.776963
21
Vouhé
PR
. Adult congenital surgery: current management. Semin Thorac Cardiovasc Surg. 2011;23(3):209–15. doi:.https://doi.org/10.1053/j.semtcvs.2011.09.003
22
Fuller
SM
,
He
X
,
Jacobs
JP
,
Pasquali
SK
,
Gaynor
JW
,
Mascio
CE
, et al.
Estimating Mortality Risk for Adult Congenital Heart Surgery: An Analysis of The Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann Thorac Surg. 2015;100(5):1728–35, discussion 1735–6. doi:.https://doi.org/10.1016/j.athoracsur.2015.07.002
23
Kovacs
AH
,
McCrindle
BW
. So hard to say goodbye: transition from paediatric to adult cardiology care. Nat Rev Cardiol. 2014;11(1):51–62. doi:.https://doi.org/10.1038/nrcardio.2013.172
24
Goossens
E
,
Stephani
I
,
Hilderson
D
,
Gewillig
M
,
Budts
W
,
Van Deyk
K
, et al.; SWITCH(2) Investigators. Transfer of adolescents with congenital heart disease from pediatric cardiology to adult health care: an analysis of transfer destinations. J Am Coll Cardiol. 2011;57(23):2368–74. doi:.https://doi.org/10.1016/j.jacc.2010.11.068
25
Yeung
E
,
Kay
J
,
Roosevelt
GE
,
Brandon
M
,
Yetman
AT
. Lapse of care as a predictor for morbidity in adults with congenital heart disease. Int J Cardiol. 2008;125(1):62–5. doi:.https://doi.org/10.1016/j.ijcard.2007.02.023
26
Schwerzmann
M
. Congenital heart disease clinics - how to keep the adult patients on board. Prog Pediatr Cardiol. 2012;34(2):113–7. doi:.https://doi.org/10.1016/j.ppedcard.2012.08.010
27
Sable
C
,
Foster
E
,
Uzark
K
,
Bjornsen
K
,
Canobbio
MM
,
Connolly
HM
, et al.; American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues: a scientific statement from the American Heart Association. Circulation. 2011;123(13):1454–85. doi:.https://doi.org/10.1161/CIR.0b013e3182107c56
28
Mackie
AS
,
Islam
S
,
Magill-Evans
J
,
Rankin
KN
,
Robert
C
,
Schuh
M
, et al.
Healthcare transition for youth with heart disease: a clinical trial. Heart. 2014;100(14):1113–8. doi:.https://doi.org/10.1136/heartjnl-2014-305748
29
Goossens
E
,
Fieuws
S
,
Van Deyk
K
,
Luyckx
K
,
Gewillig
M
,
Budts
W
, et al.
Effectiveness of structured education on knowledge and health behaviors in patients with congenital heart disease. J Pediatr. 2015;166(6):1370–6.e1. doi:.https://doi.org/10.1016/j.jpeds.2015.02.041
30
Bouchardy
J
,
Therrien
J
,
Pilote
L
,
Ionescu-Ittu
R
,
Martucci
G
,
Bottega
N
, et al.
Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120(17):1679–86. doi:.https://doi.org/10.1161/CIRCULATIONAHA.109.866319
31
Kaemmerer
H
,
Bauer
U
,
Pensl
U
,
Oechslin
E
,
Gravenhorst
V
,
Franke
A
, et al.
Management of emergencies in adults with congenital cardiac disease. Am J Cardiol. 2008;101(4):521–5. doi:.https://doi.org/10.1016/j.amjcard.2007.09.110
32
Khairy
P
,
Van Hare
GF
,
Balaji
S
,
Berul
CI
,
Cecchin
F
,
Cohen
MI
, et al.
PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart Disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm. 2014;11(10):e102–65. doi:.https://doi.org/10.1016/j.hrthm.2014.05.009
33
Lanz
J
,
Brophy
JM
,
Therrien
J
,
Kaouache
M
,
Guo
L
,
Marelli
AJ
. Stroke in Adults With Congenital Heart Disease: Incidence, Cumulative Risk, and Predictors. Circulation. 2015;132(25):2385–94. doi:.https://doi.org/10.1161/CIRCULATIONAHA.115.011241
34
Hoffmann
A
,
Chockalingam
P
,
Balint
OH
,
Dadashev
A
,
Dimopoulos
K
,
Engel
R
, et al.
Cerebrovascular accidents in adult patients with congenital heart disease. Heart. 2010;96(15):1223–6. doi:.https://doi.org/10.1136/hrt.2010.196147
35
Khairy
P
,
Aboulhosn
J
,
Broberg
CS
,
Cohen
S
,
Cook
S
,
Dore
A
, et al.; Anticoagulation Therapy in Congenital Heart Disease (TACTIC) investigators and the Alliance for Adult Research in Congenital Cardiology (AARCC). Thromboprophylaxis for atrial arrhythmias in congenital heart disease: A multicenter study. Int J Cardiol. 2016;223:729–35. doi:.https://doi.org/10.1016/j.ijcard.2016.08.223
36
Jensen
AS
,
Idorn
L
,
Nørager
B
,
Vejlstrup
N
,
Sondergaard
L
. Anticoagulation in adults with congenital heart disease: The who, the when and the how?
Heart. 2015;101(6):424–9. doi:.https://doi.org/10.1136/heartjnl-2014-305576
37
Koyak
Z
,
Harris
L
,
de Groot
JR
,
Silversides
CK
,
Oechslin
EN
,
Bouma
BJ
, et al.
Sudden cardiac death in adult congenital heart disease. Circulation. 2012;126(16):1944–54. doi:.https://doi.org/10.1161/CIRCULATIONAHA.112.104786
38
Khairy
P
,
Van Hare
GF
,
Balaji
S
,
Berul
CI
,
Cecchin
F
,
Cohen
MI
, et al.
PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Can J Cardiol. 2014;30(10):e1–63. doi:.https://doi.org/10.1016/j.cjca.2014.09.002
39
Schwerzmann
M
,
Salehian
O
,
Harris
L
,
Siu
SC
,
Williams
WG
,
Webb
GD
, et al.
Ventricular arrhythmias and sudden death in adults after a Mustard operation for transposition of the great arteries. Eur Heart J. 2009;30(15):1873–9. doi:.https://doi.org/10.1093/eurheartj/ehp179
40
Zomer
AC
,
Vaartjes
I
,
Uiterwaal
CS
,
van der Velde
ET
,
van den Merkhof
LF
,
Baur
LH
, et al.
Circumstances of death in adult congenital heart disease. Int J Cardiol. 2012;154(2):168–72. doi:.https://doi.org/10.1016/j.ijcard.2010.09.015
41
Verheugt
CL
,
Uiterwaal
CS
,
van der Velde
ET
,
Meijboom
FJ
,
Pieper
PG
,
van Dijk
AP
, et al.
Mortality in adult congenital heart disease. Eur Heart J. 2010;31(10):1220–9. doi:.https://doi.org/10.1093/eurheartj/ehq032
42
Krieger
EV
,
Valente
AM
. Heart failure treatment in adults with congenital heart disease: where do we stand in 2014?
Heart. 2014;100(17):1329–34. doi:.https://doi.org/10.1136/heartjnl-2014-305667
43
Zomer
AC
,
Vaartjes
I
,
van der Velde
ET
,
de Jong
HM
,
Konings
TC
,
Wagenaar
LJ
, et al.
Heart failure admissions in adults with congenital heart disease; risk factors and prognosis. Int J Cardiol. 2013;168(3):2487–93. doi:.https://doi.org/10.1016/j.ijcard.2013.03.003
44
Budts
W
,
Roos-Hesselink
J
,
Rädle-Hurst
T
,
Eicken
A
,
McDonagh
TA
,
Lambrinou
E
, et al.
Treatment of heart failure in adult congenital heart disease: a position paper of the Working Group of Grown-Up Congenital Heart Disease and the Heart Failure Association of the European Society of Cardiology. Eur Heart J. 2016;37(18):1419–27. doi:.https://doi.org/10.1093/eurheartj/ehv741
45
McLarry
J
,
Broberg
C
,
Opotowsky
AR
,
Kaufman
T
,
Stout
K
,
Burchill
LJ
. Defining heart failure in adult congenital heart disease. Prog Pediatr Cardiol. 2014;38(1-2):3–7. doi:https://doi.org/10.1016/j.ppedcard.2014.12.002
46
Burchill
LJ
. Heart transplantation in adult congenital heart disease. Heart. 2016;102(23):1871–7. doi:.https://doi.org/10.1136/heartjnl-2015-309074
47
Davies
RR
,
Russo
MJ
,
Yang
J
,
Quaegebeur
JM
,
Mosca
RS
,
Chen
JM
. Listing and transplanting adults with congenital heart disease. Circulation. 2011;123(7):759–67. doi:.https://doi.org/10.1161/CIRCULATIONAHA.110.960260
48
Loup
O
,
Wustmann
K
,
Martinelli
MV
,
Schwerzmann
M
,
Mohacsi
P
,
Carrel
TP
, et al.
Failing Systemic Right Ventricles With Persistent Pulmonary Hypertension: Candidates for Ventricular Assist Devices as Destination Therapy?
Ann Thorac Surg. 2017;103(2):e179–81. doi:.https://doi.org/10.1016/j.athoracsur.2016.03.080
49
Tobler
D
,
Bouchardy
J
,
Reto
E
,
Heg
D
,
Müller
C
,
Frenk
A
, et al.; SERVE trial. Effect of phosphodiesterase-5 inhibition with Tadalafil on SystEmic Right VEntricular size and function - A multi-center, double-blind, randomized, placebo-controlled clinical trial - SERVE trial - Rational and design. Int J Cardiol. 2017 May 23:S0167-5273(17)32122-8. [Epub ahead of print] doi:.https://doi.org/10.1016/j.ijcard.2017.05.079
50
Habib
G
,
Lancellotti
P
,
Antunes
MJ
,
Bongiorni
MG
,
Casalta
J-P
,
Del Zotti
F
, et al.; Document Reviewers. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075–128. doi:.https://doi.org/10.1093/eurheartj/ehv319
51
Verheugt
CL
,
Uiterwaal
CS
,
van der Velde
ET
,
Meijboom
FJ
,
Pieper
PG
,
Veen
G
, et al.
Turning 18 with congenital heart disease: prediction of infective endocarditis based on a large population. Eur Heart J. 2011;32(15):1926–34. doi:.https://doi.org/10.1093/eurheartj/ehq485
52
Kuijpers
JM
,
Koolbergen
DR
,
Groenink
M
,
Peels
KC
,
Reichert
CL
,
Post
MC
, et al.
Incidence, risk factors, and predictors of infective endocarditis in adult congenital heart disease: focus on the use of prosthetic material. Eur Heart J. 2017 Jan 8:ehw591. [Epub ahead of print] doi:.https://doi.org/10.1093/eurheartj/ehw591
53
Verheugt
CL
,
Uiterwaal
CS
,
van der Velde
ET
,
Meijboom
FJ
,
Pieper
PG
,
Vliegen
HW
, et al.
Gender and outcome in adult congenital heart disease. Circulation. 2008;118(1):26–32. doi:.https://doi.org/10.1161/CIRCULATIONAHA.107.758086
54
Lockhart
PB
,
Brennan
MT
,
Thornhill
M
,
Michalowicz
BS
,
Noll
J
,
Bahrani-Mougeot
FK
, et al.
Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc. 2009;140(10):1238–44. doi:.https://doi.org/10.14219/jada.archive.2009.0046
55
Stadelmann
P
,
Zemp
E
,
Weiss
C
,
Weiger
R
,
Menghini
G
,
Zitzmann
NU
. Dental visits, oral hygiene behaviour, and orthodontic treatment in Switzerland. Schweiz Monatsschr Zahnmed. 2012;122(2):104–26.
56
Schmidt
S
,
Ramseier-Hadorn
M
,
Thomet
C
,
Wustmann
K
,
Schwerzmann
M
. Gender-related differences in self-reported dental care in adults with congenital heart disease at increased risk of infective endocarditis. Open Heart. 2017;4(1):e000575. doi:.https://doi.org/10.1136/openhrt-2016-000575
57
Habib
G
,
Lancellotti
P
,
Antunes
MJ
,
Bongiorni
MG
,
Casalta
JP
,
Del Zotti
F
, et al.; Document Reviewers. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075–128. doi:.https://doi.org/10.1093/eurheartj/ehv319
58
Greutmann
M
,
Pieper
PG
. Pregnancy in women with congenital heart disease. Eur Heart J. 2015;36(37):2491–9. doi:.https://doi.org/10.1093/eurheartj/ehv288
59
Regitz-Zagrosek
V
,
Blomstrom Lundqvist
C
,
Borghi
C
,
Cifkova
R
,
Ferreira
R
,
Foidart
JM
, et al.; European Society of Gynecology (ESG); Association for European Paediatric Cardiology (AEPC); German Society for Gender Medicine (DGesGM); ESC Committee for Practice Guidelines. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011;32(24):3147–97. doi:.https://doi.org/10.1093/eurheartj/ehr218
60
Houben
CH
,
Spruit
MA
,
Groenen
MT
,
Wouters
EF
,
Janssen
DJ
. Efficacy of advance care planning: a systematic review and meta-analysis. J Am Med Dir Assoc. 2014;15(7):477–89. doi:.https://doi.org/10.1016/j.jamda.2014.01.008
61
Tobler
D
,
Greutmann
M
,
Colman
JM
,
Greutmann-Yantiri
M
,
Librach
LS
,
Kovacs
AH
. End-of-life in adults with congenital heart disease: a call for early communication. Int J Cardiol. 2012;155(3):383–7. doi:.https://doi.org/10.1016/j.ijcard.2010.10.050
62
Deng
LX
,
Gleason
LP
,
Khan
AM
,
Drajpuch
D
,
Fuller
S
,
Goldberg
LA
, et al.
Advance Care Planning in Adults with Congenital Heart Disease: A Patient Priority. Int J Cardiol. 2017;231:105–9. doi:.https://doi.org/10.1016/j.ijcard.2016.12.185
63
Greutmann
M
,
Tobler
D
,
Colman
JM
,
Greutmann-Yantiri
M
,
Librach
SL
,
Kovacs
AH
. Facilitators of and barriers to advance care planning in adult congenital heart disease. Congenit Heart Dis. 2013;8(4):281–8. Published online January 03, 2013. doi:.https://doi.org/10.1111/chd.12025