
Figure 1 Classification of aortic dissection according to Stanford and DeBakey (drawn by J. Gawinecka).
DOI: https://doi.org/10.4414/smw.2017.14489
Acute aortic syndrome refers to a group of interrelated life-threatening conditions and consists of aortic dissection, intramural haematoma and penetrating atherosclerotic ulcer. The incidence of acute aortic syndromes in the general population ranges from 4 to 6 cases per 100 000 person-years, but increases up to 30 or more in people older than 65 years [1–3].
The classification of the aortic dissection is based on both anatomical location of the initial tear and the time from the onset of the symptoms to the presentation at the emergency department. According to the more popular Stanford system, dissections involving the ascending aorta are classified as type A, whereas those involving only the descending aorta are classified as type B. The older DeBakey system differentiates between dissections evolving from the ascending aorta and affecting all aortic segments (type I), less extensive ones affecting only the ascending fragment (type II), and dissections affecting only descending aorta (type III) (fig. 1). With respect to the time from the onset of the symptoms, aortic dissections are divided into acute (presentation within 1 week), subacute (from 1 week to 1 month) and chronic (more than 1 month).

Figure 1 Classification of aortic dissection according to Stanford and DeBakey (drawn by J. Gawinecka).
Possible complications of aortic dissection include lethal malperfusion syndrome, aortic regurgitation, cardiac failure (myocardial infarction or cardiac tamponade) and stroke [4].
Even in elderly patients with other comorbidities, surgical repair is the method of choice for the patients presenting with acute type A aortic dissection. Patients with type B dissection are usually treated medically, unless life-threating complications, such as malperfusion syndrome, occur. The main aim of medical therapy is to reduce shear stress on the diseased aortic segment by reducing blood pressure and heart rate, as well as to relieve pain [5].
As a result of the devastating complications, the mortality rate of aortic dissection remains high. In patients with acute type A aortic dissection, the most severe form, the mortality rate amounted to 26% if they received surgery, but up to 58% if they could only be treated noninvasively because of advanced age or the presence of comorbidities [4]. However, owing to the surgical advances and improved postoperative management, the mortality dropped during the last decade to 12% [6]. The early survival of patients presenting with acute type A aortic dissection is affected by preoperative conditions such as previous aortic valve replacement, migrating chest pain, preoperative limb ischaemia, hypotension during presentation, or shock / cardiac tamponade [7]. In turn, long-term survival is influenced by preoperative renal impairment, reduced left ventricular ejection fraction and advanced age [8]. Patients with type B dissection managed medically have the lowest mortality rate of 11%, which can, however, increase up to 31% when patients have to undergo surgery because of the complications [4].
Aortic dissection arises from a tear in the aortic intima exposing the medial layer to the pulsatile blood flow. The intimal tear is frequently found in segments exposed to the greatest shear stress, namely the right lateral wall (opposite the main pulmonary artery) of the ascending aorta or in the proximal segment of the descending aorta. The progressive separation of the aortic wall layers results in the formation of a false lumen and its subsequent propagation can be followed either by aortic rupture in the case of adventitial disruption, or by re-entry back into the true lumen through another intimal tear. Aortic rupture quickly leads to exsanguination and death. In the event of blood redirection into the true lumen, creating natural fenestration, the patient can present as relatively stable with adequate perfusion. The false lumen may also end blindly in a cul-de-sac, creating a blood clot. Rarely, when thrombosis occurs very early in the event, the thrombosed false lumen is smaller than the true lumen. When thrombosis occurs late, the false lumen is usually larger than the true one. Moreover, the false lumen grows during blood clotting and further compresses the true lumen, leading to decreased systemic perfusion. The dissection can also extend into aortic branches and increase mortality risk, especially if coronary arteries are involved [9, 10].
An electron microscopy study showed that the extracellular matrix is spare in the interlamellar space of the medial layer at the dissection entry site and adjacent intact tissue, and spirally thickened collagen fibrils are often accompanied by attenuated, fragmented or disrupted elastic lamellae. Moreover, the basement membrane of the smooth muscle cells is usually thin or even lacking. In contrast, the intimal layer does not show any specific changes in the aortic dissection [11].
At the molecular level, aortic dissection is the result of remodelling of the aortic wall structure as a result of inflammation and extracellular matrix degradation. Activated macrophages infiltrate the tunica media and release matrix metalloproteinases (MMPs) and pro-inflammatory cytokines. The excessive production of MMP-1, MMP-9 and MMP-12 leads to the accelerated degradation of collagen and elastin fibres [12]. These MMPs also play a role in the pathogenesis of aortic aneurysm and Marfan syndrome [13–15]. Not only the increased release of MMPs themselves, but also the imbalance between them and their tissue inhibitors (TIMPs) promote the proteolytic dominance in aortic dissection [14, 16].
VEGF-mediated neoangiogenesis may be another process underlying aortic wall remodelling. VEGF (vascular endothelial growth factor) is the growth factor driving angiogenesis and vasculogenesis, but it also exerts proinflammatory actions. The production of VEGF is increased in the neovessels and their surrounding immune-inflammatory infiltrate of the degraded medial layer [12].
Arterial hypertension is, as described below, one of the main risk factors for aortic dissection. It can act directly as a parietal stressor and indirectly as a proinflammatory trigger, mainly by inducing macrophage recruitment and activation [17]. The hypertensive patients show elevated concentrations of proinflammatory molecules such as interleukin (IL)-6, VEGF, macrophage chemoattractant protein-1 (MCP-1), MMP-2 and MMP-9 [18–21], suggesting that hypertension promotes a proinflammatory state, which subsequently leads to the excessive extracellular matrix degeneration and culminates in the aortic dissection.
So far several risk factors for developing aortic dissection are recognised (table 1).
Table 1 Risk factors for aortic dissection.
| Male sex |
| Age >65 years |
| Hypertension |
| Smoking |
| Aneurysm |
| Congenital disorders |
| Marfan syndrome |
| Loeys-Dietz syndrome |
| Vascular Ehlers-Danlos syndrome |
| Bicuspid aortic valve |
| Inflammatory disease |
| Aortitis |
| Giant cell arteritis |
| Takayasu arteritis |
| Systemic lupus erythematous |
With an age-adjusted incidence of five versus two per 100 000 person-years, men are at higher risk of developing aortic dissection than women [1]. The sex distribution is consistent in both type A and type B dissection. Usually, women affected by aortic dissection are older and present more frequently with congestive heart failure, coma or altered mental status at hospital admission than men. Women with type A dissection also have a higher in-hospital mortality rate. This may be due to the older age, delayed presentation at the hospital or delayed diagnosis because of the less typical symptoms [22].
The incidence of aortic dissection correlates with age. The mean age at the onset of aortic dissection is approximately 65 years. Usually, patients with type A dissection are younger than those with type B dissection [1, 4]. In patients with connective tissue diseases or a bicuspid aortic valve, aortic dissection frequently occurs before 40 years of age [22]. The elderly patients suffer more frequently from other comorbidities such as hypertension, diabetes mellitus or atherosclerosis, and more often have a history of cardiac surgery or aortic aneurysm. Compared with the younger patients, they less often experience an abrupt onset of pain and pulse deficits [23].
Hypertension is considered as the most important risk factor for aortic dissection and is present in about 80% of patients with aortic dissection. In the general population, hypertension contributes 54% of population-attributable risk of acute aortic dissection, and with an incidence rate of 21 per 100 000 person-years compared with 5 in normotensive individuals [4, 24]. Patients with higher blood pressure during the 5 years before the occurrence of the aortic dissection die more often before reaching hospital than those with lower blood pressure or better hypertension control [2].
Smoking is another risk factor for developing aneurysm and aortic dissection. Compared with nonsmokers, smokers suffer from aortic dissection and thoracic aortic aneurysm twice as frequently and from abdominal aortic aneurysm even five times more frequently [2].
The annual rate of acute aortic dissection progressively increases as the aortic diameter increases, and the incidence of aortic complications reaches 30% once the aortic diameter reaches 60 mm [25]. Although aortic dilatation clearly increases the risk of aortic dissection, dilatation is not essential for dissection, and approximately 60% of acute type A aortic dissections occur at aortic diameters <55 mm [26].
Aortitis, complications caused by giant cell arteritis, Takayasu arteritis or systemic lupus erythematous, are rarer risk factors for aortic dissection, but 1 to 5% of patients with aortitis develop aortic dissection [27, 28].
The role of atherosclerosis in the development of aortic dissection remains unclear. Atherosclerosis is more common in patients with type B dissection than in those with type A dissection [29]. In general, the prevalence and extent of atherosclerosis increases with age in both general population and in patients with aortic dissection [23].
There is no association of diabetes mellitus or obesity with aortic dissection or aneurysm [2].
Marfan syndrome and other inherited connective tissue diseases, such as vascular-type Ehlers-Danlos syndrome or Loeys-Dietz syndrome, are associated with aortic dissection especially in young patients [30]. Classic Marfan syndrome is caused by fibrillin 1 (FBN1) deficiency. Studies in animal models of Marfan syndrome suggest that FBN1 mutations cause morphological changes in vascular smooth muscle cells and the release of matrix-degrading enzymes leading to elastolysis, fibre calcification, and inflammation. The progression of these pathological changes culminates in aneurysm formation and, eventually, aortic dissection [31, 32]. The prevalence of type A dissection among patients with Marfan syndrome at 60 years of age is approximately 50% [33]. The vascular-type Ehlers-Danlos syndrome is associated with defects in the synthesis of type III procollagen, which is an important regulatory element in type I collagen fibrillogenesis. The abnormal collagen type I fibrillogenesis and abnormal vascular smooth muscle cell signalling results in thinner intima media thickness with higher wall stress and increased risk of arterial dissection and rupture [34].
Mutations in genes encoding transforming growth factor β receptors 1 and 2 are responsible for Loeys-Dietz and Marfan-like syndrome. Affected patients have a high risk of aortic dissection or rupture at an early age and with aortic diameters that normally would not be predictive for these events [35].
Congenital bicuspid aortic valve is the most common cardiovascular malformation, occurring in 1 to 2% of the general population, and is a risk factor for aortic aneurysm and dissection [36]. Aortic disease and a bicuspid aortic valve are connected with each other by mechanisms similar to those described for Marfan syndrome, namely vascular matrix remodelling due to the deficient elastic fibre components, such as elastin, fibrillin or emilin, and increased matrix metalloproteinase release [37, 38]. Mutations in the following genes, among others, have been associated with bicuspid aortic valve and aortic complications: NOTCH1 (isolated and familial bicuspid aortic valve and left ventricular outflow tract defects), ACTA2 and TGF-β2 (bicuspid aortic with familial aortic aneurysm), and FNB1 (isolated bicuspid aortic valve and bicuspid aortic valve with Marfan syndrome). Moreover, family-based linkage analyses have revealed associations of the genomic regions on chromosomes 18q, 5q and 13q with bicuspid aortic valve and cardiac malformations, but no genes harbouring underlying mutations have been identified. Except for NOTCH1, the genetic associations with bicuspid aortic valve may be the result of confounding with coexisting diseases [39]. Nonetheless, the prevalence of aortic dilatation and aneurysm increases with age in patients with a bicuspid aortic valve, and the risk of acute aortic complications, such as dissection and rupture, is eight times higher in affected patients than in the general population [40, 41].
Moreover, recent genome-wide association studies showed that genetic variants in FNB1, LRP1, and ULK4 genes predispose individuals to non-familial thoracic aortic dissection [42].
Since aortic dissection is a dynamic process that may occur in any segment of the aorta, the spectrum of the clinical presentation is broad. Severe chest or back pain, described as sharp or stabbing, with abrupt onset is the most common presenting symptom of the acute aortic dissection. In less than half of the patients presenting with acute type A dissection, pulse deficit, murmur of aortic regurgitation, hypotension, syncope or other neurological findings are reported. Abdominal pain and hypertension are more frequent in the patients with type B dissection than in those with type A dissection [4]. Symptoms may mimic more common conditions, such as myocardial infarction or pulmonary embolism. However, typical physical findings can even be absent. Therefore, aortic dissection is often difficult to diagnose, and a high clinical index of suspicion is required. Nonetheless, the early and accurate diagnosis is essential for choosing the appropriate surgical or medical interventions to reduce the high lethality, which is around 1% per hour after symptom onset in untreated patients [43].
Both chest X-ray and 12-lead electrocardiogram (ECG) may be helpful in the evaluation of suspected aortic dissection, but a substantial number of patients do not show any abnormalities in these examinations (12 and 31%, respectively). In about 50% of patients, a widened mediastinum or abnormal aortic contour is present in the chest X-ray. The most common ECG finding is the presence of nonspecific ST-segment or T-wave changes. Rarely, ECG changes typical for myocardial infarction, such as ST-segment elevation or new Q waves, can be found in patients with acute type A aortic dissection [4]. According to the International Registry of Acute Aortic Dissection (IRAD) study, ECG alterations suggestive of myocardial ischaemia are likely to delay the correct diagnosis of aortic dissection [44]. Taken together, sensitivity and specificity of both chest X-ray and ECG are too low for the rule-out and rule-in of aortic dissection.
Transthoracic echocardiography (TTE) offers rapid and noninvasive assessment of several aortic segments, particularly the aortic root and proximal ascending aorta. However, it suffers from limited diagnostic sensitivity, so that negative findings on TTE do not allow any reliable exclusion of aortic dissection. It is also limited in patients with an abnormal chest wall configuration, obesity or pulmonary emphysema, and in those on mechanical ventilation. Nonetheless, owing to its broad availability, rapidity, and additional information on cardiac and aortic status, TTE has been recommended as the initial imaging modality when aortic dissection is clinically suspected [5, 45].
A systematic review on the diagnostic accuracy of transoesophageal echocardiography (TOE), contrast-enhanced computed tomography (CT), and magnetic resonance imaging (MRI) for suspected thoracic aortic dissection reports pooled sensitivity of 98 to 100% and specificity of 95 to 98% for all three imaging techniques [46].
The portable TOE is advantageous in emergency situations with time constraints and in patients with haemodynamic instability, and overcomes the limitations of the TTE. However, the quality of TOE examination depends on the observer and their experience. False positive or false negative findings can arise easily when interpreted by unskilled individuals or a single person [47]. It also suffers from the poor spatial resolution and blind spots caused by intervening anatomical structures (fig. 2).
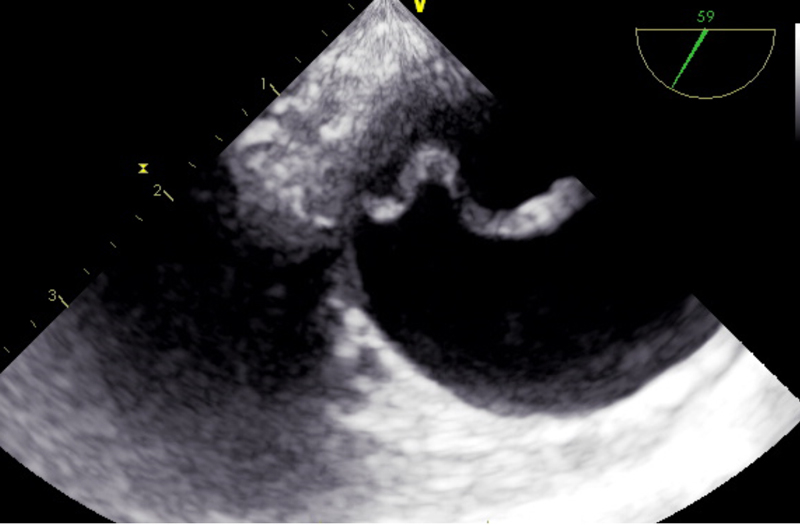
Figure 2 Transoesophageal echocardiographic image of the intimal flap in type A dissection.
MRI is considered the most accurate diagnostic technique in the assessment of aortic dissection, but is rarely used as the initial imaging technique due to the lack of availability, time delay, incompatibility with implanted metal devices, or difficulties to monitor the patient during the examination. MRI is highly suitable for serial follow-up studies in patients with known aortic disease [5].
Contrast-enhanced CT is probably the most widely used imaging technique in the diagnosis of aortic dissection. It provides fast image acquisition and processing with the ability to obtain complete 3D datasets of the entire aorta. However, CT exposes patients to ionising radiation and to contrast agents that may cause allergic reactions or renal failure [5, 46]. According to the IRAD study, prompt chest CT scan as the initial test is associated with the quickest diagnostic time [44]. Moreover, the important findings of imaging, such as extent of the dissection and regurgitation, size of the false and true lumen, involvement of the arterial branches, or presence of hematoma, can support the management (fig. 3).

Figure 3 Computed tomography scan of type A dissection with haematoma.
As yet, blood tests play only a minor role in the assessment of patients with suspected aortic dissection. D-dimers are commonly used in the diagnosis of pulmonary embolism, but they can also support diagnosis of aortic dissection. The cut-off level of 500 ng/ml widely used for ruling out pulmonary embolism can also be applied for ruling out aortic dissection within the first 24 hours after onset of symptoms [48]. A meta-analysis of the diagnostic performance of D-dimers in aortic dissection shows very high sensitivity of 97% and a rather poor specificity of 56%. However, an excellent negative likelihood ratio of 0.06 allows reliable ruling out of aortic dissection in low-risk patients who are unlikely to benefit from aortic imaging [49, 50].
About 50% of patients with aortic dissection have positive high sensitive troponin T. Similarly to an ECG suggestive of myocardial ischaemia, positive troponin T test results were found to delay the diagnosis of aortic dissection. The concomitant presence of elevated troponin and ECG abnormalities typical for myocardial infarction increases the risk of misdiagnosis and, hence, the probability of inappropriate management, such as coronary angiography or intense antithrombotic treatment [51]. Elevated cardiac troponins at the time of hospital admission for suspected acute aortic dissection are associated with an increased risk of in-hospital mortality [52].
Several inflammatory markers, like IL-6, IL-8, IL-10 or tumour necrosis factor-alpha (TNF-α), have been proposed as potentially useful in the diagnosis of the aortic dissection [53]. Although inflammation is one of the major processes involved in the development of the aortic dissection, the specificity of these biomarkers may prove too low for successful implementation as diagnostic markers in aortic dissection. Other major targets for biomarker seeking are components of the extracellular matrix. For example, circulating elastin degradation products (sELAF) are elevated in patients with aortic dissection, but they also increase with age in healthy subjects. Another limitation of sELAF is its almost negligible increase in patients with a thrombosed false lumen [54]. Both MMP-8 and MMP-12 reflect the active aortic wall remodelling in aortic dissection and appear to be more specific and sensitive biomarker candidates [55, 56]. However, more studies are needed to confirm these intriguing data. The smooth muscle myosin heavy chain, which is released after myocytic death, shows satisfactory diagnostic performance, but its rapid decrease already several hours after the onset of symptoms limits it application to the patients with early presentation [57]. Calponin, the smooth muscle analogue of troponin, has relatively low specificity and sensitivity and therefore cannot be used in clinical practice as a single biomarker [58].
The European Society of Cardiology (ESC) recommends estimating a priori risk of aortic dissection by assessing predisposing conditions (e.g., connective tissue diseases, known aortic aneurysm or aortic valve disease), pain features (abrupt onset and severe intensity), and by clinical examination (pulse deficit, systolic blood pressure difference, aortic diastolic murmur, hypotension or shock). The pre-test probability reached thereby is especially important in haemodynamically stable patients, where the diagnostic work-up is highly dependent on the risk status. In patients with low risk of aortic dissection, measurement of D-Dimer, TTE and chest X-ray should be chosen for the initial diagnostic examination. In patients with high probability of aortic dissection and inconclusive TTE results, TOE or contrast-enhanced CT are recommended as the diagnostic imaging modalities [5] (table 2).
Table 2 Comparison of diagnostic methods in aortic dissection.
| Diagnostic method | Advantages | Disadvantages |
Recommendations
of ESC [5] |
Class of recommendation* | Level of evidence† |
|---|---|---|---|---|---|
| D-dimer measurement | • Fast and easily accessible • Very high sensitivity of 97% |
• Poor specificity of 56% | • Only patients with a priori low risk of aortic dissection | IIa | B |
| Chest X-ray | • Fast and noninvasive | • Low sensitivity and specificity | • Only patients with a priori low risk of aortic dissection | IIb | C |
| Transthoracic echocardiography | • Rapid and noninvasive | • Restricted in patients with abnormal chest wall, obesity, pulmonary emphysema and mechanical ventilation • Not all aortic segments can be visualised |
• Initial imaging examination | I | C |
| Transoesophageal echocardiography | • Overcomes limitations of transthoracic echocardiography • Suitable for haemodynamically instable patients • excellent sensitivity of 95% and specificity of 95% |
• Semi-invasive and requires sedation and blood pressure control • Not feasible in patients with oesophageal diseases • Tends to be observer and experience dependent • Not all aortic segments can be visualised |
• Unstable patients with suspicion of aortic dissection | I | C |
| • Stable patients with suspicion of aortic dissection | IIa | C | |||
| Contrast-enhanced computed tomography | • Fast image acquisition • Possibility of 3D images of entire aorta • Excellent sensitivity of 100% and specificity of 98% |
• Exposure to ionising radiation and contrast agents | • Unstable or stable patients with suspicion of aortic dissection | I | C |
| • As repetitive imaging in case of initially negative finding and persistence suspicion | I | C | |||
| Magnetic resonance imaging | • Excellent sensitivity of 98% and specificity of 98% • Visualisation of entire aorta |
• Incompatible with implanted metal devices and pacemakers • Problematic patient’s monitoring during examination • Lack of widespread availability in the emergency settings |
• Stable patients with suspicion of aortic dissection | I | C |
| • As repetitive imaging in case of initially negative finding and persistence suspicion | I | C |
* Class of recommendation: I - is recommended / is indicated; IIa - should be considered; IIb - may be considered † Level of evidence: B - data derived from a single randomised clinical trial or large nonrandomised studies; C - consensus of opinion of the experts and/or small studies, retrospective studies, registries
Patients with known aortic aneurysm, genetic connective tissue disorders or a bicuspid aortic valve should undergo serial assessment of the aorta until it reaches a critical diameter at which the risk of dissection or rupture significantly increases. Elective surgery in patients with Marfan syndrome and bicuspid aortic valve is indicated when the aortic diameter exceeds 50 mm and 55 mm, respectively. Lower thresholds are to be considered if additional risks factors are present, such as a family history of dissection, size increase <3 mm/year or severe aortic regurgitation. The most suitable method for serial examination is MRI, which allows visualisation of the entire aorta and does not expose patients to ionising radiation or contrast agents. The monitoring of patients with newly diagnosed bicuspid aortic valves can also be conducted with TTE. However, when the aortic diameter reaches 45 mm or its increase >3 mm/year, the progression of dilation should be checked with the use of MRI or CT [5, 59].
Because of the extremely high lethality of 1 to 2% per hour after symptom onset in untreated patients [43], aortic dissection should have an important place in the awareness of physicians in the evaluation of emergency patients with acute chest pain. Delays in the diagnosis and treatment of aortic dissection are more frequently observed in non-tertiary hospitals with low exposure to the aortic emergencies. Continuous medical education of primary care centres on the recognition of aortic dissection, introduction of standardised protocols for the initial management of aortic dissection, and improved communication with specialised cardiovascular centres can halve the time from presentation to both confirmed diagnosis and surgical repair [60]. The reduction of deaths caused by aortic dissection also requires improvement in preventive measures, both in diagnostics to identify and monitor individuals at increased risk of aortic dissection and in preventive treatments. Imaging technologies provide a robust foundation for diagnosing and monitoring of aortic dilatation or dissection, but easily accessible and cost-effective blood tests play almost no role. Novel validated blood biomarkers have the potential to facilitate the clinical management of patients with either suspected aortic dissection or individuals at increased risk of aortic dissection or both.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Clouse WD , Hallett JW, Jr , Schaff HV , Spittell PC , Rowland CM , Ilstrup DM , et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79(2):176–80. doi:.https://doi.org/10.4065/79.2.176
2 Howard DP , Banerjee A , Fairhead JF , Perkins J , Silver LE , Rothwell PM ; Oxford Vascular Study. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation. 2013;127(20):2031–7. doi:.https://doi.org/10.1161/CIRCULATIONAHA.112.000483
3 Olsson C , Thelin S , Ståhle E , Ekbom A , Granath F . Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114(24):2611–8. doi:.https://doi.org/10.1161/CIRCULATIONAHA.106.630400
4 Hagan PG , Nienaber CA , Isselbacher EM , Bruckman D , Karavite DJ , Russman PL , et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283(7):897–903. doi:.https://doi.org/10.1001/jama.283.7.897
5 Erbel R , Aboyans V , Boileau C , Bossone E , Bartolomeo RD , Eggebrecht H , et al., The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur Heart J. 2014;35(41):2873–926. doi:.https://doi.org/10.1093/eurheartj/ehu281
6 Conway BD , Stamou SC , Kouchoukos NT , Lobdell KW , Khabbaz KR , Murphy E , et al. Improved clinical outcomes and survival following repair of acute type A aortic dissection in the current era. Interact Cardiovasc Thorac Surg. 2014;19(6):971–6. doi:.https://doi.org/10.1093/icvts/ivu268
7 Trimarchi S , Nienaber CA , Rampoldi V , Myrmel T , Suzuki T , Mehta RH , et al.; International Registry of Acute Aortic Dissection Investigators. Contemporary results of surgery in acute type A aortic dissection: The International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg. 2005;129(1):112–22. doi:.https://doi.org/10.1016/j.jtcvs.2004.09.005
8 Schoenrath F , Laber R , Maralushaj M , Henzi D , Caliskan EI , Seifert B , et al. Survival, Neurologic Injury, and Kidney Function after Surgery for Acute Type A Aortic Dissection. Thorac Cardiovasc Surg. 2016;64(2):100–7.
9 Vilacosta I , Aragoncillo P , Cañadas V , San Román JA , Ferreirós J , Rodríguez E . Acute aortic syndrome: a new look at an old conundrum. Postgrad Med J. 2010;86(1011):52–61. doi:.https://doi.org/10.1136/hrt.2008.153650
10 White A , Broder J , Mando-Vandrick J , Wendell J , Crowe J . Acute aortic emergencies--part 2: aortic dissections. Adv Emerg Nurs J. 2013;35(1):28–52. doi:.https://doi.org/10.1097/TME.0b013e31827145d0
11 Ishii T , Asuwa N . Collagen and elastin degradation by matrix metalloproteinases and tissue inhibitors of matrix metalloproteinase in aortic dissection. Hum Pathol. 2000;31(6):640–6. doi:.https://doi.org/10.1053/hupa.2000.7642
12 Del Porto F , di Gioia C , Tritapepe L , Ferri L , Leopizzi M , Nofroni I , et al. The multitasking role of macrophages in Stanford type A acute aortic dissection. Cardiology. 2014;127(2):123–9. doi:.https://doi.org/10.1159/000355253
13 Curci JA , Liao S , Huffman MD , Shapiro SD , Thompson RW . Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest. 1998;102(11):1900–10. doi:.https://doi.org/10.1172/JCI2182
14 Koullias GJ , Ravichandran P , Korkolis DP , Rimm DL , Elefteriades JA . Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann Thorac Surg. 2004;78(6):2106–10, discussion 2110–1. doi:.https://doi.org/10.1016/j.athoracsur.2004.05.088
15 Ikonomidis JS , Jones JA , Barbour JR , Stroud RE , Clark LL , Kaplan BS , et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with Marfan syndrome. Circulation. 2006;114(1, Suppl):I365–70.
16 Manabe T , Imoto K , Uchida K , Doi C , Takanashi Y . Decreased tissue inhibitor of metalloproteinase-2/matrix metalloproteinase ratio in the acute phase of aortic dissection. Surg Today. 2004;34(3):220–5. doi:.https://doi.org/10.1007/s00595-003-2683-3
17 Hahn AW , Jonas U , Bühler FR , Resink TJ . Activation of human peripheral monocytes by angiotensin II. FEBS Lett. 1994;347(2-3):178–80. doi:.https://doi.org/10.1016/0014-5793(94)00531-1
18 Chae CU , Lee RT , Rifai N , Ridker PM . Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38(3):399–403. doi:.https://doi.org/10.1161/01.HYP.38.3.399
19 Stumpf C , Jukic J , Yilmaz A , Raaz D , Schmieder RE , Daniel WG , et al. Elevated VEGF-plasma levels in young patients with mild essential hypertension. Eur J Clin Invest. 2009;39(1):31–6. doi:.https://doi.org/10.1111/j.1365-2362.2008.02056.x
20 Parissis JT , Korovesis S , Giazitzoglou E , Kalivas P , Katritsis D . Plasma profiles of peripheral monocyte-related inflammatory markers in patients with arterial hypertension. Correlations with plasma endothelin-1. Int J Cardiol. 2002;83(1):13–21. doi:.https://doi.org/10.1016/S0167-5273(02)00021-9
21 Derosa G , D’Angelo A , Ciccarelli L , Piccinni MN , Pricolo F , Salvadeo S , et al. Matrix metalloproteinase-2, -9, and tissue inhibitor of metalloproteinase-1 in patients with hypertension. Endothelium. 2006;13(3):227–31. doi:.https://doi.org/10.1080/10623320600780942
22 Januzzi JL , Isselbacher EM , Fattori R , Cooper JV , Smith DE , Fang J , et al.; International Registry of Aortic Dissection (IRAD). Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD). J Am Coll Cardiol. 2004;43(4):665–9. doi:.https://doi.org/10.1016/j.jacc.2003.08.054
23 Trimarchi S , Eagle KA , Nienaber CA , Rampoldi V , Jonker FH , De Vincentiis C , et al.; International Registry of Acute Aortic Dissection Investigators. Role of age in acute type A aortic dissection outcome: report from the International Registry of Acute Aortic Dissection (IRAD). J Thorac Cardiovasc Surg. 2010;140(4):784–9. doi:.https://doi.org/10.1016/j.jtcvs.2009.11.014
24 Landenhed M , Engström G , Gottsäter A , Caulfield MP , Hedblad B , Newton-Cheh C , et al. Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: a prospective cohort study. J Am Heart Assoc. 2015;4(1):e001513. doi:.https://doi.org/10.1161/JAHA.114.001513
25 Elefteriades JA . Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74(5):S1877–80, discussion S1892–8. doi:.https://doi.org/10.1016/S0003-4975(02)04147-4
26 Pape LA , Tsai TT , Isselbacher EM , Oh JK , O’gara PT , Evangelista A , et al.; International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter >or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2007;116(10):1120–7. doi:.https://doi.org/10.1161/CIRCULATIONAHA.107.702720
27 Nuenninghoff DM , Hunder GG , Christianson TJ , McClelland RL , Matteson EL . Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum. 2003;48(12):3522–31. doi:.https://doi.org/10.1002/art.11353
28 Gonzalez-Gay MA , Garcia-Porrua C , Piñeiro A , Pego-Reigosa R , Llorca J , Hunder GG . Aortic aneurysm and dissection in patients with biopsy-proven giant cell arteritis from northwestern Spain: a population-based study. Medicine (Baltimore). 2004;83(6):335–41. doi:.https://doi.org/10.1097/01.md.0000145366.40805.f8
29 Tsai TT , Trimarchi S , Nienaber CA . Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD). Eur J Vasc Endovasc Surg. 2009;37(2):149–59. doi:.https://doi.org/10.1016/j.ejvs.2008.11.032
30 Homme JL , Aubry MC , Edwards WD , Bagniewski SM , Shane Pankratz V , Kral CA , et al. Surgical pathology of the ascending aorta: a clinicopathologic study of 513 cases. Am J Surg Pathol. 2006;30(9):1159–68. doi:.https://doi.org/10.1097/01.pas.0000213270.38091.69
31 Bunton TE , Biery NJ , Myers L , Gayraud B , Ramirez F , Dietz HC . Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan syndrome. Circ Res. 2001;88(1):37–43. doi:.https://doi.org/10.1161/01.RES.88.1.37
32 Ramirez F , Sakai LY , Dietz HC , Rifkin DB . Fibrillin microfibrils: multipurpose extracellular networks in organismal physiology. Physiol Genomics. 2004;19(2):151–4. doi:.https://doi.org/10.1152/physiolgenomics.00092.2004
33 Détaint D , Faivre L , Collod-Beroud G , Child AH , Loeys BL , Binquet C , et al. Cardiovascular manifestations in men and women carrying a FBN1 mutation. Eur Heart J. 2010;31(18):2223–9. doi:.https://doi.org/10.1093/eurheartj/ehq258
34 Boutouyrie P , Germain DP , Fiessinger JN , Laloux B , Perdu J , Laurent S . Increased carotid wall stress in vascular Ehlers-Danlos syndrome. Circulation. 2004;109(12):1530–5. doi:.https://doi.org/10.1161/01.CIR.0000121741.50315.C2
35 Loeys BL , Schwarze U , Holm T , Callewaert BL , Thomas GH , Pannu H , et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355(8):788–98. doi:.https://doi.org/10.1056/NEJMoa055695
36 Hoffman JI , Kaplan S . The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–900. doi:.https://doi.org/10.1016/S0735-1097(02)01886-7
37 Fondard O , Detaint D , Iung B , Choqueux C , Adle-Biassette H , Jarraya M , et al. Extracellular matrix remodelling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur Heart J. 2005;26(13):1333–41. doi:.https://doi.org/10.1093/eurheartj/ehi248
38 Fedak PW , de Sa MP , Verma S , Nili N , Kazemian P , Butany J , et al. Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation. J Thorac Cardiovasc Surg. 2003;126(3):797–805. doi:.https://doi.org/10.1016/S0022-5223(03)00398-2
39 Andreassi MG , Della Corte A . Genetics of bicuspid aortic valve aortopathy. Curr Opin Cardiol. 2016;31(6):585–92. doi:.https://doi.org/10.1097/HCO.0000000000000328
40 Tadros TM , Klein MD , Shapira OM . Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119(6):880–90. doi:.https://doi.org/10.1161/CIRCULATIONAHA.108.795401
41 Michelena HI , Khanna AD , Mahoney D , Margaryan E , Topilsky Y , Suri RM , et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306(10):1104–12. doi:.https://doi.org/10.1001/jama.2011.1286
42 Guo DC , Grove ML , Prakash SK , Eriksson P , Hostetler EM , LeMaire SA , et al.; GenTAC Investigators; BAVCon Investigators. Genetic Variants in LRP1 and ULK4 Are Associated with Acute Aortic Dissections. Am J Hum Genet. 2016;99(3):762–9. doi:.https://doi.org/10.1016/j.ajhg.2016.06.034
43 Mészáros I , Mórocz J , Szlávi J , Schmidt J , Tornóci L , Nagy L , et al. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117(5):1271–8. doi:.https://doi.org/10.1378/chest.117.5.1271
44 Harris KM , Strauss CE , Eagle KA , Hirsch AT , Isselbacher EM , Tsai TT , et al.; International Registry of Acute Aortic Dissection (IRAD) Investigators. Correlates of delayed recognition and treatment of acute type A aortic dissection: the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2011;124(18):1911–8. doi:.https://doi.org/10.1161/CIRCULATIONAHA.110.006320
45 Meredith EL , Masani ND . Echocardiography in the emergency assessment of acute aortic syndromes. Eur J Echocardiogr. 2009;10(1):i31–9. doi:.https://doi.org/10.1093/ejechocard/jen251
46 Shiga T , Wajima Z , Apfel CC , Inoue T , Ohe Y . Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med. 2006;166(13):1350–6. doi:.https://doi.org/10.1001/archinte.166.13.1350
47 Shiga T , Wajima Z , Inoue T , Ogawa R . Survey of observer variation in transesophageal echocardiography: comparison of anesthesiology and cardiology literature. J Cardiothorac Vasc Anesth. 2003;17(4):430–42. doi:.https://doi.org/10.1016/S1053-0770(03)00146-0
48 Suzuki T , Distante A , Zizza A , Trimarchi S , Villani M , Salerno Uriarte JA , et al.; IRAD-Bio Investigators. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation. 2009;119(20):2702–7. doi:.https://doi.org/10.1161/CIRCULATIONAHA.108.833004
49 Shimony A , Filion KB , Mottillo S , Dourian T , Eisenberg MJ . Meta-analysis of usefulness of d-dimer to diagnose acute aortic dissection. Am J Cardiol. 2011;107(8):1227–34. doi:.https://doi.org/10.1016/j.amjcard.2010.12.027
50 Asha SE , Miers JW . A Systematic Review and Meta-analysis of D-dimer as a Rule-out Test for Suspected Acute Aortic Dissection. Ann Emerg Med. 2015;66(4):368–78. doi:.https://doi.org/10.1016/j.annemergmed.2015.02.013
51 Vagnarelli F , Corsini A , Bugani G , Lorenzini M , Longhi S , Bacchi Reggiani ML , et al. Troponin T elevation in acute aortic syndromes: Frequency and impact on diagnostic delay and misdiagnosis. Eur Heart J Acute Cardiovasc Care. 2016;5(7):61–71.
52 Vrsalovic M . Prognostic effect of cardiac troponin elevation in acute aortic dissection: A meta-analysis. Int J Cardiol. 2016;214:277–8. doi:.https://doi.org/10.1016/j.ijcard.2016.03.230
53 del Porto F , Proietta M , Tritapepe L , Miraldi F , Koverech A , Cardelli P , et al. Inflammation and immune response in acute aortic dissection. Ann Med. 2010;42(8):622–9. doi:.https://doi.org/10.3109/07853890.2010.518156
54 Shinohara T , Suzuki K , Okada M , Shiigai M , Shimizu M , Maehara T , et al. Soluble elastin fragments in serum are elevated in acute aortic dissection. Arterioscler Thromb Vasc Biol. 2003;23(10):1839–44. doi:.https://doi.org/10.1161/01.ATV.0000085016.02363.80
55 Li Y , Shao AZ , Jiang HT , Dong GH , Xu B , Yi J , et al. The prominent expression of plasma matrix metalloproteinase-8 in acute thoracic aortic dissection. J Surg Res. 2010;163(2):e99–104. doi:.https://doi.org/10.1016/j.jss.2010.05.030
56 Proietta M , Tritapepe L , Cifani N , Ferri L , Taurino M , Del Porto F . MMP-12 as a new marker of Stanford-A acute aortic dissection. Ann Med. 2014;46(1):44–8. doi:.https://doi.org/10.3109/07853890.2013.876728
57 Suzuki T , Katoh H , Tsuchio Y , Hasegawa A , Kurabayashi M , Ohira A , et al. Diagnostic implications of elevated levels of smooth-muscle myosin heavy-chain protein in acute aortic dissection. The smooth muscle myosin heavy chain study. Ann Intern Med. 2000;133(7):537–41. doi:.https://doi.org/10.7326/0003-4819-133-7-200010030-00013
58 Suzuki T , Distante A , Zizza A , Trimarchi S , Villani M , Salerno Uriarte JA , et al.; International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) Investigators. Preliminary experience with the smooth muscle troponin-like protein, calponin, as a novel biomarker for diagnosing acute aortic dissection. Eur Heart J. 2008;29(11):1439–45. doi:.https://doi.org/10.1093/eurheartj/ehn162
59 van der Linde D , Rossi A , Yap SC , McGhie JS , van den Bosch AE , Kirschbaum SW , et al. Ascending aortic diameters in congenital aortic stenosis: cardiac magnetic resonance versus transthoracic echocardiography. Echocardiography. 2013;30(5):497–504. doi:.https://doi.org/10.1111/echo.12086
60 Harris KM , Strauss CE , Duval S , Unger BT , Kroshus TJ , Inampudi S , et al. Multidisciplinary standardized care for acute aortic dissection: design and initial outcomes of a regional care model. Circ Cardiovasc Qual Outcomes. 2010;3(4):424–30. doi:.https://doi.org/10.1161/CIRCOUTCOMES.109.920140
No financial support and no other potential conflict of interest relevant to this article was reported.