Inert gas washout: background and application in various lung diseases
DOI: https://doi.org/10.4414/smw.2017.14483
Jakob
Usemannab, Sophie
Yammineb, Florian
Singerbc, Philipp
Latzinb
a
bDivision of Respiratory Medicine, Department of Paediatrics, Inselspital, Bern University Hospital,
cDivision of Respiratory Medicine,
Inert gas washout: background and application in various lung diseases
w14483
Summary
Multiple breath inert gas washout (MBW) is a lung function technique to measure ventilation inhomogeneity. The technique was developed more than 60 years ago, but not much used for many decades. Technical improvements, easy protocols and higher sensitivity compared with standard lung function tests in some disease groups have led to a recent renaissance of MBW.
The lung clearance index (LCI) is a common measure derived from MBW tests, and offers information on lung pathology complementary to that from conventional lung function tests such as spirometry. The LCI measures the overall degree of pulmonary ventilation inhomogeneity. There are other MBW-derived parameters, which describe more regional airway ventilation and enable specific information on conductive or acinar ventilation inhomogeneity. How this specific ventilation distribution is exactly related to different disease processes has not entirely been examined yet.
MBW measurements are performed during tidal breathing, making this technique attractive for children, even young children and infants. These benefits and the additional physiological information on ventilation inhomogeneity early in the course of lung diseases have led to increasing research activities and clinical application of MBW, especially in paediatric lung diseases such as cystic fibrosis (CF). In these patients, LCI detects early airway damage and enables monitoring of disease progression and treatment response. Guidelines for the standardisation of the MBW technique were recently published. These guidelines will, hopefully, increase comparability of LCI data obtained in different centres or intervention trials in children and adults.
In this non-systematic review article, we provide an overview of recent developments in MBW, with a special focus on children. We first explain the physiological and technical background to this technique with a short explanation of several methodological aspects that are important for understanding the principle behind the technique and enable high quality measurements. We then provide examples of MBW application in different lung diseases of children and adults, with regards to both clinical application and research activities. Lastly, we report on ongoing clinical trials using MBW as outcome and give an outlook on possible future developments.
Abbreviations
- AHR
-
airway hyperresponsiveness
- BTPS
-
body temperature, pressure, saturated with water
- CT
-
computed tomography
- CF
-
cystic fibrosis
- CFTR
-
cystic fibrosis transmembrane conductance regulator
- COPD
-
chronic obstructive pulmonary disease
- DLCO
-
diffusion capacity for carbon monoxide
- FEV1
-
forced expiratory volume in one second
- FVC
-
forced vital capacity
- FRC
-
functional residual capacity
- LCI
-
lung clearance index
- MBW
-
multiple-breath washout
- MR
-
moment ratio
- MRI
-
magnet resonance imaging
- PCD
-
primary ciliary dyskinesia
- Sacin
-
slope of acinar airways
- Scond
-
slope of conducting airways
Introduction
For decades, conventional spirometry has been the standard technique to assess the degree of airway obstruction in most chronic lung diseases, including cystic fibrosis (CF), asthma, and chronic lung disease of prematurity. However, there is mounting evidence that, because of its underlying physiological principle, spirometry is insensitive for the assessment of peripheral airway involvement and for the assessment of ventilation distribution. This resulted in an increased interest in gas dilution techniques, in particular multiple breath-washout (MBW), for the assessment of small airway function, i.e. efficient, homogeneous ventilation distribution [1, 2].
MBW was first described more than 60 years ago by Ward S. Fowler [3]. In his pioneering work of 1952, he compared nitrogen clearance from single breath washouts between healthy subjects and patients with cardiopulmonary disease, to assess the degree of uneven alveolar gas dilution [3]. However, the technique was little appreciated until gas analysers and computers were further developed to improve automated analysis of gas and volume signals during measurements [4, 5]. Today, the technique is returning to “prime time”, especially in the paediatric pulmonology community. Recently, an international workshop reviewed current literature on the monitoring of preschool lung disease. Besides detailed recommendations for technical standards and measurement procedures, this report suggested MBW as a promising tool in preschool children with CF, highlighting its importance [6].
In this non-systematic review article, we provide an overview of recent developments in MBW. Pubmed and the North American and European clinical trial registries were searched. Search terms were: CF, lung clearance index, lung function test, lung disease, and washout. We specifically focused on literature in children. We first explain the physiological and technical background to this technique with a short explanation of several methodological aspects important for understanding the principle behind the technique and enable high quality measurements. We then provide examples of MBW application in different lung diseases of children and adults, with regards to both clinical application and research activities. Lastly, we report on ongoing clinical trials using MBW as outcome and give an outlook on possible future developments.
Physiological background, mechanisms of ventilation inhomogeneity
The main function of the human lung is to homogeneously ventilate the lungs, enabling efficient gas exchange. During fetal lung development, the lungs grow from proximal to distal by a continuous division of the airways, which later form the unique structure of the bronchial tree. The bronchial tree consists on average of 23 bronchial generations, but gas exchange only occurs in approximately the last 9 generations. The bronchial tree resembles a self-similar, so-called fractal structure, enabling efficient gas transport. Normal ventilation distribution occurs by convection and diffusion. Three main mechanisms of ventilation inhomogeneity are currently known: (i) convection-dependent inhomogeneity in the conducting airway zone (more proximal airways); (ii) diffusion-limitation related inhomogeneity in the diffusion-dependent airway zone (distal airways, acini); (iii) interaction between convection and diffusion in an intermediate zone at the level of the diffusion-convection front, which is thought to arise at the acinar entrance [7]. The acinar compartment, the alveoli, is separated by a thin tissue layer from a capillary meshwork and forms a large surface for efficient oxygen and carbon dioxide gas exchange.
Technical background
MBW testing
Besides analysis devices, MBW tests require only a tight facemask or mouthpiece and quiet tidal breathing for 2 to 10 minutes per test, making this technique applicable across all age groups, even infancy. Measurements in infants are made in a supine position during quiet non-rapid-eye-movement sleep (or with sedation), using a face mask. In older children and adults, measurements are usually performed in a sitting position with a mouth-piece and nose clip. Differences between sitting and supine positions have been described [8] and need to be taken into account when comparing data or in longitudinal studies. To support regular breathing patterns, distraction with videos is recommended for children [9], and visual breathing pattern feedback may be useful for adolescents and adults [10]. Because time for triplicate testing can be demanding in busy outpatient clinics or for patients with advanced lung disease, promising abbreviated protocols have been proposed [11, 12].
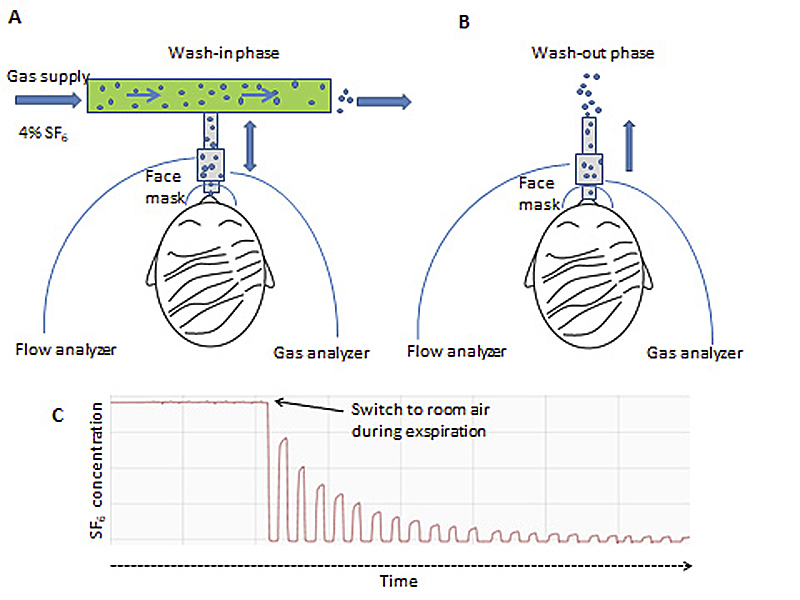
Each MBW test consists of a wash-in and a washout phase. Depending on the gas for the MBW test employed, there are in principle two different ways to perform MBW:
- When an inert extrinsic gas (4% sulphur hexafluoride; 20% helium) is used, the gas mixture is inspired until an equilibrium is reached; the washout phase (breathing room air) starts from this point of equilibrium.
- For inert intrinsic gas (nitrogen), no formal wash-in phase is required for the first of the three tests. For
Regardless of the gas used, the washout is stopped when the test gas reaches 1/40th (or 2.5% from the initial starting concentration set to 100%) of the initial gas concentration [7]. This cut-off was recently challenged, in order to improve comparability between different techniques, as the role of nitrogen is not fully understood yet [13]. The typical equipment setup for sulphur hexafluoride MBW is shown in figure 1.
MBW outcomes
Three main parameters are reported from MBW tests: the functional residual capacity (FRC), the lung clearance index (LCI) and moment ratios (MR). The FRC is the volume of air present in the lungs after tidal expiration in ventilated regions of the lungs; LCI and MR are both measures of global ventilation inhomogeneity. Besides LCI and MR, other parameters specifically assessing peripheral airway ventilation can be calculated, as detailed below.
Given that MBW setups measure inert gas concentrations and the cumulative volume required to washout the resting lung volume (FRC), the latter can be calculated. The FRC is derived from a ratio: the cumulative expired volume (CEV) of the inert gas over the difference between the end-tidal concentrations of the inert gas (Cet) measured at the start (Cetstart) and end (Cetend) of the washout. The LCI is a volume ratio, net CEV (including all gas fractions) over FRC: LCI = CEV/FRC. An increased ventilation inhomogeneity would thus result in more tidal breaths (greater net CEV) needed to wash out the inert gas, and in an increased LCI. To adjust for lung size, net CEV is divided by FRC to obtain LCI.
MR also quantify ventilation inhomogeneity, but are less commonly used. They have been described in detail elsewhere [4, 14–23]. The advantage of MR over LCI is that they can be weighted to specific parts of the washout curve.
Specific markers for peripheral lung ventilation are the slopes of alveolar phase III (SIII) of the inert gas expirogram. The first SIII value is thought to reflect ventilation inhomogeneity within diffusion-convection-dependent acinar airways (Sacin), whereas the subsequent evolution of SIII values from lung turnover 1.5 to 6.0 is thought to reflect ventilation inhomogeneity within convection-dependent conducting airways (Scond) [7, 24]. Although these indices were derived from numerical lung models [24], recent comparative data from ventilation imaging techniques are reassuring [25, 26].
MBW equipment and analysis procedures
One of the earliest and most recognised systems for MBW is based on a respiratory mass spectrometer, which allows simultaneous measurement of multiple gases at 33 Hz or higher. Drawbacks of the AMIS 2000 (Innovision, Denmark) relate to the custom design, sophisticated maintenance and costs [27]. Other customised systems have been described in detail elsewhere [7, 28]. Currently, there are at least three commercially available devices, which strongly differ in regard to the inert gas used, the gas analyser, analysis algorithms and the age group for which application is recommended (table 1).
Table 1 Currently available multiple breath washout devices.
|
Eco Medics infants
|
Eco Medics preschool and older
|
ndd EasyOne Pro LAB*
|
Innovision Innocor
|
| Method |
SF6
|
N2
|
N2
|
SF6
|
| Flow/volume measurement |
Ultrasonic flowmeter; |
Ultrasonic flowmeter |
Ultrasonic flowmeter |
Pneumotachometer |
| Tracer gas measurement |
indirect via molar mass mainstream |
Indirect via O2 and CO2 measurement |
Indirect via molar mass, O2 and CO2 measurement |
Direct via photoacoustic spectroscopy, gas reservoir bag |
| Gas concentration |
4% SF6
|
100% O2
|
100% O2
|
SF6 mixture: 0.1% or 0.2% SF6 (27.6% O2, 0.35% N2O) |
| Validation studies |
Lung model Schmidt [29], Gustafsson [30] |
Lung model and in vivo Singer [31] |
- |
In vivo Horsley [10] Lung model
and in vivo Gonem [32] |
| Methodological studies |
Anagnostopoulou [33], Latzin [34] |
Jensen [35], Summermatter [36] |
- |
Horsley [37], Downing [38], Grønbæk [39], Gonem [25], Gonem [32], Nielsen [40], Shawcross [41] |
| Application in healthy |
Fuchs [42], Anagnostopoulou [33], Gray [43] Gray [44] |
|
|
Downing [38] |
| Studies in cystic fibrosis |
Belessis [45], Simpson [46], Hall [47] |
Stanojevic [48], Stahl [49], Amin [50], Ramsey [51] |
|
Davies [52] |
| Cystic fibrosis and controls |
|
Singer [53], Poncin [54] |
Poncin [54] |
Downing [38] |
| Other disease groups |
Hulskamp [55], Latzin [56] |
Yammine [57], Boon [58], Nyilas [59], Madsen [60], Jarenback [61] |
Fuchs [62] |
Macleod [63] |
Gases for MBW testing
Depending on the choice of gas and setup, derived MBW indices differ substantially [54]. For example, helium is much lighter than sulphur hexafluoride and generates systematically higher LCI values [7]. Furthermore, in subjects with emphysematous diseases, the diffusion equilibrium in the enlarged peripheral airways differs between gases. Other aspects regarding the use of sulphur hexafluoride are its costs and limited availability, since it belongs to the most potent greenhouse gases. Applications using lower sulphur hexafluoride fractions may be more suitable for routine use.
The choice of intrinsic gas (nitrogen) for MBW has the advantage that the oxygen required for washout is widely available and affordable. Another strength is that nitrogen is present in all parts of the lung and this gas, therefore, has great sensitivity to detect abnormality compared with extrinsic gases. Further, the wash-in is with room air and without the need for a tight mouthpiece, making it much easier to apply.
There is clear evidence that breathing patterns may change during MBW. Although in adults fixed 1-L breathing protocols may have some advantages with regards to slope III standardisation and analysis [67], most studies nowadays use free tidal breathing in order to take advantage of the natural breathing pattern. This is especially important in children, as fixed breathing protocols have been shown to influence MBW outcome parameters substantially [68]. Application of 100% oxygen in MBW was shown to alter breathing patterns in infants [22], and sulphur hexafluoride induced transient hypopnoea in preterm and healthy infants [69, 70]. However, in school-age children, MBW indices were not influenced by inhalation of 100% oxygen [71]. The effect of nitrogen back-diffusion from tissue nitrogen also requires further studies.
Sample flow and gas analysis
Tidal flow and gases are usually measured within the main path of the respiratory flow (mainstream), or a continuous sample is taken from a capillary (sidestream). Sidestream sample flow (suction) may alter the analyser response and add noise to “small” signals from infants. Flows and integrated volumes have to be further corrected for BTPS (body temperature, pressure, saturated with water) [7]. The gas concentrations can then be measured directly (respiratory mass spectrometry, infrared, etc.) or indirectly (molar mass, cumulative gas fractions, etc.) [31]. Of note, usually the flow and gas signals are not sampled at the same sensor point. Signals, therefore, need to be aligned in time. Poor BTPS correction or signal misalignment can be a source of error in MBW outcomes [7, 36].
Impact of dead space on LCI
Dead space roughly consists of two compartments. Technical dead space consists of the volume of MBW hardware (mask, mouthpiece, and tubes) required to transport gases to the sensors. Anatomical and physiological dead spaces are the volume of upper and lower airways, respectively, which transport gases but do not participate in gas exchange. Technical dead space is hardware specific and affects LCI [35, 72]. The impact seems larger in younger children than in adults, and is apparently independent of lung disease. Thus, a small technical dead space of ≤2 ml/kg is recommended [73].
Software for MBW analysis
For analyses of MBW indices, commercial online and offline software is available, and several custom-made software applications exist. Offline data analysis was frequently used in the past, but it has the disadvantage that analysis is time consuming, and this limits its application in clinical settings. Several studies reported an effect on MBW indices of different software and settings [33, 36, 74]. Current commercial setups usually provide recording and analysis software on board. These applications undergo constant development and software updates need to be validated in clinical settings for reliability [75–77].
Normative data for MBW
Depending on the age of subjects and the factors mentioned above (gas, equipment, dead space, software), LCI is usually below 8.5 lung turnovers (LCI units) in healthy subjects. However, normative data for MBW measurements across different age groups are scanty [42]. Some data stem from customised setups [78], which limits their generalisability. Furthermore, data are not only system, but also gas, specific and may even be influenced by the analysis approach. The latter has been shown for both healthy individuals and patients with CF [33, 74]. Two studies reported MBW reference values for infants, recorded with common available equipment from a large Swiss [42] and African population [43] and with measurements conducted at 5 weeks postmenstrual age. Since LCI is thought to decrease throughout infancy and early childhood, then remain constant and increase in the elderly [78], these reference data cannot be applied to other age groups. There are other relevant factors that influence MBW measures, including the posture during tests (supine or seated) [8], the gas choice (sulphur hexafluoride, nitrogen) [35], dead space [34, 72, 79], as well as sedation, which may play a significant role in LCI variation with age.
Limitations of MBW testing, knowledge gaps
Limitations to MBW application relate to technical and physiological aspects. Much effort has been made to improve standardisation of MBW protocols and analyses, but there are still several unanswered questions, as mentioned above. Overcoming these knowledge gaps seems difficult, considering the, at times, poor software transparency [76]. Besides software, there are other aspects that may change MBW indices. There is evidence that interventions prior to MBW testing, such as raised volume rapid thoracoabdominal compression [80] or physiotherapy [81–83], subsequently effect MBW outcomes. Other important aspects have been outlined previously [7].
The impact of repeat LCI measurements on respiratory disease outcomes is largely unknown. Data suggest a clear association with infection burden, structural airway pathology or later pulmonary exacerbation in CF, although it remains unclear what change in LCI should prompt clinicians to intervene. As for most lung function outcome parameters, the beneficial effect of regular measurement of LCI in clinics on disease outcome has not been assessed yet. Recent data may help to establish what would constitute a clinically important change in LCI, at least in preschool children [48].
Single breath washout
There are techniques other than MBW to assess ventilation inhomogeneity: single breath washout tests, with a single or double inert tracer gas mixture. Several studies have used this technique, also in younger children. Single breath washout has been used in CF patients to detect early lung disease [84], assess response to airway clearance [85], and to study the involvement of small airways in patients with mild asthma [86], COPD [87–90], PCD [60, 91] and bronchiolitis obliterans as a complication after lung transplantation [92–94]. SBW may be attractive for clinical settings, since measurements can be completed more quickly than for MBW, and single breath washout can be used during normal tidal breathing or forced manoeuvres. Although acceptable reproducibility of this test has been reported in adults [89] and children [84], reproducibility is lower than with MBW. Several unanswered technical aspects also remain (i.e., impact of breathing pattern) [7, 95, 96], precluding its use for clinical decision making.
Application of MBW in lung diseases
Cystic fibrosis
CF is an inherited life-limiting disease with a mean prevalence of approximately 0.8/10 000 in Europe and the United States [97]. In Switzerland, newborn screening, introduced in 2011, enables early CF diagnosis and follow-up of lung function [77]. CF lung disease is characterised by mucus plugging, chronic infection and inflammation resulting in irreversible lung damage. Treatment advances have resulted in the preservation of normal forced expiratory volume in one second (FEV1) (>˗1.64 z-scores) into young adulthood, but progression of bronchiectasis may be undetected by spirometry [98]. This led to more research into detection of early airway abnormalities in CF patients with MBW, and several observational studies and clinical trials support its usefulness.
Observational studies
The majority of longitudinal data from infants and children with CF are currently obtained from two large prospective cohorts, the Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) and the London Cystic Fibrosis Collaboration (LCFC), (reviewed in [99]). There is mounting evidence that, compared with healthy controls, LCI is already abnormal shortly after birth in patients with CF [1, 100, 101]. In infancy, LCI is normal in approximately half of the infants. Interestingly, there are infants with normal LCI values in the presence of abnormal forced volume values (as assessed with the raised volume rapid thoracoabdominal compression technique) [102]. Several observational studies “tracked” LCI from preschool to school age [103, 104]. A multicentre study assessed LCI at several time points and was able to identify significant deterioration of LCI in CF over time, which was not detected by spirometry [48]. Another study found that LCI during preschool years was more likely to be abnormal than spirometry, and an abnormal LCI in preschool children predicted both abnormal LCI, and abnormal spirometry at school age [103].
Studies in infants [45], children [53, 102] and adults [105] showed that patients with evidence of a bacterial infection were more likely to have abnormal LCI values. Findings from the longitudinal study AREST-CF suggested that LCI has a more pronounced increase in infants with airway infections compared with those without, and that this increase was long lasting [46]. Several studies used MBW to monitor treatment response to antibiotic therapy in infants and adult patients with CF [49, 106, 107]. A systematic review included data from 176 exacerbations and observed an overall decrease, albeit small (≈˗3%), in LCI after antibiotic treatment. The LCI response to therapy was very heterogeneous in CF patients, and is not fully understood [106].
Clinical trials
There is an ongoing debate about whether or not MBW can be used in multicentre trials. The Cystic Fibrosis Foundation Workshop Report from 2015, for example, concluded that lack of knowledge on MBW equipment hampers routine application of MBW in clinical trials [6]. On the other hand, the European CF Society Clinical Trial Network Standardization Committee [108] suggested LCI as an outcome measure, especially in young children and those with mild CF disease.
To date, several single- and multicentre trials in CF patients that used LCI as outcome have been published. Two multicentre interventional studies investigated the treatment effect of drugs modifying the cystic fibrosis transmembrane conductance regulator (CFTR) defect in CF patients. One study enrolled patients aged >6 years, with at least one copy of the rare G551D mutation and normal FEV1, and assessed the ability of LCI to detect a treatment effect of ivacaftor. Treatment with ivacaftor resulted in significant improvement of LCI compared with placebo [52]. Another study enrolled patients aged 6 to 11 years and homozygous for F508del-CFTR, to assess the treatment effect of combined therapy with ivacaftor and lumacaftor on LCI. No changes in FEV1 were observed after 24 weeks of intervention, whereas there was significant improvement in LCI [109].
Two trials examined the usefulness of LCI in infants and preschool children to assess treatment response to hypertonic saline inhalation [50, 110]. In one study, MBW measurements were made before and after treatment with hypertonic saline (twice daily for 48 weeks) in children <6 years (n = 25). LCI decreased (improved) significantly more in the hypertonic saline group, as compared with controls [110]. Of note, the pattern of LCI change with treatment was age dependent: in a subgroup of subjects <1 year of age, LCI was in the normal range at baseline and did not change after treatment [110]. A recent study in older children (mean age 14.0 years, n = 18) investigated the short term effect of hypertonic saline inhalation [50], and found that LCI did not change after 24 hours of treatment [50].
Responsiveness of LCI was assessed in two randomised double-blind placebo-controlled trials in older children. FEV1 was not systematically affected, whereas LCI improved over a 1-month period after treatment with hypertonic saline [111], and with dornase alfa (Pulmozyme®), an enzymatic agent improving mucus clearance in CF [112].
A cross-sectional study assessed the effect of antibiotic therapy on LCI abnormalities and magnet resonance imaging (MRI) in clinically stable patients, aged 1 to 20 years. LCI and MRI were able to detect an effect of antibiotic treatment of pulmonary exacerbations [49], indicating that these tools can provide useful endpoints for intervention trials.
MBW indices and lung imaging
Several recent studies in children and infants compared the association of LCI with structural airway damage or functional correlates using lung imaging techniques, including computed tomography (CT) and MRI. Studies in infants reported poor association between LCI and bronchiectasis as assessed with CT [47, 51], but a closer correlation was found in preschool- and school-age children [51]. This is not the case for spirometry indices. A strong correlation between LCI and MRI imaging was reported in clinically stable CF patients (age range 2–20 years) [49]. This study further showed that LCI and MRI are able to distinguish disease severity levels, supporting the application of these tools for diagnostic and therapeutic monitoring. Taken together, these data support the concept of LCI as a sensitive measure of structural airway pathology. However, LCI cannot replace lung imaging yet as negative predictive values to exclude bronchiectasis appear too low, especially in younger children. An overview of MBW application in CF lung disease is given in table 2.
Table 2 Overview of studies in paediatric patients with cystic fibrosis using multiple-breath washout measurements.
|
Study description
|
Patient number
|
Age at study
|
Study type
|
Main findings
|
Reference*
|
|
CF vs controls
|
|
|
|
|
|
| Infants |
71 |
3 months |
Cross-sectional |
Higher LCI and FRC in infants with CF compared with 71 controls. |
Hoo [102] |
| Preschool / school age / adult |
40 |
2.5–5 years |
Cross-sectional |
Higher LCI in patients with CF compared with 37 controls. |
Aurora [1] |
| 48 |
4.09 (0.7) and 7.83 (1.3) years |
Prospective |
Higher LCI in CF patients at preschool age compared with 45 controls. Preschool LCI predicted abnormal LCI in CF patients at school age. |
Aurora [103] |
| 78 |
2.5–6 years |
Prospective |
LCI measurements in CF patients and 70 controls at 1, 3, 6 and 12 months detected lung function deterioration over time in CF patients. |
Stanojevic [48] |
| 60 |
7.8 (1.3) years |
Cross-sectional |
Higher LCI in CF patients compared with 60 controls. |
Owens [101] |
| 43 |
3–18 years |
Cross-sectional |
Higher LCI and MR in CF patients compared with 28 controls. |
Gustafsson [100] |
| 47 |
9.5–16.1 years |
Cross-sectional |
LCI was higher in CF patients compared with 50 controls. Comparison of LCI measured with two different MBW devices revealed poor agreement between different setups. |
Poncin [54] |
|
CF with vs without bacterial infection
|
47 |
0.32–3.24 years |
Cross-sectional |
LCI of 25 healthy subjects was lower compared with CF patients without and with bacterial infection. In CF patients, inflammatory markers from BAL correlated with LCI. |
Belessis [45] |
| 78 |
4–16 years |
Prospective |
LCI was higher in CF patients compared with 53 controls. LCI strongly correlated with Pseudomonas aeruginosa infection. |
Singer [53] |
| 108 |
0.1–2.5 years |
Prospective |
LCI measurements at three time points during 2 years revealed a long-lasting increase in LCI after pulmonary infections. Haemophilus influenzae infections had a particularly detrimental effect on lung function. |
Simpson [46] |
| 41 |
8–67 years |
Cross-sectional |
LCI was higher in CF patients compared with 6 controls. A lower colony count of aerobic/anaerobic bacteria was associated with a higher LCI in CF patients. |
O`Neill [105] |
|
Assessment of treatment
|
|
|
|
|
|
| Antibiotic treatment for pulmonary exacerbations |
|
|
Systematic review of studies published until March 2014 |
Overall, LCI decreased after antibiotic treatment but individual response was heterogeneous in CF patients. |
Sonneveld [106] |
| 26 |
1.8–19.9 years |
Prospective |
LCI was sensitive to detect response to antibiotic therapy for pulmonary exacerbations. |
Stahl [49] |
| Hypertonic saline (7%) |
25 |
1–5 years |
Observational |
Hypertonic saline twice daily for 48 weeks improved LCI. |
Subbarao [110] |
| 18 |
7–56 years |
Observational |
Hypertonic saline 5 times over 24 hours did not improve LCI. |
Amin [50] |
| 19 |
10.5 (3.1) years |
Observational |
Hypertonic saline twice daily over 4 weeks improved LCI. |
Amin [111] |
| CFTR regulator ivacaftor |
21 |
8–43 years |
Clinical trial |
Ivacaftor 150 mg twice daily for 28 days improved LCI. |
Davies [52] |
| CFTR regulators lumacaftor/ivacaftor combined |
58 |
6–11 years |
Clinical trial |
Lumacaftor 200 mg + ivacaftor 250 mg twice daily for 24 weeks improved LCI, sweat chloride and nutritional status. |
Milla [109] |
| Dornase alfa (Pulmozyme®) |
17 |
6–18 years |
Clinical trial |
For weeks 2.5 ml daily Pulmozyme® inhalation improved LCI. |
Amin [112] |
|
Lung imaging and MBW
|
|
|
|
|
|
| Magnet resonance imaging |
97 |
0.2–21.1 years |
Prospective, cross-sectional |
LCI correlated with abnormalities on MRI in infants, toddlers and children with CF. |
Stahl [49] |
| Computed tomography |
60 |
7.8 (1.3) years |
Cross-sectional |
Abnormal findings on lung CT correlated strongly with increased LCI in CF patients. |
Owens [101] |
| 49 |
8.7–112.1 weeks |
Cross-sectional |
Air trapping and bronchiectasis assessed by CT were associated with MR, but not LCI. |
Hall [47] |
| 42, 38, and 39 |
0–2, 3–6 and 7–16 years |
Cross-sectional |
Agreement between LCI and bronchiectasis in preschool and school age children, but not infants. Air trapping and LCI was only associated in infants. |
Ramsey [51] |
Wheezing
Lower respiratory tract infections, or other triggers such as allergen exposure, leading to wheezing episodes in preschool children, are highly prevalent. Some, but not all, wheezers experience worsening lung function [113] and involvement of the small airways, as assessed with MBW and lung biopsy [114]. Functional data from MBW are conflicting. One study measured LCI in 110 infants who required respiratory support during the first days of life. Measurements were performed with sedation, at approximately 5 weeks postmenstrual age. The authors found a higher LCI in infants with less (≤3%) expiratory wheezing, compared with infants with more (>3%) expiratory wheezing [115]. These results were supported in a subsequent study reporting a higher LCI in 40 infants without wheezing compared with 41 wheezing infants [72]. These findings contradict a study in preschool children, which reported an increased LCI in wheezers compared with healthy controls [114], but mean LCI differences were small (6.8 vs 6.6.) and within the normal range. Apparently, the effect of bronchodilator inhalation on LCI is somewhat paradoxical. LCI may increase (worsen) after inhaled salbutamol in controls, but not in wheezing subjects. High variability between MBW tests at baseline and heterogeneous response to bronchodilators questions the use of MBW to distinguish preschool wheezers from healthy controls [116]. To summarise, current data do not support application of MBW measurements in infants and children to characterise wheezing or to monitor treatment response.
Asthma
Asthma is the most common chronic respiratory disease in children, typically characterised by eosinophilic airway inflammation, airway hyperresponsiveness (AHR) and reversible airway obstruction. AHR is the ability of airways to narrow excessively as a result of provoking stimuli. Whereas current guidelines recommend symptom assessment and spirometry for the diagnosis of asthma [117], their value for monitoring asthma is not clear [118]. Spirometry was rather insensitive in the assessment of peripheral airways, unless severe obstruction was present [119]. LCI in asthmatic subjects was slightly elevated compared with healthy controls, but often within the normal range, and therefore not helpful in the diagnosis of asthma at the individual level [63, 120, 121]. Few studies have assessed the association between MBW measures and AHR. A study in older asthmatic subjects (age range 59–80 years) found that AHR was predicted by higher Sacin [122], and a study in asthmatic adults (age range 18–66 years) found that AHR was associated with LCI, and that treatment with inhaled corticosteroids decreased the LCI [123]. In adults with severe asthma, inhaled corticosteroid uptitration resulted in a decrease in Scond with no effect on Sacin [124]. The study further reported that those patients with increased ventilation inhomogeneity at baseline had the greatest improvement following inhaled corticosteroid dose uptitration [124]. An effect of bronchodilator response on LCI is still poorly understood. Two studies in children found no effect [63, 121], whereas another study reported that the LCI was elevated in adolescents with asthma and decreased after treatment with nebulised bronchodilator [125]. To summarise, MBW can be useful to explore the exact degree of airway damage and physiological impairment in asthma, but up to now the technique is not established for diagnosis or for monitoring treatment response in asthma.
Chronic lung diseases of prematurity
Disruption of normal lung development because of preterm birth results in complex structural changes in the airways, lung parenchyma and vasculature. This may result in smaller airway calibres and impaired alveolarisation, with a reduced number of alveoli and enlarged airspaces [126].
The functional relevance of disrupted airway development during infancy and thereafter is not fully understood. This can be partly explained by the fact that the degree of airway obstruction was underestimated by spirometry. Recent studies in former preterm infants used other outcomes, including the LCI [55–57, 127–129], FRC [44, 55, 56] and parameters specifically assessing peripheral airway damage (Scond, Sacin) [57]. Results are inconsistent, probably reflecting the heterogeneity of the disease and different measurement techniques. Abnormal values for LCI have been reported in preterm infants during infancy [55], and for former preterm infants at school age [129]. Other studies reported no difference in LCI values in former preterm infants compared with healthy subjects during early infancy [56], at 1 year [44], between 9 and 11 years [130], and between 7 and 13 years [57] of age. The last study further investigated parameters that specifically reflect peripheral airway ventilation. In that study, FEV1 and Scond were often abnormal at school age, whereas Sacin was comparable between former preterm and healthy subjects. A functionally normal alveolar compartment at school age, with functionally abnormal central conducting airways, suggest a disynaptic growth pattern of the lungs in former prematurely born children [57]. Using MBW measurements, studies assessed the development of lung volume. Prematurity was associated with a reduced FRC during early infancy [55] and at 1 year of age [44].
Primary ciliary dyskinesia
Primary ciliary dyskinesia (PCD) is a rare congenital disease characterised by defective ciliary function leading to impaired mucociliary clearance and recurrent chronic upper and lower airway infections [131]. The diagnosis of PCD is challenging because of the heterogeneous nature of the disease and poorly standardised algorithms [131]. Patients with PCD may already have reduced lung function (FEV1, forced vital capacity [FVC]) in the preschool years [132]. As in CF, spirometry may underestimate the full degree of functional impairment in early PCD lung disease [58, 59]. Recent studies reported marked peripheral airway dysfunction, assessed with MBW, in almost all patients with PCD [60, 91, 133]. Another study compared the association between LCI and FEV1 with structural abnormalities, assessed with CT, in 38 patients. The authors reported a higher concordance between LCI and structural abnormalities compared with FEV1 (83 vs 53%), suggesting that LCI measurements may be of clinical relevance in PCD patients [58]. Interestingly, studies using sulphur hexafluoride MBW did not find this association [134]. Overall, PCD lung disease is characterised by marked peripheral airway dysfunction, and MBW may be a promising tool for early detection of airway damage. Compared with CF, MBW data in PCD are scarce. Whether MBW has utility in the management and/or treatment of PCD still needs to be determined in longitudinal studies.
Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is characterised by a variable combination of airway and parenchymal abnormalities. COPD may partly develop irrespective of tobacco smoking, and much earlier in childhood than previously assumed [135, 136]. The hallmark of COPD is mainly irreversible airflow obstruction resulting from the narrowing of small conducting airways or loss of lung elastic recoil or both. In the diagnosis of airflow obstruction in COPD, simple spirometry is the main diagnostic tool, although it is recognised that none of the derived parameters are sensitive indicators of peripheral airway narrowing [137, 138].
Within a prospective study of subjects who smoked ≥10-pack years, an increase in ventilation inhomogeneity in both the conductive and acinar airways was detected by means of normal spirometry, as indicated by increased Scond and Sacin values. Moreover, both Scond and Sacin were associated with FEV1/FVC values, but the former was also associated with FEV1 and the latter with diffusion capacity for carbon monoxide (DLCO) [139]. Similar results were reported from a cross-sectional study of 57 COPD patients with ≥15-pack years. COPD patients had increased Sacin but not Scond values compared with healthy controls [61], and an association between Sacin and DLCO was also reported [89]. Taken together, these studies suggest that MBW measurements have the potential to diagnose subclinical disease at an earlier stage, separate airway from parenchymal abnormalities, and to further characterise clinical features of advanced COPD. Although good feasibility and reproducibility of washout measurements in COPD patients have been shown [89], there is still further need to validate this method for noninvasive detection of early abnormalities or peripheral or parenchymal sites [140].
Other disease groups
In addition to the application of MBW in patients with CF and other common lung diseases, recent studies have applied this technique in less frequent lung disease, of which some studies will be reported.
Bronchiolitis obliterans is a small airway disease in which chronic inflammation results in fibrotic remodelling of the peripheral airways. It can occur, for example, after exposure to toxic substances or following allogeneic haematopoietic stem cell transplantation. Early detection of bronchiolitis obliterans is important in order to prevent later lung damage. One study reported abnormal washout indices before a decrease in FEV1 was observed [141], and measurements may even be used to detect different severity grades of bronchiolitis obliterans, as recently reported [142]. In patients undergoing haematopoietic stem cell transplantation, a study of 33 clinically stable recipients provided some evidence that measurements of MBW were more sensitive than spirometry to detect bronchiolitis obliterans at an early stage [143].
Alpha-1-antitrypsin deficiency is a genetically inherited disorder resulting in emphysematous lung changes. Within a heterogeneous group of 193 patients with alpha-1-antitrypsin deficiency (age range 4–79 years), LCI was higher in patients than in controls. Furthermore, LCI was found to be abnormal in patients with normal spirometry measures, indicating that LCI identifies lung disease related to alpha-1-antitrypsin deficiency earlier than spirometry, comparable to the chronic lung diseases discussed earlier in this article [62].
Bronchiolitis is a common respiratory tract infection during early childhood. A recent study included 29 infants (mean age 3.7 months) with bronchiolitis and reported an increased LCI compared with controls [144]. Another prospective study enrolled infants below <1 year of age hospitalised with bronchiolitis due to respiratory syncytial virus infection and reported differences in FEV1, but not in LCI, between patients and controls at the age of 18 years [145].
MBW in ongoing clinical trials
Currently, there are more than 50 ongoing studies using MBW indices as outcome measures listed in the North American [146] and European [147] clinical trials registries. Most of these trials are in patients with CF or asthma, but some are in less common lung diseases. We present a selection of trials we consider interesting, although it is not a comprehensive overview.
The first trial using LCI as primary outcome in CF patients (aged 6–11 years) homozygous for the F508del mutation after treatment with combined therapy of lumacaftor/ivacaftor (Orkambi®) twice daily over 24 weeks is still ongoing [148]. The study assesses efficacy and safety of the drug, as well as treatment effects on LCI. Two further trials in children are investigating the treatment effect of 8 weeks of ivacaftor. One trial is already ongoing, aiming to recruit 50 children aged 3–5 years with different CFTR mutations to assess changes in LCI [149], and another trial will study the efficacy of ivacaftor in subjects >6 years old, focusing on a specific CFTR mutation [150].
One trial is being conducted in PCD patients within the BESTCILIA (Better Experimental Screening and Treatment for primary CILIAry dyskinesia) study. This is a European multicentre, double-blind, randomised, placebo-controlled trial with the aim to determine the efficacy of azithromycin maintenance therapy for 6 months on respiratory exacerbations in patients with PCD. This interventional trial is currently ongoing and aims to recruit 125 PCD patients, aged 7–50 years old. Besides the quality-of-life assessment, one of the main outcomes is the LCI [151]. This trial will hopefully help elucidate whether or not maintenance therapy with azithromycin has beneficial effects in patients with PCD.
Several trials assess treatment response to inhaled glucocorticoids, betamimetics or hypertonic saline in adult [152] and paediatric patients with asthma [153] and CF [154]. For example, in patients with CF aged 6–18 years of age, a randomised controlled trial is assessing the short term effects (within 24 hours post-dose) of hypertonic saline inhalation on LCI [154].
There is an observational, prospective study recruiting children with CF and asthma, but interestingly, also children with bronchiolitis obliterans and sickle cell anaemia [155]. This trial will validate the MBW device for different lung diseases in Canada, where it is not currently licensed yet. This study may also provide insights into the application of MBW in pulmonary complications in children with sickle cell disease, which has not yet been investigated.
Little is currently known of the involvement of small airways in patients with interstitial lung disease. One trial currently underway will perform MBW in adult patients with different manifestations of interstitial lung disease (Wegner’s Disease, idiopathic pulmonary fibrosis, sarcoidosis) to further understand the complex pathology and heterogeneous presentation of these diseases [156].
Conclusion
MBW has regained interest in recent years. Reasons for this renaissance relate to technical and physiological considerations. The technique is sufficiently sensitive for the early detection of lung function impairment often arising in small peripheral airways. MBW provides information that cannot currently be obtained by other lung function tests or chest imaging. MBW is especially attractive for young patients, as measurements require minimal cooperation. There is mounting evidence that the LCI is useful for assessing the extent and progression of lung disease, as well as treatment response, in patients with CF. LCI is already broadly applied in clinical care of CF patients, yet a number of unresolved questions remain. For example, there are currently poorly defined upper limits of normal for LCI values and its natural variability over time. Further, different software settings and device setups may have an effect on LCI values. Application of MBW tests in other chronic lung diseases may be attractive for research, whereas the impact on clinical management and respiratory disease outcomes require further studies.
Acknowledgement
We thank Dr Sylvia Nyilas and Dr Insa Korten for her critical reading of the manuscript and Karine Landgren Hugentobler for helping with English style.
Author contributions
All authors drafted and approved the final manuscript.
Philipp Latzin, MD, PhD, Division of Respiratory Medicine, Department of Pediatrics, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 8, CH-3010 Bern,, philipp.latzin[at]insel.ch
References
1
Aurora
P
,
Bush
A
,
Gustafsson
P
,
Oliver
C
,
Wallis
C
,
Price
J
, et al.; London Cystic Fibrosis Collaboration. Multiple-breath washout as a marker of lung disease in preschool children with cystic fibrosis. Am J Respir Crit Care Med. 2005;171(3):249–56. doi:.https://doi.org/10.1164/rccm.200407-895OC
2
Hjalmarson
O
,
Sandberg
K
. Abnormal lung function in healthy preterm infants. Am J Respir Crit Care Med. 2002;165(1):83–7. doi:.https://doi.org/10.1164/ajrccm.165.1.2107093
3
Fowler
WS
,
Cornish
ER, Jr
,
Kety
SS
. Lung function studies. VIII. Analysis of alveolar ventilation by pulmonary N2 clearance curves. J Clin Invest. 1952;31(1):40–50. doi:.https://doi.org/10.1172/JCI102575
4
Kraemer
R
,
Meister
B
. Fast real-time moment-ratio analysis of multibreath nitrogen washout in children. J Appl Physiol (1985). 1985;59(4):1137–44.
5
Robinson
PD
,
Goldman
MD
,
Gustafsson
PM
. Inert gas washout: theoretical background and clinical utility in respiratory disease. Respiration. 2009;78(3):339–55. doi:.https://doi.org/10.1159/000225373
6
Subbarao
P
,
Milla
C
,
Aurora
P
,
Davies
JC
,
Davis
SD
,
Hall
GL
, et al.
Multiple-Breath Washout as a Lung Function Test in Cystic Fibrosis. A Cystic Fibrosis Foundation Workshop Report. Ann Am Thorac Soc. 2015;12(6):932–9. doi:.https://doi.org/10.1513/AnnalsATS.201501-021FR
7
Robinson
PD
,
Latzin
P
,
Verbanck
S
,
Hall
GL
,
Horsley
A
,
Gappa
M
, et al.
Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur Respir J. 2013;41(3):507–22. doi:.https://doi.org/10.1183/09031936.00069712
8
Ramsey
KA
,
McGirr
C
,
Stick
SM
,
Hall
GL
,
Simpson
SJ
; AREST CF. Effect of posture on lung ventilation distribution and associations with structure in children with cystic fibrosis. J Cyst Fibros. 2017;S1569-1993(17)30020-6.
9
Aurora
P
,
Gustafsson
P
,
Bush
A
,
Lindblad
A
,
Oliver
C
,
Wallis
CE
, et al.
Multiple breath inert gas washout as a measure of ventilation distribution in children with cystic fibrosis. Thorax. 2004;59(12):1068–73. doi:.https://doi.org/10.1136/thx.2004.022590
10
Horsley
AR
,
Gustafsson
PM
,
Macleod
KA
,
Saunders
C
,
Greening
AP
,
Porteous
DJ
, et al.
Lung clearance index is a sensitive, repeatable and practical measure of airways disease in adults with cystic fibrosis. Thorax. 2008;63(2):135–40. doi:.https://doi.org/10.1136/thx.2007.082628
11
Yammine
S
,
Singer
F
,
Abbas
C
,
Roos
M
,
Latzin
P
. Multiple-breath washout measurements can be significantly shortened in children. Thorax. 2013;68(6):586–7. doi:.https://doi.org/10.1136/thoraxjnl-2012-202345
12
Robinson
PD
,
Stocks
J
,
Aurora
P
,
Lum
S
. Abbreviated multi-breath washout for calculation of lung clearance index. Pediatr Pulmonol. 2013;48(4):336–43. doi:.https://doi.org/10.1002/ppul.22618
13
Yammine
S
,
Lenherr
N
,
Nyilas
S
,
Singer
F
,
Latzin
P
. Using the same cut-off for sulfur hexafluoride and nitrogen multiple-breath washout may not be appropriate. J Appl Physiol (1985). 2015;119(12):1510–2. doi:.https://doi.org/10.1152/japplphysiol.00333.2015
14
Saidel
GM
,
Salmon
RB
,
Chester
EH
. Moment analysis of multibreath lung washout. J Appl Physiol. 1975;38(2):328–34.
15
Fleming
GM
,
Chester
EH
,
Saniie
J
,
Saidel
GM
. Ventilation inhomogeneity using multibreath nitrogen washout: comparison of moment ratios and other indexes. Am Rev Respir Dis. 1980;121(5):789–94.
16
Wall
MA
. Moment analysis of multibreath nitrogen washout in young children. J Appl Physiol (1985). 1985;59(1):274–9.
17
Hutchison
AA
,
Sum
AC
,
Demis
TA
,
Erben
A
,
Landau
LI
. Moment analysis of multiple breath nitrogen washout in children. Am Rev Respir Dis. 1982;125(1):28–32.
18
Kraemer
R
,
Zehnder
M
,
Meister
B
. Intrapulmonary gas distribution in healthy children. Respir Physiol. 1986;65(2):127–37. doi:.https://doi.org/10.1016/0034-5687(86)90045-9
19
Habib
RH
,
Lutchen
KR
. Moment analysis of a multibreath nitrogen washout based on an alveolar gas dilution number. Am Rev Respir Dis. 1991;144(3 Pt 1):513–9. doi:.https://doi.org/10.1164/ajrccm/144.3_Pt_1.513
20
Schibler
A
,
Hall
GL
,
Businger
F
,
Reinmann
B
,
Wildhaber
JH
,
Cernelc
M
, et al.
Measurement of lung volume and ventilation distribution with an ultrasonic flow meter in healthy infants. Eur Respir J. 2002;20(4):912–8. doi:.https://doi.org/10.1183/09031936.02.00226002
21
Aurora
P
,
Kozlowska
W
,
Stocks
J
. Gas mixing efficiency from birth to adulthood measured by multiple-breath washout. Respir Physiol Neurobiol. 2005;148(1-2):125–39. doi:.https://doi.org/10.1016/j.resp.2005.05.027
22
Singer
F
,
Yammine
S
,
Schmidt
A
,
Proietti
E
,
Kieninger
E
,
Barben
J
, et al.
Ventilatory response to nitrogen multiple-breath washout in infants. Pediatr Pulmonol. 2014;49(4):342–7. doi:.https://doi.org/10.1002/ppul.22841
23
Egger
B
,
Jost
K
,
Anagnostopoulou
P
,
Yammine
S
,
Singer
F
,
Casaulta
C
, et al.
Lung clearance index and moment ratios at different cut-off values in infant multiple-breath washout measurements. Pediatr Pulmonol. 2016;51(12):1373–81. doi:.https://doi.org/10.1002/ppul.23483
24
Verbanck
S
,
Paiva
M
. Model simulations of gas mixing and ventilation distribution in the human lung. J Appl Physiol (1985). 1990;69(6):2269–79.
25
Gonem
S
,
Hardy
S
,
Buhl
N
,
Hartley
R
,
Soares
M
,
Kay
R
, et al.
Characterization of acinar airspace involvement in asthmatic patients by using inert gas washout and hyperpolarized (3)helium magnetic resonance. J Allergy Clin Immunol. 2016;137(2):417–25. doi:.https://doi.org/10.1016/j.jaci.2015.06.027
26Bauman G, et al. Pulmonary Fourier decomposition MRI compared to multiple breath washout and spirometry: A preliminary study in Primary Ciliary Dyskinesia. ISMRM 24th Annual Meeting & Exhibition; 2016,7–13 May; Singapore. Available at: http://dev.ismrm.org/2016/2924.html
27
Arieli
R
. Mass spectrometer for respiratory research. Respir Physiol Neurobiol. 2010;170(2):183–4. doi:.https://doi.org/10.1016/j.resp.2009.12.013
28
Fuchs
SI
,
Sturz
J
,
Junge
S
,
Ballmann
M
,
Gappa
M
. A novel sidestream ultrasonic flow sensor for multiple breath washout in children. Pediatr Pulmonol. 2008;43(8):731–8. doi:.https://doi.org/10.1002/ppul.20825
29
Schmidt
A
,
Yammine
S
,
Proietti
E
,
Frey
U
,
Latzin
P
,
Riedel
T
, et al.
Validation of multiple-breath washout equipment for infants and young children. Pediatr Pulmonol. 2015;50(6):607–14. doi:.https://doi.org/10.1002/ppul.23010
30
Gustafsson
PM
,
Robinson
PD
,
Lindblad
A
,
Oberli
D
. Novel methodology to perform sulfur hexafluoride (SF6)-based multiple-breath wash-in and washout in infants using current commercially available equipment. J Appl Physiol (1985). 2016;121(5):1087–97. doi:.https://doi.org/10.1152/japplphysiol.00115.2016
31
Singer
F
,
Houltz
B
,
Latzin
P
,
Robinson
P
,
Gustafsson
P
. A realistic validation study of a new nitrogen multiple-breath washout system. PLoS One. 2012;7(4):e36083. doi:.https://doi.org/10.1371/journal.pone.0036083
32
Gonem
S
,
Singer
F
,
Corkill
S
,
Singapuri
A
,
Siddiqui
S
,
Gustafsson
P
. Validation of a photoacoustic gas analyser for the measurement of functional residual capacity using multiple-breath inert gas washout. Respiration. 2014;87(6):462–8. doi:.https://doi.org/10.1159/000357786
33
Anagnostopoulou
P
,
Egger
B
,
Lurà
M
,
Usemann
J
,
Schmidt
A
,
Gorlanova
O
, et al.
Multiple breath washout analysis in infants: quality assessment and recommendations for improvement. Physiol Meas. 2016;37(3):L1–15. doi:.https://doi.org/10.1088/0967-3334/37/3/L1
34
Latzin
P
,
Sauteur
L
,
Thamrin
C
,
Schibler
A
,
Baldwin
D
,
Hutten
GJ
, et al.
Optimized temperature and deadspace correction improve analysis of multiple breath washout measurements by ultrasonic flowmeter in infants. Pediatr Pulmonol. 2007;42(10):888–97. doi:.https://doi.org/10.1002/ppul.20674
35
Jensen
R
,
Stanojevic
S
,
Gibney
K
,
Salazar
JG
,
Gustafsson
P
,
Subbarao
P
, et al.
Multiple breath nitrogen washout: a feasible alternative to mass spectrometry. PLoS One. 2013;8(2):e56868. doi:.https://doi.org/10.1371/journal.pone.0056868
36
Summermatter
S
,
Singer
F
,
Latzin
P
,
Yammine
S
. Impact of Software Settings on Multiple-Breath Washout Outcomes. PLoS One. 2015;10(7):e0132250. doi:.https://doi.org/10.1371/journal.pone.0132250
37
Horsley
A
,
Macleod
K
,
Gupta
R
,
Goddard
N
,
Bell
N
. Enhanced photoacoustic gas analyser response time and impact on accuracy at fast ventilation rates during multiple breath washout. PLoS One. 2014;9(6):e98487. doi:.https://doi.org/10.1371/journal.pone.0098487
38
Downing
B
,
Irving
S
,
Bingham
Y
,
Fleming
L
,
Bush
A
,
Saglani
S
. Feasibility of lung clearance index in a clinical setting in pre-school children. Eur Respir J. 2016;48(4):1074–80. doi:.https://doi.org/10.1183/13993003.00374-2016
39
Grønbæk
J
,
Hallas
HW
,
Arianto
L
,
Pedersen
K
,
Thomsen
A
,
Chawes
BL
, et al.
New time-saving predictor algorithm for multiple breath washout in adolescents. Pediatr Res. 2016;80(1):49–53. doi:.https://doi.org/10.1038/pr.2016.57
40
Nielsen
N
,
Nielsen
JG
,
Horsley
AR
. Evaluation of the impact of alveolar nitrogen excretion on indices derived from multiple breath nitrogen washout. PLoS One. 2013;8(9):e73335. doi:.https://doi.org/10.1371/journal.pone.0073335
41
Shawcross
A
,
Murray
CS
,
Goddard
N
,
Gupta
R
,
Watson
S
,
Horsley
A
. Accurate lung volume measurements in vitro using a novel inert gas washout method suitable for infants. Pediatr Pulmonol. 2016;51(5):491–7. doi:.https://doi.org/10.1002/ppul.23348
42
Fuchs
O
,
Latzin
P
,
Thamrin
C
,
Stern
G
,
Frischknecht
P
,
Singer
F
, et al.
Normative data for lung function and exhaled nitric oxide in unsedated healthy infants. Eur Respir J. 2011;37(5):1208–16. doi:.https://doi.org/10.1183/09031936.00125510
43
Gray
D
,
Willemse
L
,
Visagie
A
,
Smith
E
,
Czövek
D
,
Sly
PD
, et al.
Lung function and exhaled nitric oxide in healthy unsedated African infants. Respirology. 2015;20(7):1108–14. doi:.https://doi.org/10.1111/resp.12579
44
Gray
DM
,
Turkovic
L
,
Willemse
L
,
Visagie
A
,
Vanker
A
,
Stein
DJ
, et al.
Lung Function in African Infants in the Drakenstein Child Health Study. Impact of Lower Respiratory Tract Illness. Am J Respir Crit Care Med. 2017;195(2):212–20. doi:.https://doi.org/10.1164/rccm.201601-0188OC
45
Belessis
Y
,
Dixon
B
,
Hawkins
G
,
Pereira
J
,
Peat
J
,
MacDonald
R
, et al.
Early cystic fibrosis lung disease detected by bronchoalveolar lavage and lung clearance index. Am J Respir Crit Care Med. 2012;185(8):862–73. doi:.https://doi.org/10.1164/rccm.201109-1631OC
46
Simpson
SJ
,
Ranganathan
S
,
Park
J
,
Turkovic
L
,
Robins-Browne
RM
,
Skoric
B
, et al.; AREST CF. Progressive ventilation inhomogeneity in infants with cystic fibrosis after pulmonary infection. Eur Respir J. 2015;46(6):1680–90. doi:.https://doi.org/10.1183/13993003.00622-2015
47
Hall
GL
,
Logie
KM
,
Parsons
F
,
Schulzke
SM
,
Nolan
G
,
Murray
C
, et al.; AREST CF. Air trapping on chest CT is associated with worse ventilation distribution in infants with cystic fibrosis diagnosed following newborn screening. PLoS One. 2011;6(8):e23932. doi:.https://doi.org/10.1371/journal.pone.0023932
48
Stanojevic
S
,
Davis
SD
,
Retsch-Bogart
G
,
Webster
H
,
Davis
M
,
Johnson
RC
, et al.
Progression of Lung Disease in Preschool Patients with Cystic Fibrosis. Am J Respir Crit Care Med. 2017;195(9):1216–25.
49
Stahl
M
,
Wielpütz
MO
,
Graeber
SY
,
Joachim
C
,
Sommerburg
O
,
Kauczor
HU
, et al.
Comparison of Lung Clearance Index and Magnetic Resonance Imaging for Assessment of Lung Disease in Children with Cystic Fibrosis. Am J Respir Crit Care Med. 2017;195(3):349–59.
50
Amin
R
,
Stanojevic
S
,
Kane
M
,
Webster
H
,
Ratjen
F
. A randomized controlled trial to evaluate the lung clearance index as an outcome measure for early phase studies in patients with cystic fibrosis. Respir Med. 2016;112:59–64. doi:.https://doi.org/10.1016/j.rmed.2016.01.020
51
Ramsey
KA
,
Rosenow
T
,
Turkovic
L
,
Skoric
B
,
Banton
G
,
Adams
AM
, et al.; AREST CF. Lung Clearance Index and Structural Lung Disease on Computed Tomography in Early Cystic Fibrosis. Am J Respir Crit Care Med. 2016;193(1):60–7. doi:.https://doi.org/10.1164/rccm.201507-1409OC
52
Davies
J
,
Sheridan
H
,
Bell
N
,
Cunningham
S
,
Davis
SD
,
Elborn
JS
, et al.
Assessment of clinical response to ivacaftor with lung clearance index in cystic fibrosis patients with a G551D-CFTR mutation and preserved spirometry: a randomised controlled trial. Lancet Respir Med. 2013;1(8):630–8. doi:.https://doi.org/10.1016/S2213-2600(13)70182-6
53
Singer
F
,
Kieninger
E
,
Abbas
C
,
Yammine
S
,
Fuchs
O
,
Proietti
E
, et al.
Practicability of nitrogen multiple-breath washout measurements in a pediatric cystic fibrosis outpatient setting. Pediatr Pulmonol. 2013;48(8):739–46. doi:.https://doi.org/10.1002/ppul.22651
54
Poncin
W
,
Singer
F
,
Aubriot
AS
,
Lebecque
P
. Agreement between multiple-breath nitrogen washout systems in children and adults. J Cyst Fibros. 2017;16(2):258–66.
55
Hülskamp
G
,
Lum
S
,
Stocks
J
,
Wade
A
,
Hoo
AF
,
Costeloe
K
, et al.
Association of prematurity, lung disease and body size with lung volume and ventilation inhomogeneity in unsedated neonates: a multicentre study. Thorax. 2009;64(3):240–5. doi:.https://doi.org/10.1136/thx.2008.101758
56
Latzin
P
,
Roth
S
,
Thamrin
C
,
Hutten
GJ
,
Pramana
I
,
Kuehni
CE
, et al.
Lung volume, breathing pattern and ventilation inhomogeneity in preterm and term infants. PLoS One. 2009;4(2):e4635. doi:.https://doi.org/10.1371/journal.pone.0004635
57
Yammine
S
,
Schmidt
A
,
Sutter
O
,
Fouzas
S
,
Singer
F
,
Frey
U
, et al.
Functional evidence for continued alveolarisation in former preterms at school age?
Eur Respir J. 2016;47(1):147–55. doi:.https://doi.org/10.1183/13993003.00478-2015
58
Boon
M
,
Vermeulen
FL
,
Gysemans
W
,
Proesmans
M
,
Jorissen
M
,
De Boeck
K
. Lung structure-function correlation in patients with primary ciliary dyskinesia. Thorax. 2015;70(4):339–45. doi:.https://doi.org/10.1136/thoraxjnl-2014-206578
59
Nyilas
S
,
Schlegtendal
A
,
Singer
F
,
Goutaki
M
,
Kuehni
CE
,
Casaulta
C
, et al.
Alternative inert gas washout outcomes in patients with primary ciliary dyskinesia. Eur Respir J. 2017;49(1):1600466. doi:.https://doi.org/10.1183/13993003.00466-2016
60
Madsen
A
,
Green
K
,
Buchvald
F
,
Hanel
B
,
Nielsen
KG
. Aerobic fitness in children and young adults with primary ciliary dyskinesia. PLoS One. 2013;8(8):e71409. doi:.https://doi.org/10.1371/journal.pone.0071409
61
Jarenbäck
L
,
Ankerst
J
,
Bjermer
L
,
Tufvesson
E
. Acinar ventilation heterogeneity in COPD relates to diffusion capacity, resistance and reactance. Respir Med. 2016;110:28–33. doi:.https://doi.org/10.1016/j.rmed.2015.11.005
62
Fuchs
SI
,
Schwerk
N
,
Pittschieler
K
,
Ahrens
F
,
Baden
W
,
Bals
R
, et al.
Lung clearance index for monitoring early lung disease in alpha-1-antitrypsin deficiency. Respir Med. 2016;116:93–9. doi:.https://doi.org/10.1016/j.rmed.2016.04.015
63
Macleod
KA
,
Horsley
AR
,
Bell
NJ
,
Greening
AP
,
Innes
JA
,
Cunningham
S
. Ventilation heterogeneity in children with well controlled asthma with normal spirometry indicates residual airways disease. Thorax. 2009;64(1):33–7. doi:.https://doi.org/10.1136/thx.2007.095018
64
Fuchs
SI
,
Buess
C
,
Lum
S
,
Kozlowska
W
,
Stocks
J
,
Gappa
M
. Multiple breath washout with a sidestream ultrasonic flow sensor and mass spectrometry: a comparative study. Pediatr Pulmonol. 2006;41(12):1218–25. doi:.https://doi.org/10.1002/ppul.20524
65
Fuchs
SI
,
Eder
J
,
Ellemunter
H
,
Gappa
M
. Lung clearance index: normal values, repeatability, and reproducibility in healthy children and adolescents. Pediatr Pulmonol. 2009;44(12):1180–5. doi:.https://doi.org/10.1002/ppul.21093
66
Ellemunter
H
,
Fuchs
SI
,
Unsinn
KM
,
Freund
MC
,
Waltner-Romen
M
,
Steinkamp
G
, et al.
Sensitivity of Lung Clearance Index and chest computed tomography in early CF lung disease. Respir Med. 2010;104(12):1834–42. doi:.https://doi.org/10.1016/j.rmed.2010.06.010
67
Verbanck
S
,
Paiva
M
,
Schuermans
D
,
Malfroot
A
,
Vincken
W
,
Vanderhelst
E
. Acinar and conductive ventilation heterogeneity in severe CF lung disease: back to the model. Respir Physiol Neurobiol. 2013;188(2):124–32. doi:.https://doi.org/10.1016/j.resp.2013.05.011
68
Yammine
S
,
Singer
F
,
Gustafsson
P
,
Latzin
P
. Impact of different breathing protocols on multiple-breath washout outcomes in children. J Cyst Fibros. 2014;13(2):190–7. doi:.https://doi.org/10.1016/j.jcf.2013.08.010
69
Banton
GL
,
Hall
GL
,
Tan
M
,
Skoric
B
,
Ranganathan
SC
,
Franklin
PJ
, et al.
Multiple breath washout cannot be used for tidal breath parameter analysis in infants. Pediatr Pulmonol. 2016;51(5):531–40. doi:.https://doi.org/10.1002/ppul.23326
70
Jost
K
,
Egger
B
,
Kieninger
E
,
Singer
F
,
Frey
U
,
Latzin
P
. Changes in minute ventilation after exposure to 4% sulfur hexafluoride (SF6 ) in infants. Pediatr Pulmonol. 2017;52(2):151–3. doi:.https://doi.org/10.1002/ppul.23557
71
Jost
K
,
Lenherr
N
,
Singer
F
,
Schulzke
SM
,
Frey
U
,
Latzin
P
, et al.
Changes in breathing pattern upon 100% oxygen in children at early school age. Respir Physiol Neurobiol. 2016;228:9–15. doi:.https://doi.org/10.1016/j.resp.2016.03.006
72
Schmalisch
G
,
Wilitzki
S
,
Bührer
C
,
Fischer
HS
. The lung clearance index in young infants: impact of tidal volume and dead space. Physiol Meas. 2015;36(7):1601–13. doi:.https://doi.org/10.1088/0967-3334/36/7/1601
73
Frey
U
,
Stocks
J
,
Sly
P
,
Bates
J
; European Respiratory Society/American Thoracic Society. Specification for signal processing and data handling used for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. Eur Respir J. 2000;16(5):1016–22. doi:.https://doi.org/10.1183/09031936.00.16510160
74
Foong
RE
,
Rosenow
T
,
Simpson
SJ
,
Stöklin
B
,
Gray
D
,
Pillow
JJ
, et al.
End-inspiratory molar mass step correction for analysis of infant multiple breath washout tests. Pediatr Pulmonol. 2017;52(1):10–3. doi:.https://doi.org/10.1002/ppul.23499
75
Raaijmakers
L
,
Jensen
R
,
Stanojevic
S
,
Ratjen
F
. Validation of multiple breath washout devices. J Cyst Fibros. 2017;S1569-1993(17)30018-8.
76
Mahar
RK
,
Vukcevic
D
,
King
L
,
Carlin
JB
,
Ranganathan
S
. Lack of transparency in software used to analyze multiple breath washout data. Pediatr Pulmonol. 2016;51(11):1108–10. doi:.https://doi.org/10.1002/ppul.23420
77
Anagnostopoulou
P
,
Yammine
S
,
Schmidt
A
,
Korten
I
,
Kieninger
E
,
Mack
I
, et al.
False normal Lung Clearance Index in infants with cystic fibrosis due to software algorithms. Pediatr Pulmonol. 2015;50(10):970–7. doi:.https://doi.org/10.1002/ppul.23256
78
Lum
S
,
Stocks
J
,
Stanojevic
S
,
Wade
A
,
Robinson
P
,
Gustafsson
P
, et al.
Age and height dependence of lung clearance index and functional residual capacity. Eur Respir J. 2013;41(6):1371–7. doi:.https://doi.org/10.1183/09031936.00005512
79
Benseler
A
,
Stanojevic
S
,
Jensen
R
,
Gustafsson
P
,
Ratjen
F
. Effect of equipment dead space on multiple breath washout measures. Respirology. 2015;20(3):459–66. doi:.https://doi.org/10.1111/resp.12470
80
Subbarao
P
,
Lu
Z
,
Kowalik
K
,
Brown
M
,
Balkovec
S
,
Gustafsson
P
, et al.
Changes in multiple breath washout measures after raised volume rapid thoracoabdominal compression maneuvers in infants. Pediatr Pulmonol. 2016;51(2):183–8. doi:.https://doi.org/10.1002/ppul.23220
81
Fuchs
SI
,
Toussaint
S
,
Edlhaimb
B
,
Ballmann
M
,
Gappa
M
. Short-term effect of physiotherapy on variability of the lung clearance index in children with cystic fibrosis. Pediatr Pulmonol. 2010;45(3):301–6.
82
Pfleger
A
,
Steinbacher
M
,
Schwantzer
G
,
Weinhandl
E
,
Wagner
M
,
Eber
E
. Short-term effects of physiotherapy on ventilation inhomogeneity in cystic fibrosis patients with a wide range of lung disease severity. J Cyst Fibros. 2015;14(5):627–31. doi:.https://doi.org/10.1016/j.jcf.2014.12.017
83
Grosse-Onnebrink
J
,
Mellies
U
,
Olivier
M
,
Werner
C
,
Stehling
F
. Chest physiotherapy can affect the lung clearance index in cystic fibrosis patients. Pediatr Pulmonol. 2017;52(5):625–31. doi:.https://doi.org/10.1002/ppul.23670
84
Singer
F
,
Stern
G
,
Thamrin
C
,
Abbas
C
,
Casaulta
C
,
Frey
U
, et al.
A new double-tracer gas single-breath washout to assess early cystic fibrosis lung disease. Eur Respir J. 2013;41(2):339–45. doi:.https://doi.org/10.1183/09031936.00044312
85
Abbas
C
,
Singer
F
,
Yammine
S
,
Casaulta
C
,
Latzin
P
. Treatment response of airway clearance assessed by single-breath washout in children with cystic fibrosis. J Cyst Fibros. 2013;12(6):567–74. doi:.https://doi.org/10.1016/j.jcf.2013.05.010
86
Singer
F
,
Abbas
C
,
Yammine
S
,
Casaulta
C
,
Frey
U
,
Latzin
P
. Abnormal small airways function in children with mild asthma. Chest. 2014;145(3):492–9. doi:.https://doi.org/10.1378/chest.13-0784
87
Verbanck
S
,
Schuermans
D
,
Meysman
M
,
Paiva
M
,
Vincken
W
. Noninvasive assessment of airway alterations in smokers: the small airways revisited. Am J Respir Crit Care Med. 2004;170(4):414–9. doi:.https://doi.org/10.1164/rccm.200401-037OC
88
Mikamo
M
,
Shirai
T
,
Mori
K
,
Shishido
Y
,
Akita
T
,
Morita
S
, et al.
Predictors of phase III slope of nitrogen single-breath washout in COPD. Respir Physiol Neurobiol. 2013;189(1):42–6. doi:.https://doi.org/10.1016/j.resp.2013.06.018
89
Husemann
K
,
Berg
N
,
Engel
J
,
Port
J
,
Joppek
C
,
Tao
Z
, et al.
Double tracer gas single-breath washout: reproducibility in healthy subjects and COPD. Eur Respir J. 2014;44(5):1210–22. doi:.https://doi.org/10.1183/09031936.00085713
90
Boeck
L
,
Gensmer
A
,
Nyilas
S
,
Stieltjes
B
,
Re
TJ
,
Tamm
M
, et al.
Single-Breath Washout Tests to Assess Small Airway Disease in COPD. Chest. 2016;150(5):1091–100. doi:.https://doi.org/10.1016/j.chest.2016.05.019
91
Nyilas
S
,
Singer
F
,
Kumar
N
,
Yammine
S
,
Meier-Girard
D
,
Koerner-Rettberg
C
, et al.
Physiological phenotyping of pediatric chronic obstructive airway diseases. J Appl Physiol (1985). 2016;121(1):324–32. doi:.https://doi.org/10.1152/japplphysiol.00086.2016
92
Van Muylem
A
,
Antoine
M
,
Yernault
JC
,
Paiva
M
,
Estenne
M
. Inert gas single-breath washout after heart-lung transplantation. Am J Respir Crit Care Med. 1995;152(3):947–52. doi:.https://doi.org/10.1164/ajrccm.152.3.7663808
93
Van Muylem
A
,
Verbanck
S
,
Estenne
M
. Monitoring the lung periphery of transplanted lungs. Respir Physiol Neurobiol. 2005;148(1-2):141–51. doi:.https://doi.org/10.1016/j.resp.2005.05.010
94
Riise
GC
,
Mårtensson
G
,
Houltz
B
,
Bake
B
. Prediction of BOS by the single-breath nitrogen test in double lung transplant recipients. BMC Res Notes. 2011;4(1):515. doi:.https://doi.org/10.1186/1756-0500-4-515
95
Latzin
P
,
Thompson
B
. Double tracer gas single-breath washout: promising for clinics or just a toy for research?
Eur Respir J. 2014;44(5):1113–5. doi:.https://doi.org/10.1183/09031936.00111114
96
Verbanck
S
,
Paiva
M
. Dual gas techniques for peripheral airway function: diffusing the issues. Eur Respir J. 2015;45(5):1491–4. doi:.https://doi.org/10.1183/09031936.00207514
97
Farrell
PM
. The prevalence of cystic fibrosis in the European Union. J Cyst Fibros. 2008;7(5):450–3. doi:.https://doi.org/10.1016/j.jcf.2008.03.007
98
Gustafsson
PM
,
De Jong
PA
,
Tiddens
HA
,
Lindblad
A
. Multiple-breath inert gas washout and spirometry versus structural lung disease in cystic fibrosis. Thorax. 2008;63(2):129–34. doi:.https://doi.org/10.1136/thx.2007.077784
99
Bush
A
,
Sly
PD
. Evolution of cystic fibrosis lung function in the early years. Curr Opin Pulm Med. 2015;21(6):602–8. doi:.https://doi.org/10.1097/MCP.0000000000000209
100
Gustafsson
PM
,
Aurora
P
,
Lindblad
A
. Evaluation of ventilation maldistribution as an early indicator of lung disease in children with cystic fibrosis. Eur Respir J. 2003;22(6):972–9. doi:.https://doi.org/10.1183/09031936.03.00049502
101
Owens
CM
,
Aurora
P
,
Stanojevic
S
,
Bush
A
,
Wade
A
,
Oliver
C
, et al.; London Cystic Fibrosis Collaboration. Lung Clearance Index and HRCT are complementary markers of lung abnormalities in young children with CF. Thorax. 2011;66(6):481–8. doi:.https://doi.org/10.1136/thx.2010.150375
102
Hoo
AF
,
Thia
LP
,
Nguyen
TT
,
Bush
A
,
Chudleigh
J
,
Lum
S
, et al.; London Cystic Fibrosis Collaboration. Lung function is abnormal in 3-month-old infants with cystic fibrosis diagnosed by newborn screening. Thorax. 2012;67(10):874–81. doi:.https://doi.org/10.1136/thoraxjnl-2012-201747
103
Aurora
P
,
Stanojevic
S
,
Wade
A
,
Oliver
C
,
Kozlowska
W
,
Lum
S
, et al.; London Cystic Fibrosis Collaboration. Lung clearance index at 4 years predicts subsequent lung function in children with cystic fibrosis. Am J Respir Crit Care Med. 2011;183(6):752–8. doi:.https://doi.org/10.1164/rccm.200911-1646OC
104
Kieninger
E
,
Singer
F
,
Fuchs
O
,
Abbas
C
,
Frey
U
,
Regamey
N
, et al.
Long-term course of lung clearance index between infancy and school-age in cystic fibrosis subjects. J Cyst Fibros. 2011;10(6):487–90. doi:.https://doi.org/10.1016/j.jcf.2011.07.006
105
O’Neill
K
,
Bradley
JM
,
Johnston
E
,
McGrath
S
,
McIlreavey
L
,
Rowan
S
, et al.
Reduced bacterial colony count of anaerobic bacteria is associated with a worsening in lung clearance index and inflammation in cystic fibrosis. PLoS One. 2015;10(5):e0126980. doi:.https://doi.org/10.1371/journal.pone.0126980
106
Sonneveld
N
,
Stanojevic
S
,
Amin
R
,
Aurora
P
,
Davies
J
,
Elborn
JS
, et al.
Lung clearance index in cystic fibrosis subjects treated for pulmonary exacerbations. Eur Respir J. 2015;46(4):1055–64. doi:.https://doi.org/10.1183/09031936.00211914
107
Yammine
S
,
Bigler
A
,
Casaulta
C
,
Singer
F
,
Latzin
P
. Reasons for heterogeneous change in LCI in children with cystic fibrosis after antibiotic treatment. Thorax. 2014;69(2):183. doi:.https://doi.org/10.1136/thoraxjnl-2013-204283
108
Kent
L
,
Reix
P
,
Innes
JA
,
Zielen
S
,
Le Bourgeois
M
,
Braggion
C
, et al.; European Cystic Fibrosis Society Clinical Trial Network (ECFS-CTN) Standardisation Committee. Lung clearance index: evidence for use in clinical trials in cystic fibrosis. J Cyst Fibros. 2014;13(2):123–38. doi:.https://doi.org/10.1016/j.jcf.2013.09.005
109
Milla
CE
,
Ratjen
F
,
Marigowda
G
,
Liu
F
,
Waltz
D
,
Rosenfeld
M
; VX13-809-011 Part B Investigator Group. Lumacaftor/Ivacaftor in Patients Aged 6-11 Years with Cystic Fibrosis and Homozygous for F508del-CFTR. Am J Respir Crit Care Med. 2017;195(7):912–20.
110
Subbarao
P
,
Stanojevic
S
,
Brown
M
,
Jensen
R
,
Rosenfeld
M
,
Davis
S
, et al.
Lung clearance index as an outcome measure for clinical trials in young children with cystic fibrosis. A pilot study using inhaled hypertonic saline. Am J Respir Crit Care Med. 2013;188(4):456–60. doi:.https://doi.org/10.1164/rccm.201302-0219OC
111
Amin
R
,
Subbarao
P
,
Jabar
A
,
Balkovec
S
,
Jensen
R
,
Kerrigan
S
, et al.
Hypertonic saline improves the LCI in paediatric patients with CF with normal lung function. Thorax. 2010;65(5):379–83. doi:.https://doi.org/10.1136/thx.2009.125831
112
Amin
R
,
Subbarao
P
,
Lou
W
,
Jabar
A
,
Balkovec
S
,
Jensen
R
, et al.
The effect of dornase alfa on ventilation inhomogeneity in patients with cystic fibrosis. Eur Respir J. 2011;37(4):806–12. doi:.https://doi.org/10.1183/09031936.00072510
113
Panettieri
RA, Jr
,
Covar
R
,
Grant
E
,
Hillyer
EV
,
Bacharier
L
. Natural history of asthma: persistence versus progression-does the beginning predict the end?
J Allergy Clin Immunol. 2008;121(3):607–13. doi:.https://doi.org/10.1016/j.jaci.2008.01.006
114
Sonnappa
S
,
Bastardo
CM
,
Saglani
S
,
Bush
A
,
Aurora
P
. Relationship between past airway pathology and current lung function in preschool wheezers. Eur Respir J. 2011;38(6):1431–6. doi:.https://doi.org/10.1183/09031936.00164910
115
Fischer
HS
,
Puder
LC
,
Wilitzki
S
,
Usemann
J
,
Bührer
C
,
Godfrey
S
, et al.
Relationship between computerized wheeze detection and lung function parameters in young infants. Pediatr Pulmonol. 2016;51(4):402–10. doi:.https://doi.org/10.1002/ppul.23310
116
Sonnappa
S
,
Bastardo
CM
,
Wade
A
,
Bush
A
,
Stocks
J
,
Aurora
P
. Repeatability and bronchodilator reversibility of lung function in young children. Eur Respir J. 2013;42(1):116–24. doi:.https://doi.org/10.1183/09031936.00076012
117From the Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) 2016 [cited 2017 23 Feb.]; Available from: http://www.ginasthma.org/.
118
Moeller
A
,
Carlsen
KH
,
Sly
PD
,
Baraldi
E
,
Piacentini
G
,
Pavord
I
, et al.; ERS Task Force Monitoring Asthma in Children. Monitoring asthma in childhood: lung function, bronchial responsiveness and inflammation. Eur Respir Rev. 2015;24(136):204–15. doi:.https://doi.org/10.1183/16000617.00003914
119
Horsley
A
. Lung clearance index in the assessment of airways disease. Respir Med. 2009;103(6):793–9. doi:.https://doi.org/10.1016/j.rmed.2009.01.025
120
Verbanck
S
,
Paiva
M
,
Schuermans
D
,
Hanon
S
,
Vincken
W
,
Van Muylem
A
. Relationships between the lung clearance index and conductive and acinar ventilation heterogeneity. J Appl Physiol (1985). 2012;112(5):782–90. doi:.https://doi.org/10.1152/japplphysiol.01221.2011
121
Zwitserloot
A
,
Fuchs
SI
,
Müller
C
,
Bisdorf
K
,
Gappa
M
. Clinical application of inert gas Multiple Breath Washout in children and adolescents with asthma. Respir Med. 2014;108(9):1254–9. doi:.https://doi.org/10.1016/j.rmed.2014.07.003
122
Hardaker
KM
,
Downie
SR
,
Kermode
JA
,
Berend
N
,
King
GG
,
Salome
CM
. Ventilation heterogeneity is associated with airway responsiveness in asthma but not COPD. Respir Physiol Neurobiol. 2013;189(1):106–11. doi:.https://doi.org/10.1016/j.resp.2013.07.009
123
Downie
SR
,
Salome
CM
,
Verbanck
S
,
Thompson
B
,
Berend
N
,
King
GG
. Ventilation heterogeneity is a major determinant of airway hyperresponsiveness in asthma, independent of airway inflammation. Thorax. 2007;62(8):684–9. doi:.https://doi.org/10.1136/thx.2006.069682
124
Farah
CS
,
King
GG
,
Brown
NJ
,
Peters
MJ
,
Berend
N
,
Salome
CM
. Ventilation heterogeneity predicts asthma control in adults following inhaled corticosteroid dose titration. J Allergy Clin Immunol. 2012;130(1):61–8. doi:.https://doi.org/10.1016/j.jaci.2012.02.015
125
Gustafsson
PM
. Peripheral airway involvement in CF and asthma compared by inert gas washout. Pediatr Pulmonol. 2007;42(2):168–76. doi:.https://doi.org/10.1002/ppul.20554
126
Fuchs
SI
,
Gappa
M
. Lung clearance index: clinical and research applications in children. Paediatr Respir Rev. 2011;12(4):264–70. doi:.https://doi.org/10.1016/j.prrv.2011.05.001
127
Pillow
JJ
,
Frerichs
I
,
Stocks
J
. Lung function tests in neonates and infants with chronic lung disease: global and regional ventilation inhomogeneity. Pediatr Pulmonol. 2006;41(2):105–21. doi:.https://doi.org/10.1002/ppul.20319
128
Hülskamp
G
,
Pillow
JJ
,
Dinger
J
,
Stocks
J
. Lung function tests in neonates and infants with chronic lung disease of infancy: functional residual capacity. Pediatr Pulmonol. 2006;41(1):1–22. doi:.https://doi.org/10.1002/ppul.20318
129
Lum
S
,
Kirkby
J
,
Welsh
L
,
Marlow
N
,
Hennessy
E
,
Stocks
J
. Nature and severity of lung function abnormalities in extremely pre-term children at 11 years of age. Eur Respir J. 2011;37(5):1199–207. doi:.https://doi.org/10.1183/09031936.00071110
130
Simpson
SJ
,
Logie
KM
,
O’Dea
CA
,
Banton
GL
,
Murray
C
,
Wilson
AC
, et al.
Altered lung structure and function in mid-childhood survivors of very preterm birth. Thorax. 2017;thoraxjnl-2016-208985. doi:.https://doi.org/10.1136/thoraxjnl-2016-208985
131
Lucas
JS
,
Barbato
A
,
Collins
SA
,
Goutaki
M
,
Behan
L
,
Caudri
D
, et al.
European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2017;49(1):1601090. doi:.https://doi.org/10.1183/13993003.01090-2016
132
Marthin
JK
,
Petersen
N
,
Skovgaard
LT
,
Nielsen
KG
. Lung function in patients with primary ciliary dyskinesia: a cross-sectional and 3-decade longitudinal study. Am J Respir Crit Care Med. 2010;181(11):1262–8. doi:.https://doi.org/10.1164/rccm.200811-1731OC
133
Green
K
,
Buchvald
FF
,
Marthin
JK
,
Hanel
B
,
Gustafsson
PM
,
Nielsen
KG
. Ventilation inhomogeneity in children with primary ciliary dyskinesia. Thorax. 2012;67(1):49–53. doi:.https://doi.org/10.1136/thoraxjnl-2011-200726
134
Irving
SJ
,
Ives
A
,
Davies
G
,
Donovan
J
,
Edey
AJ
,
Gill
SS
, et al.
Lung clearance index and high-resolution computed tomography scores in primary ciliary dyskinesia. Am J Respir Crit Care Med. 2013;188(5):545–9. doi:.https://doi.org/10.1164/rccm.201304-0800OC
135
Tai
A
,
Tran
H
,
Roberts
M
,
Clarke
N
,
Wilson
J
,
Robertson
CF
. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax. 2014;69(9):805–10. doi:.https://doi.org/10.1136/thoraxjnl-2013-204815
136
McGeachie
MJ
,
Yates
KP
,
Zhou
X
,
Guo
F
,
Sternberg
AL
,
Van Natta
ML
, et al.; CAMP Research Group. Patterns of Growth and Decline in Lung Function in Persistent Childhood Asthma. N Engl J Med. 2016;374(19):1842–52. doi:.https://doi.org/10.1056/NEJMoa1513737
137
Swanney
MP
,
Ruppel
G
,
Enright
PL
,
Pedersen
OF
,
Crapo
RO
,
Miller
MR
, et al.
Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63(12):1046–51. doi:.https://doi.org/10.1136/thx.2008.098483
138
Vogelmeier
CF
,
Criner
GJ
,
Martinez
FJ
,
Anzueto
A
,
Barnes
PJ
,
Bourbeau
J
, et al.
Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Eur Respir J. 2017;49(3):1700214. doi:.https://doi.org/10.1183/13993003.00214-2017
139
Verbanck
S
,
Schuermans
D
,
Paiva
M
,
Meysman
M
,
Vincken
W
. Small airway function improvement after smoking cessation in smokers without airway obstruction. Am J Respir Crit Care Med. 2006;174(8):853–7. doi:.https://doi.org/10.1164/rccm.200603-422OC
140
Brusasco
V
,
Barisione
G
,
Crimi
E
. Pulmonary physiology: future directions for lung function testing in COPD. Respirology. 2015;20(2):209–18. doi:.https://doi.org/10.1111/resp.12388
141
Reynaud-Gaubert
M
,
Thomas
P
,
Badier
M
,
Cau
P
,
Giudicelli
R
,
Fuentes
P
. Early detection of airway involvement in obliterative bronchiolitis after lung transplantation. Functional and bronchoalveolar lavage cell findings. Am J Respir Crit Care Med. 2000;161(6):1924–9. doi:.https://doi.org/10.1164/ajrccm.161.6.9905060
142
Thompson
BR
,
Ellis
MJ
,
Stuart-Andrews
C
,
Lopez
M
,
Kedarisetty
S
,
Snell
GI
, et al.
Early bronchiolitis obliterans syndrome shows an abnormality of perfusion not ventilation in lung transplant recipients. Respir Physiol Neurobiol. 2015;216:28–34. doi:.https://doi.org/10.1016/j.resp.2015.05.003
143
Lahzami
S
,
Schoeffel
RE
,
Pechey
V
,
Reid
C
,
Greenwood
M
,
Salome
CM
, et al.
Small airways function declines after allogeneic haematopoietic stem cell transplantation. Eur Respir J. 2011;38(5):1180–8. doi:.https://doi.org/10.1183/09031936.00018311
144
Stafler
P
,
Weinreb
S
,
Mussaffi
H
,
Mei-Zahav
M
,
Prais
D
,
Steuer
G
, et al.
Feasibility of multiple breath washout measurements in infants with bronchiolitis: A pilot study. Pediatr Pulmonol. 2017;52(6):763–70. doi:.https://doi.org/10.1002/ppul.23674
145
Sigurs
N
,
Aljassim
F
,
Kjellman
B
,
Robinson
PD
,
Sigurbergsson
F
,
Bjarnason
R
, et al.
Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045–52. doi:.https://doi.org/10.1136/thx.2009.121582
146ClinicalTrials.gov. [cited 2017 23 February]; Available from: https://clinicaltrials.gov.
147EU Clinical Trials Register. [cited 2017 23 February]; Available from: https://www.clinicaltrialsregister.eu.
148A Study to Evaluate the Efficacy and Safety of Lumacaftor in Combination With Ivacaftor in Subjects With CF, Homozygous for the F508del-CFTR Mutation. [cited 2017 06 March]; Available from: https://clinicaltrials.gov/ct2/show/NCT02514473.
149A Study to Evaluate Efficacy and Safety of Ivacaftor in Subjects With Cystic Fibrosis Aged 3 Through 5 Years Who Have a Specified CFTR Gating Mutation. [cited 2017 07 March]; Available from: https://clinicaltrials.gov/ct2/show/NCT02742519.
150A Study to Evaluate Efficacy of Ivacaftor in Subjects With Cystic Fibrosis Who Have a 3849 + 10KB C→T or D1152H CFTR Mutation. [cited 2017 07 March]; Available from: https://clinicaltrials.gov/ct2/show/NCT03068312.
151
Kobbernagel
HE
,
Buchvald
FF
,
Haarman
EG
,
Casaulta
C
,
Collins
SA
,
Hogg
C
, et al.
Study protocol, rationale and recruitment in a European multi-centre randomized controlled trial to determine the efficacy and safety of azithromycin maintenance therapy for 6 months in primary ciliary dyskinesia. BMC Pulm Med. 2016;16(1):104. doi:.https://doi.org/10.1186/s12890-016-0261-x
152Effects of QVAR in Smokers With Asthma (OLiVIA). [cited 2017 23 February]; Available from: https://clinicaltrials.gov/ct2/show/NCT01741285
153Changes in the Lung Clearance Index in Pediatric Patients With Asthma. [cited 2017 23 February]; Available from: https://clinicaltrials.gov/ct2/show/NCT02678949.
154A Randomized-Controlled Trial of Inhaled Hypertonic Saline (7%) to Evaluate the Lung Clearance Index. [cited 2017 23 February]; Available from: https://clinicaltrials.gov/ct2/show/NCT02276898.
155Measures of Respiratory Health (MRH). [cited 2017 23 February]; Available from: https://clinicaltrials.gov/ct2/show/NCT02657837.
156Evaluation of Novel Lung Function Parameters in Patients With Interstitial Lung Disease (ILD). [cited 2017 23 February]; Available from: https://clinicaltrials.gov/ct2/show/NCT02827734.