Osteoporosis drug treatment: duration and management after discontinuation. A position statement from the Swiss Association against Osteoporosis (SVGO/ASCO)
DOI: https://doi.org/10.4414/smw.2017.14484
Christian
Meiera, Brigitte
Uebelhartb, Bérengère
Aubry-Rozierc, Martin
Birkhäuserd, Heike A.
Bischoff-Ferrarie, Diana
Freyf, Reto W.
Kressigg, Olivier
Lamyc, Kurt
Lippunerh, Petra
Stutei, Norbert
Suhmj, Serge
Ferrarib
aDivision of Endocrinology, Diabetology and Metabolism,
bService of Bone Diseases,
cCentre of Bone Diseases,
dGynaecological Endocrinology and Reproductive Medicine,
eDepartment of Geriatrics and Aging Research,
fDivision of Rheumatology,
gUniversity Centre for Medicine of Aging, Basel Mobility Centre,
hDepartment of Osteoporosis,
iDivision of Gynaecological Endocrinology and Reproductive Medicine,
jDepartment of Orthopaedics and Traumatology, Geriatric Fracture Centre,
Osteoporosis drug treatment: duration and management after discontinuation. A position statement from the Swiss Association against Osteoporosis (SVGO/ASCO)
w14484
Summary
Antiosteoporotic drugs are recommended in patients with fragility fractures and in patients considered to be at high fracture risk on the basis of clinical risk factors and/or low bone mineral density. As first-line treatment most patients are started with an antiresorptive treatment, i.e. drugs that inhibit osteoclast development and/or function (bisphosphonates, denosumab, oestrogens or selective oestrogen receptor modulators). In the balance between benefits and risks of antiresorptive treatment, uncertainties remain regarding the optimal treatment duration and the management of patients after drug discontinuation. Based on the available evidence, this position statement will focus on the long-term management of osteoporosis therapy, formulating decision criteria for clinical practice.
Introduction
Osteoporosis is a chronic metabolic disease characterised by a progressive loss of bone mass and microarchitectural deterioration, ultimately resulting in an increased risk of fragility fractures. During recent decades remarkable advances have been made in the diagnosis and treatment of osteoporosis. Specifically, and based on the understanding of the pathogenesis of osteoporosis, new treatment options including antiresorptive and anabolic treatment modalities have been developed and introduced into clinical routine.
In treatment guidelines, antiosteoporotic drugs are recommended for patients with vertebral or nonvertebral low-trauma fractures and for patients who present with a high fracture risk based on clinical risk factors and/or low bone mineral density (BMD) [1]. As first-line treatment, most patients are started with an antiresorptive treatment, i.e., a drug that inhibit osteoclast development and/or function (bisphosphonates, denosumab or selective oestrogen receptor modulators [SERMs]). In younger women up to the age of 60 years or up to 10 years after menopause, menopausal hormone therapy is recommended [2]. Its fracture preventive effect has been shown to be significant in postmenopausal women, even those without increased fracture risk [3]. In this age group, no long-term data are available for the nonhormonal antiresorptive alternatives [2]. In the balance between benefits and risks of antiresorptive treatment, uncertainties remain regarding the optimal treatment duration and the management of patients after drug discontinuation.
Alternatively, teriparatide, a recombinant human parathyroid hormone analogue, increases the formation of new bone substance by virtue of its stimulating effects on osteoblasts [4]. In Switzerland, teriparatide is approved for second-line treatment in patients with progressive osteoporosis or glucocorticoid-induced osteoporosis. However, as a result of its pronounced anabolic effect, particularly at cancellous bone sites (such as the vertebrae), its use as a first-line treatment option is increasingly recommended in patients at high fracture risk [5].
Based on the available evidence, this position statement will focus on the long-term management of osteoporosis therapy, formulating decision criteria for clinical practice.
Treatment modalities and their mode of action
Bisphosphonates are pyrophosphate analogues that act mainly by inhibiting osteoclast-mediated bone resorption. They are characterised by a high affinity with bone and a long half-life within the skeleton [6, 7]. The identification of the receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG) as central regulators of bone metabolism has resulted in the development of denosumab, a human monoclonal antibody against RANKL. An understanding of its mode of action, as compared to that of bisphosphonates, is essential to appreciate the differences in their use. Bisphosphonates accumulate on mineralised bone surface and are internalised by mature osteoclasts. Because of their long lasting retention on bone surface (particularly so for alendronate and zoledronate), bisphosphonates have a unique residual treatment effect on bone resorption even after treatment discontinuation. In contrast, circulating denosumab inhibits the maturation of osteoclasts by binding to and inhibiting RANKL. Thereby the effect of denosumab is limited to the period of drug exposure, i.e., the duration of treatment [8]. Importantly, discontinuation of denosumab is associated with a significant bone turnover rebound and a rapid loss of bone mass [9, 10], a phenomenon that has not been observed after discontinuation of bisphosphonates [7].
The effect of menopausal hormone therapy on bone metabolism ends with the cessation of oestrogen administration. However, a long lasting decrease of fracture risk has been confirmed for all major fractures up to 16 years after discontinuation of hormone therapy [3, 11–13]. To reach this protective effect, the number needed to treat (NNT) is 7 [11].There is no reason to place mandatory limits on the duration of menopausal hormone therapy. Data from the WHI trial and other studies support safe use for at least 5 years in healthy women starting treatment before the age of 60 [2].
The mode of action of teriparatide is related to its ability to stimulate processes associated with bone formation, which are then coupled to processes associated with bone resorption, leading to an overall increase in bone remodelling, but with a positive balance towards bone formation [14]. The bone formation induced by teriparatide not only increases bone mass but also improves the microarchitecture of the skeleton, primarily the trabecular and cortical thickness, thereby leading to improved strength and increased mechanical resistance. However, teriparatide also increases intracortical bone remodelling, leading to some porosity, which may partly explain the transient decline of BMD at predominantly cortical bone sites (such as the distal radius and femoral neck). As for many antiresorptive agents (except for bisphosphonates), effects of teriparatide on bone turnover are transient and limited to the time of exposure. Hence, sequential therapy with antiresorptive drugs is needed to improve secondary mineralisation and maintain bone mass [15, 16].
Duration of treatment
Benefits and safety during long-term-treatment
There is consensus that patients with high fracture risk should receive pharmacological treatment to prevent fragility fractures. The decision for how long to treat with antiresorptive drugs is largely dependent on their long-term efficacy and safety.
A network meta-analysis of several pharmacological agents available for the prevention of fragility fractures found strong evidence to support the efficacy of bisphosphonates (alendronate, risedronate, zoledronate), denosumab and teriparatide in reducing the relative risk of vertebral fractures by 40–70% and nonvertebral fractures by 20–40% [17]. For antiresorptives, this translates into a NNT of 15–25 to prevent one vertebral fracture over 3 years of treatment. However, one has to bear in mind that the NNT depends largely on the fracture incidence in the study population, with a lower NNT in high risk patients.
Data on fracture risk reduction during long-term treatment are mainly available for antiresorptive drugs. Indeed, the use of teriparatide as an anabolic drug is limited to 24 months duration. Among antiresorptives, the results of extension studies with alendronate, risedronate and zoledronate have been analysed. Postmenopausal women treated with alendronate for 4 to 5 years in the Fracture Intervention Trial (FIT) were randomised to continue with alendronate for 5 years or switched to placebo (FLEX Study) [18]. Postmenopausal women who had already received three annual intravenous infusions of zoledronate (Horizon Study) were randomised to either continue with yearly zoledronate or switch to placebo for another 3 years (Horizon Study Extension) [19]. The results of the 10 years of therapy with alendronate and the 6 years with zoledronate were a slight reduction in clinical and/or morphometric vertebral fractures as compared with stopping alendronate after 5 years or zoledronate after 3 years, since in the latter case the incidence of vertebral fractures increased again after discontinuation of therapy. In the FLEX study, continuation of alendronate for 10 years instead of stopping after 5 years decreased nonvertebral fractures only in women whose femoral neck T-score was ˗2.5 standard deviations (SD) or less at the time of discontinuation. However, non-spine fracture incidence was similar in those who continued 10 years alendronate or 6 years zoledronate, compared with those who stopped active treatment during the extension studies [20]. Notably, these extension studies were not primarily designed for fracture outcomes, but to look at BMD changes upon continuation or discontinuation of therapy (BMD progressively declining upon treatment cessation), and were also limited in the number of patients who were enrolled long-term.
The antifracture efficacy data on long-term treatment with denosumab, up to 10 years, are reported from the Extension study of the FREEDOM trial. In the open-label Extension study, 4550 postmenopausal women with osteoporosis who previously received either denosumab (long-term treatment group) or placebo (crossover group) were assigned to receive denosumab for another 7 years. After significant reduction of vertebral and nonvertebral fractures in the denosumab-treated group as compared with placebo during the FREEDOM study (first 3 years), the yearly incidence of new vertebral fractures remained low, whereas nonvertebral fractures further significantly decreased in year 4 [21] and then remained stable (approximately 1.5% per year for both vertebral and nonvertebral fractures in years 4 to 10 of continuous therapy) [22].
In general, antiresorptive drugs have a good safety profile. The side-effects vary between drugs, but many are associated with gastrointestinal effects after intake of oral bisphosphonates or influenza-like symptoms after intravenous bisphosphonate application (e.g., zoledronic acid). Nevertheless, for bisphosphonates and denosumab rare but serious adverse events have been reported, including osteonecrosis of the jaw (ONJ) and atypical femoral fractures (AFF) [23]. ONJ is associated with oncology-dose parenteral antiresorptive therapy with bisphosphonates and denosumab. The incidence of ONJ is greatest in the oncology patient population (1–15%), where high doses of these medications are used at frequent intervals. In the osteoporosis patient population. However, the incidence of ONJ is estimated to be 0.01 to 0.025%, and increases with treatment duration. The incidence is marginally higher than the incidence in the general population (<0.001%) [24]. Atypical femoral fractures located in the subtrochanteric region and diaphysis of the femur have been reported in patients taking bisphosphonates or denosumab, but are also observed in individuals without specific bone medication. The absolute risk of AFFs in patients on bisphosphonates is extremely low, ranging from 3.2 to 50 cases per 100 000 person‐years [25]. However, long‐term use for more than 5 years may be associated with higher risk [26]. When bisphosphonates are stopped, risk of an AFF rapidly declines.
It is important to note that in treated women the absolute risk for atypical fractures is up to 100-fold less than the risk for hip fracture among untreated postmenopausal women with high fracture risk [27].
Hence, for the efficacy of bisphosphonates and denosumab to reduce hip fractures, the benefit-risk ratio of these drugs remains favourable, particularly in patients at high fracture risk.
For the decision about antiresorptive treatment duration in an individual patient, reassessment of fracture risk is essential. A patient is considered to remain at high risk of fracture if they (a) experienced hip, spine or multiple osteoporotic fractures within 5 years before and/or during therapy, (b) remain on continuous high fracture risk based on clinical judgment or comorbidities, or (c) present with persistent low BMD. Subgroup analyses of the extension studies mentioned above indicate that patients whose femoral neck (or total hip) BMD T-score remains below ˗2.5 SD after 5 years of therapy benefit most from continuing antiresorptive therapy [21, 28–30]. As age and falls are major risk factors for fracture, a T-score threshold of ˗2.0 SD for continuation of antiresorptive therapy seems reasonable in older women (>65 years) and/or in frequent fallers.
Management after discontinuation of therapy
As a rule, the effects of any drug vanish upon treatment discontinuation, so that if the disease is chronic, symptoms and complications will recur (e.g., high blood pressure and risk of stroke upon cessation of antihypertensive medicines, hyperglycaemia and related complications upon cessation of antidiabetic drugs). The same is true for most osteoporosis drugs, including oestrogens, SERMs, denosumab and teriparatide. Bisphosphonates, because of their high affinity for bone, remain in the skeleton even after the drug is discontinued, which explains the slower and/or retarded offset of effects, with a slight decrease in bone mineral density and increase in biochemical markers of bone turnover, but to levels which can remain below pretreatment levels for months to years [18, 19].
On the other hand, discontinuation of denosumab has been associated with an increase of bone turnover markers to above-baseline levels (rebound); these return to baseline levels within 2 years of treatment cessation. In parallel, BMD decreases to baseline levels within 1 to 2 years, regardless of the duration of previous therapy [10]. Recently, after a few clinical cases of vertebral fractures were reported upon cessation of denosumab therapy [31, 32], a preliminary observation in more than 1000 subjects from the FREEDOM study and its extension who discontinued denosumab or placebo, indicated that vertebral fracture incidence indeed increased nearly 4-fold within a year after denosumab was stopped [33]. However, the absolute incidence of vertebral fracture remained comparable to that observed in subjects who discontinued placebo. Nevertheless, among subjects who sustained a new vertebral fracture after discontinuing denosumab, the incidence of multiple new vertebral fractures tended to be higher than in subjects who discontinued placebo [33]. Indirect comparison of the FREEDOM results with those of the bisphosphonates extension studies (above) which showed that the risk of vertebral fractures nearly doubles upon cessation of bisphosphonate therapy, suggests that more patients may sustain severe vertebral fractures when stopping denosumab, which is consistent with the quick reversibility of its antiresorptive effects. These observations led the medical authorities in Switzerland, in agreement with the company (Amgen) to adapt the drug label in order to warn prescribers against the sudden interruption of denosumab and the need to consolidate therapy with at least one year of a non-reversible antiresorptive. Currently identified risk factors for vertebral fractures after discontinuation of denosumab include prevalent fractures, BMD loss and longer duration off therapy [32, 34]. In this context, it should be noted that some data suggest that the rebound in bone resorption and BMD can be avoided in patients previously treated with bisphosphonates, probably due to their persistence in bone tissue [35, 36].
Practical consequences
On the basis of the above mentioned data on the efficacy and safety of antiresorptive drugs, we propose an approach to aid decisions about the management of patients with osteoporosis on long-term therapy (fig. 1). Because the extension trials that suggested long-term anti-fracture efficacy based on continuation vs discontinuation of therapy were conducted exclusively in postmenopausal women, the suggested approach is restricted to the management of women with postmenopausal osteoporosis. Nevertheless, similar approaches in male osteoporosis seem reasonable. Furthermore, the suggestions on long-term osteoporosis treatment modalities described below are not evidence based, but represent interpretation of the current data. Further studies are needed to elucidate antifracture efficacies of proposed sequential treatment algorithms.
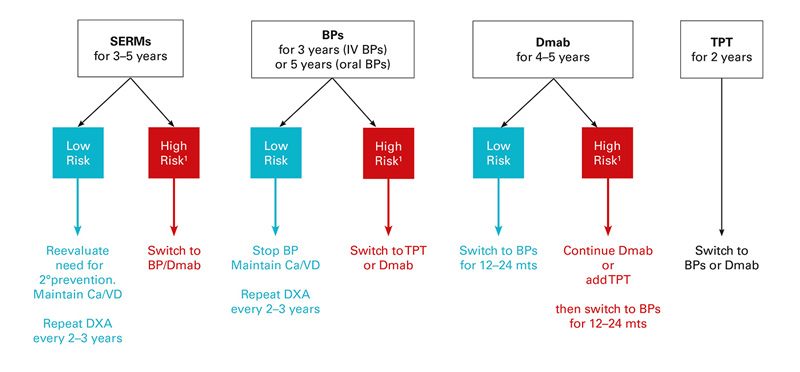
Figure 1 Approach to the management of postmenopausal women on anti-osteoporotic therapy.
1 High risk defined as (a) hip, spine or multiple fractures before or during therapy; (b) femoral neck T-score <˗2.5 SD if age <65 years, <-2.0 SD if age >65 years and/or frequent falls; (c) continuing hormone ablative therapy (e.g., aromatase inhibition, androgen deprivation therapy); (d) secondary osteoporosis, continuing glucocorticoid therapy.
BP =bisphosphonate; Ca/VD = calcium and vitamin D supplementation; Dmab, denosumab; DXA = densitometry; SERM = selective oestrogen receptor modulator; TPT = teriparatide
- Regular
- hip, spine or multiple osteoporotic fractures within 5 years before and/or during therapy;
- high fracture risk score at baseline according to FRAX
- persistent low BMD: on the basis of the above-mentioned extension trials (FLEX study, HORIZON- and FREEDOM-Extension studies), patients with a T-score persistently lower than ˗2.5 SD at the femoral neck (or <˗2.0 SD in older patients and/or frequent fallers) benefit the most from continuing therapy.
- For postmenopausal women who have been on
- For postmenopausal women who have been on
- For postmenopausal women who have been on
- In women with a favourable treatment response in which
- In patients suffering
- The anabolic effects of
- When considering the duration of and options for osteoporosis treatment, the potential contributions of poor compliance or adherence to therapy, inadequate vitamin D status, high fall risk, or new risk factors should be considered. Current guidelines recommend that treatment of any osteoporotic drug should always be supplemented with
References
1Recommendations of the Swiss Association against Osteoporosis (SVGO/ASCO). Prevention, Diagnostic and Treatment of Osteoporosis. 2015. Available from: http://www.svgo.ch/content/documents/2015/SVGO%20Empfehlungen%202015.pdf
2
Baber
RJ
,
Panay
N
,
Fenton
A
; IMS Writing Group. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric. 2016;19(2):109–50. doi:.https://doi.org/10.3109/13697137.2015.1129166
3
Manson
JE
,
Chlebowski
RT
,
Stefanick
ML
,
Aragaki
AK
,
Rossouw
JE
,
Prentice
RL
, et al.
Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–68. doi:.https://doi.org/10.1001/jama.2013.278040
4
Kraenzlin
ME
,
Meier
C
. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647–56. doi:.https://doi.org/10.1038/nrendo.2011.108
5
Meier
C
,
Lamy
O
,
Krieg
MA
,
Mellinghoff
HU
,
Felder
M
,
Ferrari
S
, et al.
The role of teriparatide in sequential and combination therapy of osteoporosis. Swiss Med Wkly. 2014;144:w13952.
6
Russell
RG
. Bisphosphonates: from bench to bedside. Ann N Y Acad Sci. 2006;1068(1):367–401. doi:.https://doi.org/10.1196/annals.1346.041
7Meier C, Kraenzlin M. Osteoporose: Therapie-Update, 2. Teil. Medikamente heute und morgen. Swiss Med Forum. 2013;13:835–40. Article in German.
8
Baron
R
,
Ferrari
S
,
Russell
RG
. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48(4):677–92. doi:.https://doi.org/10.1016/j.bone.2010.11.020
9
Miller
PD
,
Bolognese
MA
,
Lewiecki
EM
,
McClung
MR
,
Ding
B
,
Austin
M
, et al.; Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43(2):222–9. doi:.https://doi.org/10.1016/j.bone.2008.04.007
10
Bone
HG
,
Bolognese
MA
,
Yuen
CK
,
Kendler
DL
,
Miller
PD
,
Yang
YC
, et al.
Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96(4):972–80. doi:.https://doi.org/10.1210/jc.2010-1502
11
Bagger
YZ
,
Tankó
LB
,
Alexandersen
P
,
Hansen
HB
,
Møllgaard
A
,
Ravn
P
, et al.
Two to three years of hormone replacement treatment in healthy women have long-term preventive effects on bone mass and osteoporotic fractures: the PERF study. Bone. 2004;34(4):728–35. doi:.https://doi.org/10.1016/j.bone.2003.12.021
12
Watts
NB
,
Cauley
JA
,
Jackson
RD
,
LaCroix
AZ
,
Lewis
CE
,
Manson
JAE
, et al.
No Increase in Fractures after Stopping Hormone Therapy: Results from the Women’s Health Initiative. J Clin Endocrinol Metab. 2017;102(1):302–8. doi:.https://doi.org/10.1210/jc.2016-3270
13
Papadakis
G
,
Hans
D
,
Gonzalez-Rodriguez
E
,
Vollenweider
P
,
Waeber
G
,
Marques-Vidal
PM
, et al.
The Benefit of Menopausal Hormone Therapy on Bone Density and Microarchitecture Persists After its Withdrawal. J Clin Endocrinol Metab. 2016;101(12):5004–11. doi:.https://doi.org/10.1210/jc.2016-2695
14
Rizzoli
R
,
Kraenzlin
M
,
Krieg
MA
,
Mellinghoff
HU
,
Lamy
O
,
Lippuner
K
. Indications to teriparatide treatment in patients with osteoporosis. Swiss Med Wkly. 2011;141:w13297. https://smw.ch/en/article/doi/smw.2011.13297/.
15
Kaufman
JM
,
Orwoll
E
,
Goemaere
S
,
San Martin
J
,
Hossain
A
,
Dalsky
GP
, et al.
Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16(5):510–6. doi:.https://doi.org/10.1007/s00198-004-1713-3
16
Kurland
ES
,
Heller
SL
,
Diamond
B
,
McMahon
DJ
,
Cosman
F
,
Bilezikian
JP
. The importance of bisphosphonate therapy in maintaining bone mass in men after therapy with teriparatide [human parathyroid hormone(1-34)]
[human parathyroid hormone(1-34)].
Osteoporos Int. 2004;15(12):992–7. doi:.https://doi.org/10.1007/s00198-004-1636-z
17
Murad
MH
,
Drake
MT
,
Mullan
RJ
,
Mauck
KF
,
Stuart
LM
,
Lane
MA
, et al.
Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2012;97(6):1871–80. doi:.https://doi.org/10.1210/jc.2011-3060
18
Black
DM
,
Schwartz
AV
,
Ensrud
KE
,
Cauley
JA
,
Levis
S
,
Quandt
SA
, et al.; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296(24):2927–38. doi:.https://doi.org/10.1001/jama.296.24.2927
19
Black
DM
,
Reid
IR
,
Boonen
S
,
Bucci-Rechtweg
C
,
Cauley
JA
,
Cosman
F
, et al.
The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27(2):243–54. doi:.https://doi.org/10.1002/jbmr.1494
20
Adler
RA
,
El-Hajj Fuleihan
G
,
Bauer
DC
,
Camacho
PM
,
Clarke
BL
,
Clines
GA
, et al.
Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31(1):16–35. doi:.https://doi.org/10.1002/jbmr.2708
21
Ferrari
S
,
Adachi
JD
,
Lippuner
K
,
Zapalowski
C
,
Miller
PD
,
Reginster
JY
, et al.
Further reductions in nonvertebral fracture rate with long-term denosumab treatment in the FREEDOM open-label extension and influence of hip bone mineral density after 3 years. Osteoporos Int. 2015;26(12):2763–71. doi:.https://doi.org/10.1007/s00198-015-3179-x
22Bone HG. Ten years of denosumab treatment in postmenopausal women with osteoporosis: Results from the FREEDOM extension trial. In: Proceedings of the Annual Meeting of Bone and Mineral Research ASBMR 2015. 2015: Seattle, Oct 9–12.
23
Chen
JS
,
Sambrook
PN
. Antiresorptive therapies for osteoporosis: a clinical overview. Nat Rev Endocrinol. 2011;8(2):81–91. doi:.https://doi.org/10.1038/nrendo.2011.146
24
Khan
AA
,
Morrison
A
,
Hanley
DA
,
Felsenberg
D
,
McCauley
LK
,
O’Ryan
F
, et al.; International Task Force on Osteonecrosis of the Jaw. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30(1):3–23. doi:.https://doi.org/10.1002/jbmr.2405
25
Shane
E
,
Burr
D
,
Abrahamsen
B
,
Adler
RA
,
Brown
TD
,
Cheung
AM
, et al.
Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29(1):1–23. doi:.https://doi.org/10.1002/jbmr.1998
26
Dell
RM
,
Adams
AL
,
Greene
DF
,
Funahashi
TT
,
Silverman
SL
,
Eisemon
EO
, et al.
Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27(12):2544–50. doi:.https://doi.org/10.1002/jbmr.1719
27
Edwards
BJ
,
Bunta
AD
,
Lane
J
,
Odvina
C
,
Rao
DS
,
Raisch
DW
, et al.
Bisphosphonates and nonhealing femoral fractures: analysis of the FDA Adverse Event Reporting System (FAERS) and international safety efforts: a systematic review from the Research on Adverse Drug Events And Reports (RADAR) project. J Bone Joint Surg Am. 2013;95(4):297–307. doi:.https://doi.org/10.2106/JBJS.K.01181
28
Schwartz
AV
,
Bauer
DC
,
Cummings
SR
,
Cauley
JA
,
Ensrud
KE
,
Palermo
L
, et al.; FLEX Research Group. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: the FLEX trial. J Bone Miner Res. 2010;25(5):976–82. doi:.https://doi.org/10.1002/jbmr.11
29
Black
DM
,
Bauer
DC
,
Schwartz
AV
,
Cummings
SR
,
Rosen
CJ
. Continuing bisphosphonate treatment for osteoporosis--for whom and for how long?
N Engl J Med. 2012;366(22):2051–3. doi:.https://doi.org/10.1056/NEJMp1202623
30
Cosman
F
,
Cauley
JA
,
Eastell
R
,
Boonen
S
,
Palermo
L
,
Reid
IR
, et al.
Reassessment of fracture risk in women after 3 years of treatment with zoledronic acid: when is it reasonable to discontinue treatment?
J Clin Endocrinol Metab. 2014;99(12):4546–54. doi:.https://doi.org/10.1210/jc.2014-1971
31
Lamy
O
,
Gonzalez-Rodriguez
E
,
Stoll
D
,
Hans
D
,
Aubry-Rozier
B
. Severe rebound-associated vertebral fractures after denosumab discontinuation: nine clinical cases report. J Clin Endocrinol Metab. 2017;102(2):354–8
. [doi:.].https://doi.org/10.1210/jc.2016-3170
32
Anastasilakis
AD
,
Polyzos
SA
,
Makras
P
,
Aubry-Rozier
B
,
Kaouri
S
,
Lamy
O
. Clinical Features of 24 Patients With Rebound-Associated Vertebral Fractures After Denosumab Discontinuation: Systematic Review and Additional Cases. J Bone Miner Res. 2017 Feb 27. [Epub ahead of print] doi:.https://doi.org/10.1002/jbmr.3110
33Brown JP, et al. Discontinuation of denosumab and associated vertebral fracture incidence: Analysis from FREEDOM and its extension. In: Proceedings of the Annual Meeting of Bone and Mineral Research ASBMR 2016. 2016: Atlanta, Sept 16–19.
34
McClung
MR
,
Wagman
RB
,
Miller
PD
,
Wang
A
,
Lewiecki
EM
. Observations following discontinuation of long-term denosumab therapy. Osteoporos Int. 2017;28(5):1723–32. doi:.https://doi.org/10.1007/s00198-017-3919-1
35
Uebelhart
B
,
Rizzoli
R
,
Ferrari
S
. Prior exposure to bisphosphonate prevents the rebound of bone turnover markers after denosumab therapy. Osteoporos Int. 2017; (in production).
36
Leder
BZ
,
Tsai
JN
,
Jiang
LA
,
Lee
H
. Importance of prompt antiresorptive therapy in postmenopausal women discontinuing teriparatide or denosumab: The Denosumab and Teriparatide Follow-up study (DATA-Follow-up). Bone. 2017;98:54–8. doi:.https://doi.org/10.1016/j.bone.2017.03.006
37
Beaudoin
C
,
Jean
S
,
Bessette
L
,
Ste-Marie
LG
,
Moore
L
,
Brown
JP
. Denosumab compared to other treatments to prevent or treat osteoporosis in individuals at risk of fracture: a systematic review and meta-analysis. Osteoporos Int. 2016;27(9):2835–44. doi:.https://doi.org/10.1007/s00198-016-3607-6
38
Tsai
JN
,
Uihlein
AV
,
Lee
H
,
Kumbhani
R
,
Siwila-Sackman
E
,
McKay
EA
, et al.
Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013;382(9886):50–6. doi:.https://doi.org/10.1016/S0140-6736(13)60856-9
39
Leder
BZ
,
Tsai
JN
,
Uihlein
AV
,
Wallace
PM
,
Lee
H
,
Neer
RM
, et al.
Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015;386(9999):1147–55. doi:.https://doi.org/10.1016/S0140-6736(15)61120-5
