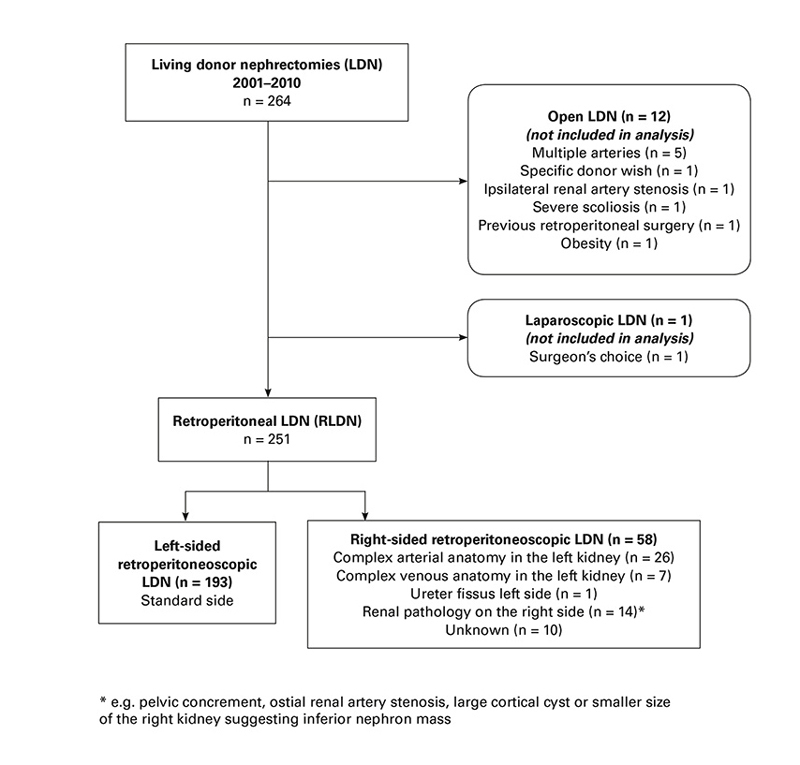
Figure 1 Selection of side of kidney for living donor nephrectomy.
DOI: https://doi.org/10.4414/smw.2017.14472
Living donor nephrectomy (LDN) is now widely performed by means of transabdominal laparoscopic or retroperitoneoscopic surgery. Transplant surgeons favour the left kidney because of the concern of complications arising from a more challenging vascular anatomy in right kidneys [1]. The shorter right renal vein has been associated with thrombosis of the renal vein [2, 3], bleeding from the caval vein in the donor [4] and the need for complex vascular reconstructions during transplantation [5]. This has led many centres to avoid right-sided transabdominal laparoscopic or retroperitoneoscopic LDN.
For the last 12 years, the kidney transplant service of the University Hospital Basel has used retroperitoneoscopic LDN as the standard procedure for all LDN. Because of the extensive expertise acquired with retroperitoneoscopic LDN and the good results obtained, we early on adopted a strategy for liberally choosing the right kidney for retroperitoneoscopic LDN. While it was shown that right-sided transabdominal laparoscopic LDN or retroperitoneoscopic LDN is safe for the donor, with complication rates comparable to left sided LDN [3, 6, 7], little is known about the intraoperative and postoperative surgical complications and the long-term outcome in the recipient. The purpose of this study was to assess whether the side of retroperitoneoscopic LDN (right or left kidney) has an impact on surgical complications and outcome in the transplant recipient.
The study was designed as a retrospective cohort study of recipients of consecutive living donor kidney transplants after retroperitoneoscopic LDN, performed at the University Hospital Basel between November 2001 (the first retroperitoneoscopic LDN at our institution) and September 2010. During this period, 13 kidneys were transplanted after LDN performed by means of open surgery (see figure 1 below). The recipients of these kidneys were excluded from analysis. Three patients contributed twice to our cohort, because they had undergone transplant failure and a second living donor transplant within the study period. For the regression analyses, only the first transplantation was considered. In one donor, right-sided retroperitoneoscopic LDN was converted to open left-sided LDN because of unexpected multiple arteries and a short renal vein on the right side. The recipient of this kidney was considered as having received a left kidney. Data collection was continued until October 2012.
Donors and recipients were evaluated prior to transplantation according to a protocol including the risks after donation for the donor, immunological and anatomical suitability. The choice whether to use the right or left kidney was made at an interdisciplinary meeting involving nephrologists, explant and transplant surgeons. Magnetic resonance (MR) angiography was the standard imaging modality for the preoperative donor work-up and was substituted by computed tomography angiography if MR was not suitable. There was no minimum length of the right renal vein for right retroperitoneoscopic LDN. Reasons for choosing the side of the kidney can be seen in the flow chart presented in figure 1. The policy governing the decision for the choice of side of kidney did not change during the study period. Retroperitoneoscopic LDN was performed as pure retroperitoneoscopic surgery with hand-assistance only for the final stages of vessel transection immediately before removing the kidney. The renal artery and vein were transected over a TA-30 stapler line. Surgery was performed by three dedicated surgeons (two urologists and one general surgeon). Details of the technique of retroperitoneoscopic LDN used have been published earlier [8, 9]. Kidney transplantation was performed via a Gibson incision in the lower abdomen, exposing the external iliac artery, the iliac vein and the bladder. Both arterial and venous anastomoses were performed end-to-side. In the case of multiple arteries or veins, either a common ostium was fashioned between the individual vessels or the individual vessels were anastomosed to the iliac vessels separately. After positioning the kidney in the iliac fossa, the graft ureter was anastomosed to the bladder using the Lich-Gregoire technique and stented with a double-J ureteric stent, which was usually removed after 6-8 weeks.
This study was approved by the institutional ethics review board.
Primary endpoint was the occurrence of at least one surgical complication in the recipient of grade 3 or higher according to the Classification of Surgical Complications by Clavien-Dindo [10], i.e., complications that required surgical, endoscopic or radiological intervention or complications that were life-threatening. Complications that were treated purely pharmacologically and did not represent life-threatening organ-dysfunction (grade 1 or 2) were not considered as they were not reported with sufficient reliability and were considered to have a negligible impact on the clinical course. All complications occurring within the first year after transplantation were included.
Secondary endpoints included creatinine clearance in the recipient, graft survival and recipient survival. Creatinine clearance in the transplant recipients was estimated with the modification of diet in renal disease formula (MDRD), and donor creatinine clearance before donation was estimated using the Cockcroft-Gault formula. For the analysis of 1-year graft function, the patients with transplant failure within the first year (n = 6) were arbitrarily assigned the lowest creatinine clearance observed in a patient with a functioning graft before requiring dialysis (glomerular filtration rate = 9.7 ml/min/1.73m2) to avoid a value of zero.
Baseline characteristics were summarised as mean (standard deviation), median (interquartile range) or number (percentages), as appropriate. Patients who received a second transplant during the observation period were considered separately in the baseline and the descriptive outcome table, but were dropped for the model analyses to avoid a model that would allow for clustering. The effect of right-sided versus left-sided retroperitoneoscopic LDN on the complication rate was assessed with a multivariable logistic regression model with Clavien-Dindo ≥3 yes vs no as binary outcome. The model was controlled for the following variables, pre-specified based on clinical judgment: donor and recipient age (per decade), donor gender, the presence of multiple renal arteries, recipient body mass index (BMI), the presence of diabetes and/or coronary heart disease and surgeon experience, expressed as the total number of kidney transplants performed as leading surgeon at the time of the transplant.
The effect of the side of the kidney on graft function 1 year after transplantation was assessed with a multivariable linear regression model. In addition to the side of the kidney and the above mentioned donor and recipient characteristics, recipient gender, the donor creatinine clearance before nephrectomy, the duration of warm ischaemia, secondary warm ischaemia time during transplantation, oral anticoagulation or antiplatelet medication at the time of transplant, the occurrence of a complication Clavien-Dindo grade ≥3 and immunological factors such as the presence of donor specific antigens in the recipient, AB0 incompatibility and human leukocyte antigen (HLA) mismatches were considered as possible confounders. Model assumptions were assessed graphically and by use of the Breusch-Pagen / Cook-Weisberg test for heteroskedasticity.
Death censored graft survival was analysed with univariate Cox proportional hazard analysis. Graft and overall survival were displayed in Kaplan-Meier graphs stratified by side of the donor kidney, presence or absence of a surgical complications Clavien-Dindo grade ≥3 and presence or absence of donor-specific antibodies (DSA).
All data were organised in Microsoft Excel. All statistical analyses and graphs were performed with Intercooled Stata Version 11.2 for Macintosh (StataCorp, College Station, Texas, USA). An alpha-level of 0.05 was considered to be statistically significant.
From 2001 to 2010, 251 living kidney transplantations after retroperitoneoscopic LDN were performed (fig. 1). Fifty-eight (23%) patients received a right kidney and 193 (77%) patients a left kidney (table 1). Donor and recipient characteristics, as well as details of surgery, are summarised in table 1. Complex vascular procedures, such as the formation of a common ostium from two renal arteries or veins, selective implantation of a polar artery or additional vein or interposition of a vein graft were more common in recipients of right kidneys (n = 16, 28%) than in recipients of left kidneys (n = 37, 19%, see table 1). The need for complex vascular procedures to deal with challenging arterial anatomy was similar in recipients of right kidneys (n = 8, 14%) and left kidneys (n = 34, 18%) but the need for complex vascular procedures to deal with challenging venous anatomy was more frequent in recipients of right kidneys (n = 8, 14%) than in recipients of left kidneys (n = 3, 2%).

Figure 1 Selection of side of kidney for living donor nephrectomy.
Table 1 Donor and recipient baseline characteristics and details of transplant surgery.
|
Total
(n = 251) |
Left kidneys
(n = 193, 77%) |
Right Kidneys
(n = 58, 23%) |
|
|---|---|---|---|
| Donor | |||
| Age in years, mean (SD) | 54 (10.4) | 54 (10.2) | 53 (10.9) |
| Male gender, n (%) | 86 (34%) | 66 (34%) | 20 (34%) |
| Creatinine clearance in ml/min/m2, mean (SD) | 103.1 (29.7) | 102.9 (29.2) | 103.8 (31.8) |
| Conversion to open nephrectomy, n (%) | 2 (1%) | 2 (1%) | 0 |
| Warm ischaemia in minutes, median (IQR)* | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 2.3 (2.0–3.0) |
| Recipient | |||
| Age in years, mean (SD) | 48 (14.0) | 48 (13.8) | 51 (14.6) |
| Male gender, n (%) | 173 (69%) | 137 (71%) | 36 (62%) |
| BMI in kg/m2, mean (SD) | 24.5 (4.1) | 24.4 (4.2) | 24.5 (3.7) |
| Anticoagulation, n (%) | 78 (31%) | 54 (28%) | 24 (41%) |
| Hypertension, n (%) | 204 (81%) | 159 (82%) | 45 (78%) |
| Diabetes, n (%) | 36 (14%) | 26 (13%) | 10 (17%) |
| Coronary heart disease, n (%) | 46 (18%) | 37 (19%) | 9 (16%) |
| Immunological characteristics | |||
| Donor specific antibodies | 34 (14%) | 26 (13%) | 8 (14%) |
| ABO-incompatibility | 27 (11%) | 19 (10%) | 8 (14%) |
| Number of HLA-Mismatches, n (%) | |||
| No mismatch | 23 (9%) | 16 (8%) | 7 (12%) |
| 1 mismatch | 12 (5%) | 10 (5%) | 2 (3%) |
| 2 mismatches | 42 (17%) | 32 (17%) | 10 (17%) |
| 3 mismatches | 65 (26%) | 53 (27%) | 12 (21%) |
| 4 mismatches | 36 (14%) | 26 (13%) | 10 (17%) |
| 5 mismatches | 54 (22%) | 42 (22%) | 12 (21%) |
| 6 mismatches | 19 (8%) | 14 (7%) | 5 (9%) |
| Details of transplant surgery | |||
| Duration in minutes, median (IQR) | 140 (124–173) | 140 (124–172) | 140 (126–186) |
| Surgeon experience†, median (IQR) | 71 (34–113) | 71 (36–117) | 68 (21–106) |
| Secondary warm ischaemia, n (%) | 19 (8%) | 13 (7%) | 6 (10%) |
| Multiple arteries, n (%) | 46 (18%) | 39 (20%) | 7 (12%) |
| Arterial common ostium, n (%) | 21 (8%) | 20 (1%) | 1 (2%) |
| Two separate arterial anastomoses, n (%) | 15 (6%) | 10 (5%) | 5 (9%) |
| Vein interposition for transplant vein, n (%) | 3 (1%) | 0 | 3 (5%) |
| Venous common ostium, n (%) | 5 (2%) | 2 (1%) | 3 (5%) |
| Complex vascular surgery for transplant artery, n (%) | 42 (17%) | 34 (18%) | 8 (14%) |
| Complex vascular surgery for transplant vein, n (%) | 11 (4%) | 3 (2%) | 8 (14%) |
* One missing value in right-sided donor kidney; † in numbers of transplantations performed
One conversion to open left-sided donor nephrectomy was required after attempted right-sided retroperitoneoscopic LDN because of unexpected multiple arteries and concurrent short vein length, and a bleeding complication in one left-sided retroperitoneoscopic LDN led to a conversion to open LDN.
A complication of grade 3 or greater according to the Clavien-Dindo classification occurred in 75 recipients (30%); details are shown in table 2. The complication rate was similar in recipients of right and left kidneys (33% vs 29%, odds ratio [OR] 0.98, 95% confidence interval [CI] 0.50, 1.94) (tables 2 and 3 ). Neither donor age, donor gender, the presence of multiple arteries, recipient age, the presence of coronary heart disease and/or diabetes nor surgeon experience were associated with the occurrence of surgical complications (table 3). After controlling for confounders, increased BMI was found to decrease the risk for surgical complications (OR 0.92, 95% CI 0.85, 1.00; p = 0.038). This result persisted also in a sensitivity analysis excluding seven underweight patients (data not shown).
Table 2 Surgical complications Clavien-Dindo ≥3, 1-year graft failure and mortality, n (%).
|
Total
(n = 251) |
Left kidneys
(n = 193, 77%) |
Right kidneys
(n = 58, 23%) |
|
|---|---|---|---|
| At least one surgical complication | 75 (30%) | 56 (29%) | 19 (33%) |
| One complication | 56 (22%) | 46 (24%) | 10 (17%) |
| More than one complications | 19 (8%) | 10 (5%) | 9 (16%) |
| Severity of surgical complication* | |||
| Grade 3a | 22 (9%) | 15 (8%) | 7 (12%) |
| Grade 3b | 48 (19%) | 37 (19%) | 11 (19%) |
| Grade 4a | 9 (4%) | 6 (3%) | 3 (5%) |
| Grade 4b | 1 (<1%) | 1 (1%) | 0 (0%) |
| Description of surgical complications | |||
| All vascular complications | 33 (13%) | 21 (11%) | 12 (21%) |
| Vascular complication with reoperation | 18 (7%) | 10 (5%) | 8 (14%) |
| Bleeding | 15 (6%) | 13 (7%) | 2 (3%) |
| Lymphocele | 14 (6%) | 9 (5%) | 5 (9%) |
| Urological complication | 17 (7%) | 10 (5%) | 7 (12%) |
| Surgical site infection** | 1 (<1%) | 1 (1%) | 0 (0%) |
| Incisional or inguinal hernia | 4 (1%) | 4 (2%) | 0 (0%) |
| Adjacent organ injury (spermatic duct) | 1 (<1%) | 1 (1%) | 0 (0%) |
| Nonsurgical complication | 10 (4%) | 7 (4%) | 3 (5%) |
| Survival | |||
| 1-year graft failure | 6 (2%) | 5 (3%) | 1 (2%) |
| 1-year mortality | 1 (<1%) | 1 (1%) | 0 (0%) |
* More than one complication per patient possible; ** definition of surgical site infection see [11]
Table 3 Logistic model for surgical complications Clavien-Dindo ≥3 (n = 248).
| Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Side of the kidney (right vs left) | 1.06 (0.55, 2.03) | 0.863 | 0.98 (0.50, 1.94) | 0.973 |
| Donor age (per decade increase) | 0.89 (0.69, 1.16) | 0.404 | 0.86 (0.65, 1.13) | 0.279 |
| Donor gender (female vs male) | 1.72 (0.94, 3.15) | 0.079 | 1.73 (0.93, 3.22) | 0.085 |
| Multiple arteries (yes vs no) | 1.20 (0.60, 2.39) | 0.601 | 1.40 (0.68, 2.87) | 0.359 |
| Recipient age (per decade increase) | 1.06 (0.87, 1.30) | 0.544 | 1.14 (0.91, 1.44) | 0.262 |
| Recipient BMI (per unit increase) | 0.95 (0.88, 1.02) | 0.136 | 0.92 (0.85, 1.00) | 0.038 |
| CHD and/or DM (vs neither CHD nor DM) | 1.32 (0.70, 2.46) | 0.390 | 1.34 (0.67, 2.69) | 0.408 |
| Surgeon experience (per 10 transplantations increase) | 0.96 (0.90, 1.01) | 0.121 | 0.96 (0.91, 1.02) | 0.160 |
BMI= body mass index; CHD = coronary heart disease, CI = confidence interval; DM = diabetes mellitus; OR = odds ratio
The 33 vascular complications comprised 10 kinking of the renal artery, 15 stenoses or thrombus at the site of the arterial anastomosis, 2 dissection of the renal artery, 1 laceration of the renal vein and 5 other vascular complications. Fifteen of these vascular complications were diagnosed and treated at the time of transplantation and thus did not lead to a reoperation. There was only one graft failure caused by a vascular complication and this occurred in the recipient of a left kidney. All the other vascular complications were successfully treated by either correction at the time of transplant, by reoperation or by balloon dilatation at a later date. Vascular complications were more common in recipients of right kidneys (n = 12, 21%) than left kidneys (n = 21, 11%). The most common vascular complication in recipients of a right kidney was kinking of the renal artery in the setting of a short renal vein, which occurred in four recipients of right kidneys (7%). This was treated either by graft repositioning or shortening and re-anastomosing the renal artery. Urological complications consisted mainly of ureteral stenosis, the majority of which were diagnosed after removal of the ureteric stent and treated by secondary ureteric stenting.
The side of the kidney was not associated with creatinine clearance one year after transplant: 50 ml/min/m2 in recipients of right kidneys, 48 ml/min/m2 in recipients of left kidneys; mean difference of 3.3 ml/min/m2, 95% CI ˗1.5, 8.1; p = 0.175, based on the multivariable model (table 4). The occurrence of a surgical complication had a significant negative impact on recipient creatinine clearance 1 year after transplant, with a mean decrease of 6 ml/min/m2 (95% CI ˗11, ˗1.1, Table 4). Other significant predictors for 1-year graft function were donor age, which was associated with an average decrease of 2.9 ml/min/m2 (95% CI ˗5.1, ˗0.6) per decade increase, and preoperative donor creatinine clearance, where a 10 ml/min/m2 increase in donor creatinine clearance corresponded to an average increase of 1.6 ml/min/m2 (95% CI 0.8, 2.4) in recipient creatinine clearance. Furthermore, surgeon experience (per 10 transplantations) was a significant predictor for an increased 1-year creatinine clearance (coefficient 0.7, 95% CI 0.3, 1.1, p = 0.001) (table 4).
Table 4 Linear regression model for recipient creatinine clearance one year after transplant (n = 2 46)*.
| Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|
|
Coefficient
(95% CI) |
p-value |
Coefficient
(95% CI) |
p-value | |
| Side (right vs left) | 3.3 (˗2.0, 8.6) | 0.216 | 3.3 (˗1.5, 8.1) | 0.175 |
| Donor age (per decade increase) | ˗5.0 (˗7.0, ˗3.0) | <0.001 | ˗2.9 (˗5.1, ˗0.6) | 0.013 |
| Donor gender (female vs male) | ˗5.5 (˗10, ˗0.9) | 0.020 | ˗3.1 (˗7.5, 1.3) | 0.168 |
| Multiple arteries in donor (yes vs no) | 0.4 (˗5.3, 6.0) | 0.901 | ˗0.8 (˗5.9, 4.4) | 0.770 |
| Donor creatinine clearance (per 10 units increase) | 2.2 (1.5, 2.9) | <0.001 | 1.6 (0.8, 2.4) | <0.001 |
| Warm ischaemia time (per minute increase) | 0.6 (˗0.4, 1.7) | 0.232 | 0.7 (˗0.2, 1.6) | 0.138 |
| Recipient age (per decade increase) | ˗0.9 (˗2.5, 0.7) | 0.266 | 0.1 (˗1.6, 1.8) | 0.911 |
| Recipient gender (female vs male) | ˗2.2 (˗7.0, 2.5) | 0.358 | ˗4.1 (˗8.9, 0.7) | 0.091 |
| Recipient BMI (per unit increase) | ˗0.2 (˗0.7, 0.4) | 0.504 | ˗0.5 (˗1.0, 0.1) | 0.080 |
| Oral anticoagulation or antiplatelet agent (yes vs no) | ˗0.4 (˗5.2, 4.4) | 0.873 | 3.5 (˗1.3, 8.2) | 0.154 |
| Donor specific antibodies (yes vs no) | ˗2.2 (˗8.8, 4.3) | 0.258 | ˗2.1 (˗8.0, 3.9) | 0.499 |
| ABO incompatible transplant (yes vs no) | 8.7 (1.7, 16) | 0.015 | 2.3 (˗4.3, 9.0) | 0.488 |
| HLA mismatches (per 1 mismatch increase) | ˗1.4 (˗2.7, ˗0.1) | 0.033 | ˗1.1 (˗2.3, 0.1) | 0.067 |
| Postoperative complications Clavien-Dindo ≥3 (yes vs no) | ˗5.6 (˗10, ˗0.9) | 0.021 | ˗6.0 (˗11, ˗1.1) | 0.016 |
| Secondary warm ischaemia (yes vs no) | 3.0 (˗5.5, 12) | 0.482 | 7.2 (˗1.5, 16) | 0.103 |
| Surgeon experience (per 10 transplantations increase) | 0.8 (0.3, 1.2) | 0.001 | 0.7 (0.3, 1.1) | 0.001 |
* In the six patients with transplant failure within the first year, the lowest creatinine clearance in the cohort (i.e. clearance of 9.7 ml/min/m2) was arbitrarily taken as the creatinine clearance at 1 year); n = 246 (251 minus three repeated transplants and minus one missing observation for warm ischaemia time and minus one patient who died)
Death-censored 1-year graft survival was 97.6% in the whole cohort and similar in right and left kidneys (98.3% vs 97.4%). The reasons for graft loss in the first year were rejection (n = 2), recurrent disease (n = 2), polyoma virus BK nephropathy (n = 1) and renal artery thrombosis because of kinking (n = 1), which occurred in a patient receiving a left kidney. There were no perioperative deaths. One patient died 3 months after transplantation, most probably of pulmonary embolism. After a median follow-up of 5 years (interquartile range 3–8 years) without any losses to follow-up, graft survival was associated with neither the side of the kidney (hazard ratio [HR] 1.42, 95% CI 0.62, 3.29; fig. 2a) nor with the occurrence of a surgical complication (HR 1.56, 95% CI 0.72, 3.42; fig. 2b). The only significant predictor of graft failure was the presence of donor-specific antibodies (HR 4.04, 95% CI 1.80, 9.08; fig. 2c).
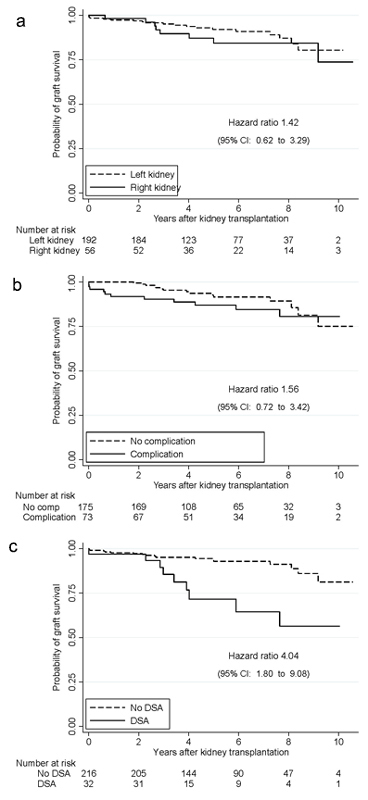
Figure 2 Death-censored graft survival according to side of kidney (a), occurrence of surgical complications (b) and presence of donor-specific antibodies (DSA) (c).
Right-sided retroperitoneoscopic LDN led to an excellent outcome in the recipient: there was no statistically significant difference in surgical complications, mortality, 1-year graft function or long term graft survival between recipients of left or right kidneys.
The strength of our study lies in its good quality of data (consecutive patient cohort, complete and long follow-up), its focus on clinically relevant parameters (surgical complications, 1-year graft function and long-term graft survival) and the adjustment of the statistical analysis for a large number of potentially relevant confounders.
The two important limitations of our study are its retrospective design and the size of the study population. However, the quality and completeness of our patient records render it unlikely that prospective data collection would have substantially altered the results. Although a study in which left or right kidneys are randomly allocated in the presence of comparable vascular anatomy is theoretically possible and has actually been performed in a small series of hand-assisted transabdominal laparoscopic LDN [12], it would appear an extremely difficult task and, given the subjective preference of transplant surgeons for the left kidney, we think it is doubtful whether such a study would be justifiable. The statistical power of our data to rule out small differences in outcome between right and left kidneys is limited. The hazard ratio confidence interval for the comparison of surgical complications after transplanting right and left kidneys (0.5, 1.94) is such that a clinically relevant difference cannot stringently be ruled out. The confidence interval of the difference in graft function between recipients of right and left kidneys (˗1.5, ˗ 8.1 ml/min/m2) does, however, make it extremely unlikely that a relevant impact on graft function would have been missed. The trend for more vascular complications in right kidneys may suggest a difference only discernible with a larger study population.
Our observations are in agreement with several single centre studies that compared recipient outcome after right versus left transabdominal laparoscopic LDN [3–6, 13–17], which all document equivalent graft survival and graft function after transplanting right kidneys. The United Network for Organ Sharing (UNOS) data analysis of 2555 right versus 25 387 left transabdominal laparoscopic LDN, however, showed a slight increase in 90-day graft failure in right kidneys (3.8 vs 2.5%) [18]. But it is doubtful whether this small difference, although statistically clearly significant, should be evidence sufficient to refrain from right-sided transabdominal laparoscopic LDN in a donor where the right kidney was more suitable, particularly as the UNOS database also showed a similarly inferior outcome for right kidneys harvested by open LDN. Data on recipient outcome after retroperitoneoscopic LDN are much scarcer [19–24]. Only three of these studies document recipient outcome specifically for the side of the kidney transplanted [19–21], again none of the studies report inferior transplant survival of right kidneys. The number of recipients of right kidneys in our study (n = 58) is considerably larger than in these studies (n = 19, 12 and 24). Furthermore, there were 23% right kidneys in our study, a rate higher than in the other studies (18%, 18% and 5%), rendering comparison between the two groups in our study more meaningful. Only two of these studies report on recipient surgical complications [20, 21]. Like us, Omoto reported a statistically nonsignificant higher complication rate in recipients of right kidneys without any impact on 1-year and 5-year graft survival. Ours is the first study comparing long-term creatinine clearance of right or left kidneys after retroperitoneoscopic LDN.
We attribute the relatively high rate of vascular complications in our series to a very liberal definition of vascular complication and to the very extensive use of intraoperative and postoperative Duplex sonography, thereby raising the sensitivity for diagnosing abnormalities that would most likely have no effect on renal function. Comparing our rate of vascular complications or overall surgical complications in the recipient to other studies may not be useful, as reported complication rates vary considerably from 10% to more than 50% [25–28]. To our knowledge, this is the first study systematically investigating potential risk factors for the occurrence of surgical complications in the recipient. Neither side of the kidney, donor age, donor gender, the presence of multiple arteries in the graft, recipient age, recipient coronary artery disease and/or diabetes nor surgeon experience were significant predictors for surgical complications. The only investigated parameter that showed a significant correlation was BMI – but to our surprise in the opposite direction to that expected: obese recipients had fewer surgical complications. It is of note that only 9.6% of our patients were obese (BMI >30 kg/m2) and only 1.2% of our patients were severely obese (BMI >35 kg/m2). Other than that, we have no explanation for this finding and have not found any studies reporting a positive effect of obesity on the occurrence of surgical complications. Whereas the occurrence of surgical complications in the recipient showed a strong correlation with inferior 1-year creatinine clearance, it did not predict inferior long-term graft survival, which only correlated with the presence of donor-specific antibodies.
We conclude from our data that if vascular anatomy or other factors suggest that the right kidney is more suitable than the left kidney, right retroperitoneoscopic LDN can be used without putting the recipient at risk for an inferior outcome. There appears to be no justification for a policy to perform all retroperitoneoscopic LDN on the left or performing right-sided LDN by open surgery. Furthermore, the observed high rate of complex vascular procedures and the high rate of vascular complications suggest that kidney transplantation should be performed by surgeons well acquainted with vascular surgical techniques.
Publication and presentation: This study has not been published prior to this submission. A part of this study has been orally presented at the Swiss Society of Surgery Annual Meeting 20–22 June 2012 in Davos, Switzerland.
Rachel Rosenthal is an employee of F. Hoffmann-La Roche Ltd. since 1 May 2014. The present study was conducted before Rachel Rosenthal joined F. Hoffmann-La Roche Ltd. and has no connection to her employment by the company. Rachel Rosenthal continues to be affiliated to the University of Basel, Switzerland.
1 Ratner LE , Fabrizio M , Chavin K , Montgomery RA , Mandal AK , Kavoussi LR . Technical considerations in the delivery of the kidney during laparoscopic live-donor nephrectomy. J Am Coll Surg. 1999;189(4):427–30. doi:.https://doi.org/10.1016/S1072-7515(99)00180-5
2 Mandal AK , Cohen C , Montgomery RA , Kavoussi LR , Ratner LE . Should the indications for laparascopic live donor nephrectomy of the right kidney be the same as for the open procedure? Anomalous left renal vasculature is not a contraindiction to laparoscopic left donor nephrectomy. Transplantation. 2001;71(5):660–4. doi:.https://doi.org/10.1097/00007890-200103150-00015
3 Saad S , Paul A , Treckmann J , Nagelschmidt M , Heiss M , Arns W . Laparoscopic live donor nephrectomy for right kidneys: Experience in a German community hospital. Surg Endosc. 2008;22(3):674–8. doi:.https://doi.org/10.1007/s00464-007-9459-6
4 Buell JF , Edye M , Johnson M , Li C , Koffron A , Cho E , et al. Are concerns over right laparoscopic donor nephrectomy unwarranted? Ann Surg. 2001;233(5):645–51. doi:.https://doi.org/10.1097/00000658-200105000-00008
5 Kay MD , Brook N , Kaushik M , Harper SJ , Bagul A , Nicholson ML . Comparison of right and left laparoscopic live donor nephrectomy. BJU Int. 2006;98(4):843–4. doi:.https://doi.org/10.1111/j.1464-410X.2006.06429.x
6 Hoda MR , Greco F , Wagner S , Heynemann H , Fornara P . Prospective, nonrandomized comparison between right- and left-sided hand-assisted laparoscopic donor nephrectomy. Transplant Proc. 2011;43(1):353–6. doi:.https://doi.org/10.1016/j.transproceed.2010.12.021
7 Tsoulfas G , Agorastou P , Ko D , Hertl M , Elias N , Cosimi AB , et al. Laparoscopic living donor nephrectomy: is there a difference between using a left or a right kidney? Transplant Proc. 2012;44(9):2706–8. doi:.https://doi.org/10.1016/j.transproceed.2012.09.019
8 Bachmann A , Wolff T , Ruszat R , Giannini O , Dickenmann M , Gürke L , et al. Retroperitoneoscopic donor nephrectomy: a retrospective, non-randomized comparison of early complications, donor and recipient outcome with the standard open approach. Eur Urol. 2005;48(1):90–6, discussion 96. doi:.https://doi.org/10.1016/j.eururo.2005.03.007
9 Sulser T , Gürke L , Langer I , Dickenmann M , Steiger J , Gasser TC , et al. Retroperitoneoscopic living-donor nephrectomy: first clinical experiences in 19 operations. J Endourol. 2004;18(3):257–62. doi:.https://doi.org/10.1089/089277904773582868
10 Dindo D , Demartines N , Clavien PA . Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. doi:.https://doi.org/10.1097/01.sla.0000133083.54934.ae
11 Mangram AJ , Horan TC , Pearson ML , Silver LC , Jarvis WR ; Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999;20(4):247–78, quiz 279–80. doi:.https://doi.org/10.1086/501620
12 Minnee RC , Bemelman WA , Maartense S , Bemelman FJ , Gouma DJ , Idu MM . Left or right kidney in hand-assisted donor nephrectomy? A randomized controlled trial. Transplantation. 2008;85(2):203–8. doi:.https://doi.org/10.1097/TP.0b013e3181601486
13 Abrahams HM , Freise CE , Kang SM , Stoller ML , Meng MV . Technique, indications and outcomes of pure laparoscopic right donor nephrectomy. J Urol. 2004;171(5):1793–6. doi:.https://doi.org/10.1097/01.ju.0000123881.76507.fe
14 Diner EK , Radolinski B , Murdock JD , Ghasemian SR . Right laparoscopic donor nephrectomy: the Washington Hospital Center experience. Urology. 2006;68(6):1175–7. doi:.https://doi.org/10.1016/j.urology.2006.08.1076
15 Husted TL , Hanaway MJ , Thomas MJ , Woodle ES , Buell JF . Laparoscopic right living donor nephrectomy. Transplant Proc. 2005;37(2):631–2. doi:.https://doi.org/10.1016/j.transproceed.2004.12.126
16 Ko EY , Castle EP , Desai PJ , Moss AA , Reddy KS , Mekeel KL , et al. Utility of the endovascular stapler for right-sided laparoscopic donor nephrectomy: a 7-year experience at Mayo Clinic. J Am Coll Surg. 2008;207(6):896–903. doi:.https://doi.org/10.1016/j.jamcollsurg.2008.07.013
17 Posselt AM , Mahanty H , Kang SM , Stoller ML , Meng MV , Roberts JP , et al. Laparoscopic right donor nephrectomy: a large single-center experience. Transplantation. 2004;78(11):1665–9. doi:.https://doi.org/10.1097/01.TP.0000144320.33956.42
18 Hsu JW , Reese PP , Naji A , Levine MH , Abt PL . Increased early graft failure in right-sided living donor nephrectomy. Transplantation. 2011;91(1):108–14. doi:.https://doi.org/10.1097/TP.0b013e3181fd0179
19 Ma L , Li G , Huang Y , Hou X , Zhao L , Wang G , et al. Retroperitoneoscopic live-donor right nephrectomy: a Chinese single center. Exp Clin Transplant. 2011;9(1):20–5.
20 Narita S , Inoue T , Matsuura S , Horikawa Y , Kakinuma H , Saito M , et al. Outcome of right hand-assisted retroperitoneoscopic living donor nephrectomy. Urology. 2006;67(3):496–500, discussion 500–1. doi:.https://doi.org/10.1016/j.urology.2005.09.064
21 Omoto K , Nozaki T , Inui M , Shimizu T , Hirai T , Sawada Y , et al. Impact of right-sided nephrectomy on long-term outcomes in retroperitoneoscopic live donor nephrectomy at single center. J Transplant. 2013;2013:546373. doi:.https://doi.org/10.1155/2013/546373
22 Tanabe K , Miyamoto N , Ishida H , Tokumoto T , Shirakawa H , Yamamoto H , et al. Retroperitoneoscopic live donor nephrectomy (RPLDN): establishment and initial experience of RPLDN at a single center. Am J Transplant. 2005;5(4 Pt 1):739–45. doi:.https://doi.org/10.1111/j.1600-6143.2004.00702.x
23 Wadström J , Biglarnia A , Gjertsen H , Sugitani A , Fronek J . Introducing hand-assisted retroperitoneoscopic live donor nephrectomy: learning curves and development based on 413 consecutive cases in four centers. Transplantation. 2011;91(4):462–9.
24 Yashi M , Yagisawa T , Nukui A , Ishikawa N , Miyamoto N , Sakuma Y , et al. Strategic hand assistance for effective and safe retroperitoneoscopic live donor nephrectomy. Transplant Proc. 2009;41(1):88–90. doi:.https://doi.org/10.1016/j.transproceed.2008.11.004
25 Burgos FJ , Pascual J , Quicios C , Marcen R , Fernández A , López Fando L , et al. Post-kidney transplant surgical complications under new immunosuppressive regimens. Transplant Proc. 2006;38(8):2445–7. doi:.https://doi.org/10.1016/j.transproceed.2006.08.192
26 Intissar H , Zoubeir S , Loubna B , Ezzaitouni F , Naima O , Rabia B , et al. [Surgical complications of renal transplantation from living donors: experience of the CHU Ibn Sina, Rabat]. Pan Afr Med J. 2010;6:20. Article in French.
27 Koçak T , Nane I , Ander H , Ziylan O , Oktar T , Ozsoy C . Urological and surgical complications in 362 consecutive living related donor kidney transplantations. Urol Int. 2004;72(3):252–6. doi:.https://doi.org/10.1159/000077125
28 Arslan G , Moray G , Bilgin N , Karamehmetoğlu M , Büyükpamukçu N , Haberal M . Early operative morbidity and mortality in 1051 consecutive kidney transplants over 20 years at our centers. Transplant Proc. 1996;28(4):2311.
Rachel Rosenthal is an employee of F. Hoffmann-La Roche Ltd. since 1 May 2014. The present study was conducted before Rachel Rosenthal joined F. Hoffmann-La Roche Ltd. and has no connection to her employment by the company. Rachel Rosenthal continues to be affiliated to the University of Basel, Switzerland.