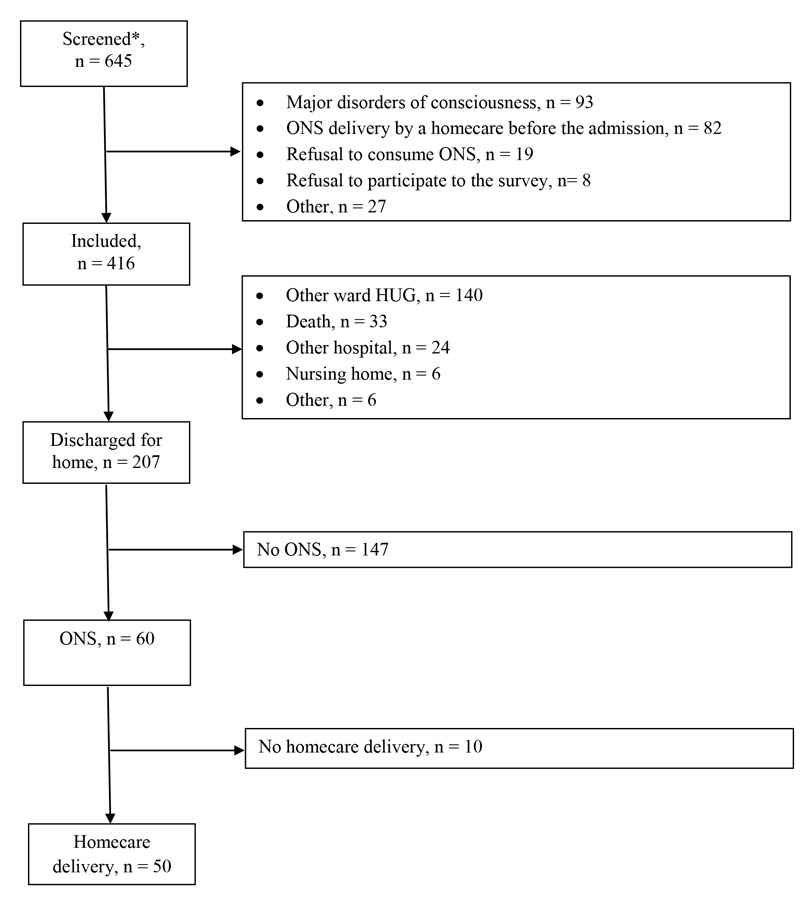
Figure 1 Selection of patients included in the survey.
* All adult patients who received ONS for the first time on their meal trays in the screened hospital units.
DOI: https://doi.org/10.4414/smw.2017.14475
Patients with an acute or chronically negative nutritional balance are at nutritional risk. They are at increased risk of infectious and noninfectious morbidity, prolonged hospital stay, high healthcare costs, and a reduced quality of life [1–5]. In Switzerland, 20 to 30% of adults admitted to hospital are at nutritional risk, defined as a Nutritional Risk Screening 2002 (NRS-2002) score ≥3 [1, 6].
Oral nutritional supplements (ONS) are medical treatments that represent the first line of nutrition intervention when patients are at nutritional risk or already malnourished. Consumption of ONS allows increased energy and protein intake, and, subsequently, weight gain or at least limitation of weight loss. Their intake is associated with improved functional capacity (strength, mobility), decreased complications (pressure ulcers, wounds, fractures, infections), rate of hospital admission and readmission and mortality and reduced costs [7–12].
Currently, in the Geneva University Hospitals, all ONS are ordered on the patients’ meal trays through a meal software (Winrest®). These orders are based either on a medical prescription or, often, on the personal initiative of the dieticians, nurses and auxiliary nurses as part of routine care.
In the ambulatory setting in Switzerland, ONS are reimbursed by the public insurance (LAMal) under conditions defined by the Swiss Society for Clinical Nutrition (SSNC). The reimbursement requires a medical prescription for the ONS and their delivery at the patient’s home by a homecare service that ensures nutritional follow-up and that can be contacted by either the physician or the patient. The homecare service needs to be accredited by the SSNC and can be chosen from those listed on the website of the SSNC (www.ssnc.ch). The indication for ONS, defined as an NRS-2002 score ≥3, must also be present. Thus, reimbursement of ONS in the ambulatory setting is a complex procedure, which may be a barrier to optimal nutritional intervention.
We hypothesised that delivery of ONS in the hospital and at home often does not rely on a medical prescription and the patients do not fulfil the indication criterion of the SSNC because there is no training in and sensitisation to clinical nutrition during undergraduate and postgraduate medical studies. We also supposed that ONS are continued at home only in the presence of a medical prescription and accredited homecare delivery, as these conditions are required for reimbursement.
This prospective survey aimed at documenting: (i) the existence of a medical prescription for ONS during hospitalisation and at discharge for home, (ii) the adequacy of the indication for ONS (NRS-2002 ≥3) during hospitalisation and at discharge for home, and (iii) the continuation or not of ONS consumption 1 month after discharge for home.
This prospective survey was performed at the Geneva University Hospitals between May 2015 and September 2016. It included all adult patients who were hospitalised in departments of medicine, surgery or rehabilitation and who received ONS for the first time on their meal trays. These patients were identified through the meal software (Winrest®). Exclusion criteria were major disorders of consciousness, delivery of ONS by a homecare service before the admission, patient refusal to take ONS and refusal to participate to the survey (see fig. 1).
The Ethical Committee of the Geneva University Hospitals (“Commission cantonale d’éthique de la recherche”) authorised the conduct of this survey without obtaining a signed consent from the patient as part of a quality of care survey supported by the General Direction of the Hospital. Investigators explained the survey to the patients who could refuse to participate. The survey was carried out in accordance with the protocol, with the guidelines of Good Clinical Practice (GCP) and, the principles enunciated in the current version of the Declaration of Helsinki.
The length of follow-up was the length of the hospitalisation at the Geneva University Hospitals, plus an additional 1 month for the patients who were discharged for home. Data were collected at the first delivery of ONS on the meal tray, at hospital discharge for home and 1 month after discharge. The types of data collected at the three time-points are detailed below.
During the hospitalisation, a research dietician was in charge of recruiting patients and collect data prospectively with the help of the hospital computer database.
At first delivery of ONS on the meal tray, the research dietician determined whether there was a medical prescription for ONS and follow-up by the nutrition team from the hospital computer database. She reported age, sex, weight, body mass index (BMI), provenance of the patient at admission (home or other care setting), area of hospitalisation and comorbidities. Comorbidities were used to calculate the Charlson Comorbidity Index (CCI) [13]. The CCI predicts the 10-year mortality for a patient with a range of comorbidities. It contains 19 categories of comorbidities assigned to a score ranging from 0 (healthy) to 37. One additional point is added for each decade of age from the age of 50 years. The research dietician also established the nutritional risk by completing the validated NRS-2002 score with the patient [14]. This score is divided into three parts including nutritional status, disease severity and age. The first scoring is allocated to impaired nutritional status (score 0 = absent, score 1 = mild, score 2 = moderate, score 3 = severe) based of three different items: BMI (kg/m2) and/or percent of weight loss and/or current food intake vs habitual food intake. The second scoring is based on the disease severity: absent (score = 0) to severe (score = 3). One additional point is added if age >70 years. A NRS-2002 score ≥3 indicates that the patient is at nutritional risk.
At hospital discharge for home, the research dietician reported, with the help of the nutrition team, whether there was a medical ONS prescription and a contact with a homecare delivery of ONS. She also calculated the NRS-2002 score with the patient.
The research dietician coordinated the data collection with the homecare providers at the patients’ homes. Data were collected on a standardised questionnaire. It involved the homecare staff, who were trained by the research dietician at the beginning of the survey.
Homecare providers recorded the person who contacted them for delivery of ONS, evaluated the patients’ knowledge about the prescription (dose and duration) and the patient’s consent for the delivery of ONS. One month after discharge for home, a dietician from the assigned homecare organisation, or the research dietician if the patient was rehospitalised, evaluated the continuation of consumption of ONS, or the timing of and reasons for its discontinuation.
Statistical analyses were done using IBM SPPS Statistics (version 22; Armonk, NY). Categorical variables are reported as frequencies and percentages, and compared between patients with and without ONS at discharge for home with chi-squared test. Continuous variables were checked for the normality of their distribution with Shapiro-Wilks tests. They were reported as mean and standard deviation or, if their distribution was not normal, as median and ranges. Age, weight and BMI were compared between patients with and without ONS at discharge for home by Wilcoxon rank sum test. A p-value <0.05 was considered significant.
Out of 645 screened patients, 416 were included (64.5%) ( fig. 1 ). The main reasons for exclusion were major disorders of consciousness (41.9%, n = 93), and ONS delivery by a homecare provider before the admission (35.8%, n = 82).

Figure 1 Selection of patients included in the survey.
* All adult patients who received ONS for the first time on their meal trays in the screened hospital units.
Baseline demographic and clinical characteristics are described in table 1. For 44.5% (n = 185) of patients, medical prescription for ONS was missing and only 39.9% (n = 166) benefited from at least one consultation by a member of nutrition team. For patients with no medical prescription for ONS and no consultation by a member of the nutrition team, ONS were distributed by the nurses or the auxiliary nurses in the ward where the patient was hospitalised.
Table 1 Demographic and clinical characteristics in the hospital.
|
At first ONS delivery on the meal tray
(n = 416) |
At discharge for home
(n = 207) |
|||
|---|---|---|---|---|
|
No ONS
(n = 147) |
ONS
(n = 60) |
p-value* | ||
| Age (year), mean (SD) | 71.7 (14.1) | 68.8 (15.8) | 69.2 (15.0) | 0.872 |
| Weight (kg), mean (SD) | 65.7 (16.6) | 64.4 (15.2) | 62.1 (17.5) | 0.359 |
| Body mass index (kg/m2), mean (SD) | 23.6 (5.2) | 23.5 (5.2) | 22.1 (5.3) | 0.079 |
| Sex (male), % | 52.6% | 50.3% | 50.0% | 0.965 |
| Patients admission, % | ||||
| Home | 42.1% | – | – | |
| Nursing home | 1.0% | – | – | |
| Other ward HUG | 53.6% | – | – | |
| Other hospital | 1.9% | – | – | |
| Other | 1.4% | – | – | |
| Area of hospitalisation, % | 0.023 | |||
| Medicine | 39.9% | 43.5% | 26.7% | |
| Rehabilitation | 51.4% | 45.6% | 66.7% | |
| Surgery | 8.7% | 10.9% | 6.7% | |
| Charlson Comorbidity Index, mean (SD) | 6.3 (3.0) | – | – | |
| Nutritional Risk Score 2002 ≥3, % | 82.7% | 63.3% | 80.0% | 0.026 |
HUG = Geneva University Hospitals; ONS = oral nutritional supplements; SD = standard deviation *Comparison of groups with vs without ONS at discharge. We used Wilcoxon rank test for age, weight and body mass index and chi-squared test for gender, area and nutritional risk score
ONS were indicated in 82.7% of patients (n = 344), based on an NRS-2002 score ≥3.
The median length of hospital stay was 18 (range 2–160) days. Out of 416 included patients, only 207 were discharged for home, and 60 of them (29%) received a medical prescription for ONS (fig. 1). Demographic and clinical characteristics of patients with and without ONS prescription at discharge for home are presented in table 1. The reasons for the absence of ONS prescription by the physician at discharge for home are described in table 2.
Table 2 Reasons for absence of oral nutritional supplement (ONS) prescription at discharge for home.
|
Number
(n = 147) |
Percent
(%) |
|
|---|---|---|
| No medical prescription during hospitalisation | 45 | 30.6 |
| Forgotten prescription by physician | 36 | 24.5 |
| No need of ONS at home according to physician or dietitian | 34 | 23.1 |
| Patient refusal | 22 | 15.0 |
| Other | 10 | 6.8 |
Of the 60 patients who received a medical prescription for ONS, a homecare service was contacted for 50 (83%), either by the hospital nutrition team (n = 47), a hospital physician (n = 2) or the general practitioner (n = 1).
After the first phone call with the patient about the order for ONS, homecare services reported that 13 out of the 50 patients (26%) were not aware of the prescription (dose and duration) and 8 patients (16%) refused any delivery. This left 42 patients for analysis 1 month after discharge.
One month after discharge for home, 30 out of the 42 patients (71.4%) were at home, whereas 8 were rehospitalised, 2 dead and 2 lost to follow-up. Most patients continued with ONS 1 month after discharge for home (76%, n = 29); the others (24%, n = 9) stopped the supplements without medical decision after a mean of 15.9 ± 12.0 days. The reasons were disgust (n = 4), costs (n = 1) (unawareness of possible reimbursement), increased blood sugar (n = 1), ran out of ONS and did not call to homecare service to order more (n = 1), switch to tube or parenteral nutrition (n = 1) and aim of nutritional therapy reached (n = 1).
This survey suggests that a medical prescription for ONS is missing for 45% of patients who receive the supplements during hospitalisation. At hospital discharge, 70% of patients who were taking the supplements during hospitalisation return back home without them, mostly because the prescription was not given. The indication for ONS, NRS-2002 score ≥3 according to the SSNC, is present for about 80% of patients who receive ONS during hospitalisation and then at discharge for home. If ONS are medically prescribed, they are continued 1 month after discharge for home.
A medical prescription for ONS during hospitalisation and at discharge for home was often absent for our patients. Recently, Streicher et al. have revealed that personal judgement of nursing staff about the nutritional state of the patient represents one of the main reasons for prescribing ONS [15]. In our survey, ONS delivery based on personal and subjective judgement of the nursing staff was one of the reasons for giving supplements, but also for the absence of a medical prescription during hospitalisation and at discharge for home. Indeed, most of the time, when the nursing staff distributed ONS to patients during hospitalisation, the physician was not informed. Consequently, they were not prescribed at discharge and the administrative work needed for their delivery to the home was not done. This lack of communication between all caregivers should be improved so that physicians prescribe ONS not only in the hospital but also at discharge for home.
Another barrier to the prescription of ONS at discharge for home seems to be the complexity of the administration needed for reimbursement. In Switzerland, ONS have been reimbursed since 2013. However, the administrative work can differ according to the insurance of the patient, making these procedures tiresome and complicated. In our survey, we have noticed that, for 25% of patients, ONS prescriptions were forgotten by the physician at discharge for home owing to a lack of time and/or knowledge of the required administrative work. Moreover, of patients with a medical prescription for ONS at discharge for home, a homecare service was not contacted for 17%, resulting in no ONS delivery to the patient. These results highlight the importance of simplifying the paperwork for ONS reimbursement, in agreement with insurance providers, and of clearly informing physicians about the procedures in order to improve the healthcare provided to patients at nutritional risk.
An interesting point raised in our survey is that 26% of patients with a medical prescription and homecare delivery of ONS were not aware of the prescription (dose and duration). ONS were thus prescribed without the patients knowing and/or understanding their benefits. This statement is reinforced by the fact that 16% of patients refused delivery of ONS, although they were prescribed. Information about the ONS consumption patterns from caregivers is of utmost importance, because it increases compliance. Indeed, in a systematic review, Hubbard et al. demonstrated that when information about ONS consumption, like “take between meals” or “take as part of medicine rounds”, was given by caregivers, compliance was higher [16]. Furthermore, a recent study has demonstrated that patients used ONS because their physician or dietitian prescribed them, and they trusted their advice [17]. A training course for physicians, focusing on the health impact of nutritional support, could help to improve the compliance of patients.
About 80% of patients who received ONS during hospitalisation and at discharge for home had an NRS-2002 score ≥3, and thus were at nutritional risk. For them, the indication for ONS according to the SSNC was fulfilled. However, 20% of patients with ONS did not have an adequate indication. This may be related to the absence of systematic and objective screening with use of the NRS-2002 in our institution and the frequent use of subjective evaluation as an indication for ONS. The absence of systematic screening may lead to inadequate treatment. Although we did not evaluate the impact of systematic screening on outcome, a previous study has shown that, in the absence of systematic screening procedures, more than 50% of patients at risk of malnutrition may not be diagnosed and treated with the most appropriate form of nutritional treatment [18]. This result suggests that physicians and nursing staff should be trained to screen for nutritional risk in the hospital in order to detect patients who are at nutritional risk and refer them to a dietician for a nutritional intervention. Systematic and objective screening would allow effective identification and subsequent treatment of patients at nutritional risk.
One month after discharge for home, compliance was good for patients with a prescription and homecare delivery of ONS (76%). Similar results were found in a systematic review evaluating compliance with ONS (consumed vs prescribed) in 33 community studies [16]. Compliance was 80% for intervention times ranging from 5 days to 1 year, with no relationship identified between duration of the intervention and compliance. Therefore, we can suppose that our patients would continue ONS for more than 1 month after discharge for home, if necessary.
Our results underlined the importance of the training of physicians and nursing staff in screening for malnutrition and the prescription of ONS. The efficacy of such a nutrition education programme in improving ONS prescribing practices has already been proven. In the Republic of Ireland, a training programme for healthcare professionals, given by a dietician and including information on malnutrition, its screening and the benefits of ONS, improved ONS prescription practices during the year after the intervention [19]. Gall et al. have also showed that the implementation of guidelines on ONS prescription for general practitioners and community nurses reduced the level of inappropriate prescription [20]. The benefits of a nutrition education programme to caregivers are numerous, and include improved screening and treatment of malnutrition, improved ONS prescription or cost reduction.
This survey has several limitations. First, no information was collected about the compliance with ONS prescription in hospital (ONS consumed vs prescribed). However, our survey first aimed to document the existence and adequacy of a medical prescription. Second, we have no information on the total number of patients in need of ONS who in fact did not get any, as we did not perform any systematic screening. Third, we defined the indication for ONS as an NRS-2002 score ≥3, which was defined by the SSNC as a criterion for reimbursement of ONS. Nevertheless, the NRS-2002 is a screening tool that established nutritional risk and not malnutrition. Finally, the collected data are representative only of one university hospital in Switzerland. The results may be different in other Swiss hospitals and hospitals in other countries. The administration involved ONS prescription and reimbursement policies are specific to each country.
Medical prescription of ONS was missing for half of the patients during hospitalisation and three quarters of the patients at discharge for home. For 80% of patients receiving ONS during hospitalisation and at discharge for home, the intervention was indicated. If a medical prescription was given, ONS were continued 1 month after discharge for home by 75% of patients.
In order to improve the medical prescription of ONS in hospital and at hospital discharge for home, evidence-based guidelines for the prescription and the indication are needed. Furthermore, in Switzerland, although the ONS are reimbursed by the insurance providers, the administration needed for reimbursement of ONS should be simplified. Further studies are needed to confirm the benefit of these suggestions especially in other Swiss hospitals with the aim of standardisation of practices.
The authors would like to thank the nutrition team and the homecare staff for their valuable help in the data collection. We also thank the nurses of the hospital wards involved in the survey for their helpful collaboration.
This survey was supported by grants from the Foundation Nutrition 2000 Plus. Claude Pichard received financial support as research grants and an unrestricted academic research grant, as well as a nonrestrictive research grant and consulting fees, from Abbott, Baxter, B. Braun, Cosmed, Fresenius-Kabi, Nestle Medical Nutrition, Novartis, Nutricia-Numico, Pfizer, and Solvay.
1 Thibault R , Makhlouf AM , Kossovsky MP , Iavindrasana J , Chikhi M , Meyer R , et al. Healthcare-associated infections are associated with insufficient dietary intake: an observational cross-sectional study. PLoS One. 2015;10(4):e0123695. doi:.https://doi.org/10.1371/journal.pone.0123695
2 Correia MI , Waitzberg DL . The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22(3):235–9. doi:.https://doi.org/10.1016/S0261-5614(02)00215-7
3 Lim SL , Ong KC , Chan YH , Loke WC , Ferguson M , Daniels L . Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin Nutr. 2012;31(3):345–50. doi:.https://doi.org/10.1016/j.clnu.2011.11.001
4 Sorensen J , Kondrup J , Prokopowicz J , Schiesser M , Krähenbühl L , Meier R , et al.; EuroOOPS study group. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr. 2008;27(3):340–9. doi:.https://doi.org/10.1016/j.clnu.2008.03.012
5 Norman K , Pichard C , Lochs H , Pirlich M . Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27(1):5–15. doi:.https://doi.org/10.1016/j.clnu.2007.10.007
6 Imoberdorf R , Meier R , Krebs P , Hangartner PJ , Hess B , Stäubli M , et al. Prevalence of undernutrition on admission to Swiss hospitals. Clin Nutr. 2010;29(1):38–41. doi:.https://doi.org/10.1016/j.clnu.2009.06.005
7 Cawood AL , Elia M , Stratton RJ . Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res Rev. 2012;11(2):278–96. doi:.https://doi.org/10.1016/j.arr.2011.12.008
8 Stratton RJ , Marinos E . A review of reviews: A new look at the evidence for oral nutritional supplements in clinical practice. Clin Nutr. 2007;2(Suppl. 1):5–23.
9 Elia M , Normand C , Norman K , Laviano A . A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in the hospital setting. Clin Nutr. 2016;35(2):370–80. doi:.https://doi.org/10.1016/j.clnu.2015.05.010
10 Elia M , Normand C , Laviano A , Norman K . A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in community and care home settings. Clin Nutr. 2016;35(1):125–37. doi:.https://doi.org/10.1016/j.clnu.2015.07.012
11 Deutz NE , Matheson EM , Matarese LE , Luo M , Baggs GE , Nelson JL , et al.; NOURISH Study Group. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin Nutr. 2016;35(1):18–26. doi:.https://doi.org/10.1016/j.clnu.2015.12.010
12 Zhong Y , Cohen JT , Goates S , Luo M , Nelson J , Neumann PJ . The Cost-Effectiveness of Oral Nutrition Supplementation for Malnourished Older Hospital Patients. Appl Health Econ Health Policy. 2017;15(1):75–83.
13 Charlson ME , Pompei P , Ales KL , MacKenzie CR . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi:.https://doi.org/10.1016/0021-9681(87)90171-8
14 Kondrup J , Allison SP , Elia M , Vellas B , Plauth M ; Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22(4):415–21. doi:.https://doi.org/10.1016/S0261-5614(03)00098-0
15 Streicher M , Themessl-Huber M , Schindler K , Sieber CC , Hiesmayr M , Volkert D . Who receives oral nutritional supplements in nursing homes? Results from the nutritionDay project. Clin Nutr. 2016;S0261-5614(16)31243-2.
16 Hubbard GP , Elia M , Holdoway A , Stratton RJ . A systematic review of compliance to oral nutritional supplements. Clin Nutr. 2012;31(3):293–312. doi:.https://doi.org/10.1016/j.clnu.2011.11.020
17 den Uijl LC , Kremer S , Jager G , van der Stelt AJ , de Graaf C , Gibson P , et al. That’s why I take my ONS. Means-end chain as a novel approach to elucidate the personally relevant factors driving ONS consumption in nutritionally frail elderly users. Appetite. 2015;89:33–40. doi:.https://doi.org/10.1016/j.appet.2015.01.016
18 Elia M , Zellipour L , Stratton RJ . To screen or not to screen for adult malnutrition? Clin Nutr. 2005;24(6):867–84. doi:.https://doi.org/10.1016/j.clnu.2005.03.004
19 Kennelly S , Kennedy NP , Corish CA , Flanagan-Rughoobur G , Glennon-Slattery C , Sugrue S . Sustained benefits of a community dietetics intervention designed to improve oral nutritional supplement prescribing practices. J Hum Nutr Diet. 2011;24(5):496–504. doi:.https://doi.org/10.1111/j.1365-277X.2011.01197.x
20 Gall MJ , Harmer JE , Wanstall HJ . Prescribing of oral nutritional supplements in Primary Care: can guidelines supported by education improve prescribing practice? Clin Nutr. 2001;20(6):511–5. doi:.https://doi.org/10.1054/clnu.2001.0479
JM participed in this survey design, recruited patients, collected, analyzed and interpreted the data, and drafted the manuscript. JA, MC, PC, CS helped to draft the manuscript. DB collected the data and helped to draft the manuscript. SG participed in the survey design, analyzed and interpreted the data and helped to draft the manuscript. LG and CP conceived the survey, participated in its design, obtained funding, analyzed and interpreted the data, and drafted the manuscript.
This survey was supported by grants from the Foundation Nutrition 2000 Plus. Claude Pichard received financial support as research grants and an unrestricted academic research grant, as well as a nonrestrictive research grant and consulting fees, from Abbott, Baxter, B. Braun, Cosmed, Fresenius-Kabi, Nestle Medical Nutrition, Novartis, Nutricia-Numico, Pfizer, and Solvay.