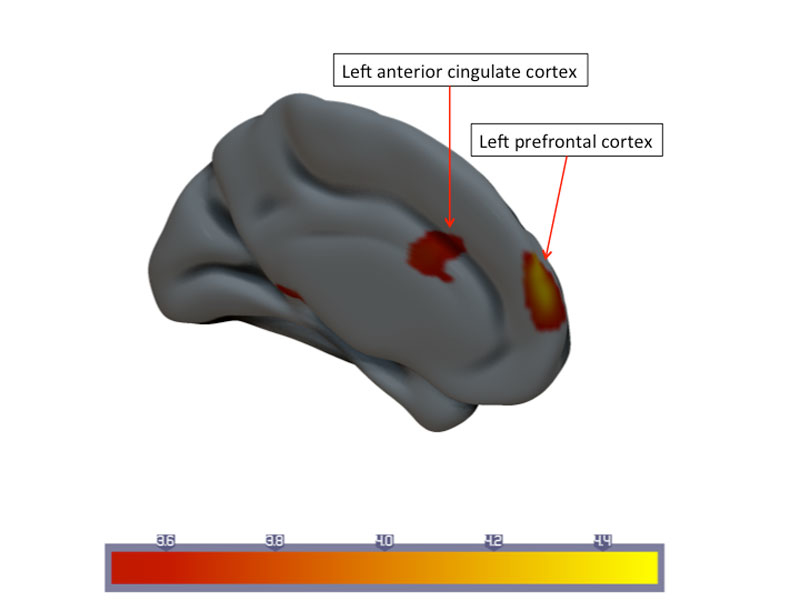
Figure 1 Statistical parametric map of longitudinal changes in mean cerebral blood flow (CBF) in the responder group. Downregulation of cortical CBF in the left prefrontal cortex and anterior cingulate cortex.
DOI: https://doi.org/10.4414/smw.2017.14454
Functional neuroimaging methods have been consistently employed for more than two decades to study neuronal correlates of chronic pain. The imaging method most frequently employed to identify brain areas involved into neuronal processing of nociception and brain pain perception is blood oxygenation level-dependent (BOLD) magnetic resonance imaging (MRI) [1].A recent meta-analysis of 138 studies of BOLD functional MRI (fMRI) use consistently identified the insular cortex, the somatosensory cortex and the anterior cingulate cortex as core areas being activated during pain perception, independently from the paradigms and populations under observation [2]. Arterial spin labelling (ASL), in contrast to BOLD fMRI, offers advantages in applications where slow varying changes in brain function are investigated [3], has considerable potential for the comparison of within- and between- subject differences associated with pain-induced state changes and baseline differences in regional cerebral blood flow (CBF) [4] and is reproducible [5]. Repeated ASL studies revealed consistent upregulation of CBF in the primary sensory cortex and posterior insula, the latter showing a CBF modulation highly correlated with pain aggravation and relief [6, 7]. Here, the ASL signal is straightforward because it reflects a single physiological process, CBF, which correlates better with the actual site of neuronal involvement than the BOLD signal [8]. Restitution of, for example, interhemispheric CBF balance related to reorganisation of the motor network after ischaemic stroke has been identified with ASL after 3 months [9], and offers a noninvasive approach to monitoring long-term attempts of the brain to reinstate physiological balance (e.g., between motor nodes after stroke or core areas of the pain matrix as a consequence of neuromodulating therapies).
We aimed to investigate the potential of ASL to monitor longitudinal CBF changes in patients with chronic pelvic pain within a randomised, placebo-controlled, double-blind single centre trial [10]. We asked if longitudinal CBF changes in core areas of the pain matrix segregate patients responding to therapy from nonresponders. We hypothesised that CBF regulation in core structures of the pain matrix (the somatosensory cortex, the insula, the anterior cingulate cortex and prefrontal cortex) are different in therapy responders and nonresponders.
In accordance with the European Association of Urology (EAU) guidelines [11], all patients with chronic pelvic pain syndrome (CPPS) enrolled into this study reported pain perceived in structures related to the pelvis for at least 3 months without proven infection or other related pathologies. All patients underwent detailed urological evaluation, including medical history, physical examination, urinalysis, determination of prostate-specific antigen (PSA), free uroflowmetry and post-void residual measurement. Chronic bacterial prostatitis was excluded by means of the Meares-Stamey 3-glass test and negative post-prostatic massage urine culture. Inclusion criteria were National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) total score ≥15, NIH-CPSI pain subscore ≥8 and unsuccessful stepwise, multimodal therapy with a minimum of one antibiotic cycle with doxycycline for at least 4 weeks, including antibiotic therapy of the sexual partner, nonsteroidal anti-inflammatory drug treatment and alpha-blocker therapy for at least 6 weeks. All patients included in the study were checked for right-handedness. Patients with urinary tract infection, post-void residual >100 ml, prostate cancer, urethral stricture, age <18 years, claustrophobia, magnetic implants (cardiac pacemakers, neurostimulators, pain/insulin pumps) were excluded.
This neuroimaging study was part of a randomised controlled trial [10] approved by the local ethics committee (Kantonale Ethikkommission Bern/3010 Bern/Switzerland/Nummer 292-07) and registered with ClinicalTrials.gov (trial registration number NCT00688506). All participants provided written informed consent before inclusion in the trial. The study conforms to the CONSORT statement.
Study participants were recruited in the urological outpatient clinic and randomly allocated to sono-electro-magnetic or sham therapy based on computer-generated random numbers with a randomisation ratio of 1:1 and a block size of 60. The trial included four clinical and two neuroimaging assessment time-points. Baseline assessments included screening and collection of baseline data followed by high-resolution structural MRI and ASL perfusion imaging of the brain. Clinical follow-up assessments were performed after 6 and after 12 weeks of therapy. MRI was repeated after 12 weeks. Data collection was closed 16 weeks after a final clinical assessment. Treatment response was defined as a decrease in four points on the NIH-CPSI [12].
All MRI scans were performed on a 3T Siemens Magnetom Trio (Erlangen, Germany) equipped with a 12-channel radio frequency head coil. All MRI examinations took place at the same time of the day, between 14:00 and 16:00. All high-resolution T1-weighted MR images were obtained with a 3D modified driven equilibrium Fourier transform (MDEFT) sequence [13] with the following parameters: repetition time TR = 7.92 ms, echo time TE = 2.48 ms, flip angle = 16°, inversion with symmetric timing (inversion time 910 ms), 256 × 224 ×176 matrix points with a field of view (FOV) of 256 mm × 224 mm yielding a nominal isotropic resolution of 1 mm3 (i.e. 1×1×1 mm), fat saturation, 12 min. total acquisition time. The functional images for the measurement of CBF with a pulsed pseudocontinuous arterial spin labelling (pCASL) technique was used with the following parameters [14, 15]: TR/TE = 4000/18 ms, FOV = 230 mm2, matrix size = 128 × 128, (isocentre of the readout slice was 90 mm above labelling plane), bandwidth 752 Hz/pixel and flip angle (FA) = 25°, post-label delay = 1500 ms; and labelling duration = 1720 ms. Sixteen axial slices (EPI readout, 3.4 mm in-plane resolution, 6 mm slice thickness and 3.0 mm gap) were positioned parallel to the bi-commissural axis, and were shifted in z-direction to cover the primary motor area of each subject. A total of 80 images, i.e., 40 pairs of label and control images, were recorded in less than 6 minutes. The sequence was recorded with 3D PACE (Siemens Erlangen, Germany) to enable prospective motion correction. Identical prescription of MR images was achieved by use of the Siemens auto-align sequence, which automatically sets up consistent slice orientation based on a standard MRI atlas.
The PCASL images were processed to examine longitudinal differences in cerebral blood flow at rest between the two cohorts. Preprocessing and analysis of MR data was performed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK: http://www.fil.ion.ucl.ac.uk/spm/software/spm8/, revision 4667) for MATLAB® (7.8.0, R2009a) and a customised Matlab code. Raw pCASL images were first realigned to remove movement artefacts during MRI acquisition. Quantification of CBF flow time series was based on the equation:
The post-label delay (PLD) (ω) = 1500 ms, labelling duration (τ) = 1720 ms, blood/tissue water partition coefficient λ = 0.9 g/ml and tagging efficiency assumed to be α = 0.95 [15]. For 3.0T, the decay time for labelled blood, T1b = 1650 ms, M0 are the equilibrium brain tissue magnetisation images. ΔM was calculated by subtraction of all realigned label and control images. Quantified CBF flow time-series and average CBF maps for the entire acquisition were calculated [16, 17]. Then the CBF images were co-registered to the individual anatomy and normalised using the individual normalisation parameters derived from segmentation (see above). T1w MDEFT images were spatially normalised with a 12-parameter affine transformation to the SPM template to correct for differences in brain size. The images were segmented, spatially normalised and modulated to the customised template in the standardised anatomic space. CBF maps were constrained to the grey matter by masking CBF images with normalised grey matter partitions that were binarised at a density threshold of 0.2. This empirical value assured that GM density values of at least 68% were included in the GM images. Finally, the CBF images were smoothed with a 3D Gaussian kernel of 12-mm full width at half maximum (FWHM) to reduce interindividual anatomical differences and increase the SNR [3]. Subsequent independent t-tests without equal variance assumption were computed for group-wise differences of the mean CBF values.
We analysed longitudinal changes in mean CBF within subjects with SPM, using a flexible factorial design with time and subject as factors, and taking scans at time-point 1 and 2 and the two time-points as conditions for each subject. Age and global mean CBF were used as nuisance covariates for perfusion analysis. The mean CBF maps were statistically analysed at an uncorrected cluster-level threshold of p <0.001 followed by a region of interest analysis of significant clusters in the pain processing pathways using small volume (e.g., volume at centre of mass coordinates was defined by a sphere of radius = 5 mm) correction (family-wise error corrected p <0.05).
Sixty patients were randomised to sono-electro-magnetic (n = 30, mean age ± standard deviation 46±14 years) or sham therapy (n = 30, mean age 42±16 years). MR datasets were incomplete in 8 of 60 patients. After 12 weeks of treatment, 31 patients responded to therapy and 21 did not. The 31 responders comprised 16 of 24 patients with active device (positive treatment response rate 67%) versus 15 of 28 patients with sham device (positive treatment response rate 54%) (p = 0.11). Treatment responders were younger than nonresponders (mean age 39±13 vs 53±13 years, p = 0.0003). Mean pain duration in the responder group was 38 days (SD 76 days) and in the nonresponder group 57 days (SD 59 days; p = 0.36). Fifty patients (23 and 27 patients with active and sham devices, respectively) were included into the ASL study; two ASL datasets (one patient with active device, one patient with sham device and both with positive treatment response) had to be excluded owing to movement artefacts and low image quality at least one of the two time-points.
To check if longitudinal CBF changes are detectable with ASL, we first investigated if longitudinal CBF changes could be observed within specific brain areas associated with pain perception in patients who received verum compared to those who received sham therapy. We identified significant longitudinal CBF upregulation in the postcentral gyrus and the insula in the nondominant right hemisphere and a CBF downregulation in the prefrontal cortex (PFC) of the left hemisphere in the verum group. The largest upregulations were observed in the postcentral gyrus (ΔCBF 5.03 ml/100g brain tissue/min, interpersonal variance at time-point 1 43.3, at time-point 2 54.3). In patients who received sham therapy, we observed increased CBF in the left dorsomedial and right dorsolateral prefrontal cortex. The largest upregulations were observed in the left dorsomedial prefrontal cortex (DMPFC) (ΔCBF 6.34 ml/100g brain tissue/min; interpersonal variance at time point 1 90.6, at time point 2 85.9).
Patients from both the verum and sham group who responded to therapy showed decreased CBF values in the anterior cingulate cortex (ACC) and left anterior prefrontal cortex, after 3 months of therapy (fig. 1).

Figure 1 Statistical parametric map of longitudinal changes in mean cerebral blood flow (CBF) in the responder group. Downregulation of cortical CBF in the left prefrontal cortex and anterior cingulate cortex.
CBF changes in the prefrontal cortex were ΔCBF ˗1.98±5.14 ml/100 g brain tissue/min for responders (interpersonal variance at time point 1 54.6, at time-point 2 54.2). Nonresponders had a ΔCBF ˗0.03±5.52 ml/100 g brain tissue/min. Longitudinal CBF changes in the ACC were ΔCBF ˗1.64±5.18 ml/100 g brain tissue/min (interpersonal variance at time-point 1 22.3, at time-point 2 27.9) for responders and ΔCBF 0.69±6.37 ml/100 g brain tissue/min for nonresponders.Significant upregulation of CBF was found in the right dorsolateral prefrontal cortex (DLPFC): ΔCBF 5.79±7.70 ml/100 g brain tissue/min for responders (interpersonal variance at time-point 1 93.6, at time-point 2 70.8), and Δ CBF 1.35±10.98 ml/100 g brain tissue/min for nonresponders (fig. 2).
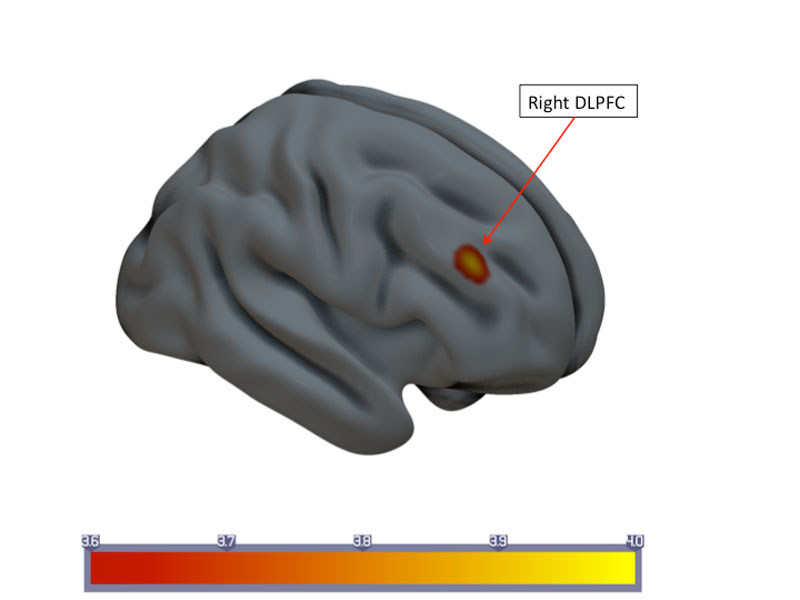
Figure 2 Statistical parametric map of longitudinal changes in mean cerebral blood flow (CBF) in the responder group. Upregulation of cortical CBF in the right dorsolateral prefrontal cortex (DLPFC).
Patients who failed to respond presented with longitudinal CBF upregulation in the right hippocampus: ΔCBF 5.10±11.94 ml/100 g brain tissue/min (interpersonal variance at time-point 1 77.7, at time-point 2 172.5) (fig. 3). In contrast, for the responders CBF changes in the same region were ΔCBF 0.79±6.89 ml/100 g brain tissue/min.
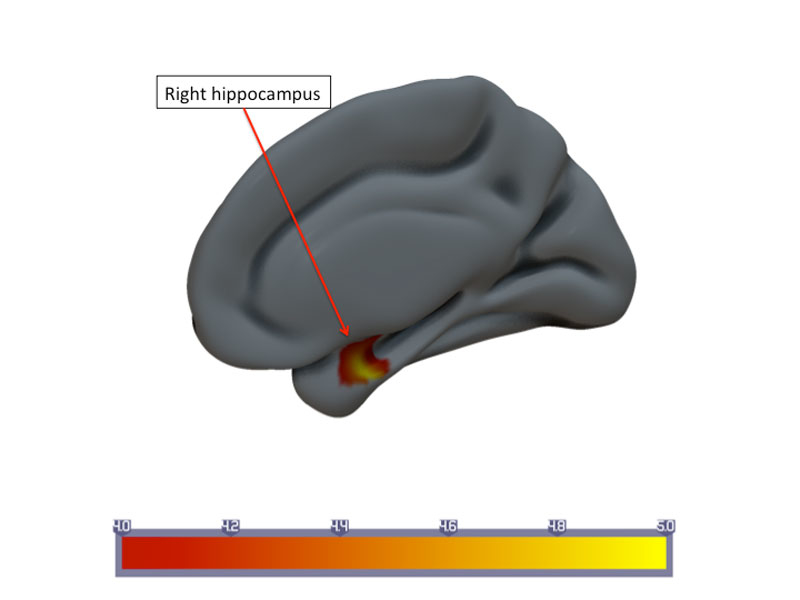
Figure 3 Statistical parametric map of longitudinal changes in mean cerebral blood flow (CBF) in the nonresponder group. Upregulation of CBF in the right hippocampus.
To relate regional CBF changes to clinical outcome, we performed a correlation analysis for responders and nonresponders with changes in CPSI total score, CPSI pain subscore, CPSI quality of life subscore and disease duration. For the whole cohort, we identified no correlation between the overall CPSI score and regional CBF changes (CPSI total score p = 0.952). After cohort segregation according to the duration of their symptoms (dichotomised into ≥1 year vs <1 year) we identified longitudinal CBF decreases within the joint clusters ACC and left anterior prefrontal cortex (Spearman´s correlation r = ˗0.900, p = 0.037) with the CPSI pain subscore in responders with a short history of CPPS (n = 8/31). CBF increases were observed in the right hippocampus (Spearman´s correlation r = ˗0.98, p = 0.01) for nonresponders (n = 4/19) with short disease duration. Patients with a disease duration >1 year revealed no correlation (n = 23, r = ˗0.117, p = 0.607 for responders; n = 15, r = ˗0.16, p = 0.954 for nonresponders). All clusters are displayed in table 1.
Table 1 Main findings after 12 weeks of treatment in the responder and nonresponder groups.
| Group comparison | Arterial spin labelling | |||||
|---|---|---|---|---|---|---|
| Anatomic location | MNI coordinates | Cluster size |
Voxel
T (degrees of freedom) |
|||
| x | y | z | ||||
| Left | Prefrontal cortex | ˗4 | 64 | 24 | 203 | 6.18 (1,29) |
| Left | Anterior cingulate cortex | ˗10 | 18 | 24 | 63 | 4.13 (1,29) |
| Right | Dorsolateral prefrontal cortex | 40 | 38 | 28 | 220 | 4.08 (1,29) |
| Right | Hippocampus | 26 | ˗4 | ˗24 | 52 | 5.24 (1,17) |
In this study, we evaluated longitudinal CBF changes related to long-term sono-electro-magnetic versus sham stimulation in brain areas jointly activated by painful stimuli. The main findings that stand out from our study are: (1) using ASL, longitudinal long-term CBF regulations are detectable in brain areas either related to nociceptive processing or cognitive control of pain perception, dependent on verum vs sham stimulation; (2) response to therapy was associated with CBF downregulation along structures related to the processing of pain-related affects and attention, whereas nonresponse was associated with CBF upregulation of pain memory. Whereas pCASL perfusion imaging has been previously used as an alternative method to measure cortical changes associated with pain-related short-term conditions, only one study has examined therapy-related long-term CBF regulations with this noninvasive and stimulus-free technique [18].We sought to find out if pCASL can be employed as a noninvasive surrogate biomarker, within a randomised controlled clinical trial that investigated therapy-related effects in patients suffering from CPPS. Sono-electro-magnetic therapy did not result in a significant improvement of clinical symptoms in the overall cohort of treatment refractory CPPS patients compared with sham therapy (the clinical results of this trial have been published elsewhere [10]), but we identified downregulation of regional CBF following 12 weeks of therapy along core structures of the pain processing matrix. These effects were observed in patients who subjectively reported an improvement of the overall CPPS score, regardless of whether they received verum or sham therapy, in the ACC and left PFC. These findings imply that neuronal correlates of subjective pain evaluation are detectable by ASL in affective-motivational and cognitive-evaluative areas of the pain matrix without the need to apply nociceptive stimuli. Although similar effects have been demonstrated on the insula and the ACC using BOLD fMRI, these studies used experimental pain stimulation to exert immediate haemodynamic responses to therapy.We assume that these CBF changes can be considered meaningful in chronic pain relief, since statistically significant downregulation in the associative pain matrix areas was only observed in CPPS subjects who had a subjective response and, moreover, changes in the nociceptive matrix were seen solely in patients within the verum cohort. By contrast, patients who failed to respond to therapy revealed longitudinal upregulation of CBF in brain areas associated with acquisition and consolidation of long-term memory of pain experience (the hippocampus [19]), and those who received sham therapy in the dorsomedial and lateral prefrontal cortex, the latter being involved into the maintenance of context information and generation of sham-related expectations and appraisals [20].In our study, longitudinal therapy-associated modulations were observed in brain areas that are crucial both for responsiveness to painful stimuli and to reduced responses to pain after sham treatment. Although our clinical trial failed to demonstrate the superiority of sono-electro-magnetic therapy compared with sham stimulation ( the between-group difference in the change of the CPSI score from baseline to 12 weeks), neuroimaging with ASL indicated that the sham stimulation engaged the endogenous pain modulation circuitry similarly to the verum application. In patients with a short duration of disease, we observed a modest correlation between CBF increases and the CPSI pain score. These correlations do not necessarily reflect causal effects of the therapy on brain activity, but may provide noninvasive information on the nature of the individual differences that predispose a person towards showing a larger or smaller therapy response. The dissociation between CBF changes and clinical response in patients with short disease duration, as compared with unresponsiveness in later stages of the disease, may further indicate duration-related limited plasticity along the network nodes engaged in successful pain relief. Structural neuroimaging using studies using voxel-based morphometry, diffusion tensor imaging and fMRI measuring functional connectivity have consistently demonstrated that CPPS may leave specific imprints in the pain matrix, predominantly in the ACC [21–23]. These areas may thus subserve as a potential target for antinociceptive interventions such as transcranial magnetic stimulation or MR-based biofeedback in patients who do not respond to standard drug regimen.Although we evaluated a well-defined patient population, there are limitations that should be addressed. From a methodological point of view, the CBF changes observed in this study must be considered only moderate (up to 6.34 ml/100 g/min). Core areas of the pain matrix such as the spinoreticular projections, hypothalamus and thalamus may have been obscured because of the low signal-to-noise ratio and spatial resolution of the pCASL sequence, making it less reliable as a method to detect CBF changes in the brain stem and deep brain nuclei. Recent developments of improved pCASL with background-suppressed 3D acquisitions (GRASE) may further improve the sensitivity and signal-to-noise ratio of this technique [24].Since our study design did not incorporate a control group without intervention, we cannot exclude the possibility that functional differences might have been observed regardless of intervention. Previous studies demonstrated placebo-related alterations of functional connectivity within core areas of the pain matrix, such as the dorsolateral PFC, ACC and dorsomedial PFC [25, 26]. Further studies using a crossover design are needed to answer this important question.Further, the influence of other covariates (such as fatigue, mood, concomitant disorders, socioeconomic status, etc.) had to be ignored owing to the limited sample size. From the perspective of daily clinical practice we aimed to assess a homogenous cohort of well-defined CPPS patients, especially regarding the strict inclusion criteria and the use of a generally accepted, disease-specific validated questionnaire.
In summary, we demonstrated therapy-related and stimulus-free longitudinal CBF changes in core areas of the pain matrix using ASL. Since baseline metabolic activity is an important index of resting brain function, it may act as a complementary noninvasive method to fMRI and single-photon emission computed tomography / positron emission tomography, especially in the longitudinal assessment of pain response in clinical trials.
The trial was supported by the Bern University Hospital. Sonodyn Corporation AG (Solothurn, Switzerland) kindly provided all stimulation devices.
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and responsibility for the decision to submit for publication. None of the authors has a potential conflict of interest relevant to this article.
1 Ogawa S , Lee TM , Kay AR , Tank DW . Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87(24):9868–72. https://doi.org/10.1073/pnas.87.24.9868
2 Jensen KB , Regenbogen C , Ohse MC , Frasnelli J , Freiherr J , Lundström JN . Brain activations during pain: a neuroimaging meta-analysis of patients with pain and healthy controls. Pain. 2016;157(6):1279–86. https://doi.org/10.1097/j.pain.0000000000000517
3 Wang J , Alsop DC , Song HK , Maldjian JA , Tang K , Salvucci AE , et al. Arterial transit time imaging with flow encoding arterial spin tagging (FEAST). Magn Reson Med. 2003;50(3):599–607. https://doi.org/10.1002/mrm.10559
4 Hodkinson DJ , Krause K , Khawaja N , Renton TF , Huggins JP , Vennart W , et al. Quantifying the test-retest reliability of cerebral blood flow measurements in a clinical model of on-going post-surgical pain: A study using pseudo-continuous arterial spin labelling. Neuroimage Clin. 2013;3:301–10. https://doi.org/10.1016/j.nicl.2013.09.004
5 Howard MA , Krause K , Khawaja N , Massat N , Zelaya F , Schumann G , et al. Beyond patient reported pain: perfusion magnetic resonance imaging demonstrates reproducible cerebral representation of ongoing post-surgical pain. PLoS One. 2011;6(2):e17096. https://doi.org/10.1371/journal.pone.0017096
6 Segerdahl AR , Mezue M , Okell TW , Farrar JT , Tracey I . The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci. 2015;18(4):499–500. https://doi.org/10.1038/nn.3969
7 Maleki N , Brawn J , Barmettler G , Borsook D , Becerra L . Pain response measured with arterial spin labeling. NMR Biomed. 2013;26(6):664–73. doi:.https://doi.org/10.1002/nbm.2911
8 Detre JA , Wang J . Technical aspects and utility of fMRI using BOLD and ASL. Clin Neurophysiol. 2002;113(5):621–34. https://doi.org/10.1016/S1388-2457(02)00038-X
9 Wiest R , Abela E , Missimer J , Schroth G , Hess CW , Sturzenegger M , et al. Interhemispheric cerebral blood flow balance during recovery of motor hand function after ischemic stroke--a longitudinal MRI study using arterial spin labeling perfusion. PLoS One. 2014;9(9):e106327. https://doi.org/10.1371/journal.pone.0106327
10 Kessler TM , Mordasini L , Weisstanner C , Jüni P , da Costa BR , Wiest R , et al. Sono-electro-magnetic therapy for treating chronic pelvic pain syndrome in men: a randomized, placebo-controlled, double-blind trial. PLoS One. 2014;9(12):e113368. https://doi.org/10.1371/journal.pone.0113368
11 Fall M , Baranowski AP , Elneil S , Engeler D , Hughes J , Messelink EJ , et al., European Association of Urology. EAU guidelines on chronic pelvic pain. Eur Urol. 2010;57(1):35–48. Published online September 08, 2009. https://doi.org/10.1016/j.eururo.2009.08.020
12 Nickel JC , Krieger JN , McNaughton-Collins M , Anderson RU , Pontari M , Shoskes DA , et al.; Chronic Prostatitis Collaborative Research Network. Alfuzosin and symptoms of chronic prostatitis-chronic pelvic pain syndrome. N Engl J Med. 2008;359(25):2663–73. Published online December 19, 2008. https://doi.org/10.1056/NEJMoa0803240
13 Deichmann R , Schwarzbauer C , Turner R . Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. Neuroimage. 2004;21(2):757–67. Published online February 26, 2004. https://doi.org/10.1016/j.neuroimage.2003.09.062
14 Dai W , Garcia D , de Bazelaire C , Alsop DC . Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60(6):1488–97. https://doi.org/10.1002/mrm.21790
15 Wu WC , Fernández-Seara M , Detre JA , Wehrli FW , Wang J . A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2007;58(5):1020–7. https://doi.org/10.1002/mrm.21403
16 Federspiel A , Müller TJ , Horn H , Kiefer C , Strik WK . Comparison of spatial and temporal pattern for fMRI obtained with BOLD and arterial spin labeling. J Neural Transm (Vienna). 2006;113(10):1403–15. Published online April 11, 2006. https://doi.org/10.1007/s00702-006-0434-5
17 Jann K , Koenig T , Dierks T , Boesch C , Federspiel A . Association of individual resting state EEG alpha frequency and cerebral blood flow. Neuroimage. 2010;51(1):365–72. Published online February 17, 2010. https://doi.org/10.1016/j.neuroimage.2010.02.024
18 Hodkinson DJ , Khawaja N , OʼDaly O , Thacker MA , Zelaya FO , Wooldridge CL , et al. Cerebral analgesic response to nonsteroidal anti-inflammatory drug ibuprofen. Pain. 2015;156(7):1301–10. https://doi.org/10.1097/j.pain.0000000000000176
19 Price TJ , Inyang KE . Commonalities between pain and memory mechanisms and their meaning for understanding chronic pain. Prog Mol Biol Transl Sci. 2015;131:409–34. https://doi.org/10.1016/bs.pmbts.2014.11.010
20 Wager TD , Atlas LY . The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 2015;16(7):403–18. https://doi.org/10.1038/nrn3976
21 Farmer MA , Chanda ML , Parks EL , Baliki MN , Apkarian AV , Schaeffer AJ . Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2011;186(1):117–24. Published online May 17, 2011. https://doi.org/10.1016/j.juro.2011.03.027
22 Mordasini L , Weisstanner C , Rummel C , Thalmann GN , Verma RK , Wiest R , et al. Chronic pelvic pain syndrome in men is associated with reduction of relative gray matter volume in the anterior cingulate cortex compared to healthy controls. J Urol. 2012;188(6):2233–7. https://doi.org/10.1016/j.juro.2012.08.043
23 Martucci KT , Shirer WR , Bagarinao E , Johnson KA , Farmer MA , Labus JS , et al. The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network-a resting-state study from the MAPP Research Network. Pain. 2015;156(9):1755–64. https://doi.org/10.1097/j.pain.0000000000000238
24 Alsop DC , Detre JA , Golay X , Günther M , Hendrikse J , Hernandez-Garcia L , et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1):102–16. https://doi.org/10.1002/mrm.25197
25 Hashmi JA , Baria AT , Baliki MN , Huang L , Schnitzer TJ , Apkarian AV . Brain networks predicting placebo analgesia in a clinical trial for chronic back pain. Pain. 2012;153(12):2393–402. https://doi.org/10.1016/j.pain.2012.08.008
26 Tétreault P , Mansour A , Vachon-Presseau E , Schnitzer TJ , Apkarian AV , Baliki MN . Brain Connectivity Predicts Placebo Response across Chronic Pain Clinical Trials. PLoS Biol. 2016;14(10):e1002570. https://doi.org/10.1371/journal.pbio.1002570
Christian Weisstanner and Livio Mordasini contributed equally to the study. Thomas M. Kessler and Roland Wiest share senior authorship.
The trial was supported by the Bern University Hospital. Sonodyn Corporation AG (Solothurn, Switzerland) kindly provided all stimulation devices.