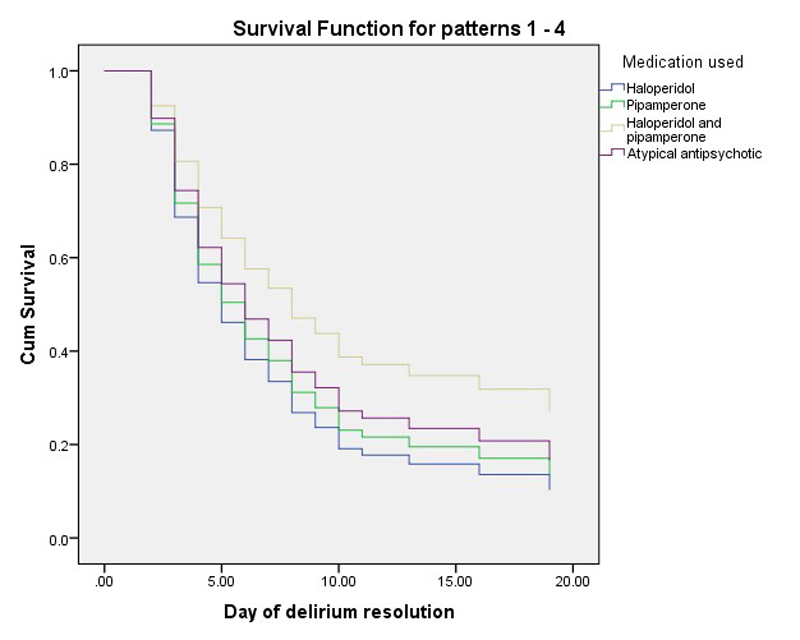
Figure 1 Survival (remission) function of pipamperone, alone and as an adjunct to haloperidol versus haloperidol and atypical antipsychotics.
DOI: https://doi.org/10.4414/smw.2017.14471
Delirium is a neuropsychiatric syndrome characterised by an abrupt onset and fluctuating disturbances in consciousness and cognition, as well as a range of noncognitive domains including disturbances in motor behaviour, emotionality and sleep-wake cycle, caused by an underlying aetiology [1, 2].
Delirium is a common occurrence over the course of hospitalisation, depending, among other factors, on the age and gender of the patient, previous episodes of delirium, pre-existing dementia and severity of illness [3–5]. Across the hospital, including the medical, surgical or general settings, delirium rates vary between 10 and 60% [5] and in the intensive care setting varies between 60 and 82% [4–7].
If not managed appropriately, delirium can exert a very adverse effect on clinical outcomes. This can include short-term consequences such as increased morbidity and mortality, and prolonged hospitalisation, as well as long-term consequences such as increased rates of cognitive decline, deterioration in functionality and institutionalisation [8, 9]. Another factor contributing to worsened delirium outcomes has been identified as delirium severity, with more severe episodes associated with worse outcomes [10, 11].
In particular, persistent delirium has been recognised to predict worse outcomes. Such persistence of delirium has been documented as long as 3 and 6 months after hospital discharge, leading to the conclusion that delirium may be much less transient than previously assumed [12–14]. Persistent delirium is also associated with a greater risk of death [15, 16]. In contrast, delirium symptoms resolving within 2 weeks have been associated with excellent functional recovery [12], and resolved delirium with decreased mortality [15].
As a result of its different psychomotor representations, delirium has been further characterised into hypoactive, hyperactive and mixed subtypes. The hypoactive subtype is characterised by decreased activity, apathy, decreased speed of activity and speech, decreased volume of speech, decreased alertness, and withdrawal; the hyperactive subtype by increased activity, loss of control of activity, restlessness, and wandering; and the mixed subtype by the occurrence of both hypoactive and hyperactive features within a given day [17].
Due to its adverse short- and long-term consequences [8, 9], delirium prevention strategies [18, 19] and, once detected, management strategies have been devised. The management approach to delirium relies on eliminating the underlying aetiology, providing environmental interventions like hearing and visual aids, frequent reorientation and, if necessary, pharmacological management [2]. Haloperidol remains the gold standard [2],but an increasing number of studies are supporting the administration of other, atypical antipsychotics. Altogether, delirium studies indicate remission rates from 40 to 90% [20, 21].
Pipamperone, a low potency antipsychotic and an antagonist of 5HT2A-C-, D2-4- and α1-2-receptors with much higher activity against the D4 and 5HT2A than D2 receptor and insignificant activity against histamine1 and muscarinic-anticholinergic (mACh) receptors [22], is recommended for the management of delirium [23, 24]. However, to date the effectiveness of pipamperone for this purpose has not yet been evaluated at all. Thus, in this study, the effectiveness of pipamperone alone and as an adjunct to haloperidol was compared with that of haloperidol alone, as well as atypical antipsychotics, in the management of delirium, both overall and its various clinical subtypes.
The aim of this study was to assess the effectiveness of pipamperone alone and as an adjunct to haloperidol in the management of delirium versus the gold standard haloperidol or atypical antipsychotics such as risperidone, olanzapine and quetiapine.
This study was approved by the ethics committee of the Canton of Zurich (KEK-ZH-Nr. 2012-0263).
All patients in this retrospective, descriptive cohort study were recruited at the University Hospital Zurich during an ongoing evaluation using the Delirium Observation Screening scale (DOS) three times daily over the first three days, in addition to initiating screening on suspicion of incident delirium. The DOS scoring was performed by nursing staff specifically trained in use of the DOS via video training material and subsequent discussion and agreement. Once the DOS indicated delirium, the short version of the Confusion Assessment Method (S-CAM) was administered by nursing staff specifically trained in the use of this scales via a mandatory training programme.
Once the diagnosis was established, the delirium management schedule was initiated and DOS scoring continued three times daily for as long as delirium was considered present.
This schedule differentiated between hypoactive and hyperactive or mixed delirium. The mainstay of management of hypoactive delirium was haloperidol, starting with a dose of 0.5 to 2 mg and increasing to a maximum of 10 mg daily, plus pipamperone 20 mg up to a maximum of 80 mg daily in those patients with anxiety, distress or restlessness. The mainstay of management of the hyperactive and mixed subtypes was pipamperone, starting at 20 to 40 mg, increasing up to a maximum of 80 mg daily. When delusions or perceptual disturbances were present, haloperidol was initiated at 0.5 to 2 mg, up to a maximum of 10 mg daily. When agitation occurred, lorazepam 0.5 to 2.5 mg, up to a daily maximum dose of 7.5 mg, was added throughout the daytime and at night. Haloperidol was administered throughout the day, whereas the focus of pipamperone administration was the night-time in order to reinforce or restore the sleep-wake cycle pattern. Once clinical improvement was evident, the delirium management schedule recommended reducing the doses of administered medications by 50%.
In addition, patients were also managed with risperidone, olanzapine and quetiapine for delirium.
In total, 192 patients were retrospectively enrolled. Inclusion criteria were the presence of delirium, active management of delirium with psychotropic medication, and DOS recordings for more than three days. Exclusion criteria were the absence of delirium, an insufficient duration of DOS recordings, and transfer to other facilities shortly after initiation of the delirium management schedule.
Once patients were included in this study, sociodemographic variables like age and gender, medical or surgical variables, as well as psychiatric diagnoses were collected. These psychiatric diagnoses were classified as cognitive disorders including dementias, addictive, psychotic and affective disorders, or other. Among management variables, psychotropic medications administered were recorded in daily doses, including all psychotropic medications in the delirium management protocol, as well as other antipsychotics and lorazepam.
Because of the nature of the protocol, various combinations of medications were possible. For the purposes of analysis, these were grouped into four categories: (1) pipamperone monotherapy; (2) pipamperone as an adjunct to haloperidol; (3) haloperidol alone accepted as the gold standard; and (4) atypical antipsychotics, including risperidone, olanzapine and quetiapine, accepted as viable alternatives to haloperidol.
Remission of delirium was defined as scores of less than 7 on three DOS measurements within 24 hours. Subtyping of delirium was based on DOS items 10, 11, and 13, a validated and reliable approach [25, 26].
Delirium rating scales implemented in this study were the Delirium Observation Screening Scale (DOS) and the short version of the Confusion Assessment Method (S-CAM).
The DOS has been validated in the screening of delirium and measurement of delirium severity [25, 26]. The scale reflects the Diagnostic and Statistical Manual of Mental Disorders fourth edition (DSM-IV) criteria of delirium and was designed to capture early delirium symptoms. The DOS has 13 items, each with a three-point rating scale (0–2), with rating possibilities of not existent, sometimes to always, and unable to assess. These items reflect disturbances in the patient’s level of consciousness [1], attention [2–4], thought processes [5, 6], orientation [7, 8], memory [9], psychomotor behaviour [10, 11, 13], and affect [12]. A total score of three or more indicates delirium.
The short version of the S-CAM [27] is a diagnostic tool validated for the assessment of delirium, reflecting the DSM III-R criteria for delirium. Items include: (1) an abrupt change in mental status; sub-items (2A) a disturbance in attention; (2B) a fluctuating course; and (2C) any inattention observed; (3) altered thought processes; and (4) depressed level of consciousness. Available ratings for items 1, 2B, 2C and 3 are yes, no, uncertain and not applicable; for sub-item 2A, not present, present in a mild form, present in a marked form, or uncertain; and for item 4, alert, hypervigilant, lethargic, stuporous, comatose, or uncertain. The diagnosis of delirium requires (1) an acute onset and fluctuating course; as well as (2) inattention and either (3) disorganised thinking or (4) an altered level of consciousness.
Data analyses were performed with the Statistical Package for the Social Sciences (SPSS) version 22 and Stata/SE 13.1 for Windows. Characteristics of the sample were summarised as mean with standard deviation for continuous variables like age, median with interquartile range for continuous variables where the distribution was skewed, and percentages for categorical variables like gender. Inter-group differences for continuous variables were identified by analysis of variance (ANOVA), in those instances in which the distribution of the data was skewed like dosing of medications, the nonparametric Kruskal-Wallis H-test, and for categorical variables by Pearson’s χ2 test.
To evaluate differences in medication administration between haloperidol and pipamperone as an adjunct to haloperidol, the Mann-Whitney U-test for independent variables was computed. To detect differences in the effectiveness of delirium management between the four medication subgroups (pipamperone alone; pipamperone as an adjunct to haloperidol; haloperidol alone; and other atypical antipsychotics), a Cox-regression model was created from the time to event, with the event set as the remission of delirium. When age and baseline delirium severity were different between the groups, these variables were included as covariates. The contrast was set on medications used and the indicator on pipamperone. The validity of the proportional hazard assumption in the Cox model was tested with Schoenfeld residuals (global test: χ2 = 1.26, p = 0.74; medication subgroups: ρ = 0.06, p = 0.47). For the three subtypes of delirium, a similar Cox-regression model was created with the indicator set on hypoactive delirium. The validity of the proportional hazard assumption in this Cox model was tested with Schoenfeld residuals (subtypes: ρ = ˗0.03, p = 0.72). Since the ‘event’ for the Cox-regression model was remission from delirium, the survival function represented the remission function and the hazard function represented the benefit function.
For all inferential tests, the significance level alpha (α) was set at p <0.05, and all tests were two-tailed.
The patient population included elderly, predominantly male patients from various medical and surgical referrals (table 1). More common psychiatric diagnoses included cognitive, addictive and affective disorders. The initial DOS scores indicated substantial impairment and the cumulative DOS scores reached 100.
Table 1 Sociodemographic, medical, psychiatric and management variables of pipamperone alone, as an adjunct to haloperidol in comparison, haloperidol and atypical antipsychotics.
|
All patients
(n = 192) |
Pipamperone
(n = 91) |
Haloperidol and pipamperone
(n = 46) |
Haloperidol
(n = 25) |
Atypical antipsychotics
(n = 30) |
p-value | |
|---|---|---|---|---|---|---|
| Age in years* | 72.1 (27.9–95.2, 13.5) |
74.4 (39.1–95.1, 10.7) |
72.8 (39.3–90.4, 11.9) |
65.7 (29.2–87.3, 17.1) |
68.2 (27.9–95.2, 17.9) |
0.008† |
| Gender in % | 0.286‡ | |||||
| Male | 63.5 | 69.2 | 60.9 | 64 | 50 | |
| Female | 36.5 | 30.8 | 39.1 | 36 | 50 | |
| Referral from, in % | 0.593‡ | |||||
| Cardiology | 29.2 | 46.2 | 28.3 | 4 | – | |
| Medicine | 16.7 | 12.1 | 19.6 | 24 | 20 | |
| Neurology | 21.4 | 20.9 | 13 | 8 | 46.7 | |
| Oncology | 15.6 | 12.1 | 26.1 | 20 | 6.7 | |
| Surgery | 8.9 | 2.2 | 8.7 | 24 | 16.7 | |
| Traumatology | 5.2 | 6.6 | 2.2 | 4 | 6.7 | |
| Other | 3.1 | – | 2.2 | 16 | – | |
| Psychiatric disorders, in % | ||||||
| Cognitive | 14.6 | 14.2 | 13 | 12 | 20 | 0.827‡ |
| Addictive | 13 | 7.7 | 8.7 | 28 | 23.3 | 0.011‡ |
| Psychotic | 3.1 | – | – | 8 | 13.3 | 0.001‡ |
| Affective | 10.9 | 6.6 | 10.9 | 20 | 16.7 | 0.183‡ |
| Other | 4.7 | 2.2 | 4.3 | 4 | 13.3 | 0.097‡ |
| DOS scores – initial 24 hours* | 15.3 (1–39, 7.2) |
13.7 (1–33, 7) |
16.4 (6–39, 7.4) |
17.8 (6–33, 7.2) |
17.8 (6–33, 7.2) |
0.030† |
| DOS scores – cumulative* | 90.7 (8–485, 76.6) |
83.1 (8–458, 77.1) |
118.3 (22–371, 81.4) |
73.8 (17–224, 52.8) |
85.4 (9–342, 77.2) |
0.041† |
| Pharmacological management with** | ||||||
| Haloperidol | 12.9 (0.5–92, 14.5) (4.3, 13.6) |
0.7 (0.5–1, 0.27) (0.5, 0.38) |
12.5 (2–92, 15.3) (7.5, 14.5) |
17.7 (2–58, 13.6) (15, 15) |
1.5 (1–2, 0.7) (1.5, –) |
<0.001§ |
| Pipamperone | 421.7 (10–2520, 355) (445, 305) |
401.1 (20–2520, 366.3) (210, 242.5) |
462.5 (10–1840, 331.7) (465, 305) |
– | – | |
| Atypical antipsychotic | ||||||
| Risperidone | 19.3 (3–41, 15.4) (11.8, 30.3) |
– | – | 32 (32) | 7.1 (1–406, 9.8) (11, 33) |
|
| Olanzapine | 57.5 (10–187.5, 66.3) (31.3, 133.8) |
– | – | – | 65 (10–187.5, 82.3) (31, 133.8) |
|
| Quetiapine | 823.6 (75–3300, 946.7) (350, 3225) |
– | – | 125 (125) | 856.9 (75–3,300, 956.8) (400, 1197) |
|
| Lorazepam | 8.4 (0.5–155, 18.5) (2, 4) |
6.3 (0.5–34, 7.3) (3, 6.6) |
25.8 (1–155, 44.6) (10.5, 20) |
7.1 (1–40, 9.8) (3.8, 5) |
0.056§ | |
| Remission of delirium, in % | 67.2 | 70.3 | 58.7 | 72 | 66.7 | 0.547‡ |
| Day of remission** | 6.3 (2–20, 3.9) (5, 5) |
6 (2–20, 4.1) (4.5, 5) |
7.4 (2–20, 3.9) (7, 5) |
5.2 (2–11, 2.5) (4, 3) |
6.4 (2–20, 4.3) (6, 5) |
0.103† |
DOS = Delirium Observation Screening scale * mean (range, standard deviation); ** mean (range, standard deviation (median, interquartile range) † ANOVA; ‡ Pearson’s chi-square; § Kruskal Wallis H-test
On average, modest amounts of pipamperone were administered, in contrast to the high maximum doses administered to some patients. The average dose of pipamperone was 421 mg, with cumulative doses of between 10 and 2520 mg administered over the course of management. With respect to haloperidol, mean doses were 13 mg, and maximum doses reached 92 mg. Corresponding doses for lorazepam were 8 and 155 mg. In two thirds of patients, remission of delirium was recorded, the mean time to remission being the sixth day of management.
Medication group differences in sociodemographic, medical, psychiatric and management variables were few (table 1). Patients in the haloperidol-alone group were younger (65.7 years) than those in any other group.
The gender distribution was equal across the groups, with male patients accounting for two thirds of the overall sample. Cognitive disorders were equally distributed between medication groups. In contrast, in those patients managed with pipamperone alone and as an adjunct to haloperidol, the prevalence of addictive disorders was lower.
At baseline, the DOS scores of the pipamperone-managed patients were lower. Altogether, modest doses of pipamperone, haloperidol, and atypical antipsychotics sufficed for the management of delirium.
When pipamperone alone was the mainstay of delirium management, an average cumulative dose of 401 mg was administered, with individual daily doses ranging from 5 to 320 mg to achieve a remission rate of 70%, on average on the sixth day of management. When pipamperone was used as an adjunct to haloperidol, comparable cumulative doses of pipamperone (463 mg) were administered, the daily range being from 10 to 320 mg. In this group, the delirium resolution rate was 58.7%, with resolution occurring, on average, on day 7. Daily doses of haloperidol ranged from 0.5 to 18 mg. Among all the medication groups, lorazepam was administered in the lowest dose in this group. In particular, much less lorazepam than haloperidol was required (4 vs 26 mg, Mann-Whitney U-test p = 0.023).
When haloperidol was administered alone, the average dose was 17.7 mg, and this resulted in more than a 70% rate of remission, which occurred, on average, on day 5. Daily doses ranged from 0.5 to 13 mg. Relative to management with pipamperone alone or as an adjunct to haloperidol, or atypical antipsychotics, higher doses of lorazepam were required to achieve symptom control in this group.
When atypical antipsychotics were the mainstay of management, on average 7 mg of risperidone, 65 mg of olanzapine, or 850 mg of quetiapine were administered daily. In this medication group, remission was achieved in nearly 60%, on average on day 6.
The management of delirium with pipamperone alone and as an adjunct to haloperidol proved to be equally effective as management with haloperidol alone or atypical antipsychotics. The omnibus test of model coefficients was significant with respect to pipamperone alone and as an adjunct to haloperidol versus haloperidol and atypical antipsychotics (p <0.001). Between pipamperone alone or as adjunct to haloperidol, haloperidol alone or atypical antipsychotics, neither the time to remission nor the hazard ratio, which represents a benefit ratio, were different (table 2, figs 1 and 2 ). However, there was a trend toward lower efficacy when pipamperone was used as an adjunct to haloperidol. Whether this trend was caused by lower dosing of haloperidol, the severity of illness or delirium, or some independent effect could not be determined. Both age and delirium severity, measured DOS scores at baseline, affected the course of delirium and time to remission.
Table 2 Variables in the equation, pipamperone alone and as an adjunct to haloperidol in the management of delirium and pipamperone in the management of its subtypes.
| B | SE | Wald | df | p-value | Hazard ratio | 95.0% CI for hazard ratio | ||
|---|---|---|---|---|---|---|---|---|
| Pipamperone alone and as adjunct to haloperidol versus haloperidol and atypical antipsychotics | Medication | |||||||
| Pipamperone | 5.173 | 3 | 0.160 | 1 | ||||
| Haloperidol | 0.267 | 0.277 | 0.927 | 1 | 0.336 | 1.306 | 0.758–2.250 | |
| Haloperidol and pipamperone | ˗0.399 | 0.232 | 2.952 | 1 | 0.086 | 0.671 | 0.425–1.058 | |
| Atypical antipsychotics | ˗0.0089 | 0.266 | 0.111 | 1 | 0.739 | 0.915 | 0.544–1.541 | |
| Baseline DOS scores | ˗0.059 | 0.014 | 17.830 | 1 | 0.000 | 0.943 | 0.918–0.969 | |
| Age | ˗0.014 | 0.006 | 4.620 | 1 | 0.032 | 0.986 | 0.974–0.999 | |
| Pipamperone in the management of the delirium subtypes | Subtype of delirium | |||||||
| Hypoactive | 1.325 | 2 | 0.516 | 1 | ||||
| Hyperactive | 0.160 | 0.426 | 0.140 | 1 | 0.708 | 1.173 | 0.509–2.706 | |
| Mixed | ˗0.246 | 0.266 | 0.851 | 1 | 0.356 | 0.782 | 0.464–1.318 | |
| B = coefficient for the constant/intercept; CI = confidence interval; df = degrees of freedom; DOS = Delirium Observation Screening scale; SE = standard error; Wald = χ2 | ||||||||

Figure 1 Survival (remission) function of pipamperone, alone and as an adjunct to haloperidol versus haloperidol and atypical antipsychotics.
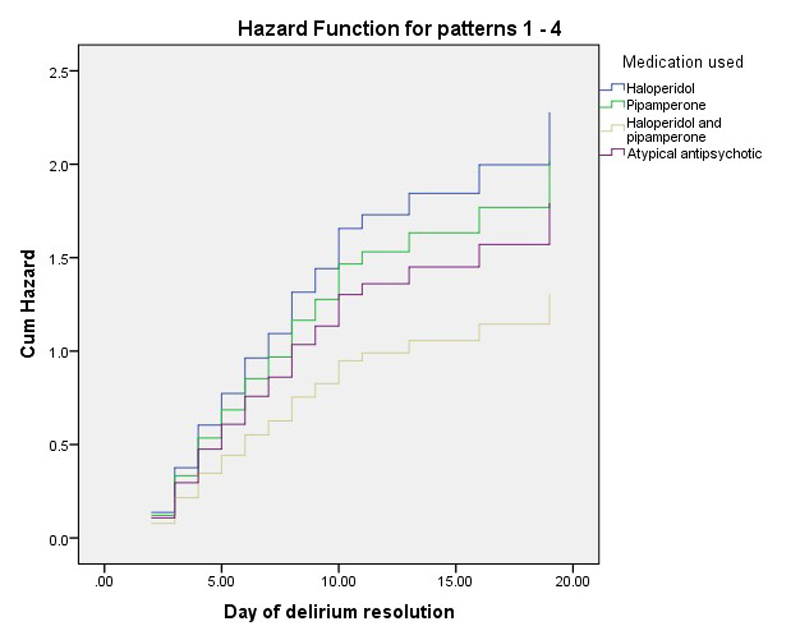
Figure 2 Hazard (benefit) function of pipamperone, alone and as an adjunct to haloperidol versus haloperidol and atypical antipsychotics.
The proportion of patients with each of the three different subtypes of delirium, among those managed with pipamperone, was 41.8% for hypoactive, 12.1% for hyperactive, and 46.2% for mixed. Comparing patients with the three subtypes, distributions for age, gender, referral service, and psychiatric diagnoses were similar (table 3).
Table 3 Sociodemographic, medical, psychiatric and management variables of pipamperone in the subtypes of delirium hypoactive, hyperactive and mixed delirium.
|
Hypoactive delirium
(n = 38) |
Hyperactive delirium
(n = 11) |
Mixed delirium
(n = 42) |
p-value | |
|---|---|---|---|---|
| Age in years* | 76. (39.1–90.6, 9.8) |
71.1 (41.7–85.3, 11.8) |
73.9 (50.2–95.1, 11) |
|
| Gender in % | ||||
| Male | 71.1 | 63.6 | 69 | 0.247† |
| Female | 28.9 | 36.4 | 31 | 0.949‡ |
| Referral from, in % | 0.247‡ | |||
| Cardiology | 47.4 | 72.7 | 38.1 | |
| Medicine | 21.1 | 9.1 | 4.8 | |
| Neurology | 13.2 | 9.1 | 31 | |
| Oncology | 10.5 | 9.1 | 14.3 | |
| Surgery | 2.6 | - | 2.4 | |
| Traumatology | 5.3 | - | 9.5 | |
| Preexisting cardiac disease, in % | 39.5 | 54.5 | 33.3 | 0.449‡ |
| Psychiatric disorders, in % | ||||
| Cognitive | 10.5 | 9.1 | 19 | 0.537‡ |
| Addictive | 5.3 | 9.1 | 9.5 | 0.868‡ |
| Psychotic | – | – | – | – |
| Affective | 10.5 | – | 4.8 | – |
| Other | 5.3 | – | – | – |
| DOS scores – initial 24 hours* | 13.1 (0–9, 2.6) |
13.3 (3–23, 6.2) | 14.4 (1–33, 7.8) | 0.699† |
| DOS scores – cumulative* | 82.6 (2–30, 6.5) |
46.2 (8–100, 28.3) |
93.3 (10–485, 83.6) |
0.198† |
| Pharmacological management with** | ||||
| Haloperidol | 0.8 (0.5–1, 0.3) (0.5, –) |
0.5 (0.5) (–) |
0.7 (0.5–1, 0.3) (0.5, –) |
0.513§ |
| Pipamperone | 434.7 (20–2520, 470.2) (369.3, 268.8) |
311.8 (160–620, 158.5) (250, 270) |
394 (40–1560, 292.7) (280, 352.5) |
0.839§ |
| Lorazepam | 5.5 (0.5–17, 3.9) (5, 5) |
3.6 (0.5–7, 3.4) (3.5, 6.1) |
7.7 (1–34, 4.3) (4, 6.8) |
0.647§ |
| Remission of delirium in % | 76.3 | 63.6 | 66.7 | 0.584‡ |
| Day of remission** | 6.1 (2–20, 4.7) (4, 2.75) |
3.9 (2–8, 1.8) (5, –) |
6.5 (2–20, 3.9) (5.5, 4.3) |
0.183† |
DOS = Delirium Observation Screening scale * mean (range, standard deviation); ** mean (range, standard deviation) (median, interquartile range) † ANOVA; ‡ Pearson’s chi-square; § Kruskal Wallis H-test
Delirium severity, measured as the initial DOS score, was similar between the subtypes, and the cumulative DOS scores – indicating the length and severity of delirium – were comparable.
Pharmacological therapy included pipamperone, as well as adjunctive haloperidol and lorazepam. The cumulative pipamperone dose ranged from 310 to 435 mg, with maximum doses reaching 1560 mg for the mixed and 2520 mg for the hypoactive subtype. Haloperidol was administered at minimal doses, ranging from 0.5 to 0.8 mg, to a maximum of 1 mg. Lorazepam was administered at doses ranging from approximately 4 to 8 mg. Altogether, the doses of pipamperone, haloperidol and lorazepam did not vary between the three subtypes of delirium.
The remission rates for delirium (as a measure of efficacy) ranged from 64 to 76% and the time to remission from approximately 4 to 7 days. Neither measure revealed differences in the management of the three subtypes of delirium.
The omnibus test of model coefficients was not significant for the assessment of pipamperone in the management of the subtypes of delirium (p = 0.51). Pipamperone was therefore equally effective in the three delirium subtypes, as indicated by similar times to remission and hazard/benefit functions.
In this first study on the effectiveness of pipamperone in the management of delirium, pipamperone alone and as an adjunct to haloperidol appeared to be as effective as haloperidol and atypical antipsychotics like risperidone, olanzapine or quetiapine. Moreover, the adjunctive use of pipamperone reduced the dose of lorazepam needed compared with haloperidol-alone patients, thereby potentially representing a lorazepam-sparing approach. The effectiveness of pipamperone also did not seem to be affected by the subtype of delirium, whether hypoactive, hyperactive or mixed.
In the current literature on antipsychotics in patients with delirium, delirium remission rates as measured with various delirium rating scales generally over the course of 1 week are between 40 and 90% [20, 21]. Haloperidol seems to be no more or less effective than atypical antipsychotics. One limitation of the existing literature is that most studies were open-label, included just a small sample of patients and did not distinguish between the various subtypes of delirium. Thus, differences in the effectiveness of antipsychotics in managing the different subtypes of delirium remain understudied [20, 21].
Although pipamperone is recommended as a standard approach to the management of delirium [23, 24], to date no published studies have evaluated its effectiveness. In this initial glimpse at pipamperone as a management for patients with delirium, it appeared to be no less effective an option than other more established drugs, as evidenced by similar rates of and times to remission. However, a trend towards lower effectiveness of pipamperone when used as an adjunct to haloperidol was noted. Whether this trend was caused by use of lower doses of haloperidol, more severe delirium (which was factored into the statistical model), or further undetermined confounding effects, warrants further study.
Within the various medication groups, the administration of pipamperone alone resulted in remission on day 6, on average, which was similar to that achieved with haloperidol alone, haloperidol and pipamperone used together, and the atypical antipsychotics, for which the average time to remission ranged from 4 to 6 days. Adding pipamperone to haloperidol, though no superior to either drug used alone in terms of overall effectiveness, did substantially lower the dose of lorazepam needed relative to haloperidol used alone. Adding pipamperone to haloperidol might be an alternative, benzodiazepine-sparing approach, decreasing their delirogenic load [28, 29].
The remission rate in the pipamperone-managed patients was 70.3%, which was not only similar to the 59 to 72% rates achieved with haloperidol alone, haloperidol plus pipamperone, and atypical antipsychotics, but also comparable to remission rates reported in the literature [20, 21].
Studies exploring the effectiveness of antipsychotics for patients with the various subtypes of delirium remain scarce. Moreover, the existing literature presents inconsistent results. For example, whereas haloperidol and risperidone have been found to be equally efficacious [30, 31], aripiprazole and olanzapine may be more effective for the hypoactive and hyperactive subtypes, respectively [32, 33]. These results suggest that pipamperone may be equally effective, irrespective of the subtype of delirium – hypoactive, hyperactive, or mixed – being managed.
Although the implemented statistical models were designed to control for confounding effects [34], some of these effects need to be mentioned. The delirium management schedule determined the medication chosen, although variations occurred. Hypoactive delirium was primarily managed with haloperidol in the presence of perceptual disturbances. Delirium in those managed with pipamperone was marginally milder. The impact of underlying diseases or type of delirium between pipamperone used alone or as an adjunct to haloperidol versus haloperidol alone or antipsychotics could not be determined. Further, although not reaching significance, the referring specialties and comorbid psychiatric disorders varied between management groups. Whether these differences affected the management outcome is not known and could not be determined.
Although this study had strengths – such as its relatively large sample size, prospective data collection despite retrospective analysis, structured approach, use of multiple scales to detect and rate the severity of delirium, thrice daily DOS scores, and the examination of several different psychotropic drugs, including lorazepam – limitations must be noted. The design of the study was retrospective. Although nursing staff were trained in the administration of the DOS via a standardised training programme, inter-rater reliability was not formally assessed. However, the DOS has been accepted as a reliable scale and its ease of use been documented [25, 26]. The allocation of medications was determined by the delirium management protocol, rather than randomised, and neither the patients nor assessors were blinded to the medications being used. Further, the number of patients managed with haloperidol or atypical antipsychotics was lower.
Although these results provide first proof for the effectiveness of pipamperone in the management of delirium, further randomised controlled studies are required to confirm these results.
Despite some methodological limitations, these initial results suggest that low-dose pipamperone, whether used alone or as an adjunct to haloperidol, may be effective in the management of delirium, irrespective of whether the delirium is hypoactive, hyperactive, or mixed. Furthermore, pipamperone administered as an adjunct to haloperidol may be benzodiazepine-sparing. The needs for randomised studies notwithstanding, these results indicated that pipamperone can be recommended as an effective drug for the management of delirium.
No financial support and no other potential conflict of interest relevant to this article was reported.
1American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. Washington, DC, American Psychiatric Association. 2000. 124–127
2 Trzepacz PT , Breitbart W , Franklin J , Levenson J , Martini R , Wang P ; American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(5, Suppl):1–20.
3 Voyer P , McCusker J , Cole MG , St-Jacques S , Khomenko L . Factors associated with delirium severity among older patients. J Clin Nurs. 2007;16(5):819–31. doi:.https://doi.org/10.1111/j.1365-2702.2006.01808.x
4 Zaal IJ , Devlin JW , Peelen LM , Slooter AJ . A systematic review of risk factors for delirium in the ICU. Crit Care Med. 2015;43(1):40–7. doi:.https://doi.org/10.1097/CCM.0000000000000625
5 Vasilevskis EE , Han JH , Hughes CG , Ely EW . Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277–87. doi:.https://doi.org/10.1016/j.bpa.2012.07.003
6 Morandi A , Jackson JC , Ely EW . Delirium in the intensive care unit. Int Rev Psychiatry. 2009;21(1):43–58. doi:.https://doi.org/10.1080/09540260802675296
7 Mehta S , Cook D , Devlin JW , Skrobik Y , Meade M , Fergusson D , et al.; SLEAP Investigators; Canadian Critical Care Trials Group. Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Crit Care Med. 2015;43(3):557–66. doi:.https://doi.org/10.1097/CCM.0000000000000727
8 Inouye SK . Delirium in hospitalized older patients. Clin Geriatr Med. 1998;14(4):745–64.
9 Inouye SK . Delirium in older persons. N Engl J Med. 2006;354(11):1157–65. doi:.https://doi.org/10.1056/NEJMra052321
10 Fong TG , Tulebaev SR , Inouye SK . Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210–20. doi:.https://doi.org/10.1038/nrneurol.2009.24
11 Marcantonio E , Ta T , Duthie E , Resnick NM . Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50(5):850–7. doi:.https://doi.org/10.1046/j.1532-5415.2002.50210.x
12 Kiely DK , Jones RN , Bergmann MA , Murphy KM , Orav EJ , Marcantonio ER . Association between delirium resolution and functional recovery among newly admitted postacute facility patients. J Gerontol A Biol Sci Med Sci. 2006;61(2):204–8. doi:.https://doi.org/10.1093/gerona/61.2.204
13 McCusker J , Cole M , Dendukuri N , Belzile E , Primeau F . Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165(5):575–83.
14 McCusker J , Cole M , Dendukuri N , Han L , Belzile E . The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med. 2003;18(9):696–704. doi:.https://doi.org/10.1046/j.1525-1497.2003.20602.x
15 Kiely DK , Marcantonio ER , Inouye SK , Shaffer ML , Bergmann MA , Yang FM , et al. Persistent delirium predicts greater mortality. J Am Geriatr Soc. 2009;57(1):55–61. doi:.https://doi.org/10.1111/j.1532-5415.2008.02092.x
16 Lee KH , Ha YC , Lee YK , Kang H , Koo KH . Frequency, risk factors, and prognosis of prolonged delirium in elderly patients after hip fracture surgery. Clin Orthop Relat Res. 2011;469(9):2612–20. doi:.https://doi.org/10.1007/s11999-011-1806-1
17 Meagher DJ , Trzepacz PT . Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5(2):75–85.
18 Abraha I , Rimland JM , Trotta F , Pierini V , Cruz-Jentoft A , Soiza R , et al. Non-Pharmacological Interventions to Prevent or Treat Delirium in Older Patients: Clinical Practice Recommendations The SENATOR-ONTOP Series. J Nutr Health Aging. 2016;20(9):927–36. doi:.https://doi.org/10.1007/s12603-016-0719-9
19 Siddiqi N , Harrison JK , Clegg A , Teale EA , Young J , Taylor J , et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563.
20 Friedman JI , Soleimani L , McGonigle DP , Egol C , Silverstein JH . Pharmacological treatments of non-substance-withdrawal delirium: a systematic review of prospective trials. Am J Psychiatry. 2014;171(2):151–9. doi:.https://doi.org/10.1176/appi.ajp.2013.13040458
21 Kishi T , Hirota T , Matsunaga S , Iwata N . Antipsychotic medications for the treatment of delirium: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. 2016;87(7):767–74. doi:.https://doi.org/10.1136/jnnp-2015-311049
22Ellenbroek BA, Cools AR. Atypical Antipsychotics. Basel: Birkhäuser; 2012.
23 Rothenhäusler HB . Klinik, Diagnostik und Therapie des nicht entzugsbedingten Delirs. Psychosom Konsiliarpsychiatr. 2008;2(3):160–7. doi:.https://doi.org/10.1007/s11800-008-0114-4
24 Rothenhäusler HB . Psychopharmakotherapie bei somatischen Patienten im Allgemeinkrankenhaus. Spektrum Psychiatrie. 2010;1:20–3.
25 Scheffer AC , van Munster BC , Schuurmans MJ , de Rooij SE . Assessing severity of delirium by the Delirium Observation Screening Scale. Int J Geriatr Psychiatry. 2011;26(3):284–91. doi:.https://doi.org/10.1002/gps.2526
26 Schuurmans MJ , Shortridge-Baggett LM , Duursma SA . The Delirium Observation Screening Scale: a screening instrument for delirium. Res Theory Nurs Pract. 2003;17(1):31–50. doi:.https://doi.org/10.1891/rtnp.17.1.31.53169
27 Inouye SK , Kosar CM , Tommet D , Schmitt EM , Puelle MR , Saczynski JS , et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160(8):526–33. doi:.https://doi.org/10.7326/M13-1927
28 Böhmdorfer B , Rohleder S , Wawruch M , van der Cammen TJ , Frühwald T , Jagsch C , et al. DEL-FINE: a new tool for assessing the delirogenic properties of drugs of relevance for European pharmacotherapy. Z Gerontol Geriatr. 2016;49(5):416–22. doi:.https://doi.org/10.1007/s00391-015-0941-9
29 Gaudreau JD , Gagnon P , Harel F , Roy MA , Tremblay A . Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23(27):6712–8. doi:.https://doi.org/10.1200/JCO.2005.05.140
30 Boettger S , Breitbart W , Passik S . Haloperidol and risperidone in the treatment of delirium and its subtypes. Eur J Psychiatry. 2011;25(2):25–57.
31 Liu CY , Juang YY , Liang HY , Lin NC , Yeh EK . Efficacy of risperidone in treating the hyperactive symptoms of delirium. Int Clin Psychopharmacol. 2004;19(3):165–8. doi:.https://doi.org/10.1097/00004850-200405000-00008
32 Boettger S , Friedlander M , Breitbart W , Passik S . Aripiprazole and haloperidol in the treatment of delirium. Aust N Z J Psychiatry. 2011;45(6):477–82. doi:.https://doi.org/10.3109/00048674.2011.543411
33 Breitbart W , Tremblay A , Gibson C . An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics. 2002;43(3):175–82. doi:.https://doi.org/10.1176/appi.psy.43.3.175
34 Pourhoseingholi MA , Baghestani AR , Vahedi M . How to control confounding effects by statistical analysis. Gastroenterol Hepatol Bed Bench. 2012;5(2):79–83.
No financial support and no other potential conflict of interest relevant to this article was reported.