A 10-year observational study of Streptococcus dysgalactiae bacteraemia in adults: frequent occurrence among female intravenous drug users
DOI: https://doi.org/10.4414/smw.2017.14469
Corinne
Ruppenab, Magnus
Rasmussenc, Carlo
Casanovaa, Parham
Sendiad
aInstitute for Infectious Diseases,
bGraduate School for Cellular and Biomedical Sciences,
cDivision of Infection Medicine, Department of Clinical Sciences,
dDepartment of Infectious Diseases, Bern University Hospital,
A 10-year observational study of Streptococcus dysgalactiae bacteraemia in adults: frequent occurrence among female intravenous drug users
w14469
Summary
Beta-haemolytic streptococci of groups C and G have become increasingly recognized as causes of invasive human infections. We reviewed clinical and molecular characteristics of Streptococcus dysgalactiae isolates that caused bacteraemia in adults from 2006 to 2015. Among 67 episodes, skin and soft-tissue infections (43%) and emm types stG62647.0 (26%) were the most frequent clinical manifestation and emm type, respectively. Nineteen (28%) episodes occurred in intravenous drug users (75% women). Our observational study shows similarities to but also differences from other reports. The former include the most frequent clinical presentations, and the most frequently found emm types. This report highlights a relatively high proportion of female intravenous drug users among S. dysgalactiae bacteraemia episodes.
Introduction
Beta-haemolytic streptococci of groups C and G (GCS and GGS) have become increasingly recognized as causes of invasive human infections [1–3]. The vast majority of GGS and GCS belong to Streptococcus dysgalactiae, of which subsp. equisimilis dominate. They cause a spectrum of disease similar to that of Streptococcus pyogenes. Previous publications reported that the most common clinical manifestations of S. dysgalactiae infections include skin and soft-tissue infections, followed by bacteraemia of unknown origin. These infections are often observed in elderly patients and those with underlying medical conditions [1–6]. Here, we investigate the demographics, comorbidities, clinical manifestations of patients who experienced S. dysgalactiae bacteraemia and presented to our centre (Bern University Hospital, Bern, Switzerland). In addition, the distribution of S. dysgalactiae emm types was determined.
Methods
Blood culture isolates from 1 January 2006 to 31 December 2015 were identified retrospectively through database searches in the laboratory of the Institute for Infectious Diseases of University of Bern, Switzerland. Available isolates were thawed from the bacterial collection stored at ˗80°C. The bacteria were identified with use of Ultraflextreme MALDI-TOF mass spectrometry (Bruker Daltonics, Bremen, Germany), using the MALDI Biotyper version 3.0 with the BDAL-5627 Database. A score ≥2.0 was required for species determination. To determine the Lancefield antigen, latex agglutination (Remel Europe Ltd., Dartford, UK) was performed. Upon identification of S. dysgalactiae bacteraemia episodes, patient charts were reviewed to obtain clinical information for each episode.
The episodes were categorised in single episode, multiple episodes (different emm type in each episode) and relapsing episodes (identical emm type in each episode). Bacteraemia that reoccurred within <1 month after the first episodes were designated to the same episode.
DNA emm type determination was performed as described previously [7]. All available isolates were subjected to emm typing as described (http://www.cdc.gov/streplab). Statistical analyses were performed using the Prism 6 software. Differences in group proportions were assessed by use of contingency tables and the chi-square test, or Fisher’s exact probability test if a frequency was smaller than 5. A two-tailed p-value of ≤0.05 was considered significant. The study was approved by the local ethics committee (2016-01614).
Results
Sixty-seven GCS and GGS isolates causing bacteraemia in 61 patients were identified in the database. Six patients had two episodes. Two isolates were not available for emm type determination. Thus, the denominators for frequency analyses were 67 episodes, 61 patients and 65 isolates (table 1 and fig. 1a). Sixty-one of them were categorised as single episodes, five as multiple episodes (different emm type in two episodes) and one as relapsing episode (identical emm type in both episodes). The demographics, distribution of gender and comorbidities are presented in table 1. The most frequent clinical manifestations were skin and soft-tissue infections, followed by bacteraemia without identified source and septic arthritis. These three entities represented 82% of the clinical manifestations. Sixteen (26%) patients with 19 (28%) episodes were intravenous drug users (IVDUs). Twelve (75%) of them were women. The difference in proportion of women between IVDUs and non-IVDUs was significant (p = 0.0003 with chi-square test).
Table 1 Patient characteristics and comorbid conditions in adult patients with Streptococcus dysgalactiae bacteraemia.
|
Characteristics
|
61 patients*
|
|
Demographics in 61 patients
|
|
| Median age (range) in years |
57.0 (2–88) |
| Women, n (%) |
23 (62.3) |
|
Bacteriology in 67 episodes
|
|
| Group C Streptococcus, n (%) |
22 (32.8) |
| Group G Streptococcus, n (%) |
45 (67.2) |
| Polymicrobial bacteraemia, n (%) |
18 (26.9) |
|
Comorbid conditions of 61 patients
|
|
| Arterial hypertension, n (%) |
26 (42.6) |
| Congestive heart failure, n (%) |
18 (29.5) |
| Diabetes mellitus, n (%) |
17 (27.9) |
| Impairment of renal function†, n (%) |
16 (26.2) |
| Intravenous drug abuse, n (%) |
16 (26.2) |
| Active or history of malignancy, n (%) |
15 (24.6) |
| Alcohol abuse, n (%) |
8 (13.1) |
| Chronic pulmonary disease‡, n (%) |
7 (11.5) |
| Peripheral arterial occlusive disease, n (%) |
6 (9.8) |
| Solid organ transplants, n (%) |
3 (4.9) |
|
Clinical manifestations in 67 episodes
|
|
| Skin- and soft-tissue infections, n (%) |
29 (43.3) |
| Erysipelas, n |
17 |
| Cellulitis, n |
3 |
| Necrotising fasciitis, n |
1 |
| Skin and/or soft tissue abscess |
6 |
| Infected foot ulcer, n |
2 |
| Bacteraemia without identified source*, n (%) |
17 (25.4) |
| Septic arthritis, n (%) |
9 (13.4) |
| Infective endocarditis, n (%) |
3 (4.5) |
| Neutropenic colitis, n (%) |
3 (4.5) |
|
Other clinical manifestations, n (%) |
6 (8.9) |
| Bacterial peritonitis, n |
2 |
| Spinal epidural abscess, n |
1 |
| Vascular graft infection, n |
1 |
| Puerperal sepsis, n |
1 |
| Osteomyelitis, n |
1 |
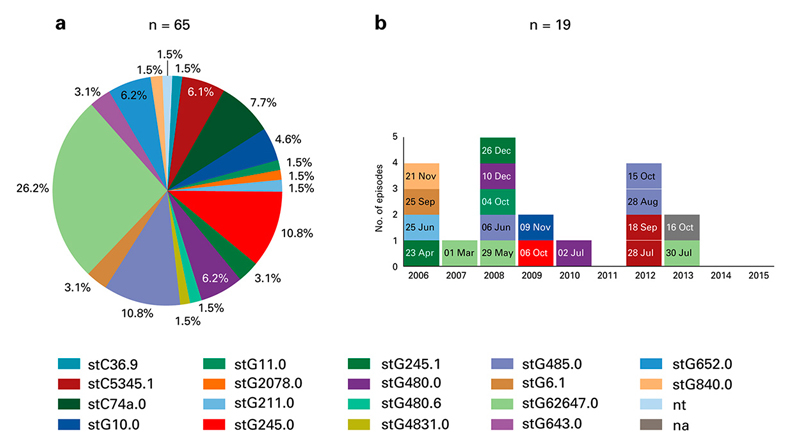
Figure 1
(a) Pie-chart with proportional distribution of 65 emm types of Streptococcus dysagalactiae isolates causing bacteraemia in adults over a 10-year period. Each colour represents one emm type. Two isolates were not available for emm type determination (na). One isolate was not typable (nt). (b) Distribution of emm types of S. dysgalactiae isolates causing 19 episodes of bacteraemia in 16 patients with intravenous drug use. Each square represents an episode, each colour a specific emm type. One isolate was not available for emm type analysis (na, grey colour). The date of bacteraemia is indicated within each square. In three female patients two episodes of bacteraemia with 67 days interval (4 Oct and 10 Dec 2008), 210 days interval (29 May and 26 Dec 2008), and 269 days interval (6 Oct 2009 and 02 Jul 2010), respectively, occurred. In 2012, four episodes in four patients (three women, one man) with two emm types was noted.
Six (9.8%) patients had a second episode that occurred between 41 to 269 days after the first episode. Three of them were IVDUs.
StG62647.0 was the most frequent emm type (26.2%), followed by stG245.0 (10.8%) and stG485.0 (10.8%) (fig. 1a). All but one isolate with emm type stG62647.0 were designated to GCS. No emm type was significantly associated with a clinical manifestation, but there was a trend association between the most frequent clinical manifestation (skin- and soft-tissue disease) and most frequent emm type (stG62647.0) (p = 0.07 with chi-square test).
In five of the six patients with two episodes, emm types were different in both episodes. This observation included three female IVDUs with 67, 210 and 269 days interval between the two episodes of bacteraemia. In one patient, the GCS bacteraemia reoccurred after 41 days, and both isolates were emm type stG62647.0. The source of relapsing bacteraemia was not found in a patient with a kidney transplant, despite extensive investigations.
The analyses of emm types among IVDUs did not show patterns of an outbreak (fig. 1b). Eleven different emm types caused 18 episodes (one isolate was not available for analyses).
Discussion
Our observational study shows similarities to but also differences from other reports. As in other publications [1–6], the most frequent clinical manifestations were skin and soft-tissue infections, bacteraemia without identified source and septic arthritis (table 1). Recurrence rate of S. dysgalactiae bacteraemia of up to 10% have been reported [8, 9]. In these cases, the second episode occurs typically ≥1 month after the first one [10]. In our series, six patients (9.8%) had a second episode of bacteraemia, but recurrence with the same emm type was observed in only one patient. Similar to our previous investigation on recurrent S. dysgalactiae bacteraemia [10], we did not identify comorbidity risk factors for a second episode of S. dysgalactiae bacteraemia.
Previous publications have highlighted that invasive S. dysgalactiae infection typically occurs in elderly patients with comorbidities [1–6, 11]. This was also the case in our series. However, we were surprised that 16 (26%) of 61 patients were IVDUs. Broyles et al. [4] reported that 6% (12 out of 212) of patients with invasive S. dysgalactiae infection in the Atlanta metropolitan area (Georgia, USA) and San Francisco Bay metropolitan area (California, USA) were IVDUs. In the 1990s, associations of IVDU and beta-haemolytic streptococcal bacteraemia were reported with group A Streptococcus (GAS) [12, 13]. Similarly, epidemics and endemic disease caused by GAS was reported in IVDUs in our geographic area (Bern, Switzerland) in 2001 [14]. Interestingly, several studies have observed that the overall incidence of invasive GCS and GGS infections has increased significantly in the last two decades (Norway [6], Denmark [15], Canada [16], Australia [17]). Because of our study design, we cannot explain the reason for the relatively high proportion of IVDU patients in our series. It is possible that the aforementioned epidemiological trend of infections due to GCS and GGS affected this risk group, also.
The female predominance within the IVDU group was also surprising and raised the speculation of an outbreak possibly transmitted via needle sharing or intimate contact.
From emm typing and temporal distribution, however, we did not see patterns of an outbreak (fig. 1b). Transmission between individuals may have happened (e.g., episodes in 2012, fig. 1b), though whole genome sequencing was not performed in the two potential episodes.
The results of molecular analyses are in line with that of others [7, 10]. StG62647.0 was the most frequent emm type and all but one of them were designated to GCS [7, 10, 18]. The trend association of skin and soft-tissue disease and stG62647.0 may be a co-incidence of the most frequent clinical manifestation and the most frequent emm type. Together with the results from our previous studies [7, 10], we found no significant indication that a specific S. dysgalactiae emm type is predominately found in a specific clinical manifestation.
Our study has limitations, including its retrospective nature and the use of medical records for data collection. Given the microbiological focus of our study, we could not analyse detailed data on the clinical course of the disease. Follow-up examinations to investigate possible transmission between IVDU patients were not possible because we were unable to contact these patients. Finally, the absolute number of isolates was relatively low, making statistically solid interpretation difficult.
In conclusion, our single-centre observation of S. dysgalactiae bacteraemia confirms clinical and molecular results from other centres, but also highlights a relatively high proportion of female IVUDs among the analysed S. dysgalactiae bacteraemia episodes. Possible routes of transmission include needle transmission or intimate contact with colonised individuals. A prospective and qualitative study is needed to investigate the transmission of GCS and GGS, and to confirm the relatively high frequency of IVDUs among patients with S. dysgalactiae bacteraemia.
Acknowledgements
We thank for the excellent technical assistance by Mrs Gisela Hovold and the important advice by Dr Malin Inghammar.
References
1
Takahashi
T
,
Ubukata
K
,
Watanabe
H
. Invasive infection caused by Streptococcus dysgalactiae subsp. equisimilis: characteristics of strains and clinical features. J Infect Chemother. 2011;17(1):1–10. doi:.https://doi.org/10.1007/s10156-010-0084-2
2
Brandt
CM
,
Spellerberg
B
. Human infections due to Streptococcus dysgalactiae subspecies equisimilis. Clin Infect Dis. 2009;49(5):766–72. doi:.https://doi.org/10.1086/605085
3
Rantala
S
. Streptococcus dysgalactiae subsp. equisimilis bacteremia: an emerging infection. Eur J Clin Microbiol Infect Dis. 2014;33(8):1303–10. doi:.https://doi.org/10.1007/s10096-014-2092-0
4
Broyles
LN
,
Van Beneden
C
,
Beall
B
,
Facklam
R
,
Shewmaker
PL
,
Malpiedi
P
, et al.
Population-based study of invasive disease due to beta-hemolytic streptococci of groups other than A and B. Clin Infect Dis. 2009;48(6):706–12. doi:.https://doi.org/10.1086/597035
5
Loubinoux
J
,
Plainvert
C
,
Collobert
G
,
Touak
G
,
Bouvet
A
,
Poyart
C
; CNR-Strep Network. Adult invasive and noninvasive infections due to Streptococcus dysgalactiae subsp. equisimilis in France from 2006 to 2010. J Clin Microbiol. 2013;51(8):2724–7. doi:.https://doi.org/10.1128/JCM.01262-13
6
Oppegaard
O
,
Mylvaganam
H
,
Kittang
BR
. Beta-haemolytic group A, C and G streptococcal infections in Western Norway: a 15-year retrospective survey. Clin Microbiol Infect. 2015;21(2):171–8. doi:.https://doi.org/10.1016/j.cmi.2014.08.019
7
Trell
K
,
Nilson
B
,
Rasmussen
M
. Species and emm-type distribution of group C and G streptococci from different sites of isolation. Diagn Microbiol Infect Dis. 2016;86(4):467–9. doi:.https://doi.org/10.1016/j.diagmicrobio.2016.09.008
8
Liao
CH
,
Liu
LC
,
Huang
YT
,
Teng
LJ
,
Hsueh
PR
. Bacteremia caused by group G Streptococci, taiwan. Emerg Infect Dis. 2008;14(5):837–40. doi:.https://doi.org/10.3201/eid1405.070130
9
Rantala
S
,
Vahakuopus
S
,
Vuopio-Varkila
J
,
Vuento
R
,
Syrjanen
J
. Streptococcus dysgalactiae subsp. equisimilis Bacteremia, Finland, 1995-2004. Emerg Infect Dis. 2010;16(5):843–6. doi:.https://doi.org/10.3201/eid1605.080803
10
Trell
K
,
Sendi
P
,
Rasmussen
M
. Recurrent bacteremia with Streptococcus dysgalactiae: a case-control study. Diagn Microbiol Infect Dis. 2016;85(1):121–4. doi:.https://doi.org/10.1016/j.diagmicrobio.2016.01.011
11
Takahashi
T
,
Asami
R
,
Tanabe
K
,
Hirono
Y
,
Nozawa
Y
,
Chiba
N
, et al.
Clinical aspects of invasive infection with Streptococcus dysgalactiae subsp. equisimilis in elderly patients. J Infect Chemother. 2010;16(1):68–71. doi:.https://doi.org/10.1007/s10156-009-0016-1
12
Navarro
VJ
,
Axelrod
PI
,
Pinover
W
,
Hockfield
HS
,
Kostman
JR
. A comparison of Streptococcus pyogenes (group A streptococcal) bacteremia at an urban and a suburban hospital. The importance of intravenous drug use. Arch Intern Med. 1993;153(23):2679–84. doi:.https://doi.org/10.1001/archinte.1993.00410230097011
13
Bernaldo de Quirós
JC
,
Moreno
S
,
Cercenado
E
,
Diaz
D
,
Berenguer
J
,
Miralles
P
, et al.
Group A streptococcal bacteremia. A 10-year prospective study. Medicine (Baltimore). 1997;76(4):238–48. doi:.https://doi.org/10.1097/00005792-199707000-00002
14
Léchot
P
,
Schaad
HJ
,
Graf
S
,
Täuber
M
,
Mühlemann
K
; Patricia Léchot, Heinz J. Schaad, S. Group A streptococcus clones causing repeated epidemics and endemic disease in intravenous drug users. Scand J Infect Dis. 2001;33(1):41–6. doi:.https://doi.org/10.1080/003655401750064059
15
Lambertsen
LM
,
Ingels
H
,
Schønheyder
HC
,
Hoffmann
S
; Danish Streptococcal Surveillance Collaboration Group 2011. Nationwide laboratory-based surveillance of invasive beta-haemolytic streptococci in Denmark from 2005 to 2011. Clin Microbiol Infect. 2014;20(4):O216–23. doi:.https://doi.org/10.1111/1469-0691.12378
16
Schwartz
IS
,
Keynan
Y
,
Gilmour
MW
,
Dufault
B
,
Lagacé-Wiens
P
. Changing trends in β-hemolytic streptococcal bacteremia in Manitoba, Canada: 2007-2012. Int J Infect Dis. 2014;28:211–3. doi:.https://doi.org/10.1016/j.ijid.2014.03.1376
17
Harris
P
,
Siew
DA
,
Proud
M
,
Buettner
P
,
Norton
R
. Bacteraemia caused by beta-haemolytic streptococci in North Queensland: changing trends over a 14-year period. Clin Microbiol Infect. 2011;17(8):1216–22. doi:.https://doi.org/10.1111/j.1469-0691.2010.03427.x
18
Pinho
MD
,
Melo-Cristino
J
,
Ramirez
M
. Clonal relationships between invasive and noninvasive Lancefield group C and G streptococci and emm-specific differences in invasiveness. J Clin Microbiol. 2006;44(3):841–6. doi:.https://doi.org/10.1128/JCM.44.3.841-846.2006
