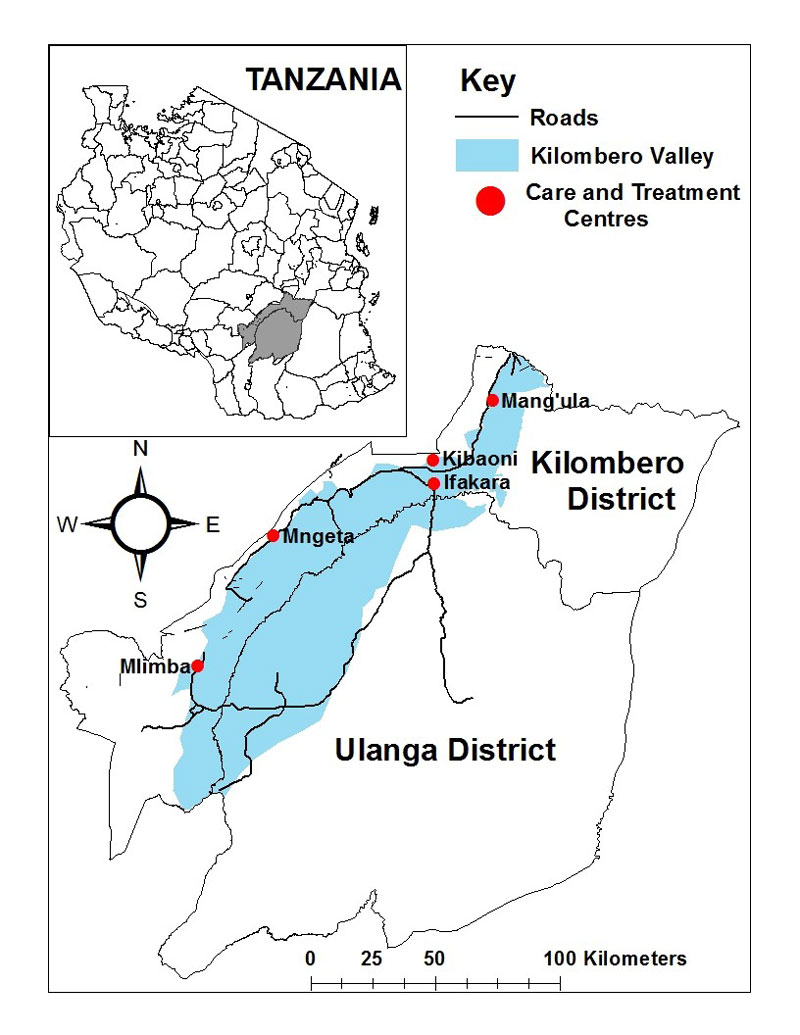
Figure 1 Catchment area of KIULARCO, Kilombero and Ulanga Districts (ArcGIS software, ESRI, USA).
DOI: https://doi.org/10.4414/smw.2017.14485
The human immunodeficiency virus / acquired immunodeficiency syndrome (HIV/AIDS) pandemic is one of the worst epidemics in human history, having affected more than 70 million people and claimed the lives of 35 million [1]. Sub-Saharan Africa (SSA) continues to bear a disproportionate share of the global HIV burden. The efforts made in the last decade have resulted in previously unimaginable access to antiretroviral treatment (ART), prevention services, and evidence-based care interventions for people living with HIV/AIDS (PLWHIV) in Africa, leading to a subsequent stabilisation of the epidemic in the continent. However, of the estimated 36.7 million PLWHIV worldwide in 2015, 70% (25.5 million) were in SSA [2]. New infections and AIDS-related deaths were also disproportionately concentrated in SSA, accounting for 65% (1.4 million) of the 2.1 million new infections and 74% (800 000) of the 1.1 million AIDS-related deaths in 2015 [2]. Remarkably, within SSA, the HIV epidemics still differ greatly between countries, with Southern and Eastern Africa being the most heavily affected regions.
Despite the success in scaling up ART, with over 17 million people treated, and the stabilisation of the epidemic in SSA, many challenges persist. ART scale-up programmes in Africa are hindered by late diagnosis of HIV infection [3], low rates of linkage into care [4], obstacles in treatment delivery [5], low availability of virological and drug resistance monitoring [6], and low long-term retention in care [7]. Other relevant challenges include logistical factors disrupting care and undermining the success of ART scale-up programmes, the neglected epidemic among key populations, and the ageing of the HIV-infected population.
In Tanzania, 1.4 million people were estimated to live with HIV in 2015 [8]. The national AIDS control programme was launched in 2004 in response to the rising epidemic and, since then, the HIV prevalence in Tanzania has declined from 7.0% to 4.7% among adults aged 15–49 years [8]. However, the HIV prevalence remains highly heterogeneous, with geographical and population variability. In addition, the HIV prevalence among key populations, such as men who have sex with men, people who inject drugs and female sex workers, remains unacceptably high at 12.3–41.0%, 34.8–42.0% and 31.4%, respectively [9]. The ART coverage in 2015 was 53% in adults and 56% in children [8]. Since 2016, Tanzania has progressively adopted the 2015 WHO recommendations of treating all PLWHIV regardless of CD4 cell counts.
Prospective cohort studies have been fundamental for describing the natural history of HIV infection, determining factors and biomarkers associated with disease progression and evaluating treatment efficacy. Given the continuously changing face of the HIV epidemic in Africa, there is a need for large representative cohort databases with comprehensive patient-level data in order to generate evidence on ART outcomes and complications to inform national and international health policy and improve HIV care and treatment nationally and globally. There are excellent HIV cohorts in SSA [10–16], but there is a paucity of observational prospective long-term cohort data on PLWHIV in Tanzania and overall in rural settings in SSA. The Tanzanian national AIDS control programme provides relevant national data with good coverage across the country, but with limited clinical depth [17–20].
In this article, we aim to describe the Kilombero and Ulanga Antiretroviral Cohort (KIULARCO) as a model for a large prospective cohort in SSA. KIULARCO is integrated within routine clinical care delivered through the national health care system, and generates daily extensive clinical and epidemiological data in real-time through an electronic data collection system tailored to the specific needs of rural sub-Saharan Africa.
KIULARCO is a single-site, open, ongoing and prospective cohort of PLWHIV based at the Chronic Diseases Clinic of Ifakara (CDCI), within the Saint Francis Referral Hospital in Ifakara, Tanzania. The CDCI was started in 2004 as a public-sector HIV care and treatment centre of the Ministry of Health and Social Welfare of the Government of Tanzania, being the first rural HIV care and treatment clinic accredited to provide HIV services through the Tanzanian national AIDS control programme. From its conception, the CDCI has received continuous support from the Swiss Tropical and Public Health Institute (Swiss TPH, Basel, Switzerland), the Ifakara Health Institute (IHI, Tanzania), the Department of Infectious Diseases and Hospital Epidemiology of the University Hospital Basel (Basel, Switzerland) and the Department of Infectious Diseases of the University Hospital Bern (Bern, Switzerland).
KIULARCO was developed originally in 2005 for monitoring and evaluating ART roll-out. The current objectives of the KIULARCO cohort are to: (i) provide patient and cohort-level information on the outcomes of HIV treatment; (ii) provide cohort-level information on opportunistic infections and non-AIDS comorbidities; (iii) evaluate aspects of HIV care and treatment that have national or international policy relevance; (iv) provide a platform for studies on improving HIV care and treatment, including clinical trials; and (v) contribute to generating local capacity to deal with the challenges posed by the HIV/AIDS pandemic in SSA.
All individuals diagnosed with HIV at the Saint Francis Referral Hospital, and those diagnosed elsewhere and coming for care at the CDCI, are invited to participate in KIULARCO. Blood samples are drawn at routine visits prior to and 3 months after ART initiation, and every 6 months thereafter, and plasma and cell pellets are cryopreserved at ˗80ºC and ˗20°C.
The Ifakara Health Institute institutional review board and the Health Research Ethics Review Committee of the National Institute for Medical Research of Tanzania provide ethical approval for KIULARCO, including for sample collection, cryopreservation and analysis of collected data. Written informed consent is sought from all participants at registration at the CDCI; for children and adolescents aged <18 years, informed consent is sought from caregivers. Data are backed up daily, stored on a secure local server and de-identified before analysis.
The rural districts of Kilombero and Ulanga, in the Morogoro region of south East Tanzania, have a population of approximately 700 000 [21] and an estimated 40 000 PLWHIV. The Saint Francis referral Hospital is the largest health facility in the Kilombero district, located in its main town, Ifakara, and serving as the referral hospital of seven primary healthcare clinics and HIV care and treatment centres (fig. 1). The Ifakara Health Institute runs two health demographic surveillance systems in the area: one rural, covering 25 villages in Ulanga and Kilombero districts, comprising 126 836 people; one urban consisting of five areas of Ifakara town, with 44 992 people [22]. The main economic activity in the Kilombero and Ulanga districts is subsistence farming, especially of rice, with smaller proportions of pastoralists who migrated into the area from the north and centre of Tanzania. In Ifakara town, the economic activity is centred on agricultural trade, farming and provision of higher education, especially in the biomedical field. There are five biomedical institutions that constitute the so-called Ifakara cluster, including besides the Ifakara Health Institute and the Saint Francis Referral Hospital: the Tanzanian Training Centre for International Health (www.ttcih.org), the Saint Francis University College on Health and Allied Sciences (sfuchas.ac.tz/ifakara/) and the Edgar Maranta School of Nursing (ifakaranursing.ac.tz).

Figure 1 Catchment area of KIULARCO, Kilombero and Ulanga Districts (ArcGIS software, ESRI, USA).
People from more than 70 ethnic groups currently reside in Ifakara, 60% of whom are in-migrants to the area. Swahili is the main language of communication and English is also spoken by professionals working in the Ifakara cluster. The population structure is typical of rural African populations, with 46% of people younger than 15 years of age [22]. The population in both districts shows net out-migration for young men, mainly to find employment in more urbanised areas, leading to difficulties in retention in care of PLWHIV. Moreover, the increasing population movements may affect HIV dynamics in the area, and the recent construction of a bridge over the Kilombero River, together with the opening of a north-south road is expected to contribute to further changes in the mid-term.
The multidisciplinary skills available at the Ifakara Health Institute, coupled with the unique position of the CDCI in a cluster of research, health service delivery and health training organisations, allow for research along the entire pipeline of intervention development to impact evaluation.
Since 2004, voluntary counselling and testing services are available to anybody willing to be tested for HIV. In addition, since 2014, all persons attending the outpatient department, antenatal clinic and hospital wards are consistently offered an HIV test through provider-initiated testing and counselling services. Group pre-test and individualised post-test counselling are given. All HIV-positive patients are invited to join KIULARCO, including non-pregnant adults, pregnant women, adolescents, children and infants. Reasons for not being enrolled include consent refusal, being regularly attended in another clinic, and foreseen short-term attendance to the CDCI. To the end of 2016, 12 185 PLWHIV have been seen at the CDCI; 9218 (76%) of whom have been enrolled into KIULARCO and 6965 (76%) of these have received ART from the clinic (fig. 2).
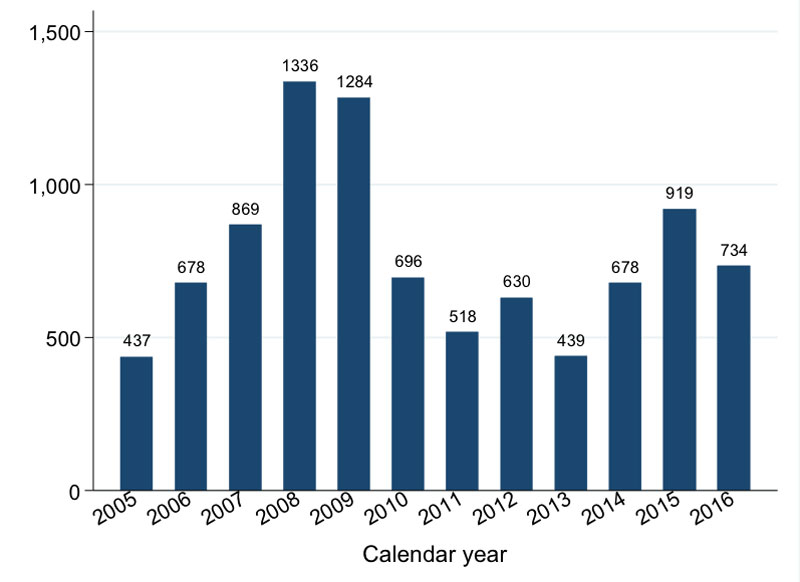
Figure 2 Number of patients enrolled per year in KIULARCO between 2005 and 2016.
The main characteristics of the adult and child populations at enrolment into the cohort are summarised in table 1.
Table 1 Summary of baseline characteristics of participants enrolled in KIULARCO from 2005 to 2016.
|
Child
(<15 years old) |
Adult
(≥15 years old) |
Total 1 | |
|---|---|---|---|
| No. | 813 (100%) | 8376 (100%) | 9218 (100%) |
| Age, years | 5 (2–8) | 38 (31–45) | 36 (29–44) |
| Sex, female 2 | 390 (48%) | 5395 (65%) | 5788 (63%) |
| Distance from home to clinic, km 3 | 25 (1–70) | 5 (1–51) | 5 (1–51) |
| Marital status, married/cohabiting 4 | – | 4466 (54%) | – |
| Education, none/primary school only 5 | – | 4444 (94%) | – |
| Pregnant 6 | – | 306 (8%) | – |
| Partner status 7 | |||
| Positive | – | 1125 (14%) | – |
| Negative | – | 586 (7%) | – |
| Not tested | – | 2395 (30%) | – |
| Unknown | – | 2888 (37%) | – |
| Not applicable | – | 861 (11%) | – |
| Disclosure of HIV status, yes 8 | – | 4668 (69%) | – |
| Time from HIV diagnosis to enrolment, days 9 | 7 (1–43) | 8 (1–49) | 8 (1–48) |
| BMI, kg/m2 10 | |||
| Underweight (<18.5) | – | 1746 (25%) | – |
| Normal (18.5–<25) | – | 4155 (60%) | – |
| Overweight (25–<30) | – | 834 (12%) | – |
| Obese (≥30) | – | 241 (3%) | – |
| WHO stage 11 | |||
| 1 | 233 (30%) | 3021 (38%) | 3261 (38%) |
| 2 | 154 (20%) | 1609 (20%) | 1770 (20%) |
| 3 | 248 (32%) | 2186 (28%) | 2439 (28%) |
| 4 | 138 (18%) | 1067 (14%) | 1210 (14%) |
| CD4 count, cells/mm3 12 | |||
| <50 | 42 (19%) | 745 (15%) | – |
| 50–99 | 14 (6%) | 609 (12%) | – |
| 100–199 | 23 (10%) | 1001 (20%) | – |
| 200–349 | 47 (21%) | 1107 (22%) | – |
| 350-499 | 24 (11%) | 715 (14%) | – |
| ≥500 | 77 (34%) | 873 (17%) | – |
| CD4% <25% 13 | 193 (67%) | – | – |
Results are number (column percentage of those with non-missing data) for categorical variables and median (interquartile range) for continuous variables. 1 Including 29 patients with unknown age who are not included in the child/adult columns (and are excluded from the age row). 2 Missing for 1 child, 26 adults, and 53 persons overall. 3 Missing for 92 children, 965 adults, and 1082 persons overall. 4 Missing for 181 adults. 5 Missing for 3634 adults. 6 Percentage is of women; missing for 1608 women. 7 Missing for 521 adults. 8 Missing for 1594 adults. 9 Missing for 51 children, 426 adults, and 505 persons overall. 10 Missing for 1400 adults. 11 As measured at first clinical visit which may be some time after registration, but 74% of participants had registration and clinical visit on same day. Missing for 40 children, 493 adults, and 538 persons overall. 12 Results for children are among the 380 children aged ≥5 years at registration. Missing for 153 children aged ≥5 years at registration and 3326 adults. 13 Results are among the 433 children aged <5 years at registration. Missing for 146 children aged <5 years at registration.
Before 2013, the data collected was limited to the parameters recommended by the Ministry of Health and Social Welfare of Tanzania for monitoring of the HIV programme. Records were kept on paper and entered into an electronic database. Examples of the early use of KIULARCO data were studies on voluntary counselling and testing [23] and ART outcomes [24]. In December 2012, an optimised data collection system was implemented, allowing for the progressive transition to a paperless clinic, effective as of June 2013. This system serves clinical data documentation and harmonisation of the data collection among CDCI clinicians, and provides the basis for analysis and a better understanding of different aspects of the daily clinical and operational challenges. The CDCI, the pharmacy and the laboratory are interconnected through this system, which has improved the efficiency of the patients’ circuits, reducing the waiting times and increasing the patients’ satisfaction. Real-time data at the time of the patient encounter is captured through a specifically designed interface based on Open Medical Record System (www.openmrs.org) to meet both clinical and research needs. Table 2 summarises the variables routinely collected in KIULARCO since 2013.
Table 2 Summary of the variables collected in KIULARCO.
| Data fields | Variable list |
|---|---|
| Sociodemographic data | Name, national ID number, gender, address, contact details, date of birth, marital status, number of children, occupation, education, date of HIV diagnosis, where referred from, and informed consent. |
| Triage data | Weight, height, blood pressure, heart rate, respiratory rate, oxygen saturation, temperature, and middle upper arm circumference, and head circumference in children |
| Clinical visit data | Mode of HIV transmission, HIV status of partner, disclosure, pregnancy, contraception, ART exposure, adherence, past medical history, alcohol use, smoking history, drug allergies, actual complaints, physical examination, ICD-10 coded clinical diagnosis, WHO stage, tuberculosis screening, immune reconstitution inflammatory syndrome, drug toxicity, treatment failure, ART prescription, destination. |
| Laboratory data | Date and time of blood collection Laboratory tests requested by the clinician Baseline and monitoring results, including CD4, full blood counts, liver and renal function tests, Cryptococcus, tuberculosis, hepatitis, syphilis, malaria and other relevant results. Localisation of the cryopreserved plasma sample Usage of the cryopreserved sample and amount left, project, investigator and date |
| Medication | ART, tuberculosis treatment, and prophylaxis regimens prescribed by the clinician and collected by the patient, date of initiation, modification or stop, reasons for treatment switching or discontinuation, pill counting |
| Outcome | Reason for stopping participation in the cohort, information on transfer to another clinic, ICD-10 coded cause of death, place of death. |
ART = antiretroviral treatment; HIV = human immunodeficiency virus; ICD-10 = International classification of Diseases, 10th edition; WHO = World Health Organization
The flow of patients at the CDCI is designed to accelerate ART initiation and to minimise losses to follow up between HIV diagnosis and treatment, which is one of the main factors affecting attrition in SSA [25]. On the day of HIV diagnosis, after post-test counselling, patients are registered at the CDCI and referred to the laboratory, where blood is withdrawn for the assessment of the baseline laboratory parameters. The baseline clinical visit is scheduled for the next working day, so the clinician has all laboratory results available. During the baseline clinical visit, a thorough medical history and physical examination are recorded (table 2). Full blood counts, liver and renal function tests, as well as screening results for opportunistic infections that may condition ART timing (mainly tuberculosis and cryptococcosis) are evaluated by the clinician. If criteria for ART initiation are met, patients receive three counselling sessions, together with a next of kin to serve as a treatment supporter, with the aim of starting ART within a week. With the recent implementation of the test and treat strategy, the first counselling visit occurs on the same day and two more sessions are provided after the start of treatment. Further clinical visits for treatment response and possible drug toxicity are scheduled at 2 weeks and 3 months after ART initiation, and every 6 months thereafter. In addition, patients are encouraged to attend the clinic in the event of any problem.
The CDCI was initially built to support the Tanzanian national AIDS control programme. Since 2013, the activities at the CDCI have been progressively expanded to take care of all out- and inpatients with HIV attended at the Saint Francis Referral Hospital, including pregnant women, adolescents, children, and HIV-exposed and HIV-infected infants. Also, the CDCI participates in the supervision and training of national and international medical students and residents and the staff of other HIV care and treatment centres in the Kilombero district, and in delivering educational activities to the community, such as radio broadcasts.
Key features of the CDCI are: (i) integration of services; (ii) paperless functioning; (iii) task shifting; and (iv) decentralisation of services.
In order to maximise the bidirectional screening and treatment of these two often fatally associated diseases (HIV and tuberculosis), in 2013 the tuberculosis clinic of Saint Francis Referral Hospital was integrated into the CDCI, which now provides outpatient and inpatient tuberculosis care. The integration of services has resulted in improved diagnostics (e.g., systematic application of WHO screening questions, improvement of procedures to provide qualitatively good sputum, and implementation of Xpert MTB/RIF for the rapid diagnosis of tuberculosis, and of sonography to detect signs of extrapulmonary tuberculosis). With this, an improvement of the ascertainment of tuberculosis cases within the clinic was noted (fig. 3), with no evidence of any change in the true prevalence in the population.
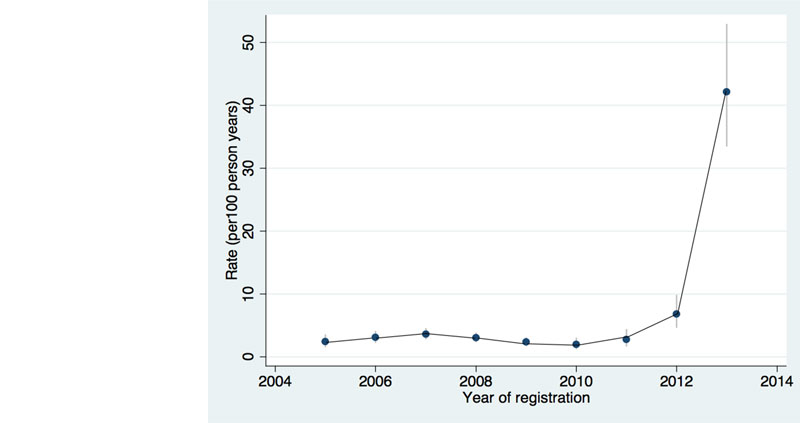
Figure 3 Incidence rate of tuberculosis ascertainment before and after the integration of HIV and tuberculosis services in 2013 (adapted from [26]).
A maternal and paediatric unit of the CDCI was created and integrated within the Reproductive and Child health Clinic of SFRH in 2013 with the following objectives: (i) to improve linkage to care of HIV-infected pregnant women; (ii) to increase uptake of the guidelines on prevention of mother-to-child transmission of HIV and early infant diagnosis; and (iii) to improve clinical outcomes of pregnant women, HIV-exposed and HIV-infected children (fig. 4) [27].
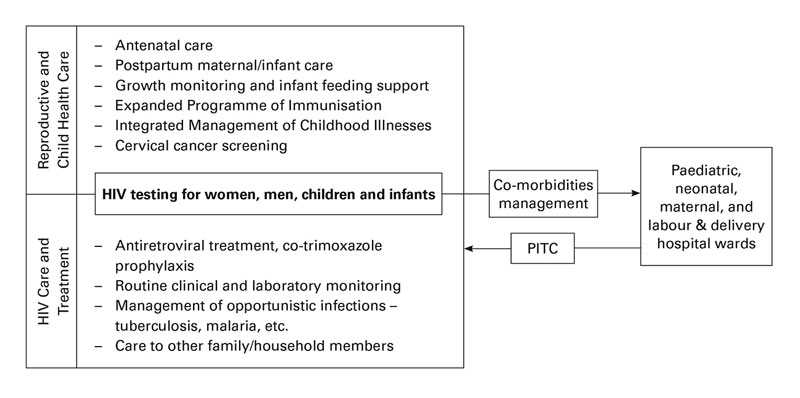
Figure 4 The One Stop Clinic of Ifakara integrates health services for HIV-infected children, pregnant women, HIV-exposed infants and their families (adapted from [27]).
HIV = human immunodeficiency virus; PITC = Provider Initiated Testing and Counselling
Since 2014, all patients admitted to the Saint Francis Referral Hospital wards without a prior HIV test are offered HIV provider-initiated testing and counselling. Medical doctors from the CDCI are fully responsible for inpatient care of those identified as HIV positive, and invite patients to enrol in KIULARCO. The same electronic data capture methods as those in the main clinic are used by CDCI staff in the wards through portable electronic devices, in order to extend the clinical benefits of this system to admitted patients and not to miss relevant information during admission (fig. 5).

Figure 5 Data capture at the medical wards. © Francesco Marzoli.
Since 2013, all patients visiting the outpatient CDCI are first seen by a registered nurse, who measures vital signs and checks for symptoms. Asymptomatic patients with no scheduled clinical visit with the medical doctor are sent directly to the pharmacy to collect refills of any current prescriptions, which are given for three months if good adherence can be ensured, until the next scheduled laboratory monitoring and clinical visit. This task-shifting from doctors to nurses has resulted in a decrease in the workload of clinicians, who are now able to focus on sicker patients. During visits with CD4 and viral load monitoring, medical doctors see patients and communicate results to the patients. To cope with increasing numbers of patients on ART without compromising efficacy, a rethinking of this system to further capacitate nurses to initiate ART and to see stable patients on ART during their monitoring visits will be needed.
The CDCI supervises peripheral HIV care and treatment centres in the Kilombero District, offering regular training and a referral system for sick patients and medical conditions that cannot be managed at peripheral health centres. In addition, a phone line has been established with the One Stop Clinic of Ifakara that offers clinical consultations by phone regarding pregnant women and children living with HIV. Further decentralisation of services for the management of stable patients will be needed to cope with the expected increase in workload resulting from the adoption of the test and treat strategy.
The CDCI provides basic laboratory services for the diagnosis and monitoring of HIV infection and comorbidities. This includes phlebotomy services, HIV rapid tests, HIV pro-viral DNA polymerase chain-reaction tests for early infant diagnosis of HIV, complete blood count, liver and renal function tests, CD4 counts, plasma HIV viral load, HIV genotyping, Xpert Mycobacterium tuberculosis / resistance to rifampicin (MTB/RIF) tests for the diagnosis of tuberculosis, cryptococcal antigen lateral flow assay, hepatitis B surface antigen, malaria blood slide and rapid test, VDRL for syphilis diagnostics, analysis of cerebrospinal and other sterile fluids originating from punctures, urine and stool analysis. All test results are entered into the electronic data collection system and can be consulted by the clinicians of the CDCI on the same day.
Research using KIULARCO data spans several relevant areas. Some examples follow.
A recent prospective study aiming to improve ART adherence identified high levels of viral suppression (91%) and adherence (over 90%) measured both by self-reported adherence and by plasma therapeutic drug monitoring among 300 adults on ART for more than 6 months [28]. Analysis of acquisition of HIV drug resistance mutations among this same population identified a 5.6% prevalence of resistance mutations [29]. In contrast, the proportion of children failing ART was 25%, and the majority harboured HIV drug-resistance mutations, with multiclass resistances in 79% of failing patients [30]. This study highlights the vulnerability of the paediatric population to treatment failure and resistance development, and the urgent need for universal viral load monitoring.
A study assessing the impact of the recent developments of the CDCI in tuberculosis ascertainment found that the integration of HIV and tuberculosis services, together with the electronic data collection and reporting system and the availability of Xpert MTB/RIF for diagnosis, resulted in a 15-fold increase in the ascertainment of tuberculosis among adults living with HIV enrolled in the cohort [26] (fig. 3). This reinforces the need for the integration of services at a national level. Studies on cryptococcosis showed a prevalence of 4.4% among adults with CD4 count below 100 cells/mm3 when cryopreserved samples were retrospectively analysed, and being cryptococcal antigen (CrAg) positive was an independent predictor of mortality [31]. This study was one of the first showing a potential survival benefit of treating cryptococcaemia with fluconazole. A subsequent prospective study conducted in KIULARCO showed a higher CrAg prevalence among inpatient adults and a reduction of mortality among CrAg-positive patients without meningitis as a result of the implementation of CrAg screening followed by fluconazole treatment [32]. A pilot CrAg screening strategy has been tested at district level [33], and a novel treatment strategy for cryptococcal meningitis tailored to rural SSA was successfully evaluated in an open-label clinical trial [34].
A study aiming to assess the burden of hypertension among KIULARCO participants aged >15 years showed a 12% prevalence at enrolment in the cohort, and an additional 10% developed hypertension during the first 6 months on ART. This study highlights the urgent need for integrating HIV and noncommunicable disease care [35]. Studies assessing the burden of liver disease identified a 9% prevalence of co-infection with hepatitis B (HBV) among adults as well as a good correlation between the HBV surface antigen rapid test and the enzyme-linked immunosorbent assay (ELISA) [36]. A subsequent study identified a high prevalence of advanced liver fibrosis and cirrhosis before ART initiation assessed through the aspartate transaminase (AST) to platelets ratio index (APRI) in the general HIV adult population, and especially among HIV-HBV co-infected individuals [37]. No cases of hepatitis delta were identified in our cohort [38].
A situation analysis in 2012 assessing the implementation of prevention of mother-to-child transmission of HIV under programmatic circumstances identified several gaps to be addressed [39]. The identification of these gaps led to the development of the One Stop Clinic of Ifakara, an integrated service delivery model of maternal and paediatric HIV services (fig. 4). This strategy resulted in an increased number of mothers and children diagnosed and linked into care, a higher detection of children with AIDS, universal treatment coverage, lower loss to follow-up, and an early mother-to-child transmission rate below the threshold of elimination of 5%. This study documents a feasible, effective and scalable model for family-centred HIV care in SSA [27].
A study describing the status of the cohort over the decade since its initiation found, in nearly 8000 individuals, 5-year mortality rates of 10 and 12% among adults and children, respectively, and corresponding lost to follow up rates of 44 and 40% [40]. These results are consistent with the national data [18] and reports from other cohorts [41]. On-going work investigates factors associated with attrition, using tracing information to correct mortality estimates for the unseen deaths among those lost to follow up.
Current and future KIULARCO projects focus on persisting challenges, such as the high rate of attrition mentioned above, the characterisation of HIV drug resistance to second-line ART, and the study of advanced HIV disease and comorbidities. KIULARCO also serves as a platform for prospective studies on several relevant topics, including the rollout of early HIV infant diagnosis in the district, ART adherence among adolescents living with HIV, acceptability and tolerance of new paediatric ART formulations, improvement of tuberculosis diagnostics, and early detection and management of cryptococcal infection. Finally, projects to up-build the test and treat strategy in the community, and linkage and retention in care are planned.
KIULARCO has several strengths that make it a valuable research asset while contributing to the strengthening of clinical care and building local capacity to deal with the challenges posed by the HIV/AIDS pandemic in rural SSA. The most important strength is the in-built managed care for patients with a research-based feedback system. The quality of clinical care, including diagnostic, treatment and laboratory aspects, are constantly analysed, enabling regular feedback to the team of the CDCI. In particular, the feedback concerning loss to follow up, ART adherence, HIV drug resistances, and opportunistic infections and comorbidities is essential to increase the quality of care. In addition, data gathered by the studies conducted within KIULARCO are used to refine the care and treatment protocols in place. Further strengths are the prospective character of the cohort study, the comprehensiveness of the data collected using standardised electronic questionnaires and the high quality of real-time patient-level data. This system allows critical questions to be answered prospectively under programmatic circumstances. Moreover, the flexibility of the data collection system allows new data collection modules to be added for substudies of interest. Remarkably, KIULARCO is the largest and most comprehensive single-site cohort in Tanzania, and among the largest in rural sub-Saharan Africa. Having large volume patient-level comprehensive clinical data is key to answering relevant questions that may be more difficult to solve through pooled data sets, which are also limited by heterogeneity between the different cohorts included. Another strength is the availability of a plasma biobank, which allows analysis of temporal trends or testing of samples retrospectively in the laboratory. Finally, this is an open, ongoing cohort, and enrolment of patients continues, making KIULARCO an invaluable platform to implement evidence-based interventions and to evaluate their impact on clinical outcomes prospectively, as well as to test new interventions through randomised clinical trials.
KIULARCO also has weaknesses. As a result of the low availability of diagnostic tools, some conditions are under-reported and need to be interpreted and analysed with caution. Also, because of the high mobility of our population, there is a high rate of silent transfer of patients to other clinics, as well as loss to follow up [40]. New systems of active follow up, decentralisation of services and community involvement will need to be implemented to cope with this important challenge that threatens the global control of the HIV epidemic.
In conclusion, KIULARCO is a dynamic and very valuable prospective observational cohort study that serves clinical and translational research and, importantly, contributes to improving the clinical setup and the wellbeing of PLWHIV attending the CDCI. In our view, the strengths of KIULARCO largely outweigh its weaknesses, and we believe that KIULARCO may serve as a model for strengthening clinical care in similar settings in SSA. Finally, in addition to improving clinical care and generating relevant information, such integrated models adapted to the loco-regional needs offer career opportunities for healthcare personnel and thus help overcoming the human resource crisis in healthcare in rural SSA.
National and international collaborations, interests and enquiries relating to KIULARCO are encouraged, and can be addressed to Prof. Christoph Hatz (Christoph.Hatz@unibas.ch), Prof. Manuel Battegay (Manuel.Battegay@usb.ch) and Mr Frederick Masanja (fmasanja@ihi.or.tz). Only relevant research questions in line with the objectives of KIULARCO will be considered. Letters of intent (LOI) are evaluated every two months by the KIULARCO scientific committee, and researchers of approved LOIs are invited to develop full proposals. Successful applicants are given access to the relevant KIULARCO data for the project by the data managers once a data sharing agreement is signed. All participating institutions who conduct analyses on the data should seek their own ethics approval at the relevant Tanzanian and international boards, using de-identified data.
Further information and contact details about the Chronic Diseases of Ifakara and the KIULARCO cohort can be found at https://www.swisstph.ch/de/projects/tanzania-chronic-disease-clinic-ifakara/ and http://ihi.or.tz/chronic-diseases-care-treatment-centre/.
We are grateful to all KIULARCO participants and staff at Saint Francis Referral Hospital. We are also grateful to Dr Marcel Stöckle who initiated KIULARCO, and to Dr Erik Mossdorf, Dr Lars Henning and other key collaborators who were involved during the history of the cohort.
The members of the KIULARCO Study Group are: Aschola Asantiel, Adolphina Chale, Diana Faini, Ingrid Felger, Gideon Francis, Hansjakob Furrer, Anna Gamell, Tracy Glass, Christoph Hatz, Speciosa Hwaya, Aneth Kalinjuma, Bryson Kasuga, Andrew Katende, Namvua Kimera, Yassin Kisunga, Thomas Klimkait, Emilio Letang, Antonia Luhombero, Ezekiel Luoga, Lameck Luwanda, Herry Mapesi, Leticia Mbwile, Mengi Mkulila, Julius Mkumbo, Margareth Mkusa, Dorcas K Mnzava, Getrud Mollel, Lilian Moshi, Germana Mossad, Dolores Mpundunga, Daimon Msami, Athumani Mtandanguo, Selerine Myeya, Sanula Nahota, Regina Ndaki, Robert Christopher Ndege, Agatha Ngulukila, Leila Samson, George Sikalengo, Marcel Tanner, Fiona Vanobberghen, and Maja Weisser.
The CDCI receives funding from the Ministry of Health and Social Welfare of the Government of Tanzania; the Government of the Canton of Basel, Switzerland; the Swiss Tropical and Public Health Institute, Basel, Switzerland; the Ifakara Health Institute, Tanzania; and TUNAJALI and, recently, USAID Boresha Afya, two Tanzanian NGOs supporting ART roll-out throughout Tanzania with funding through the United States Agency for International Development (USAID) from the President's Emergency Plan for AIDS Relief (PEPFAR) programme.
The authors certify that they have no affiliations with or involvement in any organisation or entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript.
1 World Health Organization. Global Health Observatory Data [Internet]. [cited 2017 May 3]. Available from: http://www.who.int/gho/hiv/en/
2 UNAIDS. AIDS by the numbers: AIDS is not over, but it can be. 2016 Nov. Available from: http://www.unaids.org/en/resources/documents/2016/AIDS-by-the-numbers
3 Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002-2013: a meta-analysis. Clin Infect Dis. 2015;60(7):1120–7. PubMed
4 Takuva S, Brown AE, Pillay Y, Delpech V, Puren AJ. The continuum of HIV care in South Africa: implications for achieving the second and third UNAIDS 90-90-90 targets. AIDS. 2017;31(4):545–52. doi:https://doi.org/10.1097/QAD.0000000000001340. PubMed https://doi.org/10.1097/QAD.0000000000001340
5 MacPherson P, Lalloo DG, Webb EL, Maheswaran H, Choko AT, Makombe SD, et al. Effect of optional home initiation of HIV care following HIV self-testing on antiretroviral therapy initiation among adults in Malawi: a randomized clinical trial. JAMA. 2014;312(4):372–9. doi:https://doi.org/10.1001/jama.2014.6493. PubMed https://doi.org/10.1001/jama.2014.6493
6 Inzaule SC, Ondoa P, Peter T, Mugyenyi PN, Stevens WS, de Wit TF, et al. Affordable HIV drug-resistance testing for monitoring of antiretroviral therapy in sub-Saharan Africa. Lancet Infect Dis. 2016;16(11):e267–75. doi:https://doi.org/10.1016/S1473-3099(16)30118-9. PubMed https://doi.org/10.1016/S1473-3099(16)30118-9
7 Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8(7):e1001056. doi:https://doi.org/10.1371/journal.pmed.1001056. PubMed https://doi.org/10.1371/journal.pmed.1001056
8 UNAIDS. AIDSinfo [Internet]. [cited 2017 May 3]. Available from: http://aidsinfo.unaids.org
9. Tanzanian Commission for AIDS (TACAIDS). United Republic of Tanzania. Global AIDS Response Country Progress Report. 2014.
10 Houlihan CF, Bland RM, Mutevedzi PC, Lessells RJ, Ndirangu J, Thulare H, et al. Cohort profile: Hlabisa HIV treatment and care programme. Int J Epidemiol. 2011;40(2):318–26. doi:https://doi.org/10.1093/ije/dyp402. PubMed https://doi.org/10.1093/ije/dyp402
11 Hamers RL, Oyomopito R, Kityo C, Phanuphak P, Siwale M, Sungkanuparph S, et al.; PharmAccess African PASER and TREAT Asia Studies to Evaluate Resistance. Cohort profile: The PharmAccess African (PASER-M) and the TREAT Asia (TASER-M) monitoring studies to evaluate resistance--HIV drug resistance in sub-Saharan Africa and the Asia-Pacific. Int J Epidemiol. 2012;41(1):43–54. doi:https://doi.org/10.1093/ije/dyq192. PubMed https://doi.org/10.1093/ije/dyq192
12 Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, et al. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2012;41(5):1256–64. doi:https://doi.org/10.1093/ije/dyr080. PubMed https://doi.org/10.1093/ije/dyr080
13 Funk A, Kanters S, Nansubuga M, Mwehire D, Featherstone A, Druyts E, et al. Cohort profile: the MUg Observational Cohort. Int J Epidemiol. 2012;41(6):1594–1594f. doi:https://doi.org/10.1093/ije/dys170. PubMed https://doi.org/10.1093/ije/dys170
14 Fox MP, Maskew M, MacPhail AP, Long L, Brennan AT, Westreich D, et al. Cohort profile: the Themba Lethu Clinical Cohort, Johannesburg, South Africa. Int J Epidemiol. 2013;42(2):430–9. doi:https://doi.org/10.1093/ije/dys029. PubMed https://doi.org/10.1093/ije/dys029
15 Jespersen S, Hønge BL, Oliveira I, Medina C, da Silva Té D, Correira FG, et al.; Bissau HIV Cohort study group. Cohort Profile: The Bissau HIV Cohort-a cohort of HIV-1, HIV-2 and co-infected patients. Int J Epidemiol. 2015;44(3):756–63. doi:https://doi.org/10.1093/ije/dyu201. PubMed https://doi.org/10.1093/ije/dyu201
16 Chetty T, Thorne C, Tanser F, Bärnighausen T, Coutsoudis A. Cohort profile: the Hlabisa pregnancy cohort, KwaZulu-Natal, South Africa. BMJ Open. 2016;6(10):e012088. doi:https://doi.org/10.1136/bmjopen-2016-012088. PubMed https://doi.org/10.1136/bmjopen-2016-012088
17 Somi G, Keogh SC, Todd J, Kilama B, Wringe A, van den Hombergh J, et al. Low mortality risk but high loss to follow-up among patients in the Tanzanian national HIV care and treatment programme. Trop Med Int Health. 2012;17(4):497–506. doi:https://doi.org/10.1111/j.1365-3156.2011.02952.x. PubMed https://doi.org/10.1111/j.1365-3156.2011.02952.x
18 National AIDS Control Program. (NACP, Ministry of Health and Social Welfare). Implementation of HIV/AIDS care and treatment services in Tanzania - report number 3 [Internet]. 2013 [cited 2017 May 3]. Available from: http://www.nacp.go.tz/site/publications/epidemiology-and-research-coordination
19 Kilama B. Five Years (2004-2009) Antiretroviral Treatment for HIVAIDS in Tanzania: HIV Outcomes and TB Events. In Rome, Italy; 2011.
20 Vanobberghen FM, Kilama B, Wringe A, Ramadhani A, Zaba B, Mmbando D, et al. Immunological failure of first-line and switch to second-line antiretroviral therapy among HIV-infected persons in Tanzania: analysis of routinely collected national data. Trop Med Int Health. 2015;20(7):880–92. doi:https://doi.org/10.1111/tmi.12507. PubMed https://doi.org/10.1111/tmi.12507
21 Tanzanian National Bureau of Statistics. Morogoro region. Basic demographic and socio-economic profile. [Internet]. 2016 [cited 2017 May 3]. Available from: http://www.nbs.go.tz/nbstz/index.php/english/statistics-by-subject/population-and-housing-census/697-2012-phc-regional-profiles
22 Geubbels E, Amri S, Levira F, Schellenberg J, Masanja H, Nathan R. Health & Demographic Surveillance System Profile: The Ifakara Rural and Urban Health and Demographic Surveillance System (Ifakara HDSS). Int J Epidemiol. 2015;44(3):848–61. doi:https://doi.org/10.1093/ije/dyv068. PubMed https://doi.org/10.1093/ije/dyv068
23 Mossdorf E, Stoeckle M, Vincenz A, Mwaigomole EG, Chiweka E, Kibatala P, et al. Impact of a national HIV voluntary counselling and testing (VCT) campaign on VCT in a rural hospital in Tanzania. Trop Med Int Health. 2010;15(5):567–73. doi:https://doi.org/10.1111/j.1365-3156.2010.02490.x. PubMed https://doi.org/10.1111/j.1365-3156.2010.02490.x
24 Mossdorf E, Stoeckle M, Mwaigomole EG, Chiweka E, Kibatala PL, Geubbels E, et al. Improved antiretroviral treatment outcome in a rural African setting is associated with cART initiation at higher CD4 cell counts and better general health condition. BMC Infect Dis. 2011;11(1):98–9. doi:https://doi.org/10.1186/1471-2334-11-98. PubMed https://doi.org/10.1186/1471-2334-11-98
25 Clouse K, Pettifor AE, Maskew M, Bassett J, Van Rie A, Behets F, et al. Patient retention from HIV diagnosis through one year on antiretroviral therapy at a primary health care clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2013;62(2):e39–46. doi:https://doi.org/10.1097/QAI.0b013e318273ac48. PubMed https://doi.org/10.1097/QAI.0b013e318273ac48
26 Haraka F, Glass TR, Sikalengo G, Gamell A, Ntamatungiro A, Hatz C, et al. A Bundle of Services Increased Ascertainment of Tuberculosis among HIV-Infected Individuals Enrolled in a HIV Cohort in Rural Sub-Saharan Africa. PLoS One. 2015;10(4):e0123275. doi:https://doi.org/10.1371/journal.pone.0123275. PubMed. Correction in: PLoS ONE. 2015;10(5):e0129185. https://doi.org/10.1371/journal.pone.0129185https://doi.org/10.1371/journal.pone.0123275
27 Gamell A, Glass TR, Luwanda LB, Mapesi H, Samson L, Mtoi T, et al.; KIULARCO Study Group. Implementation and Operational Research: An Integrated and Comprehensive Service Delivery Model to Improve Pediatric and Maternal HIV Care in Rural Africa. J Acquir Immune Defic Syndr. 2016;73(5):e67–75. doi:https://doi.org/10.1097/QAI.0000000000001178. PubMed https://doi.org/10.1097/QAI.0000000000001178
28 Erb S, Letang E, Glass TR, Natamatungiro A, Mnzava D, Mapesi H, et al.; Kilombero Ulanga Antiretroviral Cohort (KIULARCO) study group. Health care provider communication training in rural Tanzania empowers HIV-infected patients on antiretroviral therapy to discuss adherence problems. HIV Med. 2017;Mar 15.[Epub ahead of print] PubMed
29 Ntamatungiro AJ, Muri L, Glass TR, Erb S, Battegay M, Furrer H, et al.; KIULARCO Study Group. Strengthening HIV therapy and care in rural Tanzania affects rates of viral suppression. J Antimicrob Chemother. 2017;Apr 6. [Epub ahead of print] PubMed
30 Muri L, Gamell A, Ntamatungiro AJ, Glass TR, Luwanda LB, Battegay M, et al.; KIULARCO Study Group. Development of HIV drug resistance and therapeutic failure in children and adolescents in rural Tanzania: an emerging public health concern. AIDS. 2017;31(1):61–70. doi:https://doi.org/10.1097/QAD.0000000000001273. PubMed https://doi.org/10.1097/QAD.0000000000001273
31 Letang E, Müller MC, Ntamatungiro AJ, Kimera N, Faini D, Furrer H, et al. Cryptococcal Antigenemia in Immunocompromised Human Immunodeficiency Virus Patients in Rural Tanzania: A Preventable Cause of Early Mortality. Open Forum Infect Dis. 2015;2(2):ofv046–046. doi:https://doi.org/10.1093/ofid/ofv046. PubMed https://doi.org/10.1093/ofid/ofv046
32 Faini D, Kalinjuma A, Katende A, Mbwanji G, Mnzava D, Nyuri A, et al. Maximizing Detection and Improving Outcomes of Cryptococcosis in Rural Tanzania. In Boston, United States; 2016.
33 Mbwanji G, Faini D, Nyuri A, Katende A, Kalinjuma AV, Mnzava D, et al. Implementing CRAG Screening in HIV Patients Initiating ART in Rural HIV Clinics with Regular Absence of CD4 Testing Services in Rural Tanzania. In Paris, France; 2017.
34 Katende A, Mbwanji G, Faini D, Nyuri A, Kalinjuma AV, Mnzava D, et al. Sertraline and high dose fluconazole treatment of HIV-associated cryptococcal meningitis in Tanzania. In Seattle, United States; 2017.
35 Rodríguez-Arbolí E, Mwamelo K, Kalinjuma AV, Furrer H, Hatz C, Tanner M, et al.; KIULARCO Study Group. Incidence and risk factors for hypertension among HIV patients in rural Tanzania - A prospective cohort study. PLoS One. 2017;12(3):e0172089–14. doi:https://doi.org/10.1371/journal.pone.0172089. PubMed https://doi.org/10.1371/journal.pone.0172089
36 Franzeck FC, Ngwale R, Msongole B, Hamisi M, Abdul O, Henning L, et al. Viral hepatitis and rapid diagnostic test based screening for HBsAg in HIV-infected patients in rural Tanzania. PLoS One. 2013;8(3):e58468. doi:https://doi.org/10.1371/journal.pone.0058468. PubMed https://doi.org/10.1371/journal.pone.0058468
37 Ramírez-Mena A, Glass TR, Winter A, Kimera N, Ntamatungiro A, Hatz C, et al. Prevalence and Outcomes of Hepatitis B Coinfection and Associated Liver Disease Among Antiretroviral Therapy-Naive Individuals in a Rural Tanzanian Human Immunodeficiency Virus Cohort. Open Forum Infect Dis. 2016;3(3):ofw162. doi:https://doi.org/10.1093/ofid/ofw162. PubMed https://doi.org/10.1093/ofid/ofw162
38 Winter A, Letang E, Vedastus Kalinjuma A, Kimera N, Ntamatungiro A, Glass T, et al.; KIULARCO Study Group. Absence of hepatitis delta infection in a large rural HIV cohort in Tanzania. Int J Infect Dis. 2016;46:8–10. doi:https://doi.org/10.1016/j.ijid.2016.03.011. PubMed https://doi.org/10.1016/j.ijid.2016.03.011
39 Gamell A, Letang E, Jullu B, Mwaigomole G, Nyamtema A, Hatz C, et al. Uptake of guidelines on prevention of mother-to-child transmission of HIV in rural Tanzania: time for change. Swiss Med Wkly. 2013;143:w13775. PubMed
40 Vanobberghen F, Letang E, Gamell A, Mnzava D, Faini D, Luwanda L, et al. A decade of HIV care in rural Tanzania: trends in clinical outcomes and impact of clinic optimization in an open, prospective cohort. PLoS One. 2017; (in production).
41 Brinkhof MWG, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790. doi:https://doi.org/10.1371/journal.pone.0005790. PubMed https://doi.org/10.1371/journal.pone.0005790
The CDCI receives funding from the Ministry of Health and Social Welfare of the Government of Tanzania; the Government of the Canton of Basel, Switzerland; the Swiss Tropical and Public Health Institute, Basel, Switzerland; the Ifakara Health Institute, Tanzania; and TUNAJALI and, recently, USAID Boresha Afya, two Tanzanian NGOs supporting ART roll-out throughout Tanzania with funding through the United States Agency for International Development (USAID) from the President's Emergency Plan for AIDS Relief (PEPFAR) programme.
The authors certify that they have no affiliations with or involvement in any organisation or entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript.