Minimally invasive extracorporeal circulation: excellent outcome and life expectancy after coronary artery bypass grafting surgery
DOI: https://doi.org/10.4414/smw.2017.14474
Bernhard
Winklera, Paul Philipp
Heinischa, Grzegorz
Zuka, Katarzyna
Zuka, Brigitta
Gahla, Hans Jörg
Jennia, Alexander
Kadnera, Christoph
Huberb, Thierry
Carrela
aDepartment of Cardiovascular Surgery, Inselspital,
bDepartment of Cardiac and Vascular Surgery, HUG,
Minimally invasive extracorporeal circulation: excellent outcome and life expectancy after coronary artery bypass grafting surgery
w14474
Summary
OBJECTIVE
Coronary artery bypass grafting (CABG) remains the gold standard for complex revascularisation in multivessel disease. The concept of the minimally invasive extracorporeal circulation circuit (MiECC) was introduced to minimise pathophysiological side effects of conventional extracorporeal circulation. This study presents early and long-term outcomes after CABG with use of MiECC in a single-centre consecutive patient cohort.
METHODS
From 1 January 2005 to 31 December 2010, 2130 patients underwent isolated CABG with MiECC at our centre. We evaluated morbidity and mortality follow-up data with a median follow-up of 3.6 years. Kaplan-Meier curves and estimates of the primary end-point for all-cause mortality were compared with the life expectancy of the general population.
RESULTS
Mortality in CABG patients was comparable to the general population beginning 1 year after surgery for the whole observation period. All-cause 30-day mortality was 0.8%. The mean estimated logistic EuroSCORE and EuroSCORE II were 5.8 ± 8.6 and 3.0 ± 5.1, respectively. Mean perfusion time was 71.1 ± 23.8 min with a cross-clamp time of 44.9 ± 16.3 min. Mortality was predicted by the presence of diabetes mellitus (odds ratio [OR] 1.85, 95% confidence interval [CI] 1.40–2.46; p <0.001), peripheral arterial disease (OR 2.36, 95% CI 1.64–3.38; p <0.001), severe obstructive pulmonary disease (OR 3.21, 1.42–7.24; p = 0.005), chronic renal failure (OR 3.68, 2.49–5.43; p <0.001) and transfusion of more than one unit of erythrocyte concentrate in the perioperative period (OR 1.46, 1.09–1.95; p = 0.015). Cerebrovascular events occurred in 36 patients (1.7%).
CONCLUSION
CABG with use of MiECC is associated with a mortality rate comparable to the overall life expectancy of the general population. MiECC is the first choice for routine and emergency CABG at our centre with a 30-day mortality rate of 0.8% and a low complication rate.
Introduction
Coronary artery bypass grafting (CABG) represents the optimal revascularisation strategy for complex coronary artery disease. This treatment strategy is followed by excellent long-term survival with a lower rate of re-interventions compared with percutaneous interventions, a similar rate of major adverse cardiac and cerebrovascular events (MACCE) and, for the majority of the patients, some clear clinical and economic advantages [1, 2].
The optimal operating environment is facilitated by cardiopulmonary bypass (CPB) with arrested heart and bloodless operating field, which allows the most complete revascularisation even in the presence of very complex anatomy. Drawbacks of this technology include the triggering of a systemic inflammatory response due to the contact of blood with the foreign surfaces, as well as the requirement for priming fluid, which may result in a significant postoperative morbidity. Off-pump coronary artery bypass was one of the few answers to these problems, but was never generally accepted in the cardio-surgical community because of some intraoperative challenges, such as refractory hypotension during manipulations on the heart. Large studies and meta-analyses have not been able to demonstrate a significant benefit of off-pump surgery, but a higher rate of incomplete revascularisation leading to a more frequent rate of re-intervention was observed [3, 4].
The concept of the minimally invasive extracorporeal circulation circuit (MiECC) was introduced to combine the advantages and eliminate disadvantages of both strategies (conventional CPB and off-pump). The standard minimal invasive extracorporeal circuit consists of a centrifugal pump with a membrane oxygen exchanger; short heparin-coated tubing with biologically inert surfaces, heat exchanger, venous de-airing components and shed-blood management system [5, 6]. Developments in this field throughout the last 10 years, as well as growing experience with its use, allowed publication of the first guidelines on usage of MiECC for closed and open cardiac surgery earlier this year [5]. Despite known advantages, the percentage of coronary bypass operations performed with MiECC compared with standard CPB still remains limited [5]. At our institution, we introduced MiECC as early as 2000 and MiECC has been the standard extracorporeal circulation strategy for isolated CABG since 2005. This study summarises the experience of over two thousand operations performed in the first 5 years after implementation of MiECC at our centre, with a report on mortality and morbidity during the follow-up period in comparison with the life expectancy of the general population of Switzerland.
Materials and methods
Patient population
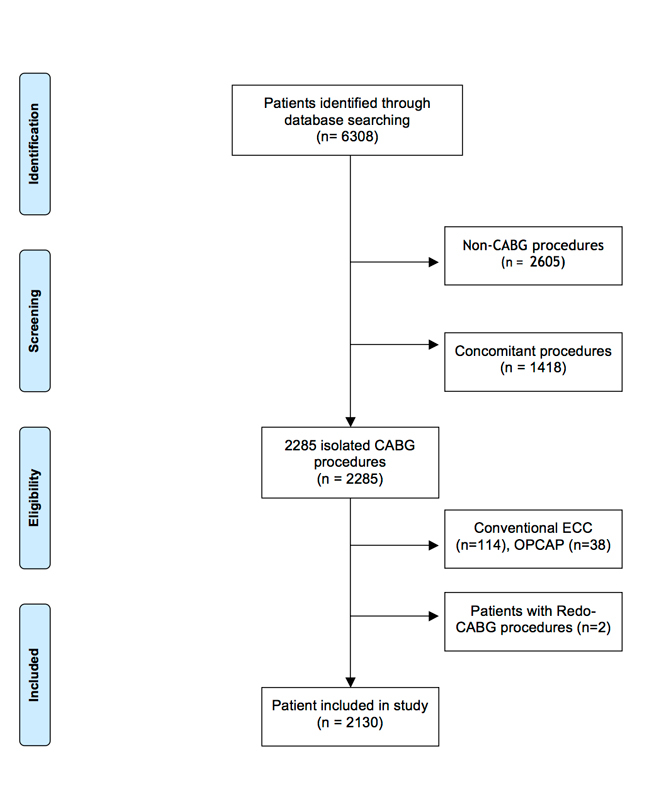
Between 1 January 2005 and 31 December 2010, 2130 patients underwent isolated, elective or emergent CABG with use of MiECC in our institution and were included in the present analysis (fig. 1). To avoid confounding factors, we excluded patients who required any concomitant cardiac or vascular procedures (for instance, carotid endarterectomy, valve surgery, etc.). Patients, or their relatives in the event of emergency, signed, on the day of admission to the hospital and prior to surgery, general informed consent for data collection and regular follow-up for the purpose of medical research. In this study a retrospective observational design was used, conforming to the STROBE checklist [7].
Operational technique and perioperative management
The typical minimally invasive cardiopulmonary circuit consists of a closed circuit, which includes the oxygenator and the pump. The circuit has no open venous reservoir like a conventional extracorporeal circulation circuit (ECC). All components of the MiECC are coated with heparin and the tubing system is significantly reduced in length when compared with the ECC. These characteristics permit a reduction of the priming volume from 1800 to 600–400 ml compared with the standard ECC and, furthermore, reduce negative side effects [3, 8, 9].
The MiECC of Maquet® (Cardiopulmonary AG, Hirrlingen, Germany) with the RotaFlow® centrifugal pump (RotaFlow, Jostra AG) with hydrophobic oxygenation membrane (Quadrox Safeline®, Maquet, Cardiopulmonary AG, Hirrlingen, Germany) were used at that time for the extracorporeal perfusion circuit. The priming volume of this system was 600 ml compared 1800 ml in standard CPB. Single shot (100 ml) crystalloid cardioplegia, as described elsewhere, was used [10]. The perfusion flow during MiECC was set to 2 litres per square meter of body surface area and, if needed, optimised by the perfusionist. Unfractionated heparin (200–300 units/kilogram body weight) was administered per institutional protocol as initial bolus to all patients and tailored to the target of activated clotting time of at least 480 seconds (ACT plus®, Medtronic©).
Data collection
Preoperative data (including gender, age, comorbidities, medication on admission, functional New York Heart Association class (NYHA), Canadian Cardiovascular Society (CCS) score, prior myocardial infarction, systolic left ventricular ejection fraction (LVEF) and perioperative risk (assessed according to the logistic EUROSCORE, additive EUROSCORE and EUROSCORE II), intraoperative data (including the number of grafted vessels, perfusion and cross-clamping time), as well as early postoperative data, were prospectively collected for the purpose of quality management at our institution (table 1).
Table 1 Baseline characteristics of the Bern cohort.
|
All patients
(n = 2130)
|
Grouped by logistic EuroSCORE
|
|
Quintile 1
(n = 433)
|
Quintile 2
(n = 425)
|
Quintile 3
(n = 430)
|
Quintile 4
(n = 419)
|
Quintile 5
(n = 423)
|
p-value
|
| Age |
65.8 (±9.8) |
56.8 (±6.7) |
62.8 (±7.7) |
68.3 (±8.2) |
70.2 (±8.6) |
71.0 (±9.6) |
0.00 |
| Female gender |
445 (20.9%) |
26 (6.0%) |
64 (15.1%) |
97 (22.6%) |
114 (27.2%) |
144 (34.0%) |
0.00 |
| BMI |
27.5 (±4.4) |
28.2 (±4.4) |
27.7 (±4.2) |
27.3 (±4.4) |
27.1 (±4.2) |
27.2 (±4.7) |
0.02 |
| Diabetes |
649 (30.5%) |
120 (27.7%) |
125 (29.4%) |
137 (31.9%) |
116 (27.7%) |
151 (35.7%) |
0.05 |
| Hypertension |
1688 (79.2%) |
328 (75.8%) |
338 (79.5%) |
342 (79.5%) |
339 (80.9%) |
341 (80.6%) |
0.35 |
| Hyperlipidaemia |
1641 (77.0%) |
351 (81.1%) |
338 (79.5%) |
319 (74.2%) |
318 (75.9%) |
315 (74.5%) |
0.05 |
| Smoking |
1269 (59.6%) |
297 (68.6%) |
273 (64.2%) |
253 (58.8%) |
237 (56.6%) |
209 (49.4%) |
0.00 |
| Previous cerebrovascular event |
126 (5.9%) |
10 (2.3%) |
20 (4.7%) |
24 (5.6%) |
37 (8.8%) |
35 (8.3%) |
0.00 |
| Arteriopathy |
356 (16.7%) |
0 (0.0%) |
41 (9.6%) |
61 (14.2%) |
104 (24.8%) |
150 (35.5%) |
0.00 |
| Renal dysfunction* |
123 (5.8%) |
6 (1.4%) |
18 (4.2%) |
21 (4.9%) |
34 (8.1%) |
44 (10.4%) |
0.00 |
| Most recent MI |
|
|
|
|
|
|
0.00 |
| <6 hours |
33 (1.5%) |
0 (0.0%) |
1 (0.2%) |
0 (0.0%) |
1 (0.2%) |
31 (7.3%) |
|
| 6–24 hours |
104 (4.9%) |
0 (0.0%) |
4 (0.9%) |
7 (1.6%) |
18 (4.3%) |
75 (17.7%) |
|
| 1–7 days |
356 (16.7%) |
1 (0.2%) |
48 (11.3%) |
60 (14.0%) |
115 (27.4%) |
132 (31.2%) |
|
| 8–21 days |
131 (6.2%) |
6 (1.4%) |
21 (4.9%) |
22 (5.1%) |
39 (9.3%) |
43 (10.2%) |
|
| 22–90 days |
96 (4.5%) |
1 (0.2%) |
24 (5.6%) |
19 (4.4%) |
29 (6.9%) |
23 (5.4%) |
|
| >90 days |
275 (12.9%) |
80 (18.5%) |
57 (13.4%) |
55 (12.8%) |
45 (10.7%) |
38 (9.0%) |
|
| No MI |
1135 (53.3%) |
345 (79.7%) |
270 (63.5%) |
267 (62.1%) |
172 (41.1%) |
81 (19.1%) |
|
| Previous PCI |
388 (18.2%) |
84 (19.4%) |
92 (21.6%) |
70 (16.3%) |
71 (16.9%) |
71 (16.8%) |
0.21 |
| NYHA class |
|
|
|
|
|
|
0.00 |
| I |
854 (40.1%) |
213 (49.2%) |
206 (48.5%) |
164 (38.1%) |
153 (36.5%) |
118 (27.9%) |
|
| II |
870 (40.8%) |
178 (41.1%) |
175 (41.2%) |
193 (44.9%) |
177 (42.2%) |
147 (34.8%) |
|
| III |
301 (14.1%) |
37 (8.5%) |
37 (8.7%) |
64 (14.9%) |
73 (17.4%) |
90 (21.3%) |
|
| IV |
105 (4.9%) |
5 (1.2%) |
7 (1.6%) |
9 (2.1%) |
16 (3.8%) |
68 (16.1%) |
|
| LVEF |
54.8 (±13.0) |
60.6 (±9.3) |
58.9 (±10.2) |
56.8 (±11.5) |
52.1 (±13.2) |
45.7 (±14.5) |
0.00 |
| Additive EuroSCORE |
4.6 (±3.3) |
0.7 (±0.7) |
2.6 (±0.5) |
4.2 (±0.6) |
5.9 (±0.8) |
9.7 (±2.5) |
0.00 |
| Logistic EuroSCORE |
5.8 (±8.6) |
1.1 (±0.2) |
1.9 (±0.3) |
3.1 (±0.5) |
5.3 (±1.0) |
17.9 (±13.3) |
0.00 |
| EuroSCORE II |
3.0 (±5.1) |
0.8 (±0.3) |
1.2 (±0.5) |
1.7 (±0.8) |
2.7 (±1.6) |
8.6 (±9.2) |
0.01 |
For each patient, we calculated the additive and the logistic EuroSCORE, as well as the EuroSCORE II, which predict the risk of 30-day all-cause mortality from preoperative factors based on a logistic regression model using age, sex, comorbid conditions, patient history and LVEF. The additive EuroSCORE ranges from 0 to about ≥40 (as age scores linearly per 5 years increment, the score is not strictly limited), the logistic EuroSCORE and EuroSCORE II range from 0.88 or 0.5, respectively, to <100, representing the risk of perioperative death in % [11–13].
The follow-up data were obtained directly through ambulatory FU or telephone interview with the patient or the general practitioner.
Herewith we documented the presence and severity of dyspnoea, angina pectoris, major cardiovascular events after the operation (myocardial infarction, cerebrovascular event, cardiovascular death, repeated revascularisation), death from any cause.
Statistical analysis
All consecutive patients undergoing surgery were included in the analysis. Missing data were not included in the final statistics (accounting for less than 10% of the patients lost for the long-term follow up). Continuous data are presented as mean ± standard deviation (SD) and categorical variables are displayed as frequency distributions (n) and simple percentages (%).
Kaplan-Meier estimates of cumulative probabilities were calculated for the primary endpoint of death for the patients operated on. We used published data about life expectancy in Switzerland, stratified for gender and year of birth, to match a corresponding record from the general cohort to each patient in the study cohort. Furthermore, the Kaplan-Meier estimates were stratified by quintiles of the logistic EuroSCORE for the whole population and by gender.
Logistic regression analysis was performed for early mortality, as well as for the adverse events such as perioperative myocardial infarction, cerebrovascular event and emergent reoperation.
Poisson regression was used to analyse skewed continuous variables such as length of hospital stay. Long term mortality was investigated using Cox proportional hazard regression. All calculations have been made using Stata 12 (College Station, Texas).
Results
Population
Out of 6308 patients operated on from 2005 to 2010, 2130 underwent isolated CABG with MiECC (33.7%) and were included in the current analysis (fig. 1). Overall, 445 patients (20.9%) were female, and the mean age was 65.8 years (±9.8 years). Dyspnoea in NYHA functional classes III and IV was reported in 403 patients (19%). Prior myocardial infarction occurred in 33 patients (2%) 6 hours before surgery, in 139 patients (7%) 24 hours earlier and in 463 patients (23%) 7 days before surgery (table 1).
Percutaneous revascularisation was attempted in 387 of those patients (18%). The estimated LVEF was normal with the mean of 54.9% (±12.9%). The estimated operative risks calculated with the standard and logistic EuroSCORE and EuroSCORE II were 4.6 (±3.3), 5.8 (±8.6) and 3.0 (±5.1), respectively.
Operative data
An arterial graft was the preferred option for revascularisation of the left anterior descending artery with a second arterial graft for the circumflex branch when appropriate and feasible, with a mean of 1.3 (±0.8) arterial and 2.0 (±1.1) venous grafts (table 2). The detailed choice of grafts is presented in table 3. The mean perfusion time was 71.1 minutes (±23.8 minutes) and the mean cross-clamp time 44.9 minutes (±16.3 minutes) (table 2).
|
All patients
(n = 2130)
|
Logistic EuroSCORE
|
|
Quintile 1
(n = 433)
|
Quintile 2
(n = 425)
|
Quintile 3
(n = 430)
|
Quintile 4
(n = 419)
|
Quintile 5
(n = 423)
|
p-value
|
| No. of arterial anastomoses |
1.3 (±0.8) |
1.7 (±0.9) |
1.5 (±0.8) |
1.2 (±0.6) |
1.2 (±0.6) |
1.1 (±0.7) |
<0.001 |
| LIMA |
2062 (96.8%) |
422 (97.5%) |
422 (99.3%) |
419 (97.4%) |
410 (97.9%) |
389 (92.0%) |
<0.001 |
| Radial |
365 (17.1%) |
139 (32.1%) |
94 (22.1%) |
50 (11.6%) |
38 (9.1%) |
44 (10.4%) |
<0.001 |
| RIMA |
200 (9.4%) |
99 (22.9%) |
53 (12.5%) |
14 (3.3%) |
15 (3.6%) |
19 (4.5%) |
<0.001 |
| Arterial grafts only |
222 (10.4%) |
92 (21.2%) |
50 (11.8%) |
30 (7.0%) |
23 (5.5%) |
27 (6.4%) |
<0.001 |
| No. of venous anastomoses |
2.0 (±1.1) |
1.5 (±1.1) |
1.9 (±1.1) |
2.1 (±1.0) |
2.3 (±1.0) |
2.2 (±1.0) |
<0.001 |
| No. of grafts |
3.3 (±0.9) |
3.2 (±0.9) |
3.3 (±0.9) |
3.3 (±0.9) |
3.4 (±0.9) |
3.3 (±0.9) |
0.091 |
| Perfusion time (min) |
71.2 (±24.0) |
68.4 (±23.2) |
70.6 (±25.4) |
71.1 (±22.3) |
72.6 (±22.1) |
73.2 (±26.6) |
0.21 |
| Cross-clamp time (min) |
44.9 (±16.3) |
44.5 (±16.5) |
45.3 (±17.2) |
44.5 (±16.3) |
45.8 (±15.3) |
44.5 (±16.3) |
0.69 |
Table 3 Initial postoperative outcome risks by the logistic EuroSCORE.
|
All patients
(n = 2130)
|
logistic EuroSCORE
|
|
Quintile 1
(n = 433)
|
Quintile 2
(n = 425)
|
Quintile 3
(n = 430)
|
Quintile 4
(n = 419)
|
Quintile 5
(n = 423)
|
p-value
|
| Troponin T (ng/ml)*
|
0.8 (±2.0) |
0.3 (±0.5) |
0.6 (±1.5) |
0.5 (±0.8) |
0.7 (±1.1) |
1.9 (±3.8) |
0.22 |
| Peak CK-MB (U/l) |
27.2 (±47) |
17.9 (±23) |
25.0 (±41) |
21.9 (±25) |
29.0 (±60) |
42.5 (±65) |
0.08 |
| Peak CK (U/l) |
815 (±1147) |
796 (±1105) |
867 (±1090) |
726 (±895) |
753 (±1513) |
935 (±1039) |
0.20 |
| Perioperative MI |
42 (2.0%) |
3 (0.7%) |
9 (2.1%) |
10 (2.3%) |
8 (1.9%) |
12 (2.8%) |
0.22 |
| New atrial fibrillation |
335 (15.7%) |
40 (9.2%) |
51 (12.0%) |
67 (15.6%) |
70 (16.7%) |
107 (25.3%) |
0.00 |
| Arrythmia†
|
45 (2.1%) |
7 (1.6%) |
4 (0.9%) |
8 (1.9%) |
7 (1.7%) |
19 (4.5%) |
0.00 |
| Permanent pacemaker |
9 (0.4%) |
0 (0.0%) |
0 (0.0%) |
4 (0.9%) |
0 (0.0%) |
5 (1.2%) |
0.01 |
| Resuscitation |
15 (0.7%) |
0 (0.0%) |
0 (0.0%) |
1 (0.2%) |
5 (1.2%) |
9 (2.1%) |
0.00 |
| Reoperation on the sternum |
22 (1.0%) |
1 (0.2%) |
3 (0.7%) |
4 (0.9%) |
7 (1.7%) |
7 (1.7%) |
0.17 |
| Pericardial effusion/tamponade‡
|
31 (1.5%) |
5 (1.2%) |
4 (0.9%) |
7 (1.6%) |
5 (1.2%) |
10 (2.4%) |
0.43 |
| Cerebrovascular event |
36 (1.7%) |
1 (0.2%) |
4 (0.9%) |
6 (1.4%) |
13 (3.1%) |
12 (2.8%) |
0.00 |
| Renal dysfunction |
|
|
|
|
|
|
0.00 |
| Without dialysis |
133 (6.2%) |
9 (2.1%) |
22 (5.2%) |
30 (7.0%) |
30 (7.2%) |
42 (9.9%) |
|
| With dialysis |
46 (2.2%) |
3 (0.7%) |
5 (1.2%) |
8 (1.9%) |
11 (2.6%) |
19 (4.5%) |
|
| Pulmonary complication |
129 (6.1%) |
14 (3.2%) |
19 (4.5%) |
27 (6.3%) |
25 (6.0%) |
44 (10.4%) |
0.00 |
| ICU stay (days) |
1.6 (±2.7) |
1.2 (±1.0) |
1.2 (±1.1) |
1.4 (±1.5) |
1.8 (±2.6) |
2.6 (±4.8) |
0.57 |
| IMC stay (days) |
1.2 (±2.7) |
0.6 (±0.8) |
0.8 (±1.2) |
1.2 (±4.2) |
1.4 (±2.3) |
1.9 (±3.2) |
0.00 |
| Length of stay (days) |
8.9 (±8.5) |
6.9 (±3.3) |
7.7 (±4.1) |
8.9 (±12.4) |
9.9 (±9.7) |
11.0 (±8.7) |
0.00 |
| 30-day mortality |
18 (0.8%) |
1 (0.2%) |
3 (0.7%) |
2 (0.5%) |
4 (1.0%) |
8 (1.9%) |
0.07 |
Outcome and survival prediction scores
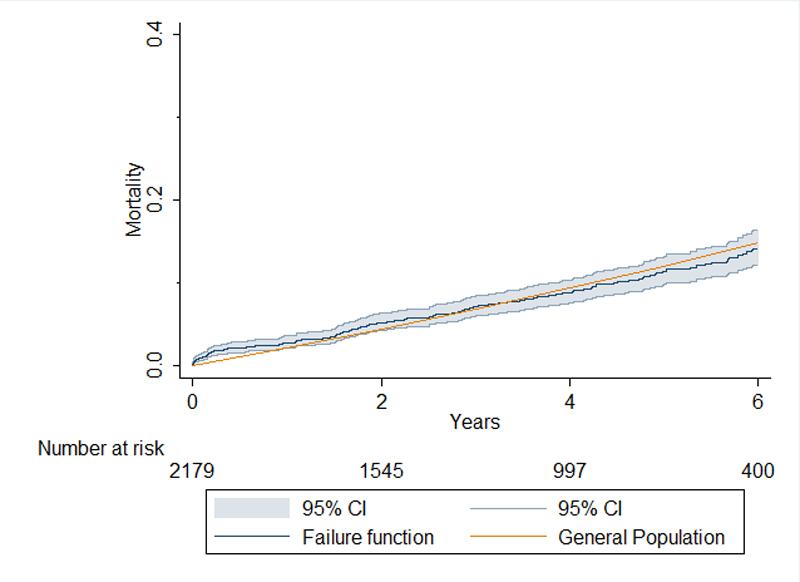
All-cause mortality was 0.8% at 30 days (table 3) and 9.1% at 6 years, comparable to the expected survival of the gender- and age-matched general Swiss population (fig. 2). Independent risk factors according to the logistic regression analysis for 30-day mortality were LVEF of less than 30% with the odds ratio (OR) of 5.03 (95% confidence interval [CI] 1.43–17.74; p = 0.012) and diabetes mellitus with OR 3.64 (95% CI 1.40–9.43; p = 0.008), whereas elective operation was protective compared with emergency procedures in this context, with an OR of 0.26 (95% CI 0.10-0.69; p = 0.015).
Perioperative myocardial infarction (table 3) occurred in 42 patients (2%). Independent predictors for myocardial infarction were peripheral arterial disease (OR 3.76, 95% CI 1.86–7.61; p <0.001), chronic renal failure (OR 3.38, 95% CI 1.47–7.78; p = 0.004), need of perioperative erythrocyte transfusion (OR 2.00, 95% CI 1.08–3.70); elective operation (OR 0.47, 95% CI 0.25–0.86; p = 0.027) represented a protective factor against postoperative morbidity. Cerebrovascular events occurred in 36 patients (1.7%) and were more frequent in the presence of the peripheral arterial disease with OR 2.99 (95% CI 1.34–6.65; p = 0.007), severe obstructive pulmonary disease (OR 6.93, 95% CI 2.00–23.98; p = 0.002) and chronic renal failure (OR 3.39, 95% CI 1.39–8.32; p = 0.008). Further perioperative findings, as well as the discharge details including length of stay are shown in table 3.
Long term mortality was predicted by the presence of diabetes mellitus with an OR of 1.85 (95% CI 1.40–2.46; p <0.001), peripheral arterial disease (OR 2.36, 95% CI 1.64–3.38; p <0.001), severe obstructive pulmonary disease (OR 3.21, 95% CI 1.42–7.24; p = 0.005), chronic renal failure (OR 3.68, 95% CI 2.49–5.43; p <0.001) and transfusion of more than one unit of erythrocyte concentrate in the perioperative period (OR 1.46, 95% CI 1.09–1.95; p = 0.015).
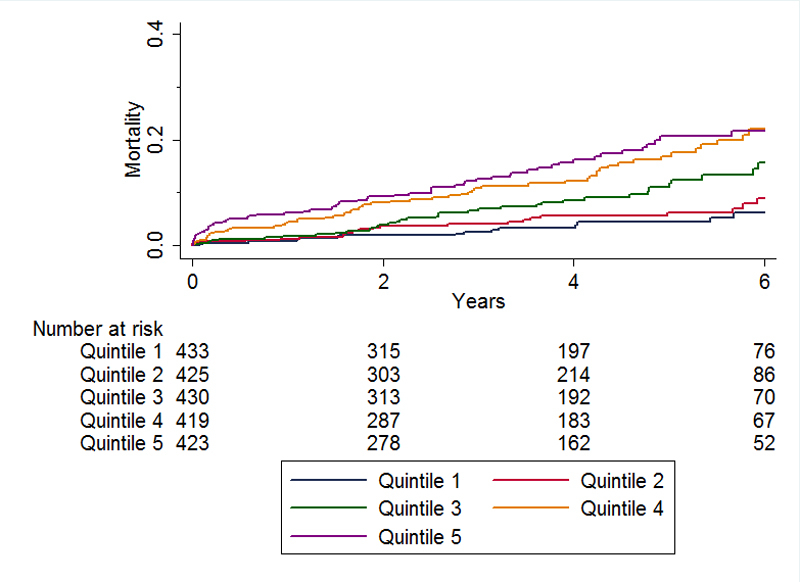
The logistic EuroSCORE predicts survival in the cohort of patients with minimised extracorporeal circulatory support during the procedure (fig. 3). However, compared with the actual observed mortality, the estimates of the logistic EuroSCORE remain grossly overestimated, especially in the late postoperative period, in both genders.
Discussion
The concept and strategy of MiECC was introduced to reduce the disadvantages of standard extracorporeal circulation. Several studies have already shown the benefits of MiECC, including low mortality, less myocardial damage, improved end-organ protection and easy application in clinical practice [3, 14]. Several meta-analyses have supported these superior results of MiECC when compared with standard extracorporeal circulation [3, 15]. Furthermore, a recent analysis has shown that MiECC does not give inferior results in comparison with off-pump coronary artery bypass.[16]. However, long-term follow-up data and large cohort studies are needed to confirm these advantages of MiECC over time.
This study presents early and long-term outcomes after CABG with MiECC in a single-centre sequential patient cohort. Long-term outcome after CABG with MiECC was compared with the life expectancy of the general Swiss population. CABG performed with use of an MiECC system for intraoperative perfusion was associated with very low early and late postoperative mortality for up to 6 years. The overall 30-day mortality after CABG with MiECC was 0.8%. Late mortality was comparable to the mortality of the country’s general population matched for age and gender. From year one after surgery the mortality rate in the patient collective was comparable to the mortality rate of the Swiss general population matched individually for year of birth and gender. The general Swiss population was chosen as the reference group for the study because of Switzerland has a high living standard and the second longest life expectancy in the world (83.4 years for both sexes) [17].
In line with contemporary publications, we attribute the low rate of early mortality and perioperative complications to an attenuated inflammatory reaction and complement activation response because of the reduced inert surfaces of the closed extracorporeal circulation system. In addition, the reduction of priming volume and minimised haemodilution can influence the onset of anticoagulation disorders [18, 19]. As a result, multiple publications have confirmed lower intraoperative haemolysis and a lower risk of bleeding, which is most pronounced among adults with a small body surface area, paediatric patients and in the setting of preoperative anaemia [20, 21].
On the other hand, higher mean arterial pressures during perfusion with an MiECC circuit as compared with CPB, and the reduced vasoactive support needed with much more preserved physiological circulation and continuous coronary flow, result in better end-organ perfusion [22, 23]. In clinical practice, this translates into superior myocardial protection and reperfusion, with significantly reduced levels of cardiac injury markers, lower incidence of postoperative atrial fibrillation, lower incidence of stroke, less haemodialysis and lower creatinine levels postoperatively, and better neurocognitive and lung function [5, 18, 19, 24–27].
Apart from the endpoints mentioned above, MiECC offered improvement of early mortality in a large meta-analysis of 2770 patients [15]. This remained true for all age groups, as well as for emergency and elective operations [28, 29]. The subjectively perceived quality of life among patients was higher in the physical and mental summary scores, compared with that observed after conventional CPB [30]. MiECC proved favourable for a fast-track strategy (defined as minimal administration of opioids, operation under normothermia, early postoperative extubation and admission to the cardiosurgical ward within 24 hours after the operation with facilitation of early recovery) with an OR of success of 3.8 in the randomised study by Anastasiadis et al. [31]. Surprisingly despite very promising results in larger cohorts, the penetration of MiECC remains low. Concerns about de-airing of the system were initially raised and addressed in one small randomised trial, which showed higher rates of cerebral gaseous emboli after aortic valve replacement with MiECC compared with standard CPB, without significant difference in the clinical outcome [32]. However, the results of Basciani et al. remain in contradiction to existing larger observational trials in which de-airing modules were implemented in the MiECC [27, 33].
An analysis of cost effectiveness in various European countries showed a cost reduction of EUR 635 in Greece, EUR 297 in Germany, EUR 1590 in the Netherlands and EUR 375 in Switzerland for the MiECC system. In the same article there was a strong tendency towards more life years gained with the minimally invasive technology [34].
The risk of mortality and perioperative complications is augmented in the presence of several risk factors included in contemporary preoperative risk estimation scores. The anticipated mortality was significantly lower in the real life cohort. These results remained in line with the recent publication of Koivisto et al. [35]. Similar findings have been shown for patients undergoing CABG with traditional CPB [36]. We therefore believe that further research is needed to optimise the risk stratification in patients operated with MiECC support, especially with the expanding indications of coronary artery stenting. In many cases, minimally invasive, fast-track operation offers an interesting alternative, especially in the patients with higher operative risk.
We acknowledge some limitations of our study. It was a retrospective observational analysis and therefore cause and effect are hard to establish. Best evidence would be guaranteed by performing a prospective randomised controlled multicentre study. Another limitation may be that in cases where the decision to use MiECC is left at the discretion of the treating physician, selection bias might occur. However, the patient characteristics are similar to those reported in randomised studies, and therefore we consider selection bias unlikely. Another shortcoming is the fact that the intervention group was matched against the same age general population and not the population operated with standard of care, namely with conventional cardiopulmonary bypass. The concept of MiECC has already been proved by other research groups and thus we decided to compare the outcome after CABG against the life expectancy of the general population to highlight the good performance of the approach at our institution.
Conclusion
MiECC shows very promising results in our patient cohort, inclusively when the long term follow up is concerned, since the latter is comparable to that of an age and gender matched general population. We thus believe that MiECC should be used in every case of on-pump CABG, when technically feasible.
Acknowledgements
The authors would like to thank the CTU (Clinical Trial Unit) Bern for their support.
Author contributions
BW and PPH contributed equally
References
1
Milojevic
M
,
Head
SJ
,
Parasca
CA
,
Serruys
PW
,
Mohr
FW
,
Morice
M-C
, et al.
TCT-164 Causes of Death after Percutaneous Coronary Intervention versus Coronary Artery Bypass Grafting in Complex Coronary Artery Disease: 5-Year follow-up of the SYNTAX trial. J Am Coll Cardiol. 2015;66(15):B60. doi:.https://doi.org/10.1016/j.jacc.2015.08.176
2
Cohen
DJ
,
Osnabrugge
RL
,
Magnuson
EA
,
Wang
K
,
Li
H
,
Chinnakondepalli
K
, et al.; SYNTAX Trial Investigators. Cost-effectiveness of percutaneous coronary intervention with drug-eluting stents versus bypass surgery for patients with 3-vessel or left main coronary artery disease: final results from the Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery (SYNTAX) trial. Circulation. 2014;130(14):1146–57. doi:.https://doi.org/10.1161/CIRCULATIONAHA.114.009985
3
Kowalewski
M
,
Pawliszak
W
,
Raffa
GM
,
Malvindi
PG
,
Kowalkowska
ME
,
Zaborowska
K
, et al.
Safety and efficacy of miniaturized extracorporeal circulation when compared with off-pump and conventional coronary artery bypass grafting: evidence synthesis from a comprehensive Bayesian-framework network meta-analysis of 134 randomized controlled trials involving 22 778 patients. Eur J Cardiothorac Surg. 2016;49(5):1428–40.
4
Winkler
B
,
Heinisch
PP
,
Gahl
B
,
Aghlmandi
S
,
Jenni
HJ
,
Carrel
TP
. Minimally Invasive Extracorporeal Circulation Circuit Is Not Inferior to Off-Pump Coronary Artery Bypass Grafting: Meta-Analysis Using the Bayesian Method. Ann Thorac Surg. 2017;103(1):342–50. doi:.https://doi.org/10.1016/j.athoracsur.2016.08.067
5
Anastasiadis
K
,
Murkin
J
,
Antonitsis
P
,
Bauer
A
,
Ranucci
M
,
Gygax
E
, et al.
Use of minimal invasive extracorporeal circulation in cardiac surgery: principles, definitions and potential benefits. A position paper from the Minimal invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact Cardiovasc Thorac Surg. 2016;22(5):647–62. doi:.https://doi.org/10.1093/icvts/ivv380
6
Anastasiadis
K
,
Antonitsis
P
,
Ranucci
M
,
Murkin
J
. Minimally Invasive Extracorporeal Circulation (MiECC): Towards a More Physiologic Perfusion. J Cardiothorac Vasc Anesth. 2016;30(2):280–1. doi:.https://doi.org/10.1053/j.jvca.2016.01.018
7
Bastuji-Garin
S
,
Sbidian
E
,
Gaudy-Marqueste
C
,
Ferrat
E
,
Roujeau
J-C
,
Richard
M-A
, et al.; European Dermatology Network (EDEN). Impact of STROBE statement publication on quality of observational study reporting: interrupted time series versus before-after analysis. PLoS One. 2013;8(8):e64733. doi:.https://doi.org/10.1371/journal.pone.0064733
8
Jenni
H
,
Rheinberger
J
,
Czerny
M
,
Gygax
E
,
Rieben
R
,
Krähenbühl
E
, et al.
Autotransfusion system or integrated automatic suction device in minimized extracorporeal circulation: influence on coagulation and inflammatory response. Eur J Cardiothorac Surg. 2011;39(5):e139–43. doi:.https://doi.org/10.1016/j.ejcts.2010.11.082
9
Immer
FF
,
Ackermann
A
,
Gygax
E
,
Stalder
M
,
Englberger
L
,
Eckstein
FS
, et al.
Minimal extracorporeal circulation is a promising technique for coronary artery bypass grafting. Ann Thorac Surg. 2007;84(5):1515–20, discussion 1521. doi:.https://doi.org/10.1016/j.athoracsur.2007.05.069
10
Kairet
K
,
Deen
J
,
Vernieuwe
L
,
de Bruyn
A
,
Kalantary
S
,
Rodrigus
I
. Cardioplexol, a new cardioplegic solution for elective CABG. J Cardiothorac Surg. 2013;8(Suppl 1):p120. doi:.https://doi.org/10.1186/1749-8090-8-S1-P120
11
Roques
F
,
Nashef
SA
,
Michel
P
,
Gauducheau
E
,
de Vincentiis
C
,
Baudet
E
, et al.
Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15(6):816–22, discussion 822–3. doi:.https://doi.org/10.1016/S1010-7940(99)00106-2
12
Roques
F
,
Michel
P
,
Goldstone
AR
,
Nashef
SAM
. The logistic EuroSCORE. Eur Heart J. 2003;24(9):881–2. doi:.https://doi.org/10.1016/S0195-668X(02)00799-6
13
Nashef
SAM
,
Roques
F
,
Sharples
LD
,
Nilsson
J
,
Smith
C
,
Goldstone
AR
, et al.
EuroSCORE II. Eur J Cardiothorac Surg. 2012;41(4):734–44, discussion 744–5. doi:.https://doi.org/10.1093/ejcts/ezs043
14
Puehler
T
,
Haneya
A
,
Philipp
A
,
Zausig
YA
,
Kobuch
R
,
Diez
C
, et al.
Minimized extracorporeal circulation system in coronary artery bypass surgery: a 10-year single-center experience with 2243 patients. Eur J Cardiothorac Surg. 2011;39(4):459–64. doi:.https://doi.org/10.1016/j.ejcts.2010.08.006
15
Anastasiadis
K
,
Antonitsis
P
,
Haidich
A-B
,
Argiriadou
H
,
Deliopoulos
A
,
Papakonstantinou
C
. Use of minimal extracorporeal circulation improves outcome after heart surgery; a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2013;164(2):158–69. doi:.https://doi.org/10.1016/j.ijcard.2012.01.020
16
Winkler
B
,
Reineke
D
,
Heinisch
PP
,
Schönhoff
F
,
Huber
C
,
Kadner
A
, et al.
Graft preservation solutions in cardiovascular surgery. Interact Cardiovasc Thorac Surg. 2016;23(2):300–9. doi:.https://doi.org/10.1093/icvts/ivw056
17World Health Organization. World health statistics 2016: monitoring health for the SDGs, sustainable development goals. Geneva: World Health Organization; 2016.
18
van Boven
W-JP
,
Gerritsen
WB
,
Driessen
AH
,
van Dongen
EP
,
Klautz
RJ
,
Aarts
LP
. Minimised closed circuit coronary artery bypass grafting in the elderly is associated with lower levels of organ-specific biomarkers: a prospective randomised study. Eur J Anaesthesiol. 2013;30(11):685–94. doi:.https://doi.org/10.1097/EJA.0b013e328364febf
19
Zangrillo
A
,
Garozzo
FA
,
Biondi-Zoccai
G
,
Pappalardo
F
,
Monaco
F
,
Crivellari
M
, et al.
Miniaturized cardiopulmonary bypass improves short-term outcome in cardiac surgery: a meta-analysis of randomized controlled studies. J Thorac Cardiovasc Surg. 2010;139(5):1162–9. doi:.https://doi.org/10.1016/j.jtcvs.2009.07.048
20
Ohata
T
,
Mitsuno
M
,
Yamamura
M
,
Tanaka
H
,
Kobayashi
Y
,
Ryomoto
M
, et al.
Minimal cardiopulmonary bypass attenuates neutrophil activation and cytokine release in coronary artery bypass grafting. J Artif Organs. 2007;10(2):92–5. doi:.https://doi.org/10.1007/s10047-007-0377-0
21
Huybregts
RAJM
,
Morariu
AM
,
Rakhorst
G
,
Spiegelenberg
SR
,
Romijn
HWA
,
de Vroege
R
, et al.
Attenuated renal and intestinal injury after use of a mini-cardiopulmonary bypass system. Ann Thorac Surg. 2007;83(5):1760–6. doi:.https://doi.org/10.1016/j.athoracsur.2007.02.016
22
van Boven
W-JP
,
Gerritsen
WB
,
Driessen
AH
,
Morshuis
WJ
,
Waanders
FG
,
Haas
FJ
, et al.
Myocardial oxidative stress, and cell injury comparing three different techniques for coronary artery bypass grafting. Eur J Cardiothorac Surg. 2008;34(5):969–75. doi:.https://doi.org/10.1016/j.ejcts.2008.07.060
23
Donndorf
P
,
Kühn
F
,
Vollmar
B
,
Rösner
J
,
Liebold
A
,
Gierer
P
, et al.
Comparing microvascular alterations during minimal extracorporeal circulation and conventional cardiopulmonary bypass in coronary artery bypass graft surgery: a prospective, randomized study. J Thorac Cardiovasc Surg. 2012;144(3):677–83. doi:.https://doi.org/10.1016/j.jtcvs.2012.05.037
24
Bauer
A
,
Diez
C
,
Schubel
J
,
El-Shouki
N
,
Metz
D
,
Eberle
T
, et al.
Evaluation of hemodynamic and regional tissue perfusion effects of minimized extracorporeal circulation (MECC). J Extra Corpor Technol. 2010;42(1):30–9.
25
Anastasiadis
K
,
Asteriou
C
,
Deliopoulos
A
,
Argiriadou
H
,
Karapanagiotidis
G
,
Antonitsis
P
, et al.
Haematological effects of minimized compared to conventional extracorporeal circulation after coronary revascularization procedures. Perfusion. 2010;25(4):197–203. doi:.https://doi.org/10.1177/0267659110373840
26
Biancari
F
,
Rimpiläinen
R
. Meta-analysis of randomised trials comparing the effectiveness of miniaturised versus conventional cardiopulmonary bypass in adult cardiac surgery. Heart. 2009;95(12):964–9. doi:.https://doi.org/10.1136/hrt.2008.158709
27
Liebold
A
,
Khosravi
A
,
Westphal
B
,
Skrabal
C
,
Choi
YH
,
Stamm
C
, et al.
Effect of closed minimized cardiopulmonary bypass on cerebral tissue oxygenation and microembolization. J Thorac Cardiovasc Surg. 2006;131(2):268–76. doi:.https://doi.org/10.1016/j.jtcvs.2005.09.023
28
Rufa
M
,
Schubel
J
,
Ulrich
C
,
Schaarschmidt
J
,
Tiliscan
C
,
Bauer
A
, et al.
A retrospective comparative study of minimally invasive extracorporeal circulation versus conventional extracorporeal circulation in emergency coronary artery bypass surgery patients: a single surgeon analysis. Interact Cardiovasc Thorac Surg. 2015;21(1):102–7. doi:.https://doi.org/10.1093/icvts/ivv103
29
Kolat
P
,
Ried
M
,
Haneya
A
,
Philipp
A
,
Kobuch
R
,
Hirt
S
, et al.
Impact of age on early outcome after coronary bypass graft surgery using minimized versus conventional extracorporeal circulation. J Cardiothorac Surg. 2014;9(1):143. doi:.https://doi.org/10.1186/s13019-014-0143-3
30
Anastasiadis
K
,
Antonitsis
P
,
Kostarellou
G
,
Kleontas
A
,
Deliopoulos
A
,
Grosomanidis
V
, et al.
Minimally invasive extracorporeal circulation improves quality of life after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2016;50(6):1196–203. doi:.https://doi.org/10.1093/ejcts/ezw210
31
Anastasiadis
K
,
Asteriou
C
,
Antonitsis
P
,
Argiriadou
H
,
Grosomanidis
V
,
Kyparissa
M
, et al.
Enhanced recovery after elective coronary revascularization surgery with minimal versus conventional extracorporeal circulation: a prospective randomized study. J Cardiothorac Vasc Anesth. 2013;27(5):859–64. doi:.https://doi.org/10.1053/j.jvca.2013.01.010
32
Basciani
R
,
Kröninger
F
,
Gygax
E
,
Jenni
H
,
Reineke
D
,
Stucki
M
, et al.
Cerebral Microembolization During Aortic Valve Replacement Using Minimally Invasive or Conventional Extracorporeal Circulation: A Randomized Trial. Artif Organs. 2016;40(12):E280–91. doi:.https://doi.org/10.1111/aor.12744
33
Anastasiadis
K
,
Argiriadou
H
,
Kosmidis
MH
,
Megari
K
,
Antonitsis
P
,
Thomaidou
E
, et al.
Neurocognitive outcome after coronary artery bypass surgery using minimal versus conventional extracorporeal circulation: a randomised controlled pilot study. Heart. 2011;97(13):1082–8. doi:.https://doi.org/10.1136/hrt.2010.218610
34
Anastasiadis
K
,
Fragoulakis
V
,
Antonitsis
P
,
Maniadakis
N
. Coronary artery bypass grafting with minimal versus conventional extracorporeal circulation; an economic analysis. Int J Cardiol. 2013;168(6):5336–43. doi:.https://doi.org/10.1016/j.ijcard.2013.08.006
35
Koivisto
S-P
,
Wistbacka
J-O
,
Rimpiläinen
R
,
Nissinen
J
,
Loponen
P
,
Teittinen
K
, et al.
Miniaturized versus conventional cardiopulmonary bypass in high-risk patients undergoing coronary artery bypass surgery. Perfusion. 2010;25(2):65–70. doi:.https://doi.org/10.1177/0267659110364443
36
Kieser
TM
,
Rose
MS
,
Head
SJ
. Comparison of logistic EuroSCORE and EuroSCORE II in predicting operative mortality of 1125 total arterial operations. Eur J Cardiothorac Surg. 2016;50(3):509–18. doi:.https://doi.org/10.1093/ejcts/ezw072