Figure 1 Demonstration of the two most common approaches through ministernotomy (image courtesy of Edwards Lifesciences Corporation).
DOI: https://doi.org/10.4414/smw.2017.14464
Aortic stenosis is the most common acquired valvular heart disease in Europe and North America. The prevalence of aortic stenosis in patients at the age 65 years or above varies between 2 and 7% [1–3]. Aortic stenosis occurs from age-related degenerative calcification or, rarely, from previous rheumatic fever in both tricuspid and bicuspid valves.
According to the recommendations of the American College of Cardiology and the American Heart Association guidelines, the treatment of choice for severe aortic valve stenosis is either surgical aortic valve replacement or, in selected (mainly older and high-risk) patients, transcatheter aortic valve implantation [4]. Surgical aortic valve replacement (SAVR) through a median sternotomy has been the treatment of choice for symptomatic, severe aortic stenosis over decades. Continuous improvement in surgical techniques and new technologies aiming at facilitating and reducing operative times allow increased performance for all patients groups, including elderly patients and patients with more comorbidities.
The literature does not provide detailed process analyses of conventional SAVR, which would add to the understanding of the most technically demanding and time-consuming parts of the operation.
Thus, we aimed to systematically analyse the different procedural steps of aortic valve replacement by using a process analysis in order to identify the most time-consuming parts during SAVR according operator experience.
Less invasive strategies, such as minimal invasive surgical techniques (fig. 1), have been developed and introduced into clinical routine with outcomes equivalent to conventional approaches [5–10]. However, minimal invasive surgical techniques are technically more demanding, with high interoperator variability in procedural duration and clinical outcomes. The reduction of surgical exposure and working space can lead to technical difficulties in accessing the aortic valve, thereby resulting in an increase in cardiopulmonary bypass time (CPB) and aortic cross-clamp time (AOx) [5, 6], which in turn might be associated with worse postoperative outcomes [11–13].

Figure 1 Demonstration of the two most common approaches through ministernotomy (image courtesy of Edwards Lifesciences Corporation).
Sutureless valve prostheses were designed to combine the advantages of transcatheter technology and surgical valve replacement. The aim of sutureless valves is to simplify the procedure and to reduce intervention time.
In the last few years, the surgical experiences with three sutureless devices have been reported: the 3f Enable valve (Medtronic, Minneapolis, MN), the Perceval S valve (Sorin Group, Saluggia, Italy) and the Intuity Valve System (Edwards Lifesciences Corporation, Irvine, CA).
Most recently the Cor-Knot device (LSI Solutions, Victor, NY, USA), a tying and cutting device, which was designed to fasten and optimise knotting especially in a minimally invasive setting, was introduced into clinical practice for valve surgery (fig. 2).

Figure 2 Intraoperative application of the Cor-Knot Device for aortic valve fixation (images courtesy of LSI SOLUTIONS®).
For the purpose of this process analysis, baseline, procedural and outcome data of 57 nonconsecutive patients undergoing isolated SAVR for severe aortic valve stenosis through complete sternotomy were prospectively collected between March 2013 and August 2015. Patients with previous cardiac surgery, the need for concomitant coronary artery bypass grafting, a minimally invasive approach or other cardiac procedures in addition to SAVR were excluded from this analysis. All patients provided written informed consent to be included in the registry with prospective follow-up assessment.
The experience of a surgeon was graded according to the total SAVR experience. Surgeons who had performed more than 100 SAVR procedures through median sternotomy were considered “more experienced”, whereas surgeons with a total experience of less than 100 SAVR procedures were considered “less experienced”, and were analysed separately. A total of 10 cardiovascular surgeons performed SAVR on the 57 patients. Five surgeons were considered more experienced.
In all patients, SAVR was performed in a standardised fashion through median sternotomy with mild hypothermia. After central cannulation and initiation of cardiopulmonary bypass circulation, the aorta was cross-clamped and cardioplegia was induced in an antegrade fashion. According to the institutional practice, a standard technique was used for the implantation of the prosthetic aortic valve. The type of aortic valve prosthesis (biological or mechanical prosthesis) was left to the discretion of the surgeon and the patient, and followed the general recommendations of the latest version of the European Society of Cardiology guidelines for the treatment of valvular heart disease [14].
After cross-clamping the study specific time measurement started using five consecutive procedural steps:
Step 1: Exposure started immediately after aortic cross-clamping and included the induction of cardioplegia and full exposure of the aortic valve.
Step 2: Resection was initiated with the start of resection of the calcified aortic valve, including the decalcification of the aortic annulus as well as the annulus measurement with the prosthesis-specific sizer in order to determine the size of the prosthesis.
Step 3: Suturing started with the first suture to be placed into the native aortic annulus.
Step 4: Tying started with the first knot of the sutures of the aortic valve prosthesis and lasted until cutting of the last suture.
Step 5: Declamping was defined as the time window after cutting the last suture until removal of the aortic cross clamp.
All time windows were measured by one dedicated person (M.N.) using a conventional stopwatch.
Statistical analysis was performed using Stata 12 (College Station, Texas, USA). Data are presented as mean and standard deviation, median with interquartile range (IQR) and number with percent, according to the type and distribution of the data. Differences between the experienced and less experienced surgeons’ operations were assessed with unpaired student t-tests, median tests or chi-squared tests, as appropriate. All p-values are two sided and p-values <0.05 were considered statistically significant.
Between March 2013 and August 2015, 57 nonconsecutive patients with symptomatic, severe aortic stenosis undergoing isolated SAVR were selectively included in this study.
Baseline clinical characteristics are presented in table 1. Patients were predominantly male (67%), with a median age of 71.0 years (IQR 65.0–76.0 years). All patients were symptomatic (23% New York Heart Association functional class III and IV) and presented with a transvalvular mean pressure gradient of 45.2 ± 16.7 mm Hg, a calculated aortic valve area of 0.8 ± 0.2 cm2 and an ejection fraction of 65.0% (57.0–65.0%). The estimated risk of mortality amounted to 1.1% (0.7–1.6%) according to the logistic EUROScore II. Overall, AOx was 50.5 ± 13.8 min. The duration of each step assessed during AOx is depicted in fig. 3 . The exposure of the aortic valve (duration 5.2 ± 2.8 min, ≈10.5% of AOx) was followed by resection of the degenerated native cusps (valve resection, 8.2 ± 4.4 min, ≈16.0% of AOx). Placement of the sutures accounted for 32.3% of AOx (16.4 ± 5.9 min) and tying the sutures in the fourth step took 9.2 ± 3.0 min (≈18.5% of AOx). The period from cutting the last suture until declamping the aorta lasted 11.4 ± 3.9 min (≈22.8% of AOx).
Table 1 Baseline clinical characteristics.
|
All patients
n = 57 |
Less experienced surgeons
n = 22 |
More experienced surgeons
n = 35 |
p-value | |
|---|---|---|---|---|
| Age (years) | 71.0 (65.0–76.0) | 69.5 (65.0–76.0) | 72.0 (63.0–76.0) | 0.166 |
| Female gender, n (%) | 19 (33) | 8 (36) | 11 (31) | 0.700 |
| Body mass index (kg/m2) | 26.2 (24.1–30.7) | 29.4 (25.2–31.9) | 25.8 (24.0–28.8) | 0.093 |
| Cardiac risk factors | ||||
| Diabetes mellitus, n (%) | 11 (19) | 6 (27) | 5 (14) | 0.227 |
| Dyslipidaemia, n (%) | 36 (63) | 14 (64) | 22 (63) | 0.953 |
| Hypertension, n (%) | 32 (56) | 13 (59) | 19 (54) | 0.722 |
| Current smoker, n(%) | 7 (12) | 2 (9) | 5 (14) | |
| Past medical history | ||||
| Coronary artery disease, n (%) | 7 (12) | 0 (0) | 7 (20) | 0.025 |
| Renal failure (GFR<60ml/min/1.73 m2), n (%) | 10 (18) | 5 (23) | 5 (14) | 0.415 |
| Previous stroke, n (%) | 2 (4) | 0 (0) | 2 (6) | 0.254 |
| Peripheral vascular disease, n (%) | 3 (5) | 1 (5) | 2 (6) | 0.847 |
| Clinical features | ||||
| Left ventricular ejection fraction (%) | 65.0 (57.0–65.0) | 65.0 (55.0–70.0) | 63.5 (58.5–65.0) | 0.153 |
| Aortic valve area (cm2) | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.483 |
| Mean transaortic gradient (mm Hg) | 45.2 ± 16.7 | 46.7 ± 10.5 | 44.3 ± 19.5 | 0.639 |
| Symptoms (New York Heart Association functional class) | ||||
| I + II, n (%) | 42 (73) | 15 (69) | 27 (77) | |
| III + IV, n (%) | 13 (23) | 5 (23) | 8 (23) | |
| Risk assessment | ||||
| EuroScore II (%) | 1.1 (0.7–1.6) | 0.9 (0.7–1.3) | 1.2 (0.7–1.7) | 0.295 |
GFR = glomerular filtration rate Data are presented as mean ± standard deviation, median (interquartile range), or n (%) as appropriate
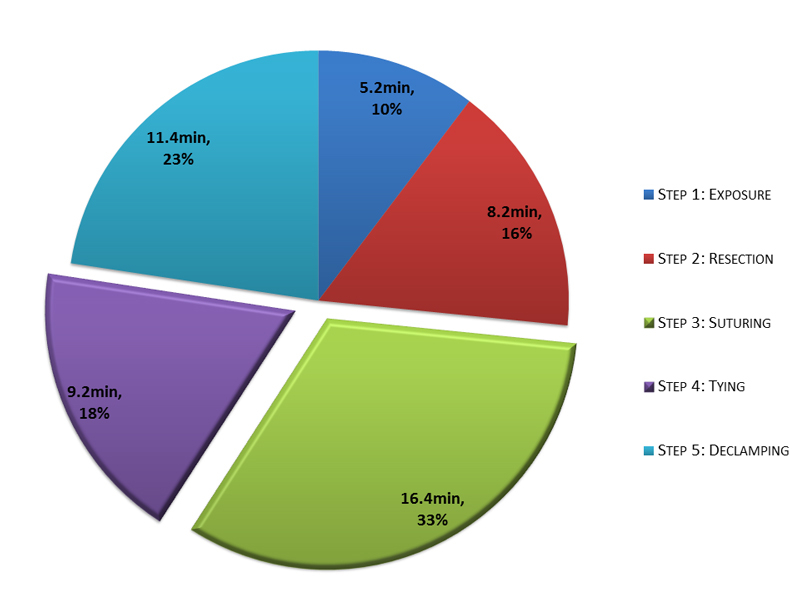
Figure 3 Distribution of the five steps of aortic cross-clamp time.
Surgeons with more experience performed 35 operations (61.4%) and needed an average of 44.1 ± 11.5 min versus 60.6 ± 11.0 min (p <0.001) for less experienced surgeons. Procedural characteristics are presented in table 2. Surgeons with more experience needed 14.0 ± 5.0 min for the suturing step and 8.4 ± 2.8 min for tying the sutures; less experienced surgeons needed 20.2 ± 5.2 min and 10.5 ± 3.0 min, respectively, (p <0.001 and p = 0.010 in comparison with more experienced surgeons). The distribution of each step is shown in fig. 4.
Table 2 Procedural characteristics and outcomes.
| All patients | p-value | Decrease* (%) | ||
|---|---|---|---|---|
|
Less experienced surgeons
n = 22 |
More experienced surgeons
n = 35 |
|||
| Procedure | ||||
| Aortic cross-clamp time (min) | 60.6 ± 11.0 (100) | 44.1 ± 11.5 (100) | <0.001 | |
| Exposure, min (% AOx) | 6.6 ± 3.7 (10.9) | 4.4 ± 1.5 (10.2) | 0.002 | 33 |
| Resection, min (% AOx) | 9.9 ± 4.5 (16.4) | 7.1 ± 4.1 (15.8) | 0.021 | 28 |
| Suturing, min (% AOx) | 20.2 ± 5.2 (33.3) | 14.0 ± 5.0 (31.6) | <0.001 | 31 |
| Tying, min (% AOx) | 10.5 ± 3.0 (17.2) | 8.4 ± 2.8 (19.2) | 0.010 | 20 |
| Declamping, min (% AOx) | 13.4 ± 3.0 (22.1) | 10.2 ± 4.0 (23.3) | 0.002 | 24 |
| Prothesis | ||||
| Perimount Magna Ease tissue prosthesis, n (%) | 18 (81.8) | 30 (85.7) | ||
| Medtronic ATS medical mechanical prosthesis, n (%) | 4 (18.2) | 5 (14.3) | ||
| Prosthesis size, mm | 23.7 ± 2.1 | 23.4 ± 2.2 | 0.55 | |
| Sutures, n | 15.9 ± 2.0 | 14.4 ± 1.8 | 0.005 | |
| Postoperative Data | ||||
| Mean transaortic gradient, mm Hg | 10.0 (8.0–17.0) | 9.5 (6.5–14.0) | 0.7 | |
| Paravalvular leak, n (%) | 1 (4.5) | – | ||
| In-hospital mortality, n (%) | 0 (0) | 0 (0) | ||
AOx = aortic cross-clamp time Data are presented as mean ± standard deviation, median (interquartile range), or n (%) as appropriate. * The average reduction in time that more experienced surgeons need as compared with less experienced surgeons.
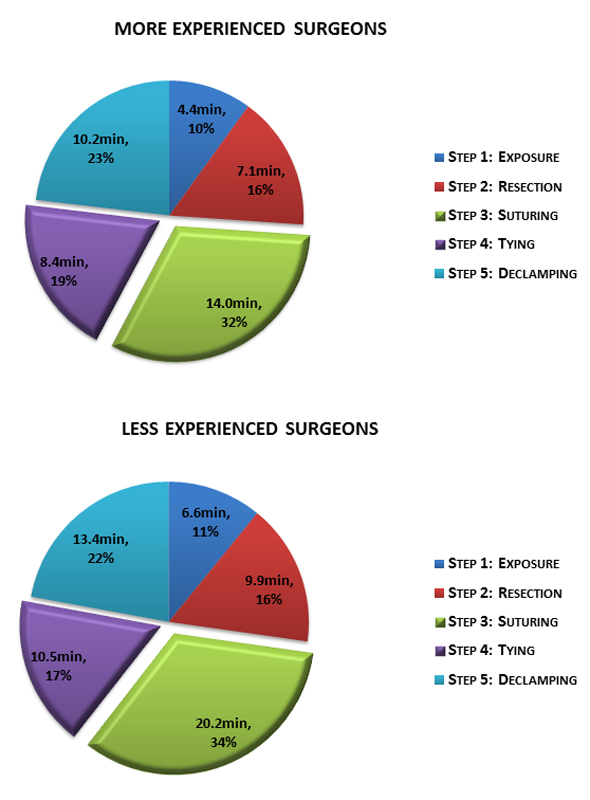
Figure 4 Comparison between less and more experienced surgeons in distribution of the five steps of aortic cross-clamp time.
The majority of patients (n = 48, 84%) received a Perimount Magna Ease tissue prosthesis (Edwards Lifesciences, Irvine, USA) and 9 patients (16%) received a Medtronic AP 360 medical mechanical prosthesis (Medtronic, Inc., Minneapolis, USA). In total a mean of 15.0 ± 2.0 sutures were used for aortic valve fixation. In six patients, three of each group, data were missing. Surgeons with more experience used a mean of 14.4 ± 1.8 sutures versus 15.9 ± 2.0 sutures (p = 0.005) used by the less experienced group. Table 3 shows the various sizes of the prosthesis and the amount of sutures used.
Table 3 Type of aortic valve prosthesis and number of sutures required.
| All Patients | ||||
|---|---|---|---|---|
| Less experienced surgeons | More experienced surgeons | |||
| n (%) | Sutures, n | n (%) | Sutures, n | |
| Edwards Lifesciences, sizes | ||||
| 19 mm | 0 (0) | 1 (3.3) | 12.0 ± 0.0 | |
| 21 mm | 4 (22.2) | 14.3 ± 0.6 | 5 (16.7) | 12.3 ± 0.5 |
| 23 mm | 6 (33.3) | 16.6 ± 3.6 | 14 (46.7) | 14.5 ± 1.1 |
| 25 mm | 5 (27.8) | 16.2 ± 0.8 | 7 (23.3) | 15.4 ± 1.9 |
| 27 mm | 3 (16.7) | 16.5 ± 0.7 | 2 (6.7) | 16.0 ± 0.0 |
| 29 mm | 0 (0) | 1 (3.3) | ||
| Medtronic, sizes | ||||
| 18 mm | 0 (0) | 1 (20) | 11 ± 0.0 | |
| 20 mm | 1 (25) | 15 ± 0.0 | 1 (20) | 13 ± 0.0 |
| 22 mm | 0 (0) | 1 (20) | 13 ± 0.0 | |
| 24 mm | 2 (50) | 15.5 ± 0.7 | 2 (40) | 15.5 ± 0.7 |
| 26 mm | 1 (25) | 17.0 ± 0.0 | 1 (20) | 17.0 ± 0.0 |
Data are presented as n (%) or mean ± standard deviation.
Overall in-hospital mortality was 0%. Follow up echocardiographic assessment was complete in 25 patients (43.9%), of whom 16 were operated on by the more experienced group and 9 by the less experienced group, and showed a mean gradient of 10.0 mm Hg (7.0–14.0 mm Hg). Postoperative data for experienced surgeons showed a mean gradient of 9.5 mm Hg (6.5–14.0) versus 10.0 mm Hg (8.0–17.0) (p = 0.70) for the less experienced group. Echocardiographic suspicion of trivial paravalvular leakage was found in one patient in the less experienced group.
This institutional process analysis investigating the different steps during conventional SAVR was able to confirm our initial expectation that the time period for prosthetic valve fixation, including placing and tying the sutures, is the most time-consuming part of the procedure at 16.4 min (32.3%) and 9.2 min (18.5%), respectively. Both account for over 50% of the cross-clamp time.
The importance of the surgeon’s experience is a well-known and significant factor that reduces AOx and may improve patient outcomes. During this process analysis, the experience of a surgeon was the most remarkable factor for the reduction of AOx. Compared with more experienced surgeons, who in our study were defined as a total aortic valve replacement experience of more than 100 procedures, surgeons with only limited experience (younger senior residents or surgical assistants still in the teaching programme) in SAVR had significantly longer AOx (60.6 ± 11.0 vs 44.1 ± 11.5 min, p <0.001). In comparison with experienced surgeons, they showed an equal distribution of the AOx between the different steps.
Interestingly, when the AOx of experienced surgeons in our study is compared with the AOx reported in the literature for procedures using sutureless aortic valve prosthesis, durations are similar despite use of the sutureless valve. AOx has been reported to be 39 ± 15 min for implanting the 3f Enable valve [15], 41.1 ± 10.6 min for the Edwards Intuity Valve System [13] and 40 ± 13.8 min for the Perceval S aortic bioprosthesis [16]; in our study, AOx during SAVR was 44.1 ± 11.5 min but went as low as 25 min, with a sutured aortic valve prosthesis and without the help of automated devices.
Another factor contributing to a longer AOx in surgeons with a lesser degree of experience might be linked to the number of sutures used for fixation of the aortic valve prosthesis. These numbers have not been reported in the literature so far. We were able to show a significantly lower number of sutures used for valve fixation by more experienced surgeons when compared with less experienced surgeons (14.4 ± 1.8 vs 15.9 ± 2.0 sutures, p = 0.005), with similar postoperative echocardiographic results in both groups: more experienced surgeons, mean gradient 9.5 mm Hg (6.5–14.0) vs less-experienced surgeons, mean gradient 10.0 mm Hg (8.0–17.0), p = 0.70.
In general, the literature reports longer CPB and AOx in SAVR through ministernotomy [5, 6, 9], although isolated studies showed no difference in CPB or AOx, but a longer total operating time [8]. Interestingly, one study even reported a shortening of CPB and AOx with the minimal-invasive techniques [7]. These different results might relate to the different sizes of the study populations or to the use of different statistical methods. Although these studies reported no difference in short- or long-term mortality, they did show a reduction of mechanical ventilation time, less blood loss and shorter intensive care unit and hospital stay in the minimal invasive patient population [5–9].
The Edwards Intuity valve system was investigated in a consecutive patient population of 152 patients with symptomatic, severe aortic stenosis. In this study, the direct sutureless fixation of the aortic bioprosthesis in the aortic annulus was able to reduce AOx by 23.5% [16]. Moreover, the surgical implantation of the Perceval S aortic bioprosthesis was able to reduce AOx by 39.4% [17] compared with standard SAVR by conventional techniques. Usage of the 3f Enable valve was able to reduce AOx by 38.5% [18]. At present there is only a single 5-year follow-up study of the 3f Enable prosthesis, showing very satisfactory clinical outcome with a reoperation rate of only 1.3%, occurrence of major paravalvular leak in only 0.8% and valve-related death in 1.6% of 141 patients. Haemodynamic parameters such as peak and mean systolic gradient were stable during 5 years of follow-up [19]. However, in some studies, sutureless valves have shown higher short-term complication rates compared with conventional SAVR [20, 21]. A further study noted that patients receiving sutureless valves had a higher rate of postoperative permanent pacemaker implantation [19].
At present, only studies on mitral valve annuloplasty with use of the automated knotting/cutting device in minimal-invasive operation have been published. These studies compared the use of the Cor-Knot devices with manually tied knots and a traditional knot pusher. Some studies report a significant reduction of CPB and AOx, as well as of the total operation time. They have shown equal morbidity and mortality [22, 23]. No studies report a comparison between Cor-Knot device and manually tied knots without the use of a knot pusher. In an ex-vivo setting, however, one study was able to show a significant time reduction compared with manual tying of sutures (12.4 vs 71.1 seconds per knot, p = 0.001) [21].
The following limitations need to be acknowledged: first, the study population was based on the experience of a single, tertiary care and teaching centre and the results may not be applicable to other centres with different procedural experience and patient selection; second, the process analysis included only a small number of patients and a larger study is required for the confirmation of our results; and finally, although patient and procedural data were collected prospectively, a certain selection bias might be present in the results of this study and cannot be excluded.
During isolated surgical aortic valve replacement for aortic valve stenosis, placing and tying the sutures accounts for 50.8% of the total aortic cross clamp time. Surgical experience impacts the five procedural steps equally. If a significant reduction in cross-clamp time might be beneficial for more complex patients (combined procedures, redo, high risk), these two steps may be shortened by automated processes.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Eveborn GW , Schirmer H , Heggelund G , Lunde P , Rasmussen K . The evolving epidemiology of valvular aortic stenosis. the Tromsø study. Heart. 2013;99(6):396–400. https://doi.org/10.1136/heartjnl-2012-302265
2 Iung B , Baron G , Butchart EG , Delahaye F , Gohlke-Bärwolf C , Levang OW , et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24(13):1231–43. https://doi.org/10.1016/S0195-668X(03)00201-X
3 Czarny MJ , Resar JR . Diagnosis and management of valvular aortic stenosis. Clin Med Insights Cardiol. 2014;8(Suppl 1):15–24. https://doi.org/10.4137/CMC.S15716
4 Nishimura RA , Otto CM , Bonow RO , Carabello BA , Erwin JP, 3rd , Guyton RA , et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):e57–185. https://doi.org/10.1016/j.jacc.2014.02.536
5 Brown ML , McKellar SH , Sundt TM , Schaff HV . Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2009;137(3):670–679.e5. https://doi.org/10.1016/j.jtcvs.2008.08.010
6 Gilmanov D , Bevilacqua S , Murzi M , Cerillo AG , Gasbarri T , Kallushi E , et al. Minimally invasive and conventional aortic valve replacement: a propensity score analysis. Ann Thorac Surg. 2013;96(3):837–43. https://doi.org/10.1016/j.athoracsur.2013.04.102
7 Neely RC , Boskovski MT , Gosev I , Kaneko T , McGurk S , Leacche M , et al. Minimally invasive aortic valve replacement versus aortic valve replacement through full sternotomy: the Brigham and Women’s Hospital experience. Ann Cardiothorac Surg. 2015;4(1):38–48.
8 Bonacchi M , Prifti E , Giunti G , Frati G , Sani G . Does ministernotomy improve postoperative outcome in aortic valve operation? A prospective randomized study. Ann Thorac Surg. 2002;73(2):460–5, discussion 465–6. https://doi.org/10.1016/S0003-4975(01)03402-6
9 Aris A , Cámara ML , Montiel J , Delgado LJ , Galán J , Litvan H . Ministernotomy versus median sternotomy for aortic valve replacement: a prospective, randomized study. Ann Thorac Surg. 1999;67(6):1583–7, discussion 1587–8. https://doi.org/10.1016/S0003-4975(99)00362-8
10 Melly L , Huber C , Delay D , Stumpe F . Chirurgische Behandlung der Aortenklappenfehler. Schweiz Med Forum. 2009;9(4):73-8. German.
11 Chalmers J , Pullan M , Mediratta N , Poullis M . A need for speed? Bypass time and outcomes after isolated aortic valve replacement surgery. Interact Cardiovasc Thorac Surg. 2014;19(1):21–6. https://doi.org/10.1093/icvts/ivu102
12 Doenst T , Borger MA , Weisel RD , Yau TM , Maganti M , Rao V . Relation between aortic cross-clamp time and mortality--not as straightforward as expected. Eur J Cardiothorac Surg. 2008;33(4):660–5. https://doi.org/10.1016/j.ejcts.2008.01.001
13 Al-Sarraf N , Thalib L , Hughes A , Houlihan M , Tolan M , Young V , et al. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int J Surg. 2011;9(1):104–9. https://doi.org/10.1016/j.ijsu.2010.10.007
14 Vahanian A , Alfieri O , Andreotti F , Antunes MJ , Barón-Esquivias G , Baumgartner H , et al.; Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33(19):2451–96. https://doi.org/10.1093/eurheartj/ehs109
15 Aymard T , Kadner A , Walpoth N , Göber V , Englberger L , Stalder M , et al. Clinical experience with the second-generation 3f Enable sutureless aortic valve prosthesis. J Thorac Cardiovasc Surg. 2010;140(2):313–6. https://doi.org/10.1016/j.jtcvs.2009.10.041
16 Kocher AA , Laufer G , Haverich A , Shrestha M , Walther T , Misfeld M , et al. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg. 2013;145(1):110–5, discussion 115–6. https://doi.org/10.1016/j.jtcvs.2012.07.108
17 Santarpino G , Pfeiffer S , Concistré G , Grossmann I , Hinzmann M , Fischlein T . The Perceval S aortic valve has the potential of shortening surgical time: does it also result in improved outcome? Ann Thorac Surg. 2013;96(1):77–81, discussion 81–2. https://doi.org/10.1016/j.athoracsur.2013.03.083
18 Permanyer E , Estigarribia AJ , Ysasi A , Herrero E , Semper O , Llorens R . The 3f Enable sutureless bioprosthesis: Early results, safeguards, and pitfalls. J Thorac Cardiovasc Surg. 2015;149(6):1578–83. https://doi.org/10.1016/j.jtcvs.2014.10.055
19 Englberger L , Carrel TP , Doss M , Sadowski J , Bartus K , Eckstein FF , et al. Clinical performance of a sutureless aortic bioprosthesis: five-year results of the 3f Enable long-term follow-up study. J Thorac Cardiovasc Surg. 2014;148(4):1681–7. https://doi.org/10.1016/j.jtcvs.2014.03.054
20 Hurley ET , O’Sullivan KE , Segurado R , Hurley JP . A Meta-Analysis Examining Differences in Short-Term Outcomes Between Sutureless and Conventional Aortic Valve Prostheses. Innovations (Phila). 2015;10(6):375–82. https://doi.org/10.1097/IMI.0000000000000221
21 Phan K , Tsai YC , Niranjan N , Bouchard D , Carrel TP , Dapunt OE , et al. Sutureless aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg. 2015;4(2):100–11.
22 Lee CY , Sauer JS , Gorea HR , Martellaro AJ , Knight PA . Comparison of strength, consistency, and speed of COR-KNOT versus manually hand-tied knots in an ex vivo minimally invasive model. Innovations (Phila). 2014;9(2):111–6, discussion 116. https://doi.org/10.1097/IMI.0000000000000051
23 Grapow MT , Mytsyk M , Fassl J , Etter P , Matt P , Eckstein FS , et al. Automated fastener versus manually tied knots in minimally invasive mitral valve repair: impact on operation time and short- term results. J Cardiothorac Surg. 2015;10(1):146. https://doi.org/10.1186/s13019-015-0344-4
No financial support and no other potential conflict of interest relevant to this article was reported.