Glycaemic, blood pressure and lipid goal attainment and chronic kidney disease stage of type 2 diabetic patients treated in primary care practices
DOI: https://doi.org/10.4414/smw.2017.14459
Antonella
Corcilloa, Edward
Pivinb, Fabrice
Lalubinb, Nelly
Pittelouda, Michel
Burnierb, Anne
Zanchiab
aService of Endocrinology, Diabetes and Metabolism, Department of Internal Medicine,
bService of Nephrology and Hypertension, Department of Internal Medicine,
Glycaemic, blood pressure and lipid goal attainment and chronic kidney disease stage of type 2 diabetic patients treated in primary care practices
w14459
Summary
INTRODUCTION
The prevalence of chronic kidney disease and diabetes is rising in Europe. These patients are at high cardiovascular and renal risk and need a challenging multifactorial therapeutic approach.
METHOD
The goal of this cross-sectional study was to examine the treatment and attainments of goals related to cardiovascular risk factors within chronic kidney disease stages in type 2 diabetic patients followed up by primary care physicians in Switzerland. Each participating physician entered into a web database the anonymised data of up to 15 consecutive diabetic patients attending her/his office between December 2013 and June 2014. Diabetes, hypertension and lipid lowering therapies were analysed, as well as glycated haemoglobin (HbA1c), blood pressure and low-density lipoprotein-cholesterol (LDL-c) levels and goal attainments by KDIGO chronic kidney disease stage 1 to 4.
RESULTS
A total of 1359 patients (mean age 66.5±12.4 years) were included by 109 primary care physicians. Chronic kidney disease stages 0–2, 3a, 3 b and 4 were present in 77.6%, 13.9%, 6.1%, and 2.4%, respectively. Average HbA1c was independent of chronic kidney disease stage and close to 7%; more than half of the patients reached the HbA1c goal. Eighty-four percent of patients were hypertensive and only 18.2% reached the then current Swiss or American Diabetes Association 2013 blood pressure goals. Despite loosening of blood pressure goals in 2015, only half of the patients reached them and most needed multiple therapies. Increased body mass index and advanced chronic kidney disease stage decreased the chance of reaching blood pressure goals. Lipid lowering therapy was prescribed in 62.1% of cases, with average LDL-c levels similar across chronic kidney disease stages. Only 42% of patients reached the LDL-c goal of <2.5 mmol/l in primary prevention and 32% reached <1.8 mmol/l in secondary prevention. Younger patients were treated significantly less aggressively than older patients (≥68 years, median age) for HbA1c, LDL-c and diastolic blood pressure control.
CONCLUSION
This cross-sectional study demonstrates that blood pressure and lipid goals are less often achieved than blood glucose control in type 2 diabetic patients followed up by primary care physicians in Switzerland. Goal attainments for HbA1c and LDL-c were not influenced by chronic kidney disease stages, in contrast to blood pressure. Reaching all three goals was rare (2.2%). There is a need for improvement in blood pressure control in advanced chronic kidney disease, whereas HbA1c goals may be loosened in the elderly and in advanced chronic kidney disease.
Introduction
Type 2 diabetes is a complex disease requiring a multifactorial approach. Since 1989, specific goals for blood glucose, blood pressure and lipid control have been elaborated by diabetes associations [1–3]. Recently these goals have been adapted to diabetic patients with chronic kidney disease (CKD) in recommendations from the Kidney Disease Outcomes Quality Initiative (KDOQI) [4], Kidney Disease: Improving Global Outcomes (KDIGO) [5], American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) [1–3]. Less stringent goals for HbA1c are now recommended in CKD patients. The Steno study was the first to emphasise the efficacy of a multifactorial approach in type 2 diabetes [6]. The group receiving intensified intervention on modifiable risk factors for cardiovascular disease had lower cardiovascular and microvascular events by around 50% compared with those receiving usual care. In the intensive therapy group, goals for HbA1c (goal <6.5%), systolic blood pressure (goal <130 mm Hg), diastolic blood pressure (goal <80 mm Hg) and total cholesterol (goal <175 mmol/l) were reached in approximately 15%, 45%, 70% and 70% of cases, respectively.
Primary care physicians (PCPs) often complain about the difficulty in reaching all goals, as time dedicated to the care of patients in an outpatient setting is confined. Goal attainments following recommendations have recently been examined in the US, in Italy, in Finland and in Canada with heterogeneous results [7–10]. For example, in a US survey, although testing rates in family practices for HbA1c, blood pressure and low-density lipoprotein cholesterol (LDL-c) were high (99.2%, 100% and 93.9%, respectively), goal attainment was not optimal. Goals were only attained in 38.9% for HbA1c (goal <7%), in 59.5% for blood pressure (goal <130/80 mm Hg) and in 71.8% for LDL-c (goal <2.6 mmol/l). This was a retrospective observational study done in a single area, showing good adherence to ADA recommendations of practitioners in clinics at Michigan State University. Although some countries have registries for diabetic patients, this are lacking in Switzerland. Thus surveys with primary care physicians are needed to examine this issue.
Objective and methods
The objective of this cross-sectional study was to examine the treatment, the CKD stages and goal attainments of type 2 diabetic patients followed up by PCPs in Switzerland. PCPs in German- (n = 16) and French-speaking (n = 4) cantons were approached for this purpose.
Data were collected from December 2013 to June 2014 by means of a cross-sectional survey of ambulatory patients visiting their physicians. The randomisation of the participating physicians was as follows: for the German and French linguistic regions of Switzerland, physicians were recruited randomly among PCPs until about 110 physicians were recruited. No physician from the Italian part of Switzerland was recruited. PCPs were asked to collect the data from up to 15 consecutive patients with type 2 diabetes, without selection of enrolled patients. The data were introduced anonymously into a web database elaborated by PNN AG (www.pnn.ch). Informed consent was obtained from each patient. Type 1 diabetes was an exclusion criterion.
Demographic and clinical data, complications related to diabetes, detailed antidiabetic, antihypertensive and lipid lowering therapies, and aspirin treatment were collected in a standardised web questionnaire. Estimated glomerular filtration rate (eGFR) was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [5]. As data on albuminuria were available for only a minority of patients, an eGFR ≥60 ml/min/1.73m2 was classified as CKD stage 0–2. Detailed antidiabetic therapy and CKD stages have been presented in a separate article [11]. Here, we present the data for attainment of HbA1c, blood pressure and LDL-c goals.
The study was accepted by the Ethics Committee of the Canton of Vaud.
Data analysis for goal attainments
Blood glucose control
The most recent values for HbA1c, creatinine and antidiabetic therapy were introduced into the database. Mean HbA1c values by KDIGO CKD stages [5] and age (<68 or ≥68 years) were examined. At initiation of the study, existing goals for HbA1c were individualised from <7% in patients at low risk of hypoglycaemia to <8% in patients with a history of severe hypoglycaemia, limited life expectancy, advanced microvascular complications and extensive comorbid conditions [5, 12]. Based on these recommendations, we examined HbA1c goal attainments of <7% in CKD stage 0–2 and <8% in CKD stage 3a, 3b and 4. Factors involved in attainment of HbA1c goals were assessed.
Blood pressure control
The most recent office blood pressure reading and antihypertensive therapy were entered into the database. Subjects were stratified by KDIGO CKD stage, and the number of antihypertensive classes analysed accordingly.
At initiation of the study, existing goals from the Swiss Hypertension Society were <130/80 mm Hg in diabetes [13]. Factors involved in blood pressure goal attainment were examined. Systolic and diastolic blood pressure levels by KDIGO CKD stages and age (<68 or ≥68 years) were examined.
Because blood pressure goal recommendations have substantially changed during recent years [14] and differed from Swiss recommendations at the time of the study, we analysed the data according to a “historical” goal of <130/80 mm Hg [13], ADA 2013 goal of <140/80 mm Hg [12], European Society of Hypertension (ESH) 2013 goal of <140/85 mm Hg [15] and the recent ADA 2015 goal of <140/90 mm Hg [2].
LDL-cholesterol goals
The most recent lipid profile and lipid lowering therapy were entered into the database. Mean LDL-c levels were analysed by KDIGO CKD stage and median age (<68 or ≥68 years). At the initiation of the study, existing Swiss goals for LDL-c in diabetes were <1.8 mmol/l in the case of end-organ damage or other cardiovascular risk factors and <2.5 mmol/l in uncomplicated type 2 diabetes [16]. We chose a goal of <1.8 mmol/l for patients with diabetes and a positive history of coronary heart disease, atherosclerosis, cerebrovascular disease and an eGFR<60ml/min. A goal of <2.5 mmol/l was chosen for others.
Statistics
Statistical analyses were conducted using STATA® version 14 (StataCorp, College Station, TX). Continuous and categorical variables were expressed as mean ± standard deviation (SD) and as number (%) of participants, respectively. Statistical significance was a 2-sided p-value of less than 0.05. We used Pearson’s correlation to assess associations between continuous variables. T-tests were performed to compare continuous variables between goal attainers and goal nonattainers. To compare the frequency of goal attainers across CKD stages we used the Pearson’s chi-squared test. We used a logistic regression to assess the associations between independent variables and goal attainment. To compare continuous variables across CKD stages we performed the nonparametric test for trend across ordered groups developed by Cuzick, which is an extension of the Wilcoxon rank-sum test [17].
Results
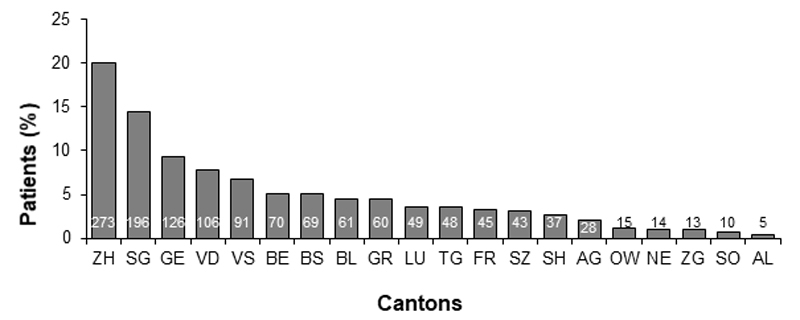
A total of 109 PCPs from 20 German- or French-speaking cantons participated in the study, with a total of 1359 patients included for analysis. Detailed geographic distribution of PCP offices has been described previously [11] (fig. 1).
Characteristics of participants according to CKD stage are presented in table 1
. There were a majority of male Caucasian patients. Average age was of 66.5±12.4 years (mean ± standard deviation) with most (57.5%) in the 60–80 year group. Mean duration of diabetes was 9.3 years and mean body mass index (BMI) 30.2 kg/m2. An eGFR ≥60 ml/min was found in 77.6% of the patients and 13.9%, 6.1% and 2.4%, respectively, were in CKD stage 3a, 3b and 4. Hypertension, dyslipidaemia and atherosclerosis were the three most reported comorbidities, present in 84%, 68% and 26% of cases, respectively. Age, duration of diabetes, sex, smoking status, diastolic blood pressure, antihypertensive therapy, lipid lowering therapy, neuropathy, retinopathy, history of atherosclerosis, cerebrovascular disease, coronary heart disease, and heart failure differed significantly by CKD stage.
Table 1 Characteristics of participants according to stage of chronic kidney disease (n = 1359).
|
Variable
|
CKD stage
|
Miss
|
p-value*
|
Total
|
|
0–2
|
3a
|
3b
|
4
|
|
Categorical, n (%)
|
|
|
|
|
|
|
|
| Total |
1057 (78.1) |
183 (13.5) |
84 (6.2) |
30 (2.2) |
5 |
|
1359 |
| Women |
430 (40.7) |
81 (44.3) |
45 (53.6) |
19 (63.3) |
2 |
0.01 |
577 (42.5) |
| Non-Caucasian |
17 (1.6) |
1 (0.5) |
2 (2.4) |
1 (3.3) |
0 |
0.53 |
21 (1.5) |
| Smoking (yes) |
136 (14.8) |
22 (12.8) |
7 (8.9) |
0 (0.0) |
158 |
0.06 |
165 (13.7) |
| Hypertension (yes) |
757 (82.6) |
151 (87.8) |
73 (92.4) |
26 (86.7) |
158 |
0.06 |
1201 (84.1) |
| Antihypertensive TTT (yes) |
782 (74.0) |
165 (90.2) |
77 (91.7) |
29 (96.7) |
0 |
<0.01 |
1056 (77.7) |
| Dyslipidaemia (yes) |
630 (68.8) |
110 (64.0) |
56 (70.9) |
23 (76.7) |
158 |
0.42 |
822 (68.4) |
| Lipid lowering TTT (yes) |
632 (59.8) |
122 (66.7) |
64 (76.2) |
23 (76.7) |
0 |
<0.01 |
844 (62.1) |
| Obesity (yes) |
471 (44.6) |
91 (49.7) |
36 (42.9) |
13 (43.3) |
0 |
0.59 |
612 (45.0) |
| Neuropathy (yes) |
111 (12.1) |
34 (19.8) |
21 (26.6) |
12 (40.0) |
158 |
<0.01 |
178 (14.8) |
| Retinopathy (yes) |
52 (5.7) |
18 (10.5) |
11 (13.9) |
4 (13.3) |
158 |
<0.01 |
85 (7.1) |
| Atherosclerosis (yes) |
206 (22.5) |
62 (36.0) |
34 (43.0) |
16 (53.3) |
158 |
<0.01 |
318 (26.5) |
| CV disease (yes) |
47 (5.1) |
17 (9.9) |
15 (19.0) |
5 (16.7) |
158 |
<0.01 |
84 (7.0) |
| CHD (yes) |
147 (16.0) |
45 (26.2) |
23 (29.1) |
13 (43.3) |
158 |
<0.01 |
228 (19.0) |
| Heart failure (yes) |
29 (3.2) |
19 (11.0) |
12 (15.2) |
8 (26.7) |
158 |
<0.01 |
68 (5.7) |
| Urogenital disease (yes) |
23 (2.5) |
9 (5.2) |
5 (6.3) |
1 (3.3) |
158 |
0.10 |
38 (3.2) |
|
Continuous (mean±SD)
|
|
|
|
|
|
|
|
| Age, yr |
64.0±11.9 |
74.5±9.0 |
76.8±9.2 |
79.0±9.4 |
0 |
<0.01 |
66.5±12.4 |
| Duration of DB, yr |
8.9±8.7 |
10.9±9.7 |
10.4±6.3 |
12.5±10.8 |
1 |
<0.01 |
9.3±8.8 |
| Number of DB drugs |
1.72±0.88 |
1.60±0.83 |
1.73±0.94 |
1.43±0.90 |
0 |
0.10 |
1.69±0.88 |
| Body mass index, kg/m2
|
30.2±5.8 |
30.4±5.3 |
30.2±5.6 |
30.3±5.3 |
1 |
0.55 |
30.2±5.7 |
| Diastolic BP, mm Hg |
80.8±9.9 |
78.8±11.3 |
77.8±12.1 |
73.7±9.2 |
0 |
<0.01 |
80.2±10.3 |
| Systolic BP, mm Hg |
136±14 |
137±15 |
138±14 |
138±16 |
0 |
0.11 |
137±14 |
| eGFR, ml/min/1.73 m2
|
87.0±15.3 |
52.7±3.9 |
37.4±4.2 |
23.8±4.4 |
5 |
– |
77.9±22.4 |
| LDL, mmol/l |
2.62±0.95 |
2.66±1.00 |
2.56±1.25 |
2.65±1.19 |
394 |
0.68 |
2.63±0.98 |
| HDL, mmol/l |
1.26±0.40 |
1.32±0.44 |
1.24±0.37 |
1.25±0.44 |
270 |
0.97 |
1.27±0.40 |
| Triglyceride, mmol/l |
1.98±1.12 |
1.92±1.00 |
2.16±1.07 |
1.92±0.89 |
275 |
0.61 |
1.97±1.09 |
| HbA1c, % |
7.12±1.22 |
7.16±1.13 |
7.16±1.20 |
7.18±1.00 |
2 |
0.40 |
7.12±1.20 |
Details of antidiabetic, antihypertensive, lipid lowering and aspirin therapies are presented in Table 2.
Table 2 Antidiabetic, antihypertensive, lipid lowering and aspirin therapies.
|
n
|
%
|
|
Antidiabetic therapy
|
1359 |
100 |
| Healthy way of life |
1037 |
76.31 |
| Pharmacological therapy |
1274 |
93.75 |
| Metformin |
1006 |
74.03 |
| DPP-4 inhibitor |
465 |
34.22 |
| Sulphonylurea |
279 |
20.53 |
| Glitazone |
41 |
3.02 |
| Glinide |
28 |
2.06 |
| Alpha glucosidase inhibitor |
4 |
0.29 |
| GLP-1 agonist |
85 |
6.25 |
| Insulin |
393 |
28.92 |
| Long acting insulin |
273 |
20.09 |
| Short and long acting insulin |
182 |
13.39 |
| Number of classes prescribed |
|
|
| 0 |
85 |
6.25 |
| 1 |
513 |
37.8 |
| 2 |
521 |
38.3 |
| 3 |
214 |
15.8 |
| 4 |
26 |
1.9 |
|
Antihypertensive therapy
|
1056 (77.7%) answers |
|
| ACE inhibitor |
420 |
39.7 |
| Angiotensin receptor blocker |
486 |
46.0 |
| Calcium antagonist |
334 |
31.6 |
| Diuretic |
483 |
45.7 |
| Beta-blocker |
354 |
33.5 |
| Other |
56 |
5.3 |
| Number of classes prescribed |
|
|
| 1 |
385 |
36.5 |
| 2 |
356 |
33.7 |
| 3 |
228 |
21.6 |
| 4 |
83 |
7.9 |
| 5 |
4 |
0.4 |
|
Lipid lowering therapy
|
844 (62.1%) answers |
|
| Statin |
793 |
94.0 |
| Fibrate |
18 |
2.13 |
| Inhibitor of cholesterol absorption |
28 |
3.3 |
| Other |
39 |
4.6 |
| Number of classes prescribed |
|
|
| 1 |
810 |
96.0 |
| 2 |
34 |
4.0 |
|
Aspirin
|
1066 (78.4%) answers |
|
|
575 |
53.9 |
Treatment and goal attainments
Glycated haemoglobin
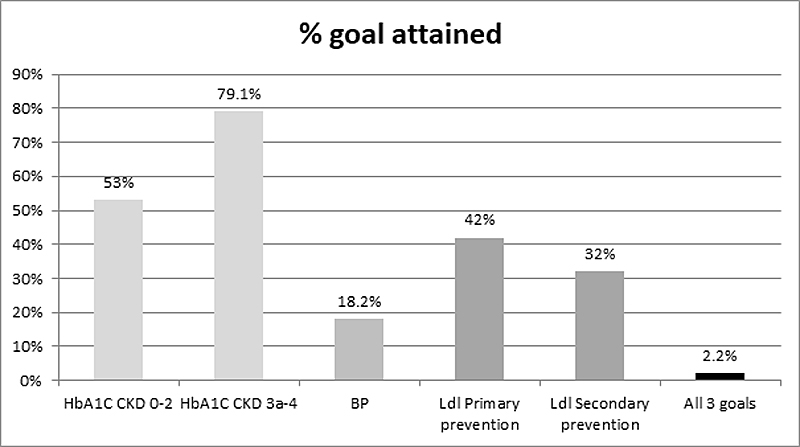
HbA1c was available for 1357 patients. Only 6.25% of subjects were on nonpharmacological therapy. The majority of subjects were on mono- (37.7%) or dual therapy (38.3%) with a clear preference for metformin (table 2). Mean (± SD) HbA1c was 7.12 ± 1.22%, 7.16 ± 1.13%, 7.16 ± 1.2% and 7.18 ± 1.0%, respectively, in subjects with stage 0–2, 3a, 3b and 4 CKD (table 1). The goal of <7% was reached in 53% of subjects with CKD stage 0–2 (and in 48.8% with CKD stage 3a–4); the goal of <8% was reached in 79.1% of patients with CKD stage 3a–4. Factors involved in HbA1c goal attainment are presented in table 3. Nonsmoking status, fewer comorbidities, increased age, shorter duration of diabetes, lower BMI, higher HDL-c and impaired renal function were factors associated with improved HbA1c goal attainment. Mean HbA1c was 7.2% in the group aged <68 y and 7.0% in the group aged ≥68y, and differed significantly (p <0.01).
Table 3 Odd ratios for glycated haemoglobin goal attainment in univariate analysis (n = 1352).
|
HbA1c goal attainment
|
|
CKD stages 0–2
(n = 1055)
|
CKD stages 3a, 3b and 4
(n = 297)
|
|
Independent variables
|
Odds ratio
(95% confidence interval)
|
p-value
|
Odds ratio
(95% confidence interval)
|
p-value
|
|
Categorical
|
|
|
|
|
| Women |
1.08 (0.84–1.38) |
0.56 |
0.90 (0.51–1.59) |
0.73 |
| Non-Caucasian |
0.48 (0.18–1.30) |
0.15 |
0.25 (0.03–1.83) |
0.17 |
| Smoking (yes) |
0.51 (0.35–0.75) |
<0.01 |
1.22 (0.44–3.35) |
0.70 |
| Hypertension (yes) |
0.55 (0.39–0.78) |
<0.01 |
1.20 (0.49–2.94) |
0.70 |
| Antihypertensive TTT (yes) |
0.58 (0.44–0.77) |
<0.01 |
1.48 (0.59–3.70) |
0.40 |
| Dyslipidaemia (yes) |
0.79 (0.60–1.05) |
0.11 |
0.70 (0.37–1.35) |
0.29 |
| Lipid lowering TTT (yes) |
0.68 (0.53–0.88) |
<0.01 |
0.99 (0.54–1.84) |
0.98 |
| Obesity (yes) |
0.67 (0.52–0.85) |
<0.01 |
0.65 (0.37–1.14) |
0.13 |
| Neuropathy (yes) |
0.47 (0.31–0.71) |
<0.01 |
1.19 (0.58–2.41) |
0.64 |
| Retinopathy (yes) |
0.40 (0.22–0.73) |
<0.01 |
0.44 (0.20–0.98) |
0.04 |
| Atherosclerosis (yes) |
0.78 (0.57–1.06) |
0.11 |
1.13 (0.62–2.07) |
0.69 |
| CV disease (yes) |
0.82 (0.46–1.48) |
0.51 |
0.63 (0.28–1.39) |
0.25 |
| CHD (yes) |
0.83 (0.58–1.18) |
0.31 |
0.91 (0.48–1.73) |
0.78 |
| Heart failure (yes) |
1.82 (0.84–3.97) |
0.13 |
0.96 (0.41–2.22) |
0.92 |
| Urogenital disease (yes) |
0.49 (0.21–1.18) |
0.11 |
1.00 (0.27–3.65) |
0.99 |
|
Continuous
|
|
|
|
|
| Age, 10 yr |
1.18 (1.06–1.31) |
<0.01 |
1.17 (0.87–1.58) |
0.30 |
| Duration of DB, yr |
0.97 (0.96–0.99) |
<0.01 |
0.97 (0.95–1.00) |
0.06 |
| Number of DB drugs |
0.44 (0.37–0.51) |
<0.01 |
0.51 (0.37–0.72) |
<0.01 |
| Body mass index, 5 kg/m2
|
0.88 (0.79–0.98) |
0.02 |
0.82 (0.64–1.06) |
0.13 |
| Diastolic BP, 10 mmHg |
0.93 (0.82–1.05) |
0.24 |
0.76 (0.59–0.98) |
0.03 |
| Systolic BP, 10 mmHg |
0.92 (0.84–1.00) |
0.05 |
0.84 (0.69–1.02) |
0.08 |
| eGFR, 10 ml/min/1.73 m2
|
0.89 (0.82–0.97) |
<0.01 |
1.13 (0.87–1.46) |
0.36 |
| LDL, mmol/l |
0.96 (0.83–1.12) |
0.62 |
1.23 (0.85–1.80) |
0.27 |
| HDL, mmol/l |
1.53 (1.08–2.17) |
0.02 |
2.78 (1.09–7.14) |
0.03 |
| Triglyceride, mmol/l |
0.76 (0.67–0.87) |
<0.01 |
0.58 (0.43–0.79) |
<0.01 |
| HbA1c, % |
– |
– |
– |
– |
Blood pressure
Overall blood pressure values were available for 1359 patients. Angiotensin II receptor blockers (ARBs), diuretics and ACE inhibitors (ACEIs) were the most prescribed antihypertensive classes (46%, 45.7% and 39.7%, respectively) followed by beta-blockers and calcium channel blockers (CCBs) (33.5% and 31.6%, respectively) (table 2). Among the 26.2% patients on dual therapy, the preferred option was ARB/diuretic (28.5%) followed by ACEI/diuretic (19.7%) and ARB/CCB (14.4%). Two patients were on inappropriate ARB/ACEI therapy. With the progression of CKD stages, the number of antihypertensive classes increased. A total of 234/1108 (21%) of patients with blood pressure ≥130/80 mm Hg were not on antihypertensive therapy (fig. 2). The number of antihypertensive drugs was the same in blood pressure goal attainers and nonattainers (1.60 vs 1.56, p = 0.6). In blood pressure goal attainers, the number of antihypertensive drugs increased with advancing CKD stage (p <0.001). After stratifying for two age groups, mean systolic and diastolic blood pressure were 136.1 mm Hg and 82.6 mm Hg, respectively, in the patients aged <68 years, and 137.0 mm Hg and 77.8 mm Hg, respectively in those aged ≥68 years (median age); for diastolic blood pressure, the difference was significant (p<0.01).
Estimated GFR was negatively associated with systolic blood pressure (p <0.01) and positively associated with diastolic blood pressure (p<0.01). Systolic blood pressure did not differ by CKD stage; however, diastolic blood pressure was lower at advanced CKD stage (nptrend p <0.001; table 3). The blood pressure goal of <130/80 mmg Hg was reached in only 18.2% of patients. Of interest, a blood pressure goal ≤130/80 mmg Hg was reached in 30% of cases, illustrating a clear digit preference for numbers ending in zero.
Factors involved in blood pressure goal attainment are presented in table 4. Presence of atherosclerosis, coronary heart disease and congestive heart disease increased goal attainment. Low BMI and low LDL-c levels were factors associated with improved blood pressure goal attainment. Recommendations for blood pressure goals have considerably changed recently and have differed between diabetes or hypertension associations. For better clarity, figure 3 presents different goals and goal attainments by CKD stage. Goal attainment increased with less stringent goals but, however, decreased with more advanced CKD. For example, the less stringent ADA 2015 goal of a blood pressure <140/90 mm Hg was reached by only 54.6%, 51.9%, 47.6%, 40%, respectively, of patients in CKD stages 0–2, 3a, 3b and 4. Goal attainment was significantly lower in advanced CKD.
Table 4 Odd ratios for blood pressure goal attainment in univariate analysis (n = 1354).
|
Independent variables
|
Odds ratio
(95% confidence interval)
|
p-value
|
|
Categorical
|
|
|
| Women |
1.10 (0.83–1.45) |
0.52 |
| Non-Caucasian |
0.75 (0.22–2.56) |
0.64 |
| Smoking (yes) |
1.12 (0.74–1.71) |
0.58 |
| Hypertension (yes) |
0.48 (0.33–0.68) |
<0.01 |
| Antihypertensive TTT (yes) |
0.72 (0.52–0.98) |
0.04 |
| Dyslipidaemia (yes) |
1.16 (0.84–1.61) |
0.37 |
| Lipid lowering TTT (yes) |
1.09 (0.82–1.46) |
0.54 |
| Obesity (yes) |
0.58 (0.44–0.78) |
<0.01 |
| Neuropathy (yes) |
1.58 (1.08–2.31) |
0.02 |
| Retinopathy (yes) |
1.67 (1.01–2.79) |
0.05 |
| Atherosclerosis (yes) |
1.64 (1.20–2.25) |
<0.01 |
| CV disease (yes) |
1.38 (0.81–2.35) |
0.24 |
| CHD (yes) |
2.13 (1.52–2.99) |
<0.01 |
| Heart failure (yes) |
3.32 (1.99–5.53) |
<0.01 |
| Urogenital disease (yes) |
0.86 (0.35–2.08) |
0.73 |
|
Continuous
|
|
|
| Age, 10 yr |
1.03 (0.92–1.15) |
0.59 |
| Duration of DB, yr |
1.02 (1.01–1.04) |
<0.01 |
| Number of DB drugs |
1.07 (0.91–1.25) |
0.41 |
| Body mass index, 5 kg/m2
|
0.78 (0.69–0.90) |
<0.01 |
| Diastolic BP, 10 mmHg |
– |
– |
| Systolic BP, 10 mmHg |
– |
– |
| eGFR, 10 ml/min/1.73 m2
|
0.96 (0.91–1.03) |
0.25 |
| LDL, mmol/l |
0.74 (0.62–0.88) |
<0.01 |
| HDL, mmol/l |
0.77 (0.52–1.15) |
0.21 |
| Triglyceride, mmol/l |
0.86 (0.73–1.01) |
0.06 |
| HbA1c, % |
0.94 (0.83–1.06) |
0.30 |
Lipids
Overall, LDL-c values were available in 965 patients. Among the 62.1% of patients on lipid lowering therapy, 94% were taking a statin. LDL-c values were similar across CKD stages of (mean ± SD): 2.62 ± 0.95 mmol/l, 2.66 ± 1.0 mmol/l, 2.56 ± 1.25 mmol/l and 2.65 ± 1.19 mmol/l, respectively, in subjects with CKD stages 0–2, 3a, 3b and 4 (table 1). In patients qualifying for primary prevention (n = 473), an LDL-c goal of <2.5 mmol/l was reached in 42% (n = 198) of cases. For those qualifying for secondary prevention (n = 407), an LDL-c goal of <1.8 mmol/l was reached in 32% of cases (n = 130) (fig. 4). LDL-c goal attainments did not change across all CKD stages.
Factors involved in LDL-c goal attainment are presented in table 5. Gender, smoking status and co-morbidities, age, duration of diabetes, DBP were factors associated with improved LDL goal attainment. Mean LDL-c was of 2.76 mmol/l in patients aged <68 years and 2.5 mmol/l in those aged ≥68 years, and differed significantly between the two age groups (p <0.01).
Table 5 Odd ratios for low-density lipoprotein goal attainment in univariate analysis (n = 937).
|
LDL goal attainment
|
|
CKD stages 0–2
n = 748)
|
CKD stages 3a, 3b and 4
(n = 189)
|
|
Independent variables
|
Odds ratio
(95% confidence interval)
|
p-value
|
Odds ratio
(95% confidence interval)
|
p-value
|
|
Categorical
|
|
|
|
|
| Women |
0.67 (0.49–0.91) |
0.01 |
0.55 (0.28–1.07) |
0.08 |
| Non-Caucasian |
0.68 (0.24–1.95) |
0.47 |
* |
* |
| Smoking (yes) |
0.56 (0.35–0.88) |
0.01 |
0.99 (0.30–3.25) |
0.98 |
| Atherosclerosis (yes) |
– |
– |
– |
– |
| Hypertension (yes) |
1.15 (0.77–1.72) |
0.48 |
1.12 (0.38–3.26) |
0.84 |
| Antihypertensive TTT (yes) |
2.04 (1.39–2.99) |
<0.01 |
4.50 (0.57–35.55) |
0.15 |
| Dyslipidaemia (yes) |
1.31 (0.94–1.83) |
0.11 |
0.62 (0.31–1.24) |
0.17 |
| Lipid lowering TTT (yes) |
2.85 (2.03–4.01) |
<0.01 |
1.78 (0.79–4.00) |
0.16 |
| Obesity (yes) |
1.29 (0.96–1.73) |
0.10 |
1.17 (0.61–2.25) |
0.63 |
| Neuropathy (yes) |
1.99 (1.26–3.15) |
<0.01 |
1.47 (0.67–3.24) |
0.33 |
| Retinopathy (yes) |
2.55 (1.29–5.06) |
0.01 |
2.40 (0.89–6.49) |
0.08 |
| CV disease (yes) |
– |
– |
– |
– |
| CHD (yes) |
– |
– |
– |
– |
| Heart failure (yes) |
2.19 (0.92–5.19) |
0.08 |
1.80 (0.70–4.65) |
0.22 |
| Urogenital disease (yes) |
1.15 (0.42–3.13) |
0.78 |
1.88 (0.51–6.97) |
0.34 |
|
Continuous
|
|
|
|
|
| Age, 10 yr |
1.20 (1.05–1.38) |
0.01 |
1.19 (0.80–1.77) |
0.40 |
| Duration of DB, yr |
1.02 (1.01–1.04) |
0.01 |
1.01 (0.98–1.04) |
0.54 |
| Number of DB drugs |
1.18 (1.00–1.40) |
0.06 |
0.86 (0.59–1.25) |
0.42 |
| Body mass index, 5 kg/m2
|
1.12 (0.99–1.27) |
0.08 |
1.15 (0.86–1.53) |
0.34 |
| Diastolic BP, 10 mm Hg |
0.77 (0.66–0.91) |
<0.01 |
0.82 (0.60–1.10) |
0.19 |
| Systolic BP, 10 mm Hg |
0.99 (0.89–1.11) |
0.89 |
0.79 (0.63–1.01) |
0.06 |
| eGFR, 10 ml/min/1.73 m2
|
0.92 (0.84–1.02) |
0.10 |
0.78 (0.59–1.04) |
0.09 |
| LDL, mmol/l |
– |
– |
– |
– |
| HDL, mmol/l |
1.04 (0.72–1.51) |
0.84 |
0.63 (0.27–1.48) |
0.29 |
| Triglyceride, mmol/l |
0.99 (0.82–1.20) |
0.91 |
0.97 (0.64–1.46) |
0.87 |
| HbA1c, % |
0.96 (0.85–1.09) |
0.51 |
1.20 (0.91–1.59) |
0.19 |
In this study, HbA1c, blood pressure and lipid goals were all achieved in only 30 patients (2.2%) (fig. 5). Characteristics by goal attainment are available in supplementary table S1 (appendix).
Discussion
The European prevalence of CKD is rising in diabetic subjects [18], and is the leading cause of end-stage renal disease (ESRD). The presence of nephropathy considerably amplifies cardiovascular risk and mortality. A multifactorial approach is thus necessary to decrease the renal function decline and cardiovascular risk. In accordance, guidelines have been released by many societies [1–4, 19] and the goal of this study was to examine whether goal attainments were reached in a primary care setting in Switzerland.
The average patient age, sex distribution, HbA1c, systolic blood pressure and LDL-c level in this survey was similar to results of other Swiss diabetic surveys [20, 21]. Our data can therefore be considered as representative of the Swiss diabetic population. To our knowledge, this is the first survey analysing the care of Swiss type 2 diabetic patients by CKD stage. Overall, this study demonstrates that the majority of subjects with type 2 diabetes are men with hypertension and lipid abnormalities. HbA1c goals are most often reached, but blood pressure goals are achieved less frequently, especially in advanced CKD stages. Among those qualifying for secondary prevention, only a third reached the recommended LDL-c goal.
The demographic characteristics of the population in this survey, are similar to other studies in Europe in terms of age group, sex and ethnicity, but differ from the US study in which sexes were equally represented and ethnicity more diverse [7–10].
HbA1c and glycaemic control
In our study, average HbA1c was independent of CKD stage and close to 7%. This was similar to the Finnish study, in which HbA1c ranged between 6.9 and 7.3% at CKD stages 2–5 [9]. Thus Swiss physicians may not differentiate their goals by CKD stage in spite of KDOQI 2012 recommendations [4]. Furthermore, older subjects (71–90 years old) were treated significantly more aggressively than younger subjects with an average HbA1c <7%. Hence these results highlight the need to improve physician awareness of the risk of hypoglycaemia in CKD and in the elderly [22].
Tight glycaemic control is beneficial in the primary prevention of nephropathy in type 1 [23, 24] and type 2 diabetes [25–27], with a decrease in the incidence of microalbuminuria and progression to macroalbuminuria, but its effect in secondary prevention is less well established. Indeed, the effect on renal function decline has been demonstrated in type 1 diabetes in the 22-year Diabetes Control and Complications Trial / Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study [28], and in subjects with type 2 diabetes after 10 years in the UK Prospective Diabetes Study (UKPDS) study [25]. Yet both studies involved intensive glucose control early in the course of diabetes. The cardiovascular and renal efficacy and safety of tight glycaemic control in type 2 diabetes patients at high cardiovascular risk needs to be carefully investigated [26, 27]. The ACCORD (Action to Control Cardiovascular Risk in Diabetes), ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) and VADT (Veteran's Affairs Diabetes Trial) trials showed an improvement in the prevention and progression of albuminuria with tight glycaemic control, but no benefits on renal function decline in high risk subjects with type 2 diabetes and cardiovascular disease. These studies, however, excluded subjects with creatinine levels over 130 to 140 µmol/l. Eventually, a post hoc analysis of the ADVANCE trial did show a significant reduction in the risk of ESRD, although numbers were low and the number needed to treat (NNT) was 445 to prevent one case of ESRD in 5 years [29]. This was recently confirmed in the ADVANCE-ON follow-up trial, which found an NNT of 109 for CKD stages 1–2 and 393 for CKD stages 3 or greater to prevent one case of ESRD over 9.9 years of follow-up [30]. A recent and important study demonstrated a decrease in new onset or worsening of nephropathy in patients with diabetes and established cardiovascular disease treated with empagliflozin, a sodium-glucose cotransporter 2 (SGLT-2) inhibitor [31]. Most experts agree that the nephroprotection provided by empagliflozin is not due to the mild improvement in blood glucose but to many other hemodynamic and metabolic effects currently under investigation [32]. As SGLT-2 inhibitors reached the market in mid-2014, no patients in this study were on this treatment.
CKD is associated with an increased risk of hypoglycaemia [33], which could increase mortality [34, 35]. The ACCORD study was prematurely stopped because of increased all-cause mortality rate in the intensive intervention arm (HbA1c goal <6%) [33]. Consequently, the KDOQI clinical practice guidelines published in 2012 recommend not targeting an HbA1c of <7% in CKD patients at risk of hypoglycaemia [4]. Likewise, the ADA recommends a personalised target based on age, comorbidities, disease duration, life expectancy, risk of hypoglycaemia, patient attitude and patient resource [1]. In our study, goal attainment was obviously easier to reach in CKD stages 3 to 4 because it was loosened to <8%.
Blood pressure
Similar to the Finnish and Canadian surveys, in this survey the large majority of subjects were hypertensive [9, 10] and only half reached the ADA 2015 goals for blood pressure and even fewer the more stringent ADA 2013 goals prevailing during the study [2]. Advanced CKD stage was associated with multiple antihypertensive drugs and reduced ADA 2015 goal attainment, as found in the Finnish population [9]. Last digit preference suggests that PCPs round measured values, which can significantly bias the analysis for goal attainment if < or ≤ are chosen for blood pressure target values. This phenomenon has been described when physicians use manual as well as automatic blood pressure measuring devices [36].
Targeting blood pressure levels is a priority in the care of patients with type 2 diabetes. Reduction in cardiovascular events with lower blood pressure has been demonstrated in a large number of randomised control trials including diabetic patients [37]. Tight systolic blood pressure control (≤130 mm Hg) decreased stroke and overt nephropathy, but not all-cause mortality, whereas less tight control (≤135 mm Hg) decreased all-cause mortality [38]. Other studies showed a decrease in the incidence and progression of nephropathy with systolic blood pressure <140 mm Hg [39–41]. Therefore, blood pressure goals have been loosened from <130/80 mm Hg before 2013 to <140/90 mm Hg (or <130/80 mm Hg in case of stage A2–A3 albuminuria) in 2015 [2]. Our study demonstrates that, despite loosening of goals, only half of patients reach them and most needed multiple therapies. The fact that a fifth of patients not at goal were not on antihypertensive drug therapy emphasises the problem of medical inertia and the need of continual PCP education.
In our study, blockade of the renin angiotensin system with ARBs or ACE inhibition and diuretics were clearly the preferred options for antihypertensive therapy. The KDIGO 2012 and KDOQI recommendations propose an ARB or ACEI as first line therapy in diabetic hypertensive subjects with albuminuria (stage A2 or more) [4, 42]. Nephroprotection with irbesartan and losartan has been demonstrated in CKD type 2 diabetic patients independent of blood pressure reduction [41, 43]. Although the KDIGO guidelines do not recommend specific second-line therapy, amlodipine could be preferred to hydrochlorothiazide because of superior efficacy in the reduction of cardiovascular events and the progression of CKD in hypertensive subjects at high cardiovascular risk on ACE inhibition [44]. In our study, we showed that multiple drug regimens were needed more with advanced CKD stages, as already demonstrated in a cross-sectional observational Swiss survey including diabetic and CKD patients visiting PCPs [45]. Multimedication and comorbidities are a cause of nonadherence to treatment [46], which could be improved by single pill combination therapy and clinical pharmacist evaluation of adverse effects and drug interactions [45].
Lipids
Lipid lowering therapy was prescribed in 62.1% of our patients, similarly to the Finnish study [9] and fewer than half of the patients reached the goal of <2.5 mmol/l for primary prevention. This rate fell to a third for those qualifying for secondary prevention. Interestingly, CKD was not a limiting factor for lipid goal attainment. Better results were reported in the Canadian survey, with 82% of type 2 diabetic patients taking lipid-lowering therapy and 57% attaining the defined LDL-c goal of ≤2.0 mmol/l [10]. To explain these low rates of success, low adherence to treatment, patient education and/or limited access to medical care were highlighted. Likewise, in a Swiss survey including 19.7% of diabetics, diabetes was a predictor for treatment nonadherence. Also, although diabetes was as a positive predictor for diagnostic adherence, it was not a predictor for overall guideline adherence, highlighting the difficulty in reaching lipid treatment goals in diabetes [47].
Patients with diabetes and CKD represent a very high cardiovascular risk group [48, 49]. The Study of Heart and Renal Protection (SHARP) demonstrated a decrease in cardiovascular events for stage 3a–5 CKD patients who were on statin/ezetimibe combination therapy. However, no significant effect was observed for mortality or the progression of renal disease [50]. Statin or statin/ezetimibe combination is recommended as the first line LDL-c therapy, regardless of the baseline values, in nondialysis diabetic CKD patients [2, 51]. Current recommendations for patients with diabetes and CKD do not specify any LDL-c goal [2, 51]. However, after initiating lipid lowering therapy, measuring LDL-c is optional to assess patient drug adherence. Doses of lipid lowering agents should be adapted to renal function as side effects are more prevalent and serious in this population [52].
Interestingly, this study showed that patients aged >68 years have significantly lower levels of LDL-c compared with younger patients. An Italian survey among diabetic CKD patients also showed that LDL-c goal was attained less often by younger adults than older adults [8]. Drug adherence is known to be lowest in younger adults, specifically in younger men. Recently, another Italian survey demonstrated a higher all-cause mortality and cardiovascular mortality in younger (<75 years) CKD diabetic patients [53], which may be due to a difficulty in reaching goals for cardiovascular risk factors in this younger population. This will need further investigation.
Limitations of the study
As our sampling method was not truly random (i.e., randomly choosing participating physicians) but based on the willingness of physicians to participate to the study, the sample studied may not be representative of the population as found in a population-based cohort study. We cannot exclude the possibility that physicians agreeing to participate in the study were more compliant with current recommendations than those who refused. Furthermore, patients selected for the data collection obviously represent a group more adherent to visits to physician offices, and men in this case may be underrepresented. This may have created a considerable bias in selection. The observational design of this study does not allow the reasons why targets were not achieved to be identified. Finally, in this study it was not possible to differentiate between non-CKD patients and patients with CKD stage 1 to 2 because of insufficient reporting of albuminuria. Devices for blood pressure measurements and the number of blood pressure measurements were not standardised. Because of the known white coat effect, the percentage of patients attaining blood pressure goals might have been underestimated. Laboratory measurements were not standardised and based on single values.
Ideally, the results of this study should be compared with those found in a population-based cohort study. Furthermore, barriers to patient and physician compliance should be assessed in order to implement strategies to improve goal attainments.
Conclusion
This cross-sectional study demonstrates that blood pressure and lipid goal attainments are less often achieved than blood glucose control in type 2 diabetic patients followed up by PCPs in Switzerland. Reaching all goals current in 2013 was close to impossible. Goal attainments for HbA1c and LDL-c were not influenced by CKD stage. HbA1c and LDL-c were less well controlled in younger individuals. There is a need for improvement in cardiovascular risk control in the younger population, blood pressure control in advanced CKD, whereas HbA1c goals may be loosened in the elderly and in advanced CKD.
Appendix Supplementary table
Table S1 Patient characteristics by goal attainment.
|
Hba1c goal attained
|
BP goal attained
|
LDL goal attained
|
|
Independent variables
|
No
|
Yes
|
No
|
Yes
|
No
|
Yes
|
|
Categorical
|
|
|
|
|
|
|
| Total |
557 |
795 |
1110 |
249 |
607 |
332 |
| Women |
228 |
346 |
467 |
110 |
280 |
116 |
| Men |
329 |
449 |
643 |
139 |
327 |
216 |
|
Continuous
|
|
|
|
|
|
|
| Age, yr |
64.1±12.0 |
68.3±12.3 |
66.4±12.0 |
66.7±13.7 |
67.0±12.6 |
68.2±10.4 |
| Body mass index, kg/m2
|
30.7± 5.3 |
29.9± 5.9 |
30.5± 5.7 |
29.0± 5.5 |
29.9± 5.8 |
30.7± 5.7 |
| Diastolic BP, mm Hg |
81±10 |
79± 10 |
82± 10 |
70± 6 |
80± 10 |
78±10 |
| Systolic BP, mm Hg |
138±15 |
136±14 |
140±12 |
119±7 |
136±14 |
136±14 |
| eGFR, ml/min/1.73 m2
|
83.5±20.5 |
73.8±22.8 |
78.2±22.2 |
76.4±23.1 |
77.7±22.5 |
79.0±20.7 |
| LDL, mmol/l |
2.63±1.02 |
2.63±0.95 |
2.68±1.00 |
2.41±0.86 |
3.14±0.82 |
1.71±0.45 |
| HDL, mmol/l |
1.22±0.40 |
1.30±0.41 |
1.27±0.41 |
1.24±0.37 |
1.28±0.37 |
1.26±0.46 |
| Triglyceride, mmol/l |
2.18±1.17 |
1.84±1.02 |
2.00±1.10 |
1.84±1.06 |
1.83±0.74 |
1.82±0.83 |
| HbA1c, % |
8.18±1.10 |
6.39±0.55 |
7.14±1.23 |
7.04±1.10 |
7.04±1.21 |
7.04±1.10 |
Author contributions
A. Corcillo Vionnet and E. Pivin contributed equally and are both first authors.
References
1
American Diabetes Association. (6) Glycemic targets. Diabetes Care. 2015;38(Suppl_1):S33–40. https://doi.org/10.2337/dc15-S009
2
American Diabetes Association. (8) Cardiovascular disease and risk management. Diabetes Care. 2015;38(Suppl_1):S49–57. https://doi.org/10.2337/dc15-S011
3
Rydén
L
,
Grant
PJ
,
Anker
SD
,
Berne
C
,
Cosentino
F
,
Danchin
N
, et al.
the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2013;34(39):3035–87. Available at: .https://doi.org/10.1093/eurheartj/eht108
4
National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60(5):850–86. https://doi.org/10.1053/j.ajkd.2012.07.005
5
KDIGO Board. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3(1, suppl):1–150.
6
Gaede
P
,
Vedel
P
,
Larsen
N
,
Jensen
GV
,
Parving
HH
,
Pedersen
O
. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–93. https://doi.org/10.1056/NEJMoa021778
7
Rao
DT
,
Sunio
LK
,
Lo
YJ
,
Gossain
VV
. Comparison of the adherence to the american diabetes association guidelines of diabetes care in primary care and subspecialty clinics. J Diabetes Metab Disord. 2015;14(1):35. https://doi.org/10.1186/s40200-015-0158-x
8
De Cosmo
S
,
Viazzi
F
,
Pacilli
A
,
Giorda
C
,
Ceriello
A
,
Gentile
S
, et al.; AMD-Annals Study Group. Achievement of therapeutic targets in patients with diabetes and chronic kidney disease: insights from the Associazione Medici Diabetologi Annals initiative. Nephrol Dial Transplant. 2015;30(9):1526–33. https://doi.org/10.1093/ndt/gfv101
9
Metsärinne
K
,
Bröijersen
A
,
Kantola
I
,
Niskanen
L
,
Rissanen
A
,
Appelroth
T
, et al.; STages of NEphropathy inType 2 Diabetes Study Investigators. High prevalence of chronic kidney disease in Finnish patients with type 2 diabetes treated in primary care. Prim Care Diabetes. 2015;9(1):31–8. https://doi.org/10.1016/j.pcd.2014.06.001
10
Leiter
LA
,
Berard
L
,
Bowering
CK
,
Cheng
AY
,
Dawson
KG
,
Ekoé
JM
, et al.
Type 2 diabetes mellitus management in Canada: is it improving?
Can J Diabetes. 2013;37(2):82–9. https://doi.org/10.1016/j.jcjd.2013.02.055
11
Lamine
F
,
Lalubin
F
,
Pitteloud
N
,
Burnier
M
,
Zanchi
A
. Chronic kidney disease in type 2 diabetic patients followed-up by primary care physicians in Switzerland: prevalence and prescription of antidiabetic drugs. Swiss Med Wkly. 2016;146:w14282.
12
American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–66. https://doi.org/10.2337/dc13-S011
13Swiss Society of Hypertension (SSH). Guidelines 2009; http://www.swisshypertension.ch/guidelines.htm.
14
Fox
CS
,
Golden
SH
,
Anderson
C
,
Bray
GA
,
Burke
LE
,
de Boer
IH
, et al.; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Quality of Care and Outcomes Research; American Diabetes Association. Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement From the American Heart Association and the American Diabetes Association. Diabetes Care. 2015;38(9):1777–803. https://doi.org/10.2337/dci15-0012
15
Mancia
G
,
Fagard
R
,
Narkiewicz
K
,
Redon
J
,
Zanchetti
A
,
Böhm
M
, et al.
2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159–219. https://doi.org/10.1093/eurheartj/eht151
16
Reiner
Z
,
Catapano
AL
,
De Backer
G
,
Graham
I
,
Taskinen
MR
,
Wiklund
O
, et al.
European Association for Cardiovascular Prevention & Rehabilitation; ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010-2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769–818. Available at: .https://doi.org/10.1093/eurheartj/ehr158
17
Cuzick
J
. A Wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. https://doi.org/10.1002/sim.4780040112
18
Kainz
A
,
Hronsky
M
,
Stel
VS
,
Jager
KJ
,
Geroldinger
A
,
Dunkler
D
, et al.
Prediction of prevalence of chronic kidney disease in diabetic patients in countries of the European Union up to 2025. Nephrol Dial Transplant. 2015;30(Suppl 4):iv113–8. https://doi.org/10.1093/ndt/gfv073
19
Bilo
H
,
Coentrao
L
,
Couchoud
C
,
Covic
A
,
De Sutter
J
,
Drechsler
C
, et al.; Guideline development group. Clinical Practice Guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR <45 mL/min). Nephrol Dial Transplant. 2015;30(Suppl 2):ii1–142. https://doi.org/10.1093/ndt/gfv100
20
Frei
A
,
Herzog
S
,
Woitzek
K
,
Held
U
,
Senn
O
,
Rosemann
T
, et al.
Characteristics of poorly controlled Type 2 diabetes patients in Swiss primary care. Cardiovasc Diabetol. 2012;11(1):70. https://doi.org/10.1186/1475-2840-11-70
21
Kaiser
A
,
Vollenweider
P
,
Waeber
G
,
Marques-Vidal
P
. Prevalence, awareness and treatment of type 2 diabetes mellitus in Switzerland: the CoLaus study. Diabet Med. 2012;29(2):190–7. https://doi.org/10.1111/j.1464-5491.2011.03422.x
22
Bodmer
M
,
Meier
C
,
Krähenbühl
S
,
Jick
SS
,
Meier
CR
. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: a nested case-control analysis. Diabetes Care. 2008;31(11):2086–91. https://doi.org/10.2337/dc08-1171
23
Nathan
DM
,
Genuth
S
,
Lachin
J
,
Cleary
P
,
Crofford
O
,
Davis
M
, et al., Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86. https://doi.org/10.1056/NEJM199309303291401
24
de Boer
IH
,
Rue
TC
,
Cleary
PA
,
Lachin
JM
,
Molitch
ME
,
Steffes
MW
, et al., Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med. 2011;171(5):412–20. https://doi.org/10.1001/archinternmed.2011.16
25
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53. https://doi.org/10.1016/S0140-6736(98)07019-6
26
Ismail-Beigi
F
,
Craven
T
,
Banerji
MA
,
Basile
J
,
Calles
J
,
Cohen
RM
, et al.; ACCORD trial group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–30. https://doi.org/10.1016/S0140-6736(10)60576-4
27
Patel
A
,
MacMahon
S
,
Chalmers
J
,
Neal
B
,
Billot
L
,
Woodward
M
, et al., ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. https://doi.org/10.1056/NEJMoa0802987
28
de Boer
IH
,
Sun
W
,
Cleary
PA
,
Lachin
JM
,
Molitch
ME
,
Steffes
MW
, et al., DCCT/EDIC Research Group. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365(25):2366–76. https://doi.org/10.1056/NEJMoa1111732
29
Shurraw
S
,
Tonelli
M
. Intensive glycemic control in type 2 diabetics at high cardiovascular risk: do the benefits justify the risks?
Kidney Int. 2013;83(3):346–8. https://doi.org/10.1038/ki.2012.431
30
Wong
MG
,
Perkovic
V
,
Chalmers
J
,
Woodward
M
,
Li
Q
,
Cooper
ME
, et al.; ADVANCE-ON Collaborative Group. Long-term Benefits of Intensive Glucose Control for Preventing End-Stage Kidney Disease: ADVANCE-ON. Diabetes Care. 2016;39(5):694–700. https://doi.org/10.2337/dc15-2322
31
Wanner
C
,
Inzucchi
SE
,
Lachin
JM
,
Fitchett
D
,
von Eynatten
M
,
Mattheus
M
, et al.; EMPA-REG OUTCOME Investigators. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375(4):323–34. https://doi.org/10.1056/NEJMoa1515920
32
Heerspink
HJ
,
Perkins
BA
,
Fitchett
DH
,
Husain
M
,
Cherney
DZ
. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation. 2016;134(10):752–72. https://doi.org/10.1161/CIRCULATIONAHA.116.021887
33
Miller
ME
,
Bonds
DE
,
Gerstein
HC
,
Seaquist
ER
,
Bergenstal
RM
,
Calles-Escandon
J
, et al.; ACCORD Investigators. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340(jan08 1):b5444. https://doi.org/10.1136/bmj.b5444
34
Bonds
DE
,
Miller
ME
,
Bergenstal
RM
,
Buse
JB
,
Byington
RP
,
Cutler
JA
, et al.
The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340(jan08 1):b4909. https://doi.org/10.1136/bmj.b4909
35
McCoy
RG
,
Van Houten
HK
,
Ziegenfuss
JY
,
Shah
ND
,
Wermers
RA
,
Smith
SA
. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35(9):1897–901. https://doi.org/10.2337/dc11-2054
36
Burnier
M
,
Gasser
UE
. End-digit preference in general practice: a comparison of the conventional auscultatory and electronic oscillometric methods. Blood Press. 2008;17(2):104–9. https://doi.org/10.1080/08037050801972881
37
Law
MR
,
Morris
JK
,
Wald
NJ
. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338(may19 1):b1665. https://doi.org/10.1136/bmj.b1665
38
Bangalore
S
,
Kumar
S
,
Lobach
I
,
Messerli
FH
. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123(24):2799–810, 9, 810. https://doi.org/10.1161/CIRCULATIONAHA.110.016337
39
UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–13. https://doi.org/10.1136/bmj.317.7160.703
40
Zoungas
S
,
de Galan
BE
,
Ninomiya
T
,
Grobbee
D
,
Hamet
P
,
Heller
S
, et al.; ADVANCE Collaborative Group. Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: New results from the ADVANCE trial. Diabetes Care. 2009;32(11):2068–74. https://doi.org/10.2337/dc09-0959
41
Bakris
GL
,
Weir
MR
,
Shanifar
S
,
Zhang
Z
,
Douglas
J
,
van Dijk
DJ
, et al., RENAAL Study Group. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163(13):1555–65. https://doi.org/10.1001/archinte.163.13.1555
42
Chapter 4: Blood pressure management in CKD ND patients with diabetes mellitus. Kidney Int Suppl (2011). 2012;2(5):363–9. https://doi.org/10.1038/kisup.2012.54
43
Lewis
EJ
,
Hunsicker
LG
,
Clarke
WR
,
Berl
T
,
Pohl
MA
,
Lewis
JB
, et al.; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60. https://doi.org/10.1056/NEJMoa011303
44
Bakris
GL
,
Sarafidis
PA
,
Weir
MR
,
Dahlöf
B
,
Pitt
B
,
Jamerson
K
, et al.; ACCOMPLISH Trial investigators. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet. 2010;375(9721):1173–81. https://doi.org/10.1016/S0140-6736(09)62100-0
45
Roas
S
,
Bernhart
F
,
Schwarz
M
,
Kaiser
W
,
Noll
G
. Antihypertensive combination therapy in primary care offices: results of a cross-sectional survey in Switzerland. Int J Gen Med. 2014;7:549–56.
46
Stemer
G
,
Zehetmayer
S
,
Lemmens-Gruber
R
. Evaluation of risk factor management of patients treated on an internal nephrology ward: a pilot study. BMC Clin Pharmacol. 2009;9(1):15. https://doi.org/10.1186/1472-6904-9-15
47
Bally
K
,
Martina
B
,
Halter
U
,
Isler
R
,
Tschudi
P
. Barriers to Swiss guideline-recommended cholesterol management in general practice. Swiss Med Wkly. 2010;140(19-20):280–5.
48
Fox
CS
,
Matsushita
K
,
Woodward
M
,
Bilo
HJ
,
Chalmers
J
,
Heerspink
HJ
, et al.; Chronic Kidney Disease Prognosis Consortium. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662–73. https://doi.org/10.1016/S0140-6736(12)61350-6
49
Tonelli
M
,
Muntner
P
,
Lloyd
A
,
Manns
BJ
,
Klarenbach
S
,
Pannu
N
, et al.; Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012;380(9844):807–14. https://doi.org/10.1016/S0140-6736(12)60572-8
50
Baigent
C
,
Landray
MJ
,
Reith
C
,
Emberson
J
,
Wheeler
DC
,
Tomson
C
, et al.; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–92. https://doi.org/10.1016/S0140-6736(11)60739-3
51
Wanner
C
,
Tonelli
M
; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85(6):1303–9. https://doi.org/10.1038/ki.2014.31
52
Tonelli
M
,
Wanner
C
; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. Lipid management in chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2013 clinical practice guideline. Ann Intern Med. 2014;160(3):182–9. https://doi.org/10.7326/M13-2453
53
Tancredi
M
,
Rosengren
A
,
Svensson
AM
,
Kosiborod
M
,
Pivodic
A
,
Gudbjörnsdottir
S
, et al.
Excess Mortality among Persons with Type 2 Diabetes. N Engl J Med. 2015;373(18):1720–32. https://doi.org/10.1056/NEJMoa1504347