Medical use of cannabis in Switzerland: analysis of approved exceptional licences
DOI: https://doi.org/10.4414/smw.2017.14463
Gablu
Kilchera, Marcel
Zwahlena, Christopher
Rittera, Lukas
Fennerab, Matthias
Eggerac
aInstitute of Social and Preventive Medicine (ISPM),
b
cSchool of Public Health and Family Medicine,
Medical use of cannabis in Switzerland: analysis of approved exceptional licences
w14463
Summary
AIMS OF THE STUDY: In recent years, the Swiss Federal Office of Public Health (FOPH) granted exceptional licenses for the medical use of cannabinoids, typically for 6 months with possible extensions. A systematic review of cannabinoids for medical use commissioned by the FOPH supports the use of cannabinoids for the treatment of chronic pain and spasticity. However, little is known about the patients treated with cannabinoids. We aimed to study medical uses of cannabinoids as part of the FOPH’s programme of exceptional licenses.
METHODS
We examined all requests for medical use of cannabinoids sent to FOPH in 2013 and 2014. A standardised data sheet was developed to extract data from the files of approved requests. We extracted the duration of the licence, the year it was granted, and the payer of the therapy. At the level of the patient we collected the date of birth, sex, region of residence, diagnosis and the indication. Ethical approval was granted by the Ethics Committee of the Canton of Bern.
RESULTS
We analysed 1193 patients licenced for cannabinoid treatment in 2013 or 2014. During 2013, 542 patients were treated under the exceptional licencing programme (332 requesting physicians) compared with 825 in 2014 (446 physicians). Over half of patients (685; 57%) were women. The mean age was 57 years (standard deviation 15.0), chronic pain (49%) and spasticity (40%) were the most common symptoms, and co-medication was reported for 39% of patients. Seventy-eight different diagnoses were recorded, including multiple sclerosis (257 patients, 22%), soft tissue disorders (119, 10%), dorsalgia (97, 8.1%), spinal muscular atrophy (65, 5.5%) and paraplegia/tetraplegia (62, 5.2%). Licence extensions were granted to 143 patients (26.4%) in 2013 and 324 patients (39.3%) in 2014. There were substantial regional variations of the rates of patients treated with cannabinoids. On average, eight patients per 100 000 residents received an exceptional licence. Most patients (1083, 91%) paid out of pocket.
CONCLUSIONS
Exceptional licences for medical use of cannabinoids have increased substantially in Switzerland, with the programme including patients with a wide range of conditions.
Introduction
Cannabinoids have been used to treat chronic pain, spasticity, nausea, and other symptoms since the middle of the 19th century [1, 2]. The chemical characterisation of the psychoactive substance delta-9-tetrahydrocannabinol (THC) in 1964 laid the foundation for the scientific study of cannabinoids [3]. Subsequently it became clear that the effects of THC are due to the activation of endogenous cannabinoid receptors within a newly discovered endocannabinoid system (ECS) [4]. More recently, the ECS has been associated with an increasing number of functional pathways, both in the central and peripheral nervous systems and in peripheral organs, and the ECS has emerged as a target of pharmacotherapy [5–10].
Endocannabinoids naturally produced in the body, for example in stress situations, modulate the ECS system and affect inflammation and the sensation of pain [7, 11]. In the 1970s, the effect of THC on pain was compared to that of codeine [12], and more recent studies showed that THC is equivalent to conventional analgesics [13]. Studies in cancer patients found that cannabinoids improved appetite, reduced emesis and nausea, and improved quality of life [14–16]. Cannabinoids have also been shown to be effective in neuropathic pain and diseases of the peripheral or central nervous system [17, 18].
The International Drug Control Conventions classify THC as an illegal, schedule I drug (together with opioids, opium derivatives, and hallucinogenic or psychedelic substances) and prohibit all use “except for scientific and very limited medical purposes by duly authorized persons” [19]. In Switzerland, the 2011 revision of the Federal Act on Narcotics and Psychotropic Substances (Narcotics Act) made it possible for the Federal Office of Public Health (FOPH) to issue exceptional licenses for the restricted medical use of some prohibited substances, including THC.
A systematic review of cannabinoids for medical use commissioned by the FOPH concluded that “there was moderate-quality evidence to support the use of cannabinoids for the treatment of chronic pain and spasticity” [20]. To look more closely at this and other uses of medical uses of cannabinoids, we studied all of the exceptional licences for medical use of cannabis issued by the Swiss FOPH in 2013 and 2014.
Materials and methods
Data source and data extraction
Physicians practising in Switzerland are required to submit a formal request to the FOPH for each patient they wish to treat with cannabinoids, either with commercially available synthetic THC (dronabinol solution with 2.5% THC) or a tincture of Cannabis sativa prepared by a pharmacist containing 5% THC [21]. The physician’s request must document the diagnosis of a severe or potentially life-threatening condition, address the benefit expected from THC treatment, and include the informed consent of the patient.
We examined all requests for medical use of cannabinoids sent to the FOPH in 2013 and 2014. We developed, tested and revised a standardised data extraction sheet to extract data from the files of approved requests. We created unique identification (ID) numbers and extracted data on the type of exceptional licence granted, the patient, and the treating physician. We extracted the duration of the licence, the year it was granted, and the payer for the therapy (out of pocket or insurance). At the level of the patient we collected the date of birth, sex, regional code, diagnosis and indication for THC use, and at the level of the physician the regional code of the surgery and the treating physician’s specialisation. We double-entered the data into a dedicated database in Epidata [22] and resolved discrepant data entries.
Definitions
We allocated patient and physician to the seven Swiss regions defined by the Nomenclature of Territorial Units for Statistics (NUTS, level II) [23]. These regions are shown in supplementary figure S1 (appendix). We used the International Statistical Classification of Diseases (ICD-10) [24] to categorise diagnoses into ten groups (ICD-10 codes in parentheses):
- Infectious and parasitic diseases (A00–B99)
- Cancer (C00–C97)
- Mental and behavioural disorders (F00–F99)
- Diseases of the nervous system (G00–G99)
- Diseases of the circulatory system (I00–I99)
- Diseases of the respiratory system (J00–J99)
- Diseases of the digestive system (K00–K93)
- Diseases of the musculoskeletal system (M00–M99)
- Injury, poisoning, and other conditions with external causes (S00–T98)
- Other diagnoses.
We used the Swiss Medical Association classification system for the specialisation and further certification of physicians [25].
Statistical methods
We used descriptive statistics (numbers and percentages) to present results of the requests and the characteristics of patients granted exceptional licences for therapeutic use of cannabinoids. Patient characteristics were taken from the first application for the same patient received in the years 2013 and 2014. We examined patient characteristics by the main symptoms pain and spasticity, and present detailed information on the underlying diagnoses reported for patients granted exceptional licenses for therapeutic use of cannabinoids. Finally, we calculated the rate of patients granted access per 100 000 residents by NUTS II region using the population statistics available from the European Union (Eurostat) [26]. We used Stata software version 14.1 (Stata Corporation, College Station, Texas, USA) for all analyses. Ethical approval was granted by the Ethics Committee of the Canton of Bern (KEK-Nr. 296/15).
Results
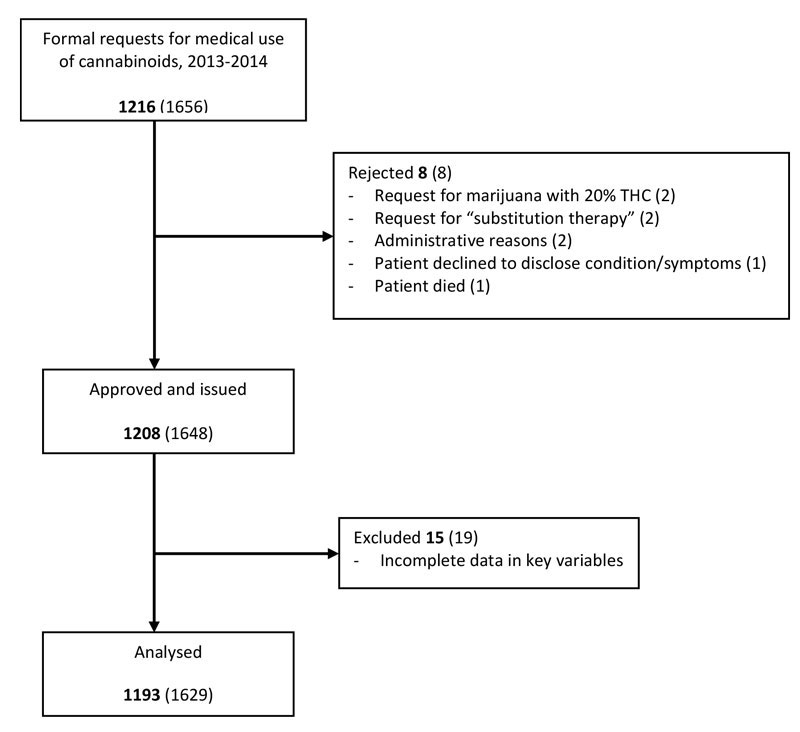
The FOPH received 1656 requests for initial exceptional licences and licence extensions to prescribe THC during 2013 and 2014. Eight requests from eight patients were rejected and 1648 licences and extensions for 1208 patients were approved. Requests were rejected for incompatibility with the law (for example, requests for “substitution therapy”) or for administrative reasons. Fifteen patients were excluded because of missing data in key variables. Thus, during these two years, 1193 unique patients received exceptional licences for treatment with cannabinoids and were included in the analyses (fig. 1). During 2013, 542 patients were treated under the exceptional licencing programme, and in 2014 the number rose to 825, including some who continued treatment begun in 2013 or earlier.
The approved licenses were requested by 332 physicians in 2013 and 446 physicians in 2014. More than half (55%) of the prescribing physicians were internal medicine specialists and 14% were neurologists. Other specialisations included 17 (2.7%) physicians who specialised in interventional pain therapy, 9 (1.4%) certified in acupuncture, and 7 (1.1%) with certificates in homeopathy.
Most patients (1083, 91%) paid for treatment out of pocket (typically amounting to US$ 400–500 per month). Only 95 patients (7.8%) were reimbursed by Switzerland's compulsory basic health insurance, with a further 15 (1.3%) reimbursed by disability or accident insurance.
Patient characteristics
The majority of the 1193 patients were women (685 patients, 57%), the mean age was 57 years (standard deviation 15 years), and most patients had neurological (580 patients, 49%) or musculoskeletal (300, 25%) conditions, or one of 19 different cancer diagnoses (121 patients, 10%). The most common complaints and indications for THC therapy were chronic pain (49%) and spasticity (40%). The characteristics of patients are summarised in table 1.
Table 1 Characteristics of patients granted exceptional licences for therapeutic use of cannabinoids by gender.
|
Variable
|
Female (n = 685)
|
Male (n = 508)
|
Total (n = 1193)
|
|
Age (years)
|
|
|
|
| 10–19 |
6 (0.9) |
8 (1.6) |
14 (1.2) |
| 20–39 |
64 (9.3) |
60 (11.8) |
124 (10.4) |
| 40–64 |
380 (54.9) |
302 (59.5) |
682 (57.2) |
| 65–79 |
187 (27.3) |
111 (21.9) |
298 (25.0) |
| 80–99 |
48 (7.1) |
27 (5.3) |
75 (6.3) |
| Mean (standard deviation), years |
58 (14.8) |
56 (15.1) |
57 (15.0) |
|
Diagnosis group (ICD-10 codes)
|
|
|
|
| Diseases of the nervous system (G00–G99) |
312 (45.6) |
268 (52.8) |
580 (48.6) |
| Diseases of the musculoskeletal system (M00–M99) |
209 (30.5) |
91 (17.9) |
300 (25.2) |
| Cancer (C00–C97) |
74 (10.8) |
47 (9.3) |
121 (10.1) |
| Injury, poisoning, and other conditions with external causes (S00–T98) |
19 (2.8) |
29 (5.7) |
48 (4.0) |
| Diseases of the circulatory system (I00–I99) |
9 (1.3) |
14 (2.8) |
23 (1.9) |
| Infectious and parasitic diseases (A00–B99) |
14 (2.0) |
8 (1.6) |
22 (1.8) |
| Mental and behavioural disorders (F00–F99) |
6 (0.9) |
11 (2.2) |
17 (1.4) |
| Diseases of the digestive system (K00–K93) |
2 (0.3) |
4 (0.8) |
6 (0.5) |
| Diseases of the respiratory system (J00–J99) |
0 (0.0) |
1 (0.2) |
1 (0.1) |
| Unclear |
40 (5.8) |
35 (6.9) |
75 (6.3) |
|
Main symptom
|
|
|
|
| Chronic pain |
365 (53.3) |
224 (44.1) |
589 (49.4) |
| Spasticity |
257 (37.5) |
219 (43.1) |
476 (39.9) |
| Neuropathic pain |
19 (2.8) |
24 (4.7) |
43 (3.6) |
| Lack of appetite |
21 (3.1) |
21 (4.1) |
42 (3.5) |
| Nausea |
15 (2.2) |
11 (2.2) |
26 (2.2) |
| Tremor |
8 (1.2) |
9 (1.8) |
17 (1.4) |
|
Comedication*
|
|
|
|
| None |
411 (60.0) |
323 (63.6) |
734 (61.5) |
| At least one drug |
274 (40.0) |
185 (36.4) |
459 (38.5) |
| Analgesics |
154 (56.2) |
83 (44.9) |
237 (51.6) |
| Muscle relaxant |
89 (32.4) |
75 (40.5) |
164 (35.7) |
| Anticonvulsant |
72 (26.3) |
48 (26.0) |
120 (26.1) |
| Immunosuppressive |
16 (5.8) |
10 (5.4) |
26 (5.7) |
| Cytotoxic agents |
13 (4.7) |
7 (3.8) |
20 (4.4) |
| Antidepressants |
20 (7.3) |
12 (6.5) |
32 (7.0) |
| Antiemetic drugs |
6 (2.2) |
3 (1.6) |
9 (2.0) |
| Parkinson medication |
1 (0.4) |
4 (2.2) |
5 (1.1) |
| Others |
6 (2.2) |
10 (5.4) |
16 (3.5) |
|
Reimbursement
|
|
|
|
| None (out of pocket) |
627 (91.5) |
456 (90.2) |
1083 (90.8) |
| Health insurance |
55 (8.0) |
40 (7.9) |
95 (7.8) |
| Disability / accident insurance |
3 (0.4) |
12 (2.4) |
15 (1.3) |
Diseases of the nervous system and spasticity were more common in men, and musculoskeletal conditions and chronic pain more common in women. The majority of patients (734, 62%) took no comedication. Among those who took at least one, the most frequently used medication was an analgesic (237 of 459 patients, 52%).
Compared with patients with spasticity, those with pain (including neuropathic pain) were older (mean age 60 vs 53 years), more likely to suffer from musculoskeletal conditions or cancer, and less likely to be reimbursed by health insurance (supplementary table S1).
Diagnoses
Multiple sclerosis was the single most common diagnosis (257 patients, 22%). A list of the most common diagnoses within the ten diagnostic groups is provided in table 2. The diagnosis was unclear in 75 patients (6.3%). A complete list of all recorded diagnoses in patients granted exceptional licences is given in supplementary table S2.
Table 2 List of most common diagnoses in patients granted exceptional licenses for therapeutic use of cannabinoids and extensions.
|
Diagnosis (ICD-10 codes)
|
Patients (n = 1193)
|
Extensions (n = 209)
|
|
Diseases of the nervous system (G00–G99)
|
580 (48.6)
|
144 (68.9)
|
| Multiple sclerosis |
257 (21.5) |
76 (36.4) |
| Spinal muscular atrophy and related syndromes |
65 (5.5) |
17 (8.1) |
| Paraplegia and tetraplegia |
62 (5.2) |
13 (6.2) |
| Hereditary and idiopathic neuropathy |
30 (2.5) |
3 (1.4) |
| Parkinson disease |
17 (1.4) |
2 (1.0) |
| Nerve root and plexus disorders |
15 (1.3) |
5 (2.4) |
| Migraine |
13 (1.0) |
0 |
| Cerebral palsy |
11 (0.9) |
4 (1.9) |
| Other disorder of central nervous systems |
10 (0.8) |
2 (1.0) |
| Hemiplegia |
10 (0.8) |
4 (1.9) |
| Other extrapyramidal and movement disorders |
9 (0.8) |
1 (0.5) |
| Epilepsy |
8 (0.7) |
1 (0.5) |
| Other polyneuropathies |
8 (0.7) |
2 (1.0) |
| Other paralytic syndromes |
8 (0.7) |
2 (1.0) |
| Huntington disease |
6 (0.5) |
1 (0.5) |
| Other headache syndromes |
6 (0.5) |
3 (1.4) |
| Disorders of trigeminal nerve |
6 (0.5) |
0 |
| Other diseases of spinal cord |
6 (0.5) |
0 |
| Other diseases of the nervous system |
33 (2.8) |
8 (3.8) |
|
Diseases of the musculoskeletal system (M00–M99)
|
300 (25.2)
|
36 (17.2)
|
| Soft tissue disorders, not elsewhere classified |
119 (10.0) |
20 (9.6) |
| Dorsalgia |
97 (8.1) |
8 (3.8) |
| Polyarthrosis |
19 (1.6) |
2 (1.0) |
| Other systematic involvement of connective tissue |
13 (1.1) |
2 (1.0) |
| Other arthritis |
12 (1.0) |
1 (0.5) |
| Other rheumatoid arthritis |
11 (0.9) |
1 (0.5) |
| Other joint disorders, not elsewhere classified |
10 (0.8) |
0 |
| Seropositive rheumatoid arthritis |
8 (0.7) |
0 |
| Other diseases of the musculoskeletal system |
11 (0.9) |
2 (1.0) |
|
Cancer (C00–C97)
|
121 (10.1)
|
7 (3.4)
|
| Breast |
29 (2.4) |
2 (1.0) |
| Lung |
18 (1.5) |
0 |
| Colon |
12 (1.0) |
0 |
| Pharynx and oesophagus |
8 (0.7) |
0 |
| Pancreas |
8 (0.7) |
1 (0.5) |
| Stomach |
6 (0.5) |
1 (0.5) |
| Prostate |
6 (0.5) |
1 (0.5) |
| Hodgkin lymphoma |
5 (0.4) |
0 |
| Other cancers |
29 (2.4) |
2 (1.0) |
|
Injury, poisoning, and other conditions with external causes (S00–T98)
|
48 (4.0)
|
8 (3.8)
|
| Complications of surgical and medical care, not elsewhere classified |
48 (4.0) |
8 (3.8) |
|
Diseases of the circulatory system (I00–I99)
|
23 (1.9)
|
1 (0.5)
|
| Cerebral infarction |
9 (0.8) |
0 |
| Other diseases of the circulatory system |
14 (1.2) |
1 (0.5) |
|
Infectious and parasitic diseases (A00–B99)
|
22 (1.8)
|
0
|
| Zoster (Herpes zoster) |
9 (0.8) |
0 |
| HIV diseases resulting in infectious and parasitic disease |
7 (0.6) |
0 |
| Other bacterial diseases, not elsewhere described |
6 (0.5) |
0 |
|
Mental and behavioural disorders (F00–F99)
|
17 (1.4)
|
2 (1.0)
|
| Tic disorder |
11 (0.9) |
1 (0.5) |
| Somatoform disorders |
5 (0.4) |
1 (0.5) |
| Recurrent depressive disorder |
1 (0.1) |
0 |
|
Diseases of the digestive system (K00–K93)
|
6 (0.5)
|
0
|
|
Diseases of the respiratory system (J00–J99)
|
1 (0.1)
|
0
|
|
Unclear
|
75 (6.3)
|
11 (5.3)
|
Extensions to exceptional licences
In most cases, licences were initially granted for 6 months. Physicians requested extensions when the effect of the treatment had, in their assessment, been satisfactory. The number of extensions increased from 143 of the 542 patients in 2013 (26.4%) to 324 of 825 patients in 2014 (39.3%). Table 2 lists the numbers of extensions per diagnosis. Multiple sclerosis patients not only were granted the most extensions, but also received the highest proportion of extensions per diagnosed patient: 76 extensions for 257 patients (29.6%). As shown in table 3, 144 of 209 total extensions (69%) were granted for diseases of the nervous system, and 36 extensions (17%) were granted for diseases of the musculoskeletal system. In terms of symptoms, 75 extensions were granted to the 514 patients (14.6%) initially reporting chronic pain, while for patients reporting spasticity, 123 of 353 patients (34.8%) were granted extensions. Unfortunately, for the patients who did not apply for extensions and stopped treatment, no further information is available in the FOPH records regarding whether cannabinoid treatment cured their disease or alleviated their symptoms, and physicians’ reports on treatment effectiveness were not required.
Table 3 Main symptoms of patients granted exceptional licences for therapeutic use of cannabinoids, separated by initial and extension licence.
|
Main symptom
|
Initial (n = 984)
|
Extension (n = 209)
|
Total (n = 1193)
|
| Chronic pain |
514 (52.2) |
75 (35.9) |
589 (49.4) |
| Spasticity |
353 (35.9) |
123 (58.9) |
476 (39.9) |
| Neuropathic pain |
38 (3.9) |
5 (2.4) |
43 (3.6) |
| Lack of appetite |
41 (4.2) |
1 (0.5) |
42 (3.5) |
| Nausea |
23 (2.3) |
3 (1.4) |
26 (2.2) |
| Tremor |
15 (1.5) |
2 (1.0) |
17 (1.4) |
One patient died during the treatment (death was not caused by cannabinoids).
Regional variation
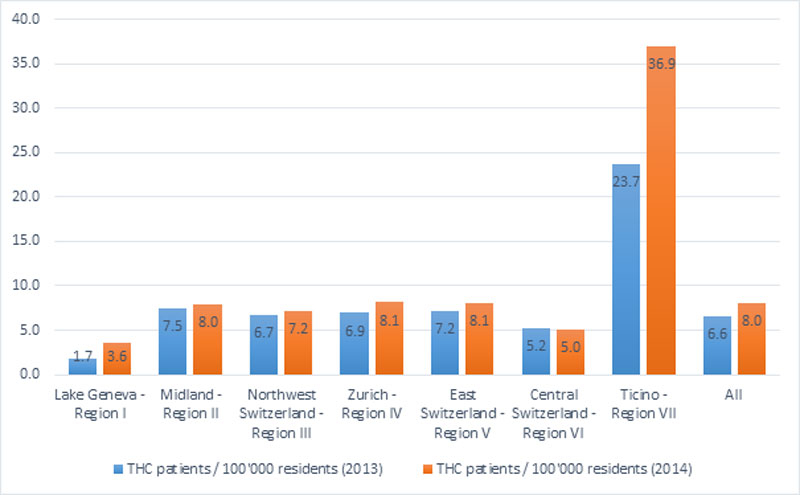
The rates of patients treated with cannabinoids per 100 000 residents increased from 2013 to 2014 in all regions, but varied substantially across the seven regions. In 2014 the rate was 3.6 in the region along the Lake of Geneva, 5.0 in Central Switzerland, and between 7.1 and 8.2 in all other regions except for the Ticino, where the rate was much higher, 36.9 per 100 000 residents (supplementary fig. S2). This could be due to a small number of frequent prescribers in this region.
Discussion
The medical use of cannabinoids through exceptional licenses increased substantially in Switzerland during the 2-year study period and involved patients with 78 different conditions. Prescriptions for neurological and musculoskeletal conditions predominated, followed by cancer. More women sought treatment with cannabinoids than men. Regionally, the cannabinoid treatment rate varied but was considerably higher in Italian-speaking Ticino than the rest of Switzerland. Additional drugs taken by patients were mostly analgesics, muscle relaxants and anticonvulsants. As for physicians, more than half of those prescribing cannabinoids specialised in internal medicine, and the next largest specialty was neurology (though internists outnumber neurologists by 4:1). Regarding payment for treatment, only one in 10 patients was reimbursed by health insurance.
Medical use of cannabinoids is growing in other countries as well (e.g., Germany, The Netherlands, United States of America, Canada, France, Spain, Israel) [27]. Table 4 compares published surveys of medical uses of cannabinoids in different countries with the current study [28–32]. Age distributions of medical cannabis users differ internationally, but overall Swiss patients are generally older than users of cannabis for medical purposes in the US, Canada and Australia [30, 31]. In the international survey conducted in 31 countries by the International Association for Cannabinoid Medicines (IACM), the mean age of cannabinoid users was 40.7 years, with 44.9% in the 41–60 age group [28]. Medical cannabis use varies by gender as well, but unlike in Switzerland, male users predominate in the US, Canada, and Australia [30, 31], and in the IACM study, 64% of patients were men [28].
Table 4 Comparison of international studies that examined medical use of cannabis.
|
Author (year)
|
Country
|
Patients (n)
|
Main symptom
|
Main diagnosis
|
| Hazekamp et al. (2013) [28] |
31 countries*
|
953 |
Back pain |
Sleep disorders |
| Ryan-Ibarra et al (2012) [29] |
USA |
350 |
Chronic pain |
Arthritis |
| Swift et al. (2005) [30] |
Australia |
128 |
Chronic pain |
Depression |
| Walsh et al. (2013) [31] |
Canada |
628 |
Chronic pain |
Arthritis |
| Ware et al. (2005) [32] |
UK |
947 |
Chronic pain |
Multiple sclerosis |
| Kilcher et al (present study) |
Switzerland |
1193 |
Chronic pain |
Multiple sclerosis |
Chronic pain figured in almost half of Swiss exceptional licences in 2013 and 2014, and worldwide the main symptom treated by medical cannabis is also pain. In addition to chronic pain, the IACM study reported treatment for anxiety (18.3%), loss of appetite (10.7%), depression (5.2%) and insomnia (5.1%) [28]. Like pain, spasticity also is a major symptom treated with cannabinoids in Switzerland, but it does not appear to be an important symptom in studies elsewhere. Of note, spasticity and chronic pain were the two indications best supported by the systematic review of the literature commissioned by the FOPH [20].
Medical cannabinoids may help lower the intake of opioid medication, as well as lower opioid overdose mortality, where the medical use of cannabis is legal [33]. A recent study found a drop in prescriptions of pharmaceuticals in several disease categories in US states that allow medical cannabinoids [34]. The numbers on concomitant drug intake in our study are low, which makes it difficult to draw conclusions. Further study is required to understand whether the use of cannabinoids leads to reductions in use of other medications.
Even as the prescription of cannabinoids is growing, the situation of physicians in North America is not always straightforward. Physicians in Canada reported they were not comfortable prescribing cannabinoids [35], and a study in the US concluded that ophthalmologists need more education about the medical use of cannabinoids to better respond to patient (mis)perceptions of the value of cannabis in glaucoma therapy [36].
The North American context differs from the Swiss, although in Switzerland, too, treatment with cannabinoids still needs clarification. In 2013, for example, Swissmedic approved Sativex®, which contains 27 mg THC and 25 mg cannabidiol per ml [37], for the treatment of medium and heavy spasticity caused by multiple sclerosis. The use of Sativex® for other indications (off-label use), however, needs an exceptional license from the FOPH. At the same time, 257 patients received exceptional licenses for cannabinoid treatment of multiple sclerosis in 2013 to 2014, and many of them were granted extensions. Similarly, 353 patients were granted licences for treatment of spasticity for which 123 extensions, 58.9% of all extensions, were approved. Since exceptional licenses ought not to be issued to supplant another effective treatment, this raises questions about the perceived effectiveness of Sativex® among both patients and physicians.
This study of patient data in the FOPH cannabinoid licencing programme, the first of its kind in Switzerland, was limited at the outset by the fact that the data do not include information such as socioeconomic status, smoking, objective treatment outcomes or reasons to stop a treatment. Information in the licences on concomitant drugs, diagnosis, and patient history was sometimes poorly reported as well. The fact that some patients may treat themselves with cannabis obtained on the black market is another issue that may have influenced and distorted our results.
These limitations highlight the need for new research on medical cannabis. A drug with long history of use, whether legal or otherwise, can accrue claims of efficacy that in time imbue it with promise that may border on that of a wonder drug. As exceptional licencing of medical cannabinoids continues to expand in Switzerland, and the recreational and medical use is legalised in an increasing number of states and countries, clear guidelines for safe and effective use must urgently be developed, based on basic research and clinical trials. It is indeed “high time for evidence-based policies” [38].
Appendix Supplementary tables and figures
Table S1 Characteristics of patients granted exceptional licences for therapeutic use of cannabinoids, by main symptoms pain and spasticity.
Variable
n (%)
|
Pain*
n = 632
|
Spasticity
n = 476
|
|
Age (years)
|
|
|
| 10–19 |
3 (0.5) |
10 (2.1) |
| 20–39 |
53 (8.4) |
64 (13.5) |
| 40–64 |
333 (52.7) |
298 (62.6) |
| 65–79 |
183 (29.0) |
92 (19.3) |
| 80–99 |
60 (9.5) |
12 (2.5) |
| Mean (standard deviation), years |
60 (15.0) |
53 (14.5) |
|
Sex
|
|
|
| Male |
248 (39.2) |
219 (46.0) |
| Female |
384 (60.8) |
257 (54.0) |
|
Diagnosis group (ICD-10 codes)
|
|
|
| Diseases of the nervous system (G00–G99) |
140 (22.2) |
427 (89.7) |
| Diseases of the musculoskeletal system (M00–M99) |
297 (47.0) |
3 (0.6) |
| Cancer (C00–C97) |
75 (11.9) |
0 |
| Injury, poisoning, and other conditions with external causes (S00–T98) |
42 (6.7) |
4 (0.8) |
| Diseases of the circulatory system (I00–I99) |
8 (1.3) |
15 (3.2) |
| Infectious and parasitic diseases (A00–B99) |
14 (2.2) |
3 (0.6) |
| Mental and behavioural disorders (F00–F99) |
4 (0.6) |
11 (2.3) |
| Diseases of the digestive system (K00–K93) |
3 (0.5) |
0 |
| Diseases of the respiratory system (J00–J99) |
1 (0.2) |
0 |
| Unclear |
48 (7.6) |
13 (2.7) |
|
Comedication†
|
|
|
| None |
384 (60.8) |
287 (60.3) |
| At least one drug |
248 (39.2) |
189 (39.7) |
| Analgesics |
206 (83.1) |
23 (12.2) |
| Muscle relaxant |
22 (8.9) |
142 (75.1) |
| Anticonvulsant |
77 (31.1) |
39 (20.6) |
| Immunosuppressive |
7 (2.8) |
18 (9.5) |
| Cytotoxic agents |
12 (4.8) |
4 (2.1) |
| Antidepressants |
21 (8.5) |
9 (4.8) |
| Parkinson medication |
1 (0.4) |
4 (2.1) |
| Others |
3 (1.2) |
13 (6.9) |
|
Reimbursement
|
|
|
| None (out of pocket) |
588 (93.0) |
415 (87.2) |
| Health insurance |
37 (5.9) |
56 (11.8) |
| Disability / accident insurance |
7 (1.1) |
5 (1.1) |
Table S2 Complete list of diagnoses in patients granted exceptional licenses for therapeutic use of cannabinoids.
|
Diagnosis (ICD-10 codes)
|
Number (%)
|
|
Diseases of the nervous system (G00–G99)
|
580 (48.6)
|
| Multiple sclerosis |
257 (21.5) |
| Spinal muscular atrophy and related syndromes |
65 (5.5) |
| Paraplegia and tetraplegia |
62 (5.2) |
| Hereditary and idiopathic neuropathy |
30 (2.5) |
| Parkinson disease |
17 (1.4) |
| Nerve root and plexus disorders |
15 (1.3) |
| Migraine |
13 (1.0) |
| Cerebral palsy |
11 (0.9) |
| Other disorder of central nervous systems |
10 (0.8) |
| Hemiplegia |
10 (0.8) |
| Other extrapyramidal and movement disorders |
9 (0.8) |
| Epilepsy |
8 (0.7) |
| Other polyneuropathies |
8 (0.7) |
| Other paralytic syndromes |
8 (0.7) |
| Huntington disease |
6 (0.5) |
| Other headache syndromes |
6 (0.5) |
| Disorders of trigeminal nerve |
6 (0.5) |
| Other diseases of spinal cord |
6 (0.5) |
| Other degenerative disease of nervous system |
5 (0.4) |
| Other disorders of peripheral nervous system |
5 (0.4) |
| Dystonia |
5 (0.4) |
| Encephalitis, myelitis and encephalomyelitis |
4 (0.3) |
| Postpolio syndrome |
3 (0.3) |
| Inflammatory polyneuropathy |
3 (0.3) |
| Polyneuropathy in diseases classified elsewhere |
3 (0.3) |
| Other demyelinating diseases of central nervous system |
2 (0.2) |
| Other neurological diseases |
3 (0.3) |
|
Diseases of the musculoskeletal system (M00–M99)
|
300 (25.2)
|
| Soft tissue disorders, not elsewhere classified |
119 (10.0) |
| Dorsalgia |
97 (8.1) |
| Polyarthrosis |
19 (1.6) |
| Other systematic involvement of connective tissue |
13 (1.1) |
| Other arthritis |
12 (1.0) |
| Other rheumatoid arthritis |
11 (0.9) |
| Other joint disorders, not elsewhere classified |
10 (0.8) |
| Seropositive rheumatoid arthritis |
8 (0.7) |
| Gonarthrosis |
4 (0.3) |
| Scoliosis |
2 (0.2) |
| Other diseases of the musculoskeletal system |
5 (0.4) |
|
Cancer (C00–C97)
|
121 (10.1)
|
| Breast |
29 (2.4) |
| Lung |
18 (1.5) |
| Colon |
12 (1.0) |
| Pharynx and oesophagus |
8 (0.7) |
| Pancreas |
8 (0.7) |
| Stomach |
6 (0.5) |
| Prostate |
6 (0.5) |
| Hodgkin lymphoma |
5 (0.4) |
| Kidney |
4 (0.3) |
| Brain |
4 (0.3) |
| Follicular lymphoma |
3 (0.3) |
| Cervix uteri |
3 (0.3) |
| Lymphoid leukaemia |
3 (0.3) |
| Rectum |
2 (0.2) |
| Uterus |
2 (0.2) |
| Ovary |
2 (0.2) |
| Bladder |
2 (0.2) |
| Multiple myeloma |
2 (0.2) |
| Anus |
1 (0.1) |
|
Injury, poisoning, and other conditions with external causes (S00–T98)
|
48 (4.0)
|
| Complications of surgical and medical care, not elsewhere classified |
48 (4.0) |
|
Diseases of the circulatory system (I00–I99)
|
23 (1.9)
|
| Cerebral infarction |
9 (0.8) |
| Sequelae of cerebrovascular disease |
5 (0.4) |
| Aortic aneurysm and dissection |
3 (0.3) |
| Other nontraumatic intracranial haemorrhage |
2 (0.2) |
| Intracerebral haemorrhage |
2 (0.2) |
| Other cerebrovascular diseases |
2 (0.2) |
|
Infectious and parasitic diseases (A00–B99)
|
22 (1.8)
|
| Zoster (Herpes zoster) |
9 (0.8) |
| HIV diseases resulting in infectious and parasitic disease |
7 (0.6) |
| Other bacterial diseases, not elsewhere described |
6 (0.5) |
|
Mental and behavioural disorders (F00–F99)
|
17 (1.4)
|
| Tic disorder |
11 (0.9) |
| Somatoform disorders |
5 (0.4) |
| Recurrent depressive disorder |
1 (0.1) |
|
Diseases of the digestive system (K00–K93)
|
6 (0.5)
|
| Ulcerative colitis |
2 (0.2) |
| Irritable bowel syndrome |
2 (0.2) |
| Others diseases of digestive system |
2 (0.2) |
|
Diseases of the respiratory system (J00–J99)
|
1 (0.1)
|
| Emphysema |
1 (0.1) |
|
Unclear
|
75 (6.3)
|
Acknowledgements
We thank Nadine Tremp for help with data entry.
Author contributions
GK initiated the study and wrote the protocol. GK, MZ and ME were responsible for the study design, data acquisition, and correspondence with the local ethical committee. GK, MZ, ME, and LF conducted the statistical analysis. GK, ME, MZ, and LF interpreted the results and GK wrote the manuscript. ME, CR, MZ and LF revised the manuscript.
References
1Fankhauser M. Haschisch als Medikament:[zur Bedeutung von Cannabis sativa in der westlichen Medizin]. SGGP; 2002.
2
Le Foll
B
,
Tyndale
RF
. Cannabinoids: Friend or foe?
Clin Pharmacol Ther. 2015;97(6):528–31. https://doi.org/10.1002/cpt.119
3
Mechoulam
R
,
Gaoni
Y
. A Total Synthesis of DL-Delta-1-Tetrahydrocannabinol, The Active Constituent of Hashish. J Am Chem Soc. 1965;87(14):3273–5. https://doi.org/10.1021/ja01092a065
4Grotenhermen F. Cannabis und Cannabinoide. Pharmakologie, Toxikologie und Ther Potential 2nd Ed Göttingen Hans Huber. 2004
5
Pertwee
RG
. Cannabinoid receptor ligands: clinical and neuropharmacological considerations, relevant to future drug discovery and development. Expert Opin Investig Drugs. 2000;9(7):1553–71. https://doi.org/10.1517/13543784.9.7.1553
6
Pertwee
RG
. Cannabis and cannabinoids: pharmacology and rationale for clinical use. Forsch Komplementarmed. 1999;6(Suppl 3):12–5. https://doi.org/10.1159/000057150
7
Lenk
R
,
Likar
R
. Cannabinoids in medicine. Wien Med Wochenschr. 2008;158(23-24):668–73. https://doi.org/10.1007/s10354-008-0619-7
8
Borgelt
LM
,
Franson
KL
,
Nussbaum
AM
,
Wang
GS
. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013;33(2):195–209. https://doi.org/10.1002/phar.1187
9
Pacher
P
,
Bátkai
S
,
Kunos
G
. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58(3):389–462. https://doi.org/10.1124/pr.58.3.2
10
Vemuri
VK
,
Makriyannis
A
. Medicinal chemistry of cannabinoids. Clin Pharmacol Ther. 2015;97(6):553–8. https://doi.org/10.1002/cpt.115
11Göbel H. Dronabinol-Therapie: Cannabis in der Medizin, chronische Schmerzen, Schmerztherapie nach Querschnittslähmung, diabetische Polyneuropathie, neuropsychiatrische Erkrankungen, Langzeittherapie bei Multipler Sklerose, Anorexie-Kachexie-Syndrom, rechtliche Aspekte. Springer; 2004.
12
Noyes
R, Jr
,
Brunk
SF
,
Avery
DA
,
Canter
AC
. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther. 1975;18(1):84–9. https://doi.org/10.1002/cpt197518184
13
Holdcroft
A
,
Maze
M
,
Doré
C
,
Tebbs
S
,
Thompson
S
. A multicenter dose-escalation study of the analgesic and adverse effects of an oral cannabis extract (Cannador) for postoperative pain management. Anesthesiology. 2006;104(5):1040–6. https://doi.org/10.1097/00000542-200605000-00021
14
Elikkottil
J
,
Gupta
P
,
Gupta
K
. The analgesic potential of cannabinoids. J Opioid Manag. 2009;5(6):341–57.
15
Johnson
JR
,
Burnell-Nugent
M
,
Lossignol
D
,
Ganae-Motan
ED
,
Potts
R
,
Fallon
MT
. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010;39(2):167–79. https://doi.org/10.1016/j.jpainsymman.2009.06.008
16
Portenoy
RK
,
Ganae-Motan
ED
,
Allende
S
,
Yanagihara
R
,
Shaiova
L
,
Weinstein
S
, et al.
Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438–49. https://doi.org/10.1016/j.jpain.2012.01.003
17
Ware
MA
,
Wang
T
,
Shapiro
S
,
Robinson
A
,
Ducruet
T
,
Huynh
T
, et al.
Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182(14):E694–701. https://doi.org/10.1503/cmaj.091414
18
Nurmikko
TJ
,
Serpell
MG
,
Hoggart
B
,
Toomey
PJ
,
Morlion
BJ
,
Haines
D
. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain. 2007;133(1-3):210–20. https://doi.org/10.1016/j.pain.2007.08.028
19United Nations Office on Drug and Crime (UNODC). Schedules of the Convention on Psychotropic Substances of 1971. In: The International Drug Control Conventions. New York: United Nations; 2013 pp 67–106.
20
Whiting
PF
,
Wolff
RF
,
Deshpande
S
,
Di Nisio
M
,
Duffy
S
,
Hernandez
AV
, et al.
Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA. 2015;313(24):2456–73. https://doi.org/10.1001/jama.2015.6358
21Fankhauser M. Station Dispensary in Langnau Emmental - Curaplant. 2016.
22EpiData Association. EpiData Software. EpiData Software. 2001.
23Federal Statistical Office (FSO). Classification of 7 territorial units of Switzerland (7 major regions)- Basic Statistics. Swiss Statistics. 2013.
24World Health Organization. WHO | International Classification of Diseases (ICD). Geneva: WHO; 2016.
25Schweizerisches Institut für ärztliche Weiter- und Fortbildung [Swiss Institute of Advanced Medical Education]. Facharzttitel und Schwerpunkte (Weiterbildung) [Specialization title and main emphasis in advanced medical education]. 2016. Available from: http://www.fmh.ch/bildung-siwf/fachgebiete/facharzttitel-und-schwerpunkte.html
26European Commission [internet]. Eurostat / Regional Statistics Illustrated. Eurostat Your key to European statistics. 2013. Available from: http://ec.europa.eu/eurostat/cache/RCI/#?vis=nuts2.labourmarket&lang=en
27Madras B. Update of cannabis and its medical use. Geneva: World Health Organization; 2015.
28
Hazekamp
A
,
Ware
MA
,
Muller-Vahl
KR
,
Abrams
D
,
Grotenhermen
F
. The medicinal use of cannabis and cannabinoids--an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45(3):199–210. https://doi.org/10.1080/02791072.2013.805976
29
Ryan-Ibarra
S
,
Induni
M
,
Ewing
D
. Prevalence of medical marijuana use in California, 2012. Drug Alcohol Rev. 2015;34(2):141–6. https://doi.org/10.1111/dar.12207
30
Swift
W
,
Gates
P
,
Dillon
P
. Survey of Australians using cannabis for medical purposes. Harm Reduct J. 2005;2(1):18. https://doi.org/10.1186/1477-7517-2-18
31
Walsh
Z
,
Callaway
R
,
Belle-Isle
L
,
Capler
R
,
Kay
R
,
Lucas
P
, et al.
Cannabis for therapeutic purposes: patient characteristics, access, and reasons for use. Int J Drug Policy. 2013;24(6):511–6. https://doi.org/10.1016/j.drugpo.2013.08.010
32
Ware
MA
,
Adams
H
,
Guy
GW
. The medicinal use of cannabis in the UK: results of a nationwide survey. Int J Clin Pract. 2005;59(3):291–5. https://doi.org/10.1111/j.1742-1241.2004.00271.x
33
Boehnke
KF
,
Litinas
E
,
Clauw
DJ
. Medical Cannabis Use Is Associated With Decreased Opiate Medication Use in a Retrospective Cross-Sectional Survey of Patients With Chronic Pain. J Pain. 2016;17(6):739–44. https://doi.org/10.1016/j.jpain.2016.03.002
34
Bradford
AC
,
Bradford
WD
. Medical Marijuana Laws Reduce Prescription Medication Use In Medicare Part D. Health Aff (Millwood). 2016;35(7):1230–6. https://doi.org/10.1377/hlthaff.2015.1661
35
St-Amant
H
,
Ware
MA
,
Julien
N
,
Lacasse
A
. Prevalence and determinants of cannabinoid prescription for the management of chronic noncancer pain: a postal survey of physicians in the Abitibi-Témiscamingue region of Quebec. CMAJ Open. 2015;3(2):E251–7. https://doi.org/10.9778/cmajo.20140095
36
Belyea
DA
,
Alhabshan
R
,
Del Rio-Gonzalez
AM
,
Chadha
N
,
Lamba
T
,
Golshani
C
, et al.
Marijuana Use Among Patients With Glaucoma in a City With Legalized Medical Marijuana Use. JAMA Ophthalmol. 2016;134(3):259–64. https://doi.org/10.1001/jamaophthalmol.2015.5209
37Swiss Drug Compendium. Product information for Sativex. Available from: http://compendium.ch/mpro/mnr/24719/html/de. 2016.
38
The Lancet Oncology. Cannabis: high time for evidence-based policies. Lancet Oncol. 2017;18(1):1. https://doi.org/10.1016/S1470-2045(16)30642-8