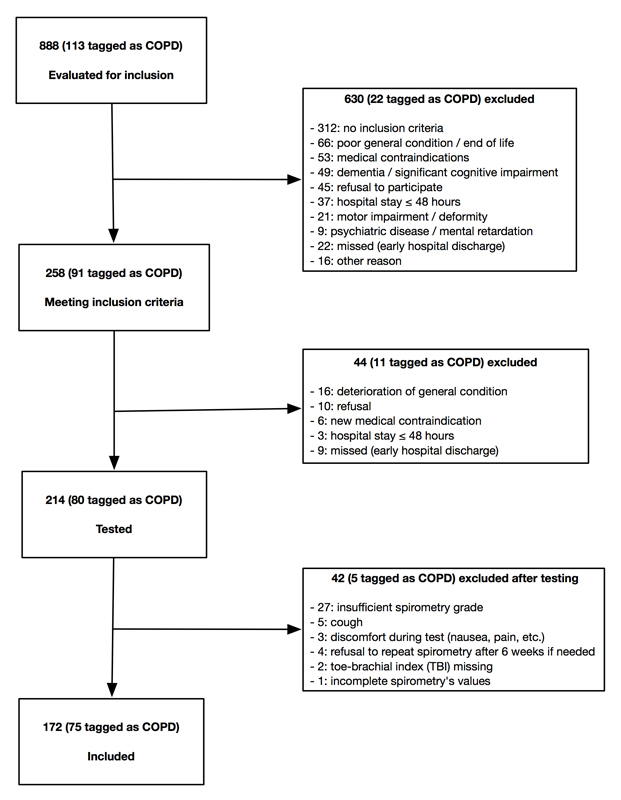
Figure 1 Patient screening and inclusion.
DOI: https://doi.org/10.4414/smw.2017.14460
Chronic obstructive pulmonary disease (COPD) is a major public health problem in our society [1], with a prevalence estimate of 2 to 15% in the Swiss population, depending on age and sex [2]. Its development depends on individual susceptibility and on exposure to well-known risk factors, which are essentially tobacco smoke and other irritant inhaled substances such as smoke produced by combustion of biomass fuel, biological dusts and several gases used in industrial work [3]. Recent data also suggest that outdoor air pollution defined by its density in certain types of particulate matter could play a role [4].
The diagnosis of COPD requires spirometry testing that shows an obstructive lung disease, defined as a forced expiratory volume in 1 second / forced vital capacity ratio (FEV1/FVC) below the lower limit of normal or below the fifth percentile of a large healthy reference group, that is not completely reversible after administration of a bronchodilator [5, 6]. Nonetheless, we notice in our daily practice of internal medicine that many patients identified in their medical records as having COPD (“tagged” as COPD) have never performed any pulmonary function test and carry a diagnosis based only on a probabilistic approach integrating the presence of well-matched symptoms and risk factors [7]. Conversely, many patients presenting with characteristic risk factors and symptoms have never been investigated. Thus, the real prevalence of COPD in our institution is unknown.
Besides, many authors support the hypothesis that chronic inflammation of the lower respiratory tract present in COPD can, if strong enough, spill over and become systemic. This is evidenced by the increased level of plasmatic markers of inflammation (leucocytes, C-reactive protein [CRP], fibrinogen, interleukins-1β, -6 and -8, and tumour necrosis factor-alpha [TNF-α]) found in patients affected by this disease, as well as various subsequent clinical manifestations such as nutritional deficiencies, osteoporosis, skeletal muscles dysfunction and increased risk of cardiovascular diseases and type 2 diabetes [8–10]. Considering these facts, and the common risk factors they share (smoking, advanced age, and outdoor air pollution [11, 12]), it is not surprising to observe an association between COPD and cardiovascular diseases such as heart failure, hypertension and atherosclerosis, particularly of the coronary arteries.
Lower limb peripheral arterial disease (PAD) is a common manifestation of atherosclerosis. Not only is it associated with great morbidity itself, but it also constitutes a powerful prospective marker of cardiovascular morbidity and mortality [13, 14]. It is now well established that PAD is not only the result of a pathological accumulation of cholesterol and thrombotic debris in arterial walls, but is above all an inflammatory disease [15]. Thus, there are numerous reasons to believe that COPD is not only associated with PAD but might also be an independent risk factor, but this remains to be demonstrated in a large prospective study. To our knowledge, this association has not been well studied.
The primary objective of this study was to determine the prevalence of confirmed COPD in patients aged 45 years or more, who were admitted in the internal medicine ward of our tertiary care hospital, and who were either tagged as having COPD or at risk for COPD. The secondary objective was to determine the prevalence of COPD-PAD association in these patients.
This was a clinical prospective, noncomparative, nonrandomised study
Between November 2013 and March 2014, every patient aged ≥45 years who was admitted into the internal medicine ward of our tertiary care hospital (HFR Fribourg, Switzerland) and whose electronic medical record contained the diagnosis of COPD, chronic bronchitis and/or lung emphysema was eligible for inclusion. Elective admissions, as well as admissions from the emergency department and intensive care unit were taken into account. These were patients tagged as COPD.
Likewise, every patient aged ≥45 years who presented at least one risk factor and one symptom of the following list was eligible for inclusion [16]: active or former history of tobacco use, passive exposure to tobacco smoke, occupational exposure to known risk factors (agriculture, inhaled chemical products, inhaled biological dusts), family history of COPD diagnosed at a young age; chronic cough, chronic sputum production, chronic dyspnoea, and frequent common colds. These were patients at risk for COPD.
Exclusion criteria were: death before having been able to participate in the study, hospital stay duration ≤48 hours, spirometry of insufficient technical quality, medical contraindication to spirometry (adapted from [17]), refusal to participate in the study, lack of discernment, refusal to perform a subsequent control spirometry if needed.
A signed informed consent was necessary to consider the inclusion of each patient in this study. This study was approved by Fribourg’s ethics committee for research (approval number 048/13-CER-FR).
During the inclusion phase, each patient who met all the required conditions for inclusion was evaluated for inclusion. The following working day after hospital admission a questionnaire checklist containing the risk factors and symptoms suggestive of COPD previously described was filled in. A first selection of patients was made to classify them as either patients tagged as COPD or patients at risk for COPD, according to the criteria used for their inclusion.
Once clinically stable, defined as absence of haemodynamic instability, significant modification of respiratory parameters and fever for 48 consecutive hours, patients were once more evaluated to exclude the occurrence of new contraindications. In the absence of any exclusion criteria, spirometry was performed, and ankle-brachial index and, if needed, toe-brachial index as described below were determined.
An EasyOneTM spirometer (NDD Medical Technologies) was used, and was calibrated before the study according to an official protocol of the company [18]. Spirometry included pre- and post-bronchodilation values (bronchodilator administered via a Vortex® spacer) and was performed according to the American Thoracic Society / European Respiratory Society (ATS/ERS) recommendations [19]. Measurements with grades A, B and C as determined by the internal software of the spirometer were considered reproducible and were included in the analysis. Measurements with grades D and F were not considered reliable and were excluded. Patients with a lung disease that required the regular administration of a long-acting bronchodilator (tiotropium bromide, indacaterol, salmeterol, formoterol) performed spirometry only after inhalation of these medications.
In order to improve screening efficiency, patients who had spirometry testing within the 6 months before their hospital admission did not need new spirometry and the results of their last test were used instead. We also used spirometry testing made in our pneumology department for patients who had been tested there during their hospital stay.
Patients who were newly diagnosed with COPD by spirometry performed during an acute illness that possibly interfered with the result (left heart failure, pneumonia, acute bronchitis) were reassessed with another spirometry test 6 weeks after discharge. This allowed exclusion of transient obstructive lung disease.
All values obtained were extracted and interpreted according to the spirometry reference equations of Kuster et al. [20].
For patients tagged as COPD, their attending physician was contacted by telephone to determine whether their diagnosis had been made using spirometry. A copy of these tests was obtained when available.
Measurements of ABI were performed with a Doppler NicoletTM EliteTM 100R probe according to a procedure previously described by one of the authors as following [21]:
ABI values between 0.9 and 1.3 were considered normal. ABI values <0.9 were considered a surrogate for PAD [22, 23]. An ABI >1.3 was considered a sign of incompressible blood vessels and was an indication to determine the toe-brachial index. This was measured with laser-Doppler in our angiology department using PeriFlux System 5000 (Perimed AB). A TBI value ≥0.6 was considered normal and a value <0.6 was considered abnormal [24–27].
ABI, as well as TBI if needed, were measured in all patients except those who had already had a lower limb revascularisation procedure by percutaneous or surgical means. These patients were all considered to have PAD. We also used results of echo-Doppler obtained in our angiology department when performed during hospital stay as a proof of PAD.
Demographic data, main and secondary diagnoses of hospital stay, past medical history and comorbid conditions, vital parameters at hospital discharge, medication list at hospital arrival and discharge were recorded from patients’ electronic medical records (see appendix).
COPD was defined as an obstructive lung disease (FEV1/FVC ratio less than the lower limit of normal) that was not completely reversible after the administration of 200 micrograms of salbutamol or any other bronchodilator of equivalent potency.
PAD was defined as an ABI value <0.9, a TBI value <0.6 or any known PAD diagnosis determined from the result of an echo-Doppler examination performed by a board certified angiologist. All patients who had had a lower limb revascularisation procedure were also considered to have from PAD.
Statistical analyses were made using JMP 12 (SAS Institute) software, from an Excel (Microsoft Office) database including data previously described.
Quantitative variables were expressed as means ± standard deviation and compared between groups with student’s t-test. Quantitative variables were expressed as percentages and absolute numbers, and compared between groups with the Fischer exact test. A nominal logistic regression examined the association between PAD and predictors, and is expressed as odds ratios (ORs) and 95% confidence intervals (95% CIs). A p-value <0.05 was considered statistically significant.
Figure 1 summarises the evaluation and inclusion processes. We evaluated 888 patients the working day following their hospital admission, of whom 113 (13%) had in their medical record a history of COPD, chronic bronchitis or lung emphysema (patients tagged as COPD). We excluded 630 patients because they did not meet the inclusion criteria or presented one or several exclusion criteria. The 258 remaining patients were re-evaluated after 48 hours of clinical stability. Of these, 44 presented new exclusion criteria. A total of 214 patients were eventually tested, of whom 40 could not be included because of a spirometry examination of insufficient grade, discomfort while performing the test or refusal to repeat spirometry after 6 weeks if needed. Amongst the 174 remaining patients, 2 were eventually excluded because a TBI was needed to confirm PAD diagnosis but could not be performed. Finally, 172 patients were included, of whom 75 (44%) were tagged as COPD. During this evaluation process, 31 patients of the cohort (3.5%) could not be evaluated, essentially because of early hospital discharge, and appear as “missed”. The definitive inclusion rate was 19.5% of all evaluated patients, and 66.5% of the patients tagged as COPD.

Figure 1 Patient screening and inclusion.
Table 1 shows the presence of COPD and its severity in all tested patients. According to our inclusion criteria, patients were classified as patients tagged as COPD or patients at risk for COPD.
Table 1 Presence of confirmed chronic obstructive pulmonary disease (COPD) and its severity according to inclusion criteria used: patients at risk for COPD vs patients tagged as COPD.
|
Confirmed COPD
(n = 81) |
No COPD
(n = 91) |
|
|---|---|---|
| Patients at risk for COPD (n = 97) |
16% (16) | 84% (81) |
| Mild | 25% (4) | |
| Moderate | 63% (10) | |
| Severe | 12% (2) | |
| Very severe | – | |
| Patients tagged as COPD (n = 75) |
87% (65) | 13% (10) |
| Mild | 1% (1) | |
| Moderate | 42% (27) | |
| Severe | 43% (28) | |
| Very severe | 14% (9) |
We found COPD in 16 (16%) of patients at risk for COPD. Newly diagnosed COPD stages were mild in 4 (25%) patients, moderate in 10 (63%) patients and severe in 2 (12%) patients. No very severe COPD was newly diagnosed.
We confirmed a COPD diagnosis in 65 (87%) patients tagged as COPD and invalidated the diagnosis in 10 (13%) patients at the time of testing. COPD stages were mild in 1 (1%) patient, moderate in 27 (42%) patients, severe in 28 (43%) patients and very severe in 9 (14%) patients
Table 2 shows the proportion of patients tagged as COPD whose diagnosis was made based on spirometry, according to a telephone survey of their attending physician.
Table 2 Diagnostic use of spirometry by attending physicians in patients tagged as chronic obstructive pulmonary disease (COPD).
| Spirometry use by attending physicians |
Patients tagged as COPD
(n = 75) |
| Yes | 73% (n = 55) |
| Confirmed COPD | 93% (n = 51) |
| No COPD | 7% (n = 4) |
| No | 27% (n = 20) |
| Confirmed COPD | 70% (n = 14) |
| No COPD | 30% (n = 6) |
We confirmed a diagnosis of COPD in 81 (47%) patients of the 172 included. Demographic characteristics, significant comorbid conditions, characteristic symptoms and risk factors for COPD (used for inclusion of patients), cardiovascular diseases and vital parameters at hospital discharge are summarised in table 3.
Table 3 Characteristics of patients with confirmed chronic obstructive pulmonary disease (COPD) vs no COPD.
|
Confirmed COPD
(n = 81) |
No COPD
(n = 91) |
p-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Mean age (years) | 69.2±10.2 | 68.3 ± 10.7 | 0.58 |
| 45–50 years | 5% (4) | 6% (5) | 1 |
| 50–59 years | 17% (14) | 11% (10) | 0.27 |
| 60–69 years | 32% (26) | 31% (28) | 0.87 |
| 70–79 years | 30% (24) | 36% (33) | 0.42 |
| ≥80 years | 16% (13) | 16% (15) | 1 |
| Males | 59% (48) | 66% (60) | 0.43 |
| Mean BMI (kg/m2) | 25.0±5.7 | 28.5±7.0 | <0.001 |
| Risk factors for COPD | |||
| Active/past history of tobacco use | 91% (74) | 76% (69) | <0.01 |
| Pack-Year | 51.5±25.7 | 42.4±29.5 | 0.05 |
| Passive smoke inhalation | 6% (5) | 14% (13) | 0.13 |
| Professional exposure (≥1 possible) | 41% (33) | 37% (34) | 0.75 |
| Agriculture | 20% (16) | 14% (13) | 0.42 |
| Biological dusts | 9% (7) | 7% (6) | 0.77 |
| Inhaled chemical gas | 20% (16) | 20% (18) | 1 |
| History of “premature” COPD | 1% (1) | 1% (1) | 1 |
| Typical symptoms of COPD | |||
| Chronic dyspnoea | 77% (62) | 80% (73) | 0.58 |
| Chronic cough | 47% (38) | 30% (27) | 0.03 |
| Chronic sputum production | 53% (43) | 26% (24) | <0.001 |
| Frequent lower airways infections | 35% (28) | 27% (25) | 0.33 |
| Cardiovascular risk factors | |||
| Hypertension | 57% (46) | 58% (53) | 0.88 |
| Hypercholesterolaemia | 58% (47) | 53% (48) | 0.54 |
| Obesity | 17% (14) | 33% (30) | 0.02 |
| Diabetes | 21% (17) | 29% (26) | 0.29 |
| Vital parameters at hospital discharge (mean±SD) | |||
| Systolic blood pressure (mm Hg) | 124±19 | 127±17 | 0.19 |
| Diastolic blood pressure (mm Hg) | 71±12 | 73±11 | 0.23 |
| Oxygen saturation (%) | 93±4 | 94±3 | <0.01 |
| Temperature (°C) | 36.5±0.5 | 36.3±0.5 | 0.02 |
| Heart rate (beats/min) | 81±14 | 77±13 | 0.10 |
| Significant comorbid conditions | |||
| Ischaemic heart disease | 36% (29) | 35% (32) | 1 |
| Heart failure | 27% (22) | 23% (21) | 0.60 |
| History of stroke/TIA | 12% (10) | 14% (13) | 0.82 |
| Chronic kidney injury | 15% (12) | 14% (13) | 1 |
| Chronic hepatic failure | 5% (4) | 8% (7) | 0.54 |
| Cancer | 33% (27) | 30% (27) | 0.63 |
| Known PAD | 21% (17) | 6% (5) | <0.01 |
| Total confirmed PAD | 43% (35) | 24% (22) | <0.01 |
BMI = body mass index; PAD = peripheral arterial disease; SD = standard deviation; TIA = transient ischaemic attack
Overall, the two groups were homogenous regarding age and sex, with a mean age of approximately 70 years and a majority of males. Patients suffering from COPD had a significantly lower body mass index (25.0±5.7 vs 28.5±7.0 kg/m2; p <0.001) and were significantly less often obese (p = 0.02). They were more often active or former smokers (p <0.01), had smoked more cigarettes (51.5±25.7 vs 42.4±29.5 pack-year; p = 0.05), more often had chronic sputum production (p <0.001) and chronic cough (p = 0.03) than patients without COPD. There was no significant difference between the two groups in prevalence of hypertension, dyslipidaemia and diabetes, nor in absolute values of blood pressure at hospital discharge. Significant but not clinically relevant differences were found for oxygen saturation and tympanic temperature at hospital discharge.
PAD was found significantly more often in patients suffering from COPD (p <0.01) than in patients without COPD. There was no significant difference in the prevalence of ischaemic heart disease, heart failure and past history of stroke of transient ischaemic attack in the two groups, nor in the other comorbid diseases studied (table 3).
Table 4 shows the prevalence of PAD and the diagnostic technique used for the diagnosis. We found PAD in 57 (33%) of included patients. This diagnosis was already known in 22 patients of whom 7 had undergone a surgical/percutaneous revascularisation procedure and were not further checked. We confirmed PAD diagnosis in 14 (93%) of the 15 remaining patients. PAD was newly diagnosed in 36 patients, from the ABI in 27 patients, TBI in 8 patients and echo-Doppler in 1 patient.
Table 4 peripheral arterial disease (PAD) prevalence and diagnostic techniques used.
|
Confirmed PAD
(n = 57) |
No PAD
(n = 115) |
|
|---|---|---|
| New diagnoses (n = 36) |
||
| ABI | 27 | 99 |
| TBI | 8 | 15 |
| Echo-Doppler | 1 | |
| Known diagnosis (n = 22) |
||
| ABI | 11 | 1 |
| Revascularisation | 7 | |
| TBI | 2 | |
| Echo-Doppler | 1 |
ABI = ankle-brachial index; TBI = toe brachial index
Overall, besides the 7 patients who already had a revascularisation procedure and did not undergo further investigation, and one patient who was diagnosed with an echo-Doppler technique, 25 (15%) patients had ABI value >1.3 and needed TBI measurement to confirm or exclude PAD.
Table 5 summarises demographic characteristics, smoking habits, cardiovascular risk factors, vital parameters at hospital discharge and significant comorbid conditions in patients with PAD or no PAD.
Table 5 Characteristics of patients with confirmed peripheral arterial disease (PAD) vs no PAD.
|
Confirmed PAD
(n = 57) |
No PAD
(n = 115) |
p-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Mean age (years) | 70.7±10.1 | 67.7±10.5 | 0.07 |
| 45–50 years | 3% (2) | 6% (7) | 0.72 |
| 50–59 years | 12% (7) | 15% (17) | 0.82 |
| 60–69 years | 30% (17) | 32% (37) | 0.86 |
| 70–79 years | 37% (21) | 31% (36) | 0.49 |
| ≥80 years | 18% (10) | 16% (18) | 0.83 |
| Males | 63% (36) | 63% (72) | 1 |
| Mean BMI (kg/m2) | 26.8±6.1 | 26.9±7.0 | 0.92 |
| Smoking habits | |||
| Active/past history of tobacco use | 93% (53) | 78% (90) | 0.02 |
| Pack-year (mean±SD) | 56.5±27.3 | 41.6±26.9 | <0.01 |
| Passive smoke inhalation | 5% (3) | 13% (15) | 0.18 |
| Cardiovascular risk factors | |||
| Hypertension | 65% (37) | 54% (62) | 0.19 |
| Hypercholesterolaemia | 60% (34) | 53% (61) | 0.42 |
| Obesity | 30% (17) | 23% (26) | 0.35 |
| Diabetes | 35% (20) | 20% (23) | 0.04 |
| Vital parameters at hospital discharge (mean±SD) | |||
| Systolic blood pressure (mm Hg) | 125±18 | 126±18 | 0.61 |
| Diastolic blood pressure (mm Hg) | 71±13 | 72±11 | 0.40 |
| Oxygen saturation (%) | 93±4 | 94±3 | 0.05 |
| Temperature (°C) | 36.3±0.5 | 36.4±0.5 | 0.18 |
| Heart rate (beats/min) | 80±12 | 78±14 | 0.41 |
| Significant comorbid conditions | |||
| Ischaemic heart disease | 46% (26) | 30% (35) | 0.06 |
| Heart failure | 39% (22) | 18% (21) | <0.01 |
| History of stroke/TIA | 11% (6) | 15% (17) | 0.49 |
| Chronic kidney injury | 25% (14) | 10% (11) | 0.01 |
| Chronic hepatic failure | 4% (2) | 8% (9) | 0.34 |
| Cancer | 25% (14) | 35% (40) | 0.22 |
| Confirmed COPD | 61% (35) | 40% (46) | <0.01 |
BMI = body mass index; COPD = chronic obstructive lung disease; SD = standard deviation; TIA = transient ischaemic attack
There were no significant differences in age, sex and vital parameters at hospital discharge. Predictors of PAD in these selected patients tagged as COPD or at risk for COPD were a history of tobacco use (p = 0.02), number of pack-years (p <0.01), presence of diabetes (p = 0.04), heart failure (p <0.01), chronic kidney injury (p = 0.01) and confirmed COPD (p <0.01). Table 6 shows results of a nominal logistic regression of these predictors and their association with PAD, where the only significant factor was ≥40 pack-years (p <0.01).
Table 6 Nominal logistic regression of the association of peripheral arterial disease with predictors.
| Odds ratio | 95% confidence interval | p-value | |
|---|---|---|---|
| ≥40* Pack-Years | 3.93 | 1.87–8.68 | <0.01 |
| Confirmed COPD | 1.88 | 0.91–3.87 | 0.09 |
| Diabetes | 1.61 | 0.68–3.75 | 0.27 |
| Heart failure | 2.03 | 0.88–4.68 | 0.10 |
| Chronic kidney disease | 2.53 | 0.89–7.31 | 0.08 |
| COPD = chronic obstructive pulmonary disease * Median value for all patients |
|||
During the evaluation phase, we managed to include 19.5% of the 888 patients aged 45 years or more who were admitted into our internal medicine ward. Patients who had chronic respiratory symptoms were likely to participate to our study as evidenced by the low observed rate of refusal. Interestingly, when aware of their COPD, patients easily accepted screening or a check-up of this disease with spirometry. We also observed that spirometry of acceptable technical grade was feasible in more than 80% of tested patients, despite the multiple potential barriers to good performance of this test, such as advanced age and serious comorbid conditions. To improve specificity, we decided to use recent Swiss reference equations [20] and the lower limit of normal for the FEV1/FVC ratio to define COPD, as most of our patients were >65 years old and Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria are known to lack specificity in this age group [5, 6, 28].
Systematic screening of COPD in at-risk patients is effective (table 1). The number needed to screen (NNS) is approximately six patients for one new diagnosis. Severity of newly diagnosed COPD tends to be mild to moderate. It seems therefore justified to propose screening spirometry to all patients who meet our inclusion criteria, when there is an opportunity. Even though this was a selected group of at-risk hospitalised patients, and NNS might be higher in an attending physician’s office, we think that spirometry should be encouraged anyway, especially since it is rarely refused by patients presenting chronic respiratory symptoms, as previously discussed. This would increase detection of patients with mild disease who have a real therapeutic option. Amongst patients tagged as COPD, the diagnosis was confirmed in 87% of the cases (table 1), and COPD tended to be more severe than with new diagnoses. Spirometry was used by their attending physicians to support the diagnosis in 73% of cases, which is a better rate than reported in previous studies [29–31], but there is still room for improvement. An incorrect diagnosis was found in 13% of cases, and was as high as 30% among patients diagnosed without spirometry (table 2). On a large scale, this could affect a considerable number of patients.
PAD was found in 33% of tested patients and was undiagnosed in more than half of them (table 4). This prevalence is similar to that of other groups of high-risk patients [32], and greater than in an unselected population in this age group [33, 34]. Furthermore, we found a significant association with confirmed COPD as compared with patients with a similar global risk profile but without COPD. The relative risk of suffering from PAD in patients with confirmed COPD was approximately 1.8 as compared with patients without COPD. However, in presence of an extremely high prevalence of tobacco use, which is significantly associated with both PAD and COPD and known to be a strong risk factor for both diseases [2, 35], it appears that COPD is not an independent predictor of PAD, as revealed by logistic regression showing smoking ≥40 pack-years as the only statistically significant predictor of PAD (table 6). Alagiakrishnan and al. found a very similar prevalence of COPD-PAD association in a small study evaluating the prevalence of asymptomatic PAD in patients with tobacco-related COPD [36]. Surprisingly, Houben-Wilke and al. reported a much smaller PAD prevalence of 8.8% in a recent large multicentre German study of COPD patients with high cardiovascular risk profile; this is even lower than the expected prevalence in an unselected population of the same age, but the authors made no comment about this fact [37].
High prevalence of coronary artery disease, heart failure, and history of stroke or transient ischaemic attack was observed, as expected in these patients with high cardiovascular risk. However, no significant association of these diseases with COPD was found (table 3). We propose that every smoker newly diagnosed with COPD be screened for PAD using ABI determination, which can be quickly and easily performed by any practitioner [21]. Furthermore, we know that PAD, whether symptomatic or not, represents a strong prospective cardiovascular marker, in particular for cardiac and cerebrovascular diseases [11]. In smokers, spirometry showing COPD constitutes a powerful test to detect higher cardiovascular risk. This seems to confirm the hypothesis that reduction in FEV1 could be used as a marker for cardiovascular risk, especially for lower-limb peripheral arterial disease [38–40].
Our study was mainly performed during winter months, when there is an epidemic peak of upper and lower respiratory tract infections that typically induce an increase in acute exacerbations of chronic lung diseases. The number of hospital admissions of patients suffering from COPD was particularly high and probably does not reflect accurately the annual average. Besides, it is likely that a selection bias exists, leading to an overrepresentation of patients suffering from severe chronic lung diseases, and therefore a greater probability of true COPD. Finally, the limited number of patients available for analysis limits the identification of statistically significant subtle differences between groups. Notably, the implication of non-tobacco-related expositional risk factors for COPD were masked by the fact that most of the patients in this study were current or former smokers.
This study shows a 9% prevalence of confirmed COPD and a 13% prevalence of patients tagged as COPD amongst the 888 patients evaluated for inclusion. Knowing that COPD diagnoses are most of the time accurate and that there is a significant number of patients suffering from COPD who are not yet diagnosed, it is reasonable to assume that the real prevalence of COPD in patients aged ≥45 years admitted in the internal medicine ward of our tertiary care hospital lies between 10% and 15%, which is the expected prevalence in this category of age [2].
Our study confirms that there is an important underdiagnosis of COPD in patients presenting typical symptoms and risk factors for this disease, with a NNS of only six patients for a new diagnosis in this selected population of hospitalised patients. Although this proportion would probably be lower in a general practitioner’s office because of the limitations discussed above, we encourage them to perform spirometry in selected patients with risk factors for COPD, as it is an effective and easy-to-use tool that is rarely refused by patients, as observed in this study.
Furthermore, in smokers tagged as COPD or at risk for COPD, spirometry not only allows confirmation of the diagnosis of obstructive lung disease, but also seems to be a useful tool to detect patients at higher cardiovascular risk, especially those suffering from PAD as shown by the significant association found with COPD. Moreover, PAD is often undiagnosed in these patients. Thus, we suggest that active screening for PAD by determining ABI should be proposed to every smoker with confirmed COPD.
First name, last name, date of birth, age, gender
Postal address, phone number
Name of attending physician and attending pulmonologist
Primary diagnosis
Any other diagnosis that might affects the spirometric values
Ischemic heart disease
Heart failure
History of stroke or transient ischaemic attack
Peripheral arterial disease
Chronic kidney injury
Chronic liver injury
Cancer (including haematological malignancies)
Name of commercial substance and dosage
We would like to thank our colleagues Daniel Périard, MD and Marie-Antoinette Rey Meyer, MD for their precious help performing toe-brachial index when needed.
The authors state that there are no outside financial support nor conflicts of interest.
1 Mathers CD , Loncar D . Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. https://doi.org/10.1371/journal.pmed.0030442
2 Bridevaux PO , Probst-Hensch NM , Schindler C , Curjuric I , Felber Dietrich D , Braendli O , et al. Prevalence of airflow obstruction in smokers and never-smokers in Switzerland. Eur Respir J. 2010;36(6):1259–69. https://doi.org/10.1183/09031936.00004110
3From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2016. Available from: http://goldcopd.org/.
4 Salvi SS , Barnes PJ . Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–43. https://doi.org/10.1016/S0140-6736(09)61303-9
5 Quanjer PH , Enright PL , Miller MR , Stocks J , Ruppel G , Swanney MP , et al. The need to change the method for defining mild airway obstruction. Eur Respir J. 2011;37(3):720–2. https://doi.org/10.1183/09031936.00135110
6 Quanjer PH , Enright PL , Miller MR , Stocks J , Ruppel G , Swanney MP , et al. The need to change the method for defining mild airway obstruction. Eur Respir J. 2011;37(3):720–2. https://doi.org/10.1183/09031936.00135110
7 Bridevaux PO , Rochat T . BPCO en 2011: y a-t-il d’autres facteurs de risque que le tabac? [COPD 2011: are there other risk factors than tobacco?.] Rev Med Suisse. 2011;7(317):2232–5.Article in French.
8 Agusti A , Soriano JB . COPD as a systemic disease. COPD. 2008;5(2):133–8. https://doi.org/10.1080/15412550801941349
9 Agustí AG , Noguera A , Sauleda J , Sala E , Pons J , Busquets X . Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(2):347–60. https://doi.org/10.1183/09031936.03.00405703
10 Mannino DM , Thorn D , Swensen A , Holguin F . Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–9. https://doi.org/10.1183/09031936.00012408
11 Finch J , Conklin DJ . Air Pollution-Induced Vascular Dysfunction: Potential Role of Endothelin-1 (ET-1) System. Cardiovasc Toxicol. 2016;16(3):260–75. https://doi.org/10.1007/s12012-015-9334-y
12 Hoffmann B , Moebus S , Kröger K , Stang A , Möhlenkamp S , Dragano N , et al. Residential exposure to urban air pollution, ankle-brachial index, and peripheral arterial disease. Epidemiology. 2009;20(2):280–8. https://doi.org/10.1097/EDE.0b013e3181961ac2
13 Golomb BA , Dang TT , Criqui MH . Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114(7):688–99. https://doi.org/10.1161/CIRCULATIONAHA.105.593442
14 Aronow WS . Association of Lower Extremity Peripheral Arterial Disease with Atherosclerotic Vascular Disease, Cardiovascular Events, and Mortality. J Cardiovasc Dis Diagn. 2014;2:e105. https://doi.org/10.4172/2329-9517.100e105
15 Libby P . Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–51. https://doi.org/10.1161/ATVBAHA.108.179705
16 Steurer-Stey C , Senn O , Pfisterer J , Karrer W , Russi EW , Müller M . BPCO: l’essentiel pour le médecin de premier recours 2013. Swiss Med Forum. 2013;13:227–30.
17 Cooper BG . An update on contraindications for lung function testing. Thorax. 2011;66(8):714–23. https://doi.org/10.1136/thx.2010.139881
18 Skloot GS , Edwards NT , Enright PL . Four-year calibration stability of the EasyOne portable spirometer. Respir Care. 2010;55(7):873–7.
19 Miller MR , Hankinson J , Brusasco V , Burgos F , Casaburi R , Coates A , et al.; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. https://doi.org/10.1183/09031936.05.00034805
20 Kuster SP , Kuster D , Schindler C , Rochat MK , Braun J , Held L , et al. Reference equations for lung function screening of healthy never-smoking adults aged 18-80 years. Eur Respir J. 2008;31(4):860–8. https://doi.org/10.1183/09031936.00091407
21 Hayoz D , Bounameaux H , Canova CR . Swiss Atherothrombosis Survey: a field report on the occurrence of symptomatic and asymptomatic peripheral arterial disease. J Intern Med. 2005;258(3):238–43. https://doi.org/10.1111/j.1365-2796.2005.01536.x
22 Rooke TW , Hirsch AT , Misra S , Sidawy AN , Beckman JA , Findeiss LK , et al.; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society for Vascular Medicine; Society for Vascular Surgery. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58(19):2020–45. https://doi.org/10.1016/j.jacc.2011.08.023
23 Reiner Z , Catapano AL , De Backer G , Graham I , Taskinen MR , Wiklund O , et al.; European Association for Cardiovascular Prevention & Rehabilitation; ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010-2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769–818. https://doi.org/10.1093/eurheartj/ehr158
24 Suominen V , Uurto I , Saarinen J , Venermo M , Salenius J . PAD as a risk factor for mortality among patients with elevated ABI--a clinical study. Eur J Vasc Endovasc Surg. 2010;39(3):316–22. https://doi.org/10.1016/j.ejvs.2009.12.003
25 Suominen V , Rantanen T , Venermo M , Saarinen J , Salenius J . Prevalence and risk factors of PAD among patients with elevated ABI. Eur J Vasc Endovasc Surg. 2008;35(6):709–14. https://doi.org/10.1016/j.ejvs.2008.01.013
26 Morimoto S , Nakajima F , Yurugi T , Morita T , Jo F , Nishikawa M , et al. Risk factors of normal ankle-brachial index and low toe-brachial index in hemodialysis patients. Ther Apher Dial. 2009;13(2):103–7. https://doi.org/10.1111/j.1744-9987.2009.00663.x
27 Park SC , Choi CY , Ha YI , Yang HE . Utility of Toe-brachial Index for Diagnosis of Peripheral Artery Disease. Arch Plast Surg. 2012;39(3):227–31. https://doi.org/10.5999/aps.2012.39.3.227
28 Janssens JP , Pache JC , Nicod LP . Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13(1):197–205. https://doi.org/10.1183/09031936.99.14614549
29 Gershon AS , Hwee J , Croxford R , Aaron SD , To T . Patient and physician factors associated with pulmonary function testing for COPD: a population study. Chest. 2014;145(2):272–81. https://doi.org/10.1378/chest.13-0790
30 Arne M , Lisspers K , Ställberg B , Boman G , Hedenström H , Janson C , et al. How often is diagnosis of COPD confirmed with spirometry? Respir Med. 2010;104(4):550–6. https://doi.org/10.1016/j.rmed.2009.10.023
31 Penña VS , Miravitlles M , Gabriel R , Jiménez-Ruiz CA , Villasante C , Masa JF , et al. Geographic variations in prevalence and underdiagnosis of COPD: results of the IBERPOC multicentre epidemiological study. Chest. 2000;118(4):981–9. https://doi.org/10.1378/chest.118.4.981
32 Hirsch AT , Criqui MH , Treat-Jacobson D , Regensteiner JG , Creager MA , Olin JW , et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–24. https://doi.org/10.1001/jama.286.11.1317
33 Meijer WT , Hoes AW , Rutgers D , Bots ML , Hofman A , Grobbee DE . Peripheral arterial disease in the elderly: The Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18(2):185–92. https://doi.org/10.1161/01.ATV.18.2.185
34 Diehm C , Schuster A , Allenberg JR , Darius H , Haberl R , Lange S , et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172(1):95–105. https://doi.org/10.1016/S0021-9150(03)00204-1
35 Agarwal S . The association of active and passive smoking with peripheral arterial disease: results from NHANES 1999-2004. Angiology. 2009;60(3):335–45. https://doi.org/10.1177/0003319708330526
36 Alagiakrishnan K , Brokop M , Cave A , Rowe BH , Wong E , Senthilselvan A . Resting and Post-Exercise Ankle-Brachial Index Measurements to Diagnose Asymptomatic Peripheral Arterial Disease in Middle Aged and Elderly Chronic Obstructive Pulmonary Disease Patients: A Pilot Study. J Clin Med Res. 2016;8(4):312–6. https://doi.org/10.14740/jocmr2493w
37 Houben-Wilke S , Jörres RA , Bals R , Franssen FM , Gläser S , Holle R , et al. Peripheral Artery Disease and Its Clinical Relevance in Patients with Chronic Obstructive Pulmonary Disease in the COPD and Systemic Consequences-Comorbidities Network Study. Am J Respir Crit Care Med. 2017;195(2):189–97. https://doi.org/10.1164/rccm.201602-0354OC
38 Hole DJ , Watt GCM , Davey-Smith G , Hart CL , Gillis CR , Hawthorne VM . Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313(7059):711–5, discussion 715–6. https://doi.org/10.1136/bmj.313.7059.711
39 Young RP , Hopkins R , Eaton TE . Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J. 2007;30(4):616–22. https://doi.org/10.1183/09031936.00021707
40 Menezes AM , Pérez-Padilla R , Wehrmeister FC , Lopez-Varela MV , Muiño A , Valdivia G , et al.; PLATINO team. FEV1 is a better predictor of mortality than FVC: the PLATINO cohort study. PLoS One. 2014;9(10):e109732. https://doi.org/10.1371/journal.pone.0109732
The authors state that there are no outside financial support nor conflicts of interest.