Invasive haemodynamic evaluation of the pulmonary circulation in pulmonary hypertension
DOI: https://doi.org/10.4414/smw.2017.14445
Alberto
Pagnamentaabc, Andrea
Azzolaad, Maurice
Beghettie f, Frédéric
Ladorce, , on behalf of the Swiss Society of Pulmonary Hypertension
aDepartment of Intensive Care Medicine of the Ente Ospedaliero Cantonale (EOC),
b
cDivision of Pneumology,
dPneumology, Department of Internal Medicine,
ePulmonary Hypertension Programme,
fPaediatric Cardiology Unit, Children’s University Hospital,
Invasive haemodynamic evaluation of the pulmonary circulation in pulmonary hypertension
w14445
Summary
The term pulmonary hypertension refers to a serious condition characterised by high pulmonary vascular pressure, mainly as a consequence of various cardiac and respiratory diseases. Current clinical classification of pulmonary hypertension considers five distinct groups. Transthoracic echocardiography represents the first and most important noninvasive screening tool for estimating the probability of pulmonary hypertension. The diagnostic approach to pulmonary hypertension is supported by a proposed algorithm, which identifies the underlying cause. The definitive diagnosis and classification of pulmonary hypertension requires invasive confirmation of an elevated pulmonary artery mean pressure during a right heart catheterisation at rest. Pulmonary artery wedge pressure assessment has a pivotal role in differentiating precapillary from postcapillary pulmonary hypertension. The correct acquisition and interpretation of invasive pulmonary haemodynamic variables play a central role, not only in confirming the diagnosis but also in prognostication and treatment decision-making. During right heart catheterisation correct zero levelling of the external pressure transducer and pressure tracing readings at end-expiration should be assured. Obese patients and patients with obstructive lung diseases require special attention, given that spontaneous positive end-expiratory intrathoracic pressures are frequently observed. Because pressure and flow determinations with a fluid-filled flow-directed thermodilution catheter are potentially insufficiently precise, it is recommended to average at least three measurements. Acute vasoreactivity testing is indicated only in selected patients. Recent data suggest that invasive pulmonary haemodynamic measurement during exercise may be more sensitive than resting haemodynamics for early diagnosis, for treatment response assessment and for prognostic purposes.
Introduction
Pulmonary hypertension (PH) is a condition frequently encountered in daily clinical practice and, despite the lack of good prevalence data, it is presumed to be the third most common cardiovascular condition after systemic hypertension and coronary artery disease [1]. The original World Health Organization (WHO) clinical classification of PH proposed in 1973 was simple and based on only two categories: primary and secondary PH [2]. Twenty-five years later at the second World Symposium on Pulmonary Hypertension (WSPH) held in Evian, France in 1998, a remarkable modification of the previous classification was adopted [3]. Five well-defined major categories of PH were created, and based on similar underlying pathophysiological mechanisms:
- Pulmonary arterial hypertension (PAH)
- Pulmonary hypertension due to left-sided heart diseases (LHD)
- Pulmonary hypertension due to chronic lung diseases and/or hypoxia
- Chronic thromboembolic pulmonary hypertension (CTEPH)
- Pulmonary hypertension with unclear multifactorial mechanisms
This new classification enabled well-designed randomised controlled trials conducted in clearly defined patient populations, which have led to the approval of eight drugs for the treatment of PAH.
At a further WSPH, the clinical classification was refined, while the general architecture of previous classifications was maintained. The most recently updated version proposed during the fifth WSPH held in Nice, France in 2013 is presented in table 1 [4]. A common classification for both paediatric and adult patients was adopted. Comparative prevalence data for the various groups are not available, but LHD, including the forms with either reduced or preserved ejection fraction, probably represents the most common cause of PH [5, 6]. Depending on the selected cohort of patients and the definition of PH used (invasive measurements vs estimation with echocardiography; different cut-off values), up to 80% of patients with LHD may have PH [7, 8], which if present negatively affects outcome [7, 9].
Table 1 Clinical classification of pulmonary hypertension.
- Pulmonary arterial hypertension
|
- Idiopathic pulmonary arterial hypertension
|
- Heritable pulmonary arterial hypertension
|
-
|
-
|
- Unknown
|
- Drug- and toxin-induced
|
- Associated with:
|
- Connective tissue disease
|
- Human immunodeficiency virus infection
|
- Portal hypertension
|
- Congenital heart diseases
|
- Schistosomiasis
|
- 1’ Pulmonary veno-occlusive diseases and/or pulmonary capillary haemangiomatosis
|
- 1’’ Persistent pulmonary hypertension of the newborn
|
- Pulmonary hypertension due to left heart diseases
|
- Left ventricular systolic dysfunction
|
- Left ventricular diastolic dysfunction
|
- Valvular disease
|
- Congenital/acquired left heart inflow/outflow tract obstruction and congenital cardiomyopathies
|
- Pulmonary hypertension due to lung diseases and/or hypoxia
|
- Chronic obstructive pulmonary disease
|
- Interstitial lung disease
|
- Other pulmonary diseases with mixed restrictive and obstructive pattern
|
- Sleep-disordered breathing
|
- Alveolar hypoventilation disorders
|
- 3.6 Chronic exposure to high altitude
|
- Developmental lung diseases
|
- Chronic thromboembolic pulmonary hypertension
|
- Pulmonary hypertension with unclear multifactorial mechanisms
|
- Haematological disorders: chronic haemolytic anaemia, myeloproliferative disorders, splenectomy
|
- Systemic disorders: sarcoidosis, pulmonary histiocytosis, lymphangioleiomyomatosis
|
- Metabolic disorders: glycogen-storage disease, Gaucher disease, thyroid disorders
|
- Others: tumoural obstruction, fibrosing mediastinitis, chronic renal failure, segmental pulmonary hypertension
|
Chronic lung diseases are considered to be the second most frequent aetiology of PH [10]. The severity of chronic obstructive pulmonary disease (COPD) seems to determine the likelihood of developing PH, which if present exerts a negative impact on survival [11]. Conversely, PH severity is only poorly correlated with lung function impairment in patients with idiopathic pulmonary fibrosis (IPF) [12]. The syndrome of combined pulmonary fibrosis and emphysema (CPFE) represents a separate entity especially predisposed to result in PH (prevalence estimates range between 30 and 50%) [13]. Recent data suggest that the annual incidence of CTEPH is about five subjects per million adult population per year, with a prior history of acute pulmonary embolism in approximately 75% of patients [14]. Reliable epidemiological data on PAH are provided by national registries and confirm that it is a rare disease with an estimated prevalence of approximately 15 to 60 cases per million adult population [15].
Haemodynamic definitions and diagnosis of pulmonary hypertension
Pulmonary hypertension is defined as an invasive pulmonary artery mean pressure (mPAP) ≥25 mm Hg obtained during right heart catheterisation at rest [16]. Normal resting mPAP values are 14 ± 3 mm Hg (mean ± standard deviation), with an upper limit of approximately 20 mm Hg [17]. A resting mPAP between 21 and 24 mm Hg is clearly above the limit of normal, but does not fulfil the criterion for a diagnosis of PH. An mPAP in this range was previously defined as “borderline PH”, a term that has now been abandoned because of its unclear clinical and prognostic significance [1, 16]. However, in a recent non-concurrent cohort study, subjects with mPAP of 21 to 24 mm Hg showed a lower 6-minute walking distance and a double prevalence of an abnormal exercise-induced pulmonary vascular response as compared with subjects with strictly normal mPAP [18]. This finding supports the concept that subjects with an mPAP of 21 to 24 mm Hg at rest represent a separate phenotype requiring close follow-up, especially in the presence of risk factors for developing PAH (connective tissue diseases, family members with idiopathic or heritable PAH) [1, 16].
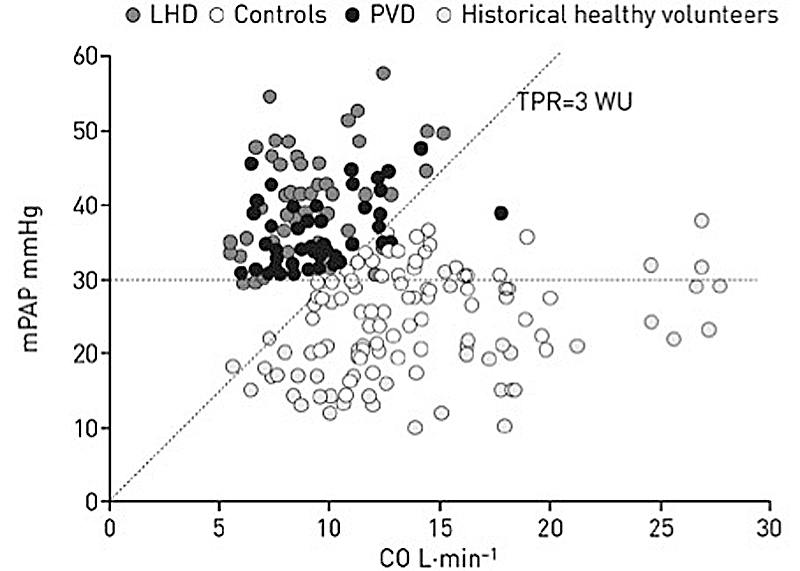
The current definition of PH considers pulmonary pressure values only at rest [16]. However, recently combined criteria for exercise-induced PH with excellent intrinsic diagnostic characteristics have been proposed as follows: mPAP >30 mm Hg with a total pulmonary vascular resistance (total PVR = mPAP / cardiac output [CO]) >3 Wood units (WU) at maximum exercise (fig. 1) [19]. This definition of PH on exercise was not integrated into the current 2015 European guidelines, and is awaiting prospective outcome validation data [1]. Unlike the general definition of PH, the definition of pulmonary arterial includes PVR, as follows: mPAP ≥25 mm Hg, pulmonary artery wedge pressure (PAWP) ≤15 mm Hg and PVR >3 WU in the absence of other precapillary aetiologies (chronic lung diseases, CTEPH and other rare diseases) [1, 16]. PVR, as opposed to total PVR, is the ratio of pressure gradient to cardiac output: PVR = (mPAP ˗ PAWP)/CO.
The term pulmonary capillary wedge pressure (PCWP) is frequently used in the medical literature. This pressure is obtained by wedging a pulmonary artery catheter with a deflated balloon, and PCWP is therefore a misleading term because it is different from effective pulmonary capillary pressure and different from PAWP. The working group on “definitions and diagnosis of PH” of the fifth WSPH suggested abandoning the term pulmonary capillary wedge pressure and preferred use of the term PAWP for pulmonary venous pressure [16].
From the haemodynamic point of view, PH is differentiated to precapillary and postcapillary forms, depending on the PAWP value. Postcapillary PH occurs in LHD and requires a PAWP above 15 mm Hg [1]. Postcapillary PH was previously subclassified into passive and reactive (or “out of proportion”) forms. Reactive postcapillary PH referred to an mPAP higher than expected from a passive backward transmission of an elevated venous pressure, as a consequence of superimposed vasoconstriction and/or vascular remodelling of the pulmonary circulation. The term reactive postcapillary PH is now abandoned, and currently two types of postcapillary PH are identified on the basis of the diastolic pressure gradient, as presented in table 2 [5].
Table 2 Haemodynamic classification of pulmonary hypertension.
|
Definition
|
Haemodynamic values*
|
Clinical category†
|
| PH |
mPAP ≥25 mm Hg |
All |
| Precapillary PH |
mPAP ≥25 mm Hg
PAWP ≤15 mm Hg |
- Pulmonary arterial hypertension
- PH due to lung diseases
- CTEPH
- PH with unclear and/or multifactorial mechanisms
|
| Postcapillary PH |
mPAP ≥25 mm Hg
PAWP >15 mm Hg |
2. PH due to left heart diseases
5. PH with unclear and/or multifactorial mechanisms |
| Isolated postcapillary PH |
DPG <7 mm Hg and/or
PVR ≤3 WU‡
|
Combined postcapillary and
precapillary PH |
DPG ≥7 mm Hg and/or PVR >3 WU‡
|
During the fifth WSPH held in 2013 it was suggested that use of the term “out of proportion” in the classification of PH due to lung diseases should be abandoned. “Out of proportion” indicated that the severity of PH was higher than expected on the basis of lung parenchymal impairment [10]. For COPD, IPF and CPFE the following definitions are currently applied: COPD/IPF/CPFE without PH (mPAP <25 mm Hg), with PH (mPAP ≥25 mm Hg) and with severe PH (mPAP ≥35 mm Hg, or mPAP ≥25 mm Hg with low cardiac index of <2.5 l/min/m2) [1].
Unfortunately, even today the diagnosis of PH is often delayed because the presenting symptoms and signs are typically nonspecific and generally related to a progressive dysfunction of right ventricle [20]. Pulmonary hypertension should be suspected in any patient with exertional dyspnoea, fatigue and impaired exercise tolerance of unexplained aetiology, chest pain, syncope and/or signs of right ventricular dysfunction. Clinical signs suggestive of PH include parasternal lift, an accentuated second heart sound, a third heart sound, a pansystolic heart murmur reflecting tricuspid regurgitation and tachypnoea without wheeze and crackles. Patients with more advanced disease developing progressive right ventricular failure present with elevated jugular venous pressure, hepatomegaly with ascites and peripheral oedema with cold extremities.
Transthoracic echocardiography (TTE) represents the first and most important noninvasive screening tool for estimating the likelihood of PH, but for a definitive diagnosis and treatment decisions right heart catheterisation is compulsory, because TTE may give inaccurate pulmonary artery pressure estimates on an individual basis [21]. Where PH is suspected, TTE should always be performed. Continuous wave Doppler assessment of peak tricuspid regurgitation velocity is the main TTE variable from which to estimate the probability of PH [1]. Additional echocardiographic signs suggestive of PH are right ventricular size and pressure overload, blood flow pattern velocity out of the right ventricle, pulmonary artery diameter and estimated right atrial pressure. These other echo “PH signs”, combined with the peak tricuspid regurgitation velocity, permit the likelihood of PH to be stratified into low, intermediate and high [21, 22].
An extensive diagnostic workup supported by a proposed algorithm is required in order to identify the underlying cause of PH [1]. When there is clinical suspicion and an intermediate to high echocardiographic probability of PH, the more prevalent clinical categories (groups 2 and 3) should be considered first. A set of additional investigations (electrocardiogram, chest radiograph, pulmonary function tests, arterial blood gas analysis and high resolution chest computed tomography) is required in order to identify the presence of group 2 (left heart diseases) or group 3 (lung diseases).
If the echocardiographic probability of PH is low, no further investigations are required and an alternative explanation for symptoms should be sought. If the diagnosis of left heart or lung diseases is confirmed and no signs of severe PH are present the appropriate treatment should be initiated without further testing, especially not right heart catheterisation. If severe PH and/or right ventricular dysfunction are present it is recommended to refer the patient to a PH expert centre for a search for the additional causes. After exclusion of group 2 and group 3 PH, a ventilation/perfusion scan is required to differentiate between CTEPH and PAH. Right heart catheterisation is compulsory for the definitive diagnosis of both entities and for treatment decisions. If the diagnosis of CTEPH is excluded by a normal or low-probability ventilation/perfusion scan, specific tests (blood chemistry and haematology, immunology, serology and ultrasonography) may be useful for identification of the individual subsets of PAH.
Best clinical practice for right heart catheterisation
Despite recent and promising advances in noninvasive techniques for measurement of the pulmonary circulation [23, 24], right heart catheterisation remains the reference standard for the confirmation of suspected PH, assessing the disease severity and thereafter evaluating prognosis and, not least, determining the response to targeted therapy during the course of the disease [1]. For group 2 and group 3 PH, right heart catheterisation is recommended only if organ transplantation is considered. It could be useful in patients with suspected PH and left heart or lung diseases in assisting the differential diagnosis and the treatment decision making.
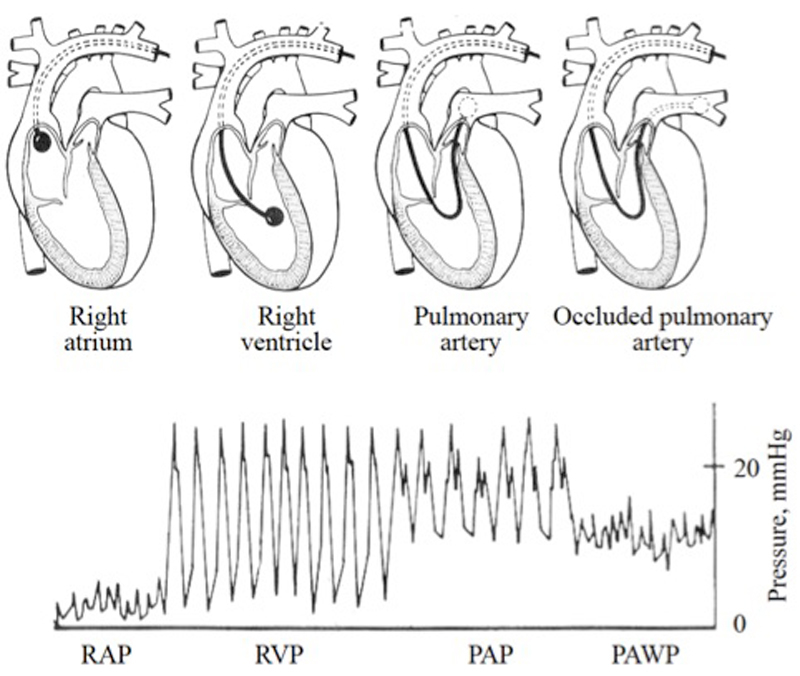
Right heart catheterisation is a safe procedure, with a reported related morbidity of 1.1% and mortality of 0.055% in patients with PH when performed in expert centres [25]. Subclavian and internal jugular veins are the preferred vascular access for the insertion of a pulmonary artery catheter. The catheter is advanced with an inflated balloon from the superior vena cava or right atrium until it reaches the wedge position (fig. 2). The frequency response of a fluid-filled pulmonary artery catheter has been generally assumed to be insufficient for accurate instantaneous pressure measurements. Recently, fluid-filled pressures determined with a pulmonary artery catheter were compared with PAP measurements made with the reference standard method (a high-fidelity micromanometer-tipped catheter), showing excellent reliability but a lack of precision [26]. Mean cardiac output is measured with a fluid-filled pulmonary artery catheter by means of the thermodilution technique, which requires several cardiac cycles and use of the Stewart-Hamilton equation [27]. In certain situations, such as severe tricuspid regurgitation and low output syndrome, this technique could provide imprecise estimations of cardiac output as compared with the direct Fick method, which is considered the reference standard but needs simultaneous measurements of oxygen uptake and arterial and mixed venous blood analysis [27]. In addition, if intracardiac shunts are suspected the direct Fick method should be preferred, because the thermodilution technique provides inaccurate cardiac output determination because of early recirculation of the injectate. The indirect Fick method may also give unreliable estimates of cardiac output and accordingly its use is discouraged [1].
In order to cope with the intrinsic random errors of pressure and flow determinations made with a fluid-filled pulmonary artery catheter, it is now clearly recommended to take several measurements (three to five with less than 10% variation among them) and average them [24]. A pulmonary artery catheter permits direct measurement of the following haemodynamic variables: PAP (systolic, diastolic and mean), PAWP, right atrial pressure (RAP), cardiac output and mixed venous oxygen saturation (SvO2); and derivation of the following variables: diastolic pressure gradient (diastolic PAP – PAWP), PVR and cardiac index (cardiac output / body surface area) [28]. PAWP is taken as a surrogate of left atrial pressure (LAP), which represents the outflow pressure of the pulmonary circulation, because LAP cannot be directly measured with a pulmonary artery catheter. A large-scale quality control study compared PAWP measurements during right heart catheterisation with concomitant LAP measurements during left heart catheterisation. Estimates of LAP by PAWP were reliable but insufficiently precise [29]. LeVarge and colleagues reported that about one third of patients with precapillary PH had an end-expiratory PAWP >15 mm Hg at a single point in time [30]. This was mainly observed in obese individuals and in patients with COPD, two populations which frequently have spontaneous positive end-expiratory intrathoracic pressure. Averaging PAWP values over several respiratory cycles in order to compensate for respiratory swings in these patients represents a valuable alternative to end-expiratory readings [31].
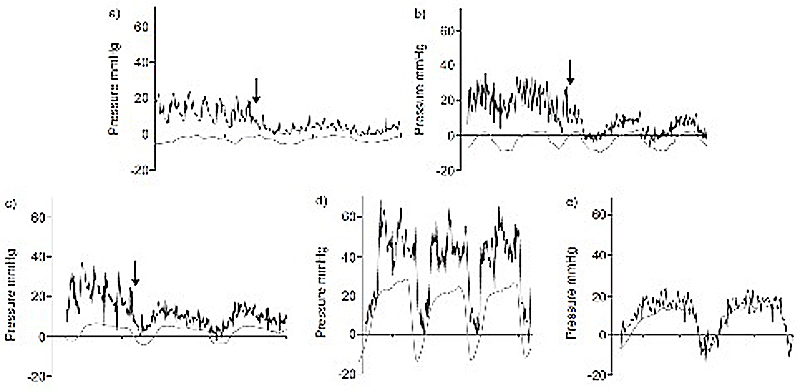
Right heart catheterisation is a demanding and time-consuming procedure, because it requires special attention and expertise for data acquisition and interpretation. Invasive haemodynamic variables should not be interpreted alone, but always integrated into the clinical context, taking into account noninvasive diagnostic investigations, especially TTE. The zero levelling of the external pressure transducer during a right heart catheterisation should be performed with the patient in the supine position at the cross-section of three transthoracic planes (midchest frontal; transverse through the fourth intercostal space; midsagittal) representing the left atrial level [31]. In order to obtain accurate and precise estimates of PAWP, the tip of pulmonary artery catheter must be positioned in West’s lung zone III and pressure tracings must be recorded at end expiration, when intrathoracic pressure approximates atmospheric pressure. Special attention should be paid to obese patients and patients with COPD, given the relevant intrathoracic pressure swings across the respiratory cycle in these subjects (fig. 3) [1, 28, 30–32].
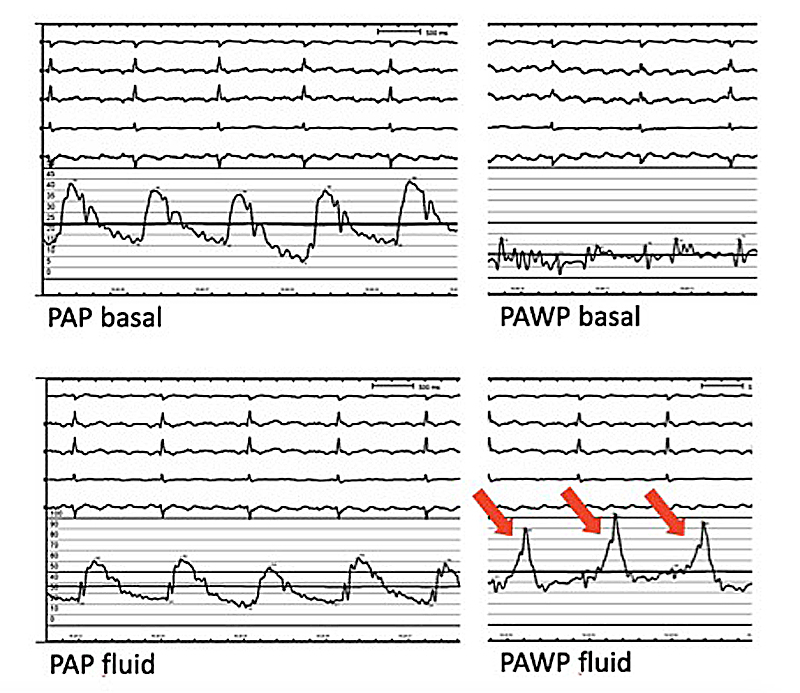
Patients with PH and LHD treated with diuretics may have a PAWP <15 mm Hg, leading to a misclassification of their PH [33]. A fluid challenge during right heart catheterisation may be an attractive strategy to unmask patients with heart failure and preserved ejection fraction (HFpEF) but PAWP <15 mm Hg at baseline (fig. 4) [34]. Unfortunately, the intrinsic diagnostic characteristics of this manoeuvre (positive and negative likelihood ratios) have not been rigorously evaluated. Recently, Robbins et al. found, in a retrospective cohort of patients primarily classified as having PAH, that rapid intravenous fluid challenge (500 ml of 0.9% NaCl over 5–10 minutes) increased PAWP >15 mm Hg in 22.2% of patients, unmasking occult pulmonary venous hypertension. Unfortunately, left ventricular end-diastolic pressure was concomitantly assessment in only a minority of patients [35]. This interesting finding, with direct consequences for PH classification and for patient selection for clinical trials in PAH, requires additional validation.
For routine assessment of PH, concomitant left heart catheterisation is currently not recommended. However, it should be seriously considered in patients with clinical risk factors for coronary artery disease or for HFpEF, in patients with echocardiographic signs of systolic and/or diastolic dysfunction and when PAWP measurement is unreliable [1]. During a right heart catheterisation, an acute pulmonary vasoreactivity test is performed in order to identify patients suitable for calcium channel blocker therapy. Currently this test is indicated only in patients with idiopathic, heritable or drug-related PAH [1]. A positive test is defined as a reduction of mPAP ≥10 mm Hg from baseline to an absolute value of mPAP ≤40 mm Hg with unchanged or increased cardiac output [16]. Vasoreactivity testing is usually performed with inhaled nitric oxide (10 to 20 parts per million). Alternatively, inhaled iloprost, intravenous epoprostenol or adenosine can be used [1].
If a cardiac left-to-right shunt is suspected and when pulmonary artery oxygen saturation is >75%, a stepwise measurement of oxygen saturation is recommended, with assessment in the superior vena cava, inferior vena cava, right atrium, right ventricle and pulmonary artery [1, 28]. A saturation difference of >7% between mixed venous saturation and the right atrium is suggestive of an atrial left-to-right shunt, whereas a difference above 5% between right atrium and right ventricle may be indicative of a ventricular left-to-right shunt [28]. Recent guidelines suggested performing right heart catheterisation in patients with PAH at regular intervals, 3 to 6 months after changes in targeted therapy, and not only in the event of clinical worsening [1]. A high RAP, low cardiac index and low SvO2 have been identified as robust independent negative prognostic factors [36, 37]. However the risk assessment in PAH should not be based only on invasive haemodynamic variables. A multidimensional approach has been proposed, which also considers clinical signs of right ventricular failure, symptom progression, WHO functional class, one measurement of exercise capacity (6-minute walking distance or cardiopulmonary exercise testing), brain natriuretic peptide levels and TTE findings [1]. Increasing evidence suggests that invasive pulmonary haemodynamics during exercise are more sensitive than resting haemodynamics in capturing treatment response in patients under targeted therapy for PAH [38, 39] and in predicting long-term outcome [40].
Conclusions
The confirmation of suspected PH requires an invasive measurement of mPAP of 25 mm Hg or above. A PAWP threshold value of 15 mm Hg is employed to differentiate between precapillary PH and postcapillary PH. Postcapillary PH is subclassified in accordance with diastolic pressure gradient. The proper use of right heart catheterisation is compulsory in order to obtain accurate and precise estimates of pulmonary vascular pressure and flow, and has direct consequences for diagnosis classification as well as for treatment decisions, and should be limited to expert PH centres. Stress testing of the pulmonary circulation during exercise is an interesting and attractive method for the evaluation of pulmonary vascular function.
References
1
Galiè
N
,
Humbert
M
,
Vachiery
JL
,
Gibbs
S
,
Lang
I
,
Torbicki
A
, et al.
2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119. https://doi.org/10.1093/eurheartj/ehv317
2Hatano S, Strasser T. Primary Pulmonary Hypertension, Report on a WHO Meeting. October 15-17, 1973, Geneva: World Health Organization, 1975
3
Fishman
AP
. Clinical classification of pulmonary hypertension. Clin Chest Med. 2001;22(3):385–91, vii. https://doi.org/10.1016/S0272-5231(05)70278-1
4
Simonneau
G
,
Gatzoulis
MA
,
Adatia
I
,
Celermajer
D
,
Denton
C
,
Ghofrani
A
, et al.
Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25, Suppl):D34–41. https://doi.org/10.1016/j.jacc.2013.10.029
5
Vachiéry
JL
,
Adir
Y
,
Barberà
JA
,
Champion
H
,
Coghlan
JG
,
Cottin
V
, et al.
Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25, Suppl):D100–8. https://doi.org/10.1016/j.jacc.2013.10.033
6
Rich
JD
,
Rich
S
. Clinical diagnosis of pulmonary hypertension. Circulation. 2014;130(20):1820–30. https://doi.org/10.1161/CIRCULATIONAHA.114.006971
7
Guazzi
M
,
Borlaug
BA
. Pulmonary hypertension due to left heart disease. Circulation. 2012;126(8):975–90. https://doi.org/10.1161/CIRCULATIONAHA.111.085761
8
Guazzi
M
. Pulmonary hypertension in heart failure preserved ejection fraction: prevalence, pathophysiology, and clinical perspectives. Circ Heart Fail. 2014;7(2):367–77. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000823
9
Ghio
S
,
Gavazzi
A
,
Campana
C
,
Inserra
C
,
Klersy
C
,
Sebastiani
R
, et al.
Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37(1):183–8. https://doi.org/10.1016/S0735-1097(00)01102-5
10
Seeger
W
,
Adir
Y
,
Barberà
JA
,
Champion
H
,
Coghlan
JG
,
Cottin
V
, et al.
Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25, Suppl):D109–16. https://doi.org/10.1016/j.jacc.2013.10.036
11
Oswald-Mammosser
M
,
Weitzenblum
E
,
Quoix
E
,
Moser
G
,
Chaouat
A
,
Charpentier
C
, et al.
Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest. 1995;107(5):1193–8. https://doi.org/10.1378/chest.107.5.1193
12
Shorr
AF
,
Wainright
JL
,
Cors
CS
,
Lettieri
CJ
,
Nathan
SD
. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J. 2007;30(4):715–21. https://doi.org/10.1183/09031936.00107206
13
Cottin
V
,
Le Pavec
J
,
Prévot
G
,
Mal
H
,
Humbert
M
,
Simonneau
G
, et al.; GERM“O”P. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J. 2010;35(1):105–11. https://doi.org/10.1183/09031936.00038709
14
Lang
IM
,
Madani
M
. Update on chronic thromboembolic pulmonary hypertension. Circulation. 2014;130(6):508–18. https://doi.org/10.1161/CIRCULATIONAHA.114.009309
15
McGoon
MD
,
Benza
RL
,
Escribano-Subias
P
,
Jiang
X
,
Miller
DP
,
Peacock
AJ
, et al.
Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62(25, Suppl):D51–9. https://doi.org/10.1016/j.jacc.2013.10.023
16
Hoeper
MM
,
Bogaard
HJ
,
Condliffe
R
,
Frantz
R
,
Khanna
D
,
Kurzyna
M
, et al.
Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25, Suppl):D42–50. https://doi.org/10.1016/j.jacc.2013.10.032
17
Kovacs
G
,
Berghold
A
,
Scheidl
S
,
Olschewski
H
. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34(4):888–94. https://doi.org/10.1183/09031936.00145608
18
Lau
EMT
,
Godinas
L
,
Sitbon
O
,
Montani
D
,
Savale
L
,
Jaïs
X
, et al.
Resting pulmonary artery pressure of 21-24 mmHg predicts abnormal exercise haemodynamics. Eur Respir J. 2016;47(5):1436–44. https://doi.org/10.1183/13993003.01684-2015
19
Hervé
P
,
Lau
EM
,
Sitbon
O
,
Savale
L
,
Montani
D
,
Godinas
L
, et al.
Criteria for diagnosis of exercise pulmonary hypertension. Eur Respir J. 2015;46(3):728–37. https://doi.org/10.1183/09031936.00021915
20
Brown
LM
,
Chen
H
,
Halpern
S
,
Taichman
D
,
McGoon
MD
,
Farber
HW
, et al.
Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL Registry. Chest. 2011;140(1):19–26. https://doi.org/10.1378/chest.10-1166
21
Rudski
LG
,
Lai
WW
,
Afilalo
J
,
Hua
L
,
Handschumacher
MD
,
Chandrasekaran
K
, et al.
Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713, quiz 786–8. https://doi.org/10.1016/j.echo.2010.05.010
22
Lang
RM
,
Badano
LP
,
Mor-Avi
V
,
Afilalo
J
,
Armstrong
A
,
Ernande
L
, et al.
Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–70. https://doi.org/10.1093/ehjci/jev014
23
D’Alto
M
,
Romeo
E
,
Argiento
P
,
D’Andrea
A
,
Vanderpool
R
,
Correra
A
, et al.
Accuracy and precision of echocardiography versus right heart catheterization for the assessment of pulmonary hypertension. Int J Cardiol. 2013;168(4):4058–62. https://doi.org/10.1016/j.ijcard.2013.07.005
24
Naeije
R
,
D’Alto
M
,
Forfia
PR
. Clinical and research measurement techniques of the pulmonary circulation: the present and the future. Prog Cardiovasc Dis. 2015;57(5):463–72. https://doi.org/10.1016/j.pcad.2014.12.003
25
Hoeper
MM
,
Lee
SH
,
Voswinckel
R
,
Palazzini
M
,
Jais
X
,
Marinelli
A
, et al.
Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006;48(12):2546–52. https://doi.org/10.1016/j.jacc.2006.07.061
26
Pagnamenta
A
,
Vanderpool
R
,
Brimioulle
S
,
Naeije
R
. Proximal pulmonary arterial obstruction decreases the time constant of the pulmonary circulation and increases right ventricular afterload. J Appl Physiol (1985). 2013;114(11):1586–92. https://doi.org/10.1152/japplphysiol.00033.2013
27
Gidwani
UK
,
Mohanty
B
,
Chatterjee
K
. The pulmonary artery catheter: a critical reappraisal. Cardiol Clin. 2013;31(4):545–65, viii. https://doi.org/10.1016/j.ccl.2013.07.008
28
Rosenkranz
S
,
Preston
IR
. Right heart catheterisation: best practice and pitfalls in pulmonary hypertension. Eur Respir Rev. 2015;24(138):642–52. https://doi.org/10.1183/16000617.0062-2015
29
Halpern
SD
,
Taichman
DB
. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest. 2009;136(1):37–43. https://doi.org/10.1378/chest.08-2784
30
LeVarge
BL
,
Pomerantsev
E
,
Channick
RN
. Reliance on end-expiratory wedge pressure leads to misclassification of pulmonary hypertension. Eur Respir J. 2014;44(2):425–34. https://doi.org/10.1183/09031936.00209313
31
Kovacs
G
,
Avian
A
,
Pienn
M
,
Naeije
R
,
Olschewski
H
. Reading pulmonary vascular pressure tracings. How to handle the problems of zero leveling and respiratory swings. Am J Respir Crit Care Med. 2014;190(3):252–7.
32
Naeije
R
,
Boerrigter
BG
. Pulmonary hypertension at exercise in COPD: does it matter?
Eur Respir J. 2013;41(5):1002–4. https://doi.org/10.1183/09031936.00173512
33
Prasad
A
,
Hastings
JL
,
Shibata
S
,
Popovic
ZB
,
Arbab-Zadeh
A
,
Bhella
PS
, et al.
Characterization of static and dynamic left ventricular diastolic function in patients with heart failure with a preserved ejection fraction. Circ Heart Fail. 2010;3(5):617–26. https://doi.org/10.1161/CIRCHEARTFAILURE.109.867044
34
Lador
F
,
Hervé
P
. A practical approach of pulmonary hypertension in the elderly. Semin Respir Crit Care Med. 2013;34(5):654–64. https://doi.org/10.1055/s-0033-1356549
35
Robbins
IM
,
Hemnes
AR
,
Pugh
ME
,
Brittain
EL
,
Zhao
DX
,
Piana
RN
, et al.
High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail. 2014;7(1):116–22. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000468
36
Humbert
M
,
Sitbon
O
,
Yaïci
A
,
Montani
D
,
O’Callaghan
DS
,
Jaïs
X
, et al.; French Pulmonary Arterial Hypertension Network. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36(3):549–55. https://doi.org/10.1183/09031936.00057010
37
Thenappan
T
,
Shah
SJ
,
Rich
S
,
Tian
L
,
Archer
SL
,
Gomberg-Maitland
M
. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J. 2010;35(5):1079–87. https://doi.org/10.1183/09031936.00072709
38
Castelain
V
,
Chemla
D
,
Humbert
M
,
Sitbon
O
,
Simonneau
G
,
Lecarpentier
Y
, et al.
Pulmonary artery pressure-flow relations after prostacyclin in primary pulmonary hypertension. Am J Respir Crit Care Med. 2002;165(3):338–40. https://doi.org/10.1164/ajrccm.165.3.2106033
39
Provencher
S
,
Hervé
P
,
Sitbon
O
,
Humbert
M
,
Simonneau
G
,
Chemla
D
. Changes in exercise haemodynamics during treatment in pulmonary arterial hypertension. Eur Respir J. 2008;32(2):393–8. https://doi.org/10.1183/09031936.00009008
40
Chaouat
A
,
Sitbon
O
,
Mercy
M
,
Ponçot-Mongars
R
,
Provencher
S
,
Guillaumot
A
, et al.
Prognostic value of exercise pulmonary haemodynamics in pulmonary arterial hypertension. Eur Respir J. 2014;44(3):704–13. https://doi.org/10.1183/09031936.00153613