Design of the Swiss Atrial Fibrillation Cohort Study (Swiss-AF): structural brain damage and cognitive decline among patients with atrial fibrillation
DOI: https://doi.org/10.4414/smw.2017.14467
David
Conenabc, Nicolas
Rodondide, Andreas
Müllerf, Juerg H.
Beerg, Angelo
Auricchioh, Peter
Ammanni, Daniel
Hayozj, Richard
Kobzak, Giorgio
Moschovitisl, Dipen
Shahm, Jürg
Schläpfern, Jan
Novako, Marcello
Di Valentinop, Paul
Erneq, Christian
Sticherlingab, Leo H.
Bonatir, Georg
Ehretm, Laurent
Rotens, Urs
Fischert, Andreas
Monschu, Christoph
Stippichv, Jens
Wuerfelw, Matthias
Schwenkglenksx, Michael
Kühneab, Stefan
Osswaldab, , for the Swiss-AF study investigators
aCardiology Division, Department of Medicine,
b
cPopulation Health Research Institute,
dInstitute of Primary Health Care (BIHAM),
eDepartment of General Internal Medicine, Inselspital, Bern University Hospital,
fDepartment of Cardiology,
gDepartment of Medicine, Cantonal Hospital of Baden and Molecular Cardiology,
hDepartment of Cardiology, Cardiocentro Ticino Lugano,
iDepartment of Cardiology, Kantonsspital St Gallen,
jDepartment of Internal Medicine, HFR-Hôpital Cantonal Fribourg,
kDepartment of Cardiology, Luzerner Kantonsspital,
lDepartment of Cardiology, Ospedale Regionale di Lugano,
mCardiology Service, Department of Medicine Specialities,
nDepartment of Cardiology,
oDepartment of Cardiology, Bürgerspital Solothurn,
pDepartment of Cardiology, Ospedale San Giovanni,
qDepartment of Biomedicine,
rNeurology Division and Stroke Centre, Department of Clinical Research,
sDepartment of Cardiology, Inselspital, Bern University Hospital,
tDepartment of Radiology, Inselspital, Bern University Hospital,
uMemory Clinic, Universitäre Altersmedizin, Felix Platter Hospital Basel,
vDivision of diagnostic and interventional neuroradiology,
w
xEpidemiology, Biostatistics and Prevention Institute,
Design of the Swiss Atrial Fibrillation Cohort Study (Swiss-AF): structural brain damage and cognitive decline among patients with atrial fibrillation
Summary
BACKGROUND
Several studies found that patients with atrial fibrillation (AF) have an increased risk of cognitive decline and dementia over time. However, the magnitude of the problem, associated risk factors and underlying mechanisms remain unclear.
METHODS
This article describes the design and methodology of the Swiss Atrial Fibrillation (Swiss-AF) Cohort Study, a prospective multicentre national cohort study of 2400 patients across 13 sites in Switzerland. Eligible patients must have documented AF. Main exclusion criteria are the inability to provide informed consent and the presence of exclusively short episodes of reversible forms of AF. All patients undergo extensive phenotyping and genotyping, including repeated assessment of cognitive functions, quality of life, disability, electrocardiography and cerebral magnetic resonance imaging. We also collect information on health related costs, and we assemble a large biobank. Key clinical outcomes in Swiss-AF are death, stroke, systemic embolism, bleeding, hospitalisation for heart failure and myocardial infarction. Information on outcomes and updates on other characteristics are being collected during yearly follow-up visits.
RESULTS
Up to 7 April 2017, we have enrolled 2133 patients into Swiss-AF. With the current recruitment rate of 15 to 20 patients per week, we expect that the target sample size of 2400 patients will be reached by summer 2017.
CONCLUSION
Swiss-AF is a large national prospective cohort of patients with AF in Switzerland. This study will provide important new information on structural and functional brain damage in patients with AF and on other AF related complications, using a large variety of genetic, phenotypic and health economic parameters.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia in the general population [1, 2]. As a result of the ongoing ageing of our population and other factors, the incidence of AF is expected to further increase in the near future [3]. Compared with individuals without AF, AF patients have an increased risk of serious complications, including death, stroke and congestive heart failure [4–7].
Cognitive dysfunction and dementia put an enormous socioeconomic burden on public health for ageing societies [8]. Recent evidence suggests an increased risk of dementia among AF patients [9–12]. For example, the relative risk (95% confidence interval) of cognitive impairment in a meta-analysis of 21 individual studies was 1.34 (1.13–1.58) in individuals with AF without a prior stroke, and 2.70 (1.82–4.00) in those with a prior stroke [11]. These data suggest that a prior stroke substantially increases the risk of cognitive impairment in AF patients. However, these studies also showed that this relationship is partly independent of clinically overt strokes, but the underlying mechanisms are currently unknown.
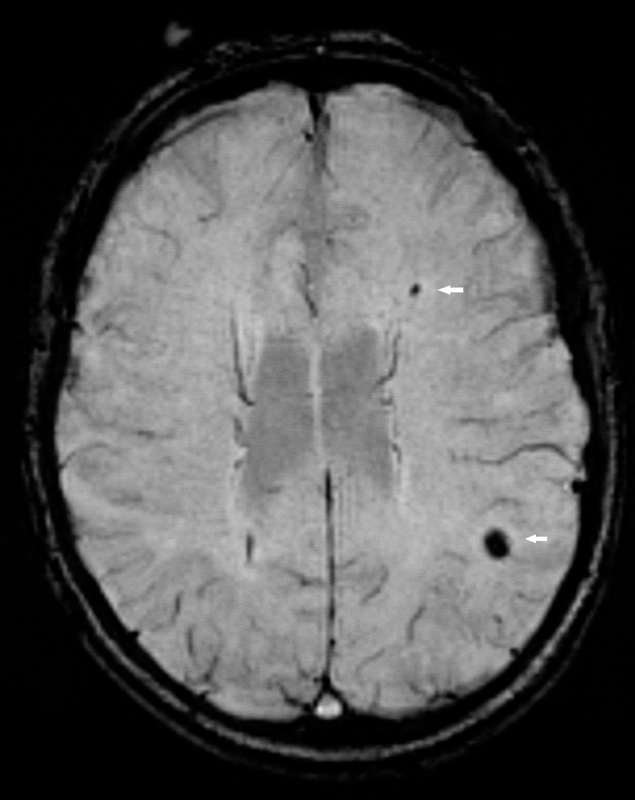
Cerebral magnetic resonance imaging (cMRI) provides detailed information on brain structure and function. The occurrence of silent cerebral infarctions (SCI), which have been found in up to 11% of individuals from the general population [13], could be mechanistically involved in the occurrence of cognitive impairment among AF patients (fig. 1). Cerebral microbleeds (fig. 2) are another structural correlate that potentially explain the link between AF and cognitive dysfunction [14], as some studies found a higher prevalence of microbleeds in patients with stroke or transient ischaemic attack (TIA) and a history of AF [15]. Other factors such as progression of the arrhythmia, concomitant diseases or medical treatments may also contribute to the cognitive decline in AF patients.
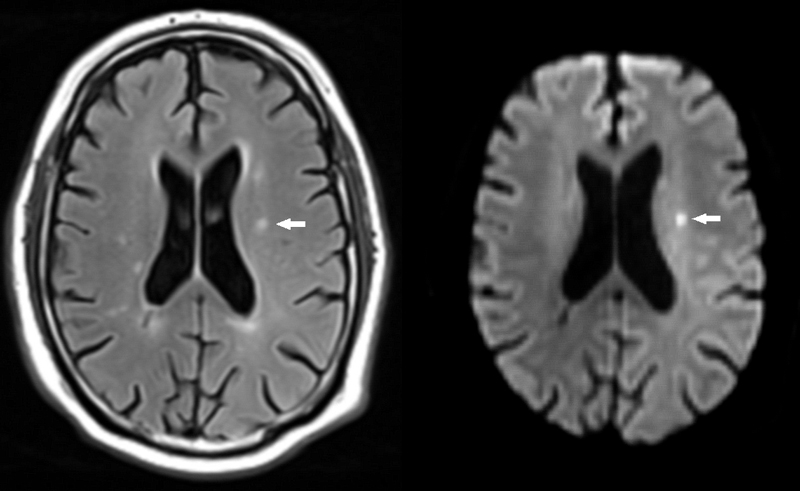
Figure 1 Small ischaemic stroke.
A small subacute ischaemic stroke is depicted on fluid attenuated inversion recovery (FLAIR, left, arrow). The lesion shows signs of restricted diffusion and is hyperintense on diffusion weighted imaging (DWI, right, arrow)
However, large systematic investigations on the relationship of cMRI parameters and cognitive decline in AF patients are currently unavailable. Therefore, one of the key aims of this large prospective cohort study is to assess the interrelationships between structural alterations on cMRI and cognitive decline among patients with established AF, in order to improve our knowledge on this crucial public health problems. This research platform will also allow us to assess differences in cognitive function and brain lesions on cMRI among patients with different AF types. In this publication, we describe the detailed methodology of the Swiss Atrial Fibrillation (Swiss-AF) cohort study.
Methods
Swiss-AF is a prospective multicentre observational cohort study across 13 centres in Switzerland. Eligible patients must be ≥65 years old and have either paroxysmal AF defined as: self-terminating AF lasting <7 days that does not require cardioversion [16] and that was documented at least twice within the last 60 months; persistent AF defined as AF sustained ≥7 days and/or requiring cardioversion, documented within the last 60 months by ECG or rhythm monitoring devices; or permanent AF (cardioversion has failed or not been attempted). The most current guidelines at the time of protocol preparation were used for Swiss-AF. In order to assess the socioeconomic aspects of AF in individuals who are potentially part of the active workforce, we aim to enrol 300 participants aged 45–65 years into the study. Main exclusion criteria for Swiss AF are the inability to provide informed consent, the presence of exclusively nonsustained episodes of secondary forms of AF (e.g., after cardiac surgery or severe sepsis) or any acute illness within the last 4 weeks. The latter group of patients is eligible for enrolment after stabilisation of their acute episode. Eligible candidates for enrolment in Swiss-AF were obtained by comprehensive screening of in- and outpatients in participating hospitals and by contacting general practitioners in the area. The study protocol has been approved by the local ethics committees, and informed written consent is obtained from each participant.
An overview of the study procedures is provided in table 1. The study case report forms have been previously validated and collect detailed information on personal characteristics, risk factors, co-morbidities, current medications, physical activity, nutrition and medication adherence. Detailed information on medical resource use is obtained. All data are collected in a standardized manner by trained study personnel. De-identified data are entered in a web-based database hosted by the Clinical Trial Unit in Basel.
Table 1 Overview of study procedures.
|
Procedure
|
Baseline
|
Year 1
|
Year 2
|
Year 3
|
Year 4+*
|
| Written informed consent |
X |
|
|
|
|
| Study case report form |
X |
X |
X |
X |
(X) |
| Clinical measures |
X |
X |
X |
X |
(X) |
| Resting electrocardiogram |
X |
X |
X |
X |
(X) |
| Outcomes |
|
X |
X |
X |
(X) |
| Cerebral MRI |
X |
|
X |
|
|
| Cognitive assessment |
X |
X |
X |
X |
(X) |
| Blood sampling |
X |
|
X |
|
|
| Disability, quality of life |
X |
X |
X |
X |
(X) |
| Health economics |
X |
X |
X |
X |
(X) |
Clinical measures
Body weight is obtained from each participant in light clothing and without shoes using a calibrated device. Body height is measured without shoes using a calibrated device. Heart rate is obtained by pulse palpation for at least 30 seconds. Blood pressure is measured in a supine position after at least 5 minutes of rest using a validated oscillometric device. Three consecutive measures are obtained, and the mean of the last two will be used for most analyses.
Resting electrocardiogram
Resting 16-lead ECG recordings of 5 minutes duration are obtained in all participants using the same ECG acquisition technology at all centres (CS-200 Excellence and CS-200 Touch, Schiller AG, Baar, Switzerland). The digital ECGs are stored with a sampling frequency of 1 kHz (signal bandwidth 0.04–387 Hz) and a resolution of 1 µV/bit. The sampling frequency is twice as high as that of standard ECG devices and will allow advanced signal processing analyses focusing on P-wave markers during sinus rhythm (morphology and variability) as well as fibrillatory markers during AF (AF amplitudes and rate, entropy, RR variability). All de-identified ECG recordings are being stored in a central ECG core laboratory at the Cardiocentro in Lugano.
Blood sampling
Non-fasting venous blood samples are collected from each patient at baseline and at 2 years of follow-up, following written standard operating procedures. Serum, plasma, whole blood and gene expression samples are obtained, processed immediately, centrifuged if necessary and aliquoted into cryotubes to be stored at ˗80°C in a centralised biobank at the University Hospital Basel. All enrolled participants are currently undergoing genotyping using the HumanCoreExome-12 BeadChip platform. More advanced genetic analyses will be planned in the future.
Cerebral magnetic resonance imaging
A standardised cMRI-protocol that does not need application of contrast agents has been implemented in all participating centres and is shown in table 2. The cMRI core protocol has been installed on an MR scanner at each participating site, at either 1.5 or 3 Tesla. Individual patients are investigated on the same scanner with identical sequence protocol at baseline and after 2 years whenever possible.
Table 2 Cerebral magnetic resonance imaging protocol.
|
MRI sequence
|
Orientation
|
Slice thickness
|
| Fluid Attenuated Inversion Recovery (FLAIR) |
axial |
3 mm |
| Diffusion weighted imaging (EPI) |
axial |
3 mm |
| Susceptibility weighted imaging (SWI, T2*) |
axial |
3 mm |
| High-resolution 3D-T1w MP-RAGE |
sagittal |
1 mm (isotropic) |
| Time of flight MR-angiography (ToF) |
axial |
0.8 mm |
Data are being checked and evaluated first on site and then transferred to the Medical Image Analysis Centre (MIAC AG) Basel, which serves as the central neuroimaging core lab. MIAC has set up an interne-tbased data transfer (webportal) in order to receive, check and accept cMRI studies from the participating sites, as well as to communicate with them. Participating sites had to perform a dummy test scan with all required sequences, including a repositioning scan for control purposes. Only after the test scan had been accepted by the MIAC, could sites proceed with the inclusion of patients. De-identified images are transferred electronically. MIAC provides quality assessment forms for all studies. All images are analysed by trained MRI technicians and validated by board-certified radiologists according to a prespecified analysis plan. Lesions are evaluated using the AMIRA software which calculates volumes and number of lesions. Atrophy measurements are calculated by means of SIENA X (normalised brain volume) and SIENA [17, 18].
Cognitive assessments
Study personnel have been trained centrally to perform a standardized neurocognitive assessment. A series of instruments has been selected based on literature review and discussion with experts, and are shown in table 3.
Table 3 Instruments for cognitive assessment.
|
Instrument name
|
Time requirement
|
| Montreal Cognitive Assessment [19] |
10 minutes |
| Trail Making Test (parts A and B) [20] |
8 minutes |
| Semantic Fluency, animals [21] |
2 minutes |
| Digit Symbol Substitution Test [22] |
3 minutes |
| Geriatric Depression Scale, 15 items [23] |
5 minutes |
The Montreal Cognitive Assessment evaluates visuospatial and executive functions, confrontation naming, memory, attention, language and abstraction [19]. Patients can obtain a maximum of 30 points and a score of <26 usually indicates cognitive impairment. The Trail Making Test, parts A and B increase the depth of assessment with respect to psychomotor speed and executive functions [20]. This series of instruments has been successfully used previously in large multicentre studies [24]. In addition, animal fluency for 1 minute [21] will assess semantic fluency – a combination of semantic memory and executive functions, which complements phonemic fluency within the Montreal Cognitive Assessment. The Digit Symbol Substitution Test (DSST) is a subtest of the Wechsler Adult Intelligence Scale [22]. The DSST assesses aspects of cognition such as information processing speed, visuomotor coordination and attention. Scores on the DSST range from zero (worst) to 90 (best). Depressive symptoms are assessed using the Geriatric Depression Scale, where a score of five or more points indicates depression [23].
Quality of life and disability
The European Quality of life-5 Dimensions questionnaire (EQ-5D) is a widely used, non-disease specific preference-based instrument to measure health related quality of life, and is used for this purpose in the current study [25]. In combination with published valuation algorithms, the EQ-5D provides a basis for the calculation of utilities, i.e., quality of life weights suitable to inform health economic evaluation studies. Disability is assessed using the Barthel Index [26].
AF-related medical resource use and costs
Health insurance claims data collected over the entire study period complement basic medical resource use information covered by the case report form for all patients, and will be used to evaluate medical resource use and medical costs. Collection of claims data is restricted to patients enrolled with four large health insurance companies and covers 40–50% of the study population. These companies were selected because of their interest in making data available for research, and because they are able to provide their data in a consistent format. One challenge that arises when Swiss claims data are used is to distinguish AF-related from non-AF-related use of medical resources. For inpatient episodes, Diagnosis-Related Group (DRG) codes will provide a basis for distinction. Given lack of International Classification of Diseases (ICD)-10 diagnosis codes on outpatient claims, outpatient services will be classified using the “Tessiner Code” (www.marcmuret.ch/tessinercode0.htm), and to-be-developed algorithms informed by drugs prescribed, services performed, and clinical events recorded in the Swiss-AF case report forms.
In addition, patient diaries are used to collect information on medical resource use outside the treating study centre in a subsample of 218 patients (13.7%), for whom health insurance claims data are also collected. The main purpose is to achieve a cross-validation of the claims data, to support the distinction of AF-related from non-AF-related resource use.
Finally, among patients aged <65 years at enrolment, a study-specific questionnaire is administered to assess days off work due to AF and potentially related conditions, changes in the actual and desired degree of employment, and early retirement, as a basis for estimating indirect costs of AF and hence the societal impact of the disease (appendix 2, available in a separate file for downloading).
Main clinical outcome measures
Main clinical outcome measures were prespecified and include stroke, systemic embolism, death, hospitalisation for heart failure, myocardial infarction and bleeding. If a clinical outcome measure is reported by the patient or detected in the medical records, detailed information is collected from the corresponding hospitals and/or treating physicians about this event. All main clinical outcome events are adjudicated by a clinical event committee that is unaware of other study-specific information. The following standardised definitions are used in Swiss-AF.
Stroke
Stroke is defined as an acute focal neurological deficit of vascular origin, with evidence of focal infarction confirmed by imaging (computed tomography or cMRI) or autopsy. Stroke is categorised as ischaemic, haemorrhagic or of unknown cause (based on computed tomography, cMRI, autopsy and other ancillary investigations). Ischaemic strokes are further classified according to the TOAST classification [27]. Fatal stroke is defined as death from any cause within 30 days after stroke.
Systemic embolism
Systemic embolism is defined as acute vascular occlusion of the extremities or any organ (kidneys, mesenteric arteries, spleen, retina or grafts) and must be documented by means of imaging, angiography, surgery or autopsy.
Mortality
The cause of death is classified as being of cardiac, stroke, any cardiovascular, bleeding, non-cardiovascular or unknown aetiology.
Major bleeding
Major bleeding is defined according to the International Society on Thrombosis and Haemostasis criteria, as clinically overt bleeding with a fatal outcome, a reduction in haemoglobin level of ≥20 g/l within 7 days, transfusion of at least two units of blood, or symptomatic bleeding in a critical area or organ (intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal) [28].
Hospitalisation for congestive heart failure
Hospitalization for congestive heart failure is defined as any unplanned hospitalisation for congestive heart failure that is associated with at least one overnight stay.
Myocardial infarction
Myocardial infarction is defined as rise and/or fall of cardiac troponin with at least one value above the 99th percentile of the upper reference limit in a clinical setting consistent with myocardial ischaemia, and with at least one of the following: symptoms of ischaemia, new significant ST-T changes or new left bundle-branch block on ECG, development of pathological Q waves in the ECG, imaging evidence of new loss of viable myocardium or new regional wall motion abnormality, identification of an intracoronary thrombus by angiography or autopsy [29].
Drug adherence
Drug adherence is a key factor in the care of patients with chronic diseases. Drug adherence in Swiss-AF is being assessed using a validated adherence questionnaire.
Sample size and power
Clinical stroke
With 280 clinical stroke events expected and assuming a β of 0.20 and an α of 0.05, the detectable stroke hazard ratio is 1.5 for a binary predictor with a prevalence of 0.4. For binary predictors with a prevalence of 0.1, the detectable stroke hazard ratio would be 1.9. Sensitivity analyses shown in figs 3a and 3b demonstrate that the detectable hazard ratio does not change substantially if the recruitment is slightly lower than projected.
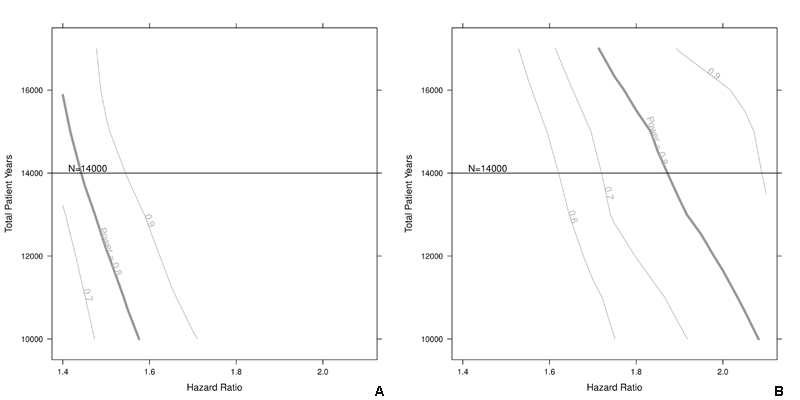
Figure 3a and 3b Sample size calculation for clinical stroke.
Sensitivity of the sample size with respect to stroke hazard ratio for a given prevalence of 0.4 (a) or 0.1 (b). The vertical lines on the intersection point of the horizontal line and the power curves indicate how to read the figures. The curve is smoothed for illustrative purposes.
Silent cerebral infarction on magnetic resonance imaging
For the cMRI endpoint of silent cerebral infarction (SCI), we assume successful completion of two cMRI studies in 2160 patients (90% of the total sample without heart device), and an SCI incidence of 10% [13], such that 216 SCI events are expected to occur. Assuming a β of 0.20 and an α of 0.05, the detectable SCI hazard ratio will be 1.5 for a binary predictor with a prevalence of 0.4. For a binary predictor with a prevalence of 0.1, the detectable SCI hazard ratio would be 2.0. Again, a slightly lower recruitment rate does only slightly change the detectable hazard ratios, as shown in figs 4a and 4b.
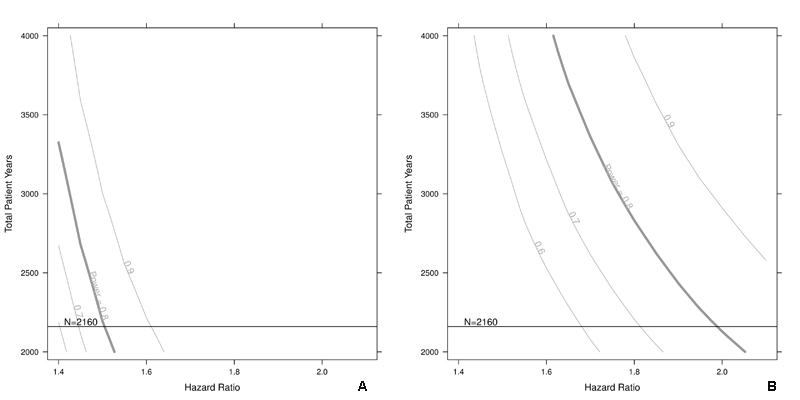
Figure 4a and 4b Sample size calculation for silent cerebral infarctions.
Sensitivity of the sample size with respect to the silent cerebral infarction hazard ratio for a given prevalence of 0.4 (a) or 0.1 (b). The vertical lines on the intersection point of the horizontal line and the power curves indicate how to read the figures. The curve is smoothed and for illustrative purposes.
Cognitive assessment
For the main cognitive assessment endpoint we will use the MoCA total score. Assuming an enrolment of 2400 patients with a complete series of questionnaires and a standard deviation of 4 for the MoCA total score [31], a 1-point difference on the MoCA score can be shown with >99% power.
Results
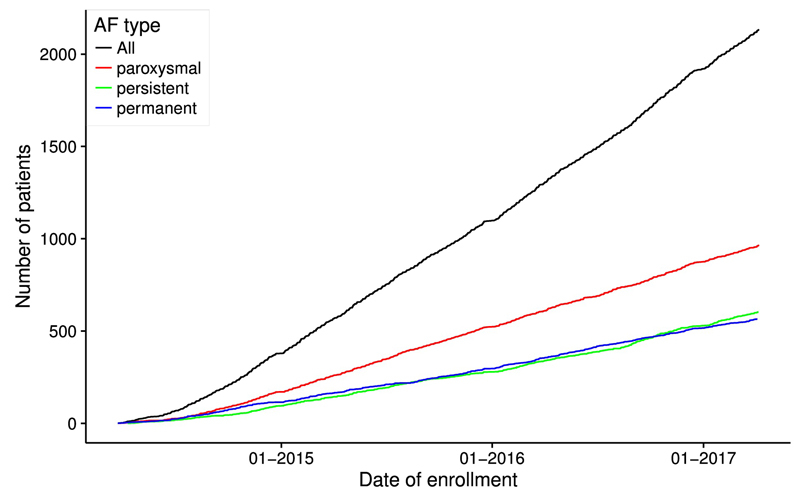
Up to 7 April 2017 the Swiss-AF study team has enrolled 2133 patients across 13 study centres into the cohort. Most centres are hospital based, although specific efforts are being made to also recruit AF patients from general practitioners. Recruitment rates are shown in figure 5. With the current recruitment rate of 15–20 patients per week, we expect that the recruitment target of 2400 patients will be reached by summer 2017.
Discussion
Swiss-AF is a large, multicentre prospective cohort study of mainly elderly patients with AF. This project will establish a large interdisciplinary platform to study the interrelationships of AF and AF progression with structural and functional brain damage over time.
We expect that several important findings will emerge from Swiss-AF. Although the increased risk of cognitive dysfunction and dementia among patients with AF is well described [9–12], its underlying mechanisms are not well understood. Not surprisingly, one meta-analysis suggested that the relative risk of incident dementia is higher among individuals with a history of clinical stroke [11], but also remained significantly increased among AF patients without a history of stroke. Our study will offer opportunities to investigate whether the residual risk is due to silent, clinically not recognisable strokes or other structural brain abnormalities such as white matter lesions, microinfarctions or microbleeds. It will be particularly important to assess the effect of microbleeds, given the clear indication for oral anticoagulation for most patients with AF. Further, we will also assess in detail the influence of known cardiovascular risk factors such as obesity and hypertension on structural and function brain parameters [32, 33].
In addition, this long term study will shed light on the impact of AF progression over time on quality of life, symptoms and various cardiovascular and non-cardiovascular outcomes among patients with non-permanent AF [5, 34]. Detailed cost and quality of life-related analyses will provide key information on the quality and cost of AF-related medical resource use in Switzerland, and provide a basis for the health economic evaluation of alternative treatment strategies. Disability, drug adherence and quality of life are important patient related metrics that will help to better inform patients with AF. Finally, the study also aims to improve understanding of underlying AF-related mechanisms and detailed genetic analyses will be a particular focus of this project.
In addition to its many strengths, there are some potential limitations of this project. First, it is important to emphasise that Swiss-AF does not have a control group of patients without AF. However, extensive resources of other cohort studies in Switzerland and internationally are available for comparison, and through discussions with respective study leaders we tried to harmonise study definitions and recording protocols as much as possible. Second, cMRI is without the application of intravenous contrast agents. This decision was made because the application of gadolinium contrast agents has been associated with rare but significant risks, would have significantly increased the study costs and the burden for the patient, and the main parameters to be studied in Swiss-AF (stroke, microbleeds, microangiopathy) do not need the application of MRI contrast.
In conclusion, Swiss-AF is a large national prospective cohort of patients with AF in Switzerland. This study will provide pertinent new information on structural and functional brain damage in patients with AF and on other AF related complications, using a large variety of genetic, phenotypic and health economic parameters.
Note after publication
(January 31, 2021) After original publication of this paper, changes were required and have been made in this version of the article. Because of a licensing issue, the test for drug adherence assessment (mentioned in the section "Drug adherence" and in reference [30] in the first version of the article) was removed from the Swiss-AF study.
Appendix 1: Swiss-AF study investigators (in addition to all co-authors)
University Hospital Basel/Basel University: Stefanie Aeschbacher, Steffen Blum, Fatih Metin, Laura Doerig, Matylda Zimny, Anna Maseli, Lucien Eggimann, Peter Egli, Andreas Fischer, Thomas Kofler, Rahel Kreuzmann, Philipp Krisai, Pascal Meyre, Christoph Muff, Christiane Pudenz, Andreas Reusser, Javier Ruperti Repilado, Aleksandra Schweizer, Anne Springer, Thomas Szucs, Stephanie van der Lely, Jan van der Stouwe, Gian Voellmin, Mirco von Rotz.
University Hospital Bern: Faculty: Drahomir Aujesky, Juerg Fuhrer, Simon Jung, Heinrich Mattle; Research fellows: Luise Adam, Carole Elodie Aubert, Martin Feller, Regula Kronenberg, Elisavet Moutzouri, Claudio Schneider, Daniel Segna; Study nurses: Evelyne Abgottspon, Tanja Flückiger, Cindy Groen, Svenja Heger, Sarah Pfaffen.
Stadtspital Triemli Zurich: Christopher Beynon, Roger Dillier, Franz Eberli, Simone Fontana, Christine Franzini, Isabel Juchli, Claudia Liedtke, Jacqueline Nadler, Thayze Obst, Xiaoye Schneider, Katrin Studerus, Dominik Weishaupt.
Kantonspital Baden: Silke Kuest, Karin Scheuch, Denise Hischier, Nicole Bonetti, Corina Bello, Henriette Isberg, Alexandra Grau, Jonas Villinger, Mary-Monica Papaux.
Cardiocentro Lugano: Adriana Anesini, Cristina Camporini, Giulio Conte, Tiziano Moccetti.
Kantonsspital St. Gallen: Roman Brenner, Manuela Forrer, Michaela Gemperle.
Hôpital Cantonal Fribourg: Mathieu Firmann, Sandrine Foucras.
Luzerner Kantonsspital: Benjamin Berte, Andrea Kaeppeli, Brigitta Mehmann, Markus Pfeiffer, Ian Russi, Kai Schmidt, Vanessa Weberndoerfer, Mabelle Young, Melanie Zbinden
EOC Lugano: Lorenzo Baldecchi, Jane Frangi, Tatiana Terrot.
University Hospital Geneva: Hervé Gallet, Elise Guillermet, Francois Lazeyras, Karl-Olof Lovblad, Patrick Perret, Cheryl Teres.
University Hospital Lausanne: Nathalie Lauriers, Marie Mean Pascual, Sandrine Salzmann.
Bürgerspital Solothurn: Nisha Arenja, Andrea Grêt, Sandra Vitelli.
EOC Bellinzona: Jane Frangi, Augusto Gallino.
St. Anna Spital Luzern: Renate Schoenenberger-Berzins.
University of Zurich: Fabienne Witassek.
Medical Image Analysis Center AG Basel: Ernst-Wilhelm Radue, Tim Sinnecker.
Clinical Trial Unit Basel: Pascal Benkert, Thomas Fabbro, Patrick Simon
Schiller AG Baar: Ramun Schmid.
Appendix 2: Employment questionnaire for patients aged <65 years
The questionnaire is available as a separate file at: https://smw.ch/en/article/doi/smw.2017.14467/
This article has been corrected - see note after publication at the end of the paper.
Swiss-AF study investigators are listed in appendix 1
References
1
Go
AS
,
Hylek
EM
,
Phillips
KA
,
Chang
Y
,
Henault
LE
,
Selby
JV
, et al.
Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–5. doi:.https://doi.org/10.1001/jama.285.18.2370
2
Krijthe
BP
,
Kunst
A
,
Benjamin
EJ
,
Lip
GY
,
Franco
OH
,
Hofman
A
, et al.
Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746–51. doi:.https://doi.org/10.1093/eurheartj/eht280
3
Miyasaka
Y
,
Barnes
ME
,
Gersh
BJ
,
Cha
SS
,
Bailey
KR
,
Abhayaratna
WP
, et al.
Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–25. doi:.https://doi.org/10.1161/CIRCULATIONAHA.105.595140
4
Benjamin
EJ
,
Wolf
PA
,
D’Agostino
RB
,
Silbershatz
H
,
Kannel
WB
,
Levy
D
. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–52. doi:.https://doi.org/10.1161/01.CIR.98.10.946
5
Conen
D
,
Chae
CU
,
Glynn
RJ
,
Tedrow
UB
,
Everett
BM
,
Buring
JE
, et al.
Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305(20):2080–7. doi:.https://doi.org/10.1001/jama.2011.659
6
Wang
TJ
,
Larson
MG
,
Levy
D
,
Vasan
RS
,
Leip
EP
,
Wolf
PA
, et al.
Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–5. doi:.https://doi.org/10.1161/01.CIR.0000072767.89944.6E
7
Wolf
PA
,
Abbott
RD
,
Kannel
WB
. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–8. doi:.https://doi.org/10.1161/01.STR.22.8.983
8
Kraft
E
,
Marti
M
,
Werner
S
,
Sommer
H
. Cost of dementia in Switzerland. Swiss Med Wkly. 2010;140:w13093.
9
Kwok
CS
,
Loke
YK
,
Hale
R
,
Potter
JF
,
Myint
PK
. Atrial fibrillation and incidence of dementia: a systematic review and meta-analysis. Neurology. 2011;76(10):914–22. doi:.https://doi.org/10.1212/WNL.0b013e31820f2e38
10
Santangeli
P
,
Di Biase
L
,
Bai
R
,
Mohanty
S
,
Pump
A
,
Cereceda Brantes
M
, et al.
Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm. 2012;9(11):1761–8. doi:.https://doi.org/10.1016/j.hrthm.2012.07.026
11
Kalantarian
S
,
Stern
TA
,
Mansour
M
,
Ruskin
JN
. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158(5 Pt 1):338–46. doi:.https://doi.org/10.7326/0003-4819-158-5-201303050-00007
12
Thacker
EL
,
McKnight
B
,
Psaty
BM
,
Longstreth
WT, Jr
,
Sitlani
CM
,
Dublin
S
, et al.
Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology. 2013;81(2):119–25. doi:.https://doi.org/10.1212/WNL.0b013e31829a33d1
13
Das
RR
,
Seshadri
S
,
Beiser
AS
,
Kelly-Hayes
M
,
Au
R
,
Himali
JJ
, et al.
Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke. 2008;39(11):2929–35. doi:.https://doi.org/10.1161/STROKEAHA.108.516575
14
van Norden
AG
,
van den Berg
HA
,
de Laat
KF
,
Gons
RA
,
van Dijk
EJ
,
de Leeuw
FE
. Frontal and temporal microbleeds are related to cognitive function: the Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) Study. Stroke. 2011;42(12):3382–6. doi:.https://doi.org/10.1161/STROKEAHA.111.629634
15
Ovbiagele
B
,
Saver
JL
,
Sanossian
N
,
Salamon
N
,
Villablanca
P
,
Alger
JR
, et al.
Predictors of cerebral microbleeds in acute ischemic stroke and TIA patients. Cerebrovasc Dis. 2006;22(5-6):378–83. doi:.https://doi.org/10.1159/000094855
16
Camm
AJ
,
Kirchhof
P
,
Lip
GY
,
Schotten
U
,
Savelieva
I
,
Ernst
S
, et al.; European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31(19):2369–429. doi:.https://doi.org/10.1093/eurheartj/ehq278
17
Smith
SM
,
Jenkinson
M
,
Woolrich
MW
,
Beckmann
CF
,
Behrens
TE
,
Johansen-Berg
H
, et al.
Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi:.https://doi.org/10.1016/j.neuroimage.2004.07.051
18
Smith
SM
,
Zhang
Y
,
Jenkinson
M
,
Chen
J
,
Matthews
PM
,
Federico
A
, et al.
Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17(1):479–89. doi:.https://doi.org/10.1006/nimg.2002.1040
19
Nasreddine
ZS
,
Phillips
NA
,
Bédirian
V
,
Charbonneau
S
,
Whitehead
V
,
Collin
I
, et al.
The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. doi:.https://doi.org/10.1111/j.1532-5415.2005.53221.x
20War Department Adjutant General’s Office. Army Individual Test Battery: Manual of Directions and Scoring. Washington D.C.: War Department Adjutant General's Office. 1944.
21
Morris
JC
,
Heyman
A
,
Mohs
RC
,
Hughes
JP
,
van Belle
G
,
Fillenbaum
G
, et al.
The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–65. doi:.https://doi.org/10.1212/WNL.39.9.1159
22Wechsler D. Wechsler Adult Intelligence Scale–Revised Manual New York: Psychological Corporation. 1981.
23Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS) Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press. 1986:165-73.
24
Lamy
A
,
Devereaux
PJ
,
Prabhakaran
D
,
Taggart
DP
,
Hu
S
,
Paolasso
E
, et al.; CORONARY Investigators. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med. 2013;368(13):1179–88. doi:.https://doi.org/10.1056/NEJMoa1301228
25
Räsänen
P
,
Roine
E
,
Sintonen
H
,
Semberg-Konttinen
V
,
Ryynänen
OP
,
Roine
R
. Use of quality-adjusted life years for the estimation of effectiveness of health care: A systematic literature review. Int J Technol Assess Health Care. 2006;22(2):235–41. doi:.https://doi.org/10.1017/S0266462306051051
26
Mahoney
FI
,
Barthel
DW
. Functional Evaluation: The Barthel Index. Md State Med J. 1965;14:61–5.
27
Amarenco
P
,
Bogousslavsky
J
,
Caplan
LR
,
Donnan
GA
,
Hennerici
MG
. Classification of stroke subtypes. Cerebrovasc Dis. 2009;27(5):493–501. doi:.https://doi.org/10.1159/000210432
28
Schulman
S
,
Kearon
C
; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–4. doi:.https://doi.org/10.1111/j.1538-7836.2005.01204.x
29
Thygesen
K
,
Alpert
JS
,
Jaffe
AS
,
Simoons
ML
,
Chaitman
BR
,
White
HD
, et al.; Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction; ESC Committee for Practice Guidelines (CPG). Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551–67. doi:.https://doi.org/10.1093/eurheartj/ehs184
31
Ball
J
,
Carrington
MJ
,
Stewart
S
; SAFETY investigators. Mild cognitive impairment in high-risk patients with chronic atrial fibrillation: a forgotten component of clinical management?
Heart. 2013;99(8):542–7. doi:.https://doi.org/10.1136/heartjnl-2012-303182
32
Conen
D
,
Tedrow
UB
,
Koplan
BA
,
Glynn
RJ
,
Buring
JE
,
Albert
CM
. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. 2009;119(16):2146–52. doi:.https://doi.org/10.1161/CIRCULATIONAHA.108.830042
33
Tedrow
UB
,
Conen
D
,
Ridker
PM
,
Cook
NR
,
Koplan
BA
,
Manson
JE
, et al.
The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J Am Coll Cardiol. 2010;55(21):2319–27. doi:.https://doi.org/10.1016/j.jacc.2010.02.029
34
Conen
D
,
Wong
JA
,
Sandhu
RK
,
Cook
NR
,
Lee
IM
,
Buring
JE
, et al.
Risk of Malignant Cancer Among Women With New-Onset Atrial Fibrillation. JAMA Cardiol. 2016;1(4):389–96. doi:.https://doi.org/10.1001/jamacardio.2016.0280