Iron substitution in the treatment of chronic heart failure
DOI: https://doi.org/10.4414/smw.2017.14453
Christine
Gstreina, Matthias R.
Meyerbc, Pablo
Anabitartea
aDivision of Cardiology, Kantonsspital Aarau,
bDivision of Cardiology, Stadtspital Triemli,
cInstitute of Primary Care,
Iron substitution in the treatment of chronic heart failure
w14453
Summary
Iron deficiency is a prevalent and clinically relevant comorbidity in up to 50% of patients with chronic heart failure (CHF). Iron deficiency in CHF patients is associated with impaired quality of life, reduced exercise capacity and increased mortality, irrespective of the presence of anaemia. It is diagnosed by determining circulating ferritin levels and transferrin saturation. Three randomised trials (CONFIRM-HF, FAIR-HF, and EFFECT-HF) of intravenous ferric carboxymaltose in the treatment of iron deficiency in CHF patients with reduced left ventricular ejection fraction demonstrated improvement of symptoms, functional capacity and quality of life. These beneficial effects were independent of the presence of anaemia. In addition, CONFIRM-HF and subsequent meta-analyses indicated that treatment of iron deficiency may reduce the rate of hospitalisations for worsening CHF. Oral iron is available at lower cost than intravenous iron, but its use did not translate into beneficial effects in CHF patients with iron deficiency. Large randomised trials designed to assess long-term clinical outcomes of iron treatment in CHF patients are pending. Current guidelines advise establishing evidence-based pharmacological and device therapy to improve symptoms and prognosis in patients with CHF. In addition, screening for iron deficiency is recommended. Intravenous ferric carboxymaltose should be considered for treating iron deficiency in ambulatory symptomatic patients with reduced left ventricular ejection fraction in order to alleviate heart failure symptoms, and to improve exercise capacity and quality of life.
Iron deficiency in chronic heart failure
Chronic heart failure (CHF) affects 1 to 2% of the adult population, with the prevalence among the elderly being greater than 10% [1]. CHF results in high morbidity and mortality despite optimal therapy [2–4]. Comorbidities have an important effect on symptoms, disease progression and prognosis in CHF [2, 5]. A critical comorbidity in CHF is iron deficiency, which affects up to 50% of patients [6]. Iron deficiency is associated with impaired quality of life, reduced exercise capacity and increased mortality, irrespective of the presence of anaemia [6–12]. It can be caused by insufficient dietary iron intake, poor intestinal iron absorption, iron loss due to gastrointestinal bleeding (often aggravated by concomitant pharmacotherapy such as nonsteroidal anti-inflammatory drugs and anticoagulants), uraemia, chronic inflammation and repeated venepunctures [13]. Iron deficiency is associated with reduced oxygen transport and utilisation in skeletal muscles. Furthermore, it is associated with activation of the sympathetic nervous system [14], and left ventricular hypertrophy and dilatation [15]. Indeed, reduced myocardial iron content was found in patients with CHF and severely reduced left ventricular ejection fraction (LVEF, 23±10%) [16], suggesting that myocardial iron depletion may play a role in the pathophysiology of the failing heart. The prevalence of iron deficiency is higher in advanced stages of CHF, and also in women and in patients with high levels of inflammatory markers (such as C-reactive protein, CRP) [17].
Iron metabolism
The normal body content of iron is about 35 to 45 mg per kilogram bodyweight in adult men, with approximately 75% bound to haemoglobin [18, 19]. Additional iron is present in myoglobin, several enzymes (e.g., cytochromes, peroxidase, catalase) and bound to its transport protein transferrin [20, 21]. Storage iron in adults is found mostly in ferritin or haemosiderin in hepatocytes and the monocyte-macrophage system in the liver, spleen, and bone marrow (fig. 1). Women have less storage iron, mainly due to regular menstrual iron loss. Iron is not only responsible for oxygen transport when bound to haemoglobin and myoglobin, but also for biochemical processes such as oxidative phosphorylation, cellular respiration and DNA synthesis [22]. Mitochondria are the key sites of cellular iron processing. They require iron in the catalysis of enzymatic reactions to generate energy stored in adenosine triphosphate, especially in cells with high energy demand such as cardiomyocytes and skeletal myocytes [20, 21, 23]. Normal loss of iron, at an estimated average of 1 to 2 mg per day, results from sloughed intestinal mucosal cells and bleeding. Iron is replaced by dietary intake. Around 10 to 15% of dietary iron is absorbed via specific transport systems expressed mainly in duodenal enterocytes [24–28]. Hepcidin, a peptide hormone synthesised in the liver, plays a major role in the regulation of iron metabolism by reducing iron release from enterocytes, hepatocytes and macrophages through inhibition of the specific transmembrane receptor (ferroprotein) [29–34]. Hepcidin synthesis is induced by proinflammatory cytokines such as interleukins 1 and 6, resulting in the reduced iron availability seen with chronic inflammation. Similarly, early stages of CHF are characterised by increased levels of circulating hepcidin. However, progressive CHF is associated with low circulating hepcidin levels [35], which are independently related to an unfavourable outcome. Thus, the pathophysiological mechanisms of iron deficiency in CHF appear to be different from those in chronic inflammatory diseases, but remain incompletely understood [35]. In that regard, a recent study has shown that messenger RNA (mRNA)-binding iron-regulatory proteins are responsible for intracellular iron availability by binding to specific noncoding sequences within an mRNA, known as iron-responsive elements. Activity of iron-regulatory proteins was significantly reduced in patients with advanced CHF and reduced iron content in the left ventricle [36].
Definition of iron deficiency in patients with chronic heart failure
Absolute iron deficiency in CHF results from depleted iron stores caused by impaired dietary iron intake, reduced gastrointestinal absorption or chronic gastrointestinal bleeding [37–40]. Serum ferritin is a good indicator for total iron stores [22]. Ferritin levels below 30 μg/l are highly suggestive of absolute iron deficiency [41]. Nevertheless, assessment based on ferritin alone may underestimate the prevalence of iron deficiency, since up to 73% of patients with advanced CHF have iron deficiency detected in bone marrow aspirates, despite predominantly normal ferritin levels (17–390 μg/l) [42]. This condition has been referred to as functional iron deficiency, which is characterised by an iron supply inadequate to maintain normal cellular functions [43–45] and frequently occurs in chronic inflammatory diseases associated with high ferritin levels regardless of body iron stores [13]. In contrast to other clinical settings, iron deficiency in CHF patients has therefore been diagnosed using higher cut-off levels for circulating ferritin and additional determination of transferrin saturation (TSAT) in clinical trials (table 1) [43]. Nonetheless, definition of iron deficiency in CHF patients remains challenging. Measurements of reticulocyte haemoglobin, hepcidin and soluble transferrin receptor (sTfR) have been proposed as more sensitive indicators to assess iron status and response to therapy. Particularly, the combination of ferritin and sTfR (transferrin receptor-ferritin index) may be the most accurate method to evaluate iron deficiency noninvasively, but has not yet been routinely used in clinical settings.
Table 1 Diagnosis of absolute and functional iron deficiency in chronic heart failure.
| Absolute iron deficiency |
Ferritin <100 μg/l |
| Functional iron deficiency |
Ferritin 100–300 μg/l and TSAT <20% |
Evidence for iron substitution in chronic heart failure
Intravenous iron
Iron substitution has been studied in CHF patients with functional and absolute iron deficiency. One of the first studies on the effects of intravenous iron substitution in CHF patients was published in 2006. In this open-label, noncomparative study, intravenous iron sucrose was administered for 5 to 17 days to anaemic CHF patients. This treatment improved symptoms and exercise capacity at 3-month follow-up [46]. Subsequently, these results were confirmed by a double-blind, randomised, placebo-controlled study including 40 patients with anaemia and symptomatic CHF, in which intravenous iron sucrose not only improved functional status, exercise capacity and quality of life, but also had beneficial effects on LVEF, plasma N-terminal pro-brain-type natriuretic peptide (NT-proBNP) and CRP levels, and hospitalisation rate [47]. Similarly, in the observer-blinded iron sucrose in heart failure (FERRIC-HF) trial, 35 patients were given intravenous iron sucrose for 16 weeks with improvement of iron status, symptoms and exercise capacity quantified by peak oxygen consumption [48]. Interestingly, an increase in peak oxygen consumption did not correlate with changes in haemoglobin levels but was associated with increased TSAT, compatible with improved circulating iron status. Salutary effects in FERRIC-HF were also observed in patients with functional iron deficiency.
The Ferinject Assessment in patients with IRon deficiency and chronic Heart Failure (FAIR-HF) study, the first randomised, double-blind, placebo-controlled multicentre trial, included 459 patients with reduced LVEF and New York Heart Association (NYHA) class II to III symptoms. Haemoglobin levels were between 9.5 and 13.5 g/dl, and iron deficiency was diagnosed when serum ferritin level was <100 μg/l, or when TSAT was <20% if serum ferritin was <300 μg/l [49]. Ferric carboxymaltose was initially given intravenously at a dose of 200 mg weekly until correction of the iron deficit. This was followed by a maintenance period, during which patients received 200 mg ferric carboxymaltose monthly, depending on the iron parameters. Treatment with intravenous ferric carboxymaltose improved symptoms, functional capacity and quality of life at week 24. Importantly, these beneficial effects were independent of the presence of anaemia. There was no significant difference in mortality or hospitalisations for cardiovascular events over 6 months. Publication of these results in 2009 led to a first weak recommendation concerning treatment of iron deficiency in the 2012 ESC heart failure guidelines [50].
Additional evidence relating to the management of iron deficiency in CHF patients with reduced ejection fraction was generated in 2014 by the ferric CarboxymaltOse evaluatioN on perFormance in patients with IRon deficiency in coMbination with chronic Heart Failure (CONFIRM-HF) trial, which had an extended observation period of 12 months [51, 52]. In CONFIRM-HF, 304 ambulatory symptomatic CHF patients with iron deficiency, an LVEF ≤45% and haemoglobin levels <15 g/dl were enrolled and randomly assigned to ferric carboxymaltose or placebo. A simplified dosing regimen based on the patients’ haemoglobin levels and bodyweight was used. During the correction phase within the first 6 weeks, iron was administered as bolus injections equivalent to 500 or 1000 mg iron up to a total of 500 to 2000 mg. In the maintenance phase, an additional dose of 500 mg of iron was given at weeks 12, 24 and 36 if ferritin and TSAT levels indicated persistent iron deficiency. As the objective primary endpoint, 6-minute walking test distance improved significantly in the iron treatment group, irrespective of the presence of anaemia. This effect was maintained until the end of the study (week 52). Importantly, the rate of hospitalisation due to worsening heart failure was reduced with iron treatment and optimised medical therapy. Additional secondary endpoints including changes in functional NYHA class, fatigue and health-related quality of life were also superior in the iron treatment group. The results of CONFIRM-HF added clinically relevant information to existing data, including selection of a more objective primary endpoint (the 6-minute walking test) compared with FAIR-HF. Furthermore, the positive effect of iron treatment was maintained for an extended period of time. The use of high single iron doses (up to 1000 mg as a bolus) was proven to be safe, offering a more clinically applicable method of therapy.
Most recently, results of the EFfect of Ferric carboxymaltose on Exercise Capacity in PaTients with iron deficiency and chronic Heart Failure (EFFECT-HF) trial have been presented. In this study, the effect of three intravenous injections of ferric carboxymaltose (day 0, week 6, and week 12) was compared with usual care in 172 patients with reduced LVEF, NYHA class II and III symptoms and iron deficiency [53, 54]. Up to week 24, treatment with ferric carboxymaltose stabilised exercise capacity measured as peak oxygen consumption, which declined in patients who received standard care irrespective of the presence of anaemia. These findings confirmed and extended the results of previous studies demonstrating salutary effects of improved body iron stores on functional capacity in CHF patients.
Two meta-analyses have evaluated the effect of intravenous iron substitution on clinical outcomes in CHF patients [55, 56]. Of note, although five clinical studies were included in each meta-analysis, most data were derived from the CONFIRM-HF and FAIR-HF trials. Both analyses suggested that intravenous iron therapy reduces the rate of hospitalisations for CHF, but there was no significant difference in all-cause mortality. However, these studies have limited power to detect a difference in mortality as a result of the short duration of follow-up. Thus, larger trials with a longer duration of follow-up are needed to determine the impact of iron therapy on clinical outcomes and mortality. Additional trials such as FAIR-HFpEF, which evaluates the effect of intravenous ferric carboxymaltose in patients with CHF and preserved left ventricular ejection fraction, are currently underway [57]. Furthermore, the FAIR-HF2 trial is designed to investigate the effect of ferric carboxymaltose on clinical endpoints in CHF patients with reduced ejection fraction [58].
Side effects of intravenous iron treatment
Regarding the treatment of iron deficiency in CHF patients, most experience relates to the use of ferric carboxymaltose and iron sucrose, which are generally well tolerated. In the two major trials (FAIR-HF and CONFIRM-HF), no severe allergic reactions after intravenous ferric carboxymaltose administration were noted [49, 51], and only few injections of ferric carboxymaltose were required for correction of the iron status (e.g., >75% of patients received no more than two injections in CONFIRM-HF). Because of serious side effects, including life-threatening anaphylactic reactions, high molecular-weight iron dextran has been largely taken from the market. However, allergic reactions have been reported with all intravenous preparations, indicating that patients should be observed during and for 30 minutes after intravenous iron application. The patient must be informed about possible nonallergic reactions, including self-limiting urticaria, palpitations, dizziness, and neck and back spasms, which occur in <1% of individuals [59]. Although less frequent than with oral iron, gastrointestinal side effects of intravenous iron treatment have also been reported [51]. In addition, correct placement of the intravenous line is crucial to avoid paravenous injections leading to longstanding skin coloration (tattooing) at the injection site.
Intravenous iron complexes are colloids consisting of iron-carbohydrate nanoparticles [60]. In animal studies, increased levels of markers for oxidative stress and inflammation have been observed with the use of certain iron preparations causing renal and hepatic damage, as well as hypotension [61–64]. Iron sucrose and ferric carboxymaltose were less prone to induce oxidative stress, but clinical data are limited. Two studies reported a transient (1–2 hours) increase of oxidative stress markers in patients on haemodialysis, accompanied by a short-time increase in lipid peroxidation after administration of iron sucrose [65, 66]. Interpretation of such data is complex because chronic haemodialysis itself induces oxidative stress, inflammation and endothelial dysfunction. Iron polymaltose and sucrose have been associated with temporary hypophosphataemia, and there are case reports of osteomalacia complicating prolonged use [67–70]. The exact mechanism is unknown, and phosphate levels have not been assessed in clinical trials on treatment of iron deficiency. Excess iron levels can also impair neutrophil and T-cell function, leading to an increased risk of microbial growth in haemodialysis patients [71]. Hence, intravenous iron therapy should be avoided in patients with active systemic infections. On the other hand, iron deficiency without adequate substitution may increase the risk of infections [71]. Taken together, the potential adverse effects mentioned above need to be taken into careful consideration when evaluating individual CHF patients for intravenous iron treatment.
Oral iron
Oral iron is usually given as ferrous (Fe[II]) salts, such as ferrous sulphate, which is widely used and available at a lower cost than intravenous iron [13]. However, the absorption of oral iron is reduced in patients with CHF because of oedema of the gastrointestinal mucosa and lower intestinal blood flow, and compliance is usually poor because of frequent gastrointestinal side effects [13]. The median required dose of iron to achieve iron repletion in iron deficiency patients with CHF is 1000 mg, and thus replenishment of iron stores with oral iron will require treatment for >6 months [13, 72]. In the placebo-controlled oral Iron Repletion effects on Oxygen UpTake in Heart Failure (IRONOUT) trial, the effects of treatment with oral iron polysaccharide (150 mg twice daily) on exercise capacity were evaluated in 225 CHF patients with NYHA class II to IV symptoms and LVEF ≤40% [54, 73]. There was no difference in the change in peak oxygen consumption after 16 weeks of iron treatment when compared with placebo. Furthermore, despite oral iron supplementation, body iron stores (ferritin levels and transferrin saturation) did not increase compared with placebo. This may be explained by reduced oral iron absorption in patients with CHF and poor compliance due to gastrointestinal side effects. Together, these results argue against the use of oral iron supplementation in CHF patients with reduced LVEF.
Oral versus intravenous iron
Only one small clinical study (IRON-HF) has compared oral with intravenous iron administration in anaemic patients with CHF [74]. At 3-month follow-up, there was an increase in peak oxygen consumption as determined by ergospirometry (mean increase, 3.5 ml/kg/min) in the intravenous, but not in the oral, iron treatment group. The difference was considered clinically relevant by the authors, although with only 18 patients analysed, the result was not statistically significant.
Since treatment of CHF usually implies the use of a complex medication regimen, a few injections as opposed to continuous oral iron treatment may be more attractive to patients, in order to reduce the number of pills taken on a regular basis. Furthermore, even though ferric carboxymaltose is more expensive than oral iron substitution, cost-effectiveness of intravenous ferric carboxymaltose therapy could be demonstrated in CHF patients [75], probably as a result of improved quality of life and fewer hospitalisations. Therefore, based on the current data, intravenous ferric carboxymaltose should be the preferred way of substitution for the treatment of iron deficiency in CHF patients.
Combination of erythropoiesis-stimulating agents and iron
In addition to iron treatment, the use of erythropoiesis-stimulating agents has been evaluated in CHF patients with anaemia. Initial studies in CHF patients with severe symptoms used a combination of erythropoietin and iron sucrose, which resulted in functional improvement, increases in LVEF and reduced hospitalisation rates in small study cohorts [76, 77]. These findings have been confirmed in a larger study including 179 CHF patients [78]. In addition, similar results were observed in randomised trials studying the use of erythropoietin in combination with oral iron and folate [79], erythropoietin and oral iron [80], or darbopoetin alpha and oral iron [81, 82] in CHF patients. The results of these studies demonstrated a functional improvement within 3 to 12 months of treatment. Subsequently, two large randomised clinical trials using darbopoetin alpha in CHF patients have been performed, the Study of Anaemia in Heart Failure Trial (STAMINA-HeFT) [83], and the Reduction of Events With Darbopoetin Alpha in Heart Failure (RED-HF) trial [84]. Both studies, which excluded patients with iron deficiency (defined as TSAT < 15%), demonstrated no effect on mortality, cardiovascular events and rate of hospitalisations. In RED-HF, an increased rate of thromboembolic events and risk of ischaemic stroke was found in patients receiving darbopoetin alpha. Possible explanations include a high target haemoglobin level (>13 g/dl) and the exclusion of patients with severe anaemia (haemoglobin <9 g/dl), who may benefit most of treatment with darbopoetin alpha. However, based on these results, the use of erythropoiesis-stimulating agents in CHF patients with anaemia is not recommended [2].
Clinical management of iron deficiency in chronic heart failure
The 2016 ESC guidelines for the management of CHF recommend evidence-based CHF therapy to improve symptoms and prognosis, including the appropriate use of angiotensin converting-enzyme (ACE) inhibitors / angiotensin receptor blockers, beta-blockers, mineralocorticoid receptor antagonists, sacubitril/valsartan, ivabradine, and device therapy (implantable cardioverter defibrillators [ICDs] and cardiac resynchronisation therapy [CRT]) in all CHF patients with reduced LVEF (class I or class IIa recommendations) [2]. These therapies have been evaluated in multiple large-scale clinical trials. Although only smaller trials on iron therapy are available, the guidelines also recommend a routine search for iron deficiency by determining ferritin levels and TSAT in all CHF patients (class I, level C recommendation). If iron deficiency is diagnosed, treatment with intravenous ferric carboxymaltose in symptomatic, ambulatory CHF patients with reduced LVEF should be considered (class IIa, level A recommendation) in order to alleviate heart failure symptoms, and improve exercise capacity and quality of life irrespective of whether anaemia is present or not. Despite these current guideline recommendations, clinical efforts to diagnose and treat iron deficiency in CHF patients remain low in Germany and Switzerland and should be strongly encouraged [85].
Even though iron therapy might be useful in early stages of CHF, the current guidelines do not indicate at which stage of disease progression the diagnosis and therapy of iron deficiency should be initiated. The majority of patients in the FAIR-HF, CONFIRM-HF and EFFECT-HF trials were already on CHF therapy with ACE inhibitors / angiotensin receptor blockers and beta-blockers, but remained in NYHA class II or III. Thus, the combination of an ACE inhibitor (or an angiotensin receptor blocker if ACE inhibitors are not tolerated) and a beta-blocker may be the minimal baseline therapy before iron deficiency should be considered in symptomatic CHF patients with reduced LVEF. Nevertheless, therapy with mineralocorticoid receptor blockers, sacubitril/valsartan, ivabradine, and devices (ICD and CRT) has to be considered at the same time.
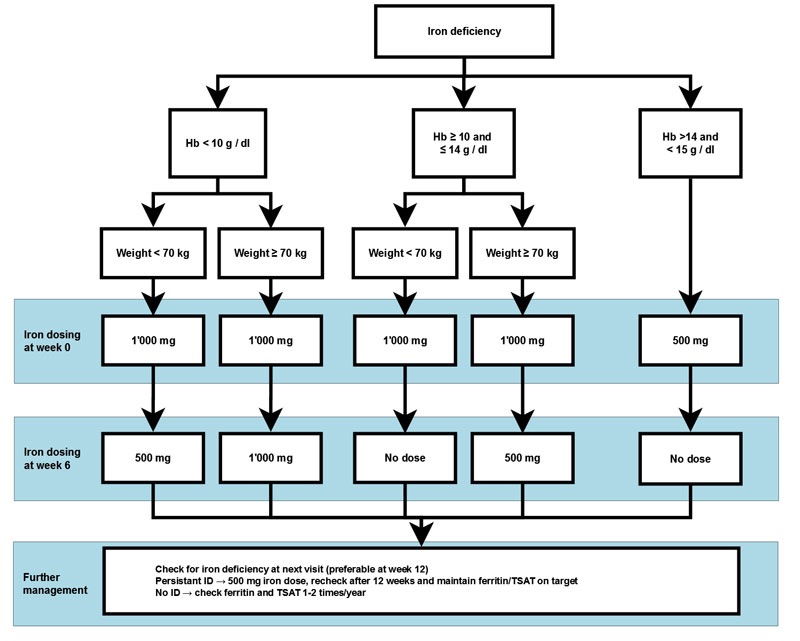
In our institution, dosing is based on the simplified regimen of the CONFIRM-HF study (fig. 2). Of note, there are no data on the safety of intravenous iron substitution in patients with CHF and haemoglobin levels >15 g/dl. However, a comprehensive work-up for potentially treatable or reversible causes (e.g., gastrointestinal bleeding) of iron deficiency is crucial if clinically suspected. Furthermore, effects of treatment that can be expected, as well as potential adverse events, need to be discussed individually with each patient.
Conclusions
Iron deficiency is highly prevalent in CHF patients and is associated – regardless of the presence or absence of anaemia – with impaired quality of life, reduced exercise capacity and increased mortality. Intravenous iron substitution improves symptoms, functional capacity and quality of life, and may reduce the risk of hospitalisation for worsening CHF in stable ambulatory patients with reduced LVEF. However, large randomised trials designed to assess long-term clinical outcomes and safety are pending. Current guidelines recommend a routine search for iron deficiency in all CHF patients. If iron deficiency is diagnosed, treatment using intravenous ferric carboxymaltose in symptomatic, ambulatory CHF patients with reduced LVEF should be considered in addition to evidence-based CHF therapy
References
1
Mosterd
A
,
Hoes
AW
. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–46. https://doi.org/10.1136/hrt.2003.025270
2
Ponikowski
P
,
Voors
AA
,
Anker
SD
,
Bueno
H
,
Cleland
JG
,
Coats
AJ
, et al.; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. https://doi.org/10.1093/eurheartj/ehw128
3
Tavazzi
L
,
Senni
M
,
Metra
M
,
Gorini
M
,
Cacciatore
G
,
Chinaglia
A
, et al.; IN-HF (Italian Network on Heart Failure) Outcome Investigators. Multicenter prospective observational study on acute and chronic heart failure: one-year follow-up results of IN-HF (Italian Network on Heart Failure) outcome registry. Circ Heart Fail. 2013;6(3):473–81. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000161
4
Hoekstra
T
,
Jaarsma
T
,
van Veldhuisen
DJ
,
Hillege
HL
,
Sanderman
R
,
Lesman-Leegte
I
. Quality of life and survival in patients with heart failure. Eur J Heart Fail. 2013;15(1):94–102. https://doi.org/10.1093/eurjhf/hfs148
5
van Deursen
VM
,
Urso
R
,
Laroche
C
,
Damman
K
,
Dahlström
U
,
Tavazzi
L
, et al.
Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014;16(1):103–11. https://doi.org/10.1002/ejhf.30
6
Klip
IT
,
Comin-Colet
J
,
Voors
AA
,
Ponikowski
P
,
Enjuanes
C
,
Banasiak
W
, et al.
Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165(4):575–582.e3. https://doi.org/10.1016/j.ahj.2013.01.017
7
Okonko
DO
,
Mandal
AKJ
,
Missouris
CG
,
Poole-Wilson
PA
. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol. 2011;58(12):1241–51. https://doi.org/10.1016/j.jacc.2011.04.040
8
Kasner
M
,
Aleksandrov
AS
,
Westermann
D
,
Lassner
D
,
Gross
M
,
von Haehling
S
, et al.
Functional iron deficiency and diastolic function in heart failure with preserved ejection fraction. Int J Cardiol. 2013;168(5):4652–7. https://doi.org/10.1016/j.ijcard.2013.07.185
9
Jankowska
EA
,
Kasztura
M
,
Sokolski
M
,
Bronisz
M
,
Nawrocka
S
,
Oleśkowska-Florek
W
, et al.
Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J. 2014;35(36):2468–76. https://doi.org/10.1093/eurheartj/ehu235
10
van Veldhuisen
DJ
,
Anker
SD
,
Ponikowski
P
,
Macdougall
IC
. Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches. Nat Rev Cardiol. 2011;8(9):485–93. https://doi.org/10.1038/nrcardio.2011.77
11
Jankowska
EA
,
Rozentryt
P
,
Witkowska
A
,
Nowak
J
,
Hartmann
O
,
Ponikowska
B
, et al.
Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail. 2011;17(11):899–906. https://doi.org/10.1016/j.cardfail.2011.08.003
12
Comín-Colet
J
,
Enjuanes
C
,
González
G
,
Torrens
A
,
Cladellas
M
,
Meroño
O
, et al.
Iron deficiency is a key determinant of health-related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail. 2013;15(10):1164–72. https://doi.org/10.1093/eurjhf/hft083
13
McDonagh
T
,
Macdougall
IC
. Iron therapy for the treatment of iron deficiency in chronic heart failure: intravenous or oral?
Eur J Heart Fail. 2015;17(3):248–62. https://doi.org/10.1002/ejhf.236
14
Turner
LR
,
Premo
DA
,
Gibbs
BJ
,
Hearthway
ML
,
Motsko
M
,
Sappington
A
, et al.
Adaptations to iron deficiency: cardiac functional responsiveness to norepinephrine, arterial remodeling, and the effect of beta-blockade on cardiac hypertrophy. BMC Physiol. 2002;2(1):1. https://doi.org/10.1186/1472-6793-2-1
15
Naito
Y
,
Tsujino
T
,
Matsumoto
M
,
Sakoda
T
,
Ohyanagi
M
,
Masuyama
T
. Adaptive response of the heart to long-term anemia induced by iron deficiency. Am J Physiol Heart Circ Physiol. 2009;296(3):H585–93. https://doi.org/10.1152/ajpheart.00463.2008
16
Maeder
MT
,
Khammy
O
,
dos Remedios
C
,
Kaye
DM
. Myocardial and systemic iron depletion in heart failure implications for anemia accompanying heart failure. J Am Coll Cardiol. 2011;58(5):474–80. https://doi.org/10.1016/j.jacc.2011.01.059
17
Jankowska
EA
,
Rozentryt
P
,
Witkowska
A
,
Nowak
J
,
Hartmann
O
,
Ponikowska
B
, et al.
Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31(15):1872–80. https://doi.org/10.1093/eurheartj/ehq158
18
Andrews
NC
. Disorders of iron metabolism. N Engl J Med. 1999;341(26):1986–95. https://doi.org/10.1056/NEJM199912233412607
19
Ganz
T
,
Nemeth
E
. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol. 2006;290(2):G199–203. https://doi.org/10.1152/ajpgi.00412.2005
20
Dunn
LL
,
Suryo Rahmanto
Y
,
Richardson
DR
. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007;17(2):93–100. https://doi.org/10.1016/j.tcb.2006.12.003
21
Cairo
G
,
Bernuzzi
F
,
Recalcati
S
. A precious metal: Iron, an essential nutrient for all cells. Genes Nutr. 2006;1(1):25–39. https://doi.org/10.1007/BF02829934
22
Brunner-La Rocca
HP
,
Crijns
HJ
. Iron i.v. in heart failure: ready for implementation?
Eur Heart J. 2015;36(11):645–7. https://doi.org/10.1093/eurheartj/ehu392
23
Beard
JL
. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131(2S-2, Suppl 2):568S–79S, discussion 580S.
24
Jankowska
EA
,
von Haehling
S
,
Anker
SD
,
Macdougall
IC
,
Ponikowski
P
. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J. 2013;34(11):816–29. https://doi.org/10.1093/eurheartj/ehs224
25
Muñoz
M
,
García-Erce
JA
,
Remacha
AF
. Disorders of iron metabolism. Part 1: molecular basis of iron homoeostasis. J Clin Pathol. 2011;64(4):281–6. https://doi.org/10.1136/jcp.2010.079046
26
Zhang
AS
,
Enns
CA
. Molecular mechanisms of normal iron homeostasis. Hematology (Am Soc Hematol Educ Program). 2009;1(1):207–14. https://doi.org/10.1182/asheducation-2009.1.207
27
Hentze
MW
,
Muckenthaler
MU
,
Galy
B
,
Camaschella
C
. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142(1):24–38. https://doi.org/10.1016/j.cell.2010.06.028
28
Anderson
GJ
,
Frazer
DM
,
McLaren
GD
. Iron absorption and metabolism. Curr Opin Gastroenterol. 2009;25(2):129–35. https://doi.org/10.1097/MOG.0b013e32831ef1f7
29
Babitt
JL
,
Lin
HY
. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis. 2010;55(4):726–41. https://doi.org/10.1053/j.ajkd.2009.12.030
30
Viatte
L
,
Vaulont
S
. Hepcidin, the iron watcher. Biochimie. 2009;91(10):1223–8. https://doi.org/10.1016/j.biochi.2009.06.012
31
Franchini
M
,
Montagnana
M
,
Lippi
G
. Hepcidin and iron metabolism: from laboratory to clinical implications. Clin Chim Acta. 2010;411(21-22):1565–9. https://doi.org/10.1016/j.cca.2010.07.003
32
Nemeth
E
,
Ganz
T
. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122(2-3):78–86. https://doi.org/10.1159/000243791
33
Handelman
GJ
,
Levin
NW
. Iron and anemia in human biology: a review of mechanisms. Heart Fail Rev. 2008;13(4):393–404. https://doi.org/10.1007/s10741-008-9086-x
34
Kemna
EH
,
Tjalsma
H
,
Willems
HL
,
Swinkels
DW
. Hepcidin: from discovery to differential diagnosis. Haematologica. 2008;93(1):90–7. https://doi.org/10.3324/haematol.11705
35
Jankowska
EA
,
Malyszko
J
,
Ardehali
H
,
Koc-Zorawska
E
,
Banasiak
W
,
von Haehling
S
, et al.
Iron status in patients with chronic heart failure. Eur Heart J. 2013;34(11):827–34. https://doi.org/10.1093/eurheartj/ehs377
36
Haddad
S
,
Wang
Y
,
Galy
B
,
Korf-Klingebiel
M
,
Hirsch
V
,
Baru
AM
, et al.
Iron-regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. Eur Heart J. 2016:ehw333; Epub ahead of print. https://doi.org/10.1093/eurheartj/ehw333
37
Sica
DA
. Pharmacotherapy in congestive heart failure: drug absorption in the management of congestive heart failure: loop diuretics. Congest Heart Fail. 2003;9(5):287–92. https://doi.org/10.1111/j.1527-5299.2003.02399.x
38
Ather
S
,
Chan
W
,
Bozkurt
B
,
Aguilar
D
,
Ramasubbu
K
,
Zachariah
AA
, et al.
Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59(11):998–1005. https://doi.org/10.1016/j.jacc.2011.11.040
39
Okonko
DO
,
Anker
SD
. Anemia in chronic heart failure: pathogenetic mechanisms. J Card Fail. 2004;10(1, Suppl):S5–9. https://doi.org/10.1016/j.cardfail.2004.01.004
40
Scharf
RE
. Management of bleeding in patients using antithrombotic agents: prediction, prevention, protection and problem-oriented intervention. Hamostaseologie. 2009;29(4):388–98.
41
Mast
AE
,
Blinder
MA
,
Gronowski
AM
,
Chumley
C
,
Scott
MG
. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44(1):45–51.
42
Nanas
JN
,
Matsouka
C
,
Karageorgopoulos
D
,
Leonti
A
,
Tsolakis
E
,
Drakos
SG
, et al.
Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol. 2006;48(12):2485–9. https://doi.org/10.1016/j.jacc.2006.08.034
43
Ebner
N
,
von Haehling
S
. Iron deficiency in heart failure: a practical guide. Nutrients. 2013;5(9):3730–9. https://doi.org/10.3390/nu5093730
44
Wish
JB
. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006;1(Suppl 1):S4–8. https://doi.org/10.2215/CJN.01490506
45
Goodnough
LT
,
Nemeth
E
,
Ganz
T
. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116(23):4754–61. https://doi.org/10.1182/blood-2010-05-286260
46
Bolger
AP
,
Bartlett
FR
,
Penston
HS
,
O’Leary
J
,
Pollock
N
,
Kaprielian
R
, et al.
Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol. 2006;48(6):1225–7. https://doi.org/10.1016/j.jacc.2006.07.015
47
Toblli
JE
,
Lombraña
A
,
Duarte
P
,
Di Gennaro
F
. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50(17):1657–65. https://doi.org/10.1016/j.jacc.2007.07.029
48
Okonko
DO
,
Grzeslo
A
,
Witkowski
T
,
Mandal
AK
,
Slater
RM
,
Roughton
M
, et al.
Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51(2):103–12. https://doi.org/10.1016/j.jacc.2007.09.036
49
Anker
SD
,
Comin Colet
J
,
Filippatos
G
,
Willenheimer
R
,
Dickstein
K
,
Drexler
H
, et al.; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361(25):2436–48. https://doi.org/10.1056/NEJMoa0908355
50
McMurray
JJ
,
Adamopoulos
S
,
Anker
SD
,
Auricchio
A
,
Böhm
M
,
Dickstein
K
, et al.; ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–847. https://doi.org/10.1093/eurheartj/ehs104
51
Ponikowski
P
,
van Veldhuisen
DJ
,
Comin-Colet
J
,
Ertl
G
,
Komajda
M
,
Mareev
V
, et al.; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36(11):657–68. https://doi.org/10.1093/eurheartj/ehu385
52
Ponikowski
P
,
van Veldhuisen
DJ
,
Comin-Colet
J
,
Ertl
G
,
Komajda
M
,
Mareev
V
, et al.
Rationale and design of the CONFIRM-HF study: a double-blind, randomized, placebo-controlled study to assess the effects of intravenous ferric carboxymaltose on functional capacity in patients with chronic heart failure and iron deficiency. ESC Heart Failure.
2014;1(1):52–8. https://doi.org/10.1002/ehf2.12006
53US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01394562. Accessed January 22, 2017.
54American College of Cardiology. Latest in cardiology. Studies Explore Use of Iron Supplements in Treating HF Patients. https://www.acc.org/latest-in-cardiology/articles/2016/11/10/15/58/wed-1145amet-ironout-effect-aha-2016. Accessed January 22, 2017.
55
Qian
C
,
Wei
B
,
Ding
J
,
Wu
H
,
Wang
Y
. The Efficacy and Safety of Iron Supplementation in Patients With Heart Failure and Iron Deficiency: A Systematic Review and Meta-analysis. Can J Cardiol. 2016;32(2):151–9. https://doi.org/10.1016/j.cjca.2015.06.009
56
Jankowska
EA
,
Tkaczyszyn
M
,
Suchocki
T
,
Drozd
M
,
von Haehling
S
,
Doehner
W
, et al.
Effects of intravenous iron therapy in iron-deficient patients with systolic heart failure: a meta-analysis of randomized controlled trials. Eur J Heart Fail. 2016;18(7):786–95. https://doi.org/10.1002/ejhf.473
57Herzzentrum Göttingen. Research. http://www.herzzentrum-goettingen.de/de/content/forschung/1980.html. Accessed January 22, 2017.
58Deutsches Zentrum für Herz-Kreislauf-Forschung e.v. https://intern.dzhk.de/Klinische-Studien2.html. Accessed January 22, 2017.
59
Miller
HJ
,
Hu
J
,
Valentine
JK
,
Gable
PS
. Efficacy and tolerability of intravenous ferric gluconate in the treatment of iron deficiency anemia in patients without kidney disease. Arch Intern Med. 2007;167(12):1327–8. https://doi.org/10.1001/archinte.167.12.1327
60
Moe
GW
,
Ezekowitz
JA
,
O’Meara
E
,
Lepage
S
,
Howlett
JG
,
Fremes
S
, et al.; Canadian Cardiovascular Society. The 2014 Canadian Cardiovascular Society Heart Failure Management Guidelines Focus Update: anemia, biomarkers, and recent therapeutic trial implications. Can J Cardiol. 2015;31(1):3–16. Erratum in: Can J Cardiol. 2016;32(3):394.https://doi.org/10.1016/j.cjca.2014.10.022
61
Geisser
P
,
Baer
M
,
Schaub
E
. Structure/histotoxicity relationship of parenteral iron preparations. Arzneimittelforschung. 1992;42(12):1439–52.
62
Toblli
JE
,
Cao
G
,
Oliveri
L
,
Angerosa
M
. Assessment of the extent of oxidative stress induced by intravenous ferumoxytol, ferric carboxymaltose, iron sucrose and iron dextran in a nonclinical model. Arzneimittelforschung. 2011;61(7):399–410. https://doi.org/10.1055/s-0031-1296218
63
Toblli
JE
,
Cao
G
,
Oliveri
L
,
Angerosa
M
. Evaluation of toxicity and oxidative stress induced by intravenous iron isomaltoside 1000 in a nonclinical model. Arzneimittelforschung. 2011;61(10):553–65.
64
Toblli
JE
,
Cao
G
,
Olivieri
L
,
Angerosa
M
. Comparison of the renal, cardiovascular and hepatic toxicity data of original intravenous iron compounds. Nephrol Dial Transplant. 2010;25(11):3631–40. https://doi.org/10.1093/ndt/gfq260
65
Garcia-Fernandez
N
,
Echeverria
A
,
Sanchez-Ibarrola
A
,
Páramo
JA
,
Coma-Canella
I
. Randomized clinical trial on acute effects of i.v. iron sucrose during haemodialysis. Nephrology (Carlton). 2010;15(2):178–83. https://doi.org/10.1111/j.1440-1797.2009.01174.x
66
Rangel
EB
,
Espósito
BP
,
Carneiro
FD
,
Mallet
AC
,
Matos
AC
,
Andreoli
MC
, et al.
Labile plasma iron generation after intravenous iron is time-dependent and transitory in patients undergoing chronic hemodialysis. Ther Apher Dial. 2010;14(2):186–92. https://doi.org/10.1111/j.1744-9987.2009.00786.x
67
Sato
K
,
Nohtomi
K
,
Demura
H
,
Takeuchi
A
,
Kobayashi
T
,
Kazama
J
, et al.
Saccharated ferric oxide (SFO)-induced osteomalacia: in vitro inhibition by SFO of bone formation and 1,25-dihydroxy-vitamin D production in renal tubules. Bone. 1997;21(1):57–64. https://doi.org/10.1016/S8756-3282(97)00084-7
68
Sato
K
,
Shiraki
M
. Saccharated ferric oxide-induced osteomalacia in Japan: iron-induced osteopathy due to nephropathy. Endocr J. 1998;45(4):431–9. https://doi.org/10.1507/endocrj.45.431
69
Schouten
BJ
,
Doogue
MP
,
Soule
SG
,
Hunt
PJ
. Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Ann Clin Biochem. 2009;46(2):167–9. https://doi.org/10.1258/acb.2008.008151
70
Okada
M
,
Imamura
K
,
Fuchigami
T
,
Omae
T
,
Iida
M
,
Nanishi
F
, et al.
[2 cases of nonspecific multiple ulcers of the small intestine associated with osteomalacia caused by long-term intravenous administration of saccharated ferric oxide]. Nippon Naika Gakkai Zasshi. 1982;71(11):1566–72.Article in Japanese. https://doi.org/10.2169/naika.71.1566
71
Ishida
JH
,
Johansen
KL
. Iron and infection in hemodialysis patients. Semin Dial. 2014;27(1):26–36. https://doi.org/10.1111/sdi.12168
72
Filippatos
G
,
Farmakis
D
,
Colet
JC
,
Dickstein
K
,
Lüscher
TF
,
Willenheimer
R
, et al.
Intravenous ferric carboxymaltose in iron-deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR-HF trial. Eur J Heart Fail. 2013;15(11):1267–76. https://doi.org/10.1093/eurjhf/hft099
73US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02188784. Accessed January 22, 2017.
74
Beck-da-Silva
L
,
Piardi
D
,
Soder
S
,
Rohde
LE
,
Pereira-Barretto
AC
,
de Albuquerque
D
, et al.
IRON-HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol. 2013;168(4):3439–42. https://doi.org/10.1016/j.ijcard.2013.04.181
75
Hofmarcher
T
,
Borg
S
. Cost-effectiveness analysis of ferric carboxymaltose in iron-deficient patients with chronic heart failure in Sweden. J Med Econ. 2015;18(7):492–501. https://doi.org/10.3111/13696998.2015.1029491
76
Silverberg
DS
,
Wexler
D
,
Blum
M
,
Keren
G
,
Sheps
D
,
Leibovitch
E
, et al.
The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35(7):1737–44. https://doi.org/10.1016/S0735-1097(00)00613-6
77
Silverberg
DS
,
Wexler
D
,
Sheps
D
,
Blum
M
,
Keren
G
,
Baruch
R
, et al.
The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001;37(7):1775–80. https://doi.org/10.1016/S0735-1097(01)01248-7
78
Silverberg
DS
,
Wexler
D
,
Blum
M
,
Tchebiner
JZ
,
Sheps
D
,
Keren
G
, et al.
The effect of correction of anaemia in diabetics and non-diabetics with severe resistant congestive heart failure and chronic renal failure by subcutaneous erythropoietin and intravenous iron. Nephrol Dial Transplant. 2003;18(1):141–6. https://doi.org/10.1093/ndt/18.1.141
79
Mancini
DM
,
Katz
SD
,
Lang
CC
,
LaManca
J
,
Hudaihed
A
,
Androne
AS
. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003;107(2):294–9. https://doi.org/10.1161/01.CIR.0000044914.42696.6A
80
Palazzuoli
A
,
Silverberg
D
,
Iovine
F
,
Capobianco
S
,
Giannotti
G
,
Calabrò
A
, et al.
Erythropoietin improves anemia exercise tolerance and renal function and reduces B-type natriuretic peptide and hospitalization in patients with heart failure and anemia. Am Heart J. 2006;152(6):1096.e9–15. https://doi.org/10.1016/j.ahj.2006.08.005
81
Ponikowski
P
,
Anker
SD
,
Szachniewicz
J
,
Okonko
D
,
Ledwidge
M
,
Zymlinski
R
, et al.
Effect of darbepoetin alfa on exercise tolerance in anemic patients with symptomatic chronic heart failure: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2007;49(7):753–62. https://doi.org/10.1016/j.jacc.2006.11.024
82
van Veldhuisen
DJ
,
Dickstein
K
,
Cohen-Solal
A
,
Lok
DJ
,
Wasserman
SM
,
Baker
N
, et al.
Randomized, double-blind, placebo-controlled study to evaluate the effect of two dosing regimens of darbepoetin alfa in patients with heart failure and anaemia. Eur Heart J. 2007;28(18):2208–16. https://doi.org/10.1093/eurheartj/ehm328
83
Ghali
JK
,
Anand
IS
,
Abraham
WT
,
Fonarow
GC
,
Greenberg
B
,
Krum
H
, et al.; Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation. 2008;117(4):526–35. https://doi.org/10.1161/CIRCULATIONAHA.107.698514
84
Swedberg
K
,
Young
JB
,
Anand
IS
,
Cheng
S
,
Desai
AS
,
Diaz
R
, et al.; RED-HF Committees; RED-HF Investigators. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368(13):1210–9. https://doi.org/10.1056/NEJMoa1214865
85
Wienbergen
H
,
Pfister
O
,
Hochadel
M
,
Michel
S
,
Bruder
O
,
Remppis
BA
, et al.; RAID-HF (Registry Analysis of Iron Deficiency–Heart Failure) REGISTRY Study Group. Usefulness of Iron Deficiency Correction in Management of Patients With Heart Failure [from the Registry Analysis of Iron Deficiency-Heart Failure (RAID-HF) Registry]. Am J Cardiol. 2016;118(12):1875–80. Published online September 15, 2016. https://doi.org/10.1016/j.amjcard.2016.08.081