The search for atrial fibrillation and its impact on public health
DOI: https://doi.org/10.4414/smw.2017.14447
Anna
Lam, Eleni
Goulouti, Laurent
Roten
Department of Cardiology, Inselspital,
The search for atrial fibrillation and its impact on public health
w14447
Summary
Atrial fibrillation may be clearly symptomatic and is easily amenable to state-of-the-art treatment, most importantly oral anticoagulation therapy for the prevention of thromboembolism. However, atrial fibrillation may also go unnoticed for long periods in many patients. This silent or subclinical atrial fibrillation is nevertheless associated with thromboembolic risk just like clinically evident atrial fibrillation. Early detection of atrial fibrillation in patients at increased thromboembolic risk and consequent oral anticoagulation therapy may have a significant impact on public health. This review focuses on screening recommendations for atrial fibrillation and on the impact of silent atrial fibrillation in various clinical scenarios.
Introduction
Atrial fibrillation is an endemic disease affecting 3% of the adult population with a prevalence of about 1% at the age of 60 years, increasing to >15% at the age of 85 years [1]. The number of patients affected by atrial fibrillation is expected to double by the year 2050 because of the aging population [2]. Atrial fibrillation is the leading cause of ischaemic stroke and is highly prevalent in stroke patients, with prevalence increasing steeply with age [3]. Atrial fibrillation is not only associated with an increased risk of stroke, but also increases the risk of heart failure and all-cause mortality [4]. Atrial fibrillation has also been linked to silent brain lesions, as well as cognitive impairment and dementia [5, 6]. Once atrial fibrillation is diagnosed, oral anticoagulation therapy is initiated in most patients to prevent thromboembolic events, thereby significantly lowering morbidity and mortality [7].
However, atrial fibrillation is often clinically silent and therefore not diagnosed or only discovered late, at the time of an event such as an ischaemic stroke [8, 9]. Silent atrial fibrillation is a challenging problem for public health: early identification and treatment of affected subjects, maybe even subjects at risk of atrial fibrillation, might further lower morbidity and mortality.
Screening for atrial fibrillation in the general population
Back in 2012, atrial fibrillation guidelines recommended only opportunistic screening for atrial fibrillation in patients aged ≥65 years by pulse palpation followed by an electrocardiogram in the case of an irregular pulse. With this simple community screening approach, atrial fibrillation can be identified in about 1.4% of subjects not previously known to have atrial fibrillation [10]. And according to the guidelines, oral anticoagulation therapy should be considered in all of these patients, as thromboembolic risk is elevated in patients aged ≥65 years [7]. Since 2012, recommendations for atrial fibrillation screening have evolved significantly as a result of various studies, which will be presented hereafter (table 1).
Table 1 Recommendations for screening for atrial fibrillation according to the 2016 European Society of Cardiology guidelines for the management of atrial fibrillation.
|
Recommendations
|
Class*
|
Level†
|
| Opportunistic screening for AF is recommended by pulse taking or ECG rhythm strip in patients >65 years of age. |
I |
B |
| In patients with TIA or ischaemic stroke, screening for AF is recommended by short-term ECG recording followed by continuous ECG monitoring for at least 72 hours. |
I |
B |
| It is recommended to interrogate pacemakers and ICDs on a regular basis for AHRE. Patients with AHRE should undergo further ECG monitoring to document AF before initiating AF therapy. |
I |
B |
| In stroke patients, additional ECG monitoring by long-term noninvasive ECG monitors or implanted loop recorders should be considered to document silent atrial fibrillation. |
IIa |
B |
| Systematic ECG screening may be considered to detect AF in patients aged >75 years, or those at high stroke risk. |
IIb |
B |
More advanced, stepwise community screening was tested in a Swedish study: screening of a population aged 75–76 years with at least one additional stroke risk factor by means of 12-lead ECG, and, if negative, by repeat hand-held ECG identified new atrial fibrillation in an additional 1% and 7.4% of subjects, respectively [11]. The same group thereafter expanded the stepwise screening programme to include 7173 subjects aged 75–76 years, irrespective of additional stroke risk factors. In this study, new atrial fibrillation was diagnosed by ECG in 0.5% of the cases, and by repeat hand-held ECG in 2.5% [12]. In the above-mentioned study, the probability of diagnosing new atrial fibrillation was higher in subjects with vascular disease, additional stroke risk factors or a high body mass index [12]. Another approach would therefore be to focus screening efforts on populations known to be at higher risk of atrial fibrillation. Additional parameters that may prove to be useful to triage patients for screening are echocardiographic, laboratory and ECG results. For instances, patients with a dilated left atrium, prolonged electromechanical delay, increased high-sensitive C-reactive protein (CRP), brain-type natriuretic peptide (BNP), high-sensitive cardiac troponin or a high number of premature atrial complexes are more often found to have atrial fibrillation during follow-up [13–19].
Nevertheless, all screening efforts for atrial fibrillation are futile unless patients with positive screening are treated as recommended by the guidelines. To put screening efforts into perspective, in the Swedish study mentioned above, 2.1% of the population had known but untreated atrial fibrillation (half of them thereafter started oral anticoagulation therapy), compared with 3% of newly diagnosed atrial fibrillation cases [12]. To improve public health, screening efforts are certainly needed. However, treatment of all patients known to have atrial fibrillation according to guidelines should be ensured in the first place.
Whether aggressive screening and treatment of atrial fibrillation will indeed improve outcome may also be answered by our Swedish colleagues. They plan to compare the outcome of screened and, if required, treated subjects with the remaining population through the Swedish patient registry [20]. In the meanwhile, systematic ECG screening of patients aged >75 years or at high risk of ischaemic stroke has received a Class IIb indication in current atrial fibrillation guidelines (table 1).
Atrial fibrillation in ischaemic stroke patients
As discussed above, the prevalence of atrial fibrillation in the general population rises steeply with age. This is also true among ischaemic stroke patients, with the difference that prevalence rates of atrial fibrillation are about four-fold higher in each age group compared with the general population [8]. Many patients with known atrial fibrillation presenting with ischaemic stroke have received either no anticoagulation or sub-therapeutic anticoagulation [8, 21]. These patients also have worse outcome from ischaemic stroke than patients with atrial fibrillation and therapeutic anticoagulation presenting with ischaemic stroke [21]. Again, primary emphasis from a public health standpoint should be to treat patients known to have atrial fibrillation according to guidelines.
Monitoring of ischaemic stroke patients for the presence of atrial fibrillation is nevertheless mandatory: an admission ECG will yield about 7.7% of new atrial fibrillation cases, in-hospital monitoring an additional 5.1% of cases and ambulatory Holter monitoring 10.7% [9]. Extending monitoring to 30 days with event-triggered recording following an ischaemic stroke has yielded 16.1% of new atrial fibrillation cases [22]. With the implantation of a loop recorder, the rate of new atrial fibrillation cases increases up to 30% after 3 years [23]. The overall atrial fibrillation detection rate after ischaemic stroke including admission ECG, in hospital and ambulatory monitoring may add up to well over 20% of cases [9]. As a result, screening for atrial fibrillation after an ischaemic stroke by short-term ECG recording followed by continuous ECG monitoring up to 72 hours is a class I indication in the current atrial fibrillation guidelines (table 1). Additionally, prolonged noninvasive monitoring or implantation of a loop recorder after an ischaemic stroke is a class IIa indication.
Importantly, screening of atrial fibrillation in ischaemic stroke patients should not be restricted to cryptogenic stroke patients or patients presenting with a most probable origin for embolic stroke according to the TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification, because competing causes of ischaemic stroke are frequently present [24].
Studies are currently in the recruiting phase that randomise ischaemic stroke patients without an atrial fibrillation diagnosis to oral anticoagulation therapy versus aspirin (Rivaroxaban Versus Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients With Recent Embolic Stroke of Undetermined Source [NAVIGATE ESUS;ClinicalTrials.gov identifier: NCT02313909] and Dabigatran Etexilate for Secondary Stroke Prevention in Patients With Embolic Stroke of Undetermined Source [RE-SPECT ESUS; NCT02239120]). If these studies prove superiority of oral anticoagulation therapy, screening for atrial fibrillation after an ischaemic stroke may be less important in the future.
Atrial fibrillation in silent stroke patients
The prevalence of silent brain lesions is age dependent and generally reported in the range of 10 to 20% in most studies, with an annual incidence of 2 to 4% [25, 26]. Silent brain lesions are not harmless: patients with silent brain lesions have an increased risk of dementia and a steeper decline in cognitive function than those without such lesions [27]. The presence of silent brain lesions also increases the risk of new silent brain lesions three-fold, and the risk of symptomatic ischaemic stroke two-fold [26, 28].
On the other hand, brain imaging studies comparing patients with and without atrial fibrillation but no history of ischaemic stroke have reported an important association of atrial fibrillation with silent brain lesions (odds ratio 2.62, 95% confidence interval 1.81–3.80]) [5]. The prevalence of silent brain lesions on computed tomography and magnetic resonance imaging among patients with atrial fibrillation in these studies has been reported to be 22 and 40%, respectively [5]. Whether such silent brain lesions in atrial fibrillation patients are cardioembolic in origin or whether their coexistence with atrial fibrillation is simply a reflection of shared risk factors remains to be proven.
Atrial fibrillation is probably the most important cause of cardioembolic, typically large and clinically relevant, ischaemic strokes. Nevertheless, cardioembolic strokes can certainly also occlude smaller vessels, depending on the diameter of the embolus. Although causes other than atrial fibrillation may be responsible for a bigger proportion of smaller ischaemic strokes than of larger strokes, atrial fibrillation may still be an important and underestimated player.
To date, no study has rigorously investigated the presence of atrial fibrillation in patients with silent brain lesions. As a consequence, prolonged rhythm monitoring to search for atrial fibrillation is nowadays routinely performed in ischaemic stroke patients, but not in patients with silent brain lesions.
Atrial high-rate episodes a cardiac implantable electronic devices
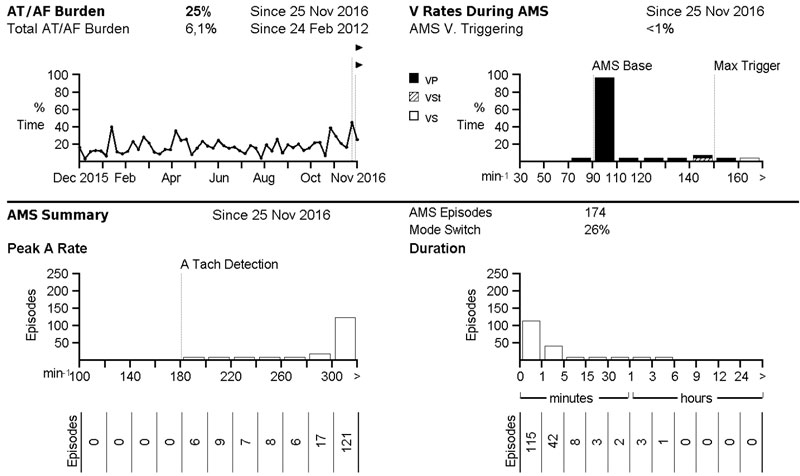
The number of patients with cardiac implantable electronic devices including an atrial lead is steadily growing. These devices permit continuous monitoring for atrial high-rate episodes exceeding a programmable or device-specific duration. If this function is enabled, a notification will appear upon device interrogation if an episode fulfilling programmed criteria occurred (fig. 1). Such notifications may also be transmitted daily by remote monitoring if this feature is enabled, allowing for continuous, almost real-time arrhythmia screening. Several studies have reported an association of atrial high-rate episodes with clinically diagnosed atrial fibrillation, as well as increased risk of ischaemic stroke and even mortality [29–31]. Interestingly, atrial high-rate episodes have also been reported to be an independent predictor for silent brain lesions [32].
Different cut-offs have been used, but generally a duration exceeding 5 to 6 minutes is accepted as a criterion for relevant atrial high-rate episodes (by definition rates have to be >180 bpm). Current guidelines have also been adapted accordingly (table 1) [7]. In the event of atrial high-rate episodes, the presence of atrial fibrillation should be verified on a corresponding electrogram from the device (fig. 2) or on a standard ECG or rhythm strip, and anticoagulation should be initiated according to guidelines. Verification of atrial fibrillation is important, because atrial high-rate episodes may also correspond to noise or far field electrograms, etc. On an individual basis, patients with atrial high-rate episodes at high stroke risk may also be anticoagulated without electrogram or ECG verification of atrial fibrillation. However, the benefit of anticoagulation in patients with atrial high-rate episodes is currently less well established than in patients with atrial fibrillation. Several studies currently underway are testing the benefit of oral anticoagulation therapy in patients with atrial high rate episodes (e.g. Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation [ARTESiA; NCT01938248] and Non vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes [NOAH]; NCT02618577). However, much has still to be learned about the causal relationship between atrial high-rate episodes and thromboembolism: many thromboembolic events in the studies mentioned above were not temporally associated with atrial high-rate episodes [33, 34]. Finally, it also has to be emphasised that patients with cardiac implantable electronic devices represent a very select patient population with advanced cardiac disease, and these findings cannot be generalised to other populations.
Atrial fibrillation as a causative or surrogate factor for thromboembolic risk
As discussed above, atrial high-rate episodes and atrial fibrillation are associated with ischaemic stroke, but the temporal relationship is intriguingly loose. This raises the question of whether atrial fibrillation is in fact the cause or rather a surrogate factor of increased thromboembolic risk. Interestingly, both the CHA2DS2-VASc score as well as the CHADS2 score can predict ischaemic stroke events in populations without known atrial fibrillation with similar accuracy to that in populations with atrial fibrillation, although the event rate is somewhat lower [35–37]. On the other hand, frequent premature atrial contractions are also an independent predictor of ischaemic stroke, even when subjects are censored in the event of an atrial fibrillation diagnosis [38]. Subjects with a CHA2DS2-VASc score ≥2 and frequent premature atrial contractions are reported to have an ischaemic stroke risk of 2.4%, very similar to historical populations with atrial fibrillation [38].
Subjects with higher CHA2DS2-VASc scores certainly have more advanced vascular disease, which may also increase thromboembolic risk independently of atrial fibrillation. However, except the rare cases of primary electrical disease, patients with atrial fibrillation have some kind of atrial cardiomyopathy leading to atrial fibrillation [39]. According to Virchow’s triad, haemodynamic changes, endothelial injury and hypercoagulability all contribute to thrombosis. The increased risk of thromboembolism attributed to atrial arrhythmias may partly be explained by associated haemodynamic changes. But the occurrence of premature atrial contractions, atrial high-rate episodes and eventually atrial fibrillation may also be interpreted as a surrogate of more advanced atrial fibrosis and inflammation, i.e., atrial cardiomyopathy leading to endothelial injury and hypercoagulability, and resultant increased thromboembolic risk.
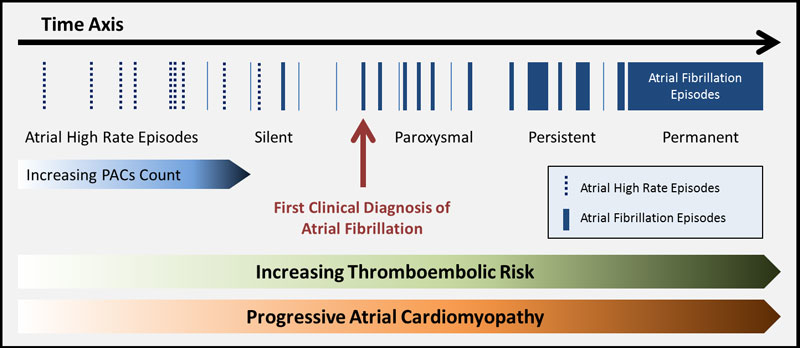
Most probably, atrial fibrillation is both a causative and a surrogate factor of increased thromboembolic risk (fig. 3). Maybe expanding the CHA2DS2-VASc score to also include premature atrial contractions or a diagnosis of atrial fibrillation would serve well as a more general approach for thromboembolic risk calculation in the general population. To our knowledge, no studies are currently planned to randomise patients with a high CHA2DS2-VASc score but without atrial fibrillation to anticoagulation therapy versus no therapy, except the studies of patients after ischaemic stroke discussed above. But with the advent of the novel oral anticoagulants, the time for such studies is probably not so far away anymore.
References
1
Heeringa
J
,
van der Kuip
DA
,
Hofman
A
,
Kors
JA
,
van Herpen
G
,
Stricker
BH
, et al.
Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949–53. https://doi.org/10.1093/eurheartj/ehi825
2
Naccarelli
GV
,
Varker
H
,
Lin
J
,
Schulman
KL
. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104(11):1534–9. https://doi.org/10.1016/j.amjcard.2009.07.022
3
Buchwald
F
,
Norrving
B
,
Petersson
J
. Atrial Fibrillation in Transient Ischemic Attack Versus Ischemic Stroke: A Swedish Stroke Register (Riksstroke) Study. Stroke. 2016;47(10):2456–61. https://doi.org/10.1161/STROKEAHA.116.013988
4
Stewart
S
,
Hart
CL
,
Hole
DJ
,
McMurray
JJ
. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113(5):359–64. https://doi.org/10.1016/S0002-9343(02)01236-6
5
Kalantarian
S
,
Ay
H
,
Gollub
RL
,
Lee
H
,
Retzepi
K
,
Mansour
M
, et al.
Association between atrial fibrillation and silent cerebral infarctions: a systematic review and meta-analysis. Ann Intern Med. 2014;161(9):650–8. https://doi.org/10.7326/M14-0538
6
Santangeli
P
,
Di Biase
L
,
Bai
R
,
Mohanty
S
,
Pump
A
,
Cereceda Brantes
M
, et al.
Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm. 2012;9(11):1761–8. https://doi.org/10.1016/j.hrthm.2012.07.026
7
Kirchhof
P
,
Benussi
S
,
Kotecha
D
,
Ahlsson
A
,
Atar
D
,
Casadei
B
, et al.
2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962. https://doi.org/10.1093/eurheartj/ehw210
8
McGrath
ER
,
Kapral
MK
,
Fang
J
,
Eikelboom
JW
,
Conghaile
A
,
Canavan
M
, et al., Investigators of the Ontario Stroke Registry. Association of atrial fibrillation with mortality and disability after ischemic stroke. Neurology. 2013;81(9):825–32. https://doi.org/10.1212/WNL.0b013e3182a2cc15
9
Sposato
LA
,
Cipriano
LE
,
Saposnik
G
,
Ruíz Vargas
E
,
Riccio
PM
,
Hachinski
V
. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14(4):377–87. https://doi.org/10.1016/S1474-4422(15)70027-X
10
Lowres
N
,
Neubeck
L
,
Redfern
J
,
Freedman
SB
. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Haemost. 2013;110(2):213–22. https://doi.org/10.1160/TH13-02-0165
11
Engdahl
J
,
Andersson
L
,
Mirskaya
M
,
Rosenqvist
M
. Stepwise screening of atrial fibrillation in a 75-year-old population: implications for stroke prevention. Circulation. 2013;127(8):930–7. https://doi.org/10.1161/CIRCULATIONAHA.112.126656
12
Svennberg
E
,
Engdahl
J
,
Al-Khalili
F
,
Friberg
L
,
Frykman
V
,
Rosenqvist
M
. Mass Screening for Untreated Atrial Fibrillation: The STROKESTOP Study. Circulation. 2015;131(25):2176–84. https://doi.org/10.1161/CIRCULATIONAHA.114.014343
13
Stahrenberg
R
,
Edelmann
F
,
Haase
B
,
Lahno
R
,
Seegers
J
,
Weber-Krüger
M
, et al.
Transthoracic echocardiography to rule out paroxysmal atrial fibrillation as a cause of stroke or transient ischemic attack. Stroke. 2011;42(12):3643–5. https://doi.org/10.1161/STROKEAHA.111.632836
14
De Vos
CB
,
Weijs
B
,
Crijns
HJ
,
Cheriex
EC
,
Palmans
A
,
Habets
J
, et al.
Atrial tissue Doppler imaging for prediction of new-onset atrial fibrillation. Heart. 2009;95(10):835–40. https://doi.org/10.1136/hrt.2008.148528
15
Peña
JM
,
MacFadyen
J
,
Glynn
RJ
,
Ridker
PM
. High-sensitivity C-reactive protein, statin therapy, and risks of atrial fibrillation: an exploratory analysis of the JUPITER trial. Eur Heart J. 2012;33(4):531–7. https://doi.org/10.1093/eurheartj/ehr460
16
Dewland
TA
,
Vittinghoff
E
,
Mandyam
MC
,
Heckbert
SR
,
Siscovick
DS
,
Stein
PK
, et al.
Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med. 2013;159(11):721–8. https://doi.org/10.7326/0003-4819-159-11-201312030-00004
17
Beaulieu-Boire
I
,
Leblanc
N
,
Berger
L
,
Boulanger
JM
. Troponin elevation predicts atrial fibrillation in patients with stroke or transient ischemic attack. J Stroke Cerebrovasc Dis. 2013;22(7):978–83. https://doi.org/10.1016/j.jstrokecerebrovasdis.2012.01.008
18
Shibazaki
K
,
Kimura
K
,
Fujii
S
,
Sakai
K
,
Iguchi
Y
. Brain natriuretic peptide levels as a predictor for new atrial fibrillation during hospitalization in patients with acute ischemic stroke. Am J Cardiol. 2012;109(9):1303–7. https://doi.org/10.1016/j.amjcard.2011.12.022
19
Wallmann
D
,
Tüller
D
,
Kucher
N
,
Fuhrer
J
,
Arnold
M
,
Delacretaz
E
. Frequent atrial premature contractions as a surrogate marker for paroxysmal atrial fibrillation in patients with acute ischaemic stroke. Heart. 2003;89(10):1247–8. https://doi.org/10.1136/heart.89.10.1247
20
Friberg
L
,
Engdahl
J
,
Frykman
V
,
Svennberg
E
,
Levin
LA
,
Rosenqvist
M
. Population screening of 75- and 76-year-old men and women for silent atrial fibrillation (STROKESTOP). Europace. 2013;15(1):135–40. https://doi.org/10.1093/europace/eus217
21
Hylek
EM
,
Go
AS
,
Chang
Y
,
Jensvold
NG
,
Henault
LE
,
Selby
JV
, et al.
Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349(11):1019–26. https://doi.org/10.1056/NEJMoa022913
22
Gladstone
DJ
,
Spring
M
,
Dorian
P
,
Panzov
V
,
Thorpe
KE
,
Hall
J
, et al.; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370(26):2467–77. https://doi.org/10.1056/NEJMoa1311376
23
Sanna
T
,
Diener
HC
,
Passman
RS
,
Di Lazzaro
V
,
Bernstein
RA
,
Morillo
CA
, et al.; CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–86. https://doi.org/10.1056/NEJMoa1313600
24
Grond
M
,
Jauss
M
,
Hamann
G
,
Stark
E
,
Veltkamp
R
,
Nabavi
D
, et al.
Improved detection of silent atrial fibrillation using 72-hour Holter ECG in patients with ischemic stroke: a prospective multicenter cohort study. Stroke. 2013;44(12):3357–64. https://doi.org/10.1161/STROKEAHA.113.001884
25
Fanning
JP
,
Wong
AA
,
Fraser
JF
. The epidemiology of silent brain infarction: a systematic review of population-based cohorts. BMC Med. 2014;12(1):119. https://doi.org/10.1186/s12916-014-0119-0
26
Vermeer
SE
,
Koudstaal
PJ
,
Oudkerk
M
,
Hofman
A
,
Breteler
MM
. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2002;33(1):21–5. https://doi.org/10.1161/hs0102.101629
27
Vermeer
SE
,
Prins
ND
,
den Heijer
T
,
Hofman
A
,
Koudstaal
PJ
,
Breteler
MM
. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–22. https://doi.org/10.1056/NEJMoa022066
28
Gupta
A
,
Giambrone
AE
,
Gialdini
G
,
Finn
C
,
Delgado
D
,
Gutierrez
J
, et al.
Silent Brain Infarction and Risk of Future Stroke: A Systematic Review and Meta-Analysis. Stroke. 2016;47(3):719–25.
29
Glotzer
TV
,
Hellkamp
AS
,
Zimmerman
J
,
Sweeney
MO
,
Yee
R
,
Marinchak
R
, et al.; MOST Investigators. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107(12):1614–9. https://doi.org/10.1161/01.CIR.0000057981.70380.45
30
Healey
JS
,
Connolly
SJ
,
Gold
MR
,
Israel
CW
,
Van Gelder
IC
,
Capucci
A
, et al.; ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120–9. https://doi.org/10.1056/NEJMoa1105575
31
Glotzer
TV
,
Daoud
EG
,
Wyse
DG
,
Singer
DE
,
Ezekowitz
MD
,
Hilker
C
, et al.
The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2(5):474–80. https://doi.org/10.1161/CIRCEP.109.849638
32
Benezet-Mazuecos
J
,
Rubio
JM
,
Cortés
M
,
Iglesias
JA
,
Calle
S
,
de la Vieja
JJ
, et al.
Silent ischaemic brain lesions related to atrial high rate episodes in patients with cardiac implantable electronic devices. Europace. 2015;17(3):364–9. https://doi.org/10.1093/europace/euu267
33
Brambatti
M
,
Connolly
SJ
,
Gold
MR
,
Morillo
CA
,
Capucci
A
,
Muto
C
, et al.; ASSERT Investigators. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129(21):2094–9. https://doi.org/10.1161/CIRCULATIONAHA.113.007825
34
Daoud
EG
,
Glotzer
TV
,
Wyse
DG
,
Ezekowitz
MD
,
Hilker
C
,
Koehler
J
, et al.; TRENDS Investigators. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm. 2011;8(9):1416–23. https://doi.org/10.1016/j.hrthm.2011.04.022
35
Mitchell
LB
,
Southern
DA
,
Galbraith
D
,
Ghali
WA
,
Knudtson
M
,
Wilton
SB
; APPROACH investigators. Prediction of stroke or TIA in patients without atrial fibrillation using CHADS2 and CHA2DS2-VASc scores. Heart. 2014;100(19):1524–30. https://doi.org/10.1136/heartjnl-2013-305303
36
Welles
CC
,
Whooley
MA
,
Na
B
,
Ganz
P
,
Schiller
NB
,
Turakhia
MP
. The CHADS2 score predicts ischemic stroke in the absence of atrial fibrillation among subjects with coronary heart disease: data from the Heart and Soul Study. Am Heart J. 2011;162(3):555–61. https://doi.org/10.1016/j.ahj.2011.05.023
37
Ntaios
G
,
Lip
GY
,
Makaritsis
K
,
Papavasileiou
V
,
Vemmou
A
,
Koroboki
E
, et al.
CHADS2, CHA2S2DS2-VASc, and long-term stroke outcome in patients without atrial fibrillation. Neurology. 2013;80(11):1009–17. https://doi.org/10.1212/WNL.0b013e318287281b
38
Larsen
BS
,
Kumarathurai
P
,
Falkenberg
J
,
Nielsen
OW
,
Sajadieh
A
. Excessive Atrial Ectopy and Short Atrial Runs Increase the Risk of Stroke Beyond Incident Atrial Fibrillation. J Am Coll Cardiol. 2015;66(3):232–41. https://doi.org/10.1016/j.jacc.2015.05.018
39
Goette
A
,
Kalman
JM
,
Aguinaga
L
,
Akar
J
,
Cabrera
JA
,
Chen
SA
, et al.
EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Heart Rhythm. 2017;14(1):e3–40.