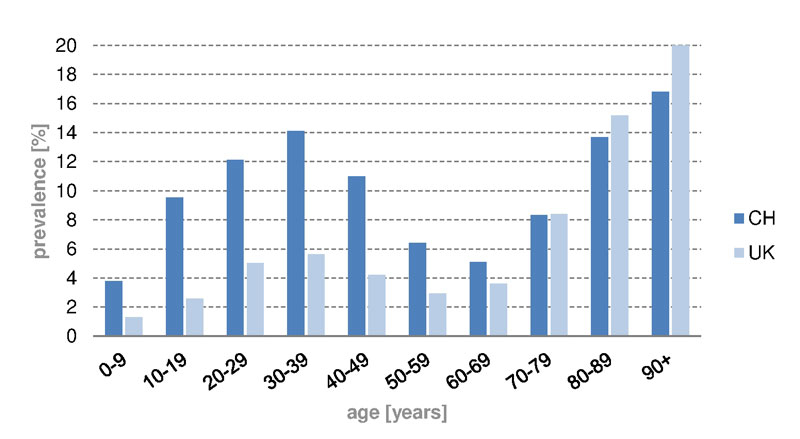
Figure 1 Prevalence of iron substitution in different age groups in Switzerland and the UK, 2012 to 2014.
DOI: https://doi.org/10.4414/smw.2017.14444
Iron deficiency is the most common nutritional disorder in the world and the only nutrient deficiency that is common in all industrialised nations [1, 2]. The prevalence of iron deficiency in Europe has been reported to be around 12 to 40% [3]. In the UK, 21% of female teenagers between 11 and 18 years, and 18% of women between the ages of 16 and 64 years are iron deficient [4]. The prevalence of iron deficiency in Switzerland is not well known. A recent study reported a prevalence of 50% (serum ferritin cut-off 22 μg/l) in a sample of healthy female hospital employees in Switzerland; with a lower cut-off of 15 μg/l, the prevalence was still 33% [3]. Another recent Swiss study assessed iron deficiency in hospitalised patients and found a prevalence of 4.9% (cut-off: ferritin <15 μg/l) [5], and screening in young Swiss soldiers yielded a prevalence of 16.8% (cut-off: ferritin <30 μg/l) [6]. In 2013, the iron deficiency prevalence in Portugal was reported to be 16.7% (cut-off: ferritin <15 μg/l) in the total population, and 19.8% in females [7]. Serum ferritin is the most powerful test to detect iron deficiency and the best indicator of a response to an intervention to treat it. The cut-off level of serum ferritin varies between 12 and 15 μg/l [1, 2].
Iron deficiency is thought to be the most common cause of anaemia [1, 8, 9]. The World Health Organization defines anaemia as a haemoglobin level below 13 g/dl in men over 15 years of age, below 12 g/dl in nonpregnant women over 15 years, and below 11 g/dl in pregnant women [1, 10].
It is estimated that in the UK 3% of men and 8% of women suffer from iron deficiency anaemia [11]. It is further estimated that in the developed world around 2 to 5% of adult men and postmenopausal women have iron deficiency anaemia [10]. In Switzerland, 15% of a sample of healthy female hospital employees [3] and 1.0% of young Swiss soldiers [6] were diagnosed with iron deficiency anaemia, and another Swiss study reported a high co-occurrence of iron deficiency and iron deficiency anaemia [5]. The prevalence of iron deficiency anaemia in Germany was reported to be 2.9% (cut-off: ferritin <15 μg/l) for the total population, and of 4.1% for females in 2011 [12]. In 2013, a high iron deficiency anaemia prevalence of 5.8% (cut-off: ferritin <15 μg/l) in the total population, and of 6.4% in females was found in Portugal [7]. A large nationwide population-based study published incidence rates of iron deficiency anaemia (cut-off: ferritin <15 μg/l) for Germany, Belgium, Spain and Italy [12]. The authors reported the highest incidence rate in 2011 in Germany and Spain, with rates of 12.42 and 14.14 per 1000 person-years, respectively. The incidence rates for females in Germany and Spain were reported to be 17.27 and 22.14 per 1000 person-years, respectively. Table 1 gives an overview of the iron deficiency / iron deficiency anaemia prevalence.
Table 1 Overview of iron deficiency/iron deficiency anaemia prevalence in different studies.
| Study | ID prevalence | IDA prevalence | Serum ferritin cut-off |
|---|---|---|---|
| Schuepbach et al. [3] | 50% of female hospital employees | 15% of female hospital employees | ID: < 22 μg/l IDA: < 22 μg/l |
| Heath et al. [4] |
|
|
|
| Hug et al. [5] | 4.9% of hospitalised patients | <15 μg/l | |
| Schleiffenbaum et al. [6] | 16.8% of young Swiss soldiers | 1% of young Swiss soldiers | ID: <30 μg/l IDA: <30 μg/l |
| Fonseca et al. [7] |
|
|
|
| British Society of Gastroenterology [10] | 2–5% of adult men and postmenopausal women | ||
| Ruston et al. [11] |
|
|
|
|
|
|
ID = Iron deficiency; IDA = Iron deficiency anaemia
However, not all anaemic people are iron deficient, and iron deficiency may occur without anaemia. Iron supplementation is the most common therapeutic option currently used in developing countries to treat iron deficiency, as well as to treat existing iron deficiency anaemia. It is importance to correct iron deficiency in populations at high risk of iron deficiency and anaemia, and to prevent anaemia by providing iron supplementation. However, supplementation for the prevention of iron deficiency anaemia and supplementation to correct it must be distinguished; the dose to prevent iron deficiency anaemia in women of childbearing age in populations with a high prevalence of anaemia (>40%) is 60 mg/day for 3 months; the dose of iron recommended to treat iron deficiency anaemia in adults is 120 mg/day for 3 months [1]. Women of childbearing age, not only pregnant women in whom the prevalence of iron deficiency anaemia is 14% [12], are the target group for supplementation for the prevention of iron deficiency. Therapeutic supplementation should be covered by the healthcare delivery system [1].
To date there are no data available on the correlation between iron deficiency anaemia and iron supplementation in Switzerland. According to the Swiss Compendium [13], treatment with parenteral iron drugs (e.g., Ferinject®) is accepted for use restricted to the treatment of iron deficiency when oral iron preparations are ineffective or cannot be used. The diagnosis must be based on laboratory tests. Using claims data from the largest health insurance group in Switzerland and general practitioner-based data from the UK, we quantified iron drug use in Switzerland and described patterns of use of oral and parenteral iron supplementation (multivitamins are excluded) between 2012 and 2014 in the Swiss market and in the UK health system.
We conducted a descriptive, bi-national study using claims data from the Swiss health insurance Helsana Group [14] and from the Clinical Practice Research Datalink (CPRD) [15] covering the time span between 2012 and 2014. The Helsana Group insures some 1.9 million inhabitants in Switzerland. All health insurance companies in Switzerland are private, there is no national health insurance system, but health insurance is mandatory for everybody living in Switzerland. In collaboration with Helsana, we have used claims data for descriptive analyses of health resource utilisation (HRU) in Switzerland [16, 17]. The recorded data include patient demographics such as age and gender, postal code of residence, and drug prescriptions (including dose, galenic formulation and pack size). Patients’ personal characteristics such as smoking habits or weight / body mass index (BMI), as well as laboratory data, symptoms, ambulatory diagnoses or medical resource use during hospitalisations are not recorded in the database. In 2012, Switzerland introduced a payment system based on diagnosis-related groups (DRGs) for acute-somatic inpatient care. The SwissDRG system is based on the German G-DRG version of 2008 [18, 19]; Swiss DRG codes are available from the database. The database is located at Helsana, and researchers had access to an anonymised dataset encompassing the relevant patients and the relevant parameters for this analysis.
The CPRD, formerly known as the General Practice Research Database (GPRD) [15], was established in 1987. It is a large UK-based database providing healthcare information on some 10 million patients in the UK; it is one of the largest databases of longitudinal medical records from primary care in the world. The CPRD has previously been described in detail [20, 21]. In the UK, general practitioners (GPs) are responsible for primary healthcare and for referrals to specialists. They have been trained to record information on demographics, medical diagnoses, laboratory values and drug prescriptions, as well as patient referrals and hospital admissions, using standard coding systems. The medical diagnoses are recorded as Read codes. The GPs generate prescriptions directly from the computer, and this information is automatically transcribed into the individual computerised patient records. They contain the drug name, instructions for use, route of administration, dose and number of tablets for each prescription. For complete information, individual patient records may be assessed. Additionally, the CPRD holds information regarding lifestyle variables such as BMI, alcohol consumption and smoking. We extracted the relevant patients and data parameters for this analysis from the fully anonymised database.
The study was approved by ISAC, the Independent Scientific Advisory Committee for Medicines and Healthcare products Regulatory Agency (MHRA) database research (protocol number 15_080R).
We identified all individuals who received at least one prescription for a drug coded in the anatomic therapeutic chemical classification system (ATC) as class B03A (oral and parenteral iron drugs; multivitamins are excluded) between 2012 and 2014. We then excluded all patients with a diagnosis of cancer (except nonmelanoma skin cancer) at any time in the record. In the CPRD, drugs are identified with gemscript codes. The date of the first iron prescription during the study period was considered the index date. We quantified the number of oral and parenteral iron prescriptions, as well as the number of ferritin and haemoglobin tests 12 and 24 months prior to the index date. We calculated the prevalence of iron drug use (oral/parenteral/both) for years 2012, 2013 and 2014, as well as the cumulative prevalence in 2012 to 2014. In addition, we assessed the time interval between the index prescription and the previous and subsequent iron drug prescriptions.
In a sensitivity analysis, we restricted the study population to “new” users of oral or parenteral iron supplementations; new users must not have received another oral or parenteral iron prescription for at least 180 days before the index date. We assessed whether these patients had any serum ferritin and/or haemoglobin tests prior to the “new” iron drug prescription.
An additional analysis distinguished between different parts of Switzerland according to language region (German, French or Italian). The list of cantons representing the three language regions is displayed as footnote in table 2.
We additionally stratified the numbers and proportions of drug users by age group (0–9, 10–19…90+) and gender (male/female). These analyses were prespecified and planned a priori. We focused on women of childbearing age because of the higher prevalence of iron deficiency anaemia in this subgroup [12]. We calculated frequency distributions using the software programs Stata MP 13.
Within the Helsana claims database, the 3-year prevalence for iron deficiency was 9.4%, and the 1-year prevalence was 4.5% in 2014, which reflects a slight increase of 0.3% since 2012. In contrast, in the UK the 3-year prevalence for iron deficiency was 4.4% and the 1-year prevalence 2.6% in 2014, reflecting an increase of 0.2% since 2012. Women (CH 16.0%, UK 6.9%) had a substantially higher prevalence of diagnosed iron deficiency than men (CH 2.6%, UK 1.7%). We further observed a marked difference in the use of parenteral iron supplementation. In Switzerland around 1.9% of all insured persons received an infusion at some point in time during the study period in the ambulatory setting, whereas in the UK only 0.002% received iron intravenously, as recorded by the GPs. Since 2012, use of parenteral iron supplementation has slightly (+0.2%) increased in Switzerland, but remained stable at a low level in the UK. On the other hand, oral iron supplementation has increased both in Switzerland and in the UK by about 0.2% during the same time period. In Switzerland, iron supplementation tended to be slightly higher in the French-speaking part of Switzerland (5.4% in 2014) than in the German- (5.0% in 2014) or Italian- (5.0% in 2014) speaking parts, whereas use of parenteral iron supplementation tended to be slightly higher in the German-speaking part (2.1% in 2014) compared with the Italian- (1.9% in 2014:) and the French- (1.6% 2014:) speaking parts (table 2).
Table 2 Prevalence of oral and parenteral iron supplementation in Switzerland (percentage of insured people).
| Region | 2012 | 2013 | 2014 | Change since 2012 |
|---|---|---|---|---|
| German part of Switzerland | ||||
| Oral iron supplementation | 3.2 | 3.3 | 3.4 | +0.2 |
| Parenteral iron supplementation | 2.0 | 2.1 | 2.1 | +0.1 |
| Total | 4.8 | 4.9 | 5.0 | +0.2 |
| French part of Switzerland | ||||
| Oral iron supplementation | 4.0 | 4.1 | 4.2 | +0.2 |
| Parenteral iron supplementation | 1.3 | 1.5 | 1.6 | +0.3 |
| Total | 5.0 | 5.2 | 5.4 | +0.4 |
| Italian part of Switzerland | ||||
| Oral iron supplementation | 3.1 | 3.3 | 3.4 | +0.4 |
| Parenteral iron supplementation | 1.5 | 1.8 | 1.9 | +0.4 |
| Total | 4.3 | 4.7 | 5.0 | +0.7 |
|
Overall Switzerland
Oral iron supplementation Parenteral iron supplementation |
3.2 1.8 |
3.3 1.9 |
3.4 1.9 |
+0.2 +0.2 |
| Total | 4.6 | 4.8 | 4.9 | +0.3 |
German part: Aargau, Appenzell Innerrhoden, Appenzell Ausserrhoden, Bern, Basel-Landschaft, Basel-Stadt, Glarus, Gaubünden, Luzern, Nidwalden, Obwalden, St Gallen, Schaffhausen, Solothurn, Schwyz, Thurgau, Uri, Zug, Zürich French part: Fribourg, Geneva, Jura, Neuchâtel, Vaud, Valais Italian part: Ticino
In Switzerland, the iron (III)-hydroxide polymaltose complex Maltofer® was the most frequent oral iron preparation used (11.7%). In contrast to other oral iron medications, it should be taken with food ingestion for better gastrointestinal tolerability [13]. Maltofer® was followed by iron (II) sulphate without folic acid (9.3%), and by a combination with folic acid (8.8%). Among parenteral iron preparations, iron carboxymaltose (Ferinject®) was the most commonly used preparation (86.3%).
In Switzerland (fig. 1), iron supplements were mostly given to patients between the ages of 20 and 49 years (prevalence 12.4%) and after the age of 80 years, with peak prevalence of 14.1% between 30 and 39 years, and of 16.8% above 90 years. In the UK, iron supplements were less likely given to younger people, but more often to the elderly as compared with Switzerland. Compared with the UK (fig. 2), oral iron supplements were prescribed more to women (CH 11.0%, UK 6.9%) and less often to men (CH 1.7%, UK 1.9%) in Switzerland. Furthermore, children in Switzerland were more frequently treated with oral iron than in the UK (children up to the age of 9 years: CH 3.8%, UK 1.3%).

Figure 1 Prevalence of iron substitution in different age groups in Switzerland and the UK, 2012 to 2014.

Figure 2 Prevalence of oral iron substitution in different age groups for both sexes in Switzerland (left) and the UK (right), 2012 to 2014.
In Switzerland, iron infusions were given less often than oral iron drugs (fig. 3). The age curves of parenteral and oral administration had similar shapes, with a peak at age above 90 years (oral 11.6%, parenteral 5.2%). Intravenous iron applications were more likely to be given to the 40 to 49 year age group (4.5%); oral supplementation, was more common in the age range of 30 to 39 years (10.1%). In women (fig.4) the peak prevalence of iron infusion was reached in the age group 40 to 49 years (8.8%), and in men the peak was in the group aged over 90 years (5.0%). In children up to the age of 9 years almost no iron infusions were administered in Switzerland, and none in the UK. Owing to lack of studies [13], parenteral iron supplementation is not recommended in children. In Switzerland and in the UK (supplementary figs S1 and S2 , appendix), oral iron supplementation was given mainly to women of childbearing age (12–49 years) between 30 and 39 years old (prevalence CH 20.4%, UK 10.7%). On the other hand, parenteral iron supplementation was given mainly to women aged 40 to 49 years. However, iron infusions in the UK were rare, even in women of childbearing age (prevalence CH 8.8%, UK 0.005%).
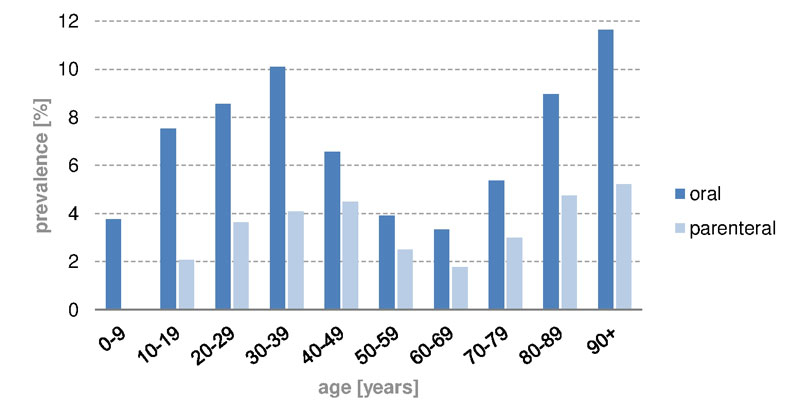
Figure 3 Prevalence of oral and parenteral iron substitution in different age groups in Switzerland, 2012 to 2014.

Figure 4 Prevalence of parenteral iron substitution in different age groups for both sexes in Switzerland, 2012 to 2014.
The sensitivity analysis of newly started oral and parenteral iron substitutions (table 3 ) showed that in both Switzerland and the UK, laboratory parameters were measured more often before starting a new parenteral therapy (CH 87.9%, UK 87.1%) than before a new oral therapy (CH 73.8%, UK 78.2%). Ferritin was in general measured more frequently in Switzerland (oral 67.2%, parenteral 86.6%) than in the UK (oral 43.3%, parenteral 65.5%), while haemoglobin tests before a new parenteral iron therapy were rare in Switzerland (oral 14.9%, parenteral 11.7%) and more frequent in the UK (oral 77.4%, parenteral 85.6%). Testing both ferritin and haemoglobin was more frequent in the UK (oral 42.6%, parenteral 64.0%) than in Switzerland (oral 8.3% parenteral 10.3%).
Table 3 Laboratory parameters (ferritin, haemoglobin) measured within 180 days before iron substitution (percentage of patients treated).
| Laboratory values | Switzerland (%) | United Kingdom (%) |
|---|---|---|
| Oral iron substitution | ||
| Haemoglobin or ferritin | 73.8 | 78.2 |
| Ferritin only | 67.2 | 43.3 |
| Haemoglobin only | 14.9 | 77.4 |
| Haemoglobin and ferritin in combination | 8.3 | 42.6 |
| Parenteral iron substitution | ||
| Haemoglobin or ferritin | 87.9 | 87.1 |
| Ferritin only | 86.6 | 65.5 |
| Haemoglobin only | 11.7 | 85.6 |
| Haemoglobin and ferritin in combination | 10.3 | 64.0 |
The practice of iron substitution is quite different in Switzerland and in the UK. In 2014, iron application overall was more frequent in Switzerland than in the UK. The prevalence of parenteral iron infusions was about 1000 times higher in Switzerland than in the UK in the ambulatory setting. We cannot rule out the possibility that we missed infusions administered in hospitals in the UK; however the same holds true for Switzerland. This observation of a substantially higher frequency intravenous application of iron in ambulatory care may be explained by the fact that GPs in the UK administer iron infusions rarely, and iron infusions administered in specialised clinics or hospitals are not comprehensively recorded in the CPRD. However, despite some possibly missing data in the CPRD, a substantial difference seems to exist in the frequency of iron infusions administered in Switzerland and in the UK in the ambulatory setting.
The overall prevalence of iron supplementation in Switzerland, 4.9% in our data, was consistent with the iron deficiency prevalence of 4.9% reported in a recent Swiss study [5], in which a cut-off of serum ferritin of <15 μg/l was used.
The frequency of laboratory measurement of ferritin and haemoglobin for patients who were newly started on iron supplementation was lower in Switzerland than in the UK. In theory, assessment of haemoglobin is required to justify iron infusions and to find the appropriate iron dose [13], but these results were not always available in Switzerland. Between 2006 and 2010, haemoglobin parameters were assessed more frequently than ferritin 90 days before iron treatment in Switzerland [22].
To our knowledge, large studies assessing the prevalence of iron deficiency in Switzerland are lacking [3, 5, 6]. However, in relation to the iron deficiency prevalence reported in the international literature [3, 4, 7, 11, 12], the number of patients treated with iron seems to be low. A recently published study from Australia investigated whether people with iron deficiency anaemia were more likely than “healthy” people to get iron supplementations [23]. They concluded that there was a mismatch between the number of people who got iron supplementation and the number of people in need. They found evidence that iron supplementation is dependent on socioeconomic status, but less associated with risk factors for iron deficiency anaemia, a pattern that was similar in other studies [24–26]. Thus, estimating the prevalence of iron deficiency or of iron deficiency anaemia in Switzerland by taking iron supplementation as a proxy may not be reliable.
Iron infusions in Switzerland are popular because administration is relatively quick and easy, the rate of adverse drug reactions, for carboxymaltose (Ferinject®), for example, is low and intravenous application eliminates concerns over poor adherence, which may be a challenge where oral iron supplementation is associated with adverse gastrointestinal effects.
The strengths of our study include the substantial number of individuals with iron supplementation from different regions of Switzerland, as well as from the UK, with several years of prior recorded drug history. Claims data tend to encompass drug use comprehensively; furthermore, medications had not only been prescribed, but actually dispensed by a pharmacy or physician. In the UK prescriptions are recorded by the GP, but it is not known whether the prescription was filled. In addition, it is intriguing to compare iron supplementation between two countries with different health systems – the Swiss health system with a mix of private and public characteristics versus the National Health Service in the UK.
Our study has several limitations. Because we used claims data, we identified only individuals who received prescriptions for the iron preparations of interest, but there is also use of over-the-counter oral iron supplements. The same is also true for the analysis of UK data. However, such use is likely to be minimal compared with longer-term use prescribed by doctors, which occurs when iron deficiency anaemia is diagnosed. Another limitation of the study is that although Swiss claims data do provide information on whether and/or when laboratory test was performed, they do not provide information on the actual laboratory value (e.g., ferritin, haemoglobin). Thus, we were not able to address the question of whether iron supplementation was justified in a given individual patient or not. Similar to the Swiss data, a limitation of the CPRD database is that we may have missed iron infusions administered in hospitals.
In conclusion, iron supplementation is more common in Switzerland than in the UK, particularly the application of parenteral iron infusions.
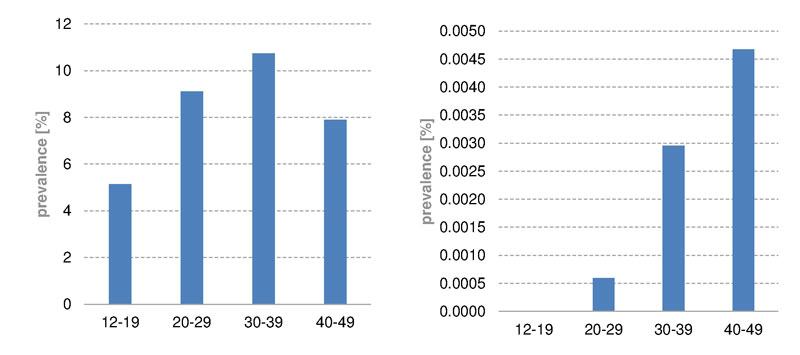
Figure S1 Prevalence of oral (left) and parenteral (right) iron substitution of women of childbearing age in the UK, 2012 to 2014.
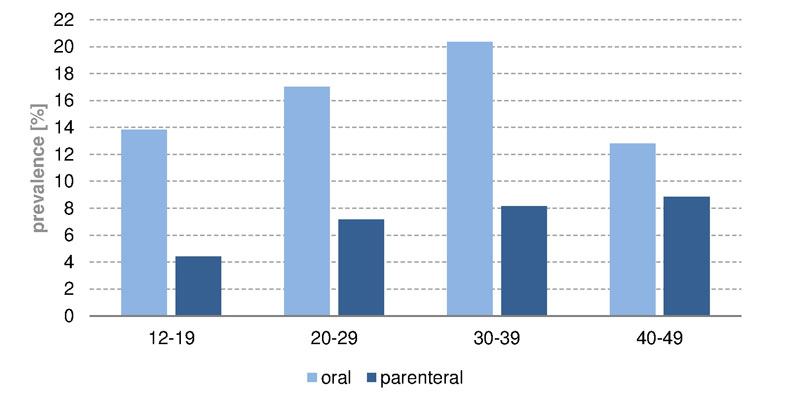
Figure S2 Prevalence of oral and parenteral iron substitution of women of childbearing age in Switzerland, 2012 to 2014.
The authors thank Mathias Früh and Pascal Egger for their technical support and computer programming.
The project was partially funded by Helsana Group, Zürich, Switzerland. BLH has received funds for investigation of iron deficiency and iron deficiency anaemia by Vifor Pharma AG. All authors meet the ICMJE requirements for authorship, and each author confirms that the manuscript represents honest and original work. All versions of the manuscript were reviewed and approved by all authors. The data have partially been reported in a document in German language in Switzerland produced by the Helsana insurance group (Helsana Arzneimittelreport). All authors declare that they have no conflict of interest.
1United Nations Children’s Fund, United Nations University, World Health Organization. Iron Deficiency Anaemia - Assessment, Prevention and Control. WHO/NHD/01.3. Geneva: World Health Organization; 2005.
2World Health Organization, Centers for Disease Control and Prevention. Assessing the Iron Status of Populations. 2nd edition. Geneva: World Health Organization; 2007.
3 Schuepbach RA , Bestmann L , Béchir M , Fehr J , Bachli EB . High Prevalence of Iron Deficiency among Educated Hospital Employees in Switzerland. Int J Biomed Sci. 2011;7(2):150–7.
4 Heath AL , Fairweather-Tait SJ . Clinical implications of changes in the modern diet: iron intake, absorption and status. Best Pract Res Clin Haematol. 2002;15(2):225–41. https://doi.org/10.1053/beha.2002.0208
5 Hug BL , Tichelli A , Benkert P , Stirnimann G , Schifferli JA . Diagnosis and treatment of iron deficiency in medical inpatients at a Swiss tertiary university referral hospital: a retrospective observational cohort study of clinical practice. Swiss Med Wkly. 2013;143:w13847. https://smw.ch/en/article/doi/smw.2013.13847/
6 Schleiffenbaum BE , Schaer DJ , Burki D , Viollier AF , Viollier E , Stettler ER , et al. Unexpected high prevalence of metabolic disorders and chronic disease among young male draftees--the Swiss Army XXI experience. Swiss Med Wkly. 2006;136(11-12):175–84. https://smw.ch/en/article/doi/smw.2006.11266/
7 Fonseca C , Marques F , Robalo Nunes A , Belo A , Brilhante D , Cortez J . Prevalence of anaemia and iron deficiency in Portugal: the EMPIRE study. Intern Med J. 2016;46(4):470–8. https://doi.org/10.1111/imj.13020
8World Health Organization Institutional Repository for Information Sharing. The prevalence of anemia in women. 1992. Available from: http://apps.who.int/iris/handle/10665/58994.
9World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. WHO/NMH/NHD/MNM/11.1. Geneva; World Health Organization: 2011.
10 Goddard AF , James MW , McIntyre AS , Scott BB ; British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60(10):1309–16. https://doi.org/10.1136/gut.2010.228874
11Ruston D, Hoare J, Henderson L, Swan G. The National Diet & Nutrition Survey : adults aged 19 to 64 years. Vol. 4, Nutritional status (anthropometry and blood analytes), blood and blood pressure and physical activity. London: Her Majesty’s Stationery Office; 2004.
12 Levi M , Rosselli M , Simonetti M , Brignoli O , Cancian M , Masotti A , et al. Epidemiology of iron deficiency anaemia in four European countries: a population-based study in primary care. Eur J Haematol. 2016;97(6):583–93. https://doi.org/10.1111/ejh.12776
13Documed AG. Compendium [Internet]. [cited 2016 Feb 3]. Available from: www.documed.ch
14Helsana Gruppe. Helsana [Internet]. [cited 2016 Aug 31]. Available from: http://www.helsana.ch/
15Clinical Practice Research Datalink [Internet]. [cited 2016 Jul 20]. Available from: http://www.cprd.com/intro.asp
16Biétry F, Pitzurra R, Schwenkglenks M, Meier C. Helsana-Arzneimittelreport. 2014.
17Biétry F, Schur N, Pfeil A, Schwenkglenks M, Meier C. Helsana Arzneimittelreport. 2015.
18 Mehra T , Müller CTB , Volbracht J , Seifert B , Moos R . Predictors of High Profit and High Deficit Outliers under SwissDRG of a Tertiary Care Center. PLoS One. 2015;10(10):e0140874. https://doi.org/10.1371/journal.pone.0140874
19Busse R, Geissler A, Quentin W, Wiley M (eds). Diagnosis-Related Groups in Europe: Moving towards transparency, efficiency. 1st Edition. Maidenhead, England: McGraw-Hill, Open University Press; 2011.
20 Walley T , Mantgani A . The UK General Practice Research Database. Lancet. 1997;350(9084):1097–9. https://doi.org/10.1016/S0140-6736(97)04248-7
21 Wood L , Martinez C . The general practice research database: role in pharmacovigilance. Drug Saf. 2004;27(12):871–81. https://doi.org/10.2165/00002018-200427120-00004
22 Giger M , Achermann R . Ambulante Eisensubstitution in der Schweiz – Kostensteigerung infolge venöser Applikation [Iron substitution in outpatients in Switzerland: Increase of costs associated with intravenous administration]. Z Evid Fortbild Qual Gesundhwes. 2013;107(4-5):320–6. https://doi.org/10.1016/j.zefq.2012.12.023
23 Callander EJ , Schofield DJ . Is there a mismatch between who gets iron supplementation and who needs it? A cross-sectional study of iron supplements, iron deficiency anaemia and socio-economic status in Australia. Br J Nutr. 2016;115(4):703–8. https://doi.org/10.1017/S0007114515004912
24 Bodnar LM , Scanlon KS , Freedman DS , Siega-Riz AM , Cogswell ME . High prevalence of postpartum anemia among low-income women in the United States. Am J Obstet Gynecol. 2001;185(2):438–43. https://doi.org/10.1067/mob.2001.115996
25 Bodnar LM , Cogswell ME , Scanlon KS . Low income postpartum women are at risk of iron deficiency. J Nutr. 2002;132(8):2298–302.
26 Kim JY , Shin S , Han K , Lee K-C , Kim J-H , Choi YS , et al. Relationship between socioeconomic status and anemia prevalence in adolescent girls based on the fourth and fifth Korea National Health and Nutrition Examination Surveys. Eur J Clin Nutr. 2014;68(2):253–8. https://doi.org/10.1038/ejcn.2013.241
The project was partially funded by Helsana Group, Zürich, Switzerland. BLH has received funds for investigation of iron deficiency and iron deficiency anaemia by Vifor Pharma AG. All authors meet the ICMJE requirements for authorship, and each author confirms that the manuscript represents honest and original work. All versions of the manuscript were reviewed and approved by all authors. The data have partially been reported in a document in German language in Switzerland produced by the Helsana insurance group (Helsana Arzneimittelreport). All authors declare that they have no conflict of interest.