Leucopenia associated with metamizole: a case-control study
DOI: https://doi.org/10.4414/smw.2017.14438
aDivision of Clinical Pharmacology and Toxicology,
bDivision of Haematology,
cRegional Pharmacovigilance Centre,
dCentre of Primary Health Care,
Leucopenia associated with metamizole: a case-control study
w14438
Lea S Blasera, Hala Hassnaa, Sarah Hofmanna, Andreas Holbrob, Manuel Haschkea,c, Alexandra E Rätz Bravoa,c, Andreas Zellerd, Stephan Krähenbühla,c, Anne B. Taegtmeyera,c
AIMS OF THE STUDY: The aim of this study was to identify possible risk factors for the development of leucopenia associated with metamizole use.
METHODS: A retrospective case-control study was performed. Cases of metamizole-associated leucopenia managed at a single centre (2005–2013) were characterised and compared with matched controls who took metamizole without developing complications.
RESULTS: Fifty-seven cases and 139 controls were identified. Of the cases, 32 were postoperative and these were compared to age-, sex- and ward-matched postoperative controls (n = 64). The remaining cases (n = 25) were compared to sex-matched, non-postoperative controls (n = 75). The number of patients with a positive allergy history was higher among postoperative cases than controls (p = 0.004) as was the number with previous leucopenic episodes (p = 0.03). The prevalence of diagnosed hepatitis C infection was 9% among all cases compared with 1% among all controls (p = 0.005). The use of concomitant cytostatic agents (even at immunosuppressive doses) was significantly higher among non-postoperative cases than controls (p = 0.011). There was no association between renal function and the development of leucopenia.
CONCLUSIONS: A history of allergies, previous leucopenic episodes, hepatitis C infection and concomitant cytostatic agents are possible risk factors for leucopenia associated with metamizole use.
Introduction
Metamizole (dipyrone) is an old antipyretic and analgesic drug whose mechanism of action is not completely known, but postulated by some investigators to be similar to that of nonsteroidal anti-inflammatory drugs [1]. Metamizole was first licensed in Switzerland in 1949 [2]. Its safety profile – in particular the risk of blood disorders including agranulocytosis – is controversial. This has led many countries to withdraw or withhold metamizole from the market. Australia withdrew metamizole in 1964, USA in 1977, Singapore in 1978 and Sweden in 1974 [3, 4]. Sweden reintroduced metamizole in 1995, only to withdraw it again in 1999 on account of agranulocytosis cases [4]. However, in other countries such as Switzerland and Germany, metamizole is still frequently used and has even gained market share in recent years [5, 6], most likely because of its good overall clinical efficacy and tolerability. Routine monitoring of blood counts while under metamizole therapy is not common practice and is not included in the drug label. The drug label, however, clearly states that patients should be made aware of this adverse drug reaction and that they should seek medical attention if they develop fever, cold symptoms, mucositis or sore throat [7]. The mechanism by which metamizole causes blood disorders has not yet been fully elucidated. Available data suggest an immunological process, as well as direct toxicity towards the progenitor cells in the bone marrow [8].
In the 1980s, a large population-based case-control study examined the risk of agranulocytosis or aplastic anaemia during treatment with several drugs, including metamizole [9]. The study found that the incidence of metamizole-induced agranulocytosis varied markedly between countries. Little is known, however, about the underlying risk factors for developing blood cell disorders under metamizole. If strong risk factors are found, these could be used to identify patients for whom metamizole should be avoided. Conversely, patients who do not possess identified risk factors may benefit from the advantages of metamizole over other analgesics such as paracetamol and nonsteroidal anti-inflammatory drugs. Metamizole would therefore be applied in a safer, more targeted fashion.
A number of studies have characterised cases of metamizole-induced white blood cell disorders [4–6]; however as far as we know, no case-control studies have been performed. In the recently published study by Huber and colleagues, data from a prospective case-control surveillance study were used, but the presented results only contain data pertaining to the cases [6].
The aim of the present study was, therefore, to compare cases of metamizole-associated leucopenia with control patients to identify possible risk factors for developing this complication and thus to improve our knowledge about the risk-benefit profile of metamizole.
Methods
This retrospective, descriptive case-control study examined cases of metamizole-associated leucopenia that were managed at the University Hospital in Basel, Switzerland, between April 2005 and August 2013. Cases were either postoperative or non-postoperative. In order to avoid confounding by selection, cases were compared with postoperative and non-postoperative controls. Postoperative cases were compared with age-, sex-, and ward-matched postoperative control patients who had received metamizole without complication between 2005 and 2013. Non-postoperative cases were compared with sex-matched control patients who had received metamizole for at least 4 weeks between 2001 and 2014 in primary care settings in the Basel area without complication. The study was approved by the local ethics committee “Ethikkommission Beider Basel” (protocol number EKBB 2013/130). As data were analysed anonymously, no patient informed consent was required.
Selection and assessment of cases
The electronic medical records of the University Hospital Basel were screened for the keywords “metamizole” or “Novalgin” in combination with “agranulocytosis”, “neutropenia” or “leucopenia”. Additionally, the in-patient referrals to the haematology department which included the keyword “Novalgin” were screened for metamizole-associated leucopenia. Cases of leucopenia (leucocyte count below 3.5 × 109/l) occurring during metamizole exposure and prompting the discontinuation of metamizole were included. The definition of leucopenia, a leucocyte count below 3.5 × 109/l, was in accordance with the local population reference range set by the diagnostic haematology laboratory, University Hospital Basel. All procedures were performed according to the standard operating procedure of our accredited laboratories (ISO/IEC 17025 and ISO 15189).
We evaluated demographic data such as age at the time of diagnosis of the adverse drug reaction (ADR) and body mass index (BMI), underlying diseases, history of immediate- and delayed-type hypersensitivity reactions (“allergy”), comedication, drug administration information (dose, route, frequency) as well as duration of metamizole therapy, latency time of the ADR, laboratory findings and the outcome and treatment of the ADR for each case. Comedication with drugs known to be associated with acute agranulocytosis (level 1 evidence according to Andersohn and colleagues) were taken into consideration when assessing ADR causality [10] and were named “potentially myelotoxic”. The concomitant use of cytostatic agents (which cause dose-dependent myelotoxicity) such as cancer chemotherapeutic drugs or immunosuppressant-dose methotrexate (maximum 30 mg/week oral or subcutaneous) or azathioprine (maximum 5 mg/kg body weight / day) was also recorded. We assessed ADR causality according to the Naranjo ADR probability scale, which includes assessment of other possible causes such as comorbidities and comedication [11]. Only cases with an at least “possible” causality assessment according the Naranjo scale were evaluated further.
Using the available dosage information, therapy and ADR dates, we calculated the daily dose, cumulative dose, duration of treatment, latency time and duration of the ADR. The latency time was calculated as the period between the metamizole start date and the date of ADR onset according to laboratory assessments as defined above. If metamizole was prescribed as an “as required” medication and metamizole intake and dosage regimen were not known with confidence, the daily dose was determined as the mean of the minimal daily dose (500 mg) and the individual’s maximal prescribed daily dose (14 of 57 cases).
Full blood counts (haemoglobin, leucocyte and platelet counts) were recorded at the start date of the metamizole therapy, during metamizole therapy (controls) or when the leucocyte count reached its nadir (cases). Since metabolites of metamizole are mainly renally excreted and their accumulation in renal impairment might be a risk factor for the development of leucopenia, glomerular filtration rate (GFR) was estimated (Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] formula [12]) at the start of metamizole therapy and at the start of the ADR (cases) or before and during metamizole therapy (controls).
Selection and assessment of controls
Postoperative controls
The electronic medical records of the University Hospital Basel were screened for postoperative patients treated between 2005 and 2013 who received metamizole as postoperative analgesia and were discharged with a prescription for daily metamizole until resolution of pain. A discharge prescription for metamizole was taken to indicate good clinical drug tolerability (as confirmed by the absence of fever, sore throat, cold symptoms or mucositis). Specifically, these patients were not known to develop any haematological complications. Two age- (±5 years), sex-, and ward-matched controls per postoperative case were randomly chosen from the list of all possible controls (n = 21 381) by use of a list randomiser provided by random.org [13]. We evaluated demographic data (sex, age at last intake, BMI), underlying diseases, history of allergy, comedication, and if available, laboratory findings (full blood count and GFR) before and during metamizole therapy. Comedication was evaluated in the same manner as for the cases. Cumulative metamizole dose and duration of treatment were not known because these patients were discharged home with a metamizole prescription.
Non-postoperative controls
Non-postoperative controls were selected from an outpatient setting to ensure selection of patients who had been regularly exposed to metamizole for at least 4 weeks without developing haematological complications. The minimum duration of metamizole intake in this non-postoperative control group was chosen according to the latency time data from our previous pharmacovigilance study [5]. In that study, 84% of the cases occurred within 28 days of metamizole treatment. General practitioners in the Basel area were asked to identify patients who had taken metamizole continuously for at least 28 days at a dosage of at least 500 mg per day and who had not developed any obvious blood disorders. As a result of the rarity of such controls, it was only possible to sex-match them with the cases. Data were collected from the medical records.
Using the available dosage information and therapy dates, we calculated the daily dose, cumulative dose and duration of treatment of the corresponding episode of metamizole intake. If metamizole was prescribed as an “as required” medication, and metamizole intake and dosage regimen were not known with confidence, the same method as described above was used. If the amount of metamizole that the patient obtained was known (dispensing practices), this was used for the calculation of the cumulative and daily dose. When only the month and year of the start date was reported, we counted the recorded month as a full month (30 days). In cases where several episodes of more than 28 days of metamizole intake with breaks between the episodes took place, only the longest episode was evaluated. The same laboratory values as in the case group were recorded, but at the nearest available date before the start date of metamizole as well as the newest values during the metamizole therapy.
Statistical analysis
Microsoft Office Excel (Version 2010, Redmond, Washington, USA) and GraphPad PRISM (Version 6, La Jolla, California, USA) were used for descriptive analyses. For variables with normally distributed numerical values, the arithmetic mean and standard deviation were calculated. For variables without normally distributed values, median and range were determined. In cases where the current body weight was missing, the last known value (last observation carried forward method) was used to calculate the BMI. Significance of differences between the cases and controls were assessed using univariate and multivariate conditional logistic regression, the chi-square, Fisher exact test for small numbers, Mann-Whitney-U and Wilcoxon signed- rank tests as appropriate. Differences were considered as significant when p ≤0.05. Multivariate conditional logistic regression models were constructed for postoperative and non-postoperative groups individually and for pooled data using variables that were significantly associated with the development of leucopenia in the univariate analyses (p ≤0.05), using STATA 8.0 (College Station, USA).
Results
Fifty-seven cases of metamizole-associated leucopenia managed at the University Hospital Basel between April 2005 and August 2013 were included according to the predefined inclusion criteria. Of these, 32 were postoperative and 25 non-postoperative. Of the 25 non-postoperative cases, 14 developed leucopenia prior to and 11 during hospitalisation. Sixty-four age-, sex- and ward-matched postoperative controls and 75 sex-matched non-postoperative control patients from general practice settings were identified. In total, 543 general practitioners were approached, 19 of whom provided 93 patients, from which 75 could be sex-matched to non-postoperative cases on a 1:3 basis.
Patient and metamizole treatment characteristics
Table 1 summarises the patient characteristics. The median age of the non-postoperative controls was higher than that of the non-postoperative cases (79 vs 51 years, p <0.001). In keeping with the older age, the number of diagnoses and total number of comedications per patient were also higher among those controls than in non-postoperative cases. In contrast, among postoperative cases, the total numbers of diagnoses and comedications were higher than among the age-, sex- and ward-matched controls.
In the non-postoperative control group, metamizole was solely taken orally, whereas in the corresponding case group, 20% received intravenous metamizole (table 2). As also shown in table 2, the mean daily metamizole dose did not differ significantly between non-postoperative cases and controls and was within the recommended range. The median treatment duration in the postoperative case group was 6 days and approximately two thirds of the cases took metamizole for 1 to 7 days. The median treatment duration in the non-postoperative case group was 13 days, with one third taking metamizole for 1 to 7 days. The non-postoperative controls took metamizole per definition for at least 28 days, one third for even longer than 1 year.
Characteristics of the leucopenia
Figure 1 shows the leucocyte and neutrophil values for the case and control subgroups; all cases experienced a distinct fall in leucocyte and neutrophil counts. Leucocyte counts measured during treatment with metamizole were available for 72% of postoperative controls. Leucocyte counts before and during metamizole exposure were available for 53% and 52% of postoperative and non-postoperative controls, respectively. Among non-postoperative controls, 69% had a during-treatment leucocyte count result, all of which were above the lower limit of the reference range. No trend in haemoglobin and platelet counts was seen (fig. 2).
Table 3 shows the ADR characteristics among the cases. No cases fulfilled the criteria for a “definite” causality assessment according the Naranjo scale. The three cases that had a recurrence of the ADR on rechallenge would, however, have been classified as “certain” if the WHO–UMC Probability Scale had been applied [14].
The ADR appeared during the first week of treatment in 40% and during the first 2 months in 93% of all cases. Nineteen patients (33%) were known to have been treated with granulocyte colony-stimulating factor (G-CSF).
Four cases in the study had a fatal outcome. In two of them, metamizole was assessed as probably being causal and in the other two as possibly causal because concomitant methotrexate and azathioprine, respectively, were also suspected. All fatal cases were women (22, 71, 75 and 76 years old). One patient in the case group was recently re-exposed to metamizole with fatal outcome [15].
Comorbidities and their association with the ADR
Figure 3 shows the course of the GFR at the start of metamizole therapy and at the start of the ADR in the case groups. In the cases, the GFR did not change significantly between these two time points. Among controls, an increase and a decrease in GFR were seen in the postoperative and non-postoperative groups, respectively. These differences were, however, not clinically relevant. Three patients in the case group (one postoperative and two non-postoperative cases) and one patient in the non-postoperative control group had pre-existing severe renal impairment (GFR <30 ml/min/1.73 m2) at the start of the metamizole therapy. However, the GFR increased or remained clinically stable during the metamizole therapy in all cases (ranging from 16–61.2, 24.4–34.0, 21.5–28.3 and 27.3–22.0 ml/min/1.7 3m2, respectively).
Patients with a history of allergy were analysed in more detail (table 4). The number of known medication and non-medication allergies was significantly higher in the postoperative case group compared with the corresponding control group (p = 0.003 and 0.026, respectively). The corresponding odds ratios (OR) for developing leucopenia if a medication or other type of allergy was present were 5.44 (95% confidence interval [CI] 1.76– 6.8) and 11.13 (95% CI 1.33–92.5), respectively. Specifically, the number of cases with beta-lactam allergies was significantly higher (p = 0.03). Among non-postoperative cases and controls, there was a non-statistically significant trend towards a higher prevalence of non-medication allergies among cases compared with controls (table 4).
Patients with a history of previous leucopenias were also analysed in more detail. One patient (a postoperative case) had a pre-existing bicytopenia (recurrent episodes of anaemia and neutropenia) of unknown origin. The neutropenia re-emerged under metamizole and resolved again after stopping metamizole. In three cases (two postoperative and one non-postoperative), a pre-existing recurrent pancytopenia of uncertain origin had previously been diagnosed. In all of these cases, the leucocyte or neutrophil counts fell from a value within the normal range to a value below the lower limit of normal under metamizole. Three other cases had a pre-existing haematological condition (one patient had haemophilia B, one patient had an isolated factor VII deficiency and another chronic myelocytic leukaemia).
Five patients in the case group (three postoperative and two non-postoperative cases) and one control patient were hepatitis C positive. Hepatitis C infection was not the reason for admission in any of these cases. When data from all cases and all controls together were analysed, there were significantly more patients with underlying hepatitis C infection among cases than controls. The OR for developing leucopenia under metamizole among subjects with an underlying hepatitis C infection was 11.59 (95% CI 1.35–99.78, p = 0.026). After adjustment of the model for other variables that were significantly associated with leucopenia in the univariate analysis of the pooled data, namely presence of an allergy, concomitant use of cytostatic agents, number of comedications and number of chronic diseases, the OR was 35 (95% CI 2.99–409, p = 0.005). One patient with an underlying hepatitis C infection was co-infected with hepatitis B and another with hepatitis B and human immunodeficiency virus (HIV). No other viral infectious diseases were known to be present in either the case or the control groups.
Comedications and their association with the adverse drug reaction
The total number of patients concomitantly treated with potentially myelotoxic comedication was similar in all groups (p = 0.86 postoperative cases vs controls and p = 0.09 non-postoperative cases vs controls; table 1). Potentially myelotoxic and cytostatic comedications are listed in detail in table 5. In 54% of all cases, comedication other than metamizole could potentially have also caused or contributed to the ADR; however, these were taken into consideration when determining the Naranjo score. The number of patients treated with concomitant cytostatic agents did not differ significantly between the postoperative case and control group (table 1). However, in the non-postoperative groups, a greater proportion of cases than controls received concomitant cytostatic agents (p = 0.011).
Multiple conditional logistic regression analyses showed history of allergy and number of comedications to be independently associated with leucopenia in the setting of postoperative metamizole exposure (OR 3.39, 95% CI 1.08–10.65; p = 0.037 and 1.16, 95% CI 1.0–1.34; p = 0.045, respectively). In the comparison of non-postoperative cases with controls, independent variables associated with leucopenia were concomitant use of cytostatic drugs and number of chronic diseases (OR 15.84, 95% CI 1.82–137.15; p = 0.012 and 0.24 95% CI 0.1–0.59, p = 0.002, respectively).
Discussion
This retrospective study of metamizole-associated leucopenia identified a number of possible risk factors, namely a history of allergy, previous leucopenic episodes, infection with hepatitis C and concomitant use of cytostatic agents (including at immunosuppressive doses), which might allow targeted and safer metamizole use. This is of particular interest in view of the increasing use of metamizole in central European countries such as Switzerland and Germany.
Previous population-based studies of drug-induced agranulocytosis found a high incidence among older patients [16, 17]. Firm conclusions, however, can only be drawn if the exposure data of specific age groups are known, and the current study does not provide this.
The opposing findings regarding the total number of diagnoses and concomitant medication among postoperative and non-postoperative cases and controls probably reflects the inability to age-match in the non-postoperative groups. As the findings are in opposition, we do not believe the total number of diagnoses or total number of comedications are risk factors for the development of metamizole-associated leucopenia; however, their role cannot be completely excluded. Clearly, a larger study is needed.
The fact that the daily doses in the non-postoperative case and control groups were similar and that postoperative controls were exposed to higher daily doses during hospitalisation compared with postoperative cases is evidence against a typical dose-dependent toxic effect; rather it favours toxicity associated with the presence of immunological or metabolic susceptibility factors in affected patients. The median latency time of 11 days between the start of the metamizole therapy and the onset of the ADR (see table 3) lies within the 7 to 14 day latency time found in a previous analysis of pharmacovigilance data [5]. Similarly, the ADR appeared within the time-frame previously described in the pharmacovigilance data study of 1417 reported cases of metamizole-induced white blood cell disorders [5] and in agreement with other previous studies [10, 18]. These findings would support routine blood count monitoring during the first weeks of metamizole therapy, but this needs to be evaluated in a prospective, randomised fashion. Prior to this, the optimum time points and frequency of blood count monitoring should be determined. The benefits and costs of a screening programme for metamizole-induced leucopenia would also have to be considered prospectively.
The duration of the ADR was approximately 5 days, independent of the use of G-CSF, which is similar to the findings of Navarro-Martinez and colleagues [19]. This finding has to be interpreted in the light of the fact that the more severe cases tended to preferentially receive G-CSF, despite the paucity of data supporting the benefit of G-CSF in this situation.
No changes in GFR were seen under metamizole and there was no difference in GFR between the case and control groups (see fig. 3). Renal impairment was, therefore, not found to be a risk factor for the development of metamizole-induced leucopenia in the present study.
If the mechanism of toxicity has an immunological component, another possible risk factor could be a susceptibility to allergies, which our findings from postoperative patients support. The lack of association between allergy history and the development of leucopenia in association with metamizole among non-postoperative cases compared with controls may be a chance finding or reflect the different data-recording practices in hospitals and in general practice. The postoperative groups – all of which were inpatients in the same hospital – were subject to the same documentation process, so their data are more comparable. New concepts of immunological mechanisms, like the p-i-concept in which drugs or metabolites interact directly with T-cells, may help understanding of the mechanisms of the toxicity, which cannot easily be categorised into a classical known mechanism [20]. A genetic predisposition, which could not be examined in this study, may also be relevant: a genetic study of five patients who recovered from metamizole-induced agranulocytosis found an association with the presence or absence of specific human leucocyte antigen (HLA) types [21]. Similarly, other drugs known to cause agranulocytosis, such as thiourea antithyroid drugs, have recently been investigated in a genome-wide association study and patients possessing the HLA-B*38:02:01 genotype were found to be at higher risk of developing this adverse drug reaction [22]. The role of genetic factors in the predisposition to developing leucopenia under metamizole requires further study.
Of special interest were the cases with pre-existing haematological conditions, especially previous leucopenia. In two of the four patients with previous leucopenia, the likeliest cause was liver disease due to HCV infection. In the other two patients, no reason for the peripheral leucopenia could be found in a bone marrow study. Whether underlying haematological conditions make patients more vulnerable to the haematotoxic effects of metamizole cannot be conclusively assessed here. However, these data suggest that pre-existing haematological conditions are a possible risk factor.
Our observation regarding an increased prevalence of hepatitis C infection in patients who developed a metamizole-associated leucopenia compared with controls is in keeping with the increased risk of ADRs in the setting of viral infection seen with other drugs [23]. An increased risk for developing ADRs has been shown for HIV, hepatitis C, Epstein-Barr virus, herpes simplex virus, human herpesvirus and cytomegalovirus infection [24–27]. Asymptomatic carriers of hepatitis B with normal liver function tests were found to have impaired clearance of metamizole via oxidative pathways compared with healthy controls, leading to increased exposure to the 4-methylaminoantipyrine metabolite (carriers and controls all had slow the acetylator phenotype) [28]. In Western Europe, the prevalence of hepatitis C infection is estimated as 2.4% [29]. Overall, our case group showed a prevalence of 9% and the control group a prevalence of 1%. The exact mechanisms whereby viral infections cause an increased risk of ADR are not fully known, but could be any combination of a reduction in immune tolerance, increased antigenicity or altered drug metabolism [23]. Furthermore, the chronic viral infection itself may be associated with altered blood counts, including leucopenia [30], and hepatitis C virus has been found to be present in the bone marrow of some infected patients [31]. It is likely that bone-marrow toxic agents and hepatitis C virus are co-risk factors for developing leucopenia; however, this requires further study.
Regarding the co-administration of other bone-marrow toxic drugs, it is not always possible to separate out the impact the different drugs have on the ADR. This led to 54% of the cases receiving comedications that may or may not have contributed to the ADR. However, the administration of non-chemotherapeutic, potentially myelotoxic comedication, as given by Andersohn and colleagues, was not different between both types of cases and controls (see table 1). The use of beta-lactam antibiotics, particularly at high doses and for a long duration, is known to be associated with the development of agranulocytosis [32]. Concomitant use of cytostatic agents was more common among cases than their respective controls. Among non-postoperative patients this difference was statistically significant, indicating that the concomitant use of cytostatic agents might be a risk factor for the development of a white blood cell disorder under metamizole therapy. In the pharmacovigilance data study, coadministration of methotrexate was identified as a risk factor for a fatal outcome [5]. In this study, two fatalities involving concomitant immunosuppressive-dose methotrexate and azathioprine, respectively, occurred.
Retrospective studies are associated with a number of limitations. The problem of missing data is one important aspect and may have led to the inclusion of control patients who might have gone on to develop a white blood cell disorder under metamizole, especially in the postoperative control group. Furthermore, the limited number of study subjects, due to the rarity of the ADR, as well as the limited number of patients who take metamizole on a long-term basis reduces study power and precluded optimal matching. It is also possible that some cases and data were missed because of varying documentation policies. The inclusion of possible rather than only probable or confirmed cases according to the Naranjo scoring system may have led to the inclusion of some cases where metamizole was not the likeliest cause of the white blood cell disorder. Lastly, the true prevalence of hepatitis C was not known, as hepatitis C testing is not routinely performed in either the in-patient or general practice setting. Large-scale, prospective studies are needed in order to resolve these limitations.
Conclusion
A history of allergy, previous leucopenic episodes, infection with hepatitis C and concomitant use of cytostatic agents (including at immunosuppressive doses), but not impaired renal function, might be risk factors for metamizole-induced leucopenia and require further study.
Table 1 Patient characteristics.
|
Post-OP cases
(n = 32)
|
Post-OP controls
(n = 64)
|
p-value
|
Non-post-OP cases
(n = 25)
|
Non-post-OP controls
(n = 75)
|
p-value
|
| Age in years at ADR diagnosis (cases) or at last intake (controls); median (range) |
58 (16–82) |
58 (16–82) |
0.87a
|
51 (20–84) |
79 (41–98) |
<0.001a
|
| Female; number (%) |
17 (53) |
34 (53) |
1b
|
19 (76) |
57 (76) |
1b
|
| Body mass index in kg/m2; median (range) |
25 (20–45)
n = 18 |
26 (18–56)
n = 56 |
0.69a
|
21 (18–47)
n = 11 |
28 (16–40)
n = 51 |
0.18 a
|
| Co-morbidities |
|
|
|
|
|
|
| Total number of diagnoses; median (range) |
5 (1–9) |
2 (0–10) |
0.004c
|
4 (1–9) |
6 (2–11) |
0.002c
|
| Number of chronic diseases; median (range) |
2 (0–5) |
2 (0–10) |
0.27c
|
3 (0–6) |
4 (1–9) |
<0.001c
|
| Number of patients with known allergy; number (%) |
16 (50) |
12 (19) |
0.004c
|
8 (32) |
18 (24) |
0.389c
|
| Previous leucopenic episodes; number (%)e
|
3 (9) |
0 (0) |
0.03d
|
1 (4) |
0 (0) |
0.25d
|
| Underlying Hepatitis C infection; number (%)f
|
3 (9) |
1 (2) |
0.12c
|
2 (8) |
0 (0) |
0.061d
|
| Comedication (see also table 5) |
|
|
|
|
|
|
| Total number of comedications; median (range)] |
9 (1–18) |
5 (1–18) |
0.001c
|
6 (1–18) |
9 (0–19) |
0.048c
|
| Patients concomitantly receiving non-chemotherapeutic potentially myelotoxic comedication; number (%)g
|
9 (28) |
19 (30) |
0.86c
|
6 (24) |
33 (44) |
0.09c
|
| Patients concomitantly receiving cytostatic agents; number (%)h
|
3 (9) |
2 (5) |
0.23c
|
6 (24) |
3 (4) |
0.011c
|
Table 2 Characteristics of metamizole therapy.
|
Post-OP cases
(n = 32)
|
Post-OP controls
(n = 64)
|
Non-post-OP cases
(n = 25)
|
Non-post-OP controls
(n = 75)
|
| Formulation |
|
|
|
|
| Oral; number (%) |
12 (38) |
64 (100) |
14 (56) |
75 (100) |
| Intravenous; number (%) |
12 (38) |
10 (16)a
|
5 (20) |
0 (0) |
| Unknown; number (%) |
8 (25) |
0 (0) |
6 (24) |
0 (0) |
| Daily dose in g; mean (SD) |
2.19 (1.22)
n = 27 |
3.36 (1.03)
n = 40b
|
1.99 (1.27)
n = 17 |
1.76 (0.82) |
| Cumulative dose in g; mean (SD) |
13.50 (0.5–94)
n = 25 |
na |
21.6 (1–82.5)
n=14 |
316 (24–3109) |
| Treatment duration in days; median (range) |
6 (1–59) n= 29 |
na |
13 (1–365)
n = 16 |
187 (32–2192) |
| Number (%) of patients treated for: |
|
|
|
|
| 1–7 days |
18 (62) |
na |
5 (31) |
n/a |
| 8–30 days |
8 (28) |
na |
8 (50) |
n/a |
| 31–60 days |
3 (10) |
na |
1 (6) |
11 (15) |
| 61–120 days |
0 (0) |
na |
1 (6) |
18 (24) |
| 121–180 days |
0 (0) |
na |
0 (0) |
6 (8) |
| 180–365 days |
0 (0) |
na |
1 (6) |
14 (19) |
| >365 days |
0 (0) |
na |
0 (0) |
26 (35) |
Table 3 Characteristics of the white blood cell disorders (all cases).
|
All cases
(n = 57)
|
|
Naranjo Causality assessment; number (%)
|
|
| Definite |
0 (0) |
| Probable |
20 (35) |
| Possible |
37 (65) |
|
Rechallenge information; number (%)
|
|
| Known positive rechallenge |
3 (5) |
| Known negative rechallenge |
2 (4) |
| Rechallenge with unknown outcome |
1 (2) |
| No known rechallenge |
51 (89) |
|
Timing of ADR (n = 49)
|
|
| During therapy; number (%) |
31 (63) |
| 1–7 days after last application; number (%) |
14 (29) |
| 8–14 days after last application; number (%) |
2 (4) |
| >14 days after last application; number (%) |
2 (4) |
| Latency timea in days; median (range)
|
11 (1–368) |
| ADR duration in days; median (range)
|
|
| With G-CSF (n = 16) |
5.5 (1–20) |
| Without G-CSF (n = 21) |
5 (1–21) |
| Unknown whether G-CSF given or not (n = 12) |
5 (1–51) |
| Time between ADR onset and nadir in days; median (range)
|
1 (0–36) |
| Time between nadir and recovery in days; median (range)
|
4 (1–19) |
| With G-CSF (n = 16) |
3.5 (1–19) |
| Without G-CSF (n = 21) |
4 (1–13) |
| Unknown whether G-CSF given or not (n = 12) |
4 (1–16) |
| Time between stopping metamizole and recovery in days (in cases where ADR occurred during metamizole therapy); median (range)
|
|
| With G-CSF (n = 9) |
5 (1–29) |
| Without G-CSF (n = 15) |
4 (1–19) |
| Unknown whether G-CSF given or not (n = 2) |
6.5 (6–7) |
|
Degree of leucopenia (n = 56)
|
|
| Leucocytes 2–3.5 × 109/l; number (%) |
18 (32) |
| Leucocytes 1–2 × 109/l; number (%) |
22 (39) |
| Leucocytes <1 × 109/l: number (%) |
16 (29) |
|
Degree of neutropenia (n = 55)
|
|
| Neutrophils 1–1.3 × 109/l; number (%) |
10 (18) |
| Neutrophils 0.5–1 × 109/l; number (%) |
19 (35) |
| Neutrophils <0.5 × 109/l; number (%) |
26 (47) |
|
Outcome; number (%)
|
|
| Recovered |
53 (93) |
| Died |
4 (7) |
|
Post-OP cases
(n = 32)
|
Post-OP controls
(n = 64)
|
p-valuea
|
Non-post-OP cases
(n = 25)
|
Non-post-OP controls
(n = 75)
|
p-valuea
|
| Patients with known medication allergies; number (%) |
13 (41) |
10 (16) |
0.003a
|
6 (24) |
15 (20) |
0.67a
|
| Median number of trigger drugs in patients with medication allergies (range) |
3 (1–6) |
1 (1–3) |
|
1 (1–2) |
1 (1–4) |
|
| Trigger drugs; number (%) |
|
|
|
|
|
|
| Beta-lactams: |
|
|
|
|
|
|
| Total |
9 (28) |
6 (9) |
0.028a
|
3 (12) |
8 (11) |
0.84a
|
| With skin reaction |
5 |
2 |
|
2 |
4 |
|
| With anaphylactic reaction (dyspnoea) |
1 |
– |
|
1 |
– |
|
| With unknown reaction |
3 |
4 |
|
– |
4 |
|
| Other antibiotics |
4 |
1 |
|
– |
3 |
|
| NSAID |
4 |
4 |
|
1 |
5 |
|
| Opioids |
1 |
– |
|
1 |
2 |
|
| Others |
5 |
3 |
|
2 |
6 |
|
| Patients with known non-medication allergy; number (%) |
7 (22) |
3 (5) |
0.026a
|
4 (16) |
3 (4) |
0.06a
|
| Allergic rhinitis |
4 |
– |
|
1 |
1 |
|
| Other allergies |
5 |
3 |
|
3 |
1 |
|
Table 5 Comedication known to be associated with agranulocytosis.
|
Post-OP cases
(n = 32)
|
Post-OP controls
(n = 64)
|
Non-post-OP cases
(n = 25)
|
Non-post-OP controls
(n = 75)
|
| Total number of comedication |
286 |
368 |
185 |
688 |
| Patients concomitantly receiving cytostatic agents; number (%) |
|
|
|
|
| Methotrexate (low-dose) |
2 (6) |
3 (5) |
3 (12) |
2 (3) |
| Azathioprine |
– |
– |
2 (8) |
– |
| Gemcitabine |
– |
– |
– |
1 (1) |
| Sorafenib |
– |
– |
1 (4) |
– |
| Ipilimumab |
1 (3) |
– |
– |
– |
| Cisplatin/5-fluorouracil |
– |
– |
1 (4) |
– |
| Patients concomitantly receiving non-chemotherapeutic potentially myelotoxic comedicationa; number (%) |
|
|
|
|
| Clopidogrel |
1 (3) |
2 (3) |
1 (4) |
3 (4) |
| Diclofenac |
– |
– |
– |
3 (4) |
| Fluoxetine |
1 (3) |
– |
– |
3 (4) |
| Ibuprofen |
5 (16) |
13 (20) |
4 (16) |
3 (4) |
| Ramipril |
2 (6) |
4 (6) |
2 (8) |
15 (20) |
| Spironolactone |
1 (3) |
1 (2) |
– |
7 (9) |
| Phenytoin |
1 (3) |
1 (2) |
– |
– |
| Coprescribed drugs for which an association with the ADR could not be ruled out; number (%) |
|
|
|
|
| Beta-lactam antibiotics |
|
|
|
|
| Amoxicillin/Clavulanic acid |
1 (3) |
3 (5) |
1 (4) |
1 (1) |
| Tazobactam/Piperacillin |
8 (25)b
|
1 (2) |
1 (4) |
– |
| Flucloxacillin |
2 (6)b
|
– |
1 (4) |
– |
| Cephalosporin |
1 (3) |
2 (3) |
– |
– |
| Not specified |
1 (3) |
– |
1 (4) |
– |
| Other antibiotics |
|
|
|
|
| Ciprofloxacin |
4 (13) |
3 (5) |
1 (4) |
1 (1) |
| Moxifloxacin |
1 (3) |
– |
– |
– |
| Vancomycin |
7 (22) |
– |
– |
– |
| Tobramycin |
1 (3) |
– |
– |
– |
| Proton-pump inhibitor use |
|
|
|
|
| Any proton-pump inhibitor |
18 (56) |
37 (58) |
15 (60) |
50 (67) |
| Other drugs |
|
|
|
|
| Quetiapine |
1 (3) |
2 (3) |
1 (4) |
6 (8) |
| Acetylsalicylic acidc
|
– |
9 (14) |
1 (4) |
22 (29) |
| Mesalazine |
– |
– |
1 (4) |
– |
| Rifampicin |
2 (6) |
– |
– |
– |
| Interferon |
– |
– |
1 (4) |
– |
| Gabapentin |
– |
1 (2) |
1 (4) |
3 (4) |
| Mirtazapine |
– |
2 (3) |
1 (4) |
4 (5) |
| Haloperidol |
1 (3) |
– |
– |
– |
| Caspofungin |
1 (3) |
– |
– |
– |
| Pregabalin |
– |
2 (3) |
1 (4) |
7 (9) |

Figure 1 Leucocyte and neutrophil counts before starting metamizole and at time of diagnosis of ADR (case groups A–D) or at last follow-up while taking metamizole (control groups E–H). Red lines indicate upper and lower reference values (10–3.5 × 109/l for leucocytes and 6.7–1.3 × 109/l for neutrophils).
ADR = adverse drug reaction; post-OP = postoperative
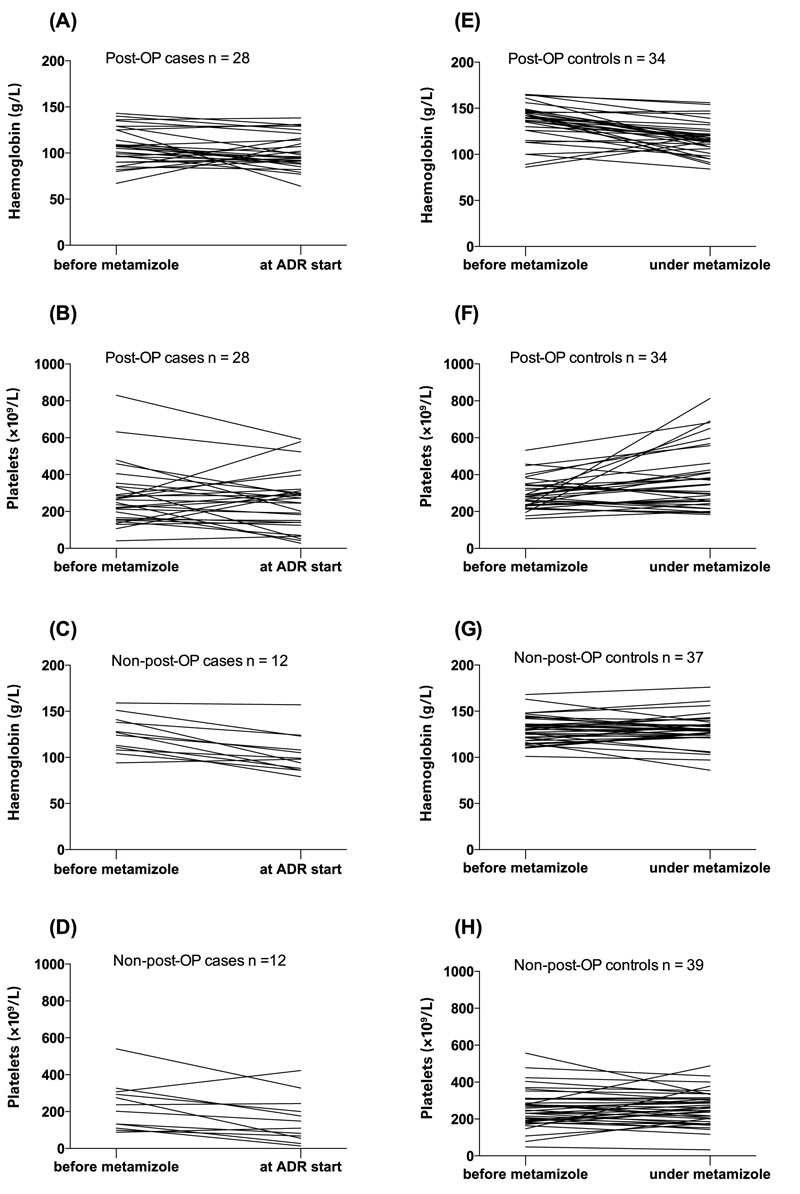
Figure 2 Haemoglobin and platelet counts before starting metamizole and at time of diagnosis of ADR (case group A–D) or at last follow-up while taking metamizole (control group E–H).
ADR adverse drug reaction; post-OP = postoperative
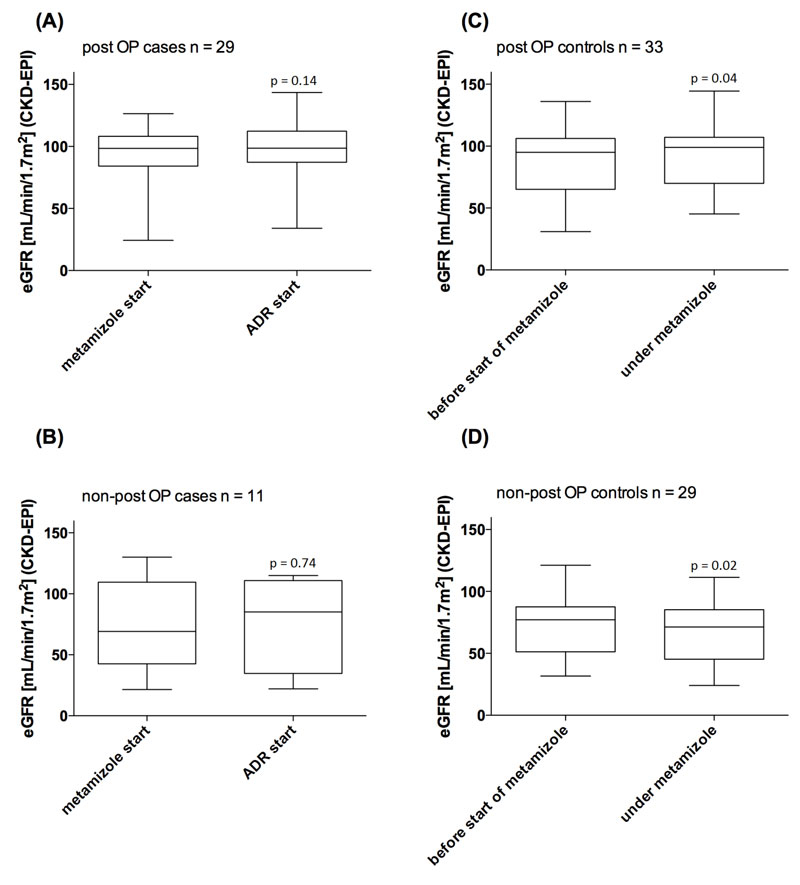
Figure 3 Estimated glomerular filtration rate (eGFR) at the start of metamizole therapy and at ADR start (case groups, left side), eGFR before the start of metamizole therapy and under metamizole therapy (control groups, right side), compared with use of the Wilcoxon signed-rank test.
ADR adverse drug reaction; post-OP = postoperative
Acknowledgements
The authors would like to cordially thank all collaborating general practitioners (including Doctors Beat Biedermann, Mathis Grehn, Edy Riesen, Peter Sigg, Radu Vornicel-Schwenck, and colleagues). A special thanks also to the Institute of Primary Health Care, University of Basel, Switzerland for the important collaboration. Thanks also to the Senglet-Foundation, the foundation for the promotion of young pharmaceutical researchers in Basel, for their financial support (LSB).
Anne Taegtmeyer MD, PhD, Clinical Pharmacology and Toxicology, University Hospital Basel, Schanzenstrasse 55, CH-4031 Basel, Switzerland, anne.taegtmeyer[at]usb.ch
References
1 Hinz B, Cheremina O, Bachmakov J, Renner B, Zolk O, Fromm MF, et al. Dipyrone elicits substantial inhibition of peripheral cyclooxygenases in humans: new insights into the pharmacology of an old analgesic. FASEB J. 2007;21(10):2343–51. PubMed http://dx.doi.org/10.1096/fj.06-8061com
https://doi.org/10.1096/fj.06-8061com
2 Zugelassene Praeparate. Erweiterte Praeparatliste. Available from https://www.swissmedic.ch/arzneimittel/00156/00221/00222/00230/index.html?lang=de. Last accessed 14th February 2017
3 Chan TY, Chan AW. Aminopyrine-induced blood dyscrasias--still a problem in many parts of the world. Pharmacoepidemiol Drug Saf. 1996;5(4):215–9. PubMed http://dx.doi.org/10.1002/(SICI)1099-1557(199607)5:4<215::AID-PDS208>3.0.CO;2-5
https://doi.org/10.1002/(SICI)1099-1557(199607)5:4<215::AID-PDS208>3.0.CO;2-5
4 Hedenmalm K, Spigset O. Agranulocytosis and other blood dyscrasias associated with dipyrone (metamizole). Eur J Clin Pharmacol. 2002;58(4):265–74. PubMed http://dx.doi.org/10.1007/s00228-002-0465-2
https://doi.org/10.1007/s00228-002-0465-2
5 Blaser LS, Tramonti A, Egger P, Haschke M, Krähenbühl S, Rätz Bravo AE. Hematological safety of metamizole: retrospective analysis of WHO and Swiss spontaneous safety reports. Eur J Clin Pharmacol. 2015;71(2):209–17. PubMed http://dx.doi.org/10.1007/s00228-014-1781-z
https://doi.org/10.1007/s00228-014-1781-z
6 Huber M, Andersohn F, Sarganas G, Bronder E, Klimpel A, Thomae M, et al. Metamizole-induced agranulocytosis revisited: results from the prospective Berlin Case-Control Surveillance Study. Eur J Clin Pharmacol. 2015;71(2):219–27. PubMed http://dx.doi.org/10.1007/s00228-014-1777-8
https://doi.org/10.1007/s00228-014-1777-8
7 Product Information Novalgin®, sanofi-aventis (schweiz) ag, 1214 Vernier/GE. Available from http://www.swissmedicinfo.ch. Last accessed 14th February 2017.
8 Tesfa D, Keisu M, Palmblad J. Idiosyncratic drug-induced agranulocytosis: possible mechanisms and management. Am J Hematol. 2009;84(7):428–34. PubMed http://dx.doi.org/10.1002/ajh.21433
https://doi.org/10.1002/ajh.21433
9 Anonymous. Risks of agranulocytosis and aplastic anemia. A first report of their relation to drug use with special reference to analgesics. The International Agranulocytosis and Aplastic Anemia Study. JAMA. 1986;256(13):1749–57. PubMed http://dx.doi.org/10.1001/jama.1986.03380130077032
https://doi.org/10.1001/jama.1986.03380130077032
10 Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146(9):657–65. PubMed http://dx.doi.org/10.7326/0003-4819-146-9-200705010-00009
https://doi.org/10.7326/0003-4819-146-9-200705010-00009
11 Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45. PubMed http://dx.doi.org/10.1038/clpt.1981.154
https://doi.org/10.1038/clpt.1981.154
12 Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. PubMed http://dx.doi.org/10.7326/0003-4819-150-9-200905050-00006
https://doi.org/10.7326/0003-4819-150-9-200905050-00006
13 Haahr M. List randomizer. Available from: https://www.random.org/. Last ascessed 08.10.2015.
14 Adverse drug reaction and causality assessment scales. WHO. Available from: http://who-umc.org/Graphics/24734.pdf. Last accessed 22.02.2016.
15 Zeiner E, Blaser LS, Tisljar K, Heim D, Taegtmeyer A. Fatale Agranulozytose nach Metamizol-Reexposition. Praxis (Bern). 2015;104(3):151–4. http://dx.doi.org/10.1024/1661-8157/a001916
https://doi.org/10.1024/1661-8157/a001916
16 Ibáñez L, Vidal X, Ballarín E, Laporte JR. Population-based drug-induced agranulocytosis. Arch Intern Med. 2005;165(8):869–74. PubMed http://dx.doi.org/10.1001/archinte.165.8.869
https://doi.org/10.1001/archinte.165.8.869
17 Théophile H, Bégaud B, Martin K, Laporte JR, Capella D. Incidence of agranulocytosis in Southwest France. Eur J Epidemiol. 2004;19(6):563–5. PubMed http://dx.doi.org/10.1023/B:EJEP.0000032371.97823.85
https://doi.org/10.1023/B:EJEP.0000032371.97823.85
18 Ibáñez L, Vidal X, Ballarín E, Laporte JR. Agranulocytosis associated with dipyrone (metamizol). Eur J Clin Pharmacol. 2005;60(11):821–9. PubMed http://dx.doi.org/10.1007/s00228-004-0836-y
https://doi.org/10.1007/s00228-004-0836-y
19 Navarro-Martínez R, Chover-Sierra E, Cauli O. Non-chemotherapy drug-induced agranulocytosis in a tertiary hospital. Hum Exp Toxicol. 2016;35(3):244–50. PubMed http://dx.doi.org/10.1177/0960327115580603
https://doi.org/10.1177/0960327115580603
20 Pichler WJ, Naisbitt DJ, Park BK. Immune pathomechanism of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127(3, Suppl):S74–81. PubMed http://dx.doi.org/10.1016/j.jaci.2010.11.048
https://doi.org/10.1016/j.jaci.2010.11.048
21 Vlahov V, Bacracheva N, Tontcheva D, Naumova E, Mavrudieva M, Ilieva P, et al. Genetic factors and risk of agranulocytosis from metamizol. Pharmacogenetics. 1996;6(1):67–72. PubMed http://dx.doi.org/10.1097/00008571-199602000-00005
https://doi.org/10.1097/00008571-199602000-00005
22 Cheung CL, Sing CW, Tang CS, Cheng VK, Pirmohamed M, Choi CH, et al. HLA-B*38:02:01 Predicts Carbimazole/Methimazole-Induced Agranulocytosis. Clin Pharmacol Ther. 2015;99(5):555–61. doi:10.1002/cpt.309. PubMed
23 Levy M. Role of viral infections in the induction of adverse drug reactions. Drug Saf. 1997;16(1):1–8. PubMed http://dx.doi.org/10.2165/00002018-199716010-00001
https://doi.org/10.2165/00002018-199716010-00001
24 Ahluwalia J, Abuabara K, Perman MJ, Yan AC. Human herpesvirus 6 involvement in paediatric drug hypersensitivity syndrome. Br J Dermatol. 2015;172(4):1090–5. PubMed http://dx.doi.org/10.1111/bjd.13512
https://doi.org/10.1111/bjd.13512
25 Bonfanti P, Valsecchi L, Parazzini F, Carradori S, Pusterla L, Fortuna P, et al.; Coordinamento Italiano Studio Allergia e Infezione da HIV (CISAI) Group. Incidence of adverse reactions in HIV patients treated with protease inhibitors: a cohort study. J Acquir Immune Defic Syndr. 2000;23(3):236–45. PubMed http://dx.doi.org/10.1097/00126334-200003010-00004
https://doi.org/10.1097/00126334-200003010-00004
26 Duval X, Journot V, Leport C, Chêne G, Dupon M, Cuzin L, et al.; Antiprotease Cohort (APROCO) Study Group. Incidence of and risk factors for adverse drug reactions in a prospective cohort of HIV-infected adults initiating protease inhibitor-containing therapy. Clin Infect Dis. 2004;39(2):248–55. PubMed http://dx.doi.org/10.1086/422141
https://doi.org/10.1086/422141
27 Guitton E, Montastruc JL, Lapeyre-Mestre M; French Network of Pharmacovigilance Centres. Influence of HCV or HBV coinfection on adverse drug reactions to antiretroviral drugs in HIV patients. Eur J Clin Pharmacol. 2006;62(3):243–9. PubMed http://dx.doi.org/10.1007/s00228-005-0080-0
https://doi.org/10.1007/s00228-005-0080-0
28 Levy M, Leibowich I, Zylber-Katz E, Ilan Y, Granit L, Sviri S, et al. Impairment of the metabolism of dipyrone in asymptomatic carriers of the hepatitis B virus. Clin Pharmacol Ther. 1997;62(1):6–14. PubMed http://dx.doi.org/10.1016/S0009-9236(97)90145-4
https://doi.org/10.1016/S0009-9236(97)90145-4
29 Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–42. PubMed http://dx.doi.org/10.1002/hep.26141
https://doi.org/10.1002/hep.26141
30 Kedia S, Bhatt VR, Rajan SK, Tandra PK, El Behery RA, Akhtari M. Benign and Malignant Hematological Manifestations of Chronic Hepatitis C Virus Infection. Int J Prev Med. 2014;5(Suppl 3):S179–92. PubMed
31 Abou El Azm AR, El-Bate H, Abo-Ali L, Mansour N, Ghoraba H, Salem ML. Correlation of viral load with bone marrow and hematological changes in pale patients with chronic hepatitis C virus. Arch Virol. 2012;157(8):1579–86. PubMed http://dx.doi.org/10.1007/s00705-012-1321-z
https://doi.org/10.1007/s00705-012-1321-z
32 Medrano-Casique N, Tong HY, Borobia AM, Carcas AJ, Frías J, Ramírez E. Non-Chemotherapy-Induced Agranulocytosis Detected by a Prospective Pharmacovigilance Program in a Tertiary Hospital. Basic Clin Pharmacol Toxicol. 2015;117(6):399–408. PubMed http://dx.doi.org/10.1111/bcpt.12418
https://doi.org/10.1111/bcpt.12418


